* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download respiration_DSE_revi..
Butyric acid wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Mitochondrion wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Electron transport chain wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Phosphorylation wikipedia , lookup
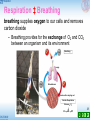
Basal metabolic rate wikipedia , lookup
Photosynthesis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Biochemistry wikipedia , lookup
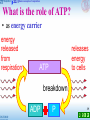
Microbial metabolism wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
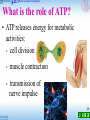
1 Food like corn can provide energy for the body. 2 The sugar in it can also undergo fermentation (發酵) to produce an alcohol. 3 The alcohol can be used as a fuel to power vehicles. 4 1 How does our body obtain energy from the food we eat 5 2 How is alcohol produced from corn by fermentation 6 3 The sugar in corn is made by photosynthesis. What is the relationship between respiration and photosynthesis 7 22.1 Basic concepts of respiration What is respiration? 8 22.1 Basic concepts of respiration What is respiration? • when food is burnt, it reacts with oxygen (oxidation 氧化): heat O2 light CO2 + H2O glucose 9 22.1 Basic concepts of respiration What is respiration? • when food is burnt, it reacts with oxygen (oxidation 氧化): - one step reaction - takes place anywhere - no enzyme involved - fast and violent reaction 10 22.1 Basic concepts of respiration What is respiration? • the large amount of heat released in burning kills living cells organisms undergo respiration (呼吸作用) the process by which organisms release energy from food through the controlled oxidative breakdown of food 11 Photosynthesis and respiration provide energy for life Sunlight energy – Cellular respiration makes ATP and consumes O2 – During the oxidation of glucose to CO2 and H2O ECOSYSTEM Photosynthesis in chloroplasts CO2 Glucose + + H2O O2 Cellular respiration in mitochondria ATP (for cellular work) Heat energy 12 Respiration ‡ Breathing breathing supplies oxygen to our cells and removes carbon dioxide – Breathing provides for the exchange of O2 and CO2 between an organism and its environment O2 Breathing CO2 Lungs CO2 Bloodstream O2 Muscle cells carrying out Cellular Respiration Glucose + O2 Figure 6.2 CO2 + H2O + ATP 13 22.1 Basic concepts of respiration What is respiration? • glucose is the most common substrate heat ATP O2 CO2 + H2O glucose (in the cell) 14 22.1 Basic concepts of respiration What is respiration? • respiration: - a series of reactions - takes place in all living cells all the time - controlled by many enzymes - slow and gradual reactions 15 22.1 Basic concepts of respiration What is respiration? • overall equation: glucose C6H12O6 Glucose + 6 CO2 O2 enzymes O2 Oxygen gas 6 CO2 Carbon dioxide H2O + 6 H2O Water energy + ATPs Energy 16 The human body uses energy from ATP for all its activities ATP powers almost all cellular and body activities Table 6.4 17 22.1 Basic concepts of respiration What is the role of ATP? • as energy carrier energy released from respiration ATP phosphorylation (磷酸化) P ADP 18 22.1 Basic concepts of respiration What is the role of ATP? • as energy carrier energy released from respiration releases energy to cells ATP breakdown ADP P 19 22.1 Basic concepts of respiration What is the role of ATP? • ATP releases energy for metabolic activities: - cell division - muscle contraction - transmission of nerve impulse 20 22.1 Basic concepts of respiration What is the role of ATP? • ATP releases energy for metabolic activities: - synthesis of biomolecules amino acids protein - absorption of food molecules or minerals by active transport 21 22.1 Basic concepts of respiration Types of respiration 1 Aerobic respiration (需氧呼吸) • requires oxygen • glucose is completely broken down • a large amount of energy is released 22 22.1 Basic concepts of respiration Types of respiration 2 Anaerobic respiration (缺氧呼吸) • does not require oxygen • glucose is only partly broken down • much less energy is released • products are different from aerobic respiration 23 22.2 Site of respiration • some reactions occur in the cytoplasm, some in the mitochondria 24 22.2 Sites of respiration Adaptive features of a mitochondrion 3D model • bound by a double membrane • outer membrane controls the movement of substances outer membrane 25 