* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
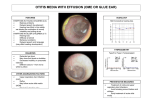
Download Ear manipulations help model neuroplasticity limitations
Subventricular zone wikipedia , lookup
Neuroregeneration wikipedia , lookup
Caridoid escape reaction wikipedia , lookup
Multielectrode array wikipedia , lookup
Electrophysiology wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Sensory cue wikipedia , lookup
Axon guidance wikipedia , lookup
Nervous system network models wikipedia , lookup
Microneurography wikipedia , lookup
Synaptic gating wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Synaptogenesis wikipedia , lookup
Sound localization wikipedia , lookup
Central pattern generator wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Optogenetics wikipedia , lookup
Circumventricular organs wikipedia , lookup
Development of the nervous system wikipedia , lookup
Neuroanatomy wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
University of Iowa Iowa Research Online Theses and Dissertations Fall 2013 Ear manipulations help model neuroplasticity limitations Karen Louise Elliott Thompson University of Iowa Copyright 2013 Karen Louise Elliott Thompson This dissertation is available at Iowa Research Online: http://ir.uiowa.edu/etd/4969 Recommended Citation Thompson, Karen Louise Elliott. "Ear manipulations help model neuroplasticity limitations." PhD (Doctor of Philosophy) thesis, University of Iowa, 2013. http://ir.uiowa.edu/etd/4969. Follow this and additional works at: http://ir.uiowa.edu/etd Part of the Biology Commons EAR MANIPULATIONS HELP MODEL NEUROPLASTICITY LIMITATIONS by Karen Louise Elliott Thompson A thesis submitted in partial fulfillment of the requirements for the Doctor of Philosophy degree in Biology in the Graduate College of The University of Iowa December 2013 Thesis Supervisor: Professor Bernd Fritzsch Graduate College The University of Iowa Iowa City, Iowa CERTIFICATE OF APPROVAL _______________________ PH.D. THESIS _______________ This is to certify that the Ph.D. thesis of Karen Louise Elliott Thompson has been approved by the Examining Committee for the thesis requirement for the Doctor of Philosophy degree in Biology at the December 2013 graduation. Thesis Committee: ___________________________________ Bernd Fritzsch, Thesis Supervisor ___________________________________ Douglas Houston ___________________________________ Michael Dailey ___________________________________ Joshua Weiner ___________________________________ Daniel Weeks To my husband, Nathan, and my son, Noah ii ACKNOWLEDGMENTS I would like to first thank my husband, Nathan, for his continued support for me and helping me pursue my goals. I would also like to thank my advisor, Dr. Bernd Fritzsch, for his guidance throughout my years in graduate school. In addition, I would like to thank members of the Fritzsch lab, both past and present: Jeremy Duncan, Israt Jahan, Jennifer Kersigo, Benjamin Kopecky, Hannah Maher, Ning Pan, and Tian Yang. Finally, I would like to thank members of my committee: Dr. Bernd Fritzsch, Dr. Michael Dailey, Dr. Douglas Houston, Dr. Daniel Weeks, and Dr. Joshua Weiner for their guidance, suggestions, and support. iii TABLE OF CONTENTS LIST OF TABLES ............................................................................................................. vi LIST OF FIGURES .......................................................................................................... vii LIST OF ABBREVIATIONS .......................................................................................... viii CHAPTER I INTRODUCTION ..........................................................................................1 Connecting Sensory Organs with the Central Nervous System .......................1 Inner Ear Anatomy and Function .....................................................................2 Neurosensory Development of the Inner Ear ...................................................4 Evolution of the Inner Ear ................................................................................7 Motor Neuron Targeting .................................................................................10 Inner Ear Sensory Neuron Guidance ..............................................................15 Hindbrain Neurons..........................................................................................20 Experimental Approach for Generating and Manipulating a ‘Novel’ Sensory System ...............................................................................................27 CHAPTER II METHODS .................................................................................................28 Animals ...........................................................................................................28 GFP mRNA injections ....................................................................................28 Dextran amine injections ................................................................................29 Transplantations ..............................................................................................29 Swimming Behavior .......................................................................................31 Dextran amine label ........................................................................................32 Lipophilic dye label ........................................................................................32 Quantification of percent overlap of sensory neurons in the vestibular nucleus of ‘three-eared’ frogs .........................................................................34 In situ hybridization ........................................................................................35 Immunohistochemistry ...................................................................................36 Plastic imbedding for light and transmission electron microscopy ................37 Three-dimensional reconstruction of Mauthner cells .....................................37 Dendritic Branching Analysis.........................................................................38 CHAPTER III TRANSPLANTATION OF XENOPUS LAEVIS EARS REVEALS THE ABILITY TO FORM AFFERENT AND EFFERENT CONNECTIONS WITH THE SPINAL CORD .............................................39 Abstract ...........................................................................................................39 Introduction.....................................................................................................40 Results.............................................................................................................42 Assessment of transplantations................................................................42 Transplanted ears develop hair cells and ganglia ....................................43 Afferent innervation of transplanted ears ................................................44 Efferent innervation of transplanted ears ................................................45 Cranial and lateral line nerves develop nearly normal ............................47 Discussion .......................................................................................................47 iv CHAPTER IV TRANSPLANTATION OF XENOPUS LAEVIS TISSUES TO DETERMINE THE ABILITY OF MOTOR NEURONS TO ACQUIRE A NOVEL TARGET ...................................................................63 Abstract ...........................................................................................................63 Introduction.....................................................................................................64 Results.............................................................................................................67 Completion of eye removal and assessment of transplantation success .....................................................................................................67 Afferent innervation of transplanted ears ................................................69 Efferent innervation of transplanted ears ................................................70 Innervation of transplanted somite, eye muscle, and jaw muscle ...........72 Innervation of transplanted heart and liver ..............................................73 Discussion .......................................................................................................74 CHAPTER V GENERATION OF ‘THREE-EARED’ FROGS REVEALS MOLECULAR AND ACTIVITY-BASED GUIDANCE OF CENTRAL PROJECTIONS ...........................................................................91 Abstract ...........................................................................................................91 Introduction.....................................................................................................92 Results.............................................................................................................95 Success of transplantation .......................................................................95 Analysis of Swimming Behavior.............................................................96 Analysis of Afferent Projections to the Hindbrain ..................................97 Discussion .......................................................................................................99 CHAPTER VI EAR MANIPULATIONS REVEAL A CRITICAL PERIOD OF HINDBRAIN DEPENDENCE ON THE EAR FOR DEVELOPMENT AND SURVIVAL ........................................................................................109 Abstract .........................................................................................................109 Introduction...................................................................................................110 Results...........................................................................................................115 Effect of stage of ear removal on the degree of ear regeneration ..........115 Effect of stage of ear removal on the presence of the Mauthner cell ....115 Effect of stage of ear removal on the dendritic branching of surviving Mauthner cells .......................................................................116 Effect of increasing afferent input on the dendritic branching of Mauthner cells .......................................................................................120 Discussion .....................................................................................................121 Viability of the Mauthner cell depends on ear input .............................122 Dendrite growth and branching depends on vestibular afferents ..........123 CHAPTER VII CONCLUSIONS AND FUTURE DIRECTIONS .................................132 Motor Neuron Acquisition............................................................................132 Inner Ear Sensory Neuron Guidance ............................................................135 Hindbrain Neurons........................................................................................143 Proposed Mechanism ....................................................................................148 REFERENCES ................................................................................................................152 v LIST OF TABLES Table 3.1. Success of transplantation of otic placodes. .....................................................53 Table 3.2. Formation of residual ears as related to success of transplantation. .................54 Table 4.1. Success of tissue transplantation.......................................................................80 Table 5.1. Analysis of tadpole swimming behavior. .......................................................102 vi LIST OF FIGURES Figure 3.1. Stage 46 X. laevis showing transplanted ears. .................................................55 Figure 3.2. Development of the transplanted ear. ..............................................................57 Figure 3.3. Afferent and efferent innervation of transplanted ears. ...................................59 Figure 3.4. Development of cranial and lateral line nerves. ..............................................61 Figure 4.1. Stage 46 Xenopus laevis. .................................................................................81 Figure 4.2. Afferent projections to transplanted ears. ........................................................83 Figure 4.3. Efferent projections to transplanted ears. ........................................................85 Figure 4.4. Transplanted muscle tissue. .............................................................................87 Figure 4.5. Transplantated tissue lacking nicotinic acetylcholine receptors such as heart and liver. ..........................................................................................................89 Figure 5.1. Stage 46 Xenopus laevis 'three-eared' frogs. .................................................103 Figure 5.2. Inner ear afferent projections.........................................................................105 Figure 5.3. Overlap and segregation of inner ear afferents from transplanted and native ears. ..............................................................................................................107 Figure 6.1. Success of ear removal. .................................................................................126 Figure 6.2. Mauthner cell survival. ..................................................................................128 Figure 6.3. Dendritic development of Mauthner cells following ear manipulation.........130 Figure 7.1. Proposed mechanism to guide swimming behavior in three-eared frogs. .....150 vii LIST OF ABBREVIATIONS ACh Acetylcholine Akt Protein Kinase B Atoh1 Atonal homolog 1 Atoh7 Atonal homolog 7 BDNF Brain Derived Neurotrophic Factor bHLH basic Helix-Loop-Helix BMP Bone Morphogenetic Protein Chrna9 Acetylcholine Receptor subunit α9 Chrna10 Acetylcholine Receptor subunit α10 CNS Central Nervous System E Embryonic day Eya1 Eyes absent homolog 1 FGF Fibroblast growth factor Fgf3 Fibroblast growth factor 3 Fgf8 Fibroblast growth factor 8 Fgf10 Fibroblast growth factor 10 Gata3 GATA binding protein 3 GFP Green Fluorescent Protein Islet-1 Insulin gene enhancer protein 1 LIM LIM homeodomain miR MicroRNA MMR Marc’s Modified Ringers Solution viii MuSK Muscle Specific Receptor Tyrosine Kinase MyoVI Myosin VI nAChR Nicotinic Acetylcholine Receptor Neurod1 Neuronal differentiation 1 Neurog1 Neurogenin 1 NGS Normal Goat Serum NT-3 Neurotrophic Factor 3 P Postnatal day Pax2 Paired box gene 2 Pax5 Paired box gene 5 Pax6 Paired box gene 6 Pax8 Paired box gene 8 PaxB Paired box gene B PBS Phosphate Buffered Saline PFA Paraformaldehyde Rapsyn Receptor-Associated Protein of the Synapse RGC Retinal Ganglion Cell RIC-3 Resistance to Inhibitors of Cholinesterase 3 Six1 Sine oculus-related homeobox 1 homolog Sox3 SRY-box containing gene 3 TrkB Tyrosine Kinase B VAChT Vesicular Acetylcholine Transporter VOR Vestibulo-Ocular Reflex ix Wnt Wingless-related MMTV Integration Site x 1 CHAPTER I INTRODUCTION Connecting Sensory Organs with the Central Nervous System Sensory organs send information about the outside world to specified nuclei in the central nervous system (CNS). In turn, the CNS sends information back to certain sensory organs to modulate the incoming signal. For example, the retina of many vertebrates receives a centrifugal or efferent innervation (Fritzsch, 1991) and this is even more conserved for the vertebrate ear (Fritzsch, 1999, Köppl, 2011). As sensory organs, such as the ear, diversified over time to change from a vestibular to a mixed vestibular/auditory system, new connections evolved to process that information distinctly. This process of forming contacts between the CNS and sensory organs or subsystems is incompletely understood. The inner ear, one of the sensory organs located in the head, evolved in early vertebrates (Retzius, 1881, Fritzsch and Straka, 2013). The inner ear transforms mechanical stimulation of sensory-transducing hair cells into electrical impulses to transmit sound, gravity, balance, and acceleration information through afferent neurons to the hindbrain which in turn sends a signal back to the inner ear through efferent neurons to modulate the periphery. How these afferent and efferent processes of neurons navigate to the hindbrain or hair cells, respectively, is not well understood at the molecular level beyond a small set of experimental data (Simmons et al., 2011, Yang et al., 2011). It has been suggested that efferents to the ear are rerouted facial branchial motor neurons that 2 may do so because of the unique expression of one or more transcription factors (Simmons et al., 2011). In my thesis I examine the ability of the nervous system to adapt to a ‘novel’ vestibular system through transplantation of frog inner ears. Specifically, I ask the following questions: 1) Can inner ear hair cells recruit any motor neuron as an efferent innervation through their conserved nicotinergic acetylcholine receptor? 2) Can inner ear afferents find their targets in the hindbrain if they enter through a different cranial nerve or enter into a foreign territory? 3) What mechanisms do inner ear afferents use to find their targets in the hindbrain? 4) How will alteration of afferent input, either by ear removal or addition of ‘extra’ ears, affect neurons in the CNS dedicated to inner ear sensory input? My thesis will first provide a background on the inner ear anatomy, neurosensory development and evolution, followed by motor neuron acquisition and sensory neuron guidance. Finally I will discuss how sensory neurons can shape second-order neurons in the hindbrain that influence motor control. Inner Ear Anatomy and Function The inner ear is comprised of the vestibular and auditory systems. The more dorsal, vestibular portion contains sensory epithelia for the perception of gravity, balance, and acceleration, whereas the more ventral, auditory portion contains sensory epithelia for the perception of sound. The sensory epithelia contain mechanosensory hair cells and supporting cells. The hair cells transduce mechanical stimulation of their stereocilia into electrical signals that are relayed to the brain through afferent (sensory) neurons (Torres 3 and Giráldez, 1998, Rubel and Fritzsch, 2002, Fritzsch and Beisel, 2003). The brain, in turn, sends signals through efferent (motor) neurons to modulate the sensitivity of the hair cells (Simmons et al., 2011) that are highly conserved across vertebrates (Köppl, 2011). The vestibular sensory epithelia consist of the utricle and saccule for the perception of linear acceleration and gravity and the three semicircular canals: anterior crista, posterior crista, and horizontal crista for the perception of angular acceleration. The utricle and saccule maculae are covered by calcium carbonate crystals, known as otoconia. The otoconia are partially imbedded in a matrix that is tethered to the kinocilium of the underlying hair cells. This dense otoconial complex provides inertia to generate shearing forces on the hair cells in response to linear acceleration or gravity (Riley and Phillips, 2003, Lundberg et al., 2006). Each semicircular canal cristae is located within the ampulla associated with the respective semicircular canal. The long hair cell stereocilia within each crista are covered by a gelatinous material. Movement of endolymph fluid within the canal moves the gelatinous material and deflects the hair cell stereocilia (Lewis et al., 1985, Riley and Phillips, 2003) The auditory sensory epithelia consist of the cochlea in mammals or the basilar papilla in most other organisms. Frogs and other amphibians have an amphibian papilla in addition to the basilar papilla. Furthermore, in amphibians, the saccule also has an auditory role in addition to a vestibular one (Bever et al., 2003, Riley and Phillips, 2003, Quick and Serrano, 2005). Fish, amphibians, and reptiles also have a lagena, which may provide auditory information in addition to gravitational information (Lewis and Narins, 1999, Bever et al., 2003). 4 Neurosensory Development of the Inner Ear The vertebrate inner ear arises from a single otic placode adjacent to rhombomeres 5 and 6 of the hindbrain (Fritzsch et al., 1998, Rinkwitz et al., 2001, Riley and Phillips, 2003, Groves, 2005, Ohyama et al., 2007, Schlosser, 2010, Pieper et al., 2011). The otic placode originates as part of a pan-placodal domain (Groves, 2005, Ohyama et al., 2007, Schlosser, 2010, Pieper et al., 2011). The pan placodal domain is an area of non-neuronal ectoderm bordering the neural plate that gives rise to the various sensory placodes in the vertebrate head (Schlosser, 2010). This pan placodal domain is defined by Six1, Eya1 and Pax2/8 expression (Bouchard et al., 2010) and these genes are suggested to promote generic placodal properties (Schlosser and Ahrens, 2004, Schlosser, 2005, 2010). The pan-placodal domain is later subdivided into the anterior and posterior placodal area; the otic placode originating from the latter (Schlosser and Ahrens, 2004, Schlosser, 2010). The otic placode is induced by signals from the underlying paraxial mesoderm and the adjacent neural plate (Yntema, 1950, Gallagher et al., 1996, Torres and Giráldez, 1998, Rinkwitz et al., 2001, Groves, 2005). Members of the fibroblast growth factor (FGF) family, such as Fgf3, Fgf8, and Fgf10, have been shown to play a role in otic placode induction, although the specific FGF varies across vertebrates (Groves, 2005, Schlosser, 2010). Mice mutant for these Fgf genes have absent or reduced otic placodes and overexpression of FGFs in chick, mouse, Xenopus laevis, and zebrafish results in otic vesicle expansion and extra otic vesicles (Lombardo et al., 1998, Vendrell et al., 2000, Alvarez et al., 2003, Pauley et al., 2003, Wright and Mansour, 2003, Groves, 2005, Schimmang, 2007). These FGFs induce Pax8, Pax2, and Sox3 5 expression, among others, in the otic placode (Hans et al., 2004, Solomon et al., 2004, Mackereth et al., 2005, Sun et al., 2007). Since FGFs also play a role in epibranchial and lateral line placode induction (Baker and Bronner-Fraser, 2001, Sun et al., 2007, Schlosser, 2010), additional factors are needed to further specify the otic placode. Other factors, such as Wnts, bone morphogenetic proteins (BMPs), and Notch, have been shown to play an additional role in otic placode induction (Ladher et al., 2000, Rinkwitz et al., 2001, Solomon et al., 2004, Ohyama et al., 2006, Ohyama et al., 2007, Park and Saint-Jeannet, 2008, Urness et al., 2010). In Xenopus, the otic placode is first visibly recognized at stage 21 (Nieuwkoop and Faber, 1994), though otic placode markers, for example Pax8 and Pax2, are expressed earlier (Schlosser and Ahrens, 2004). Following induction, the otic placode undergoes invagination to form a cup and later closes off to form the otic vesicle that is completely separated from the overlying ectoderm (Alvarez et al., 1989, Torres and Giráldez, 1998, Rinkwitz et al., 2001, Fekete and Wu, 2002, Bever et al., 2003). In Xenopus, the vesicle forms by stage 27 and has separated from the ectoderm by stage 28 (Nieuwkoop and Faber, 1994). All inner ear cell types: hair cells, supporting cells, and inner ear afferents arise from the otic vesicle (Fekete and Wu, 2002). Afferent sensory neurons that will innervate vestibular and auditory sensory epithelia differentiate and delaminate relatively early from the otic vesicle (Torres and Giráldez, 1998, Rubel and Fritzsch, 2002, Riley and Phillips, 2003, Alsina et al., 2009) following upregulation of neurogenin 1(Neurog1) and Neuronal differentiation 1 (Neurod1) (Ma et al., 1998, Ma et al., 2000, Jahan et al., 2010a). Expression of the earliest neuronal marker, Neurog1, is first upregulated in the otic placodes at stage 20-22 in Xenopus embryos (Nieber et al., 2009). The otic, or eighth, 6 ganglion (VIII) is first detected by stage 31 in Xenopus (Nieuwkoop and Faber, 1994, Quick and Serrano, 2005) and axons reach the hindbrain by stage 34 in axolotls (Fritzsch et al., 2005a), and at comparable stages in Xenopus. The ganglion will later separate into the vestibular and auditory ganglia (Torres and Giráldez, 1998). After the otic vesicle has formed, the walls undergo growth and folding to create a series of interconnected chambers that will eventually form the various components of the inner ear (Riley and Phillips, 2003). The initial process of forming the ear is relatively conserved across most vertebrates, but differences arise in the formation of specific epithelia in various organisms. The formation of the pars superior, comprised of the utricle and semicircular canals, is highly conserved in vertebrates, whereas the formation of the pars inferior, comprised of the saccule and auditory component(s), has more variation across different animals (Riley and Phillips, 2003). In general, the endolymphatic duct, which is used to maintain proper levels of endolymph in the inner ear (Riley and Phillips, 2003), is the first structure to develop (Morsli et al., 1998, Bever et al., 2003, Riley and Phillips, 2003) and in Xenopus, this occurs by stage 37 (Quick and Serrano, 2005). A single sensory epithelia patch develops in the otic vesicle and eventually separates into the individual components (Fritzsch et al., 2001a). From this sensory patch, the semicircular canal cristae, utricle, and saccule are the first to develop followed by the auditory sense organ(s) (Morsli et al., 1998, Bever et al., 2003, Riley and Phillips, 2003, Quick and Serrano, 2005). In a study involving paint filling of Xenopus ears, the formation of the semicircular canals is initiated at stage 43 and is completed by stage 47; the utricle and saccule are prominent by stage 46, the amphibian papilla and basilar papilla are prominent by stage 47, and lagena is prominent by stage 49-50 (Bever 7 et al., 2003). However, histological evidence puts sensory epithelia development at slightly earlier stages for each (Paterson, 1949, Quick and Serrano, 2005). The earliest hair cells, observed by F-actin staining for stereocilia bundles, are observed as early as stage 31 in Xenopus and the appearance of a separate pars superior and pars inferior are formed by stage 42 (Quick and Serrano, 2005). It is also at this time when the animals begin to swim (Quick and Serrano, 2005). Between stages 45 and 47, the vestibular sensory epithelia components differentiate and between stages 47 and 50, the auditory sensory epithelia components differentiate (Quick and Serrano, 2005). Evolution of the Inner Ear Evolution of new cells, tissues, and organs likely occurs as a duplication event at the sensory cell level, followed by segregation and diversification (Duncan and Fritzsch, 2012) comparable to the well-known molecular events. The perception of gravity, movement, and sound by the inner ear depends upon the mechanosensory hair cell. It is thought that the mechanosensory cell is at the base of the evolution of the ear and other mechanosensory systems (Duncan and Fritzsch, 2012). In other words, the ciliated mechanosensory cell existed prior to the evolution of the inner ear. The ancestor for the mechanosensory cell is the single-celled choanoflagellates. These choanoflagellates have a single kinocilium surrounded by microvilli (Fritzsch et al., 2007, Fritzsch and Straka, 2013). Similarly, during development, the vertebrate hair cell initially forms a central kinocilium surrounded by stereocilia, but later adopts the asymmetric organization of a polarized kinocilium and stereocilia (Duncan and Fritzsch, 2012). Mechanosensory cells exist in other non-vertebrates such as ascidians, molluscs, cnidarians, and insects; each 8 serving a unique purpose, and are most likely homologous to the vertebrate hair cell (Burighel et al., 2011). The sister group to vertebrates within Deuterostomes are the tunicates (Delsuc et al., 2006, Burighel et al., 2011). In this group, the ascidians have two organs, the capsular organ and the coronal organ. The capsular organ has a sensory macula containing 5-6 cilia-containing primary sensory cells (contain both cilia and an axon) that are believed to perceive vibrations. These capsular organs were thought to have evolved from isolated or clustered primary sensory cells aggregating into the organ. Due to their location, organization and basic function, these primary mechanosensory cells are thought to be homologous with the vertebrate inner ear (Burighel et al., 2011). In fact, these ascidian cells originate from a Pax2/5/8-expressing region, similar to that of the inner ear (Wada et al., 1998, Bassham et al., 2008, Bouchard et al., 2010). The other organ, the coronal organ, consists of a row of ciliated secondary sensory cells that make both afferent and efferent synapses with neurons (Burighel et al., 2003, Burighel et al., 2011). These ciliated cells are flanked by supporting cells (Burighel et al., 2011). This organization is quite similar to that of the vertebrate inner ear, which consists of separate secondary mechanosensory cells and neurons rather than a single primary sensory cell (Fritzsch and Beisel, 2004, Pan et al., 2012). It is thought that the secondary mechanosensory cells and neurons are derived from the primary mechanosensory cell (Duncan and Fritzsch, 2012). The derivation of a separate mechanosensory hair cell and neuron from an ancestral primary mechanosensory cell with an axon is thought to have arisen from the duplication and diversification of the basic helix-loop-helix (bHLH) atonal transcription 9 factor. Atonal bHLH genes evolved with multicellular organisms (Seipel et al., 2004) and are associated with photo-and mechanosensor development (Fritzsch et al., 2007). The atonal bHLH family consists of Atonal homolog 1 (Atoh1), Neurog1 and Neurod1. The ancestral atonal gene multiplied and split giving rise to Atoh1 and Neurog1 (Fritzsch and Beisel, 2003, 2004). The newly-generated genes could then be assigned novel functions (Fritzsch and Beisel, 2001, 2004, Pan et al., 2012). In invertebrates, the primary mechanosensory cells that contain an axon rely on atonal for their development. In vertebrates, hair cells depend on Atoh1 for their development and the afferent neurons depend on Neurog1 (Ma et al., 1998, Bermingham et al., 1999, Ma et al., 2000, Fritzsch et al., 2010). It is thought that the sensory precursor cells underwent an additional round of cell division to give rise to both the hair cell through Atoh1 expression and the sensory neuron through Neurog1 expression, thus suggesting a clonal relationship between the two (Fritzsch and Beisel, 2003, 2004). In addition, neurons must upregulate Neurod1 to differentiate as neurons (Jahan et al., 2013). Neurod1 inhibits Atoh1 expression and in the absence of Neurod1, some neurons differentiate as hair cells (Jahan et al., 2010b). It is thought that hair cells then became restricted to the placodal region to form an aggregation of hair cells in a sensory epithelia that over time would evolve to form the first ear (Duncan and Fritzsch, 2012). The earliest ear likely existed as a single sensory epithelia, likely a gravistatic sensor, found in most free-swimming animals (Markl, 1974, Fritzsch et al., 2007, Duncan and Fritzsch, 2012) and it is from this simple gravistatic-sensing ear that all other vertebrate ears arose. Of the vertebrate ears, the ear of the hagfish is the most simple and thought to be the closest to the ancestral ear (Fritzsch and Beisel, 2004). To achieve up 10 to the nine distinct sensory epithelia in gymnophionans (Fritzsch and Wake, 1988), or any of the more derived vertebrate ears, duplication, segregation, and specialization of the existing sensory epithelia occurred (Fritzsch et al., 2007). Motor Neuron Targeting The vertebrate inner ear receives two kinds of fibers: afferents that come from placodally-derived sensory neurons (Rubel and Fritzsch, 2002) and efferents which come from brainstem neurons (Simmons, 2002, Simmons et al., 2011). Whereas afferents carry information from the ear to the brain, efferents provide a feedback loop to modify mechanical stimuli information processing at the periphery. In mammals, efferents project bilaterally from their origin in the hindbrain, through the vestibulocochlear nerve, to innervate both inner ears; however, in other vertebrates, projections are mostly ipsilateral, but with some contralateral innervation (Holt et al., 2011). The origin of the auditory efferents in mammals is the superior olivary complex, whereas the vestibular efferents originate outside the vestibular nucleus, near the facial nerve, in the hindbrain (Brown, 2011, Holt et al., 2011). All other non-mammalian vertebrates have a single population of efferent neurons originating partially within or near the facial motor nucleus in the hindbrain (Holt et al., 2011, Köppl, 2011). In amphibians and other lateral line-containing vertebrates, the efferent nucleus referred to as the octavolateralis nucleus and gives rise to efferents innervating both the lateral line neuromasts as well as the vestibular and auditory hair cells (Hellmann and Fritzsch, 1996, Fritzsch, 1999, Holt et al., 2011). The close proximity of the efferents to the facial branchial motor neurons (Roberts and Meredith, 1992) and their similarities during development, has led to the 11 suggestion that the inner ear efferents are rerouted facial branchial motor neurons (Fritzsch and Nichols, 1993). The inner ear efferents and the facial branchial motor neurons both exit the cell cycle near the floorplate in rhombomere 4 of the hindbrain