* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 1 - UPenn School of Engineering and Applied Science
Survey
Document related concepts
Subventricular zone wikipedia , lookup
Multielectrode array wikipedia , lookup
Optogenetics wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Brain-derived neurotrophic factor wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Neural engineering wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Development of the nervous system wikipedia , lookup
Node of Ranvier wikipedia , lookup
Neuroanatomy wikipedia , lookup
Synaptogenesis wikipedia , lookup
Transcript
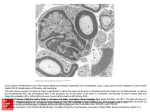
Introduction Historically, the challenge of spinal cord injury (SCI) was seemingly insurmountable until experiments by Aguayo and David 1 challenged the myth that neurons of the central nervous system (CNS) were not capable of regeneration as were neurons of the peripheral nervous system (PNS). In recent years, research has bolstered the idea that the central nervous system (CNS) can regenerate and has made clinical therapy of CNS damage not an unreasonable goal for the next 20 years. A cursory review of the CNS regeneration literature identified two general themes in current research. First, successful CNS regeneration requires the promotion of pro-regeneration factors and inhibition of anti-regeneration factors. Second, practical therapies will depend on a multi-step process to restore CNS function. Philip Horner and Fred Gage identified some of the crucial steps needed to repair damaged axons and restore function: 1) damaged neurons must be viable when treatment is given, 2) axonal growth and extension must be encouraged, 3) repaired axons must enervate the appropriate target effector cells and 4) synaptic structure and function must be repaired5. Strategies for CNS regeneration are just beginning to explore combinations of the steps that will reverse the damaged CNS. Spinal cord injury (SCI) injury can be classified into two modes: acute injury in which blunt trauma compresses or severs the spinal cord and gradual injury in which disease or injury initiates a complex biochemical pathway that ends in CNS impairment. Girardi et al. called these modes of CNS injury primary and secondary injury cascades7. In fact, Horner and Gage recognized that secondary injury would be more difficult to treat because of “chronic pathological sequelae.”5 Accordingly, CNS damage that results in loss of specific cell populations without chronic pathology has been the most commonly used model for CNS damage in animals. These damaged neuronal cells (and accessory cells) must be enticed to repair themselves otherwise the damaged cells will undergo cell death and result in a net loss in the total number of cells. Experimentally, several types of cells have been used to encourage neuronal cell survival in animal models. They include fetal tissue grafts, embryonic stem cells, multi-potent stem cells (from adults), Schwann cells (that are normally found in the peripheral nervous system but not the CNS), and olfactory ensheathing cells. Another important area of research that must be applied to CNS regeneration is the study of the normal biochemical signaling that occurs in adult neurons to maintain cell number and integrity. There is strong data that suggested neurotrophic factors (NTFs) provide pro-survival signals to neurons and that deprivation of these signals results in cell death, primarily through apoptosis. For example, deprivation of neurotrophic factors (NTFs) resulted in apoptosis, but, over-expression of Bcl-2, an anti-apoptotic protein, rescued these neurons from cell death2. NTFs that stimulate axonal growth have been used in many different models to facilitate CNS regeneration. One of the major problems using NTFs is simply the number neurotrophic protein families and the number of molecules in these families. Horner and Gage listed ten families of proteins, each family with several members, that have been studied as potential candidates for nerve regeneration. In addition, it is known that different glial cells secrete different NTFs suggesting differential utilization of these factors by different cell subpopulations. This complexity means that the identities, the combinations, and the concentrations of these factors need to be studied and may not be the same for all types of CNS pathology. Unfortunately, no complete picture exists of which NTFs are required and which are dispensable, what combinations of NTFs will be most efficacious, what concentrations of NTFs are necessary, and what temporal requirements exist for the NTFs. Obviously, the fewer the factors needed, the more practical and more cost