22.2 Sites of respiration Adaptive features of a mitochondrion • inner membrane is highly folded provides a large surface area to pack more enzymes inner membrane 26 22.2 Sites of respiration Adaptive features of a mitochondrion • mitochondrial matrix (基質) provides a fluid medium for reactions to take place • it also contains enzymes mitochondrial matrix 27 22.2 Sites of respiration Adaptive features of a mitochondrion • most energy in food is released inside mitochondria active cells contain many mitochondria muscle cells 28 22.2 Sites of respiration 22.1 2 Identify various structures of the mitochondrion 29 22.3 Aerobic respiration • takes place in the presence of oxygen • three stages: glycolysis (糖酵解) Krebs cycle (克雷伯氏循環) oxidative phosphorylation (氧化磷酸化) 30 Food are highly reduced Cells tap energy from foods by oxidization Energy are tapped when electrons “falling” from organic fuels to oxygen – Electrons lose potential energy • During their transfer from organic compounds to oxygen 31 An overview of cellular respiration NADH High-energy electrons carried by NADH NADH FADH2 and GLYCOLYSIS Glucose Pyruvate CITRIC ACID CYCLE OXIDATIVE PHOSPHORYLATION (Electron Transport and Chemiosmosis) Mitochondrion Cytoplasm ATP Substrate-level phosphorylation CO2 ATP CO2 Substrate-level phosphorylation ATP Oxidative phosphorylation – When glucose is converted to carbon dioxide • It loses hydrogen atoms, which are added to oxygen, producing water Loss of hydrogen atoms (oxidation) C6H12O6 + 6 O2 6 CO2 + Glucose 6 H2O + Energy (ATP) Gain of hydrogen atoms (reduction) Figure 6.5A 33 Dehydrogenase removes electrons (in hydrogen atoms) from fuel molecules (oxidation) • And transfers them to NAD+ (reduction) Oxidation H NAD+ Dehydrogenase Reduction NADH 2H + + 2H Figure 6.5B O + 2H H O + 2e + H+ (carries 2 electrons) 34 – NADH passes electrons to an electron transport chain – As electrons “fall” from carrier to carrier and finally to O2 • Energy is released in small quantities / controlled NADH NAD + H + ATP 2e + Controlled release of energy for synthesis of ATP 2e 2 H + 1 2 O2 H2O 35 22.3 Aerobic respiration Glycolysis • occurs in the cytoplasm • does not require oxygen 36 Glycolysis harvests chemical energy by oxidizing glucose to pyruvate – In glycolysis, ATP is used to energize a glucose molecule – Which is split into two molecules of pyruvate 2 NAD+ 2 NADH + 2 H+ Glucose 2 Pyruvate 2 ADP Figure 6.7A +2 P 2 ATP 37 A summary of glycolysis 38 Bad news: glycolysis involves 10 complicated steps! 39 40 Good news: that’s all you need to know in DSE! • overall equation: glucose (6-C) 2 pyruvate (3-C) 41 Good news: that’s all you need to know in DSE! • overall equation: 2 NAD+ glucose (6-C) 2 NADH 2 pyruvate (3-C) 42 Good news: that’s all you need to know in DSE! • overall equation: 2 NAD+ glucose (6-C) 2 ADP + 2 P 2 NADH 2 pyruvate (3-C) 2 ATP 43 Good news: that’s all you need to know in DSE! • overall equation: 2 NAD+ glucose (6-C) 2 ADP + 2 P 2 NADH 2 pyruvate (3-C) 2 ATP transported to mitochondrion 44 22.3 Aerobic respiration ‘Energy investment’ phase of glycolysis - Breakdown of glucose to triose phosphate glucose (6-C) 2 ATP 2 ADP + P 2 triose phosphate (3-C) 45 22.3 Aerobic respiration ‘Energy payoff’ phase of glycolysis - Oxidation of triose phosphate to pyruvate 2 triose phosphate (3-C) 2 NAD+ 2 NADH 4 ADP + 4 P 4 ATP 2 pyruvate (3-C) 46 22.3 Aerobic respiration Glycolysis - Oxidation of triose phosphate to pyruvate 2 triose phosphate (3-C) 2 NAD+ 2 NADH as hydrogen 4 ATPcarrier 2 pyruvate (3-C) 47 22.3 Aerobic respiration ‘Energy payoff’ phase of glycolysis - Production of ATP 2 triose phosphate (3-C) 4 ADP + 4 P 4 ATP 2 pyruvate (3-C) 48 Glycolysis produces ATP by substrate-level phosphorylation high energy phosphate- carrying molecules are produced in the conversion of TP to pyruvate a phosphate group is transferred from the high energy phosphate- carrying molecules to ADP 49 Substrate level phosphorylation high energy molecule high energy molecule 50 Substrate level phosphorylation high energy molecule lower energy molecule lower energy molecule high energy molecule The conversion of phosphoenolpyruvate to pyruvate is another example of substrate level phosphorylation. 