and later migrate to their final location (Simmons et al., 2011). Although their nuclei migrate away from each other, the inner ear efferents and facial branchial motor neuron axons run together through the facial genu before diverging to the eighth and seventh cranial nerves, respectively (Simmons et al., 2011). In the absence of inner ear afferent neurons, the inner ear efferents project with the facial nerve (Ma et al., 2000). Inner ear efferents may be guided to reach the ear by the transcription factor, GATA3, which is uniquely expressed in the inner ear efferents and is absent from the facial branchial motor neurons (Karis et al., 2001). Knock out of Gata 3 results in most inner ear efferents projecting along the facial nerve (Duncan et al., 2011). Thus the efferent population was likely rerouted from the facial branchial motor neurons after the emergence of Gata3 and of the inner ear. In vertebrates, the ear develops at the rostral boundary of the anteriormost, first forming somite (Cooke, 1978, Chung et al., 1989, Huang et al., 1997) and paraxial mesoderm anterior to the ear does not form somites (Noden and Francis-West, 2006) but may form somitomeres. Furthermore, in vertebrates, the most rostral somite is reduced in size to accommodate the ear. In animals without ears, such as amphioxus, somites are found more rostrally (Bardet et al., 2005). Taken together, these data could be interpreted to imply that in vertebrates, the ear develops in place of a somite or somitomere. If true, the facial branchial motor neurons that were destined to innervate the somite- or 12 somitomere-derived muscle fibers may have been rerouted to innervate the ear when the ear evolved in ancestral vertebrates (Fritzsch et al., 2007). That a subpopulation of facial branchial motor neurons was able to acquire a novel target, the inner ear, proposes a bigger question of how motor neurons acquire a novel target in general. Synaptic contacts likely originated in evolution as connections between sensory-motor neurons and muscle tissue in diploblastic animals, such as jellyfish, (Seipel et al., 2004) forming a monosynaptic reflex network from a sensory cell directly to a motor cell. As animal cell types diversified, the sensory-motor interface evolved in complexity, forming muscle fibers, motor neurons, sensory neurons, and interneurons (Fritzsch and Glover, 2007). As tissues diversified in the course of evolution, motor neurons acquired novel targets. Vertebrate motor neurons have evolved to form synapses on a variety of targets originating from developmentally different sources including mesoderm-derived muscle fibers, epithelial placode-derived neurosensory cells and neurons (i.e. the inner ear hair cells and neurons), and neural crest-derived autonomic ganglia (Eisen, 1999, Fritzsch, 1999, Takano-Maruyama et al., 2010, Simmons et al., 2011). Of all cranial motor neurons, only the facial nerve innervates targets from three different developmental origins: branchial arch-derived muscle by branchial motor neurons, neural crest-derived ganglia by visceral motor neurons, and placodally-derived inner ear hair cells by inner ear efferents (Fritzsch and Northcutt, 1993). While the inner ear efferents are now classified as separate from the facial nerve as discussed above, their proposed origin as rerouted facial branchial motor neurons merges them with this group. The glossopharyngeal nerve also innervates more than one tissue type: muscle, ganglia, 13 and in aquatic vertebrates such as Xenopus laevis, hair cells of the placodally-derived posterior lateral line (Hellmann and Fritzsch, 1996). Given the ability of some motor neurons to innervate targets from a variety of origins, the possibility exists that conserved core molecular machinery exists that allows multiple motor neuron types to recognize a particular target. However, some tissues are only innervated by one motor neuron type, even when other motor neuron types project to proximal territories as is the case with the innervation of the trapezius muscle by the spinal accessory nerve rather than spinal motor neurons (Boord and Sperry, 1991, Sienkiewicz and Dudek, 2010, Dudek et al., 2011). This suggests that there must be some limitation to the potential promiscuity of motor neurons for targets of different embryological origin. In order for a vertebrate effector cell to be able to respond to input from a motor neuron, it must have the ability to detect acetylcholine (ACh) input. One thing in common that all targets receiving direct motor input have is the presence of nicotinic ACh receptors (nAChRs) (Zuo et al., 1999, Vernino et al., 2009). nAChRs are comprised of a pentamer of various subunits: α, β, δ, ε, and γ (Tsunoyama and Gojobori, 1998, Jones and Sattelle, 2004, Albuquerque et al., 2009). Of these subunits, the α subunit is required for the receptor to bind ACh (Jones and Sattelle, 2004). The original α sequence has diversified, giving rise to the 10 different isoforms present today (Jones and Sattelle, 2004). Of the 10 α subunits, α9 and α10 are the most ancestral (Sgard et al., 2002, Franchini and Elgoyhen, 2006, Katz, 2011, Lipovsek et al., 2012). The α9 and α10 subunits are expressed only in the ear, pituitary gland, lymphocytes, and keratinocytes (Sgard et al., 2002, Katz, 2011, Kawashima et al., 2012) and activation of nAChRs containing these subunits results in cell hyperpolarization or desensitization instead of 14 depolarization as with other nAChRs (Elgoyhen and Katz, 2012, Kawashima et al., 2012, Lipovsek et al., 2012). The more derived α subunit, α1, is present in muscle tissue (Tsunoyama and Gojobori, 1998, Albuquerque et al., 2009, Katz, 2011, Elgoyhen and Katz, 2012). The remaining α subunits are expressed in neurons (Tsunoyama and Gojobori, 1998, Albuquerque et al., 2009, Katz, 2011, Elgoyhen and Katz, 2012). Given that all motor neuron targets contain nAChRs, and tissues that are not directly innervated by motor neurons do not contain these receptors, it is likely that the presence of nAChRs and associated molecules to form synaptic contacts is necessary for general motor neuron development, much like Islet-1 (Ericson et al., 1992, Inoue et al., 1994). However, while the presence of nAChRs might be necessary for motor innervation, it is not sufficient as nAChR-containing lymphocytes are never innervated. Instead, in lymphocytes, ACh present in the bloodstream binds to nAChRs and muscarinic AChRs to modulate immune function by downregulation or upregulation of proinflammatory cytokines respectively (Kawashima et al., 2012). In contrast, tissues that do receive direct motor innervation, such as muscle, autonomic neurons, and inner ear hair cells, contain three accessory molecules: muscle specific receptor tyrosine kinase (MuSK), receptor-associated protein of the synapse (rapsyn), and a transmembrane protein RIC-3. These molecules have been shown to be important for synapse formation (Apel et al., 1997, Sanes et al., 1998, Lin et al., 2001, Osman et al., 2008) as they are necessary for receptor assembly and clustering at the neuromuscular junction (Apel et al., 1997, Sanes et al., 1998, Lin et al., 2001). In addition, rapsyn and RIC-3 have been shown to be necessary and sufficient for functional nAChRs containing α9 and α10 at the cell surface of inner ear hair cells (Osman et al., 2008). Thus, not only are nAChRs 15 required for motor neuron innervation, but accessory molecules are needed as well. From this it follows that the specific α nicotinergic subtype present in the accessory moleculecontaining target tissue, together with the specific LIM code in different motor neuron populations (Tsuchida et al., 1994), could determine the degree of normal motor neuron to target specificity or plasticity, in addition to other unknown molecular contributions. Inner Ear Sensory Neuron Guidance Vestibular and auditory neurons delaminate from the developing otic vesicle (Torres and Giráldez, 1998, Rubel and Fritzsch, 2002, Riley and Phillips, 2003, Alsina et al., 2009) and migrate away to form the vestibular and auditory ganglia (Maklad and Fritzsch, 2003). The neurons differentiate as bipolar neurons, sending their dendrites and axons to the inner ear hair cells and to the CNS, respectively (Fritzsch et al., 2002). Vestibular sensory neuron axons enter the hindbrain at the level of the lateral vestibular nucleus, bifurcate, and send an ascending branch to the superior vestibular nucleus and the cerebellum and a descending branch to the medial and inferior vestibular nuclei (Büttner-Ennever, 1992). Auditory sensory neurons enter the cochlear nuclei, bifurcate, and send axons to the anteroventral cochlear nucleus, the posteroventral cochlear nucleus, and the dorsal cochlear nucleus (Rubel and Fritzsch, 2002). The auditory sensory neurons project to the cochlear nuclei in a tonotopic manner that maintains the frequencyspecific organization of the cochlea (Rubel and Fritzsch, 2002). Similar to the highlyspecific organization of the auditory nuclei, vestibular sensory neurons innervating the various sensory epithelia partially segregate (Kevetter and Perachio, 1986, Maklad and Fritzsch, 1999, 2003), though it is not a complete segregation as fibers from different sensory epithelia do overlap in the vestibular nuclei (Birinyi et al., 2001, Maklad and 16 Fritzsch, 2002). It is possible that the partial overlap functions to integrate semicircular canal input with that of the utricle and/or saccule for a given movement (BüttnerEnnever, 1992, Maklad and Fritzsch, 2002, Straka et al., 2002, Maklad and Fritzsch, 2003, Newlands and Perachio, 2003) to allow for precise and coordinated compensatory eye, head, and body movements (Büttner-Ennever, 1992, Maklad and Fritzsch, 2002). While much is known about where the inner ear vestibular sensory neurons project to, the mechanisms used by these neurons to project to their specific location in the vestibular nuclei remain largely unknown. The eye has been extensively studied and is developmentally related to the ear through shared molecular processes. For example, jellyfish express PaxB in both the statocyst and eye (Kozmik et al., 2003). PaxB has evolved into Pax 6 and Pax 2/5/8 (Fritzsch and Piatigorsky, 2005, Galliot et al., 2009). The highly-conserved Pax 6 is involved in eye development across nearly all species (Gehring and Ikeo, 1999), whereas Pax 2/5/8 is involved in ear development (Pfeffer et al., 1998, Heller and Brändli, 1999, Bouchard et al., 2010). Elimination of Pax 6 or Pax 2/5/8 leads to the absence or severe reduction of the eye and ear, respectively (Hoge, 1915, Gehring and Ikeo, 1999, Bouchard et al., 2010). Retinal ganglion cells (RGCs) also depend upon Pax 2 for proper guidance (Torres et al., 1996, Mansouri et al., 1999). In addition to Pax, the bHLH Atonal transcription factor is important for both eye and ear development. In Drosophila melanogaster, atonal is expressed in both photoreceptors and chordotonal organs (Jarman et al., 1995). Atonal has since duplicated and diversified. In the eye, Atoh7 is expressed in the RGCs and is important for their development (Kanekar et al., 1997, Brown et al., 1998, Brown et al., 2002, Skowronska-Krawczyk et al., 2009). Overexpression of Atoh7 17 in Xenopus resulted in an increase in RGCs (Brown et al., 1998) and inactivation of Atoh7 in zebrafish and mouse results in the near absence of RGCs in the retina (Brown et al., 2001, Kay et al., 2001). In the ear, Atoh1 is important for hair cell formation, whereas other atonal family members, Neurog1 and Neurod,1 are important for the development of sensory neurons (Fritzsch et al., 2010). Conditional deletion of Neurod1 in the mouse inner ear of mice resulted in a reduction in neuron number, aberrant central projections, and transformation of neurons into hair cells as a result of disinhibition of Atoh1 (Jahan et al., 2010a, Jahan et al., 2010b). These data suggest that Neurod1 plays a role in axon guidance to central targets, in addition to its role in neuronal differentiation, which is supported by the ability of Neurod1 to replace Atoh7 in retinal ganglion cells for normal central projections (Mao et al., 2008). The molecular similarities between the ear and the eye would suggest that the ear may use similar mechanisms as the eye for guiding axons to their proper locations in the CNS. In the eye, vertebrates use concentration gradients of EphA and ephrin-A along the nasal-temporal axis of the retina and anterior-posterior axis of the superior colliculus/tectum to guide retinal ganglion axons to the appropriate region of the CNS (Clandinin and Feldheim, 2009) to maintain appropriate representation of the visual field. Eph receptors and their ligands, ephrins, are involved in axon guidance, among other roles. Binding of ephrin to an Eph receptor generally results in repulsion (Kullander and Klein, 2002). In the eye, EphA concentrations are the greatest in the temporal retina and lowest in the nasal retina. In the superior colliculus/tectum, ephrin-A concentrations are higher in the posterior and lower in the anterior. Thus, RGCs from the temporal retina, containing high EphA receptor are repulsed by the higher concentration of ephrin-A in 18 the posterior superior colliculus/tectum and terminate in the anterior superior colliculus/tectum, where ephrin-A concentrations are low. In contrast the RGCs from the nasal retina are not repulsed by ephrin-A and terminate in the posterior superior colliculus/tectum (Clandinin and Feldheim, 2009). The retinal projections to the superior colliculus/tectum are further refined in some vertebrates to account for input from both eyes. In higher mammals, (e.g. cats and primates), the eyes are located in the front of the head, resulting in a significant overlap of part of the visual field between the two eyes (Schmidt and Tieman, 1985, Leamey et al., 2009). This overlap of the visual field results in the segregation of eye-specific projections to the visual cortex into stripes, referred to as ocular dominance columns (Hubel and Wiesel, 1977, Wiesel, 1982), whereas when there is no overlap of the visual field, there is no formation of ocular dominance columns (Schmidt and Tieman, 1985). The formation of ocular dominance columns can be induced in lower vertebrates with lateral eyes, (i.e. frogs and fish), by transplantation of an extra eye such that two eyes project to the same tectum (Constantine-Paton and Law, 1978, Springer and Cohen, 1981, Constantine-Paton, 1982, Easter, 1983). Various experiments have demonstrated that the formation of ocular dominance columns is primarily activity-based (Hubel and Wiesel, 1965, Swindale, 1981, Meyer, 1982, Swindale and Cynader, 1983, Boss and Schmidt, 1984, Mower et al., 1984, Reh and Constantine-Paton, 1985) and presents a compromise between the molecularly-specified targeting and the individual activity of the neurons. It seems logical to suggest that the ear may use both molecular and activity-based mechanisms to guide cochlear afferents (Rubel and Fritzsch, 2002) and projections from the various end organs of the vestibular system (Maklad and Fritzsch, 2003, Newlands et 19 al., 2003) to the proper location in the CNS, comparable to the eye. Ephs and Ephrins have been identified in the auditory system and have been demonstrated in mice and chicken to play some role in axon guidance (Allen-Sharpley and Cramer, 2012, AllenSharpley et al., 2013, Defourny et al., 2013). Ephs and Ephrins have also been shown to be expressed in vestibular neurons and their peripheral vestibular hair cell targets (Bianchi and Liu, 1999), though a specific role in vestibular guidance, especially centrally, has not been determined. Support for molecular targeting in the vestibular system comes from selective labeling of various vestibular sensory epithelia. Maklad and Fritzsch (2002) demonstrated that vestibular afferents made no large errors, such as targeting extra-vestibular areas. This initial targeting was activity-independent as observations were made prior to the onset of vestibular sensation (Maklad and Fritzsch, 2002, Maklad and Fritzsch, 2003, Maklad et al., 2010). However, activity-based guidance may play a later role in refining projections since changes in the pattern of connectivity in the vestibular nucleus from sensory neurons innervating individual sensory epithelia occur around the onset of a functional vestibular system (Maklad and Fritzsch, 2003). Exposure of rats to microgravity resulted in decreased branching in the vestibular nucleus from the gravity-sensing saccule (Ronca et al., 2000, Fritzsch et al., 2001a), which suggests that activity plays a role in refining vestibular processes. Thus the hypothesis is that sensory neurons may first be guided by molecular mechanisms to the vestibular nuclei, which are further refined by the specific activity from each sensory epithelia with particular head and/or body movements. 20 Hindbrain Neurons Once inside the hindbrain, vestibular afferents from the various inner ear sensory epithelia synapse on neurons in the vestibular nuclei. Vestibular nuclei neurons project both caudally to the spinal cord and rostrally to the cerebellum and extraocular motor nuclei where they are involved in several reflexes with the proprioceptive and visual systems to maintain balance, posture, and gaze during movement. To maintain balance and posture, vestibular neurons project rostrally to spinal motor neurons, which synapse on extensor and flexor muscles in the neck and on extensor muscles of the limbs to control head and body movements (Glover, 1996, Uchino and Kushiro, 2011). To maintain proper gaze, vestibular nuclei neurons project both contralaterally and ipsilaterally to neurons in the extraocular motor nuclei neurons to coordinate eye movement in a process known as the vestibulo-ocular reflex (VOR) (Glover, 2003, Straka, 2010). The VOR is an open loop system: vestibular sensory neuron to vestibular nuclei neuron to extraocular neuron. The loop is closed by relay of the retinal image back to the extraocular motor nuclei where they also integrate the intended body movement from the spinal cord and actual body movement from the vestibular nucleus (Straka, 2010). In addition, vestibular neurons project to the floccular lobe of the cerebellum (Uchino and Kushiro, 2011). Purkinje cells in the flocculus receive head and eye movement information via the parallel fibers and retinal image motion through the climbing fibers where they play a role in VOR learning (Gittis and du Lac, 2006). Auditory neurons synapse on neurons in the cochlear nuclei. From there, cochlear nuclei neurons project to the superior olivary complex to localize sound and to the lateral lemniscus and superior colliculus, which are part of the auditory pathway leading to the 21 cortex (Cant and Benson, 2003). In addition, cochlear nuclei neurons project to the ventrolateral medullary nucleus to control autonomic functions (Kamiya et al., 1988), and in some mammals, to the pontine and medullary reticular formation to control cardiovascular and respiratory function as well as an auditory startle response (Cant and Benson, 2003). Neurons in the cochlear nuclei are dependent upon input from the inner ear for their survival (Levi-Montalcini, 1949, Parks, 1979, 1981, Goodman and Model, 1988, Fritzsch, 1990). Unilateral removal of the inner ear early in chick development led to a reduction in the volume and number of neurons in the cochlear nuclei, as compared to the unoperated side, after embryonic day (E) 11 (Levi-Montalcini, 1949). The cochlear nuclei neurons continued to die over time. By E21, there were 32% and 82.5% fewer neurons than observed on E11 in two of the cochlear nuclei (Levi-Montalcini, 1949). This reduction in volume and number of neurons was later confirmed and extended (Parks, 1979, Ryugo and Parks, 2003). The time point at which the number of neurons in the cochlear nuclei began to diminish corresponded to the onset of afferent activity via action potentials (Jackson et al., 1982). Blocking action potentials by application of tetrodotoxin to the sensory neurons resulted in a reduction of volume and number of neurons in the cochlear nuclei, similar to that seen with ear ablation (Pasic and Rubel, 1989, Sie and Rubel, 1992). Together, these data would suggest that most of the initial neuronal development occurs in the hindbrain without excitatory input from primary sensory neurons and that later, without this activity and/or activity-related neurotrophin release, cell death and atrophy of remaining hindbrain neurons occurs (Rubel and Fritzsch, 2002). 22 However, other studies have shown that this dependence on a presynaptic neuron is not permanent, which would imply that there is a critical period for cell survival (Rubel and Fritzsch, 2002). Removal of the cochlea in chicks 2 and 6 weeks post-hatch resulted in a 30% loss of cochlear nucleus magnocellularis neurons (Born and Rubel, 1985), similar to that seen when the ear was ablated at embryonic stages (Parks, 1979); however, in adult chickens (66 weeks post-hatch), there was no significant loss of cochlear nucleus neurons following cochlear removal (Born and Rubel, 1985). Similar critical periods exist in mammals. Removal of the cochlea in postnatal day (P) 5 mice resulted in the loss of 61% of neurons in the cochlear nucleus compared with only 1% loss when the cochlea was removed at P14 (Mostafapour et al., 2000). In gerbils, removal of the cochlea prior to P7 resulted in cell death in 45-88% of cochlear nucleus neurons, whereas removal of the cochlea after P9 resulted in virtually no cell death (Tierney et al., 1997) The loss of susceptibility to presynaptic neuron ablation occur around the time of hearing onset, P12 in gerbils (Woolf and Ryan, 1984, 1985, Tierney et al., 1997), which supports at least an early necessity of activity for cell survival. As mentioned previously, early embryonic removal of the chick ear resulted in death of most cochlear nuclei and severe atrophy of the remaining neurons (Levi-Montalcini, 1949, Parks, 1979); however, virtually no cell death occurred in the vestibular nuclei (Levi-Montalcini, 1949), whose neurons exit the cell cycle much earlier (Altman and Bayer, 1980), suggesting they may be past the critical period at the time these manipulations took place. In this context it is important to note that even though early ear removal results in significant cochlear nucleus cell loss, some cells do survive. Unfortunately, the reason for why some cells die and others do not is not well understood. 23 The leading hypothesis to explain why some post-synaptic neurons survive while others die following early afferent deprivation is that two competing intracellular responses are initiated following afferent loss: apoptotic-like and survival pathways (Garden et al., 1994, Hyde and Durham, 1994a). This hypothesis is supported by several studies. For example, early processes of cellular degradation following afferent loss are constant across all cells, not just some (Steward and Rubel, 1985, Born and Rubel, 1988, Garden et al., 1994, Garden et al., 1995, Kelley et al., 1997), and oxidative enzyme activity first increases and then decreases following afferent loss (Durham and Rubel, 1985, Durham et al., 1993, Hyde and Durham, 1994b). Thus the cells that survive depend upon the effectiveness of the survival pathway to overcome apoptosis. Afferent neurons rely on neurotrophic factors provided by the hair cells of the ear and the target neurons for cell survival (Fritzsch et al., 2001b). In the ear, the neurotrophic factors are BDNF and NT-3, and loss of BDNF or NT-3 in the inner ear results in embryonic sensory neuron cell death (Fritzsch et al., 2001b). Changes in the amount of receptor for BDNF, Tyrosine Kinase B (TrkB), occur in the cochlear nucleus following ear ablation (Suneja and Potashner, 2002). The nature of the neurotrophic substance by which afferents support cochlear nucleus neurons has not been determined, although neurotrophic factors have been suggested to play a role. Levi-Montalcini proposed that loss of ‘neurotrophic’ support from the afferents following ear removal may cause the cell death in the cochlear nucleus (Levi-Montalcini, 1949). Surviving cells must either receive enough neurotrophic support elsewhere for survival or are past a critical phase when inner ear sensory neurons provide support. 24 These above-mentioned studies have increased our understanding of the importance of primary sensory input on higher-order neuron survival; however, these studies looked only at whole populations of cells when determining the critical period of cell survival and when determining why some cells survive while others do not following ear removal. Furthermore, due to technical limitations, ears can be removed in the chick only embryonically, and in the mouse, only postnatally, which prevents a complete analysis of the loss of afferent input over a longer period of time in these species. In fact, mice genetically engineered to lose all neurons (Ma et al., 2000) do not survive past birth and thus cannot be used to study the effect of neuronal loss in postnatal development. In addition, the effects on higher-order neurons following ear removal at early embryonic placodal stages have not been well studied, largely due to the unpredictable tendency of partial or complete otic placode regeneration (Levi-Montalcini, 1949, Waldman et al., 2007). In the hindbrain of the frog, an animal easily accessible across all developmental time points due to its external development, exists a single pair of cells that receive inner ear input, the Mauthner cells. Developmental effects of ear removal can thus be studied on this single neuron. Mauthner cells are a pair of large, easily identifiable, reticulospinal neurons at the level of the ear in the hindbrain of many aquatic animals (Herrick, 1914, Bartelmez, 1915). These cells are an important component of the escape reflex (Korn and Faber, 2005). Inner ear vestibular and auditory neurons of fish and premetamorphic amphibians form synapses on the lateral dendrite of the ipsilateral Mauthner cell. The Mauthner cell axon crosses the midline of the hindbrain and projects caudally to synapse onto contralateral spinal motor neurons to activate the C-start escape response (Korn and 25 Faber, 2005, Sillar, 2009). In addition to the ear, Mauthner cells also receive input from the lateral line and the eye that can add to the C-start escape response (Zottoli et al., 1987, Bezgina et al., 2000, Korn and Faber, 2005, Sillar, 2009). Several studies suggest that the Mauthner cell is dependent upon input for its development and/or survival. A study in axolotls (Ambystoma mexicanum) has shown the absence of the Mauthner cell in one-third of embryos following otic vesicle ablation at stage 27 (Piatt, 1969). In one embryo observed by Goodman and Model following ear ablation, the entire Mauthner cell was absent (1988). While Piatt (1969) suggested that the inner ear afferents are important for, and often a decisive factor for, the development of the Mauthner cell, Goodman and Model (1988) suggested that surgical perturbations may be the cause of the occasional Mauthner cell absence. In contrast to early ear removal, no Mauthner cells were absent when the ear was extirpated at later stages (stage 34), although dendritic branching was reduced (Kimmel et al., 1977, Fritzsch, 1990). Since early ear removal, before the critical period, results in significant loss of the cochlear nuclei neurons (Levi-Montalcini, 1949, Parks, 1979, Born and Rubel, 1985, Tierney et al., 1997, Mostafapour et al., 2000). These data would suggest that removal of the ear at the earlier stages (Piatt, 1969, Goodman and Model, 1988) prevented the differentiation and/or survival of the Mauthner cell, hinting at a critical period for afferent innervation on Mauthner cell survival and development. The inner ear not only affects the development of the Mauthner cell, but may have a role in the development of the lateral dendrite as well. In a study in axolotls, removal of the ear of midtailbud animals (stage 28-30) prior to the outgrowth of the VIIIth nerve resulted in significant reduction of dendritic branching of the Mauthner cell lateral 26 dendrites in areas normally receiving vestibular input (Goodman and Model, 1988). Ear removal at a slightly later stage in axolotls (stage 34) also resulted in reduction of dendritic branching (Kimmel et al., 1977), though the degree of dendritic loss across other stages has not been investigated. Removal of otic vesicles in zebrafish resulted in reduced branching of the Mauthner cell lateral dendrites (Kimmel, 1982). Likewise, ablation of the otic vesicle in stage 38 X. laevis embryos resulted in reduced Mauthner cell lateral dendrites (Fritzsch, 1990). The eye synapses on another Mauthner cell dendrite, the ventral dendrite. Enucleation of the eye resulted in delayed development of the Mauthner cell and diminished ventral dendrite volume in X. laevis and goldfish (Bezgina et al., 1999, Bezgina et al., 2000, Grigor'eva et al., 2010, Mikheeva et al., 2011). Increase in the lateral dendrite size in combination with reduction in the ventral dendrite was observed occasionally following eye enucleation (Grigor'eva et al., 2010, Grigorieva et al., 2012). Blocking nerve impulse activity in axolotls by grafting to them tetrodotoxin-containing newts did not affect Mauthner cell dendritic branching patterns, suggesting that that innervation itself, rather than neural activity, is important for dendritic development (Goodman and Model, 1990). However, spontaneous local synaptic vesicle release could still play a role in this tetrodotoxin model. Taken together, these data suggest that the ingrowing axons stimulate growth and development of the dendrites. This is further supported by a study in axolotls where an additional ear was transplanted rostral to the native ear. The Mauthner cells in these animals were claimed to display enhanced branching of the lateral dendrite (Goodman and Model, 1988) although no quantitative data to support this conclusion were provided. 27 Experimental Approach for Generating and Manipulating a ‘Novel’ Sensory System Ears from the frog, Xenopus laevis, were either transplanted from the same embryo or removed from a different same staged embryo to generate a ‘novel’ sensory system or remove an existing one, respectively. The manipulated ears provide data to better understand how the ear becomes innervated during development and, by logical inference, through evolution. Specifically, ears were transplanted caudally to the trunk to replace a somite or rostrally to the orbit to replace the eye to test whether any motor neuron can become an efferent to the inner ear. To further elucidate the properties of motor innervation, other tissues were transplanted to test for the ability of any tissue to receive motor input. In addition, ears were transplanted from a donor to a host embryo rostral to the native ear to create a three-eared frog with two tandem ears on one side. These ears were transplanted either in the native orientation to maintain similar activity between the two ears or rotated by 90 degrees to set up differential activity between the two ears in order to determine the extent to which molecular cues and activity-based guidance directs sensory axons to their locations within the vestibular nucleus. Finally, ears were removed or additional ears were transplanted to determine the effects of loss of input or additional input on the developing hindbrain target neuron, the Mauthner cell. Ears were removed over a time course to establish a potential critical period of necessity of input for Mauthner cell survival. Detailed descriptions and results of these experiments will be outlined in the following chapters. 28 CHAPTER II METHODS Animals Xenopus laevis embryos were obtained through induced breeding using human gonadotropin injection and fertilized with a sperm suspension in 1X Marc’s Modified Ringer’s Solution (MMR, see below), pH 7.8. Fertilized embryos were maintained in stock cultures in Petri dishes. Other than during manipulations, embryos were kept in 90mm Petri dishes containing 0.1X MMR (diluted from 1x MMR) until stage 46. X. laevis stages were as described by Nieuwkoop and Faber (1994). GFP mRNA injections For synthesis of green fluorescent protein (GFP) mRNA, plasmid template (pβGFP/RN3P) was linearized using SfiI and transcribed using T3 RNA polymerase from the mMessage mMachine kit (Ambion). Protocol was followed according to manufacturer’s directions. The jelly coat was removed using 2% cysteine in 0.1X MMR (diluted from 1X MMR) shortly after fertilization for embryos used in injections. Embryos were placed in a Ficoll solution (2% Ficoll 400, GE/Pharmacia, in 0.5X MMR) for 5 min. GFP mRNA was diluted with distilled water so that the final amount injected was 1 ng. Embryos were injected at the 2 to 4 cell stage using a calibrated glass needle controlled by a PicoInjector (Harvard Apparatus, Holliston, MA). Injections were made into each cell, keeping the total amount of mRNA per embryo constant. 