* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download DNA methylation
United Kingdom National DNA Database wikipedia , lookup
Genealogical DNA test wikipedia , lookup
X-inactivation wikipedia , lookup
Human genome wikipedia , lookup
DNA damage theory of aging wikipedia , lookup
Genomic library wikipedia , lookup
No-SCAR (Scarless Cas9 Assisted Recombineering) Genome Editing wikipedia , lookup
Genetic engineering wikipedia , lookup
Genome evolution wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Epitranscriptome wikipedia , lookup
DNA vaccination wikipedia , lookup
Point mutation wikipedia , lookup
Molecular cloning wikipedia , lookup
Nucleic acid double helix wikipedia , lookup
Long non-coding RNA wikipedia , lookup
DNA supercoil wikipedia , lookup
Cre-Lox recombination wikipedia , lookup
Genomic imprinting wikipedia , lookup
Oncogenomics wikipedia , lookup
Cell-free fetal DNA wikipedia , lookup
Extrachromosomal DNA wikipedia , lookup
Histone acetyltransferase wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Non-coding DNA wikipedia , lookup
Designer baby wikipedia , lookup
Epigenetics of depression wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Genome editing wikipedia , lookup
DNA methylation wikipedia , lookup
Primary transcript wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Microevolution wikipedia , lookup
Epigenetic clock wikipedia , lookup
History of genetic engineering wikipedia , lookup
Helitron (biology) wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Bisulfite sequencing wikipedia , lookup
Transgenerational epigenetic inheritance wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Epigenetics of diabetes Type 2 wikipedia , lookup
Behavioral epigenetics wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Cancer epigenetics wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Epigenetics wikipedia , lookup
Epigenetics in stem-cell differentiation wikipedia , lookup
Epigenetics in learning and memory wikipedia , lookup
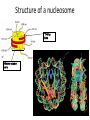
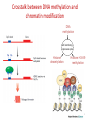
REGULATION OF GENE EPRESSION IN EUKARYOTES The organizational structure of an eukaryotic cell determines the mode of gene regulation: Chromatin packaging into nucleosomes and other organized structures → possible control at the chromatin structure level Compartmentalization of the cell→ need of internal signaling system to communicate between different compartments Multicellular organism→ need of intercellular communication system Differentiation of a totipotent cell into different cell types during body formation → spatial and temporal regulation The organizational structure of an eukaryotic cell determines the mode of gene regulation : Due to organizational characteristics of eukaryotic cell and organism, and the spatial and temporal separation of transcription and translation, the regulation of gene expression in eukaryotes can be exerted at more levels than in prokaryotes,. CONTROL AT THE LEVEL OF CHROMATIN AND GENOME STRUCTURE Chromatin structure and organization fundamentally affect gene expression by changing the chromatin structure, especially its compaction state. The degree of chromatin compaction essentially relies on histone modifications and DNA methylation. Active chromatin regions usually contain high rate of acetylated histones and unmethylated DNA whereas inactive regions are associated with nonacetylated histones and methylated DNA. Histone modifications and DNA methylation constitute the base of a special mechanism of gene expression control called epigenetic inheritance. Epigenetic inheritance refers to inherited gene expression pattern independent of modifications in DNA sequence. It concerns alternative heritable expression of genes that occur throughout the whole life of an organism and usually expand to its offspring. Epigenetic inheritance is essential to the normal development of eukaryotes. Some phenomena considered as epigenetic regulation involve X chromosome inactivation and genomic imprinting. Epigenetic inheritance is crucial for normal embryonic development, plays important roles in cancerogenesis and other biological processes. What is Epigenetics? • Study of heritable changes in gene function that do not involve changes to the nucleotide sequence of DNA • When a cell undergoes mitosis or meiosis, the epigenetic information is stably transmitted to the subsequent generation • Epigenetic controls add an ‘extra layer’ of transcriptional control Epigenetics was coined by C. H. Waddington in 1942. Epigenesis is an old word which has more recently been used to describe the differentiation of cells from their initial totipotent state in embryonic development. When Waddington coined the term the physical nature of genes and their role in heredity was not known; he used it as a conceptual model of how genes might interact with their surroundings to produce a phenotype. Robin Holliday defined epigenetics as "the study of the mechanisms of temporal and spatial control of gene activity during the development of complex organisms.