51 Glycolysis Free energy level of intermediates and net energy gain: 52 A summary of glycolysis 53 22.3 Aerobic respiration Glycolysis • overall equation: 2 NAD+ glucose (6-C) 2 ADP + 2 P 2 NADH 2 pyruvate (3-C) 2 ATP transported to mitochondrion 54 The link reaction - before entering the Krebs cycle: When pyruvate enters a mitochondrion it is converted to acetylCoA. Coenzyme A (CoA) is a large molecule (and a vitamin) that acts as a coenzyme. The conversion of pyruvate to acetylCoA is an coupled oxidationreduction reaction in which high energy electrons are removed from pyruvate and end up in NADH. The three carbon pyruvate is split into CO2 and the two carbon acetate. 55 The link reaction - before entering the Krebs cycle: The Link reaction (between glycolysis and Citric acid cycle) – Prior to the citric acid cycle – Enzymes process pyruvate, releasing CO2 and producing NADH and acetyl CoA NAD+ NADH + H+ 2 CoA Pyruvate 1 3 CO2 Acetyl CoA (acetyl coenzyme A) Coenzyme A 56 Fate of pyruvate – with oxygen 57 22.3 Aerobic respiration Krebs cycle • occurs in the mitochondrial matrix • two main steps: 1 Combination of acetyl-CoA with 4-C compound 2 Regeneration of 4-C compound 58 Bad news: Krebs cycles is very complicated! 59 Good news: Krebs cycle can be understood in a simple way! 1 Combination of acetyl-CoA with 4-C compound acetyl-CoA (2-C) 4-C compound CoA 6-C compound 60 22.3 Aerobic respiration Krebs cycle 2 Regeneration of 4-C compound 4-C compound 6-C compound ATP ADP + P FADH FAD 2 CO2 3 NAD+ 3 NADH 61 Krebs cycle CoA For each turn of the cycle Acetyl CoA CoA 2 carbons enter cycle 4C intermediate Two CO2 molecules are released 6C Citric acid NADH + H+ CO2 NAD+ The energy yield is one ATP, CITRIC ACID CYCLE leaves cycle NAD+ 1 4C intermediate NADH ADP + + H+ P 5 FADH2 three NADH, and one FADH2 ATP 2 5C intermediate / alpha-keto acid FAD CO2 leaves cycle 4C intermediate 4 NADH + H+ NAD+ 3 1 2 3 4 5 62 22.3 Aerobic respiration Krebs cycle • each glucose molecule generates two pyruvate molecules a total of six NADH, two FADH2 and two ATP are formed 63 22.3 Aerobic respiration Oxidative phosphorylation • occurs on the inner membrane of the mitochondrion (cristae) • two main steps: 1 Regeneration of NAD+ and FAD 2 Formation of ATP 64 22.3 Aerobic respiration Oxidative phosphorylation 1 Regeneration of NAD+ and FAD intermembrane space inner membrane mitochondrial matrix 65 22.3 Aerobic respiration Oxidative phosphorylation 1 Regeneration of NAD+ and FAD intermembrane space inner membrane e- electron carrier mitochondrial matrix NADH NAD+ 66 22.3 Aerobic respiration Oxidative phosphorylation 1 Regeneration of NAD+ and FAD e- e- NADH NAD+ FADH2 FAD H2O H+ e+ O 67 22.3 Aerobic respiration Oxidative phosphorylation 2 Formation of ATP e- e- NADH NAD+ ADP+P ATP eFADH2 FAD H+ + O H2O 68 22.3 Aerobic respiration Oxidative phosphorylation 2 Formation of ATP • one NADH can generate three ATPs • one FADH2 can generate two ATPs 69 An ad of a pharmaceutical product 70 Vitamins & energy metabolism Which vitamin group and How they are involved in cellular energy metabolism?71 Vitamins Involved in Energy Metabolism Vitamins and minerals Are required for proper metabolism Do not directly provide energy Often function as coenzymes The B-complex vitamins are especially important for energy metabolism. B-complex Vitamins: Thiamin (Vitamin B1) Coenzyme thiamin is required for carbohydrate metabolism Beriberi: deficiency of thiamin resulting in muscle wasting and nerve damage, heart failure B-complex Vitamins: Riboflavin (Vitamin B2) Part of coenzymes involved in oxidation-reduction reactions Milk is a good source of riboflavin B-complex Vitamins: Niacin Nicotinamide and nicotinic acid Coenzyme assists with the metabolism of carbohydrates and fatty acids Good sources: meat, fish, poultry, enriched bread products Toxicity can result from supplements 72 Net Energy Production from Aerobic Respiration 73 Chemiosmosis (reference) – Electrons from NADH and FADH2 • Travel down the electron transport chain to oxygen, which picks up H+ to form water – Energy released by the redox reactions • Is used to pump H+ into the space between the mitochondrial membranes (intermembrane space) 74 22.3 Aerobic respiration Oxidative phosphorylation Formation of ATP…… HOW? e- e- NADH NAD+ ADP+P ATP eFADH2 FAD H+ + O H2O 75 The ‘chemiosmosis’ theory 76 The ‘chemiosmosis’ theory 77 Mitochondrion Structure • This drawing shows a mitochondrion cut lengthwise to reveal its internal membrane. Intermembrane Space Cristae 78 Matrix 78 This drawing shows a close-up of a section of a mitochondrion. Chemiosmotic Phosphorylation