29 Dextran amine injections Dextran amine (Fluorescein, 3000MW (Fritzsch, 1993)) was dissolved in water to make a 1% solution. The jelly coat was removed as described above. Embryos were placed in the Ficoll solution (2% Ficoll) for 5 min prior to injection. Embryos were injected at the one cell stage using a glass needle controlled by a Pico-Injector. Transplantations All transplantations were performed in 1x MMR pH 7.6-7.8, diluted from 10x stock (1M NaCl, 18mM KCl, 20mM CaCl 2 , 10mM MgCl 2 , 150mM Hepes) at room temperature. To determine the ability of various motor neurons to acquire a novel target, tissues were transplanted. For ear to trunk transplantations, otic placodes from the right side of stage 24-26 embryos were removed and transferred ipsilaterally more caudal, replacing a somite. For ear to orbit transplantations, otic placodes from the right side of stage 24-26 embryos were removed using fine tungsten needles and transferred ipsilaterally to the orbit, replacing the eye. In addition, otic placodes were removed from stage 24-26 embryos previously injected with GFP mRNA to label donor tissue and transplanted to the orbit of a non-injected host embryo of the same stage to replace the eye. To check the success rate of the ear transplantations, the ear was removed and reimplanted, but rotated by approximately 180°. Those rotated ears were monitored and scored as above (Table 3.1). In contrast to the caudal transplantation our success rate of normal differentiation of rotated ears was greater (approximately 92% vs. 67%). For somite transplantations, donor somite tissue from stage 24-25 embryos previously injected with GFP was removed using fine needles and transplanted to the orbit of a host stage 24-25 embryo to replace the eye. Alternatively, donor somite tissues from stage 30 24-25 embryos were placed in a Fluorescein dextran amine solution (10,000MW, 10%) for 5 min, rinsed and transplanted into host stage 25 embryos to replace the ear. For eyeto-muscle transplantations, donor eyes and surrounding tissue from stage 25 embryos previously injected with GFP were removed using fine needles and transplanted to the trunk of a host stage 25 embryo to replace approximately three to four somites (the size of the eye). For jaw muscle transplantations, donor jaw muscle from stage 45-46 donor embryos previously injected with dextran amine (1%) was removed and processed into small pieces. A small jaw muscle fragment was transplanted to the trunk of a host stage 25 embryo to replace a somite. For heart transplantations, donor heart tissue from stage 27 embryos, some having been previously injected with GFP mRNA, was removed using fine needles and transplanted to the orbit of a host stage 24-26 embryo to replace the eye. For liver transplantations, donor liver tissue from anesthetized (0.02% Benzocaine) (Crook and Whiteman, 2006) stage 42 embryos was removed and transplanted to stage 24-26 embryos to replace the eye. To determine whether inner ear sensory neurons use molecular and activity-based cues, ‘three-eared’ frogs were generated. Single otic placodes from donor X. laevis embryos were transplanted rostral to the native ear in host embryos between stages 25-27. Otic placodes were transplanted either in the native orientation or rotated by 90 degrees. The rationale being that ears in alignment should have little difference in activity, whereas when one is rotated by 90 degrees, the sensory epithelia will be offset from each other and there should be differential activity between the two ears in tandem. To study the effects of ear removal on the Mauthner cell, single otic placodes or otic vesicles were removed from stage 24, 25, 26, 27, 28, 30, 32, 34, 36, 38, and 40 31 embryos. Single otic placodes or vesicles were removed from stage 24-26 embryos and then immediately replaced to serve as a surgical control. Embryos were kept in 1xMMR for about 10-15 min to promote healing before transfer to 0.1xMMR. Healing was confirmed visually as a fusion of the ectoderm superficial to the otocyst with the ectoderm of the insertion side. Animals were reared and the transplantation was checked daily for continued growth. Appearance of otoconia was monitored and compared with the remaining untouched control ear. Success of ear to trunk transplantation was scored as three levels of success as follows: normal ear development with formation of otoconia; formation of an ear without recognizable otoconia; no ear development (Table 3.1). Completeness of transplanted ear formation in the orbit was observed to determine the success of transplantations (Fig. 4.1A-4.1C). Success of GFP-positive ear to orbit transplantations was observed as the presence of a third ear (Fig. 4.1D) and was confirmed to contain GFP using an epifluorescence microscope. Success of other tissue transplantations was monitored (Fig. 4.1E-4.1H). Movies were recorded of beating hearts in the orbit using a camera mounted to a dissecting microscope (Leica). Prior to fixation, swimming behavior was observed and dextran amine dyes were injected where applicable (see below). After embryos reached stage 46, they were anesthetized in 0.02% Benzocaine and fixed in 10% paraformaldehyde (PFA) by immersion. Swimming Behavior Animals were dropped into a 3”x4” swimming arena to observe their initial startle and swimming behavior. All initial behaviors were recorded and scored as the following: 32 normal (swimming upright), swiming on side, swimming upside down, swimming vertical, spinning, looping (Ferris wheel formation), spiraling (corkscrew formation). Dextran amine label Texas Red, rhodamine, or fluorescein 3000 molecular weight dextran amines were used to backfill from the spinal cord to the transplanted ear, from the transplanted ear to the spinal cord, and from the spinal cord into the hindbrain. Dextran amines were dissolved in distilled water and recrystallized on a tungsten needle (Fritzsch, 1993). Stage 46 embryos were anesthetized in 0.02% benzocaine (Crook and Whiteman, 2006) For dye filling of afferents and efferents to the ear, a small cut was made into the spinal cord adjacent to the transplanted ear and the dextran amine dye was applied. For transganglionic filling of afferents and retrograde filling of efferents, a small opening was made into the transplanted ear and the dextran amines were applied to the sensory epithelial surface inside the ear, which rapidly filled the entire ear vesicle with dye (Fig. 3.1I). For labeling of the Mauthner cell in the hindbrain, a small cut was made into the spinal cord rostral to the hindbrain and dextran amine dye was applied. The embryos were transferred to the above outlined 0.1X MMR buffer in a Petri dish to rinse off excess dye and then were transferred to a fresh Petri dish for 1-3h, depending upon the distance the dye needed to travel (2mm/h (Fritzsch, 1993)). The wash dish was changed frequently. Finally, the embryos were re-anesthetized and fixed in 4 or 10% PFA by immersion. Lipophilic dye label For ears transplanted caudally to the trunk, lipophilic dye-soaked filter paper (Fritzsch et al., 2005b, Tonniges et al., 2010) was used to backfill from the spinal cord to 33 the transplanted ear. Small pieces of dye-soaked filter paper were implanted into the spinal cord immediately rostral (NeuroVue™ Green) and caudal (NeuroVue™ Maroon) to the transplanted ear (Fig. 3.1H). For other embryos, small pieces of flattened dyesoaked filter paper (NeuroVue™ Red) were implanted immediately ventral to the spinal cord, entering from the contralateral side so not to destroy the nerves innervating the ear (Fig. 3.1J). For this injection, the dye would only contact the spinal motor neurons exiting the ventral spinal cord. For some embryos in which dextran amines were injected into the ear, lipophilic dye (NeuroVue™ Maroon) was implanted rostral and caudal to the ear into the nerves innervating the muscle to backfill to the spinal cord. For tissues transplanted to the orbit, small pieces of dye-soaked filter paper were flattened and implanted longitudinally into the midbrain (NeuroVue™ Maroon) and transversely into the hindbrain (NeuroVue™ Red) at the level of the native ear (Fig. 4.2A), thus primarily filling the oculomotor nerve as it decussates the midline of the midbrain and the trigeminal nerve as it exits the hindbrain respectively. Both oculomotor and trigeminal nerves normally send projections to or near the orbit and thus near the transplanted tissue in the orbit. For some embryos, dye-soaked filter papers were implanted into the native and transplanted ears to label fibers projecting to the brain. For tissues transplanted to the trunk, small pieces of dye-soaked filter paper (NeuroVue™ Red) were flattened and implanted immediately ventral to the spinal cord as described above for the ear to trunk transplantation. For somite tissue transplanted to replace the ear, small pieces of dyesoaked filter paper were implanted transversely into the hindbrain at the level of the trigeminal nerve (NeuroVue™ Red) and at the level of the vagus nerve (NeuroVue™ Maroon). For three-eared frogs, small pieces of dye soaked filter paper were flattened 34 and implanted into the native ear (NeuroVue™ Maroon) and transplanted ear (NeuroVue™ Red) to label fibers projecting to the hindbrain. Dyes were allowed to diffuse at room temperature for 15-24 hours, 36°C for 15-18 hours, or 60°C for 7 hours. Filter paper was removed prior to imaging. Embryos or dissected tissues were mounted on a slide in glycerol and images were taken with a Leica TCS SPE confocal microscope or TCS SP5 multi-photon confocal microscope. Quantification of percent overlap of sensory neurons in the vestibular nucleus of ‘three-eared’ frogs The percent overlap of sensory neurons in the vestibular nucleus of ‘three-eared’ frogs when the transplanted ear was either in the native orientation or rotated by 90 degrees was calculated using the Leica software. Three optical sections were selected for each animal, near the top, middle, and bottom of the stack. A region of interest line was drawn perpendicularly through the sensory neurons at the approximate midpoint rostralcaudal of the descending tract of the vestibular nucleus. Using the line profile intensity tool, an intensity histogram was provided for the channels representing the two populations of sensory neurons, belonging to the native and transplanted ear. The medial and lateral boundaries of the two histograms were determined manually by using the boundaries generated from a threshold omitting the bottom 25% of the relative intensity. Animals in which either of the fluorescent signals was weak, making it difficult to generate an accurate intensity profile, were not included in the analysis. The percent overlap was calculated by determining the percent of the narrower histogram contained within the wider histogram. The percent overlap obtained for each of the three optical sections was pooled to provide a percent overlap per animal. Animals were pooled to 35 provide the mean percent overlap for the two orientations of ‘three-eared’ frogs. The means and standard errors were calculated. Students t-test was used as a test for significance. In situ hybridization Whole-mount In situ hybridization for micro RNA (miR)-183 and miR-124 was performed on control and transplanted ears from stage 46 embryos as previously described (Pauley et al., 2003, Pierce et al., 2008). Briefly, locked nucleic acid probe for miR-183 and miR-124 (Integrated DNA Technologies, Coralville, IA) was labeled with digoxigenin (Roche Diagnostics GmbH, Mannheim, Germany). Fixed embryos were bleached overnight in 2ml Eppendorf tubes containing 3% hydrogen peroxide. Embryos were re-fixed with 4% PFA, washed in RNAse free PBS 3 times, digested with proteinase K treatment for 40 min for miR-183 or 5 min for miR-124 and then fixed again in 4%PFA. The embryos were washed again with PBS. After 1h incubation in hybridization mix at 60°C, 200 μl salmon sperm and 100ng riboprobe were added and the embryos were kept overnight at 60°C. Embryos were then washed with 2X SSC and then PBS, after which 2 μl RNAse A Enzyme (5mg/ml, 83U/mg) was added and embryos were kept at 37°C for 90 min. Embryos were then washed several times with 1X Wash solution (Roche), then incubated in 1X blocking buffer (Roche) for 1h and finally overnight in anti-digoxigenin antibody conjugated with alkaline phosphatase (Roche; diluted 1:2000 in 1X blocking buffer). The antibody solution was discarded and replaced by 1X wash solution once per hour and then kept overnight in wash solution. Embryos were rinsed with 1X detection buffer (Roche) and then detected with BM Purple. 36 Immunohistochemistry Control and transplanted ears, somites, hearts, and liver were immunostained with antibody against acetylated tubulin (Farinas et al., 2001), myosin (Myo) VI (Kaiser et al., 2008), and vesicular acetylcholine transporter (VAChT) (de Castro et al., 2009). Hindbrains were immunostained with antibody against 3A10 to label reticular neurons, including the Mauthner cell(s) (Liu et al., 2003, Patten et al., 2007). Since the 3A10 antibody did not label all of the dendrites filled with dextran amine dye (Fig. 5.3A-A”), this antibody was used only to confirm the presence or absence of the Mauthner cell. Cranial and lateral line nerves were immunostained with antibody against acetylated tubulin. Embryos were defatted in 70% ethanol from 30 min to overnight and then rehydrated in PBS for 1h. Embryos were blocked with 500μl 1% Triton X-100 and 500μl 5% normal goat serum (NGS) for one hour. Embryos were washed 3 times briefly in PBS and then incubated in either tubulin primary antibody (1:800; Cell Signaling Technology), Myo VI (1:400; Proteus Biosciences; a specific hair cell marker), VAChT (1:500; Sigma; identifies motor neuron terminals), or 3A10 (1:500; Developmental Studies Hybridoma Bank, University of Iowa) in diluted block (0.01% Triton X-100, 0.25% NGS in PBS) for 72h. Embryos were washed in PBS 3 times for an hour each and blocked for an hour. Embryos were then washed 3 times briefly in PBS and incubated in species-specific secondary antibody (1:500; Alexa) for 24h in the diluted block solution. Embryos were washed 3 times for an hour each. The embryos or tissues of interest were mounted on slides in glycerol. Confocal images were taken with a Leica TCS SPE confocal microscope or TCS SP5 multi-photon confocal microscope. 37 Plastic embedding for light and transmission electron microscopy Transplanted ears along with surrounding muscle tissue were fixed in 2.5% glutaraldehyde overnight and then washed in 0.1M phosphate buffer three times. Ears were then fixed in 1% osmium tetroxide for one hour and washed again in 0.1M phosphate buffer. Ears were then dehydrated in 70% ethanol overnight. Next, ears were further dehydrated in absolute ethanol with 5 washes, 10 min each, then put in a 1:1 solution of absolute ethanol to propylene oxide for 5 min, and finally propylene oxide, 5 washes, 10 min each. Ears were then infiltrated with a 1:1 solution of propylene oxide to resin overnight. Next, the propylene oxide was allowed to evaporate for 4 hrs and the resin was poured into a mold. This was incubated at 60°C for 24 hrs. Sections were cut with an ultratome using a diamond blade, 2μm thick for light microscopy and 100nm thick for electron microscopy. Thick sections were counterstained for light microscopic imaging with Stevenel’s blue (2% potassium permanganate and 1.3% methylene blue) at 60°C (del Cerro et al., 1980). Ultrathin sections were counterstained with uranyl acetate and lead citrate and imaged in a Jeol 1230 TEM microscope. Three-dimensional reconstruction of Mauthner cells Embryos injected with dextran amines into the spinal cord were used for threedimensional (3D) reconstruction of Mauthner cells (Kopecky et al., 2012). Briefly, brains were mounted ventral-side up in glycerol on a microscope slide. Confocal z-series images at 1-3 µm were taken of the hindbrain using a Leica TCS SP5 confocal microscope. Z-series stacks were loaded into Amira Version 5.4 software for manual segmentation and volume rendering as described previously (Kopecky et al., 2012). 38 Dendritic Branching Analysis The total number of terminal branches for each Mauthner cell lateral dendrite was counted. Those with the greatest and the least difference between left and right Mauthner cells were removed in all populations as outliers for the calculations of mean and standard error. The actual difference between left and right Mauthner cells was counted by subtracting the number of branches on the left from the right Mauthner cell. In addition, the absolute difference was calculated by subtracting the smaller branch number from the larger regardless of side. Students paired t-test was used to determine whether a significant difference in branch number occurred between control (left) and treated (right) Mauthner cells. A Bonferonni multiple comparison adjustment was performed following an ANOVA on the differences between left and right Mauthner cells across all groups. Sholl analysis (Sholl, 1953) was performed to determine the branching pattern of the lateral dendrite of Mauthner cells. Concentric circles spaced 25 µm were drawn around the cell soma. The number of lateral dendritic branches that crossed each circle were counted for the right Mauthner cell. Students t-test was used as a test for significance. 39 CHAPTER III TRANSPLANTATION OF XENOPUS LAEVIS EARS REVEALS THE ABILITY TO FORM AFFERENT AND EFFERENT CONNECTIONS WITH THE SPINAL CORD 1 Abstract Previous comparative and developmental studies have suggested that the cholinergic inner ear efferent system derives from developmentally redirected facial branchial motor neurons that innervate the vertebrate ear hair cells instead of striated muscle fibers. Transplantation of Xenopus laevis ears into the path of spinal motor neuron axons could show whether spinal motor neurons could reroute to innervate the hair cells as efferent fibers. Such transplantations could also reveal whether ear development could occur in a novel location including afferent and efferent connections with the spinal cord. Ears from stage 24-26 embryos were transplanted from the head to the trunk and allowed to mature to stage 46. Of 109 transplanted ears, 73 developed with otoconia. The presence of hair cells was confirmed by specific markers and by general histology of the ear, including TEM. Injections of dyes ventral to the spinal cord revealed motor innervation of hair cells. This was confirmed by immunohistochemistry and by electron microscopy structural analysis, suggesting that some motor neurons rerouted to innervate the ear. Also, injection of dyes into the spinal cord labeled vestibular ganglion cells in transplanted ears indicating that these ganglion cells connected to the spinal cord. These nerves ran together with spinal nerves innervating 1 This chapter is adapted from Elliott KL, Fritzsch B (2010) Transplantation of Xenopus laevis ears reveals the ability to form afferent and efferent connections with the spinal cord. Int. J. Dev. Biol. 54: 1443-1451. 40 the muscles, suggesting that fasciculation with existing fibers is necessary. Furthermore, ear removal had little effect on development of cranial and lateral line nerves. These results indicate that the ear can develop histologically normal in a new location, complete with efferent and afferent innervations to and from the spinal cord. Introduction The vertebrate inner ear receives two kinds of fibers: afferents that come from placodally-derived sensory neurons (Rubel and Fritzsch, 2002) and efferents which come from brainstem neurons (Simmons, 2002). Whereas afferents carry information from the ear to the brain, efferents provide a feedback loop to modify mechanic stimuli information processing at the periphery. Efferents have been described in all vertebrates (Fritzsch, 1999) and the extensive branching of single fibers to reach both lateral line and inner ear hair cells has been extensively studied in amphibians (Hellmann and Fritzsch, 1996). The nature of these efferent cells has long been enigmatic and they have been referred to as reticular formation cells or branchial motor neurons given their close proximity in many vertebrates to the facial branchial motor neurons (Roberts and Meredith, 1992). More recent developmental studies have shown that efferent innervation of the ear is derived from facial branchial motor neurons (Fritzsch and Nichols, 1993) and that efferents may be guided to reach the ear by the transcription factor Gata3 that is uniquely expressed in these motor neurons and nearby reticular formation neurons (Karis et al., 2001). In vertebrates, the ear develops at the rostral boundary of the anteriormost, first forming somite (Cooke, 1978, Chung et al., 1989, Huang et al., 1997) and paraxial mesoderm anterior to the ear does not form somites (Noden and Francis-West, 2006) but may form somitomeres. Furthermore, in 41 vertebrates, the most rostral somite is reduced in size to accommodate the ear. Combined these data could be interpreted to imply that in vertebrates, the ear develops in place of a somite or somitomere. If true, the facial branchial motor neurons destined to innervate the somite- or somitomere-derived muscle fibers may have been rerouted to innervate the ear when the ear evolved in ancestral vertebrates (Fritzsch et al., 2007). This raises the question as to whether there is a unique property of cranial motor neurons to innervate hair cells of the ear or whether any motor neuron can innervate the ear if placed in its trajectory. In other words, is formation of efferents to the ear a chance event that happened to capture facial branchial motor neurons simply because the ear evolved in the trajectory of these motor neurons? Supporting evidence comes from the lateral line system in which branchial motor neurons associated with the glossopharyngeal nerve become efferent to the hair cells of the posterior lateral line (Hellmann and Fritzsch, 1996). In the present study, we transplanted Xenopus laevis ears into the path of spinal motor neuron axons, revealing that spinal motor neurons have the ability to innervate the ear as efferent fibers and end on hair cells instead of muscle cells and probably modify hair cell function via the now well characterized nicotinergic acetylcholine receptors expressed in hair cells (Sugai et al., 1992, Jagger et al., 2000, Katz et al., 2004, Derbenev et al., 2005). Such transplantations could also reveal the potential for normal ear development in a novel location, including afferent connection with the spinal cord, a part of the CNS that never receives inner ear afferent innervations which is normally restricted to the alar plate of the brainstem (Maklad and Fritzsch, 2003). 42 Results Assessment of transplantations Success of transplantations is shown in Table 3.1 and in Figure 3.1. Residual ear formation is shown in Table 3.2 for embryos in which the success of transplantation and ear regeneration were monitored. In some cases in which only a transplanted vesicle without otoconia formed, a new otic vesicle was observed in the native location (Fig. 3.1E). This was more frequent in transplantations of earlier embryos (stage 24) than in later stages (stage 26). It remains unclear whether this indicates a residual capacity for parts of the otocyst to regenerate. However, in many cases, the residual native ‘ear’ consisted of nothing more but an endolymphatic duct filled with calcium carbonate crystals (Fig. 3.1C and 3.1I). In transplanted ears which formed otoconia, 75% did not form a residual ear nor endolymphatic duct in the original location (Fig. 3.1B and 3.1H). However, formation of a residual native ear in transplants that had developed otoconia occasionally occurred (17%). To determine whether manipulation of the otic placode affects further development, ears were removed, rotated 180°, and replaced. Even though for transplantations we sought to maintain orientation, it was not always the case and often the ears underwent some degree of rotation. Thus rotation of the ear and replacing it back into the head replicated many of the manipulations performed during transplantation and could serve as an additional control for the ability of a manipulated ear to develop normally. Success of ear rotations is shown in Table 3.1. Eight of the 13 ears were normal or near normal in appearance (Figs. 3.1D and 3.1G) when compared with control embryos (Fig. 3.1A). Otoconia in nearly half of the ears were even found to be re- 43 oriented so that they resumed native orientation. In the chick, anterior-posterior specification occurs after the formation of the otic placode (Bok et al., 2005), thus this might explain the re-orientation of rotated X. laevis ears. Only one ear rotation failed to form otoconia. Thus we conclude that our manipulations themselves have little effect on normal ear development. Transplanted ears develop hair cells and ganglia To assess normal development of the sensory epithelia in ears containing otoconia, in situ hybridization for miR-183, a conserved hair cell marker of ears (Pierce et al., 2008), was performed on ears containing otoconia. In the untransplanted ears, miR-183 was localized in the sensory end organs: the utricle, saccule, and anterior, horizontal and posterior canals as well as in the placodally-derived ganglion cells: Trigeminal, Vestibular and Vagus (Fig. 3.2A). Transplanted ears were also miR-183 positive in the utricle, saccule, the 3 semicircular canals, and in the placodally-derived vestibular ganglion cells (Fig. 3.2B). To confirm the presence of hair cells, transplanted ears were immunostained with antibody against myoVI and acetylated tubulin. MyoVIpositive hair cells in patches of sensory epithelia were observed in transplanted ears (Fig. 3.2C), although some sensory epithelia contained only a few hair cells. These hair cells had kinocilia positive for tubulin (Fig. 3.2D). Serial microtome sections of plasticimbedded transplanted ears also revealed hair cells with apical specializations as seen with a light microscope (Fig. 3.2F) and with transmission electron microscopy (Fig. 3.2G). These data suggest that the transplanted ears developed relatively normally with all sensory epithelia present that occur at that stage. 44 To confirm the presence of sensory ganglia in the transplanted ears, in situ hybridization for miR-124 was performed on transplanted ears. MiR-124 is expressed in the central nervous system (Mishima et al., 2007) and in inner ear vestibular ganglion cells (Weston et al., 2006). Cells positive for miR-124 were present in transplanted ears (data not shown). To further confirm the presence of ganglion cells, otic placodes from embryos injected with GFP were transplanted into uninjected individuals. Daily observation using a dissection microscope equipped with epifluorescence revealed GFPpositive delaminating ganglion cells migrating away from the ear. After a week, these delaminated cells were imaged with confocal microscopy (Fig. 3.2E), indicating that the ganglion cells originate from the transplanted otic placode and migrate outward during embryo development. Afferent innervation of transplanted ears Dextran amine and lipophilic dyes were injected into the spinal cord of embryos (Fig. 3.1H) containing otoconia in their transplanted ears to label innervations of these ears. Both types of dye labeled ganglion cells in the transplanted ear (Figs. 3.2E, 3.3A and 3.3B). The lack of dye in some ganglion cells in Figures 3.2E and 3.3A indicates that not all ganglion cells sent projections to the spinal cord. Ganglion cells making connection with the spinal cord appear to do so by fasciculation with existing nerves innervating the surrounding muscle tissue (Figs. 3.3A and 3.3B). In addition to making projections to the spinal cord, immunohistochemistry for acetylated tubulin revealed fibers (presumably from ganglion cells) closely apposed to hair cells (Fig. 3.3C). Transmission electron microscopy confirmed the presence of afferent terminals on hair cells (Fig 3.3K). 45 Efferent innervation of transplanted ears To determine whether spinal motor neurons project to hair cells in the transplanted ear, several methods were used. Transplanted ears were injected with Rhodamine dextran amine dyes to backfill to the spinal cord (Fig 3.1I). In addition, for some embryos Flurosceine dextran amine dye or lipophilic dye (NeuroVue™ maroon) was applied rostral and caudal to the ear to label motor neurons that innervate muscle tissue to determine if any uncut nerves outside of the ear took up Rhodamine dextran amine that might then give a false positive; these cells would be double labeled. Cell bodies in the ventral spinal cord positive for only Rhodamine dextran amines were present in 10 of 16 ventral spinal cords examined, suggesting that motor neurons can innervate the ear if placed in their trajectory (Fig. 3.3D). To further demonstrate that motor neurons do innervate the ear, lipophilic dye was injected immediately ventral to the spinal cord from the contralateral side as to not damage the ipsilateral nerves innervating the ear (Fig. 3.1J). The dye would only come into contact with spinal motor neurons exiting the spinal cord via ventral roots and thus any nerves labeled in the transplanted ear should be motor neurons that had rerouted to innervate the ear. Lipophilic dye label was observed in a few nerve fibers innervating several of the transplanted ears (Figs 3.2E and 3.3G). In one example, lipophilic-dye filled nerve fibers that traveled around the ear on each side and also filled nerve fibers which sent projections to the hair cells (Fig. 3.3G). To further visualize the extent to which this ear was innervated, beyond the contribution from the spinal motor neurons, this ear was immunostained with antibody against tubulin. This revealed more extensive, presumably afferent innervations (Fig. 3.3H). The deeper ventral roots observed in 46 Figure 3.3G were faintly stained with tubulin, but not easily detected in the collapsed image (Fig. 3.3H). The reduction of staining of deeper neurons is likely due to the inability of the antibody to fully penetrate the tissue. In another example, a few of the ganglion cells sent projections along the presumed motor nerves as they colocalized with the dye (Fig. 3.2E, see G*) therefore, it was necessary to confirm motor innervation even when co-localization with ganglion cells was not apparent as in Figure 3.3G. To confirm that motor neurons innervate some transplanted ears, antibody against VAChT was used to show motor terminals on hair cells. Since antibody against VAChT had not yet been characterized in amphibians, we tested it on lateral line neuromasts and hair cells in untransplanted ears, two sources with known motor innervation (Will, 1982). The neuromasts and control ears were found to have puncta positive for VAChT on the hair cells (Figs. 3.3E and 3.3F). In the transplanted ears, we found terminals positive for VAChT on hair cells (Figs. 3.3I and 3.3J). Specifically, the transplanted ear that had shown extensive spinal motor neuron innervation when dye was injected ventral to the spinal cord (Fig. 3.3G) also had VAChT-positive terminals on hair cells that co-localized with areas labeled by the lipophilic dye (Fig. 3.3I). To further validate the presence of efferent terminals on hair cells, transplanted ears that were positive for VAChT terminals on hair cells were imbedded in resin and sectioned for transmission electron microscopy. Efferent terminals were confirmed on hair cells by the presence of axon terminals containing vesicles (Fig. 3.3L), thus demonstrating that spinal motor neurons can innervate an ear if placed in its trajectory. 