* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Gas Chromatography: Analyzing Alkene Isomers David L. Flanigan
Survey
Document related concepts
Asymmetric induction wikipedia , lookup
George S. Hammond wikipedia , lookup
Discodermolide wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Kinetic resolution wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Hydroformylation wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Transcript
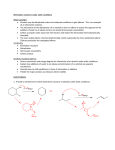
Gas Chromatography: Analyzing Alkene Isomers David L. Flanigan CHM2211L February 20, 2004 Introduction: In this experiment a secondary alcohol (2-methyl-2-butanol) was dehydrated using a dilute solution of sulfuric acid. The reaction resulted in a mixture of alkenes that was purified by distillation and analyzed using gas chromatography. Theoretical Background: Alcohols can be dehydrated using catalytic acid conditions to give alkenes. H+ OH R H 2O R Alkenes Alcohols Water Ease of alcohol dehydration follows the general trend: 3° > 2° > 1°. This trend is based on the fact that most acid catalyzed alcohol dehydrations proceed through the E1 mechanism. In order for the elimination to occur a good leaving group and a base sufficiently strong enough to remove a β-proton must be present. The hydroxyl group is not a good leaving group. To enhance its leaving potential it must be transformed into a good leaving group. This is accomplished by using strong mineral acids. The strong acid donates a proton (Brønstead-Lowry acid) to the oxygen of the hydroxyl group which is the proton acceptor (Brønstead-Lowry base). R O H OH2 H R poor LG H O H H2O good LG Good leaving groups must be weak bases after leaving and water is a good example. In the presence of heat and an ionizing solvent the leaving group departs and a carbocation is formed. This is the aspect of the dehydration that allows 3° alcohols to dehydrate easier than 1° based on ease of carbocation formation. H O R -H2O H R carbocation When the carbocation is formed the β-proton is now activated toward removal resulting in elimination H R H2O R alkene The β-proton is removed by water forming the alkene and regenerating the acid catalyst. Some alcohols can form mixtures of alkenes upon dehydration. For example, when 2-methyl-2-butanol is exposed to catalytic acid dehydration conditions two products are formed. They are 2-methyl-2-butene and 2-methyl-1-butene. H3O+ HO 2-methyl-2-butanol 2-methyl-1-butene 2-methyl-2-butene This is caused by two different types of β-proton that are susceptible to removal upon carbocation formation. H 2O Ha Hb Upon removal of proton Ha the disubstituted alkene is formed. When proton Hb is removed the trisubstituted alkene is formed. H 2O Ha Hb The ratio of products formed is based on the stability of alkenes formed. Since the more highly substituted alkene is more stable it is formed as the major product as stated by Saytzeff’s Rule. The product mixture can then be analyzed using gas chromatography to confirm the product distribution. Data and Results: Contents of reaction flask: Product distilled at 28-40°C Theoretical Yield Calculation: 1.0 mL water 0.5 mL conc. H2SO4 1.1 mL of 2-methyl-2-butanol (den. = 0.805, MW = 88.15) 1.1 mL alcohol X 0.805 g alcohol 1 mL alcohol X 1 mol alcohol 88.15 g alcohol Theoretical Yield: Actual Yield : X 1 mol alkene 1 mol alcohol X 70.1 g alkene 1 mol alkene = 0.70 g alkene 0.70 g alkene mixture 0.52 g alkene mixture Percent Yield Calculation: % Yield = 0.52 g alkene mix 0.70 g alkene mix % Yield: Boiling Point: GC Data: 2-methyl-1-butene 2-methyl-2-butene X 100 = 74% 74% 2-methyl-1-butene 2-methyl-2-butene RT 3.489 4.176 31 °C 39 °C Area Area % 25961682 16.375 132578322 83.625 Discussion/Conclusion: When 2-methyl-2-butanol is subject to acid catalyzed dehydration conditions two alkene products are formed. Based on the gas chromatography data the ratio of 2-methyl-2-butene to 2-methyl-1-butene is approximately 84:16. It was predicted that two different alkene products would form upon dehydration of an alcohol and that the more substituted alkene would be the major product formed. This data also confirms that Saytzeff’s Rule holds true.