H+ H+ Matrix (inside) H+ H+ Intermembrane Space H+ H+ H+ H+ Outside Matrix H+ H+ H+ H+ 79 H+ Pumps within the membrane moves hydrogen ions from the matrix to the intermembrane space creating a concentration gradient. H+ H+ Outside Matrix (inside) H+ H+ H+ 00 H+ H+ H+ H+ H+ Intermembrane Space Matrix H+ H+ H+ H+ H+ 80 H+ Menu This process requires energy – from passing of e- along ETC H+ H+ Outside Matrix (inside) H+ H+ H+ H+ H+ H+ H+ H+ Intermembrane Space Matrix H+ H+ H+ 81 H+ A high concentration of hydrogen ions in the intermembrane space creates a gradient for diffusion of H+ back to the matrix. H+ Chemiosmotic Phosphorylation Outside Matrix (inside) H+ H+ H+ H+ H+ H+ H+ Intermembrane Space Matrix H+ H+ H+ 82 H+ the hydrogen ions pass through this protein (called ATP Chemiosmotic synthase) as they return to the matrix down the Phosphorylation diffusion gradient. + H H+ Outside Matrix (inside) H+ H+ H+ H+ H+ H+ H+ Intermembrane Space Matrix H+ H+ H+ H+ 83 H+ H+ H+ Matrix (inside) Chemiosmotic Phosphorylation H+ H+ H+ ATP H+ Intermembrane Space H+ H+ ADP + Pi Outside H+ H+ H+ H+ ATP synthase produces ATP by phosphorylating ADP. The + energy needed to produce ATP comesHfrom hydrogen ions forcing their way into the matrix as they pass through the + H ATP synthase. 84 ATP synthase produces ATP using energy from the proton gradient https://www.youtub e.com/watch?v=lRl TBRPv6xM 85 Chemiosmotic Phosphorylation • Chemiosmotic phosphorylation is used by the mitochondrion to produce ATP. The energy needed to initially pump H+ ions into the intermembrane space comes from glucose. The entire process is called cellular respiration. • The chloroplast also produces ATP by chemiosmotic phosphorylation. The energy needed to produce ATP comes from sunlight. 86 86 Chloroplast Structure • The chloroplast is surrounded by a double membrane. • Molecules that absorb light energy (photosynthetic pigments) are located on disk-shaped structures called thylakoids. • The interior portion is the stroma. Stroma Double membrane Thylakoids 87 87 A Thylakoid H+ H+ H+ H+ H+ H+ H+ H+ H+ H+ H+ H+ H+ H+ H+ H+ In order to synthesize ATP, hydrogen ions must first be pumped into the thylakoid. This process requires energy. 88 88 A Thylakoid H+ H+ H+ H+ H+ H+ H+ H+ H+ H+ H+ H+ H+ H + H+ H+ A concentration gradient of hydrogen ions is established. The chemical gradient can be used as an energy source for producing ATP. 89 89 Chemiosmotic Phosphorylation H+ hydrogen ions force through this protein (ATP synthase) as they return to the stroma. H+ H+ H+ H+ H+ ADP + Pi H+ H+ H+ H+ H+ H+ H+ H+ H+ ATP H+ 90 ATP synthase produces ATP by phosphorylating+ADP. The energy H comes from hydrogen ions forcing their way into the stroma as they pass 90 through the ATP synthase Phosphorylation • We have just discussed two different forms of phosphorylation: – Substrate-level phosphorylation – Chemiosmotic phosphorylation (involves the ETC) • We saw that chemiosmotic phosphorylation occurred in both the mitochondria (during cellular respiration) and in the chloroplast (during photosynthesis). These two processes are sometimes given separate names: – Oxidative phosphorylation (in mitochondria) – Photophosphorylation (in chloroplast) 91 91 Chemiosmosis: Chloroplasts vs. Mitochondria Similarities: In both organelles –Redox reactions of electron transport chains generate a H+ gradient across a membrane –Involves ATP synthase which uses this proton-motive force to make ATP Difference: –use different sources of energy to accomplish this (proton gradient). Chloroplasts use light energy (photophosphorylation) and mitochondria use the chemical energy in organic molecules (oxidative phosphorylation). 92 Chemiosmosis – In chemiosmosis, the H+ diffuses back through the inner membrane through ATP synthase complexes --Driving the synthesis of ATP H+ H+ H+ H+ + . H H+ Protein complex H+ Electron carrier Intermembrane space H+ H+ ATP synthase Inner mitochondrial membrane FADH2 Electron flow FAD NAD+ NADH + H 1 O + 2 H+ 2 2 + Mitochondrial matrix H H+ Electron Transport Chain H2O ADP + P ATP H+ Chemiosmosis 93 OXIDATIVE PHOSPHORYLATION Evidences supporting Chemiosmosis Certain poisons interrupt critical events in cellular respiration Cyanide, Rotenone Oligomycin carbon monoxide Block the movement of electrons H+ H+ H+ H+ Block the flow of through ATP synthase H+ H+ H H+ H+ H+ Allow H+ to leak through the membrane + ATP Synthase DNP FADH2 FAD 1 O 2 + 2 H+ 2 NAD+ NADH H+ H+ H2O ADP + P ATP H+ Electron Transport Chain Figure 6.11 Chemiosmosis 94 22.3 Aerobic respiration Oxidative phosphorylation 2 Formation of ATP (through oxidative phosphorylation) Glycolysis: 2 NADH Pyruvate to acetyl-CoA: 2 NADH Krebs cycle: 6 NADH 2 FADH = 6 ATP = 6 ATP = 22 ATP Total: 34 ATP 95 22.3 Aerobic respiration Let’s summarize the overall process of aerobic respiration. 