47 Cranial and lateral line nerves develop nearly normal To assess the effect otocyst removal had on the development of the cranial nerves and lateral line nerves, heads from both control embryos and those having an ear transplanted were immunostained with antibody against acetylated tubulin. Figure 3.4A shows the normal distribution of the cranial nerves. In embryos in which an ear was removed, near normal distribution of the cranial nerves was observed although the cranial nerves that would normally circle the ear now traverse the empty space (Fig. 3.4B and 3.4C). Similar effects were observed with the lateral line nerves. Compared to control embryos (Figs. 3.4D and 3.4F), in transplanted embryos the lateral line neuromasts spread out to cover the space not occupied by the ear (Fig. 3.4E, 3.4G, and 3.4H). In some instances, and more often when the ear was transplanted during the later stages, the parietal lateral line neuromasts failed to develop or were reduced (Fig. 3.4E). Otherwise the development of the lateral line nerves in transplanted embryos was normal and showed almost no quantitative differences in the number of neuromasts, in particular the supraorbital line (10.1 ± 0.5 neuromasts posterior to the parietal line for the control side versus 10.2 ± 0.5 for the transplanted side (mean ± standard error of the mean, n = 11). Discussion Our results confirm and extend previous studies on ear transplantation (Fritzsch, 1996, Fritzsch et al., 1998, Groves, 2005) in amphibians but add the hitherto unstudied aspect of afferent and efferent innervations. Below we will first discuss the degree of development we achieved in transplanted Xenopus ears, followed by a discussion of our tracing data. 48 Evidence that these transplanted ears could develop relatively normally in a novel location is the presence of hair cells in the transplanted ears, identified by morphology as well as in situ hybridization and immunohistochemistry for hair cell markers. This suggests that the epithelial end organs all develop and that either their development is likely not dependent upon the tissue surrounding the placode or that it was specified prior to transplantation, as recent work in chicks has demonstrated that the otic ectoderm becomes specified prior to placodal formation (Abelló et al., 2010). On the other hand, while care was taken to remove only the otic placode, it cannot be ruled out that some surrounding mesoderm and ectoderm was also transplanted, thus influencing further development of the ear. Furthermore, in the GFP-labeled ears, while migration of ganglion cells was observed, it is also possible that some of the green cells observed surrounding the ear may be transplanted mesoderm in addition to the GFP-labeled delaminating ganglion cells. Taken together, these sets of data confirm that welldeveloped sensory epithelia form with hair cells. Thus, many transplanted ears develop fully normal with respect to otoconia formation, hair cell differentiation and segregation of several sensory epithelia (Fritzsch and Wake, 1988). That the transplanted ear can develop normally would suggest that the fates of the cells in the otic placode were determined by stage 24-26. In most of the embryos where only a vesicle formed on the trunk, a partial or near normal ear redeveloped in the original location. Waldman et al. (2007), using X. laevis, ablated otic placodes at various stages to determine the ability of the surrounding tissue to regenerate an ear. Placodes ablated at early stages (21-23) were able to regenerate completely normal ears whereas beyond stage 28, the ear rarely regenerates. Ablation at stages 24-27 sometimes resulted 49 in regenerated, although abnormal ears (Waldman et al., 2007). In a study with salamanders, ablation of the otic placode could result in regeneration of the inner ear, but only when ablation was done at early stages (Kaan, 1926). Therefore, in the present study, it could be that in the embryos in which an ear redeveloped, the embryo was at a stage in which the remaining tissue was competent to regenerate an ear. On the other hand, it is also possible that since the transplanted placode only formed a vesicle on the trunk and not a complete ear, that not the entire placode was completely transplanted. Waldman et al. (2007) also demonstrated that partial ablations could result in regeneration of the ears in some instances. Therefore, it could also be that the partial placode remaining in the head regenerated an ear. The fact that the partial placode in the trunk did not form a complete ear might be due to the novel environment that it developed in and that perhaps additional cues from the head might be necessary for restoring the otocyst. Support for the latter possibility, that residual ears formed when the placode was not completely transplanted, was that most of the transplanted ears that contained otoconia did not have a residual ear in the native location (Fig. 3.1B). Whereas, for transplanted ears containing only a vesicle and no otoconia or when no transplant was observed, some component of the residual ear, whether it be just the endolymphatic duct or an entire ear, formed in the native location (Figs. 3.1C and 3.1E). In addition to the ability of the ears to develop in a novel location, many ears were also innervated. Dye injections into the spinal cord or transplanted ears revealed afferent and efferent innervations respectively. Using these dye injections, we observed that some afferent ganglion cells sent projections to the hair cells and to the spinal cord along trajectories that likely do not bear any informational molecules used by inner ear 50 afferents to navigate during development to reach the hindbrain (Rubel and Fritzsch, 2002). Furthermore, the random dispersal of delaminating ganglion cells away from the ear is also explained by an absence of these normal guidance molecules as there did not appear to be any formal organization of their migration (Fig. 3.2E). The observation that some, but not all, ganglion cells were filled with dye when injections were made into the spinal cord (Figs. 3.2E and 3.3A), suggests that connections to the spinal cord happen by chance and are only made if the growing ganglion cell axons happen to fasciculate with existing nerves. However, since many ganglion cells axons were apposed to hair cells, synaptic connections are likely made thus indicating that the mechanism for hair cell innervation develops normal in the novel location. Immunostaining of transplanted ears with antibody against VAChT revealed puncta on the basal surface of the hair cells, demonstrating the presence of motor terminals on these hair cells. For transplanted ears that were also labeled from dye injections ventral to the spinal cord, VAChT puncta were found only on hair cells that were in close proximity to the labeled spinal motor neuron projections (Figs. 3.3G and 3.3I). Furthermore, transmission electron microscopy confirmed efferent terminals on hair cells in transplanted ears as the presence of many synaptic vesicles in the axon terminal is a property of efferent axons, but is not found in afferent axons (Jones and Eslami, 1983, Simmons, 2002). The reduced integrity of the membranes was a result of utilizing ears previously processed for immunohistochemistry to confirm the presence of VAChT. Thus, the evidence presented here of efferent innervation of the transplanted ear demonstrates that some spinal motor neurons have the ability to reroute to innervate a new target if the new target replaces the previous target and is in the direct trajectory of 51 the growing axon. This finding is consistent with the hypothesis that efferent innervation of the ear arose in evolution from rerouted facial branchial motor neurons. Finally, immunohistochemistry for acetylated tubulin was performed to determine the effect, if any, transplantation had on the development of the cranial and lateral line nerves of the head. The results presented here demonstrated that ear transplantation had little to no effect on further cranial and lateral line development other than, for both the cranial nerves and lateral line neuromasts, they spread out to either traverse or fill in, respectively, the space left void by the ablated ear (Fig. 3.4). It is possible that for the lateral line neuromasts, either the ear suppresses expansion of the anterior and posterior lateral lines and that, in the absence of the ear, the neuromasts spread out to cover that space, or simply as the skin closes following the removal of the ear, the lateral line placode is stretched as the wound heals. One aberrant effect that was occasionally observed was the absence of the parietal lateral line on the transplanted side (Fig 3.4E). In X. laevis, the lateral line placodes is formed by about stage 24 (Winklbauer and Hausen, 1983). At this stage, the inducibility of surrounding tissue to form the lateral line begins to decline (Schlosser, 2002). Therefore any disruption of the placode at stage 24 likely results in reinduction of surrounding tissue to form a new placode, whereas at stage 26, the surrounding tissue is less inducible, resulting in a possible loss of neuromasts in some places, such as the parietal line. In conclusion, the results presented here demonstrate the potential for a motor neuron to reroute to innervate a novel target, supporting the idea that efferents may have arisen from rerouted facial branchial motor neurons during the evolutionary formation of the ear. Such rerouting to novel targets can be induced by ablation of innervations of a 52 given muscle fiber inducing the attraction of nearby motoneuron axons to expand to denervated targets in X. laevis (Fritzsch and Sonntag, 1990, 1991), mice (Fritzsch et al., 1995, Porter and Baker, 1997) and man (Engle et al., 1997, Miyake et al., 2008). We cannot exclude such near range attractive signaling from denervated hair cells. However, since hair cell innervation by spinal motor neurons occurred only when the hair cells were in the direct trajectory of the growing axons, this suggests that the rerouting of the facial branchial motor neurons occurred as a chance event when the ear developed in their trajectory. Future tests will determine whether reinnervation of the ear can occur by other cranial motor neurons and thus reflects a general motor neuron ability to engage in synaptic innervations in placode derived hair cells. 53 Table 3.1. Success of transplantation of otic placodes. Development of Development of ear No development of ear with otoconia without otoconia ear Transplanted ears (109) 73* 29* 7 Rotated ears (13) 12 1 0 Note: Numbers of transplants or rotated ears performed are indicated in parentheses. *Examples of transplants with and without otoconia are shown in Figures 1B/1F and 1C respectively. 54 Table 3.2. Formation of residual ears as related to success of transplantation. Residual ear Development of Development of No development of formation transplanted ear with transplanted ear transplanted ear otoconia without otoconia No residual ear 30 0 0 ED only 3 10 0 Residual ear, no ED 7 0 0 Residual ear + ED* 0 2 3 Note: ED, endolymphatic duct * Residual formation of ear plus endolymphatic duct is shown in Figure 1E. 55 Figure 3.1. Stage 46 X. laevis showing transplanted ears. (A) Control, untransplanted embryo, ears are circled in black. (B) Embryo with a transplanted ear containing otoconia, outlined in a dotted red line. (C) Embryo with a transplanted empty vesicle, outlined in a dotted red line. (D) Embryo that had its ear rotated, outlined in a dotted yellow line. (E) Untransplanted ear, left, and residual ear formation, right. (F) Higher magnification of transplanted ear in B. (G) Higher magnification of rotated ear in D. (H) Lipophilic dye injections into the spinal cord rostral and caudal to the ear. (I) Dextran amine dye injection into the transplanted ear. (J) Lipophilic injection ventral to the spinal cord and contralateral to the ear. Native, unmanipulated ears are labeled. U, utricle; S, saccule; ED, endolymphatic duct. Asterisks indicate sites of dye injections. Scale bar is 0.5 mm for A-D,H,I and 0.25 mm for E,F,G,J. 56 57 Figure 3.2. Development of the transplanted ear. (A) MiR-183 in sensory epithelia in the native ear and in surrounding cranial ganglia as well as ganglia next to the ablated ear (i.e. Trigeminal ganglia, V). (B) Lateral view of the transplanted ear with miR-183 positive sensory epithelia and ganglion cells; anterior is to the right. (C) Acetylated tubulin (green) and MyoVI (red) immunostaining of a transplanted ear showing hair cells. (D) Magnification of region in C show kinocilia (white arrows) on hair cells. (E) Green fluorescent protein (GFP)-labeled transplanted ear reveals GFP-positive delaminating sensory ganglion cells (G), a few of which (G*) are colocalized with lipophilic dye (red). Lipophilic dye injections were ventral to the spinal cord. (F) 2μm thick transverse section through the dorsal and lateral view of transplanted ear showing a sensory epithelium with hair cells and an endolymphatic duct. (G) Transmission electron micrograph of two hair cells in the transplanted ear with synaptic ribbons (black arrows). The boxed areas are shown at higher magnification in Figure 3.3K and 3.3L. U, utricle; S, saccule; Ac, anterior canal crista; Hc, horizontal canal crista; Pc, posterior canal crista; G, ganglion cell(s); HC, hair cells K, kinocilium; S, stereocilia; ED, endolymphatic duct. Scale bar is 0.5 mm in A,B; 25 µm in C,D; 50 µm in E, 0.1 mm in E inset; 10 µm in F; and 5 µm in G. 58 59 Figure 3.3. Afferent and efferent innervation of transplanted ears. (A) Transplanted ear that had dextran amine (red) injected into the spinal cord. Autofluorescence of the tissue is green. Inset: higher magnification of boxed area. (B) Transplanted ear that had lipophilic dyes injected into the spinal cord rostral (red) and caudal (green) to the ear. (C) Acetylated tubulin immunostaining (red) of boxed area in B showing ganglion cells apposed to hair cells. Autofluorescence of the tissue is green. (D) Cells in the ventral spinal cord positive for dextran amine dyes that had been injected into the transplanted ear. (E) Neuromasts in the skin showing vesicular acetylcholine transporter (VAChT)positive terminals on the hair cells. Autofluorescence is blue. (F) Hair cells in a control ear immunostained for acetylated tubulin and VAChT. (G) Transplanted ear that had lipophilic dye injected ventral to the spinal cord show nerve fibers extending to the ear. (H) Transplanted ear in G immunostained for acetylated tubulin. (I) Transplanted ear in G immunostained for acetylated tubulin and VAChT. Inset: higher magnification of boxed area. Red arrows indicate VAChT immunostaining. (J) Transplanted ear immunostained for acetylated tubulin and VAChT. Inset: single optical section of VAChT staining (red arrows) at the base of hair cells. (K) Electron micrograph of an afferent terminal on a hair cell. A synaptic ribbon (arrow) is observed on the hair cell above the afferent terminal (L) Electron micrograph of an efferent terminal with vesicles (circled) making a synapse on a hair cell. These TEM images were obtained from ears previously processed for immunohistochemistry to confirm the presence of VAChT, hence poor preservation of membranes. GC, ganglion cell; HC, hair cell; MN, motor neuron cell body; NM, neuromasts; Aff, afferent terminal; Eff, efferent terminal; V, vesicles; Mt, mitochondria. Scale bar is 100 µm in A,B,C,D,G,H; 25 µm in E,F,I,J and 1 µm K,L. 60 61 Figure 3.4. Development of cranial and lateral line nerves. Acetylated tubulin immunostaining of cranial nerves in a control embryo (A) and in embryos in which the ear was transplanted (B, C). (D) Acetylated tubulin (green) immunostaining of lateral line nerves in the cranial skin of a control embryo (E) Acetylated tubulin (green) and Hoechst (blue) immunostaining of a later-stage transplanted embryo. Acetylated tubulin (green) immunostaining of lateral line nerves in the cranial skin of a control embryo (F) and an embryo in which the ear was transplanted (G). (H) Higher magnification of boxed area in E. Areas where native ears are present and where ablated ears had occupied are circled with solid and dotted lines, respectively. Native ears are labeled ‘Ear’. Eyes (A,B) and skin over the eyes (D,E,F,G) are labeled ‘Eye’. Cranial nerves are labeled with Roman Numerals. LL, lateral line ; U, utricle; S, saccule; L, lagena; Ac, anterior canal; Hc, horizontal canal; Pc, posterior canal. Scale bar is 250 µm. 62 63 CHAPTER IV TRANSPLANTATION OF XENOPUS LAEVIS TISSUES TO DETERMINE THE ABILITY OF MOTOR NEURONS TO ACQUIRE A NOVEL TARGET 2 Abstract The evolutionary origin of novelties is a central problem in biology. At a cellular level this requires, for example, molecularly resolving how brainstem motor neurons change their innervation target from muscle fibers (branchial motor neurons) to neural crest-derived ganglia (visceral motor neurons) or ear-derived hair cells (inner ear and lateral line efferent neurons). Transplantation of various tissues into the path of motor neuron axons could determine the ability of any motor neuron to innervate a novel target. Several tissues that receive direct, indirect, or no motor innervation were transplanted into the path of different motor neuron populations in Xenopus laevis embryos. Ears, somites, hearts, and lungs were transplanted to the orbit, replacing the eye. Jaw and eye muscle were transplanted to the trunk, replacing a somite. Applications of lipophilic dyes and immunohistochemistry to reveal motor neuron axon terminals were used. The ear, but not somite-derived muscle, heart, or liver, received motor neuron axons via the oculomotor or trochlear nerves. Somite-derived muscle tissue was innervated, likely by the hypoglossal nerve, when replacing the ear. In contrast to our previous report on ear innervation by spinal motor neurons, none of the tissues (eye or jaw muscle) was innervated when transplanted to the trunk. Taken together, these results suggest that 2 This chapter is adapted from Elliott KL, Houston, DW, Fritzsch B (2013) Transplantation of Xenopus laevis tissues to determine the ability of motor neurons to acquire a novel target. PLoS ONE 8(2): e55541. doi:10.1371/journal.pone0055541. 64 there is some plasticity inherent to motor innervation, but not every motor neuron can become an efferent to any target that normally receives motor input. The only tissue among our samples that can be innervated by all motor neurons tested is the ear. We suggest some possible, testable molecular suggestions for this apparent uniqueness. Introduction Synaptic contacts likely originated in evolution as connections between sensorymotor neurons and muscle tissue in diploblastic animals, such as jellyfish, (Seipel et al., 2004) forming a monosynaptic reflex network from sensory cell directly to effector cell. As animal cell types diversified, the sensory-motor interface evolved in complexity, forming muscle fibers, motor neurons, sensory neurons, and interneurons (Fritzsch and Glover, 2007). As tissues diversified in the course of evolution into the over 200 cell types recognized in metazoans, motor neurons acquired novel targets during this diversification process. As new targets evolved to provide novel synaptic contacts, motor neurons evolved into novel sub-categories (Fritzsch and Northcutt, 1993, Murakami et al., 2004, Dufour et al., 2006). Although all motor neurons share a common developmental transcription factor, Islet 1 (Ericson et al., 1992, Inoue et al., 1994), they have developed unique LIM code molecular signatures for each population (Tsuchida et al., 1994). Vertebrate motor (efferent) neurons have evolved to form synapses on a variety of targets originating from developmentally different sources including mesoderm-derived muscle fibers, epithelial placode-derived neurosensory cells and neurons (i.e. inner ear hair cells and neurons), and neural crest-derived autonomic ganglia (Eisen, 1999, Fritzsch, 1999, Takano-Maruyama et al., 2010, Simmons et al., 2011). Among all cranial motor neurons, motor neurons of the facial nerve are unique in that 65 they innervate targets from three different developmental origins: branchial arch-derived muscle by branchial motor neurons, neural crest-derived ganglia by visceral motor neurons, and placodally-derived inner ear hair cells by inner ear efferents (Fritzsch and Northcutt, 1993). Although the efferents to the inner ear hair cells project in mammals along the vestibulocochlear nerve, it has been shown that efferent innervation of the inner ear is ontogenetically derived from the facial branchial motor neurons, as inner ear efferent neurons exit the cell cycle in the same area as the facial branchial motor neurons in early embryonic mice prior to segregation of the two neuron populations (Fritzsch and Nichols, 1993, Köppl, 2011, Simmons et al., 2011) and can project with the facial nerve in the absence of afferent neurons (Ma et al., 2000). Only one other branchial motor nerve, the glossopharyngeal nerve, innervates more than one tissue type: muscle, ganglia, and in aquatic vertebrates such as Xenopus laevis, hair cells of the placodally-derived posterior lateral line (Hellmann and Fritzsch, 1996). Given the ability of some motor neurons to innervate targets from a variety of origins, the possibility exists that there is conservation of the core molecular machinery that allows for recognition of a particular target by multiple motor neuron types. In contrast to the ability for few populations of motor neurons to innervate a variety of targets, most tissue types have only one source of motor input, even when other motor neuron types project to adjacent territories. Such is the case with the innervation of the trapezius muscle by the spinal accessory nerve rather than spinal motor neurons (Boord and Sperry, 1991, Sienkiewicz and Dudek, 2010, Dudek et al., 2011). Further support for specificity between motor neurons and targets includes studies in some vertebrates showing that spinal motor neurons can reinnervate the correct muscles 66 following nerve transection (Landmesser, 1980). In addition, spinal motor neurons can find the correct muscle following anterior-posterior reversal of a few lumbar spinal cord segments, demonstrating innervation selectivity (Lance-Jones and Landmesser, 1980, Landmesser, 1980). However, there must be some degree of plasticity in the system, as the oculomotor nerve has been shown to innervate the lateral rectus muscle, a normal target of the abducens nerve, in Duane’s retraction syndrome where the abducens nerve is absent (Demer et al., 2007) or the abducens nerve expands its territory when the oculomotor nerve is absent (Fritzsch et al., 1995). Furthermore, motor neurons were able to reroute to innervate a novel target, as demonstrated by Xenopus laevis spinal motor neurons rerouting to innervate hair cells of an ear transplanted to the trunk to replace a somite (Elliott and Fritzsch, 2010). This result indicates the presence of a common molecular denominator between some targets of motor neurons, in this case between the inner ear hair cells and somites, that allows for recognition by the same population of spinal motor neurons. It is likely that the ability of different motor neurons to form synaptic contacts with hair cells of the inner ear uses an evolutionary conserved molecular mechanism for target recognition and for maintenance of innervation. Whether this applies to other motor neuron populations or other tissues is not yet known. Thus, our goal is to determine the extent to which various nerves can gain affinity for a novel target. In the present study, we expand upon the previous X. laevis transplantation study (Elliott and Fritzsch, 2010) by transplanting X. laevis ears, which are directly innervated by motor (efferent) neurons into the path of various cranial motor neurons: oculomotor, trochlear, trigeminal, and, abducens to test for further acceptance of motor neuron 67 innervation by hair cells. In addition, other tissues that receive direct motor innervation (somite-derived muscle and branchial arch-derived eye muscle and jaw muscle) were transplanted into the path of various cranial motor neurons or into the path of spinal motor neurons, respectively. Finally, tissues that are not directly innervated by motor neurons (heart, liver) were transplanted into the path of oculomotor, trochlear, and abducens motor neurons. Transplantation of the ear to the orbit revealed that motor neurons destined to innervate the muscles for eye movement could innervate hair cells of the transplanted ear if placed in their trajectory; however, comparable transplantations of other tissues revealed no innervation in somite-derived muscle, hearts, and livers transplanted to the orbit. In addition, neither eye muscle nor jaw muscle transplanted to the trunk to replace a somite was supplied by axons from spinal motor neurons. However, when somite-derived muscle was transplanted to replace an ear, there was supply of axons from what appears to be the hypoglossal nerve. Together these data suggest that the ear differs from other motor neuron targets in that it can receive motor neuron axons from a variety of sources and thus has a built in ability for novel motor neuron innervation. Results Completion of eye removal and assessment of transplantation success Completeness of transplanted tissue formation was scored to determine the effectiveness of transplantations (Figure 4.1A-1H; Table 4.1). Success for all tissues was defined as the detection of transplanted tissue, whereas completeness of transplantation was defined as the amount and/or degree of normality of transplanted tissue. Of the 159 successful ear to orbit transplantations (out of 173), almost two-thirds of the ears 68 transplanted to the orbit contained otoconia (n = 97), whereas others were just a vesicle and lacked otoconia (n = 62) (Figure 4.1B-4.1C). Hair cells were present in ears that lacked otoconia (data not shown). Only ears containing otoconia were considered more complete ears and were used for further analysis. Removal of the lens placode and developing eye still lead to the regrowth of parts of the eye in all but 36 of the 159 successful ear to orbit transplantations (Compare inset in Fig. 4.1A with insets in Figs. 4.1B and 4.1C). Eye muscles, which develop from surrounding mesoderm, accompanied residual eyes. All embryos containing any remaining portion of the eye were used for further analysis. Success of muscle tissue transplantation (somite, eye muscle, and jaw muscle) was also quantified by the presence of transplanted tissue (Figure 4.1D-4.1F). All but one transplant (out of 10) involving somites, whether to the orbit or otic region, were successful. Eyes with attached muscle transplanted to the trunk were present in 6 of 7 embryos. Attempts to transplant only eye muscle was not successful as no extra muscle was detected in the trunk when eye muscle was transplanted without the eye. Jaw muscle transplanted to the trunk was present in 12 of 13 embryos. Embryos with larger muscle transplants were used for further analysis. Success of heart and liver transplantations was also investigated (Figure 4.1G-4.1H). There was variation in the amount of heart tissue that developed in the orbit ranging from none detectable to a large, beating heart (Fig. 4.1G). Heart tissue was present in 23 of 31 transplants. Slightly less than half (10 of 23) of the hearts transplanted were beating regularly and apparently autonomously in the orbit. One transplanted heart beat at 130 beats per min (Fig. 4.1G), which was near the rate of native hearts (146 beats per min, n = 7). All 10 embryos containing beating 69 transplanted hearts were used for further analysis. Liver tissue was present in the orbit in all 13 transplants and all were used for further analysis. Afferent innervation of transplanted ears Lipophilic dyes implanted into the brain revealed sensory vestibular ganglion cells, detected by their cell bodies, labeled with dyes from both midbrain (blue) and hindbrain (red) implantations (Fig. 4.2A) when the ear was transplanted to the orbit. Most delaminating sensory ganglion cells sent projections along the nearby trigeminal nerve and into the hindbrain (Figs. 4.2B and 4.2C). Fewer ears sent projections along the oculomotor nerve and into the midbrain (Fig. 4.2D). Of the 25 transplanted ears analyzed with lipophilic dye tracing, 23 had lipophilic dye labeling of axons projecting to the transplanted ears. Twenty-one of these 23 ears had lipophilic dye labeling of sensory vestibular ganglion cells. Of these, 16 sent sensory axons along the trigeminal nerve only, 2 had ganglion cell axons projecting along the oculomotor nerve only, and 3 had projections along both trigeminal and oculomotor. However, not all ganglion cells may have sent projections to the hindbrain or midbrain as was evident in the transplanted ears labeled with GFP (Fig. 4.2C). In these ears (n=4), some GFP-positive ganglion cells did not project to the brain areas where dye was implanted (Fig. 4.2C) as they showed no lipophilic dye label. It is not known where these unlabeled ganglion cells project to and if they reach the brain at all. Afferent axons that do reach the brain, as observed following implantations of lipophilic dye into the ear, project along either the trigeminal oculomotor or optic nerve to enter the brain; however, there was no consistency between different transplants in the trajectory of afferent projections (Fig. 4.2F-I). Of the eighteen ears in the orbit labeled with lipophilic dyes that sent afferents to the brain, one projected 70 to the forebrain, midbrain, and hindbrain, five projected to the forebrain and midbrain, three to the midbrain alone, five to the midbrain and hindbrain, and four to the hindbrain alone. Afferent axons from three of the transplanted ears appeared to project to the ipsilateral vestibular nucleus (Fig. 4.2I, 4.2I’), a native target of the ear. These data suggest that projections of inner ear afferents primarily grow randomly along adjacent nerves with little evidence for preferences. Efferent innervation of transplanted ears Efferent innervation of transplanted ears from the oculomotor and trochlear nerves was demonstrated with lipophilic dye implantation into the midbrain. Of the 25 embryos implanted with lipophilic dyes into their brains, 9 transplanted ears had projections to them from the oculomotor nerve (Fig. 4.3A) and 1 from the trochlear nerve (Fig. 4.3F). These projections were without obvious co-labeling of vestibular sensory ganglion cells, though a pure motor innervation required additional confirmation (see below). In 8 of 9 embryos in which the oculomotor nerve sent axons to the transplanted ear, the surrounding eye muscle was also innervated; the remaining embryo had no eye or eye muscles remaining to be innervated. In the embryo in which the trochlear nerve sent axons to the transplanted ear, eye muscles were present, but were not innervated. For the 13 embryos lacking oculomotor or trochlear nerve projections to the transplanted ear but had regrowth of part of the eye, the motor nerves innervated only the eye muscles. The most noticeable difference between embryos whose transplanted ears had projections to them and those whose did not was the position of the transplanted ear relative to the native eye muscle. All transplanted ears imaged that were more medial than the eye muscle received projections from motor nerves (5 of 5 ears). Half of the transplanted 71 ears that were equidistant from the brain as the eye muscle received projections from motor nerves (5 of 10 ears). Finally none of the ears located lateral to the eye muscles were innervated (0 of 8 ears). These data imply that the ear is as good a substrate for motor neurons as are eye muscles with the decisive difference being driven by the relative position following a simple first encountered, first innervated rule. It was necessary to confirm that there were indeed motor neuron axons projecting to the transplanted ear and not just afferent projections of sensory ganglion cells from the ear back to the midbrain along the oculomotor nerve, since ganglion cells were shown to occasionally project along the oculomotor nerve into the midbrain (Figs. 4.2D, 4.3I). Sensory ganglion cells were detected in these ears by the existence of neuronal cell bodies in a putative vestibular ganglion that were also labeled with lipophilic dye. Their axons could be traced back to the oculomotor nerve (Fig. 4.3I), or in other cases were indistinguishable from the nerve itself (Fig. 4.2D). We classified ears as receiving projections from oculomotor or trochlear nerve based on the absence of lipophilic labeling of sensory ganglion cells. These ear transplants without lipophilic dye-labeled neuronal cell bodies along the oculomotor or trochlear nerves were selected for further analysis. To further confirm that these axons projecting to the transplanted ear were of motor origin, we used an antibody against VAChT. Our previous data demonstrated that ears containing VAChT-positive axon terminals on hair cells observed with electron microscopy, had motor neurons with synaptic vesicles terminating at the base of hair cells (Elliott and Fritzsch, 2010), thus VAChT is a good indicator of motor innervation. We tested 12 ears that had lipophilic label when dye was inserted into the midbrain: 7 with projections from the oculomotor or trochlear nerve without labeled ganglion cells and 5 72 that had ganglion cells that were labeled (Fig. 4.2D) to serve as a control since these were not expected to have VAChT labeling. VAChT-positive motor axon terminals were confirmed on hair cells in the 7 transplanted ears determined to have motor neuron innervation based on lipophilic labeling (Figs. 4.3C-4.3E, 4.3H). The 5 ears that had ganglion cells labeled by midbrain lipophilic dye implantation (Figs. 4.2D, 4.3I) were not positive for VAChT (Fig. 4.3K). Immunohistochemistry for tubulin revealed innervation of the transplanted ears, demonstrating that axons from the oculomotor nerve was at least a subset of the total innervation of the ear, the remainder likely being afferents (Figs. 4.3B, 4.3G). Innervation of transplanted somite, eye muscle, and jaw muscle Somite-derived muscle transplanted to the orbit failed to be innervated by oculomotor or trochlear nerves (0 of 14 transplants) as demonstrated by absence of innervation when lipophilic dyes were implanted into the midbrain. Even when the somite-derived muscle was located medial to the native eye muscles as was the case for 3 transplants, the oculomotor nerve bypassed the somite-derived muscle to innervate the remaining eye muscles (Figs. 4.4A-4.4C). In contrast, when the somite-derived muscle was transplanted to the otic region to replace the ear, 3 of 5 transplants showed some projections of possibly motor neuron axons to the somite-derived muscle, likely by the hypoglossal nerve. This suggestion derives from implantations of lipophilic dyes implanted into the hindbrain rostral and caudal to the transplanted somite (Fig. 4.4D). The remaining 2 transplants were not innervated. Eye muscle transplanted with the eye to the trunk failed to be innervated by spinal motor nerves (0 of 4 transplants) as demonstrated when lipophilic dyes were implanted 73 ventral to the spinal cord to label motor neurons as they exit the spinal cord (Fig. 4.4E). In addition, jaw muscle transplanted to the trunk failed to be innervated by spinal motor nerves (0 of 9 transplants) as demonstrated when lipophilic dyes were implanted ventral to the spinal cord (Figure 4.4F). For both eye muscle and jaw muscle transplants, the spinal motor neuron axons exiting the spinal cord navigated around the transplanted tissue and innervated neighboring native somite-derived muscle. Innervation of transplanted heart and liver Lipophilic dyes implanted into the midbrain and hindbrain revealed some axons projecting to transplanted heart tissue. Seven of 9 hearts had projections from the trigeminal nerve, as observed