effective the regeneration strategy will be, but, it is not at all certain that such a simple solution is possible. Review of Papers In two papers3,4, Xu et al. introduced and then expanded on a model of unilateral CNS repair using an acrylonitrile/vinyl chloride mini-channel that was seeded with Schwann cells from the sciatic nerve in a rat model. The authors created a hemisected model of CNS damage by cutting the right half of the spinal cord at the eighth thoracic vertebra. The therapeutic approach included restoration of the dura, a thin membrane that surrounds the CNS, as one feature that distinguished this approach from others. The authors argued that this restored the physiological environment of the CNS and may contribute to the success of the graft by restoring cerebrospinal fluid circulation. Because 50-60% of CNS injuries in humans occur in the cervical region7, mini-channels are a viable treatment strategy, compared to larger channels, because of the limited space available in the cervical region of the spinal cord. In the first paper, Xu at al. demonstrated that the Schwann cell-seeded mini-channel system (and the factors secreted by the transplanted Schwann cells) allowed axons from transected neurons to grow into and through the mini-channel, emerge on the other side of the mini-channel, and integrate into the caudal segment of the rat CNS. Unfortunately, the number of axons capable of performing these steps was too few to expect significant restoration of motor and/or sensory function lost due to the hemi-section. Therefore, the authors hypothesized that the addition of NTFs, which have demonstrated the ability to induce axonal growth and regeneration in various models, would improve the model by increasing the number of axons in the mini-channel and the host spinal cord. In the first paper, a non-degradable mini-channel plus extracellular matrix or the mini-channel plus extracellular matrix plus transplanted Schwann cells were the two experimental groups. In the second paper, combinations of NTFs were delivered to the caudal side of the mini-channel graft using mini-osmotic pumps, in addition to the seeded mini-channels. This review will focus on the data from the second paper but refer to data from the first paper when it contributes to the discussion. Five experimental groups were included in the second paper: Experimental Group Mini- Matrigel Schwann channel BDNF$ NT-3& PBS Number of cells MG@ + BDNF/NT-3 SC# + PBS SC + BDNF SC + NT-3 SC + BDNF/NT-3 Animals 4 4 4 3 8 (@=Matrigel, #=Schwann cell, $=brain derived neurotrophic factor, &=neurotrophin-3) Photographs of the critical, sequential steps in the surgical procedure were shown in Figure 2. The spinal cord was exposed after careful layer-by-layer dissection of the area between the seventh and the ninth thoracic vertebrae. The spinal cord was exposed (after the dura was carefully preserved for suturing at the end of the operation) and the spinal cord was transected which resulted in a 2.5-2.8 mm lesion. The lesion was located at the eighth thoracic vertebra. The mini-channel graft was transplanted into the lesion site and the two stumps of the spinal cord were placed into the open ends of the 3 mm mini-channel. Next, an Alset miniosmotic pump cannula was placed 2.5 mm from the caudal end of the minichannel. The Alset mini-osmotic pumps were designed to release 5 ug of factor(s)/day for a total of 28 days (0.83 ug/ul of human brain derived neurotrophic factor [BDNF] and neurotrophin-3 [NT-3]). Although human BDNF and NT-3 were administered to the rats, sequence comparison, using the BLAST algorithm, of BDNF (gi 6978569 for rat and gi 4502393 for human) and NT-3 (gi 205774 for rat and gi 189303 for human) amino acid sequences confirmed 95% sequence identity. Finally, all the dissected layers were closed with 10-0 silk suture. Thirty days after mini-channel transplantation, histology of transverse sections from the middle of the mini-channels demonstrated that new tissue had grown into the channel, creating what the authors called a tissue cable. This cable represented Schwann cells transplanted with the mini-channel but also in-growth of new cells and cellular structures. Normally, an individual nerve fiber (one single neuron) is located in a bundle with many other nerve fibers. (A nerve is a actually a bundle of nerve fibers.) A nerve fascicle is the next level of organization and consists of a bundle of nerve fibers surrounded by the perineurium. The perineurium consists of concentric layers of flattened cells that alternate with layers of fine, collagenous, longitudinal fibers. The fascicle is easily identified histologically. The next level of organization is