“ Thus epigenetic can be used to describe anything other than DNA sequence that influences the development of an organism. The molecular basis of epigenetics is complex. It involves modifications of the activation of certain genes, but not the basic structure of DNA. Additionally, the chromatin proteins associated with DNA may be activated or silenced. This accounts for why the differentiated cells in a multi-cellular organism express only the genes that are necessary for their own activity. Epigenetic changes are preserved when cells divide. Most epigenetic changes only occur within the course of one individual organism's lifetime, but, if a mutation in the DNA has been caused in sperm or egg cell that results in fertilization, then some epigenetic changes are inherited from one generation to the next. This raises the question of whether or not epigenetic changes in an organism can alter the basic structure of its DNA, a form of Lamarckism. Specific epigenetic processes include paramutation, bookmarking, imprinting, gene silencing, X chromosome inactivation, position effect, reprogramming, transvection, maternal effects, the progress of carcinogenesis, many effects of teratogens, regulation of histone modifications and heterochromatin, and technical limitations affecting parthenogenesis and cloning. Epigenetic research uses a wide range of molecular biologic techniques to further our understanding of epigenetic phenomena, including chromatin immunoprecipitation, fluorescent in situ hybridization, methylation-sensitive restriction enzymes, DNA adenine methyltransferase identification (DamID) and bisulfite sequencing. Furthermore, the use of bioinformatic methods is playing an increasing role (computational epigenetics). Three major epigenetic processes we will discuss • DNA Methylation • Histone modifications • RNA-mediated phenomena DNA methylation is a biochemical process that is important for normal development in higher organisms. It involves the addition of a methyl group to the 5 position of the cytosine pyrimidine ring or the number 6 nitrogen of the adenine purine ring (cytosine and adenine are two of the four bases of DNA). This modification can be inherited through cell division. DNA methylation is a crucial part of normal organismal development and cellular differentiation in higher organisms. DNA methylation stably alters the gene expression pattern in cells such that cells can "remember where they have been" or decrease gene expression; for example, cells programmed to be pancreatic islets during embryonic development remain pancreatic islets throughout the life of the organism without continuing signals telling them that they need to remain islets. DNA methylation is typically removed during zygote formation and reestablished through successive cell divisions during development. However, the latest research shows that hydroxylation of methyl group occurs rather than complete removal of methyl groups in zygote. DNA Methylation Most well-studied epigenetic tag/mark; best understood epigenetic cause of disease Conserved across various kingdoms of life SAM – S-adenosylmethionine SAH – S-adenosylhomocystine So, G, A, T, C…. and the fifth base, mC in mammalian genome 13 Distribution of DNA methylation • In mammals, in the context of CpG dinucleotides (plants have other types too) • Methylated CpGs are associated with silenced DNA, eg. Transposons, inactive X chromosome, imprinted genes • “CpG islands”, associated with promoters of 40% of mammalian genes, are generally free of methylation eg. housekeeping genes, tissue-specific genes 14 DNA methyltransferases (DNMTs) 2 major classes of enzymes in mammalian systems De novo methylases Maintenance methylase Mouse knockouts of these genes tell us they are necessary for the survival and proper development of the organism. 15 In mammalian cells, DNA methylation occurs mainly at the C5 position of CpG dinucleotides and is carried out by two general classes of enzymatic activities – maintenance methylation and de novo methylation. Maintenance methylation activity is necessary to preserve DNA methylation after every cellular DNA replication cycle. Without the DNA methyltransferase (DNMT), the replication machinery itself would produce daughter strands that are unmethylated and, over time, would lead to passive demethylation. DNMT1 is the proposed maintenance methyltransferase that is responsible for copying DNA methylation patterns to the daughter strands during DNA replication. It is thought that DNMT3a and DNMT3b are the de novo methyltransferases that set up DNA methylation patterns early in development. DNMT3L is a protein that is homologous to the other DNMT3s but has no catalytic activity. Instead, DNMT3L assists the de novo methyltransferases by increasing their ability to bind to DNA and stimulating their activity. Finally, DNMT2 (TRDMT1) has been identified as a DNA methyltransferase homolog, containing all 10 sequence motifs common to all DNA methyltransferases; however, DNMT2 (TRDMT1) does not methylate DNA but instead methylates cytosine-38 in the anticodon loop of aspartic acid transfer RNA. How does DNA methylation affect gene transcription? Unmethylated (or hypomethylated) promoter allows gene transcription Methylated CpGs block binding of TFs; hence, transcription blocked Me-CpG binding proteins also preclude TF binding to the promoter region Other ways too… 18 Role of DNA methylation • Tight control for maintaining gene silencing (vertebrate genes are less “leaky” compared to bacterial) • Transcriptional silencing of transposons (‘genome defense’ model) • Genomic imprinting – one of the alleles of a gene is silenced, depending on the parent of origin • X inactivation – all but one of the X chromosomes in female is inactivated – methylation of the inactive X copy 19 Three major epigenetic processes • DNA Methylation • Histone modifications • RNA-mediated phenomena 20 For gene expression, eukaryotic DNA must be decompacted to become accessible to transcription initiators. The decompaction process is ensured by nucleosome modifiers. Nucleosome modifiers are classified into two groups : 1. Enzymes that modify the amino-terminal tails of histones such as histone deacetylases, histone acetylases and histone methyltransferases 2. Remodeler complexes that “loosen” the interaction between DNA and histones Modifications of the chromatin can activate gene expression by two ways : 1. “Loosening” the chromatin structure, thus liberating binding sites for regulatory proteins 2. Enhancing the binding of some particular regulatory proteins to the modified chromatin Structural organization of the genome Unless the genome is accessible by the transcription machinery of the cell, the genome cannot be functional! Hence, the utilization of the biological information in the genome is dependent on the chromatin organization. 