by dye implantations into the hindbrain (Fig. 4.5A). Five of the 9 hearts had projections from oculomotor neurons, as observed by implantations into the midbrain (Fig. 4.5B); however closer examination of these latter hearts showed that oculomotor neurons may be terminating on autonomic ganglia associated with the heart rather than on heart muscle itself (Fig. 4.5C). Thus, there were no clear examples of direct heart muscle innervation by motor neurons. Likewise, the heart beat was not changing when tadpoles moved around, suggesting limited effectiveness of oculomotor neurons to change the autonomous heartbeat frequency, when compensatory eye movements are initiated (Straka et al., 2009, Rössert et al., 2011). Lipophilic dyes implanted into the hindbrain revealed very little innervation of the transplanted liver and no innervation of the liver was observed when lipophilic dyes were implanted into the midbrain. If there were apparent projections to the liver from the hindbrain, it was from a subset of trigeminal nerve axons. Trigeminal projections to the liver occurred in 11 of the 13 transplants. In 7 livers a subset of axons from the 74 trigeminal just passed over the surface of the liver (Fig. 4.5E). The oculomotor nerve failed to innervate the transplanted liver in the 13 transplanted livers imaged and if it approached the liver, it would pass around or over to innervate the nearby eye muscles, if the latter were present (Fig. 4.5D and 4.5E). Discussion The results here extend our previous work (Elliott and Fritzsch, 2010) by demonstrating that, not only spinal somatic motor neurons, but other subsets of motor neurons such as oculomotor motor neurons can also reroute and innervate the hair cells of the transplanted ear. In addition, we have tested for the ability of other targets and nontargets of motor neurons to receive novel innervation when similarly transplanted. Here we will discuss the range of innervation of novel tissues and its likely implications. Using lipophilic dye implantations, we were able to demonstrate the ability of the oculomotor nerve, and in one case the trochlear nerve, to extend motor neuron axons to hair cells of an ear transplanted to the orbit to replace the eye. This finding, together with that of Elliott and Fritzsch (2010), that spinal somatic motor neurons can innervate the ear when their normal target is no longer present, supports the idea that facial branchial motor neurons may have been rerouted to innervate the ear when the ear evolved in place of somites or somitomeres in ancestral vertebrates (Fritzsch and Nichols, 1993, Fritzsch et al., 2007, Simmons et al., 2011). Together, these data demonstrate that spinal, branchial, oculomotor and thus likely any motor neuron have the ability to become an efferent to the ear; however, whether this is also true for visceral motor neurons projecting axons to neural crest derived ganglia (Fritzsch and Northcutt, 1993, TakanoMaruyama et al., 2010) remains to be seen. Unlike the ear, transplantation of somite, 75 heart, and liver tissue into the orbit did not result in innervation by either the oculomotor or the trochlear nerve, with the exception of the autonomic parasympathetic ganglia associated with the heart which received axons from the oculomotor nerve in a few cases, possibly reflecting the parasympathetic component of the oculomotor nerve. Overall, this suggests that not all motor neurons can become efferents to any target, even if that tissue normally receives motor innervation, for example the somites. This is in line with previous reports of selective reinnervation of eye muscles after trochlear nerve transection which showed that in Xenopus, the trajectory of the nerve combined with the timing of innervation formation, determines the pattern of innervation by only one ocular nerve or several (Fritzsch and Sonntag, 1990). Unlike the facial branchial motor neurons which can innervate a variety of tissue types, including hair cells as efferents (Simmons et al., 2011), the oculomotor and trochlear motor neurons normally innervate only specific eye muscles, with the exception of a parasympathetic branch of the oculomotor nerve that innervates the parasympathetic ciliary ganglion (Adams et al., 2008). It is possible that this branch of the oculomotor was responsible for the innervation of parasympathetic ganglia associated with the heart tissue when transplanted to the orbit. The inability of the oculomotor or trochlear axons to innervate somite-derived muscle tissue or of the spinal motor neurons to innervate branchial-arch-derived muscle tissue are likely due to differences in the origin of the tissues and may reflect an inability of cranial nerves to supply somite-derived tissue and of spinal motor neurons to supply branchial-arch-derived tissue. Such is the case with the trapezius muscle in normal development. The branchial-arch-derived trapezius muscle sits in the trajectory of spinal 76 motor neurons, yet is innervated by motor neuron axons of the spinal accessory nerve or by a branch of the vagus (Boord and Sperry, 1991, Sienkiewicz and Dudek, 2010, Dudek et al., 2011). Thus, it seems plausible that cranial motor neurons and spinal motor neurons rely on unique molecular signatures adapted by their target tissues to prevent cross-innervation at the head-neck boundary. What this signature is remains unclear. Data on experimental reorganization of ocular innervation in cases of loss of abducens or oculomotor innervation (Fritzsch et al., 1995) suggests that some hierarchy of crossinnervation possibilities exist that need to be further investigated. The ability of the hypoglossal motor axons to innervate somite-derived muscle tissue transplanted to the region previously occupied by the ear may reflect the natural ability of the hypoglossal motor neurons to innervate the somite-derived tongue muscle (Leperchey, 1979). In aquatic organisms, such as X. laevis, the tongue is absent (Cannatella and de Sá, 1993). The hypoglossal nerve, without a target, may degenerate as nerve or motor neurons have not been found in adult X. laevis (Nikundiwe and Nieuwenhuys, 1983); however, given the chance to innervate a target of similar origin (somite-derived muscle), the hypoglossal motor neurons apparently does so at least transiently. In a sense, motor neuron axons supplies of somite-derived muscle via the hypoglossal nerve, also recapitulates the original efferent innervation paradigm of the ear previously suggested which forced some facial motor neurons to innervate the ear in the absence of their likely somite derived original target (Fritzsch, 1999). As was observed in our previous work (Elliott and Fritzsch, 2010), afferents from transplanted ears can project to a novel area in the CNS. Although most afferents projected along the trigeminal nerve into the hindbrain as seen from both hindbrain and 77 ear implantations with lipophilic dyes, some afferents from ears transplanted to the orbit projected back to the midbrain along the oculomotor or optic nerve. From there, the occasional axons projected into the forebrain. It appears that these afferents fasciculated with the nearest cranial nerve. That more axons followed the trigeminal nerve than any of the other nerves may be due to the larger territory of trigeminal projections than that of the others (Borges and Casselman, 2010). However, even with the same entry point into the brain along a given cranial nerve, there was no consistency once inside the brain for afferent axon projections. The ability of the oculomotor and trochlear nerves to send projections to hair cells of the ear but not the other tissues directly suggests that there is something unique about the ear to allow for cross-innervation. One thing in common that all targets receiving direct motor input have is the presence of nicotinic acetylcholine receptors (nAChRs) (Zuo et al., 1999, Vernino et al., 2009). nAChRs are comprised of a pentamer of various subunits: α, β, δ, ε, and γ (Tsunoyama and Gojobori, 1998, Jones and Sattelle, 2004, Albuquerque et al., 2009). Of these subunits, the α subunit is required for the receptor to bind ACh (Jones and Sattelle, 2004). The original α sequence has diversified, giving rise to the 10 different isoforms present today (Jones and Sattelle, 2004). Of the 10 α subunits, α9 and α10 are the most diverged (Sgard et al., 2002, Franchini and Elgoyhen, 2006, Katz, 2011, Lipovsek et al., 2012). Hair cells contain these most divergent of nAChR alpha subunits, α9 and α10 (Sgard et al., 2002, Franchini and Elgoyhen, 2006, Katz, 2011, Lipovsek et al., 2012). It is possible that all motor neurons retain the ability to form synapses on α9 and α10-containg nAChRs. Mice in which the gene encoding the α9 nAChR subunit (Chrna9) or α10 nAChR subunit (Chrna10) was knocked out showed 78 that these receptors were in part necessary for the development of synaptic connections between the olivocochlear efferents and the inner ear hair cells (Vetter et al., 1999, Vetter et al., 2007, Katz, 2011). In mice lacking either α9 or α10 nAChR subunits, efferent synaptic contacts were larger in size but reduced in number compared to wild type littermates (Vetter et al., 1999, Vetter et al., 2007). No α9 or α10 double null mouse efferent innervation has been reported, leaving it open whether absence of both receptors eliminates all efferent synaptogenesis on hair cells. We are currently planning to knockdown both α9 and α10 nAChR subunits in X. laevis and transplant ears from these embryos into control embryos, either replacing the native ear or transplanting to the trunk to replace a somite or the orbit to replace the eye. The prediction would be that there is no efferent innervation of hair cells by any motor neurons, including an absence of hair cell innervation in the untransplanted ear. Future work would require also misexpressing α9 and α10 nAChR subunits in tissues that did not receive motor innervation when transplanted to either the orbit or trunk such to determine whether it is these nAChRs that allow for hair cell innervation by other motor neuron types. In conclusion, the results presented here demonstrate the potential for a motor neuron to reroute to target a novel tissue that is transplanted into its trajectory; however, the nervous system is not completely plastic and not every motor neuron can interact with any target. Our data suggest that it is only the ear (and possibly parasympathetic ganglia) that can receive motor input from any motor neuron when placed in the trajectory of all motor neuron types tested here. The next step is to determine what properties present in the ear that are lacking in the other tissues transplanted that allow for synaptic formation by motor neurons. As previously mentioned, one strong candidate is the presence of the 79 ancestral α9 and α10 nAChR subunits in the ear. Additionally there could be other unique components involved in synaptic formation as well as short range guidance cues to guide axons to the hair cells. Determining these factors may provide an additional understanding of the evolution of motor neuron innervation specificity of distinct peripheral targets. Furthermore, the insights generated here may be applicable in helping individuals with motor neuron damage regain function by rerouting other motor neurons to the denervated target tissue. 80 Table 4.1. Success of tissue transplantation. Transplantation Transplanted Tissue Transplanted Tissue Total Present Absent Ear to Orbit 159 14 173 Somite to Orbit 9 1 10 Eye/Eye muscle to Trunk 6 1 7 Jaw muscle to Trunk 12 1 13 Heart to Orbit 23 8 31 Liver to Orbit 13 0 13 Note: Success of transplantation is defined as the visual detection of transplanted tissue. 81 Figure 4.1. Stage 46 Xenopus laevis. (A) Embryo with a transplanted ear containing otoconia completely replacing the eye. Inset shows higher magnification of the transplanted ear. (B) Embryo with a transplanted ear containing otoconia medial to the reformed eye. A residual ear (RE) regrew in the native location. Inset shows higher magnification of the transplanted ear and remaining portion of the eye (circled). (C) Embryo with a transplanted, empty vesicle medial to the eye, which has formed a secondary eye more caudal. A residual ear regrew in the native location. Inset shows higher magnification of the transplanted ear and remaining portion of the eye (circled). (D) Embryo with transplanted GFP-expressing somite-derived muscle tissue medial to the eye. (E) Embryo with transplanted somite-derived muscle tissue to replace the ear. (F) Embryo with a transplanted donor GFP-expressing eye to the trunk, replacing a somite. Inset shows the GFP expression in the transplanted eye and surrounding transplanted eye muscle. (G) Embryo with a transplanted heart completely replacing the eye. (H) Embryo with a transplanted liver completely replacing the eye. Native, unmanipulated ears are labeled ‘Ear’ and are circled with a black dotted line. Eyes, native and reformed, are indicated by ‘Eye’. Arrows indicate transplanted tissues; transplanted ears are circled in addition with a white dotted line. Scale bar is 1 mm in A, B, C, F, H; 0.5 mm in D, E. 82 83 Figure 4.2. Afferent projections to transplanted ears. (A) Embryo showing implantation of lipophilic dyes into the midbrain (blue) and hindbrain (red). The transplanted ear is noted by the arrow. (B) Transplanted ear with ganglion cells (GC) projecting to hair cells (HC) in the inner ear and along the trigeminal nerve (V, red) back to the hindbrain. The optic nerve (II) is green. (C) Transplanted ear labeled with GFP reveals delaminated ganglion cells (GC), some of which project back to the brain (*) along the trigeminal nerve (V) as noted by colocalization with lipohilic dye. Other ganglion cells (**) did not colocalize with lipophilic dyes. (D) Transplanted ear labeled with GFP reveals delaminated ganglion cells (GC) which project back to the brain along the oculomotor nerve (III). Inset is higher magnification of boxed area showing the GFP labeled otic ganglion cells. (E) Embryo demonstrating implantations of lipophilic dyes into the native ear (blue) and transplanted ear (red, arrow). (F-I) Brains from embryos following lipophilic implantation into the native ear (green) and transplanted ear (red) reveal variation in afferent projections from the transplanted ear. Note: some of the lipophilic dye-labeled projections are from cranial nerves that were labeled transcellularly from the afferents. (I’) Stack of eight z-series confocal images from I showing hindbrain projections from the transplanted ear to the alar plate, probably the vestibular nucleus. Scale bar is 1 mm in A and E, 50 µm in B and C, 100 µm in D, F, G, H, I, and I’. 84 85 Figure 4.3. Efferent projections to transplanted ears. (A) Implantations of lipophilic dyes into the midbrain (green) and hindbrain (red) revealed axon projections from the oculomotor nerve (III) to hair cells of the transplanted ear (circled). (B) Immunohistochemistry for tubulin of the ear in A shows all innervation. (C-E) Immunohistochemistry for VAChT (red) confirms motor terminals on hair cells (HC) of boxed areas in B. Insets show higher magnification of VAChT staining at the base of hair cells. (C’) Single z-series images at the base of the hair cells (lower left) showing VAChT-positive terminals and at the apex (upper right) devoid of VAChT staining. (F) Implantations of lipophilic dyes into the midbrain (green) and hindbrain (red) revealed axon projections from the trochlear nerve (IV) to hair cells in the transplanted ear (circled). Afferent axons projected along the trigeminal nerve to the hindbrain, demonstrated by the colocalization of ganglion cells (GC) with the red lipophilic dye. (G) Immunohistochemistry for tubulin of the ear in G shows all innervation. (H) Immunohistochemistry for VAChT (red) confirms motor terminals on hair cells of boxed area in G. Inset shows higher magnification of VACHT staining at the base of hair cells. (I) Implantations of lipophilic dyes into the midbrain (green) and hindbrain (red) revealed ganglion cells (GC) projecting along the oculomotor nerve (III). For this ear, the oculomotor nerve innervated the eye muscles ventral to the transplanted ear. (J) Immunohistochemistry for tubulin of the ear in I shows all innervation. (K) Immunohistochemistry for VAChT (red) shows the absence of motor terminals on hair cells (HC) of boxed area in I. Scale bar is 100 µm in A, B, F, G, I, J; 25 µm in C, C’, D, E,H, K. 86 87 Figure 4.4. Transplanted muscle tissue. (A) Implantations of dye into the midbrain (red) and hindbrain (blue) revealed axon projections from oculomotor nerve to the eye muscles but not to the transplanted somite-derived muscle (GFP, green). (B-C) Single z series showing innervation to the eye muscle but not the transplanted somite-derived muscle (GFP, green). (D) Implantations of dye into the hindbrain at the level of the trigeminal (red) and the glossopharyngeal, vagus, and hypoglossal (green) revealed axons projecting to transplanted somite-derived muscle tissue, likely from the hypoglossal nerve. (E) Implantations of dye ventral to the spinal cord revealed spinal motor neuron innervation of surrounding somite-derived muscle but not to the GFP-positive eye muscle (green) transplanted with the eye. (F) Implantations of dye ventral to the spinal cord revealed spinal motor neuron innervation of surrounding somite-derived muscle, but not to the jaw muscle transplanted from a dextran-injected embryo. Scale bar is 100 µm. 88 89 Figure 4.5. Transplantated tissue lacking nicotinic acetylcholine receptors such as heart and liver. (A) Implantations into the midbrain (red) and hindbrain (blue) revealed trigeminal innervation of the transplanted GFP-positive heart. The oculomotor (III) only innervated nearby eye muscle tissue. (B) Example of axons from the oculomotor nerve (III) projecting to a transplanted heart in addition to eye muscle. (C) Immunohistochemistry for tubulin (green) and VAChT (red) demonstrate that axons from the oculomotor nerve project to ganglion cells associating with the heart but not on the heart muscle itself. (D) Implantations into the midbrain (green) and hindbrain (red) revealed no axons projecting to the liver. (E) Implantations into the midbrain (green) and hindbrain (red) showed nerve fibers passing over the liver, but not innervating it. Transplanted tissue is circled. Scale bar is 50 µm in A, C; 100 µm in B, D, E. 90 91 CHAPTER V GENERATION OF ‘THREE-EARED’ FROGS REVEALS MOLECULAR AND ACTIVITY-BASED GUIDANCE OF CENTRAL PROJECTIONS Abstract As new sensory organs, such as the ear, arose in evolution, connections between the sensory system and CNS were formed. How these inner ear neurons find their central target in the hindbrain to transmit sound and movement information is not yet known. Much more is known about the developmentally-related visual system. In the eye, retinal ganglion cells use both molecular cues (Eph/Ephrin) and activity-based mechanisms (formation of ocular dominance columns) to guide axons to the proper location in the CNS. In order to determine whether the ear also uses molecular mechanisms and activity-based guidance, we generated ‘three-eared’ frogs. An additional ear was transplanted rostral to the native ear, either maintaining the native orientation, or rotating it by 90 degrees. The rationale was that in the native orientation, the gravistatic and angular acceleration-detecting sensory epithelia would be in line and thus there should be little or no difference in activity between the two ears. On the other hand, when one ear is rotated 90 degrees, its sensory epithelia will be offset from the native ear and will not respond the same to a given stimulus. Swimming behavior of tadpoles indicated that embryos in which the transplanted ear was in line with the native ear swam more normally, whereas embryos in which the transplanted ear was rotated by 90 degrees swam aberrantly. Injections of lipophilic die tracers indicated that afferent axons from the two ears projected to overlapping areas when the transplanted ear was in line with the 92 native ear. In contrast, when the transplanted ear was rotated by 90 degrees, afferent axons from the two ears were segregated from each other, forming ‘vestibular dominance columns’ reminiscent of the ocular dominance columns formed from varying activity between the two eyes. These results suggest that afferents of transplanted ears rotated 90 degrees interfere with vestibular processing as indicated by the aberrant swimming. In addition, the partial overlap and segregation of afferent innervation in the two ‘threeeared’ frog models implies that both molecular and activity-based mechanisms affect central projections. Introduction During normal development, the central nervous system (CNS) makes very stereotyped connections with peripheral sensory targets allowing for proper transmission of information. Such evolutionarily stable connections project to molecularly specified locations and nuclei within the brain (Brodmann, 1909). For instance, retinal ganglion cells project to the superior colliculus/tectum (Clandinin and Feldheim, 2009), olfactory receptor neurons to the olfactory bulb (Adam and Mizrahi, 2010), vestibular afferents to the vestibular nucleus and cerebellum (Barmack, 2003), and auditory afferents to the auditory nucleus (Meredith, 1988). While these connections are rather refined in organisms that possess these sensory systems now, the brain has evolved such connections and sensory organs over time. How those sensory organs and their projections evolved in vertebrates is unknown. Proper connections need to be made from a novel sensory system to the CNS to allow meaningful interpretation of the data provided by the sensory organ. In the wellstudied visual system, projections of the retinal ganglion cells to the superior 93 colliculus/tectum are guided by molecular cues in order to produce a topographical map of the environment. Vertebrates use concentration gradients of the EphA receptor and its ligand, ephrin-A, along the nasal-temporal axis of the retina and anterior-posterior axis of the superior colliculus/tectum to guide retinal ganglion axons to the appropriate region of the CNS (Clandinin and Feldheim, 2009) to maintain appropriate representation of the visual field. These projections to the superior colliculus/tectum are further refined in some vertebrates to account for input from both eyes. In higher mammals, (i.e. cats and primates), the eyes are located in the front of the head, resulting in a significant overlap of part of the visual field between the two eyes (Schmidt and Tieman, 1985, Leamey et al., 2009). The same part of the visual field from both eyes project to the same area in the CNS, resulting in the segregation of eye-specific projections to the visual cortex into stripes, referred to as ocular dominance columns (Hubel and Wiesel, 1977, Wiesel, 1982). In animals with laterally-located eyes, there is no or very limited overlap of the visual field and there is no formation of ocular dominance columns (Schmidt and Tieman, 1985). The formation of ocular dominance columns can be induced in lower vertebrates with lateral eyes, (i.e. frogs and fish), by transplantation of an extra eye such that two eyes project to the same tectum (Constantine-Paton and Law, 1978, Springer and Cohen, 1981, Constantine-Paton, 1982, Easter, 1983). Various experiments have demonstrated that the formation of ocular dominance columns is primarily activity-based (Hubel and Wiesel, 1965, Swindale, 1981, Meyer, 1982, Swindale and Cynader, 1983, Boss and Schmidt, 1984, Mower et al., 1984, Reh and Constantine-Paton, 1985) and presents a compromise between the difference in activity between the two eyes and the same molecular map for both eyes. 94 Much less is known about axon guidance in the ear. The ear has been shown to be developmentally related to the eye. In Jellyfish, Pax B is expressed in both the eye and the statocyst (Kozmik et al., 2003). PaxB has evolved into Pax 6 and Pax 2/5/8 (Fritzsch and Piatigorsky, 2005, Galliot et al., 2009). Pax 6 is involved in eye development across nearly all species (Gehring and Ikeo, 1999), whereas Pax 2/5/8 is involved in ear development (Pfeffer et al., 1998, Heller and Brändli, 1999, Bouchard et al., 2010). In addition, in Drosophila melanogaster, atonal is expressed in both photoreceptors and chordotonal organs (Jarman et al., 1995). Atonal has since duplicated and diversified. In the eye, Atoh7 is expressed in the RGCs and is important for their development (Kanekar et al., 1997, Brown et al., 1998, Brown et al., 2002, SkowronskaKrawczyk et al., 2009), whereas in the ear, Atoh1 is important for hair cell formation, and other atonal family members, Neurog1 and Neurod1 are important for the development of sensory neurons (Fritzsch et al., 2010). Given these developmental similarities between the eye and ear and other similarities including the ability to form a space map (topographical map in the visual system, tonotopic in the auditory system) and similar cortical plasticity in cross-modal innervation studies (Mao et al., 2011), we hypothesized that the ear may use both molecular and activity-based mechanisms to order cochlear afferents (Rubel and Fritzsch, 2002) and terminate specified projections from the various end organs of the vestibular system (Maklad and Fritzsch, 2003, Newlands et al., 2003) comparable to the eye. To test this hypothesis, we generated for the present study ‘three-eared’ frogs to determine whether the ear uses molecular and activity-based cues for axon guidance by transplanting an additional ear rostral to the native ear, either maintaining its orientation 95 or rotating it by 90 degrees in the horizontal plane. The rationale is that in the native orientation, the gravistatic and angular acceleration-detecting sensory end organs would be in line and thus there should be little or no difference in activity between the two ears. On the other hand, when one ear is rotated 90 degrees, its sensory end organs will be offset from the native ear and will not respond the same way to a given stimulus. If the axons are guided using molecular cues only, afferents from both ears should project to the same areas in the brain with little segregation between axons from the two ears, regardless of the orientation of the transplanted ear. If there is both molecular and activity-based guidance, then in animals in which the transplanted ear was in line with the native ear, afferents should project as above; however, when the transplanted ear was rotated 90 degrees, afferents should project to the same areas in the brain, but with segregation between axons, forming “vestibular dominance columns” reminiscent of the ocular dominance columns formed from varying activity between the two eyes. Results Success of transplantation Success for ear transplantation was defined as the detection of a third ear, whereas completeness of transplantation was defined as the amount and/or degree of normality of transplanted tissue. Of the 195 successful ear transplantations in which a third ear could be identified, 86% of the ears transplanted contained otoconia (n = 167), whereas 14% were just a vesicle and lacked otoconia (n = 28). Of the 167 ears that contained otoconia, 105 were nearly indistinguishable from a normal ear, showing two otocnia-bearing maculae and three semicircular canal cristae. In 49 transplantations, the transplanted ear fused with the native ear, forming a larger ear with multiple sets of otoconia. Fusion 96 occurred more frequently with earlier transplantations (stage 25 vs stage 27). Transplanted ears were not detected in 17 animals. Only animals in which a complete third ear formed (Fig. 5.1), containing otoconia over the utricle and saccule, and either in the normal orientation (n = 38) or rotated by 90 degrees (n = 55), were used for further analysis. Analysis of Swimming Behavior Initial swimming behaviors, normal (swimming upright), swimming on side, swimming upside down, swimming vertical, spinning, looping (Ferris wheel formation), and spiraling (corkscrew formation), were determined in animals for animals in which the third ear was in line with the native ear or rotated by 90 degrees (Table 5.1). In animals in which the transplanted ear was in the native orientation, animals primarily swam upright. Slightly less than half (45%) had one additional swimming behavior, though most of the time was spent swimming upright (data not shown). Of these 45%, only two animals showed more than one additional swimming behavior. The most common behavior, besides normal swimming, in these animals was spinning. This was usually one of the first behaviors observed when the embryos were dropped into the arena, and often they quickly re-oriented themselves. This initial spinning behavior is also seen in approximately half of control, unmanipulated animals. Also for control animals, most time was spent swimming upright (data not shown). The remaining behaviors were seen in 3% to 11% of animals with the transplanted ear in the native orientation. In animals in which the transplanted ear was rotated by 90 degrees, most animals were able to eventually swim upright (93%), but not all. Normal upright swimming was the first behavior observed in 31% of these animals. For those that were able to swim 97 upright at some point, all but two displayed additional swimming behaviors. Of the animals displaying additional swimming behaviors besides normal swimming, 43% showed at least two additional swimming behaviors. The most common behavior, besides normal swimming, in these animals was swimming on the side (49% of animals), swimming vertically (42% of animals), and spinning (35% of animals). The remaining behaviors were seen in 5% - 23% of animals. The swimming behavior of most animals in which the transplanted ear was rotated by 90 degrees is similar to animals with unilateral ear ablation, resulting in an ear only on one side. As with animals with a rotated transplanted ear, most animals with an ablated ear were able to swim upright (85% of animals); however, of the ablated ear animals eventually able to swim upright, 71% displayed at least two additional swimming behaviors, which was a little higher than the 43% of animals with a rotated transplanted ear. The most common behaviors in animals with only one ear, besides the ability to eventually swim upright, were spinning and swimming on the side (70% and 60% of animals, respectively). Analysis of Afferent Projections to the Hindbrain Lipophilic dyes implanted into the native and transplanted ears of fourteen animals revealed afferent projections in the hindbrain. The transplanted ear projected either with the native VIIIth ganglion, completely with its own ‘VIIIth’ ganglion, or in a mixture of both with the native and with their own ‘VIIIth’ ganglion (Fig. 5.2). In one animal, the transplanted ear, in the native orientation, projected entirely with the native VIIIth ganglion, although fibers from the two ears were mostly segregated within the ganglion (Fig. 5.2A). In seven animals, the transplanted ear projected with its own ‘VIIIth’ ganglion (Fig. 5.2B). Five of these animals had rotated ears; two had ears in the 98 native orientation. In the remaining six animals, the transplanted ear projected partially with the native ganglion and partially with its own ganglion (Figs. 5.2C and 5.2D). Two of these animals had rotated ears; four had ears in the native orientation. Axons from the additional ‘VIIIth’ ganglion from the transplanted ears entered the hindbrain at the approximate level of the trigeminal ganglion. Afferents from all seven embryos with ears transplanted in line with the native ear project to the same area in the hindbrain with nearly complete overlap of fibers from the two ears (Figs. 5.3A and 5.3B). In contrast afferents from all seven embryos with ears transplanted 90 degrees offset from the native ear project in general to the same area in the hindbrain, but with nearly complete to complete segregation of fibers from the two ears (Figs. 5.3C and 5.3D). Projections into the hindbrain from the rotated ears were always located medial to the native ear projections (Figs. 5.3C and 5.3D), but lateral to the trigeminal tract. The percent overlap of sensory neurons from the native and transplanted ears was calculated from intensity histograms obtained for the two sensory neuron populations (Figs. 5.3E-5.3F). When the transplanted ears were in line with the native ear, the percent overlap of the narrower histogram with the wider histogram was 97.7 ± 2.3% (n = 5) (Fig. 5.3G). The range of overlap for a single optical section in these animals was between 66-100%. When the transplanted ears were rotated by 90 degrees with respect to the native ear, the percent overlap of the narrower histogram with the wider histogram was 21.3 ± 6.4 (n = 5) (Fig. 5.3G). The difference in overlap profiles between animals with normally oriented transplanted ears compared with rotated transplanted ears was 99 significant (p<0.0001). The range of overlap for a single optical section in these animals was between 0-57%. Discussion The results presented here on ‘three-eared’ frogs establish the basic mechanisms used by inner ear afferents to project to the proper location in the hindbrain. In addition, we have examined the functionality of the third ear to influence the swimming behavior of the tadpoles. The transplanted ear developed completely normally in 105 of 195 cases, supporting our previous data in frogs and data from chicks showing future sensory epithelia have already been specified by the otic placode stage (Abelló et al., 2010, Elliott and Fritzsch, 2010). The fusion of the transplanted ear and native ear may be a sideeffect of the early transplantation, as this was observed less frequently when the ear was transplanted at stage 27 rather than at stage 25. Using lipophilic dyes injected into the native ear and transplanted ear, we showed that both ears, regardless of orientation project to the same approximate region of the hindbrain. This suggests that initial guidance of inner ear sensory afferents is molecularbased. While the exact molecular nature of the guidance remains unknown, the most logical candidate is the Eph/Ephrin system used by RGCs to guide axons to the tectum/superior colliculus to maintain the visual field. Ephs and Ephrins have been shown to play some role in axon guidance in the auditory system (Allen-Sharpley and Cramer, 2012, Allen-Sharpley et al., 2013, Defourny et al., 2013). Given that Ephs and Ephrins are also expressed in vestibular neurons (Bianchi and Liu, 1999, Cowan et al., 2000), suggests that Ephs and Ephrins may play a role in guiding sensory afferents to the 100 proper location centrally in the vestibular nucleus, though this would need to be confirmed. While sensory neurons from the two ears projected to the same approximate region, the precise targeting depended upon the orientation of the transplanted ear with respect to the native ear. The nearly complete overlap in projections from the two ears when the transplanted ear was in the native orientation and the nearly complete segregation of projections from the two ears when the transplanted ear was rotated by 90 degrees suggest that activity-based mechanisms also play a role in axon guidance in the vestibular system. The nearly complete segregation of sensory axons in the vestibular nucleus when the transplanted ear was rotated by 90 degrees formed ‘vestibular dominance columns’ reminiscent of the ocular dominance columns found in the visual system naturally (Hubel and Wiesel, 1977, Wiesel, 1982) and experimentally ‘three eyed frogs’ (Constantine-Paton and Law, 1978, Springer and Cohen, 1981, Constantine-Paton, 1982, Easter, 1983) as a result of differential activity between two eyes. This activitybased guidance likely refines the initially set up by molecular-based guidance. Initial targeting by vestibular afferents is thought to be molecular since initial observations of vestibular afferent targeting were made prior to the onset of vestibular sensation (Maklad and Fritzsch, 2002, Maklad and Fritzsch, 2003, Maklad et al., 2010). Later changes in the pattern of vestibular nucleus connectivity from the various sensory epithelia occur around the onset of a functional vestibular system (Maklad and Fritzsch, 2003). The ability of ‘three-eared’ frogs to swim normally depended upon the manipulation, which suggests that projections into the vestibular nucleus from the transplanted ear are functional. Animals in which the transplanted ear was in alignment 101 swam similar to control, unmanipulated, animals most of the time. In contrast, animals in which the transplanted ear was rotated by 90 degrees displayed more aberrant swimming, similar to that of animals with only one ear. These interpretations are consistent with our control experiments in which we remove and reinsert one ear, forcing afferents to reestablish their connections. Such manipulations result in completely normal swimming behavior indistinguishable from unmanipulated animals. Given that the latter manipulations results in asymmetrical and mismatched gravitational sensation suggests that bilateral symmetry in sensory epithelia orientation is required for proper sensing of the animals’ orientation to proper guide its swimming. 