the epineurium that consists of multiple nerve fascicles or a nerve trunk encapsulated by connective tissue, blood vessels, and lymphatics6. Gross histology in Figure 3 A showed that the tissue cable in the channel was well vascularized and recapitulated an epineurium-like structure, including multiple fascicles, blood vessels, and lymphatics. Magnification of the transverse section in Figure 3 B showed individual myelinated axons surrounded by connective tissue. In the first paper, the authors included a photomicrograph of normal epineurium that was more organized than the tissue cables in the mini-channels. So even though many of the features of the epineurium were present, there was a visible difference in the degree of organization between the tissues. The electron micrograph in Figure 5 A confirmed the presence of myelinated axons and unmyelinated axons in the mini-channel tissue cable. In the first paper, pre-labeling of transplanted Schwann cells demonstrated that the Schwann cells located in the mini-channel were from donor cells not host cells. So, presumably, the Schwann cells that surround the axons that have grown into the mini-channel, depicted in Figure 5 C, were from transplanted Schwann cells rather than host cells, although the data is not shown. In the next experiment, the numbers of axons that grew into the minichannels from the various experimental groups were quantified by simply counting the number of axons. The results from the first and second paper are summarized in the table below: Paper Experimental Group Avg. # of myelinated axons SEM Number of Animals First MG 185 72.3 4 SC 1004 126 11 10 6 4 SC + PBS 734 139 4 SC + BDNF 907 121 4 SC+ NT-3 861 254 4 SC + BDNF/NT-3 935 323 3 Second MG + BDNF/NT-3 The MG + BDNF/NT-3 group only had an average of 10 axons in the mini-channel which was much smaller than the findings in the first paper. No explanation for this observation was given, although the two groups are not strictly identical. The Schwann cells were critical for attracting the damaged axons into the mini-channel, even though these cells are not native to the CNS. However, the addition of either BDNF (907) or NT-3 (861) did not significantly increase the average number of axons in the mini-channel compared to the SC + PBS control (734). Also, the combination of the BDNF and NT-3 (935) did not function in an additive manner much less a synergistic manner, on the average number of axons in the minichannel. Although the NTFs were added to the caudal side of the mini-channel with the hope of increasing the number axons in the mini-channel, the NTFs, alone or in combination, did not significantly increase the number of axons located in the mini-channel. Next, anterograde transport was used to measure the extent of axon integration into the host spinal cord on the caudal side of the graft. Two compounds were used for anterograde transport, Phasellus vulgaris- leucoagglutinin (PHA-L) and biotinylated-dextran amine (BDA). The compounds were injected 3 mm from the rostral end of the mini-channel and traveled in the anterograde direction, down the axon from the cell body of the neuron towards the synapse. A partial summary of the anterograde transport experiments from the first and second papers is in the table below: Paper Experimental Group Max. Distance (mm) Numbers of Animals First MG ~0 4 of 4 SC ~3 4 of 101 MG + BDNF/NT-3 ~0 N.I.C. SC + PBS Limited N.I.C. SC + BDNF ~6 2 of 4 SC + NT-3 ~4.5 2 of 4 SC + BDNF/NT-3 ~6 4 of 5 Second N.I.C.=not indicated clearly 1: This represents two different groups, each group receiving PHA-L at different times. During the anterograde transport studies, the left side of the spinal cord was transected prior to administration of PHA-L or BDA to prevent the compounds from traveling around the mini-channel rather that through the mini-channel. Histology demonstrated the absence of stained axons on the left hemi-section of the spinal cord. In the first paper, the maximum distance of axon integration, measured from the caudal end of the channel, was ~3 mm. In the second paper, with the addition of NTFs, alone or in combination, the average distance was increased approximately two-fold. In the SC + BDNF/NT-3 group, there was a steep drop-off in the number of axons as the distance from the caudal end of the mini-channel increased. However, Figure 9 also clearly showed the substantial increase in the maximum distance traveled. Furthermore, the axons actually extended beyond the location of the osmotic pump cannula (2.5 mm from the caudal end of the mini-channel) by approximately 3.5 mm, suggesting that the distance may be increased further if the NTF gradient can be provided over longer