* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 19_lecture
General circulation model wikipedia , lookup
2009 United Nations Climate Change Conference wikipedia , lookup
Media coverage of global warming wikipedia , lookup
Global warming controversy wikipedia , lookup
Climate change in the Arctic wikipedia , lookup
Climate change in Tuvalu wikipedia , lookup
Effects of global warming on humans wikipedia , lookup
Effects of global warming on human health wikipedia , lookup
Climate change and agriculture wikipedia , lookup
Mitigation of global warming in Australia wikipedia , lookup
Climate change and poverty wikipedia , lookup
Fred Singer wikipedia , lookup
Scientific opinion on climate change wikipedia , lookup
United Nations Climate Change conference wikipedia , lookup
Global warming hiatus wikipedia , lookup
Future sea level wikipedia , lookup
Attribution of recent climate change wikipedia , lookup
Effects of global warming on oceans wikipedia , lookup
Climate change in the United States wikipedia , lookup
Global Energy and Water Cycle Experiment wikipedia , lookup
Surveys of scientists' views on climate change wikipedia , lookup
Effects of global warming on Australia wikipedia , lookup
Instrumental temperature record wikipedia , lookup
United Nations Framework Convention on Climate Change wikipedia , lookup
Years of Living Dangerously wikipedia , lookup
Global warming wikipedia , lookup
Solar radiation management wikipedia , lookup
Climate change, industry and society wikipedia , lookup
Public opinion on global warming wikipedia , lookup
Politics of global warming wikipedia , lookup
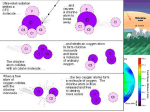
Chapter 19 Global Change Global Change Global change- any chemical, biological or physical property change of the planet. Examples include cold temperatures causing ice ages. Global climate change- changes in the climate average temperature of the Earth. Global warming- one aspect of climate change, the warming of the oceans, land masses and atmosphere of the Earth. Greenhouse Gases Natural Water vapor Volcanic eruptions Carbon dioxide Methane Nitrous oxide Ozone-O3 simulation Anthropogenic Causes of Greenhouse Gases Burning of fossil fuels-NOx, SO4, CO, CO2 Agricultural practices-all the above & CH4 Deforestation-increases CO2 and CH4 Landfills-CH4 Industrial production- CFC’s Chloroflourocarbons Feedbacks Increasing CO2 Concentrations We reached 400ppm in 2014! We reached 400.9ppm in 2015! Use the graph to Determine the net change in atmospheric CO2 concentration between 100,000 YA & present day levels. 1. Read the graph to find the CO2levels for 100,000 years ago and for the present day. 100,000 years ago: CO2 levels were about 230 ppm (An accepted range of answers would be 220ppm to 235ppm) Present day: CO2 levels are about 390 ppm (An accepted range of answers would be 380ppm to 395ppm) 2. subtract the 100,000 years ago from present day 390 ppm – 230 ppm = 160 ppm increase of CO2 concentration over past 100,000 years Formation of ozone O2 + O =O3 1 CFC molecule breaks up 100,000 O3 molecules If there were 200 CFC’s how many O3 molecules would be broken up? Stratospheric Ozone The stratospheric ozone layer exists roughly 45-60 kilometers above the Earth. Ozone has the ability to absorb ultraviolet radiation and protect life on Earth. Formation and Breakdown of Ozone First, UV-C radiation breaks the bonds holding together the oxygen molecule , leaving two free oxygen atoms: O2 + UV-C -> O2 Sometimes the free oxygen atoms result in ozone: O2 + O -> O3 Ozone is broken down into O2 and free oxygen atoms when it absorbs both UV-C and UV-B ultraviolet light: O3 + UV-B or UV-C -> O2 + O Anthropogenic Contributions to Ozone Destruction First, chlorine breaks ozone’s bonds and pulls off one atom of oxygen, forming a chlorine monoxide molecule and O2: O3 + Cl -> ClO + O2 Next, a free oxygen atoms pulls the oxygen atom from ClO, liberating the chlorine and creating one oxygen molecule: ClO + O -> Cl + O2 One chlorine atom can catalyze the breakdown of as many as 100,000 ozone molecules before it leaves the stratosphere. How CFC break up O3 Polar Vortex • • • CIRCULATING WINDS ISOLATE COLD AIR traps WARM AIR inside the circulating vortex When ambient AIR IS -80C CLOUDS form of WATER & NITRIC ACID. This acidic vapor allows CFC’S to breakdown into CHLORINE