102 Table 5.1. Analysis of tadpole swimming behavior. Animal Treatment Control (n = 28) One Ear (n = 20) Normal Third Ear (n = 38) Rotated Third Ear (n = 55) Upright Side 28 17 38 51 1 12 4 27 Swimming Behavior Vertical Upside Spin Down 1 0 10 5 9 14 1 3 12 12 23 19 Spiral Loop 0 8 1 7 0 7 1 5 Note 1: Numbers in parenthesis are the total number of animals for each condition. Note 2: Numbers represent total animals observed for each swimming behaviors. Some animals displayed multiple behaviors and are included in each total. 103 Figure 5.1. Stage 46 Xenopus laevis 'three-eared' frogs. (A) Embryo with a transplanted third ear in the native orientation. (B) Embryo with a transplanted ear rotated 90 degrees from the native ear. (C) Higher magnification of the natively-oriented transplanted ear and the right native ear in A. (D) Higher magnification of the 90 degree rotated transplanted ear and the right native ear in B. (E) Three-dimensional reconstruction of a 90 degree rotated transplanted ear next to the native ear. Endolymphatic space is magenta, endolymphatic duct is cyan, and the hair cells are green. Native, unmanipulated ears are labeled ‘Ear’ and are circled with a black dotted line. Transplanted ears are indicated with a white arrow and are circled with a white dotted line. U, utricle; S, sacule. Blue and yellow arrows indicate ear orientation. Scale bar is 0.5 mm. 104 105 Figure 5.2. Inner ear afferent projections. (A) Animal in which inner ear afferents projected entirely together in the VIIIth ganglion. (B) Animal in which inner ear afferents from the natively-oriented transplanted ear projected in their own ‘VIIIth’ ganglion and entered the hindbrain separate from the inner ear afferents from the native VIIIth ganglion. (C) Animal in which the inner ear afferents from the 90 degrees rotated transplanted ear entered the hindbrain from both in its own ‘VIIIth’ ganglion and with the native VIIIth ganglion. (D) Animal in which the inner ear afferents leave the nativelyoriented transplanted ear both in its own ‘VIIIth’ ganglion and along with the native VIIIth ganglion. Green arrowheads indicate projections from the transplanted ear. Red arrowheads indicate projections from the native ear. Scale bar is 100 µm. 106 107 Figure 5.3. Overlap and segregation of inner ear afferents from transplanted and native ears. (A-B) Hindbrain from two animals in which the transplanted ear was in the native orientation showing overlap of sensory neurons from the native (red) and transplanted (green) ears. (C-D) Hindbrain from two animals in which the transplanted ear was rotated by 90 degrees with respect to the native ear showing segregation of sensory neurons from the native (red) and transplanted (green) ears. (E) Intensity histogram from an animal with the transplanted ear in line with the native ear shows an overlap of intensity profiles in a single optical section. (F) Intensity histogram from an animal with the transplanted ear rotated by 90 degrees with respect to the native ear shows a segregation of intensity profiles in a single optical section. (G) Mean percent overlap and standard error of sensory neurons from the native and transplanted ears for animals in which the transplanted ear was in line with or rotated by 90 degrees with respect to the native ear. Numbers represent the number of animals. Each animal is the mean of measurements taken from 3 different optical sections. ***, p<0.001. Scale bar is 25 µm. 108 109 CHAPTER VI EAR MANIPULATIONS REVEAL A CRITICAL PERIOD OF HINDBRAIN DEPENDENCE ON THE EAR FOR DEVELOPMENT AND SURVIVAL Abstract Many second-order neurons rely on afferents from sensory organs during a critical period for survival and differentiation. Research describing these effects has mostly focused on whole populations of neurons, rather than a single cell. We studied the effects of partial deafferentation on a single identifiable cell in the hindbrain of a frog, the Mauthner cell. Some previous work hinted on a critical period in Mauthner cell viability without ear input while others found that surviving Mauthner cells had reduced branching of the lateral dendrite. The suggestion that axons from the ear stimulate the growth and development of the dendrites is further supported when transplantation of an additional ear allegedly increased dendritic branching. Extirpation of the ear at various stages of Xenopus laevis development defines a critical period of progressively reduced dependency of Mauthner cells on afferents lasting to about stage 36. In addition, earlier ear removal results in a greater reduction in the number of dendritic branches as compared with later ear removal. However, some rudimentary lateral dendrites form, indicating a limited autonomous development of the lateral dendrite. The greater reduction in the number of dendrites when the ear was removed at earlier stages indicates that inner ear afferents play a role in further dendritic development. The latter is confirmed quantitatively in frogs receiving a third ear: the lateral dendrite is larger and shows significantly more branches than the control dendrite of the unmanipulated side. 110 Introduction The ablation of a primary sensory organ results in hypoplasia of higher-order neuronal centers (Harrison, 1935). Early studies in amphibians demonstrated that removal of an eye results in a reduction in the size of the opposite midbrain and removal of the nasal pit results in a reduction in the olfactory center in the forebrain (Harrison, 1935). In addition, several studies have shown that removal of the ear and associated ganglion neurons prior to axonal outgrowth alters the development of various target neurons in the hindbrain (Levi-Montalcini, 1949, Parks, 1979, 1981, Goodman and Model, 1988, Fritzsch, 1990). Removal of an ear during embryonic development lead to the eventual reduction in the volume and number of neurons in the cochlear nuclei (LeviMontalcini, 1949, Parks, 1979, Ryugo and Parks, 2003) at the time point at which afferent activity could be detected (Jackson et al., 1982). Blocking afferent activity with tetrodotoxin resulted in a reduction of volume and number of neurons in the cochlear nuclei, similar to that seen with ear ablation (Pasic and Rubel, 1989, Sie and Rubel, 1992). Together, these data would suggest that most of the neuronal development occurs without excitatory activity and that later, without this activity and/or activity related neurotrophin release, cell death and atrophy of remaining neurons occurs (Rubel and Fritzsch, 2002). This dependence on a presynaptic neuron for survival is not permanent, implying there is a critical period in which input is necessary for cell survival (Rubel and Fritzsch, 2002). In gerbils, cochlea removal before postnatal day (P) 7 resulted in cell death in 45-88% of cochlear nucleus neurons, whereas removal after P9 resulted in virtually no cell death (Tierney et al., 1997). Other species also have critical periods, but with varying critical time points and degrees of cell loss (Born and Rubel, 1985, 111 Mostafapour et al., 2000). In addition, while removal of the ear early resulted in death of most cochlear nuclei and severe atrophy of the remaining neurons (Levi-Montalcini, 1949, Parks, 1979), virtually no cell death occurred in the vestibular nuclei (LeviMontalcini, 1949). Vestibular nuclei neurons exit the cell cycle much earlier than cochlear nuclei neurons (Altman and Bayer, 1980), suggesting that the vestibular nuclei neurons may be past the critical period at the time these manipulations took place. Even though early ear removal results in significant cochlear nucleus cell loss, some cells do survive. Unfortunately, the reason for why some cells die and others do not is unclear. The leading hypothesis to explain why some post-synaptic neurons survive while others die following early afferent deprivation is that two competing intracellular responses are initiated, apoptotic-like and survival pathways, following afferent loss, and one pathway overtakes the other (Garden et al., 1994, Hyde and Durham, 1994a). This hypothesis is supported by several pieces of data. For example, early degradative processes following afferent loss are constant across all cells, not just some (Steward and Rubel, 1985, Born and Rubel, 1988, Garden et al., 1994, Garden et al., 1995, Kelley et al., 1997), and oxidative enzyme activity first increases and then decreases following afferent loss (Durham and Rubel, 1985, Durham et al., 1993, Hyde and Durham, 1994b). Thus the cells that survive depend upon the effectiveness of the survival pathway to overcome apoptosis. Levi-Montalcini proposed that loss of ‘neurotrophic’ support from the afferents following ear removal may cause the cell death in the cochlear nucleus (Levi-Montalcini, 1949); however, the exact molecular nature of the factors by which afferents support cochlear nucleus neurons is still unknown. 112 These above-mentioned studies have increased our understanding of the importance of primary sensory input on second-order neuron survival; however, these studies looked only at whole populations of cells when determining the critical period of cell survival and determining why some cells survive while others do not following ear removal. Furthermore, due to technical limitations, ears can be removed in the chick only embryonically, and in the mouse, only postnatally, which prevents a comprehensive analysis of the loss of afferent input over a longer period of time in these species. While afferents or ears can be genetically removed in mice, the resulting mutants are typically not viable blocking investigations on long term auditory nucleus viability (Ma et al., 2000). To overcome limitations, we have studied the effects of early otic placode/ear extirpation at various developmental stages, focusing on a single cell in the hindbrain of the externally developing frog, the Mauthner cell. Mauthner cells are a pair of large, easily identifiable, reticulospinal neurons at the level of the ear in the hindbrain of many aquatic animals (Herrick, 1914, Bartelmez, 1915) that are an important component of the escape reflex (Korn and Faber, 2005). Inner ear vestibular and auditory neurons of premetamorphic amphibians and fish form synapses on the lateral dendrite of the ipsilateral Mauthner cell, which in turn synapses on contralateral spinal motor neurons to activate the C-start escape response (Korn and Faber, 2005, Sillar, 2009). In addition to the ear, Mauthner cells also receive input from the lateral line and the eye that can add to the C-start escape response (Zottoli et al., 1987, Bezgina et al., 2000, Korn and Faber, 2005, Sillar, 2009). A study in axolotls has shown the absence of the Mauthner cell in one-third of embryos following otic vesicle ablation at stage 27 (Piatt, 1969); however, all Mauthner cells were present when the ear was 113 extirpated at a later stage (stage 34), although dendritic branching was reduced (Kimmel et al., 1977). In one embryo observed by Goodman and Model following ear ablation, the entire Mauthner cell was absent (1988). While Piatt (1969) suggested that the inner ear afferents are important for, and often a decisive factor for, the development of the Mauthner cell, Goodman and Model (1988) suggested that surgical perturbations may be the cause of the occasional Mauthner cell absence. Since early ear removal, before the critical period, results in significant loss of the cochlear nuclei neurons (Levi-Montalcini, 1949, Parks, 1979, Born and Rubel, 1985, Tierney et al., 1997, Mostafapour et al., 2000), we reason that removal of the ear at the earlier stages (Piatt, 1969, Goodman and Model, 1988) may have prevented the differentiation and/or survival of the Mauthner cell, indicating a yet to be defined critical period for a single cell. The inner ear not only affects the development of the Mauthner cell, but may also have a role in the development of the lateral dendrite that receives ear afferents. In a study in axolotls (Ambystoma mexicanum), removal of the ear of midtailbud (stage 2830) prior to the outgrowth of the VIIIth nerve resulted in significant reduction of dendritic branching of the Mauthner cell lateral dendrites in areas normally receiving vestibular input (Goodman and Model, 1988). Ear removal at a slightly later stage in axolotls (stage 34) also resulted in reduction of dendritic branching (Kimmel et al., 1977). Likewise, removal of otic vesicles in zebrafish resulted in reduced branching of the Mauthner cell lateral dendrites (Kimmel, 1982) and ablation of the otic vesicle in stage 38 X. laevis embryos resulted in reduced Mauthner cell lateral dendrites (Fritzsch, 1990). The ear is not the only sensory system that, when removed, affects dendritic branching of the Mauthner cell. Enucleation of the eye resulted in delayed development of the Mauthner 114 cell and diminished ventral dendrite volume in X. laevis and goldfish (Bezgina et al., 1999, Bezgina et al., 2000, Grigor'eva et al., 2010, Mikheeva et al., 2011). Increase in the lateral dendrite size in combination with reduction in the ventral dendrite was observed occasionally following eye enucleation (Grigor'eva et al., 2010, Grigorieva et al., 2012). Blocking nerve impulse activity in axolotls by grafting to them tetrodotoxincontaining newts did not affect Mauthner cell dendritic branching patterns, suggesting that that innervation itself, rather than neural activity, is important (Goodman and Model, 1990), the latter being in stark contrast to later experiments using other means to block activity (Pasic and Rubel, 1989, Sie and Rubel, 1992). Taken together, these data suggest that the ingrowing axons from the ear stimulate growth and development of the dendrites. This is further supported by a study in axolotls where an additional ear was transplanted rostral to the native ear. The Mauthner cells in these animals displayed unspecified ‘enhanced branching’ of the lateral dendrite (Goodman and Model, 1988). In the present study, we alter the sensory input to the developing Mauthner cell in various ways in order to determine how sensory input shapes the development of the cell and of its lateral dendrites. First, we explore the effects of otic placode/vesicle extirpation on the developing Mauthner cells and their lateral dendrites by removing the ear at both early stages (stage 24-26), when the ear is a placode, and later stages (stage 27-40), when the ear has become a closed vesicle, in X. laevis. We also explore the effects of additional otic placode transplantations on the developing Mauthner cell and lateral dendrite. This study defines the critical period during which the Mauthner cell is dependent upon input from the ear for cell survival. In addition, this study determines the dependence on input from the ear for lateral dendrite development and also the degree of 115 autonomous development of the lateral dendrite in the absence of the ear or with diminished afferent input. Results Effect of stage of ear removal on the degree of ear regeneration Removal of ears at otic placode stages (stages 24-26) led to regrowth of a part or of nearly all of the ear by stage 46 in over one-third of the embryos (Fig. 6.1A), the remaining embryos did not have ear regrowth (Fig. 6.1D), as confirmed with tubulin and Myo VI staining (Fig. 6.1E). The amount of ear regrowth in these early stages varied, ranging from an endolymphatic duct, to a small otic vesicle, or to a nearly complete ear. Removal of ears at otic vesicle stages (stages 27-40) resulted in fewer instances in which the ear regrew in embryos by stage 46 as compared to ears removed at placode stages (stages 24-26); most had no ear regrowth when the ear was removed at stage 27 and older. In these later stages, if there was any regrowth, it was nearly always just the endolymphatic duct. No regrowth of any part of the ear was detected in embryos in which the ear was removed at stages 38-40. These data confirm previous work indicating placodal induction extends over a lengthy period (Yntema, 1950). Effect of stage of ear removal on the presence of the Mauthner cell Prior to determining the effect of ear removal on the Mauthner cell, we wanted to establish that the physical process of ear removal has no effect on the survival of the Mauthner cell, as was previously suggested (Goodman and Model, 1988). In order to test this, ears were removed between stages 24-26, and immediately replaced. The replaced ear developed normally in 23 of 25 embryos (Compare Fig. 6.1C with Fig. 6.1B). For 116 one embryo, the replaced ear had a single otoconia, the other was only a vesicle. The Mauthner cell was present at stage 46 for all embryos examined in which their ears were removed and replaced immediately (n = 14) (Figure 6.2B) and was morphologically similar to that of control animals (Figure 6.2A). The stage of ear removal had an effect on the development and/or maintenance of the Mauthner cell. The earlier the stage of ear removal, the less likely it was that the ipsilateral Mauthner cell would be present at stage 46 (Figure 6.2C). To confirm that the absent Mauthner cell was not an artifact of incomplete Dextran amine dye application, an antibody against reticular neurons, including the Mauthner cell, 3A10, was also used (Figs. 6.2D-D”). To determine the percentage of Mauthner cell survival, the number of animals with a Mauthner cell present on both the ablated side as well as control side was divided by the total number of animals analyzed in each group (Fig. 6.2C). When the ear was removed at otic placode stages, between stages 24 to 26, the Mauthner cell was present on the ablated side only 38% of the time (n = 91). When the ear was removed at early otic vesicle stages, between stages 27 to 30, the Mauthner cell was present on the ablated side 64% of the time (n = 39). When the ear was removed at later otic vesicle stages, the Mauthner was present on the ablated side 95% of the time (n = 40). The Mauthner cell was always present when the ear was removed between stages 36 to 40 (n = 24). Effect of stage of ear removal on the dendritic branching of surviving Mauthner cells Prior to determining the effect ear removal has on dendritic branching of the lateral dendrite of surviving Mauthner cells, we wanted to establish the innate variability 117 in dendritic branching between left and right Mauthner cells within the same animal as well as variability in dendritic branching between animals for control animals and for those whose ear was removed and then immediately replaced. The total numbers of dendritic branch terminals were counted for three-dimensionally (3D) reconstructed left and right Mauthner cells (Figs. 6.3B-6.3E) obtained from confocal images of dextran amine dye-labeled cells. 3D reconstructions were made from dextran amine-labeled Mauthner cells as opposed to 3A10-labeled cells due to a more complete filling of the dendrites (Figs. 6.3A-6.3A”). The mean number of dendritic branches in the lateral dendrite of control animals was 22.2 ± 1.8 for the left Mauthner cell and 21.1 ± 1.4 for the right (n = 14) (Figure 3F). There was no significant difference between left and right Mauthner cell dendritic branch number in control animals (p > 0.05). There was more variation in the number of dendritic branches between different control animals than within the same animal. The range of dendritic branches between control animals was from 11 branches to 33 branches for a single Mauthner cell. The greatest difference between the dendritic branch number of left and right Mauthner cells within the same animal was 7 branches. Both the difference, subtracting the number of branches on the left from the right Mauthner cell, and the absolute difference, subtracting the smaller branch number from the larger regardless of side, were calculated. The mean difference and mean absolute difference between left and right Mauthner cell dendritic branch number were 1.1 ± 1.2 and 3.9 ± 0.6 (n = 14), respectively. For animals in which the right ear was removed and immediately replaced, the mean number of dendritic branches in the lateral dendrite was 23.9 ± 1.9 for the left Mauthner cell and 19.7 ± 1.3 for the right (n = 14) (Figure 6.3F). Though the Mauthner 118 cell on the side in which the ear was removed and immediately replaced had fewer dendritic branches compared with the control side, there was no significant difference between left and right Mauthner cell dendritic branch number when one ear was removed and immediately replaced (p > 0.05). The mean difference and mean absolute difference between left and right Mauthner cell dendritic branch number for embryos in which the right ear was removed and immediately replaced were 4.1 ± 2.1 and 6.3 ± 1.6 (n = 14), respectively. These data would suggest that the surgical procedure to remove an ear has little effect on Mauthner cell lateral dendrite development. Importantly, no animal with ear replacement had lost the ipsilateral Mauthner cell despite the fact that needed manipulations exceed simple otocyst removal. When ears were removed at any of the stages, the dendritic branching of the ipsilateral Mauthner cell lateral dendrite was always reduced compared to the control side. This reduction in branching for the ipsilateral Mauthner cells was significant for each of the stages of ear removal: early placode, early otic vesicle, and later otic vesicle (p < 0.001 for each). For animals in which the right ear was removed at placode stages (stages 24-26), the mean number of dendritic branches in the right lateral dendrite was 4.9 ± 0.6 compared with 23.1 ± 3.1 branches on the control side (n = 14) (Figure 6.3F). For animals in which the right ear was removed at early otic vesicle stages (stages 27-30), the mean number of dendritic branches in the right lateral dendrite was 7.3 ± 1.0 compared with 21.3 ± 2.5 branches on the control side (n = 14) (Figure 6.3F). For animals in which the right ear was removed at later otic vesicle stages (stages 31-40), the mean number of dendritic branches in the right lateral dendrite was 8.8 ± 0.9 compared with 23.8 ± 2.2 branches on the control side (n = 14) (Figure 6.3F). The difference in 119 branch number between left and right Mauthner cells was calculated by subtracting the number of dendritic branches of the right Mauthner cell from the left Mauthner cell. These differences were compared across groups using an ANOVA. Following a Bonferonni multiple comparison adjustment, there was no significant difference between the control and remove and replace groups(p > 0.05). While there was a trend showing a more severe reduction in dendritic branching at the earliest stage of ear removal when compared to ear removal at later stages, no stage of ear removal was significantly different from each other (p < 0.05); however, all stages of ear removal were significantly different from the control and remove and replace groups (p < 0.005). These data suggest that the lateral dendrite of surviving Mauthner cells may depend over a lengthy period on vestibular input for normal development. Sholl Analysis was performed to compare branching patterns. The numbers of dendritic crossings for Mauthner cells from animals in which the ipsilateral ear had been removed and replaced were significantly less than control Mauthner cells for 25 µm and 50 µm distances away from the soma (p<0.05), but were similar 75 µm away from the soma and beyond (Fig. 6.3G). The numbers of crossings for Mauthner cells from animals in which the ipsilateral ear had been removed were fewer than control (Fig. 6.3G). The number of crossings for Mauthner cells from animals in which the ipsilateral ear had been removed at otic placode stages (stages 24-26) was significantly fewer than the number of crossings for control Mauthner cells at 25 µm and 50 µm distances away from the soma (p<0.05). The number of crossings for Mauthner cells from animals in which the ipsilateral ear had been removed at early otic vesicle stages (stages 27-30) was significantly fewer than the number of crossings for control Mauthner cells at 25 µm and 120 50 µm distances away from the soma (p<0.05). The number of crossings for Mauthner cells from animals in which the ipsilateral ear had been removed at later otic vesicle stages (stages 31-40) was significantly fewer than the number of crossings for control Mauthner cells at only 50 µm away from the soma (p<0.05). While there were no crossings in control right side Mauthner cells beyond 100 µm away from the soma, four animals with removed ears had crossings at 125 µm away from the soma, though only by one dendrite. Effect of increasing afferent input on the dendritic branching of Mauthner cells The addition of an ear rostral to the native ear (Fig. 6.1F) lead to growth of the vestibular nerve fibers of these ears into the brain that mostly end in the vestibular fiber tract and nuclei with the unmanipulated control ear (Fig. 6.1G). In those cases were afferents of the ‘third’ transplanted ear reached into the brain there was a significant increase in dendritic branching in the ipsilateral Mauthner cell of approximately 30% more branches (p < 0.001). The mean number of dendritic branches in the right lateral dendrite was 31.1 ± 1.7 compared with 22.5 ± 2.1 branches on the control side (n = 10) (Fig. 6.3F). These data suggest that the upper limit of lateral dendrite branching is determined by yet to be understood interactions of all vestibular afferent fibers with the growing lateral dendrite. Sholl Analysis was also performed. The numbers of dendritic crossings for Mauthner cells from animals in which an additional ear was transplanted rostral to the native ear were significantly more than Mauthner cells from control animals between 75 µm and 100 µm away from the soma, at which point there were no additional crossings in 121 control Mauthner cells (Fig. 6.3G) (p<0.05). In one animal with an extra ear, crossings were detected 175 µm away from the soma. Five additional animals with an extra ear had crossings 150 µm away from the soma. Discussion The results presented here extend previous work on studying the effects of ear removal on second order neurons in the hindbrain by focusing on a single hindbrain neuron, the Mauthner cell. Below, the effect of the stage of ear removal on the regrowth of the ear will be addressed. In addition, the effect of ear removal on Mauthner cell survival as well as the effect of ear removal or addition of an extra ear on the dendritic development of the Mauthner cell will be put in perspective. The later the stage of ear removal, the increased likeliness was that all or part of the ear would not regenerate. Ablation of ears in embryos between stages 24-27 had previously been shown to have the capacity to regenerate a new ear or partial ear (Waldman et al., 2007). Similar to this study, our results showed complete or partial ear regeneration during these stages. Beyond stage 27 the ear rarely regenerated; if there was any part of the ear present, it was nearly always only the endolymphatic duct. We did not find any instance of regeneration beyond stage 38. That the ear regenerated more often when removed at the early placode stages indicates that the remaining tissue was competent to regenerate a new ear (Waldman et al., 2007), consistent with earlier suggestions (Yntema, 1950). Alternatively, while care was taken to remove the entire ear, it cannot be ruled out that a small portion was left behind. This may be the likely scenario for endolymphatic ducts being present at later stages of ear removal, when the tissue is not believed to be as likely to regenerate (Waldman et al., 2007). 