96 22.3 Aerobic respiration 97 22.3 Aerobic respiration Overall equation: C6H12O6 6 O2 enzymes 6 CO2 6 H2O 38 ATP 98 Want to watch a video? 99 22.3 Aerobic respiration Different stages of aerobic respiration: 1 Glycolysis occurs in cytoplasm . • Glucose is split into two molecules of triose phosphate using energy from ATP 100 22.3 Aerobic respiration Different stages of aerobic respiration: 1 Glycolysis occurs in cytoplasm . • Triose phosphate is oxidized to pyruvate ; NADH and ATP are formed 101 22.3 Aerobic respiration Different stages of aerobic respiration: 1 Glycolysis occurs in cytoplasm . • Net amount of ATP formed: 2 102 22.3 Aerobic respiration Different stages of aerobic respiration: 2 Conversion of pyruvate to acetyl-CoA occurs in mitochondrial matrix . • Pyruvate is converted to acetyl-CoA; carbon dioxide and NADH are formed 103 22.3 Aerobic respiration Different stages of aerobic respiration: 2 Conversion of pyruvate to acetyl-CoA occurs in mitochondrial matrix . • Net amount of ATP formed: 0 104 22.3 Aerobic respiration Different stages of aerobic respiration: 3 Krebs cycle occurs in mitochondrial matrix. • Acetyl-CoA combines with a 4-C compound to form a 6-C compound 105 22.3 Aerobic respiration Different stages of aerobic respiration: 3 Krebs cycle occurs in mitochondrial matrix. • The 6-C compound is oxidized step by step to regenerate 4-C compound; carbon dioxide, NADH, FADH and ATP are formed 106 22.3 Aerobic respiration Different stages of aerobic respiration: 3 Krebs cycle occurs in mitochondrial matrix. • Net amount of ATP formed: 2 107 22.3 Aerobic respiration Different stages of aerobic respiration: 4 Oxidative phosphorylation occurs in inner membrane of mitochondrion. • NADH and FADH lose hydrogen . They are oxidized to regenerate NAD and FAD 108 22.3 Aerobic respiration Different stages of aerobic respiration: 4 Oxidative phosphorylation occurs in inner membrane of mitochondrion. • The oxidation of NADH and FADH releases energy to form ATP by phosphorylation 109 22.3 Aerobic respiration Different stages of aerobic respiration: 4 Oxidative phosphorylation occurs in inner membrane of mitochondrion. • Hydrogen is finally accepted by oxygen to form water 110 22.3 Aerobic respiration Different stages of aerobic respiration: 4 Oxidative phosphorylation occurs in inner membrane of mitochondrion. • Net amount of ATP formed: 36 111 22.4 Anaerobic respiration • does not require oxygen • all reactions occur in the cytoplasm only • starts with glycolysis but will not proceed to the Kerbs cycle and oxidative phosphorylation 112 22.4 Anaerobic respiration How does anaerobic respiration occur? 113 Fate of pyruvate depends on the availability of oxygen 114 Fermentation is an anaerobic alternative to aerobic respiration – Without O2 as the final electron acceptor, oxidative phosphorylation stops, Krebs cycle cannot proceed…. why? – Under anaerobic conditions, many kinds of cells • Can use glycolysis alone to produce small amounts of ATP 115 Glycolysis harvests chemical energy by oxidizing glucose to pyruvate – In glycolysis, ATP is used to energize a glucose molecule – Which is split into two molecules of pyruvate 2 NAD+ 2 NADH + 2 H+ Glucose 2 Pyruvate 2 ADP Figure 6.7A +2 P 2 ATP 116 Fermentation can generate ATP from glucose by substrate-level phosphorylation as long as there is a supply of NAD+ to accept electrons. • In aerobic respiration, NAD+ is regenerated in ETC • Without O2, ETC stops working!!!!!! •If the NAD+ pool is exhausted, glycolysis shuts down also! 117 In aerobic respiration, NAD+ is regenerated thro ETC, with O2 as the final e acceptor. Fermentation is an anaerobic alternative to aerobic respiration to regenerate NAD+ 118 In lactic acid fermentation • 2 NADH is oxidized to NAD+ as pyruvate is reduced to lactate NAD+ 2 2 NADH NADH 2 NAD+ GLYCOLYSIS 2 ADP + 2 P 2 ATP 2 Pyruvate 2 Lactate Glucose Figure 6.13A 119 22.4 Anaerobic respiration 1 Formation of lactic acid (乳酸) in muscles glucose (6-C) 2 ADP + 2 P 2 NAD 2 ATP 2 NADH 2 pyruvate (3-C) glycolysis 120 22.4 Anaerobic respiration 1 Formation of lactic acid (乳酸) in muscles Reduced 2 pyruvate (3-C) 2 NADH 2 NAD+ oxidized 2 lactic acid (3-C) 121 22.4 Anaerobic respiration 1 Formation of lactic acid (乳酸) in muscles Reduced 2 pyruvate (3-C) 2 NADH oxidized 2 NAD+ oxidized 2 lactic acid (3-C) Reduced 122 22.4 Anaerobic respiration 1 Formation of lactic acid (乳酸) in muscles • produces only two ATP through glycolysis • simple and can supply energy quickly 123 22.4 Anaerobic respiration 1 Formation of lactic acid (乳酸) in muscles • the formation of lactic acid by anaerobic respiration is called lactic acid fermentation (乳酸發酵) • overall equation: glucose energy (2 ATP) 2 lactic acid 124 22.4 Anaerobic respiration 1 Formation of lactic acid (乳酸) in muscles • anaerobic respiration provides additional energy in a very short time allows muscles to contract more powerfully and at a higher rate 125 22.4 Anaerobic respiration 1 Formation of lactic acid (乳酸) in muscles • lactic acid formed builds up in muscles and causes pain muscle fatigue (肌肉疲勞) 126 22.4 Anaerobic respiration amount of O2 breathed in 1 Formation of lactic acid (乳酸) in muscles rest exercise recovery rest time • after doing strenuous exercise, our breathing remains deep for some time 127 22.4 Anaerobic respiration amount of O2 breathed in 1 Formation of lactic acid (乳酸) in muscles oxygen debt (氧債) rest exercise recovery rest time • extra oxygen is used to break down lactic acid 128 22.4 Anaerobic respiration amount of O2 breathed in 1 Formation of lactic acid (乳酸) in muscles oxygen debt (氧債) rest exercise recovery rest time • lactic acid is broken down to CO2 and water or converted to glycogen 129 In alcohol fermentation • NADH is oxidized to NAD+ while converting pyruvate to CO2 and ethanol NAD+ 2 2 2 NADH NADH 2 NAD+ GLYCOLYSIS 2 ADP + 2 Glucose P 2 2 ATP 2 Pyruvate CO2 released 2 Ethanol Figure 6.13B Figure 6.13C 130 22.4 Anaerobic respiration 2 Formation of ethanol and carbon dioxide in yeast glucose (6-C) 2 ADP + 2 P 2 NAD 2 ATP 2 NADH 2 pyruvate (3-C) glycolysis 131 22.4 Anaerobic respiration 2 Formation of ethanol and carbon dioxide in yeast 2 pyruvate (3-C) 2 NADH 2 CO2 2 NAD 2 ethanol (2-C) 132 22.4 Anaerobic respiration 2 Formation of ethanol and carbon dioxide in yeast • the formation of ethanol by anaerobic respiration is called alcoholic fermentation (酒精發酵) • overall equation: glucose energy (2 ATP) 2 ethanol 2 CO2 133 Brewing of wine involves alcoholic fermnetation 134 How to Measure the rate of respiration of a small animal ? 135 A respirometer How about plants? 136 22.4 Anaerobic respiration Applications of anaerobic respiration • the brewing of beer makes use of the alcohol formed when yeast ferments the sugar in barley (大麥) 137 22.4 Anaerobic respiration Applications of anaerobic respiration • the brewing of wine makes use of the alcohol formed when yeast ferments the sugar in grape juice 138 22.4 Anaerobic respiration Applications of anaerobic respiration • CO2 formed by alcoholic fermentation in yeast helps raise dough in breadmaking 139 22.4 Anaerobic respiration Applications of anaerobic respiration • yoghurt contains lactic acid formed by anaerobic respiration in bacteria 140 22.4 Anaerobic respiration Applications of anaerobic respiration • lactic acid formed by anaerobic respiration in bacteria helps coagulate milk to form cheese 141 22.4 Anaerobic respiration Applications of anaerobic respiration • ethanol formed by the fermentation of sugar in crops can be used as a fuel to power vehicles 142 22.4 Anaerobic respiration 1 Anaerobic respiration in skeletal muscles: Glucose undergoes glycolysis and is oxidized to pyruvate . NADH and ATP are formed in the process. Pyruvate is reduced to lactic acid by NADH . 143 22.4 Anaerobic respiration 2 Anaerobic respiration in muscles provides additional energy in a very short time for muscle contraction . 144 22.4 Anaerobic respiration 3 During strenuous exercise, the lactic acid formed by anaerobic respiration accumulates in muscles and causes muscle fatigue . 145 22.4 Anaerobic respiration 3 We keep breathing deeply after exercise to take in extra oxygen . It is used to remove all lactic acid by breaking it down to carbon dioxide and water or converting it to glycogen . 146 22.4 Anaerobic respiration 4 Anaerobic respiration in yeast: Glucose undergoes glycolysis and is oxidized to pyruvate. NADH and ATP are formed in the process. Pyruvate is reduced to ethanol by NADH. Carbon dioxide is released in the process. 