distances. In addition to distance measurements, some observations about the axonal structures were made. In the SC + NT-3 group (Figure 6), anterograde-transport staining showed axonal arborization or branching of the terminal ends of the axons. The staining also identified bouton-like bodies consistent with synapse formation. In the SC + BDNF group (Figure 7), anterograde-transport staining also identified bouton-like structures. The authors also noted fascicle-like structures in this group using toluidine blue staining. Unfortunately, no staining for known neurotransmitters was included in the paper. This relatively simple experiment would have reinforced the argument that the bouton-like structures were consistent with synapse formation. Also, Figure 7 H identified a particularly long axon stained in the host spinal cord. The tissue is a three-dimensional object but the histological sections only represent two dimensions. Transport studies using a dye or compound monitored using three dimensional radiological equipment would be an exciting way to track the length and continuity of the axons that re-integrate into the host spinal cord. This experiment would be more informative but may be impractical. Finally in the SC + BDNF/NT-3 group (Figure 8), staining identified bundles longitudinal axons in a fascicle-like structure. Normally, neurons or nerve fibers are bundled together in a fascicle, so Figure 8 D demonstrated the recapitulation of this level of CNS structure. (See Figure 10 for a surprisingly simple but informative summary of the data.) Conclusions The progression of any research area is composed of small incremental changes published in the scientific literature, until a critical mass is reached when a more complete understanding is achieved. Although the critical mass in CNS regeneration research has not been reached, Xu et al. have created an exciting model of CNS regeneration that provided a few new steps in the process. The Schwann cell-seeded mini-channels with NTFs provided survival signals to the transected neurons and promoted axonal repair and growth. Although the addition of NTFs did not increase the number of axons in the mini-channel, it did increase the distance the axons integrated into the host spinal cord. Some questions still need to be addressed. For example, the authors noted that only anterograde transport was used in the second paper because the majority of axons in the minichannel were derived from neurons on the rostral side of the mini-channel. Can improvements be made to increase the numbers of axons from neurons on the caudal side of the mini-channel? Were the concentration and duration of NTFs physiologically relevant? How long do the re-integrated axons survive in the host spinal cord? Do the bouton-like bodies secrete neurotransmitters? How can function be assessed in this experimental model? What other NTFs could be used in this system? Since Schwann cells were required to attract damaged axons into the mini-channel, would concatenation of the seeded mini-channels attract the axons exiting the caudal end of one mini-channel into the adjacent mini-channel? These and other questions need answers if Horner and Gages’ prediction of clinical trials in ten years is to come true. End Notes 1. Axonal Elongation into Peripheral Nervous System “Bridges” after Central Nervous System Injury in Adult Rats. Science. 214: 931-933, 1981. 2. The Relationship Between Neuronal Survival and Regeneration. Annual Review of Neuroscience. 23:579-612, 2000. 3. Regrowth of Axons into the Distal Spinal Cord through a Schwann cell-seeded Mini-channel Implanted into Hemisected Adult Rat Spinal Cord. European Journal of Neuroscience. 11: 1723-1740, 1999. 4. Neurotrophins BDNF and NT-3 Promote Axonal Re-entry into the Distal Host Spinal Cord through Schwann Cell-seeded Mini-channels. European Journal of Neuroscience. 13:257-268, 2001. 5. Regenerating the Damaged Central Nervous System. Nature. 407: 963-970, 2000. 6. Stedman’s Medical Dictionary. 25th Ed. 1990. Williams and Wilkins, Baltimore, MD. 7. Advances and Strategies for Spinal Cord Regeneration. Tissue Engineering in Orthopedic Surgery. 31: 465-471, 2000. Progress in a Schwann cell-seeded Mini-channel Model of CNS Regeneration Eugene H. Kang BE553 Dr. Keith Gooch Friday 27 April 2001 Included articles: Regrowth of Axons into the Distal Spinal Cord through a Schwann cell-seeded Mini-channel Implanted into Hemisected Adult Rat Spinal Cord. European Journal of Neuroscience. 11: 1723-1740, 1999. Neurotrophins BDNF and NT-3 Promote Axonal Re-entry into the Distal Host Spinal Cord through Schwann Cell-seeded Mini-channels. European Journal of Neuroscience. 13:257-268, 2001.