* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Marketing Operations Manager, International
Affiliate marketing wikipedia , lookup
Food marketing wikipedia , lookup
Marketing communications wikipedia , lookup
Product planning wikipedia , lookup
Target audience wikipedia , lookup
Marketing research wikipedia , lookup
Ambush marketing wikipedia , lookup
Marketing channel wikipedia , lookup
Multi-level marketing wikipedia , lookup
Sports marketing wikipedia , lookup
Target market wikipedia , lookup
Digital marketing wikipedia , lookup
Viral marketing wikipedia , lookup
Integrated marketing communications wikipedia , lookup
Guerrilla marketing wikipedia , lookup
Direct marketing wikipedia , lookup
Youth marketing wikipedia , lookup
Advertising campaign wikipedia , lookup
Marketing plan wikipedia , lookup
Marketing strategy wikipedia , lookup
Marketing mix modeling wikipedia , lookup
Multicultural marketing wikipedia , lookup
Green marketing wikipedia , lookup
Street marketing wikipedia , lookup
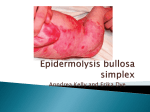
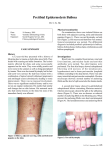
Job Description Marketing Operations Manager, International Company Background (for external only) Amicus Therapeutics (NASDAQ:FOLD) is a biotechnology company at the forefront of therapies for rare and orphan diseases. The Company has a robust pipeline of novel, first-in-class treatments in development for a broad range of human genetic diseases, including lysosomal storage disorders (LSDs) and Epidermolysis Bullosa (EB). Amicus is building a leading biotechnology company in rare diseases, and is currently in the pre-commercial stage focusing on the following areas: • Migalastat for Fabry Disease Migalastat is an oral personalized medicine intended to treat Fabry disease in patients who have amenable genetic mutations. Amicus has built a commercial organization in the EU that is prepared to launch migalastat upon approval. The EMA's review of the MAA for migalastat is currently in progress. Amicus anticipates an opinion from the Committee for Medicinal Products for Human Use (CHMP) in early 2016. • Novel ERT for Pompe Disease (ATB200 + Chaperone) Amicus has initiated the clinical study ATB200-02 in Pompe patients with a novel treatment paradigm that consists of ATB200, a uniquely engineered recombinant human acid alpha-glucosidase (rhGAA) enzyme with an optimized carbohydrate structure to enhance uptake, administered with a pharmacological chaperone (AT2221) to improve activity and stability. Amicus anticipates dosing of first patients in the clinical study early in 2016. • SD-101 for Epidermolysis Bullosa (EB) SD-101 is a novel, late-stage, proprietary topical treatment and potential first-to-market therapy for EB. This investigational product was granted FDA breakthrough therapy designation in 2013 based on results from a Phase 2a study for the treatment of lesions in patients suffering with EB. SD-101 is the first-ever treatment in EB clinical studies to show improvements in wound closure across all major EB subtypes. SD-101 is currently being investigated in a Phase 3 study (SD-005) to support global regulatory submissions. As such Amicus now wishes to appoint a Senior Manager, International Marketing Operations who will work with the global marketing team and the regional management team to enable effective execution of global strategies across the international organisation. Major Activities and Responsibilities Drive the execution of the brand strategy for the EU+ region; signal market / products trends, measure brand strategy success, provide recommendations and adjust execution to drive optimal product performance. Support local affiliates in brand planning Develop and manage execution plans in the region including management of allocated budget Support global marketing in developing marketing strategies for approved brands as well as products in development. Guide, support and advise local commercial teams while they implement / execute the marketing strategies for approved brands. Reporting Line The Senior Manager, International Marketing Operations will have a dual reporting line to the Vice President, Global Marketing (based in the US) and the Senior Director, International Commercial Operations Qualifications and Background Requirements Able to work in a fast-paced environment with minimal supervision at times. Capable to answer (make decisions) the demands of customers (internal and external) quickly, accurately and professionally. Excellent analytical, strategic thinking and problem-solving skills. Experience in translating strategies into actions that deliver successful results Proven success in influencing without direct hierarchical structures Strong presentation skills to senior management. Strong social competencies and presentation skills with peers Sales experience preferred Educational Requirements University degree Personal Qualities A passion for making a difference in the lives of people living with rare and devastating diseases A strong customer focus Ability and willingness to travel (30-50%) and work from affiliate offices across the region Professional work Experience 3-4 years’ experience in a similar role with Proven success in marketing role in the biopharma industry Location Amicus Therapeutics international headquarters, Gerrards Cross, UK