147 22.4 Anaerobic respiration 5a Similarities of aerobic and anaerobic respiration: Both release energy from the oxidative breakdown of organic substances . 148 22.4 Anaerobic respiration 5a Similarities of aerobic and anaerobic respiration: Both transfer energy to the energy carrier ATP , and some energy is lost as heat . 149 22.4 Anaerobic respiration 5a Similarities of aerobic and anaerobic respiration: Both consist of a number of reactions controlled by enzymes . 150 22.4 Anaerobic respiration 5b Differences between aerobic and anaerobic respiration: Aerobic respiration occurs in cytoplasm and mitochondria while anaerobic respiration occurs only in cytoplasm . 151 22.4 Anaerobic respiration 5b Differences between aerobic and anaerobic respiration: Aerobic respiration requires oxygen but anaerobic respiration does not. 152 22.4 Anaerobic respiration 5b Differences between aerobic and anaerobic respiration: In aerobic respiration, organic substances are completely broken down into carbon dioxide and water . 153 22.4 Anaerobic respiration 5b Differences between aerobic and anaerobic respiration: But in anaerobic respiration, organic substances are partly broken down to form lactic acid or ethanol and carbon dioxide. 154 22.4 Anaerobic respiration 5b Differences between aerobic and anaerobic respiration: In aerobic respiration, 38 ATP per glucose molecule is formed (a larger amount of energy is released). 155 22.4 Anaerobic respiration 5b Differences between aerobic and anaerobic respiration: In anaerobic respiration, 2 ATP per glucose molecule is formed (a much smaller amount of energy is released). 156 22.4 Anaerobic respiration 6 Alcoholic fermentation in yeast is used in brewing beer and wine , raising dough in bread-making and producing ethanol as a biofuel. 157 22.4 Anaerobic respiration 6 Lactic acid fermentation in bacteria is used in making yoghurt and cheese. 158 Connection between molecular breakdown and synthesis • Cells use many kinds of organic molecules as fuel for cellular respiration 159 – Carbohydrates, fats, and proteins can all fuel cellular respiration • When they are converted to molecules that enter glycolysis or the citric acid cycle Food, such as peanuts Carbohydrates Fats Sugars Glycerol Proteins Fatty acids Amino acids Amino groups Glucose G3P Pyruvate Acetyl CoA CITRIC ACID CYCLE OXIDATIVE PHOSPHORYLATION (Electron Transport and Chemiosmosis) GLYCOLYSIS Figure 6.14 ATP 160 Energy metabolism of carbohydrates, fats, and proteins 161 • Fats are first digested into gly_____ and f____ acids. glycerol and fatty acids A fat molecule 162 glycerol can be converted into to triose phosphate / PGAL Which enters glycolytic pathway 163 • Fatty acids are broken down into 2 carbon residues which combine with Coenzyme A and becomes acetyl COA and enters the K____ cycle 164 • Proteins must first be digested to individual a____ acids. • Amino acids that will be catabolized must have their amino groups removed via deamination or transamination. 165 166 • The carbon skeletons are modified by enzymes and enter as intermediaries into glycolysis or the citric acid cycle, depending on their structure. 167 • The carbon skeletons are modified by enzymes and enter as intermediaries into glycolysis or the citric acid cycle, depending on their structure. 168 • Catabolism of energy-giving foods: 169 Intermediates from glycolysis and the citric acid cycle are used as raw materials for making complex organic substances - The biosynthesis of organic substances 170 Intermediates from glycolysis and the citric acid cycle are used as raw materials for making complex organic ATP needed to drive biosynthesis Substances ATP -The biosynthesis of CITRIC organic substances ACID GLUCOSE SYNTHESIS Acetyl Pyruvate CoA G3P Glucose CYCLE Amino groups Amino acids Fatty Glycerol Sugars acids Proteins Fats Carbohydrates Cells, tissues, organisms 171 The fuel for respiration ultimately comes from photosynthesis – All organisms • Can harvest energy from organic molecules – Plants • make these molecules from inorganic sources by the process of photosynthesis Figure 6.16 172 22.5 Relationship between respiration and photosynthesis • exchange of molecules between respiration and photosynthesis bridges the flow of energy from the environment to organisms 173 22.5 Relationship between respiration and photosynthesis