122 Viability of the Mauthner cell depends on ear input By using two independent methods to label neurons, Dextran amine dye tracers and immunohistochemistry, we have shown that the development and/or survival of the Mauthner cell is dependent upon the presence of the ear. Moreover, the ability of the Mauthner cell to survive depends on the stage at which the ear is removed. The earlier in development that the Mauthner cell is deprived of input from the ear, the more likely the cell does not survive. The Mauthner cell was always observed beyond stage 36, indicating that stages earlier than stage 36 are in a critical period of dependence on the ear for survival. Stage 36 corresponds with the stage at which afferents from the ear were observed projecting into the hindbrain of axolotls (Fritzsch et al., 2005a). As with neurons in the cochlear nucleus (Levi-Montalcini, 1949, Parks, 1979, Ryugo and Parks, 2003), not all Mauthner cells are absent following ear ablation. Perhaps, as has been proposed for neurons in the cochlear nucleus (Garden et al., 1994, Hyde and Durham, 1994a), both the apoptotic and survival pathways are initiated in the Mauthner cell and whether the cell survives or not depends upon which pathway overtakes the other. Clearly, our data are consistent with earlier suggestions of a dependency of the Mauthner cell on the ear. Moreover, since we always found a Mauthner cell when we simply removed and replaced the ear, we reject the suggestion that loss of Mauthner cells is simply a function of ear removal manipulation (Goodman and Model, 1988). The Mauthner cell provides a unique opportunity to study initiation of degeneration as a consequence of partial and delayed denervation in a single cell. 123 Dendrite growth and branching depends on vestibular afferents Our data suggests that the significant reduction in branching following ear removal was a result of the loss of input from the ear and not from the physical process of ear removal, since there was no significant difference in the total number of dendritic branches of the lateral dendrite between left and right Mauthner cells when the ear had been removed and immediately replaced on the right side. The numbers of dendritic crossings from the Sholl analysis were less for Mauthner cells in which the ear was removed and immediately replaced when compared with Mauthner cells from control animals for shorter distances from the soma. Since there was no significant difference in total numbers of dendrites, we conclude the effect of ear removal itself has a potential effect on dendritic morphology, but not as much on the total number of dendritic branches. Perhaps the slightly different morphology was due to a short delay in the arrival of sensory afferents from ears that were removed and immediately replaced as compared to sensory neuron afferents from control ears, though this has not been confirmed. In all cases in which the ear was removed and not replaced, the dendritic branching on the ablated side was always less than the dendritic branching on the unoperated side. Furthermore, in all cases in which an extra ear was transplanted rostral to the native ear, there was increased dendritic branching on the side with an extra ear compared with the unoperated side. Taken together, these quantitative data suggest that the degree of dendritic branching is related to the number of presynaptic sensory afferents, consistent with earlier exclusively qualitative findings (Kimmel et al., 1977, Kimmel, 1982, Goodman and Model, 1988, Fritzsch, 1990). Our study investigates not 124 only at the effect of ear removal on dendritic branching, but also the effect of the timing of ear removal. The earlier the ear was removed, the fewer dendritic branches were present, suggesting that the degree of dendritic development depends upon the time at which the ear was removed. While there was no significant difference between the three stages of ear removal when comparing the difference between left and right Mauthner cells following ear removal, there was a trend in which the number of dendritic branches was slightly higher when the ear was removed at later otic vesicle stages (stages 31-40) compared with early otic vesicle stages (stages 27-30), and the number of dendritic branches was slightly higher when the ear was removed at early otic vesicle stages (stages 27-30) than when the ear was removed at placodal stages (stages 24-26). Since the otic ganglion is only recognized after stage 31 (Nieuwkoop and Faber, 1994), the slightly more dendritic branches in the earlier otic vesicle stages (stages 27-30) than in the otic placode stages (stages 24-26) may indicate that there are other mechanisms for dendritic development in addition to direct innervation from the presynaptic sensory afferents. That any dendrites developed in these two groups in the complete absence of any innervation could support additional mechanisms such as short range diffusible factors released from the ear or alternatively suggest that there is some autonomous development of dendritic branches in the direction of the future sensory neurons, but that contacts between the dendrites and sensory neurons are necessary for further development and maintenance of the dendritic branches. The increase in dendritic branching following the addition of an extra ear rostral to the native ear was also observed in axolotls (Goodman and Model, 1988), though not quantified. An increase in dendritic branching indicates that additional sensory neurons 125 entering the hindbrain from the transplanted ear stimulate the growth of additional dendritic branches. That the number of dendritic branches on the side with the extra ear was not double the number on the control side suggests that an upper limit of dendritic branching may occur. This could be tested by grafting additional ears to a single side. 126 Figure 6.1. Success of ear removal. (A) Percentage of animals with any form of ear regrowth at each stage of ear removal. (B) Control Xenopus laevis at stage 46. (C) Embryo in which the right ear was removed and replaced. (D) Embryo in which the right ear was removed. (E) Immunohistochemistry for acetylated tubulin showing cranial nerves (roman numerals) and myoVI showing the absence of the ear on the right side as indicated by the absence of hair cells. (F) Embryo in which an additional ear was added rostral to the native right ear. (G) Lipophilic dye labeling showing sensory neuron projections from both the native (red) and transplanted (green) ears into the vestibular nucleus in the hindbrain. Ears are circled and labeled, Ear. Scale bar is 0.5 mm in B-D and F; 200 µm in E; 25 µm in G. 127 128 Figure 6.2. Mauthner cell survival. (A) Dextran amine dye-labeled Mauthner cells in a control animal. (B) Dextran amine dye-labeled Mauthner cells in an animal in which the right ear was removed and immediately replaced. (C) Percentage of Mauthner cells present on the right side at the different stages of ear removal. (D) Dextran amine dyelabeled and (D’) 3A10 antibody immunohistochemistry showing the absence of the ipsilateral Mauthner cell following the removal of the right ear at stage 28. (D’’) Merge of D and D’. M, Mauthner cell. Scale bar is 50 µm. 129 130 Figure 6.3. Dendritic development of Mauthner cells following ear manipulation. (A) Dextran amine dye labeling and (A’) 3A10 immunohistochemistry of a Mauthner cell (M) showing filling of dendrites by dextran amine dye. (A”) Merge of A and A’. (B) 3D reconstruction of a pair of Mauthner cells from a control embryo. (C) 3D reconstruction of a pair of Mauthner cells in which the right ear was removed and immediately replaced show little difference in the number of dendritic branches between Mauthner cells. (D) 3D reconstruction of a pair of Mauthner cells from an animal in which the right ear was removed at stage 26 show a reduction in dendritic branching in the ipsilateral Mauthner cell. (E) 3D reconstruction of a pair of Mauthner cells from an animal in which an additional ear was transplanted rostral to the native ear at stage 26 show an increase in dendritic branching in the ipsilateral Mauthner cell. (F) Number of dendritic branches following ear removal or ear addition. Dark shaded bars are left (control) Mauthner cells, light shaded bars are right (treated) Mauthner cells. ***, p<0.001. (G) Sholl Analysis of the Mauthner cells on the right (treated) side in F. The number of dendritic branch crossings were counted at 25 µm intervals. Scale bar is 50 µm. 131 132 CHAPTER VII CONCLUSIONS AND FUTURE DIRECTIONS As outlined in Chapter 1, in order for a novel sensory system to become functional, it needs to send information it gathers about the outside world to the CNS for processing. In turn, the CNS will send information back to several sensory organs to modulate the incoming signal. How new sensory organs evolved over time and how the process of forming contacts between the CNS and the newly evolving sensory organs develops has been poorly understood. The goal of this work was to understand how the nervous system adapts to a novel sensory system through transplantation of frog inner ears to specifically understand how inner ear hair cells acquire motor innervation, how inner ear sensory neurons find their CNS targets, and how manipulation of the inner ear affects CNS targets of sensory neurons. Motor Neuron Acquisition It has been suggested that the motor neurons innervating the inner ear hair cells as efferents are rerouted facial branchial motor neurons (Fritzsch and Nichols, 1993) given their close proximity of the efferents to the facial branchial motor neurons (Roberts and Meredith, 1992) and similarities with the efferents during development (Simmons et al., 2011). The inner ear develops at the rostral boundary of the anteriormost, first forming somite (Cooke, 1978, Chung et al., 1989, Huang et al., 1997) and in animals without ears, such as amphioxus, somites are found more rostrally, indicating that the vertebrate ear develops in place of a somite or somitomere. This implies that the facial branchial motor neurons that were destined to innervate the somite- or somitomere-derived muscle fibers may have been rerouted to innervate the ear (Fritzsch et al., 2007). We first tested this 133 theory by transplanting otic placodes caudally to the trunk to replace a somite, recapitulating the likely original scenario that arose in vertebrates during ear evolution. These transplanted ears grew normally in their novel location in the trunk and in some instances their hair cells received motor neuron terminals, as was shown by immunostaining with VAChT antibody and confirmed with electron microscopy (Chapter 3). That spinal motor neurons could reroute to innervate hair cells of a transplanted ear as efferents indicates that the original rerouting of facial branchial motor neurons to the inner ear of vertebrates is a possible scenario. Given that spinal motor neurons only contacted hair cells when the ear was transplanted in the direct trajectory of growing axons suggests that the initial innervation of the vertebrate ear by rerouted facial branchial motor neurons was likely a chance occurrence and became a permanent fixture through selection of those genes responsible for rerouting a subset of facial motor neurons to the ear. Neurons that lose their target, and subsequent neurotrophic factors, often degenerate and die (Gould and Enomoto, 2009). An alternate target, the ear, would have provided neurotrophic support (Fritzsch et al., 2004, Green et al., 2012) to maintain the displaced facial branchial motor neurons. The ability to become an efferent to the inner ear is not a unique property of neurons once destined to innervate somite-derived tissues, since the ear transplanted to the orbit to replace the eye receives motor neuron terminals on hair cells from both the oculomotor and trochlear nerves (Chapter 4), suggesting that any motor neuron has the ability to become an efferent to hair cells of the inner ear. However, this is a property unique to the inner ear as other targets of motor innervation are not always crossinnervated by other motor neurons when transplanted (Chapter 4). That any target of 134 motor innervation is not innervated by any motor neuron is likely due to differences in the origin of the tissues and may reflect an inability of cranial nerves to innervate somitederived tissue and of spinal motor neurons to innervate branchial-arch-derived tissue. This is in line with the selective innervation of the trapezius muscle. The branchial-archderived trapezius muscle is located in the trajectory of spinal motor neurons; however, it is not innervated by them but by the spinal accessory nerve or by a branch of the vagus (Boord and Sperry, 1991, Sienkiewicz and Dudek, 2010, Dudek et al., 2011), suggesting that cranial motor neurons and spinal motor neurons rely on unique molecular signatures to prevent cross-innervation at the head-neck boundary. The ability of all nerves tested to directly innervate hair cells of the ear but rarely innervate other tissues directly suggests that there is something unique about the ear to allow for all tested cross-innervations. Hair cells contain the most primitive nAChR alpha subunits, α9 and α10 (Sgard et al., 2002, Franchini and Elgoyhen, 2006, Katz, 2011, Lipovsek et al., 2012). Perhaps all motor neurons retain the ability to form synapses on the primitive α9 and α10-containg nAChRs in addition to the more-derived versions specific to that motor neuron type. Mice in which the gene encoding the α9 nAChR subunit (Chrna9) or α10 nAChR subunit (Chrna10) was knocked out showed that these receptors were in part necessary for the development of synaptic connections between the olivocochlear efferents and the inner ear hair cells (Vetter et al., 1999, Vetter et al., 2007, Katz, 2011). In mice lacking either α9 or α10 nAChR subunits, efferent synaptic contacts were larger in size but reduced in number compared to wild type littermates (Vetter et al., 1999, Vetter et al., 2007). No α9 or α10 double null mouse efferent innervation has been reported, leaving it open whether absence of both receptors 135 eliminates all efferent synaptogenesis on hair cells. Future directions would be to knockdown both α9 and α10 nAChR subunits in X. laevis and transplant ears from these embryos into control embryos, either replacing the native ear or transplanting to the trunk to replace a somite or the orbit to replace the eye. The prediction for both would be that there is no efferent innervation of hair cells by any motor neurons, including an absence of hair cell innervation in the untransplanted ear. Future work would require also misexpressing α9 and α10 nAChR subunits in tissues that did not receive motor innervation when transplanted to either the orbit or trunk such to determine whether it is these nAChRs that allow for hair cell innervation by other motor neuron types. Additionally there are likely other unique components involved in synaptic formation as well as short range guidance cues that will guide any motor axon to hair cells. A comparison of deep sequencing profiles of ears and of the other tissues transplanted to foreign areas may give insights into the molecular properties that are either present in the ear and lacking in the other tissues transplanted or properties that are uniquely expressed in only the somatic-derived tissue but not in the branchial arch derived tissue, or vice versa, but are also expressed in the ear. Candidate genes would be those with known roles in short-range axon guidance or synaptic formation. A test for functionality would be to knock these genes down in X. laevis and again transplant ears from these embryos into control embryos to either replace the native ear, to the trunk to replace the somite, or to the orbit to replace the eye. Inner Ear Sensory Neuron Guidance The mechanism by which inner ear vestibular afferents project to their specific targets in the vestibular nuclei is not well known. What is known is that Neurod1 is 136 necessary for normal vestibular afferent projection. In mice lacking Neurod1, vestibular afferents project to cochlear nuclei in addition to vestibular nuclei (Jahan et al., 2010a). Transplantation of the ear to the orbit resulted in sensory neurons projecting along existing nerves into the brain (Chapter 4). Sensory neurons from the transplanted ear projected either along the trigeminal nerve into the hindbrain or along the optic or oculomotor nerve into the midbrain and forebrain. Of those fibers that projected along the trigeminal nerve, some were able to reach the vestibular nucleus. However, the inability of other sensory neurons entering the midbrain to find the vestibular nucleus indicates the absence of long-range diffusible signals. Based on the differential ability of afferents to reach vestibular nuclei only upon entering into the hindbrain, it is plausible that any molecular cues are at most short-ranged. Since all vestibular afferents of transplanted ‘third’ ears will end up in the vestibular nuclei, no matter their entry point into the hindbrain alar plate, we can reject the alternative assumption that they reach the vestibular nucleus by pure chance. If this were the case we would have encountered many animals with afferents from transplanted ‘third’ ears being associated with fiber tracts other than the vestibular nucleus. In animals in which vestibular afferents enter the midbrain, there was no consistency in sensory neuron projections. This indicates a random interaction of vestibular afferents with local neurons, indicating axons were unable to re-organize these neurons to form a ‘vestibular nucleus’. This is in contrast to the olfactory system where transplantation of olfactory placode results in the ability of olfactory sensory neurons to form glomeruli in any location in the brain that has been tested (Graziadei et al., 1978, Graziadei et al., 1979, Graziadei and Samanen, 1980, Magrassi and Graziadei, 1985, 137 Morrison and Graziadei, 1996) with the notable exception of the hindbrain. A critical experiment would be to swap olfactory eptihelia with ears to see how each of these placodal derived neurons can handle the challenge of interacting with a very different set of target neurons. Given the developmental similarities between the ear and the eye, we hypothesized that the ear may use the same mechanisms as the eye: molecular cues, as suggested above, and activity-based guidance, to route inner ear afferents to the proper location in the hindbrain. The inconsistencies in sensory neuron guidance when the ear was transplanted to the orbit made this difficult to test. Instead, we tested whether molecular mechanisms and activity-based guidance played a role in guiding inner ear sensory neurons by transplanting an additional ear immediately rostral to the native ear, either in the native orientation or rotated by 90 degrees to generate ‘three-eared’ frogs (Chapter 5). Transplanting the ear rostral to the native ear puts the transplanted ear in the territory of any potential molecular cues that may be guiding native inner ear sensory neurons to the vestibular nucleus as the vestibular nucleus extends through all rhombomeres and continuous into the cerebellum as much as the vestibular afferents do. Furthermore, the addition of an ear rostral to the native ear would force two ears to the same side hindbrain, much like two eyes either normally, or experimentally (ConstantinePaton and Law, 1978), project to the same superior colliculus/tectum. This allows us to determine whether the sensory neurons are guided to the same territory as the native sensory neurons, indicating a molecular mechanism. By transplanting the ears either in the native orientation or rotated by 90 degrees allows us to determine whether activitybased mechanisms play a role in fiber competition as well. The rationale is that when 138 rotated 90 degrees, the sensory epithelia would not be aligned between the two ears in tandem and they would respond differently to the same stimulus, essentially setting up a differential activity scenario. The transplanted ears in either orientation projected to the same approximate area of the hindbrain as the native ear (Chapter 5), suggesting molecular cues play a role in axon guidance in the vestibular system. In addition, when the transplanted ear was in alignment with the native ear, there was nearly complete overlap of sensory axons from the two ears in the vestibular nucleus. In contrast to this, when the transplanted ear was rotated by 90 degrees compared with the native ear, there was nearly complete segregation of sensory axons from the two ears in the vestibular nucleus (Chapter 5), forming ‘vestibular dominance columns’ reminiscent of the ocular dominance columns found in the visual system naturally (Hubel and Wiesel, 1977, Wiesel, 1982) and in experimentally generated three eared frogs (Constantine-Paton and Law, 1978, Springer and Cohen, 1981, Constantine-Paton, 1982, Easter, 1983). These data suggest that in addition to molecular mechanisms, activity also plays a role in axon guidance in the vestibular system. The addition of an additional ear, rostral to the native ear, in a sense, recapitulates ear evolution. It appears that ear evolution starts with the duplication of a sensory epithelia, followed by further spatial segregation and diversification (Duncan and Fritzsch, 2012). In this experiment, we have effectively duplicated the sensory epithelia of the ear by adding a second ear. Early studies proposed that a common placode gave rise to the inner ear and lateral line and that neurons from these tissues project to the same nuclei in the hindbrain (Duncan and Fritzsch, 2012). However, it is now known that not only do the inner ear and lateral line originate from their own placode (Schlosser, 139 2010), but they project to molecularly-distinct regions within the hindbrain (Fritzsch et al., 2006, Duncan and Fritzsch, 2012). Furthermore, neurons of the trigeminal and solitary tract also project to molecularly-specified regions within the hindbrain (Qian et al., 2001). It has been shown that hindbrain nuclei boundaries are established by differential bHLH gene expression (Fritzsch et al., 2006) and that axons from the various sensory systems project to these molecularly-defined regions from the start (Fritzsch et al., 2005a), indicating that sensory afferents are guided to these regions, possibly by reading molecular cues from the brain. The same bHLH genes that regulate neuronal and hair cell development, Neurog1 and Atoh1, establish boundaries for the vestibular and auditory nuclei, respectively (Fritzsch et al., 2006). Thus the genes that are important for inner ear neurosensory development are again utilized for axon targeting in the brain. Conditional deletion of Neurod1, a bHLH gene downstream of Neurog1, resulted in vestibular and cochlear sensory afferents projecting to both vestibular and cochlear nuclei (Jahan et al., 2010a). While all vestibular neurons project entirely to the vestibular nuclei, within the vestibular nuclei, there is a partial overlap in projections from the different sensory epithelia (Birinyi et al., 2001, Maklad and Fritzsch, 2002, Straka et al., 2002). However, it is thought that the partial overlap functions to integrate semicircular canal input with that of the utricle and/or saccule for any given movement (Büttner-Ennever, 1992, Maklad and Fritzsch, 2002, Straka et al., 2002, Maklad and Fritzsch, 2003, Newlands and Perachio, 2003). While overlap exists in the vestibular nuclei, it is not a complete overlap, as some vestibular neurons innervating the various sensory epithelia partially segregate (Kevetter and Perachio, 1986, Maklad and Fritzsch, 1999, 2003), likely due to 140 differential activities set up by the perceptions of different movements. That both the transplanted and native ears project to the same approximate area suggests that during the evolution of a new inner ear sensory epithelia, molecular cues initially guide the sensory afferents to the same area of the brain. However, once a duplicated sensory epithelia has acquired a novel function, for example the perception of linear acceleration in an different direction upon formation of a perpendicular gravistatic sensory epithelia or sound perception by the basilar papilla upon loss of otoconia from a gravistatic function, the sensory afferents from the new inner ear epithelia project to a unique location in the brain within the molecularly-defined boundary for those neurons (Duncan and Fritzsch, 2012, Fritzsch and Straka, 2013). Inner ear sensory afferents apparently are guided to the vestibular area which forms a likely molecularly defined space that captures all vestibular afferents. The transplanted and rotated ears mimic the diversification of a newly evolved sensory epithelia, in this case perception in a different orientation. The segregation of afferents from these rotated ears from the native ears into ‘vestibular dominance columns’ should logically mimic similar processes when additional epithelia form. In other words, I suggest that as sensory epithelia diversified in evolution to have unique perceptions, it is the differential activity in these epithelia that drove the afferents to project to unique locations within the molecularly-specified vestibular region. The formation of ‘vestibular dominance columns’ is comparable to ocular dominance columns. In the retina projection of the eye, the formation of ocular dominance columns is a compromise between the molecularly-specified targeting of each eye to a specific location in the tectum/superior colliculus (Clandinin and Feldheim, 141 2009) and the differences in retinal activity between the two eyes (Hubel and Wiesel, 1977, Wiesel, 1982). In contrast to the ear, for the retina projection, the molecular basis is well understood. In the eye, concentration gradients of EphA and ephrin-A along the nasaltemporal axis of the retina and anterior-posterior axis of the superior colliculus/tectum guide retinal ganglion axons to the appropriate region of the CNS (Clandinin and Feldheim, 2009). Given that Ephs and ephrins have been shown to play some role in axon guidance in the auditory system (Allen-Sharpley and Cramer, 2012, Allen-Sharpley et al., 2013, Defourny et al., 2013) and that Ephs and ephrins are expressed in vestibular neurons and their peripheral vestibular hair cell targets (Bianchi and Liu, 1999, AllenSharpley et al., 2013), suggests that Ephs and ephrins may play a role in guiding sensory afferents to the proper location centrally in the vestibular nucleus. It has recently been shown that expression of different Eph classes in auditory and vestibular neurons conbributes to boundary formation in the hindbrain for the two populations of neurons (Allen-Sharpley et al., 2013), but whether or how Ephs and ephrins play a role in vesbibular axon pathfinding centrally has not been established. Future directions would be to determine the specific Ephs and ephrin(s) expressed in the frog vestibular nucleus. Likely candidates would be those of the three ephrin-B ligands or ephrin-A5 since vestibular axons have been shown to express the EphB2 receptor (Himanen et al., 2004, Siddiqui and Cramer, 2005, Allen-Sharpley et al., 2013). Of these ligands, ephrin-B2 has been shown to be expressed in auditory and vestibular nuclei in mice (Liebl et al., 2003). Since misexpression of EphA4 also affected the boundary of the vestibular nucleus (Allen-Sharpley et al., 2013), ephrin-A ligands may also guide vestibular axons and their 142 expression centrally would also be determined. The ephrin(s) expressed in the vestibular nucleus would be knocked down or misexpressed in one half of a X. laevis embryo and the axon targeting of the vestibular nuclei by vestibular sensory axons on the manipulated side would be compared with the vestibular axon targeting of the vestibular nuclei on the unaltered side to determine whether Ephs and ephrins play a role in neuronal guidance in the vestibular system as in the auditory system (Allen-Sharpley and Cramer, 2012). Initial targeting by vestibular afferents is thought to be molecular since initial observations of vestibular afferent targeting were made prior to the onset of vestibular sensation (Maklad and Fritzsch, 2002, Maklad and Fritzsch, 2003, Maklad et al., 2010). This would logically imply that the activity-based guidance refines the initially set-up molecular-based guidance. Support comes from observed changes in the pattern of vestibular nucleus connectivity around the onset of a functional vestibular system (Maklad and Fritzsch, 2003). This order of axon guidance could be experimentally demonstrated using ears transplanted from X. laevis embryos previously injected with tau GFP mRNA (Brand, 1995) or tdTomato mRNA (Blackiston and Levin, 2013). Otic placodes from embryos injected with tau GFP and otic placodes from embryos injected with tdTomato would be transplanted in tandem into an uninjected host, either both in the native orientation, or one rotated by 90 degrees from the other. The daily growth and projection of the sensory afferents could be monitored with confocal microscopy. I would expect to see afferents from the two ears projecting to the same area initially in both conditions, but only in those with an ear rotated by 90 degrees, over time see segregation of the axons. 143 The ability of ‘three-eared’ frogs to swim normally depended upon the manipulation. Animals in which the transplanted ear was in alignment swam similar to control, unmanipulated, animals most of the time. In contrast, animals in which the transplanted ear was rotated by 90 degrees displayed more aberrant swimming, similar to that of animals with only one ear (Chapter 5). Given that the latter manipulations results in asymmetrical and mismatched gravitational sensation suggests that bilateral symmetry in sensory epithelia orientation is required for proper sensing of the animals’ orientation to proper guide its swimming. A proposed mechanism for how the specific ear manipulations leads to the swimming behavior observed will be discussed in the last section. Hindbrain Neurons It has long been known that ablation of the inner ear results in a reduction in the number and volume of neurons in the cochlear nucleus (Levi-Montalcini, 1949, Parks, 1979, Ryugo and Parks, 2003) at the time point at which afferent activity could be detected (Jackson et al., 1982). It has been shown that activity is necessary for neuronal survival (Pasic and Rubel, 1989, Sie and Rubel, 1992) but only for a certain critical period, after which there is little to no reduction in the number and volume of cochlear nucleus neurons following ear ablation (Born and Rubel, 1985, Tierney et al., 1997, Mostafapour et al., 2000). Rather than focus on an entire population of neurons as was done in these studies, we instead looked at the effect of ear removal on a single target neuron, the Mauthner cell. The Mauthner cell is one of the first cells to become postmitotic at stage 10-12 in Xenopus laevis (Vargas-Lizardi and Lyser, 1974), several stages before the onset of ear development (Schlosser, 2010). Previous data had shown that ear 144 removal results in the occasional loss of the Mauthner cell, indicating a dependence on the ear for cell survival after its initial development. In surviving Mauthner cells, a decrease in dendritic branching was observed following ear removal (Piatt, 1969, Kimmel et al., 1977, Kimmel, 1982, Goodman and Model, 1988, Fritzsch, 1990). However, these studies only looked at a few time points. From the few time points available, we hypothesized that we would see a critical period of dependence upon the ear for Mauthner cell survival. By using two independent methods to label neurons, Dextran amine dye tracers and immunohistochemistry, we have shown that the Mauthner cell is dependent upon the presence of the ear for its development and/or survival, but only for a critical time in development (Chapter 6). The earlier in development that ear is removed, the more likely the Mauthner cell does not survive. The Mauthner cell was always present after stage 36, indicating that by stage 36, the critical period of dependence on the ear for survival has ended. Stage 36 corresponds with the stage at which afferents from the ear were observed projecting into the hindbrain of axolotls (Fritzsch et al., 2005a). The exact nature of the early support provided by the ear to the Mauthner cell for survival and/or maintenance is unknown. Loss of an ear prior to any vestibular sensory neuron outgrowth often results in the loss of a Mauthner cell. This suggests that a short-range diffusible factor released by the ear itself is a possible candidate. Future directions would be to determine the nature of this factor promoting cell survival and/or maintenance. I would look at expression profiles of known shortrange diffusible factors expressed by the inner ear, for example the FGFs. Fgf10 is nearly exclusively expressed in the otic placode and otic vesicle ranging from stages 23 through 40 in Xenopus laevis (Lea et al., 2009). To test whether FGF10 plays a role in 145 Mauthner cell survival/ and or maintenance, otic placodes would be removed at stages 24-26, when the Mauthner cell was most absent, and beads soaked in FGF10 would be inserted in its place to determine whether FGF10 can functionally rescue the Mauthner cell in the absence of the ear. Not all Mauthner cells are absent following ear ablation, as was observed for neurons in the cochlear nucleus following ear ablation (Levi-Montalcini, 1949, Parks, 1979, Ryugo and Parks, 2003). Perhaps, as has been proposed for neurons in the cochlear nucleus (Garden et al., 1994, Hyde and Durham, 1994a), both the apoptotic and survival pathways are initiated in the Mauthner cell and whether the cell survives or not depends upon which pathway overtakes the other. The Mauthner cell provides a unique opportunity to study initiation of these pathways in a single cell that could potentially be applied to any neuron. Future directions would be to look at markers for survival/apoptosis, such as activated Akt or cleaved caspace-3 (Ferrer and Planas, 2003, Zhang et al., 2011), in Mauthner cells following ear removal at the early placodal stages (stages 24-26), when Mauthner cell absence was high, and at early otic vesicle stages (stages 27-30) when there was more Mauthner cell survival. Both of these stages are within the critical period of dependence of the ear for cell survival. Following ear removal, groups of animals will be observed daily for survival or apoptotic pathway presence in the Mauthner cell. I would expect to see initial upregulation of both apoptotic and survival pathway markers and over time, more apoptotic in stages 24-26 removal, more survival in stages 27-30 removal. For animals in which the Mauthner cell survived following ear removal, the dendritic branching on the ablated side was always less than the dendritic branching on 146 the unoperated side. Furthermore, in all cases in which an extra ear was transplanted rostral to the native ear, there was increased dendritic branching on the side with an extra ear compared with the unoperated side. Together, these data suggest that the degree of dendritic branching is related to the number of presynaptic sensory afferents, supporting the few earlier findings (Kimmel et al., 1977, Kimmel, 1982, Goodman and Model, 1988, Fritzsch, 1990). The timing of ear removal may be relevant. The earlier the ear was removed, the fewer dendritic branches were present, suggesting that the degree of dendritic development depends upon the time at which the ear was removed. While there was no significant difference between the three stages of ear removal when comparing the difference between left and right Mauthner cells following ear removal, there was a trend in which the number of dendritic branches was slightly higher when the ear was removed at later otic vesicle stages (stages 