23 Structure of a nucleosome ~146 bp DNA Histone octamer core 24 Post-translational histone modifications The amino-terminal tails of nucleosomal histones protrude from the DNA and are subject to covalent modifications. These modifications include lysine acetylation, lysine and arginine methylation, serine and threonine phosphorylation, ADP-ribosylation, and ubiquitination. Histone lysine methylation can have different effects depending on the residue that is modified: methylation of histone H3 at Lys4 (H3K4) is associated with gene activation, whereas methylation of H3K9, H3K27, and H4K20 generally correlates with transcriptional repression. The roles of H3K36 and H3K79 methylation remain elusive; indeed, these modifications are associated with both transcriptional activation and repression. Different modifications of histone amino-terminal tails constitute the so-called 'histone code‘ . Indeed, specific combinations of histone modifications can alter chromatin structure to allow transcription or to repress it, either reversibly or stably. Chromatin modifications confer a unique identity on the nucleosomes involved. The composite pattern of modifications regulates the binding and activities of other chromatin-associated components. Indeed, modifications of histones at a specific nucleosome very likely influence subsequent modifications, regulated by both cis and trans mechanisms. Characterizing such modifications could provide insight into the roles of chromatin-binding proteins Post-translational histone modifications A = acetylation M = methylation P = phosphorylation U = ubiquitination 27 Consequences of tail modifications • Higher order chromatin structure is affected eg. Addition of acetyl groups (-ve) neutralizes the positive charge on lysine => affinity of the histone to bind tightly to DNA is reduced => chromatin becomes less compact => transcription of the associated gene is favored Vice versa for deacetylation (the gene is repressed) • Other proteins are attracted to these sites of odifications….which, in turn, affect gene expression 28 Enzymes catalyze these covalent tail modifications • Histone Acetyl Transferases (HATs) function as large, multiprotein complexes, eg. SAGA, ADA complexes (yeast), TFTC complexes (humans); associated with transciptional activation. • Histone Deacetylases (HDACs) part of multiprotein complexes, eg.Sin3, NuRD; associated with transcriptional repression. • Histone Methyl Transferases (HMTs) • Histone Demethylases 29 Comparing chromatin types Transcriptionally active chromatin/euchromatin Transcriptionally inactive chromatin/ heterochromatin Chromatin conformation Open, extended conformation Highly condensed conformation DNA CpG methylation Relatively unmethylated, especially at promoter regions Methylated, including at promoter regions Histone acetylation Acetylated histones Deacetylated histones Histone methylation H3-K4me3, R17me2 H3-K9me 30 Crosstalk between DNA methylation and chromatin modification DNA methylation Self-reinforcing repressive cycle Histone deacetylation Histone H3-K9 methylation 31 Three major epigenetic processes • DNA Methylation • Histone modifications • RNA-mediated phenomena 32 RNA interference (RNAi) is a system within living cells that takes part in controlling which genes are active and how active they are. Two types of small RNA molecules – microRNA (miRNA) and small interfering RNA (siRNA) – are central to RNA interference. RNAs are the direct products of genes, and these small RNAs can bind to other specific RNAs (mRNA) and either increase or decrease their activity, for example by preventing a messenger RNA from producing a protein. RNA interference has an important role in defending cells against parasitic genes – viruses and transposons – but also in directing development as well as gene expression in general. RNA interference (RNAi) causes gene silencing RNAi initiates heterochromatin formation in fission yeast and DNA methylation in plants. 34 Epigenetics in human disease Association with various cancers – stomach, kidney, colon, pancreas, liver, uterus, lung and cervix ICF syndrome Fragile X syndrome Angelman’s syndrome Rett Syndrome HUMAN “EPIGENOME” PROJECT Coffin-Lowry Syndrome 35 BIOLOGICAL MEANINGS OF EPIGENETIC INHERITANCE Epigenetic inheritance play crucial roles in normal growth and development of multicellular eukaryotic organisms : In embryonic development, epigenetic abnormalities can lead to genetic disorders such as Prader Willi and Angelman syndromes. Babies with Prader-Willi and Angelman syndromes are born with both alleles expressed, an abnormal active paternal allele (Prader-Willi) or an abnormal active maternal allele (Angelman) of the same gene. In Assisted Reproductive Technologies, epigenetic inheritance is thought to be associated with abnormal embryonic development due to loss of maternal/paternal selective allele expression and high rate of embryonic losses. It is thought that imprinting is a tentative of the mother to protect herself from her fetus. Silencing of maternal alleles limit the fetus growth. DNA hypermethylation cause tumor suppressot gene silencing whereas DNA hypomethylation favorize oncogene expression. These are the cause of many cancer types, e.g the aberrant methylation pattern of Igf2 and H19 genes give rise to simultaneous expression of maternal and paternal alleles and are the cause of many human cancers. Demethylating agents and agents promoting histone acetylation constitute possible therapeutic approaches for certain cancers. Epigenetic control is thought to be used by cells to silencing some regions in the genome containing repetitive “useless” DNA, e.g inserted “foreign” (viral) sequences (transposon). Most of these transposons are methylated