Exchange of molecules light H2O H2O photochemical reactions oxidative phosphorylation chloroplast mitochondrion O2 O2 174 22.5 Relationship between respiration and photosynthesis Exchange of molecules CO2 CO2 Calvin cycle Krebs cycle glycolysis glucose pyruvate 175 22.5 Relationship between respiration and photosynthesis Flow of energy oxygen glucose photosynthesis CO2 water respiration 176 22.5 Relationship between respiration and photosynthesis In energy transformation • ATP acts as the energy carrier energy stored in organic compounds ATP light energy energy for cellular metabolism ADP +P ADP +P ATP 177 22.5 Relationship between respiration and photosynthesis In energy transformation • ATP acts as the energy carrier energy stored in photosynthesis organic ADP compounds +P ATP light energy energy for cellular metabolism ADP +P respiration ATP 178 22.5 Relationship between respiration and photosynthesis Differences between respiration and photosynthesis: 1 Site of occurrence: Respiration occurs in all living cells while photosynthesis occurs in chloroplast-containing cells 179 22.5 Relationship between respiration and photosynthesis Differences between respiration and photosynthesis: 2 Type of metabolism: In respiration, catabolism occurs. Organic food is broken down by oxidation to release energy 180 22.5 Relationship between respiration and photosynthesis Differences between respiration and photosynthesis: 2 Type of metabolism: In photosynthesis, anabolism occurs. Organic food is built up by reduction to store energy 181 22.5 Relationship between respiration and photosynthesis Differences between respiration and photosynthesis: 3 Energy change: In respiration, chemical energy in food is converted to ATP and heat 182 22.5 Relationship between respiration and photosynthesis Differences between respiration and photosynthesis: 3 Energy change: In photosynthesis, light energy from the sun is converted to chemical energy in food 183 22.5 Relationship between respiration and photosynthesis Differences between respiration and photosynthesis: 4 Cyclic reactions: In Krebs cycle of respiration, carbon dioxide is removed from the substrate and ATP , NADH and FADH are formed 184 22.5 Relationship between respiration and photosynthesis Differences between respiration and photosynthesis: 4 Cyclic reactions: In Calvin cycle of photosynthesis, carbon dioxide is fixed into the cycle by a 5-C compound and NADPH and ATP are used 185 22.5 Relationship between respiration and photosynthesis Differences between respiration and photosynthesis: 5 Formation of ATP: In respiration, ATP is formed in glycolysis, Krebs cycle and oxidative phosphorylation 186 22.5 Relationship between respiration and photosynthesis Differences between respiration and photosynthesis: 5 Formation of ATP: In photosynthesis, ATP is formed in photophosphorylation 187 22.5 Relationship between respiration and photosynthesis Differences between respiration and photosynthesis: 6 Hydrogen donor: In respiration, NADH and FADH are the hydrogen donors while in photosynthesis, water is the hydrogen donor 188 22.5 Relationship between respiration and photosynthesis Differences between respiration and photosynthesis: 7 Final hydrogen acceptor: In respiration, oxygen is the final hydrogen acceptor while in photosynthesis, a 3-C compound in Calvin cycle is the final hydrogen acceptor 189 1 How does our body obtain energy from the food we eat? Our body releases energy stored in food by respiration. The energy is used to form ATP which drives all cellular activities. 190 2 How is alcohol produced from corn by fermentation? Sugar in corn is converted to ethanol by alcoholic fermentation in yeast. 191 3 The sugar in corn is made by photosynthesis. What is the relationship between respiration and photosynthesis? Respiration and photosynthesis together allow the flow of energy in the ecosystem. 192 Respiration is requires oxygen does not require oxygen anaerobic aerobic respiration respiration oxidative breakdown of food 193 oxidative breakdown of food releases chemical energy mostly as heat some stored in ATP 194 aerobic respiration anaerobic respiration both involve glycolysis occurs in cytoplasm 195 glycolysis if aerobic, then followed by Kerbs cycle occur in oxidative phosphorylation mitochondria 196 glycolysis if anaerobic, then followed by formation of lactic acid occur in formation of ethanol and carbon dioxide cytoplasm 197