SUNLIGHT returns in the SPRING so it increases the rate of Ozone depletion Polar Vortex Anthropogenic Contributions to Ozone Destruction Certain chemicals can break down ozone, particularly chlorine. The major source of chlorine in the stratosphere is a compound known as chlorofluorocarbons (CFCs) CFCs are used in refrigeration and air conditioning, as propellants in aerosol cans and as “blowing agents” to inject air into foam products like Styrofoam. Anthropogenic Contributions to Ozone Destruction When CFCs are released into the troposphere they make their way to the stratosphere. The ultraviolet radiation present has enough energy to break the bond connecting chlorine to the CFC molecule. which can then break apart the ozone molecules. Depletion of the Ozone Layer Global Ozone concentrations had decreased by more than 10%. In the Antarctic it can be as much as 70% Depletion was greatest at the poles Decreased stratospheric ozone has increased the amount of UVB radiation that reaches the surface of Earth. Emissions Developed V. Developing Look at per/capita Global Temperatures since 1880 Sea ice change Interactives NASA Since 1880 temperatures have increased 0.8°C. However, some have declined. Temperatures and Greenhouse Gas Concentrations in Past 400,000 Years Habitable Planet chapter 13 video first 16’ No one was around thousands of years ago to measure temperatures so we use other indirect measurements. Some of these are Changes in species compositions Chemical analyses of ice Measurement of CO2 gas in ice cores Ocean sediments can tell what past ocean temperature and CO2 levels were like Video clip Consequences to the Environment Because of Global Warming Melting of polar ice caps, Greenland and Antarctica Melting of many glaciers around the world Melting of permafrost Rising of sea levels due to the melting of glaciers and ice sheets and as water warms it expands Heat waves Cold spells Change in precipitation patterns Increase in storm intensity Shift in ocean currents What is the ITCZ Where does it take up a larger area? What are the effects of this shift? Consequences to Organisms Wild plants and animals can be affected. The growing season for plants has changed and animals have the potential to be endangered/threatened if they can’t move to better climates. Humans may have to relocate, some diseases like those carried by mosquitoes could increase and there could be economic consequences. The Controversy of Climate Change The fundamental basis of climate change- that greenhouse gas concentrations are increasing and will lead to global warming is not in dispute among 98% climate scientists What is unclear is how much world temperatures will increase for a given change in greenhouse gases, depends on feedback loops. Loss of moisture Ground water recharge-drought, flooding Salinization of soil-infiltrate of sea water along coastal areas Higher CO2 increases growth rate. Soil can absorb it. Increased soil temperature Putting It Together We know that an increase in CO2 in the atmosphere causes a greater capacity for warming through the greenhouse effect. When the Earth experiences higher temperatures, the oceans warm and cannot contain as much CO2 gas and, as a result, they release CO2 into the atmosphere. The Kyoto Protocol 1997, nations went to Kyoto, Japan to discuss how best to control the emissions contributing to global warming. agreement -emissions of greenhouse gases from all industrialized countries will be reduced to 5.2% below 1990 levels by 2012. The U.S. did NOT sign-Developing nations did not have emission limits imposed by the protocol. Update! U.N. Agreement COP21 (conference of parties, 21st meeting) Key highlights Limit warming to 1.5 C above pre-industrial levels. Reduce deforestation, encourage sustainable forestry Developed countries collect funds to assist developing countries System to verify reductions by countries Balance between anthropogenic output and sinks Avert, minimize loss & damages by countries Meet every 5 years with new reduction limits (used to be 10 years) Carbon Sequestration taking CO2 out of the atmosphere include storing carbon in agricultural soils or retiring agricultural land and allowing it to become pasture or forest-return carbon sink captured CO2 would be compressed and pumped into abandoned oil wells or the deep ocean.