* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download CH19 Aldehydes and Ketones
Survey
Document related concepts
Elias James Corey wikipedia , lookup
Bottromycin wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Aldol reaction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Hydroformylation wikipedia , lookup
Discodermolide wikipedia , lookup
Petasis reaction wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Asymmetric induction wikipedia , lookup
Transcript
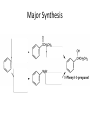
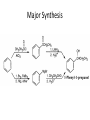
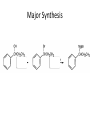
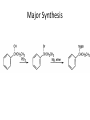
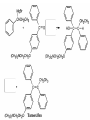
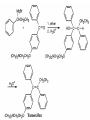
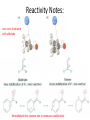
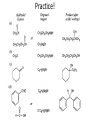
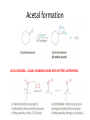
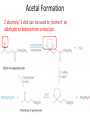
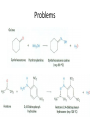
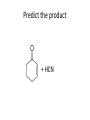
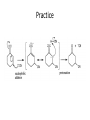
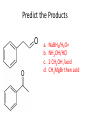
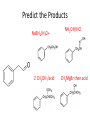
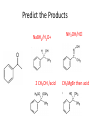
Ketones, Aldehydes CH21 PS CLASS Recall the many times we’ve synthesized these! I command thee. • Oxidation of R-OH – (Periodinane, CrO3/Na2Cr2O7) • Hydration of Alkynes (keto-enol tautomerism) – H3O+/HgSO4, BH3/H2O2,OH-, etc… • Friedel-Crafts ACYLation of Aromatics – (acid halide + AlCl3) REACTIONS OF ALDEHYDES/KETONES • Oxidation of Aldehydes • Nucleophilic Additions (overview) – Hydride (H-) and Grignard (R-) as Nucleophiles – Water addition (hydrate/diol/geminal diol formation) – Alcohol addition (acetal formation) – Amine addition (imine formation) • Conjugate Nucleophilic Addition Reactions Major Synthesis Major Synthesis Major Synthesis Major Synthesis Major Synthesis Major Synthesis Major Synthesis Oxidation of Aldehydes Where [O] = CrO3 among others. Nucleophilic Addition Rxns Slightly different mechanisms in acid or base. Neutral vs. Negatively charged Nucleophile General picture (basic): Reactivity Notes: Less steric hindrance with aldehyde Benzaldehyde less reactive due to resonance stabilization. Reduction of Ketones/Aldehydes Addition of Grignard Reagent Grignard Reagents + carbonyls Practice! COME UP WITH PAIRS, More than one answer for some. Practice! Hydrate formation/ Geminal Diols Basic vs. Acidic BASIC: Strong nucleophile attacks, as in Nu- ACIDIC: Carbonyl is converted to a stronger ELECTROPHILE as CABON BECOMES MORE POSITIVE. Problem Acetal formation ACID CATALYZED… AGAIN, CARBONYL MADE INTO BETTER ELECTROPHILE. Acetal Formation Acetal Formation Acetal Formation 2 alcohols/ 1 diol can be used to ‘protect’ an aldehyde or ketone from a reaction. Nuc. Addition of Amines to form: Imines (bisaya ka dong?) Amine must have 2 protons, RNH2 Problems Predict the Products! Problems Conjugate Nucleophilic Addition Rxns Conjugate Nucleophilic Addition Rxns THE DOUBLE BOND IS EFFECTIVELY “POLARIZED” INTO A NEGATIVE REGION THAT’S RESONANCE STABILIZED, AND A POSITIVE REGION AT THE BETA-CARBON. Predict the product + HCN Practice Predict the Products a. b. c. d. NaBH4/H3O+ NH2OH/HCl 2 CH3OH /acid CH3MgBr then acid Predict the Products NaBH4/H3O+ 2 CH3OH /acid NH2OH/HCl CH3MgBr then acid Predict the Products NaBH4/H3O+ 2 CH3OH /acid NH2OH/HCl CH3MgBr then acid