31-40) compared with early otic vesicle stages (stages 27-30), and early otic vesicle stages (stages 27-30) being slightly higher than when the ear was removed at placodal stages (stages 24-26). The dendritic branching in the latter two groups occurs prior to the formation of the otic ganglion in stage 31 (Nieuwkoop and Faber, 1994). That there were slightly more dendritic branches in the earlier otic vesicle stages (stages 27-30) than in the otic placode stages (stages 24-26) may indicate the presence of other mechanisms for dendritic development in addition to direct innervation from the presynaptic sensory afferents. The development of dendrites in general in these two groups in the complete absence of any innervation could support the presence of additional mechanisms or alternatively suggest that there is some autonomous development of dendritic branches toward the future sensory neurons, but 147 that contacts with the neurons are necessary for further development and maintenance of the dendritic branches. The increase in dendritic branching following the addition of an extra ear rostral to the native ear was also proposed in axolotls (Goodman and Model, 1988), though the authors did not quantify the exact increase in dendritic branching. Using neuroanatomical techniques we quantify this here for the first time and show that indeed by all measures an extra ear causes an increased lateral dendrite in Mauthner cells. The increase in Mauthner cell dendritic branching on the side with an extra ear indicates that additional sensory neurons entering the hindbrain from the transplanted ear stimulate the growth of additional dendritic branches. That the number of dendritic branches on the side with the extra ear was not double the number on the control side suggests that an upper limit of dendritic branching may occur. Future directions would be to transplant ears both rostral and caudal to the native ear, thus further increasing the number of sensory afferents from the current study, and determining the percent increase in the number of dendritic branches of the ipsilateral Mauthner cell compared to the contralateral control Mauthner cell. If the percent increase is similar, then it would indicate there is a maximum increase in the number of dendritic branches possible for a given Mauthner cell. Alternatively, molecular knockdown of neuronal development could be achieved through inhibition of Neurod1 function, thereby generating ears with a continuous set of reduced afferents to establish quantitative relationships with the Mauthner cell dendrites beyond our data. 148 Proposed Mechanism As mentioned previously, afferents from three-eared frogs are guided to their proper locations using both molecular and activity-based mechanisms. Projections into the hindbrain from the two ears guide the swimming of the tadpoles, such that when the transplanted ear is in alignment with the native ear, afferents from both ears overlap in the vestibular nucleus and the animal swims relatively normally. In contrast, when the transplanted ear is rotated by 90 degrees from the native ear, afferents from both ears segregate in the vestibular nucleus and the animal shows more aberrant swimming behaviors. Furthermore, we have shown that the dendritic branching of a target neuron of inner ear afferents, the Mauthner cell, is increased with the addition of an ear. Blocking afferent activity has been shown not to affect dendritic branching in amphibians (Goodman and Model, 1990), therefore the dendritic branching pattern is independent of the orientation of the ear and should be similarly increased for animals with both normally-orientated or rotated transplanted ears. Thus, we propose that the molecular cues guide the inner ear sensory neurons to target neurons (Fig. 7.1A). The increased dendritic branching from the additional ear allows for contacts from both ears (Figs. 7.1B and 7.1C). When the ears are in alignment with each other, the incoming signal from the ears would be the same and the cell would respond (Fig. 7.1B); however, when the transplanted ear is rotated, the incoming signals would likely be in opposition, essentially cancelling each other out (Fig. 7.1C). Thus, an additional but rotated ear would have the same effect as having no ear on one side and 149 would explain the aberrant behavior seen similarly for both three-eared frogs with a rotated transplanted ear and one-eared frogs. Future directions would be to test this model by determining whether ear manipulations determine the initial direction of movement following low-level startle stimulation using a high speed camera. The Mauthner cell is an important component of the C-start escape response (Korn and Faber, 2005). Inner ear vestibular and auditory neurons of premetamorphic amphibians and fish form synapses on the lateral dendrite of the ipsilateral Mauthner cell, which in turn synapses on contralateral spinal motor neurons to activate the C-start escape response (Korn and Faber, 2005, Sillar, 2009). We would expect to see that in control animals, there is no bias in the initial direction of the startle response since the incoming signal to the Mauthner cell and its dendritic branching should be symmetrical. In animals in which the ear was removed, generating one-eared animals, there should be a bias in turning toward the ablated side. This would be a result of the contralateral Mauthner cell firing due to the absence of signal reaching the ipsilateral Mauthner cell. In animals in which the transplanted ear is in alignment with the native ear, we would expect that if there is a bias in direction, it would be in the direction opposite the transplanted ear since the ipsilateral Mauthner cell should have increased dendritic branching and may have a slightly higher tendency to fire than the contralateral Mauthner cell. In contrast, in animals in which the transplanted ear is rotated by 90 degrees, if the incoming signals cancel each other out, than like the oneeared animals, the initial direction should be a bias toward the ablated side. 150 Figure 7.1. Proposed mechanism to guide swimming behavior in three-eared frogs. (A) In normal, 2-eared embryos, molecular cues guide the inner ear sensory neurons to target neurons. (B-C) The increased dendritic branching from the additional ear allows for contacts from both ears. (B) The transplanted ear (green) is in alignment with the native ear (red) and sensory neurons overlap. When the ears are in alignment with each other, the incoming signal from the ears would be the same and the cell would respond. (C) The transplanted ear (green) is rotated 90 degrees with respect to the native ear (red) and sensory neurons segregate, but still contact dendrites of the same cell. When the transplanted ear is rotated, the incoming signals would likely be in opposition, essentially cancelling each other out. Thus, an additional but rotated ear would have the same effect as having no ear on one side. 151 152 REFERENCES Abelló G, Khatri S, Radosevic M, Scotting PJ, Giráldez F, Alsina B (2010) Independent regulation of Sox3 and Lmx1b by FGF and BMP signaling influences the neurogenic and non-neurogenic domains in the chick otic placode. Developmental Biology 339:166-178. Adam Y, Mizrahi A (2010) Circuit formation and maintenance—perspectives from the mammalian olfactory bulb. Current Opinion in Neurobiology 20:134-140. Adams ME, Linn J, Yousry I (2008) Pathology of the Ocular Motor Nerves III, IV, and VI. Neuroimaging clinics of North America 18:261-282. Albuquerque EX, Pereira EFR, Alkondon M, Rogers SW (2009) Mammalian Nicotinic Acetylcholine Receptors: From Structure to Function. Physiological Reviews 89:73-120. Allen-Sharpley MR, Cramer KS (2012) Coordinated Eph-ephrin signaling guides migration and axon targeting in the avian auditory system. Neural Dev 7:29. Allen-Sharpley MR, Tjia M, Cramer KS (2013) Differential Roles for EphA and EphB Signaling in Segregation and Patterning of Central Vestibulocochlear Nerve Projections. PLoS One 8:e78658. Alsina B, Giraldez F, Pujades C (2009) Patterning and cell fate in ear development. The International Journal of Developmental Biology 53:1503-1513. Altman J, Bayer SA (1980) Development of the brain stem in the rat. III. Thymidineradiographic study of the time of origin of neurons of the vestibular and auditory nuclei of the upper medulla. J Comp Neurol 194:877-904. Alvarez IS, Martín-Partido G, Rodríguez-Gallardo L, González-Ramos C, Navascués J (1989) Cell proliferation during early development of the chick embryo otic anlage: Quantitative comparison of migratory and nonmigratory regions of the otic epithelium. The Journal of comparative neurology 290:278-288. Alvarez Y, Alonso MT, Vendrell V, Zelarayan LC, Chamero P, Theil T, Bösl MR, Kato S, Maconochie M, Riethmacher D, Schimmang T (2003) Requirements for FGF3 and FGF10 during inner ear formation. Development 130:6329-6338. Apel ED, Glass DJ, Moscoso LM, Yancopoulos GD, Sanes JR (1997) Rapsyn is required for MuSK signaling and recruits synaptic components to a MuSK-containing scaffold. Neuron 18:623-635. Baker CVH, Bronner-Fraser M (2001) Vertebrate Cranial Placodes I. Embryonic Induction. Developmental Biology 232:1-61. Bardet P-L, Schubert M, Horard B, Holland LZ, Laudet V, Holland ND, Vanacker J-M (2005) Expression of estrogen-receptor related receptors in amphioxus and zebrafish: implications for the evolution of posterior brain segmentation at the invertebrate-to-vertebrate transition. Evolution & Development 7:223-233. 153 Barmack NH (2003) Central vestibular system: vestibular nuclei and posterior cerebellum. Brain Research Bulletin 60:511-541. Bartelmez GW (1915) Mauthner's cell and the nucleus motorius tegmenti. The Journal of comparative neurology 25:87-128. Bassham S, Canestro C, Postlethwait J (2008) Evolution of developmental roles of Pax2/5/8 paralogs after independent duplication in urochordate and vertebrate lineages. BMC Biology 6:35. Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY (1999) Math1: an essential gene for the generation of inner ear hair cells. Science 284:1837-1841. Bever MM, Jean YY, Fekete DM (2003) Three-dimensional morphology of inner ear development in Xenopus laevis. Developmental Dynamics 227:422-430. Bezgina E, Moshkov D, Nikitin V, Savel'eva L, Uteshev V (2000) The morphogenesis of mauthner neurons in tadpoles of the common frog after early unilateral enucleation of the eye. Neuroscience and Behavioral Physiology 30:521-524. Bezgina EN, Moshkov DA, Nikitin VA, Savel'eva LN, Uteshev VK (1999) Morphogenesis of Mauthner neurons of Xenopus laevis tadpoles following early unilateral eye enucleation. Morfologiia 115:49-52. Bianchi LM, Liu H (1999) Comparison of Ephrin-A ligand and EphA receptor distribution in the developing inner ear. The Anatomical Record 254:127-134. Birinyi A, Straka H, Matesz C, Dieringer N (2001) Location of dye-coupled second order and of efferent vestibular neurons labeled from individual semicircular canal or otolith organs in the frog. Brain research 921:44-59. Blackiston DJ, Levin M (2013) Ectopic eyes outside the head in Xenopus tadpoles provide sensory data for light-mediated learning. The Journal of Experimental Biology 216:1031-1040. Bok J, Bronner-Fraser M, Wu DK (2005) Role of the hindbrain in dorsoventral but not anteroposterior axial specification of the inner ear. Development 132:2115-2124. Boord RL, Sperry DG (1991) Topography and nerve supply of the cucullaris (trapezius) of skates. Journal of Morphology 207:165-172. Borges A, Casselman J (2010) Imaging the trigeminal nerve. European Journal of Radiology 74:323-340. Born D, Rubel E (1988) Afferent influences on brain stem auditory nuclei of the chicken: presynaptic action potentials regulate protein synthesis in nucleus magnocellularis neurons. The Journal of Neuroscience 8:901-919. Born DE, Rubel EW (1985) Afferent influences on brain stem auditory nuclei of the chicken: Neuron number and size following cochlea removal. The Journal of comparative neurology 231:435-445. 154 Boss V, Schmidt J (1984) Activity and the formation of ocular dominance patches in dually innervated tectum of goldfish. The Journal of Neuroscience 4:2891-2905. Bouchard M, de Caprona D, Busslinger M, Xu P, Fritzsch B (2010) Pax2 and Pax8 cooperate in mouse inner ear morphogenesis and innervation. BMC Dev Biol 10:89. Brand A (1995) GFP in Drosophila. Trends in Genetics 11:324-325. Brodmann K (1909) Vergleichende Lokalisationslehre der Grosshirnrinde. Leipzig: Barth. Brown CM (2011) Anatomy of Olivochochlear Neurons. In: Auditory and Vestibular Efferents(Ryugo, D. K. et al., eds) New York: Springer. Brown NL, Dagenais SL, Chen C-M, Glaser T (2002) Molecular characterization and mapping of ATOH7, a human atonal homolog with a predicted role in retinal ganglion cell development. Mammalian Genome 13:95-101. Brown NL, Kanekar S, Vetter ML, Tucker PK, Gemza DL, Glaser T (1998) Math5 encodes a murine basic helix-loop-helix transcription factor expressed during early stages of retinal neurogenesis. Development 125:4821-4833. Brown NL, Patel S, Brzezinski J, Glaser T (2001) Math5 is required for retinal ganglion cell and optic nerve formation. Development 128:2497-2508. Burighel P, Caicci F, Manni L (2011) Hair cells in non-vertebrate models: Lower chordates and molluscs. Hearing Research 273:14-24. Burighel P, Lane NJ, Fabio G, Stefano T, Zaniolo G, Carnevali MDC, Manni L (2003) Novel, secondary sensory cell organ in ascidians: In search of the ancestor of the vertebrate lateral line. The Journal of comparative neurology 461:236-249. Büttner-Ennever JA (1992) Patterns of Connectivity in the Vestibular Nucleia. Annals of the New York Academy of Sciences 656:363-378. Cannatella DC, de Sá RO (1993) Xenopus Laevis as a Model Organism. Systematic Biology 42:476-507. Cant NB, Benson CG (2003) Parallel auditory pathways: projection patterns of the different neuronal populations in the dorsal and ventral cochlear nuclei. Brain Research Bulletin 60:457-474. Chung H-M, Neff AW, Malacinski GM (1989) Autonomous death of amphibian (Xenopus laevis) cranial myotomes. Journal of Experimental Zoology 251:290299. Clandinin TR, Feldheim DA (2009) Making a visual map: mechanisms and molecules. Current Opinion in Neurobiology 19:174-180. Constantine-Paton M (ed.) (1982) The retinotectal hookup: The process of neural mapping. New York: Alan R. Liss. 155 Constantine-Paton M, Law M (1978) Eye-specific termination bands in tecta of threeeyed frogs. Science 202:639-641. Cooke J (1978) Somite abnormalities caused by short heat shocks to pre-neurula stages of Xenopus laevis. Journal of Embryology and Experimental Morphology 45:283294. Cowan CA, Yokoyama N, Bianchi LM, Henkemeyer M, Fritzsch B (2000) EphB2 guides axons at the midline and is necessary for normal vestibular function. Neuron 26:417-430. Crook AC, Whiteman HH (2006) An Evaluation of MS-222 and Benzocaine as Anesthetics for Metamorphic and Paedomorphic Tiger Salamanders (Ambystoma tigrinum nebulosum). American Midland Naturalist 155:417-421. de Castro BM, De Jaeger X, Martins-Silva C, Lima RF, Amaral E, Menezes C, Lima P, Neves CM, Pires RG, Gould TW, Welch I, Kushmerick C, Guatimosim C, Izquierdo I, Cammarota M, Rylett RJ, Gomez MV, Caron MG, Oppenheim RW, Prado MA, Prado VF (2009) The vesicular acetylcholine transporter is required for neuromuscular development and function. Mol Cell Biol 29:5238-5250. Defourny J, Poirrier A-L, Lallemend F, Mateo Sánchez S, Neef J, Vanderhaeghen P, Soriano E, Peuckert C, Kullander K, Fritzsch B, Nguyen L, Moonen G, Moser T, Malgrange B (2013) Ephrin-A5/EphA4 signalling controls specific afferent targeting to cochlear hair cells. Nat Commun 4:1438. del Cerro M, Cogen J, del Cerro C (1980) Stevenel's Blue, an excellent stain for optical microscopical study of plastic embedded tissues. Microscopica acta 83:117-121. Delsuc F, Brinkmann H, Chourrout D, Philippe H (2006) Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature 439:965968. Demer JL, Clark RA, Lim K-H, Engle EC (2007) Magnetic Resonance Imaging Evidence for Widespread Orbital Dysinnervation in Dominant Duane’s Retraction Syndrome Linked to the DURS2 Locus. Investigative Ophthalmology & Visual Science 48:194-202. Derbenev AV, Linn CL, Guth PS (2005) Muscarinic ACh Receptor Activation Causes Transmitter Release From Isolated Frog Vestibular Hair Cells. Journal of Neurophysiology 94:3134-3142. Dudek A, Sienkiewicz W, Marczak M, Kaleczyc J (2011) Immunohistochemical properties of motoneurons supplying the trapezius muscle in the rat. Polish Journal of Veterinary Sciences 14:199-205. Dufour HD, Chettouh Z, Deyts C, de Rosa R, Goridis C, Joly J-S, Brunet J-F (2006) Precraniate origin of cranial motoneurons. Proceedings of the National Academy of Sciences 103:8727-8732. Duncan JS, Fritzsch B (2012) Evolution of Sound and Balance Perception: Innovations that Aggregate Single Hair Cells into the Ear and Transform a Gravistatic Sensor into the Organ of Corti. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology 295:1760-1774. 156 Duncan JS, Lim KC, Engel JD, Fritzsch B (2011) Limited inner ear morphogenesis and neurosensory development are possible in the absence of GATA3. The International Journal of Developmental Biology 55:297-303. Durham D, Matschinsky FM, Rubel EW (1993) Altered malate dehydrogenase activity in nucleus magnocellularis of the chicken following cochlea removal. Hearing Research 70:151-159. Durham D, Rubel EW (1985) Afferent influences on brain stem auditory nuclei of the chicken: Changes in succinate dehydrogenase activity following cochlea removal. The Journal of comparative neurology 231:446-456. Easter SS (1983) Postnatal neurogenesis and changing connections. Trends in Neurosciences 6:53-56. Eisen JS (1999) Patterning motoneurons in the vertebrate nervous system. Trends in Neurosciences 22:321-326. Elgoyhen AB, Katz E (2012) The efferent medial olivocochlear-hair cell synapse. Journal of Physiology-Paris 106:47-56. Elliott KL, Fritzsch B (2010) Transplantation of Xenopus laevis ears reveals the ability to form afferent and efferent connections with the spinal cord. Int J Dev Biol 54:1443-1451. Engle EC, Goumnerov BC, McKeown CA, Schatz M, Johns DR, Porter JD, Beggs AH (1997) Oculomotor nerve and muscle abnormalities in congenital fibrosis of the extraocular muscles. Annals of Neurology 41:314-325. Ericson J, Thor S, Edlund T, Jessell T, Yamada T (1992) Early stages of motor neuron differentiation revealed by expression of homeobox gene Islet-1. Science 256:1555-1560. Fekete DM, Wu DK (2002) Revisiting cell fate specification in the inner ear. Curr Opin Neurobiol 12:35-42. Ferrer I, Planas AM (2003) Signaling of Cell Death and Cell Survival Following Focal Cerebral Ischemia: Life and Death Struggle in the Penumbra. Journal of Neuropathology & Experimental Neurology 62:329-339. Franchini LF, Elgoyhen AB (2006) Adaptive evolution in mammalian proteins involved in cochlear outer hair cell electromotility. Molecular Phylogenetics and Evolution 41:622-635. Fritzsch B (1990) Experimental reorganization in the alar plate of the clawed toad, Xenopus laevis. I. Quantitative and qualitative effects of embryonic otocyst extirpation. Brain Res Dev Brain Res 51:113-122. Fritzsch B (1991) Ontogenetic clues to the phylogeny of the visual system. In: The Changing Visual System, vol. 222 (Bagnoli, P. and Hodos, W., eds), pp 33-49 New York: Springer. 157 Fritzsch B (1993) Fast axonal diffusion of 3000 molecular weight dextran amines. Journal of Neuroscience Methods 50:95-103. Fritzsch B (1996) Development of the labyrinthine efferent system. Annals of the New York Academy of Sciences 781:21-33. Fritzsch B (1999) Ontogenetic and Evolutionary Evidence for the Motoneuron Nature of Vestibular and Cochlear Efferents. In: The Efferent Auditory System: Basic Science and Clinical Applications(Berlin, C., ed), p 31: Singular Publishing Group, Inc. Fritzsch B, Barald K, Lomax M (1998) Early embryology of the vertebrate ear. In: Development of the Auditory System(Rubel, E. W. et al., eds), pp 80-145 New York: Springer-Verlag. Fritzsch B, Beisel KW (2001) Evolution and development of the vertebrate ear. Brain Res Bull 55:711-721. Fritzsch B, Beisel KW (2003) Molecular conservation and novelties in vertebrate ear development. Curr Top Dev Biol 57:1-44. Fritzsch B, Beisel KW (2004) Keeping sensory cells and evolving neurons to connect them to the brain: molecular conservation and novelties in vertebrate ear development. Brain Behav Evol 64:182-197. Fritzsch B, Beisel KW, Jones K, Farinas I, Maklad A, Lee J, Reichardt LF (2002) Development and evolution of inner ear sensory epithelia and their innervation. J Neurobiol 53:143-156. Fritzsch B, Beisel KW, Pauley S, Soukup G (2007) Molecular evolution of the vertebrate mechanosensory cell and ear. Int J Dev Biol 51:663-678. Fritzsch B, Eberl DF, Beisel KW (2010) The role of bHLH genes in ear development and evolution: revisiting a 10-year-old hypothesis. Cell Mol Life Sci 67:3089-3099. Fritzsch B, Glover JC (2007) Evolution of the deuterostome central nervous system: an intercalation of developmental patterning processes with cellular specification processes. . In: Evolution of Nervous Systems vol. 2 (Kaas, J. H., ed), pp 1-24 Oxford: Academic Press. Fritzsch B, Gregory D, Rosa-Molinar E (2005a) The development of the hindbrain afferent projections in the axolotl: evidence for timing as a specific mechanism of afferent fiber sorting. Zoology (Jena) 108:297-306. Fritzsch B, Maklad A, Bruce LL, Crapon de Caprona MD (2001a) Development of the ear and of connections between the ear and the brain: is there a role for gravity? Advances in Space Research 28:595-600. Fritzsch B, Muirhead KA, Feng F, Gray BD, Ohlsson-Wilhelm BM (2005b) Diffusion and imaging properties of three new lipophilic tracers, NeuroVue(TM) Maroon, NeuroVue(TM) Red and NeuroVue(TM) Green and their use for double and triple labeling of neuronal profile. Brain Research Bulletin 66:249-258. 158 Fritzsch B, Nichols DH (1993) DiI reveals a prenatal arrival of efferents at the differentiating otocyst of mice. Hear Res 65:51-60. Fritzsch B, Nichols DH, Echelard Y, McMahon AP (1995) Development of midbrain and anterior hindbrain ocular motoneurons in normal and Wnt-1 knockout mice. J Neurobiol 27:457-469. Fritzsch B, Northcutt RG (1993) Cranial and spinal nerve organization in amphioxus and lampreys: evidence for an ancestral craniate pattern. Acta Anat (Basel) 148:96109. Fritzsch B, Pauley S, Feng F, Matei V, Nichols DH (2006) The molecular and developmental basis of the evolution of the vertebrate auditory system. International Journal of Comparative Psychology 19:1-24. Fritzsch B, Piatigorsky J (2005) Ancestry of photic and mechanic sensation? Science 308:1113-1114; author reply 1113-1114. Fritzsch B, Silos-Santiago I, Farinas I, Jones KR (2001b) Neurotrophins and neurotrophin receptors involved in supporting afferent inner ear innervation. In: Neurobiology of the Neurotrophins(Mocchetti, I., ed), pp 149-163 Johnson City, TN: FP Graham Publishing Co. Fritzsch B, Sonntag R (1990) Oculomotor (N III) motoneurons can innervate the superior oblique muscle of Xenopus after larval trochlear (N IV) nerve surgery. Neurosci Lett 114:129-134. Fritzsch B, Sonntag R (1991) Sequential double labelling with different fluorescent dyes coupled to dextran amines as a tool to estimate the accuracy of tracer application and of regeneration. Journal of Neuroscience Methods 39:9-17. Fritzsch B, Straka H (2013) Evolution of vertebrate mechanosensory hair cells and inner ears: toward identifying stimuli that select mutation driven altered morphologies. J Comp Physiol A 1-14. Fritzsch B, Tessarollo L, Coppola E, Reichardt LF (2004) Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance. In: Progress in Brain Research, vol. Volume 146 (Luigi, A. and Laura, C., eds), pp 265-278: Elsevier. Fritzsch B, Wake MH (1988) The Inner-Ear of Gymnophione Amphibians and its Nerve Supply a Comparative-Study of Regressive Events in a Complex Sensory System (Amphibia, Gymnophiona). Zoomorphology 108:201-217. Gallagher BC, Henry JJ, Grainger RM (1996) Inductive Processes Leading to Inner Ear Formation duringXenopusDevelopment. Developmental Biology 175:95-107. Galliot B, Quiquand M, Ghila L, de Rosa R, Miljkovic-Licina M, Chera S (2009) Origins of neurogenesis, a cnidarian view. Developmental Biology 332:2-24. Garden G, Canady K, Lurie D, Bothwell M, Rubel E (1994) A biphasic change in ribosomal conformation during transneuronal degeneration is altered by inhibition of mitochondrial, but not cytoplasmic protein synthesis. The Journal of Neuroscience 14:1994-2008. 159 Garden GA, Redeker-DeWulf V, Rubel EW (1995) Afferent influences on brainstem auditory nuclei of the chicken: Regulation of transcriptional activity followiqg cochlea removal. The Journal of comparative neurology 359:412-423. Gehring WJ, Ikeo K (1999) Pax 6: mastering eye morphogenesis and eye evolution. Trends in Genetics 15:371-377. Gittis AH, du Lac S (2006) Intrinsic and synaptic plasticity in the vestibular system. Current Opinion in Neurobiology 16:385-390. Glover JC (1996) Development of Second-Order Vestibular Projections in the Chicken Embryoa. Annals of the New York Academy of Sciences 781:13-20. Glover JC (2003) The development of vestibulo-ocular circuitry in the chicken embryo. Journal of Physiology-Paris 97:17-25. Goodman L, Model P (1988) Superinnervation enhances the dendritic branching pattern of the Mauthner cell in the developing axolotl. The Journal of Neuroscience 8:776-791. Goodman L, Model P (1990) Eliminating afferent impulse activity does not alter the dendritic branching of the amphibian Mauthner cell. J Neurobiol 21:283-294. Gould TW, Enomoto H (2009) Neurotrophic Modulation of Motor Neuron Development. The Neuroscientist 15:105-116. Graziadei PP, Levine RR, Graziadei GA (1978) Regeneration of olfactory axons and synapse formation in the forebrain after bulbectomy in neonatal mice. Proceedings of the National Academy of Sciences 75:5230-5234. Graziadei PPC, Levine RR, Monti Graziadei GA (1979) Plasticity of connections of the olfactory sensory neuron: Regeneration into the forebrain following bulbectomy in the neonatal mouse. Neuroscience 4:713-727. Graziadei PPC, Samanen DW (1980) Ectopic glomerular structures in the olfactory bulb of neonatal and adult mice. Brain Res 187:467-472. Green SH, Bailey E, Wang Q, Davis RL (2012) The Trk A, B, C's of Neurotrophins in the Cochlea. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology 295:1877-1895. Grigor'eva EE, Shtanchaev RS, Mikahailova GZ, Tiras NR, Moshkov DA (2010) Correlation between the sizes of the indivudual parats of goldfish Mauthner neuron and its integral function after eye enucleation. Morfologiia 137:10-15. Grigorieva E, Shtanchaev R, Mikhailova G, Tiras N, Moshkov D (2012) Correlation between the Sizes of Individual Parts of Mauthner Neurons in Goldfish and Their Integral Function after Enucleation of the Eye. Neuroscience and Behavioral Physiology 42:52-57. Groves AK (2005) The induction of the Otic Placode. In: Development of the Inner Ear(Kelley, M. et al., eds), pp 10-84 New York: Springer. 160 Hans S, Liu D, Westerfield M (2004) Pax8 and Pax2a function synergistically in otic specification, downstream of the Foxi1 and Dlx3b transcription factors. Development 131:5091-5102. Harrison RG (1935) The Croonian Lecture: On the Origin and Development of the Nervous System Studied by the Methods of Experimental Embryology. Proceedings of the Royal Society of London Series B, Biological Sciences 118:155-196. Heller N, Brändli AW (1999) Xenopus Pax-2/5/8 orthologues: Novel insights into Pax Gene evolution and identification of Pax-8 as the earliest marker for otic and pronephric cell lineages. Developmental Genetics 24:208-219. Hellmann B, Fritzsch B (1996) Neuroanatomical and histochemical evidence for the presence of common lateral line and inner ear efferents and of efferents to the basilar papilla in a frog, Xenopus laevis. Brain Behav Evol 47:185-194. Herrick CJ (1914) The medulla oblongata of larval amblystoma. The Journal of comparative neurology 24:343-427. Himanen J-P, Chumley MJ, Lackmann M, Li C, Barton WA, Jeffrey PD, Vearing C, Geleick D, Feldheim DA, Boyd AW, Henkemeyer M, Nikolov DB (2004) Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling. Nat Neurosci 7:501-509. Hoge MA (1915) Another Gene in the Fourth Chromosome of Drosophila. The American Naturalist 49:47-49. Holt JC, Lysakowski A, Goldberg JM (2011) The Efferent Vestibular System. In: Auditory and Vestibular Efferents(Ryugo, D. K. et al., eds) New York: Springer. Huang R, Zhi Q, Ordahl P, Christ B (1997) The fate of the first avian somite. Anatomy and Embryology 195:435-449. Hubel DH, Wiesel TN (1965) Binocular interaction in striate cortex of kittens reared with artifical squint. Journal of Neurophysiology 28:1041-1059. Hubel DH, Wiesel TN (1977) Functional architecture of macaque monkey visual cortex. Proceedings of the Royal Society of London B 198:1-59. Hyde G, Durham D (1994a) Increased deafferentation-induced cell death in chick brainstem auditory neurons following blockade of mitochondrial protein synthesis with chloramphenicol. The Journal of Neuroscience 14:291-300. Hyde GE, Durham D (1994b) Rapid increase in mitochondrial volume in nucleus magnocellularis neurons following cochlea removal. The Journal of comparative neurology 339:27-48. Inoue A, Takahashi M, Hatta K, Hotta Y, Okamoto H (1994) Developmental regulation of Islet-1 mRNA expression during neuronal differentiation in embryonic zebrafish. Developmental Dynamics 199:1-11. 161 Jackson H, Hackett JT, Rubel EW (1982) Organization and development of brain stem auditory nuclei in the chick: Ontogeny of postsynaptic responses. The Journal of comparative neurology 210:80-86. Jagger DJ, Griesinger CB, Rivolta MN, Holley MC, Ashmore JF (2000) Calcium signalling mediated by the alpa 9 acetylcholine receptor in a cochlear cell line from the Immortomouse. J Physiol 527:49-54. Jahan I, Kersigo J, Pan N, Fritzsch B (2010a) Neurod1 regulates survival and formation of connections in mouse ear and brain. Cell and tissue research 341:95-110. Jahan I, Pan N, Kersigo J, Fritzsch B (2010b) Neurod1 suppresses hair cell differentiation in ear ganglia and regulates hair cell subtype development in the cochlea. PLoS One 5:e11661. Jahan I, Pan N, Kersigo J, Fritzsch B (2013) Beyond generalized hair cells: Molecular cues for hair cell types. Hearing Research 297:30-41. Jarman AP, Sun Y, Jan LY, Jan YN (1995) Role of the proneural gene, atonal, in formation of Drosophila chordotonal organs and photoreceptors. Development 121:2019-2030. Jones AK, Sattelle DB (2004) Functional genomics of the nicotinic acetylcholine receptor gene family of the nematode, Caenorhabditis elegans. BioEssays 26:39-49. Jones DG, Eslami H (1983) An ultrastructural study of the development of afferent and efferent synapses on outer hair cells of the guinea pig organ of Corti. Cell and tissue research 231:533-549. Kaan HW (1926) Experiments on the development of the ear of Amblystoma punctatum. Journal of Experimental Zoology 46:13-61. Kaiser CL, Chapman BJ, Guidi JL, Terry CE, Mangiardi DA, Cotanche DA (2008) Comparison of activated caspase detection methods in the gentamicin-treated chick cochlea. Hearing Research 240:1-11. Kamiya H, Itoh K, Yasui Y, Ino T, Mizuno N (1988) Somatosensory and auditory relay nucleus in the rostral part of the ventrolateral medulla: A morphological study in the cat. The Journal of comparative neurology 273:421-435. Kanekar S, Perron M, Dorsky R, Harris WA, Jan LY, Jan YN, Vetter ML (1997) Xath5 Participates in a Network of bHLH Genes in the Developing Xenopus Retina. Neuron 19:981-994. Karis A, Pata I, van Doorninck JH, Grosveld F, de Zeeuw CI, de Caprona D, Fritzsch B (2001) Transcription factor GATA-3 alters pathway selection of olivocochlear neurons and affects morphogenesis of the ear. J Comp Neurol 429:615-630. Katz E (ed.) (2011) Cholinergic inhibition of hair cells. New York: Springer. Katz E, Elgoyhen AB, Gomez-Casati ME, Knipper M, Vetter DE, Fuchs PA, Glowatzki E (2004) Developmental regulation of nicotinic synapses on cochlear inner hair cells. J Neurosci 24:7814-7820. 162 Kawashima K, Fujii T, Moriwaki Y, Misawa H (2012) Critical roles of acetylcholine and the muscarinic and nicotinic acetylcholine receptors in the regulation of immune function. Life Sciences 91:1027-1032. Kay JN, Finger-Baier KC, Roeser T, Staub W, Baier H (2001) Retinal Ganglion Cell Genesis Requires lakritz, a Zebrafish atonal Homolog. Neuron 30:725-736. Kelley MS, Lurie DI, Rubel EW (1997) Rapid regulation of cytoskeletal proteins and their mRNAs following afferent deprivation in the avian cochlear nucleus. J Comp Neurol 389:469-483. Kevetter GA, Perachio AA (1986) Distribution of vestibular afferents that innervate the sacculus and posterior canal in the gerbil. The Journal of comparative neurology 254:410-424. Kimmel CB (1982) Development of synapses on the Mauthner neuron. Trends in Neurosciences 5:47-50. Kimmel CB, Schabtach E, Kimmel RJ (1977) Developmental interactions in the growth and branching of the lateral dendrite of Mauthner's cell (Ambystoma mexicanum). Developmental Biology 55:244-259. Kopecky BJ, Duncan JS, Elliott KL, Fritzsch B (2012) Three-dimensional reconstructions from optical sections of thick mouse inner ears using confocal microscopy. Journal of Microscopy 248:292-298. Köppl C (2011) Evolution of the Octavolateral Efferent System. In: Auditory and Vestibular Efferents, vol. 38 (Ryugo, D. K., ed), pp 217-259 New York: Springer. Korn H, Faber DS (2005) The Mauthner Cell Half a Century Later: A Neurobiological Model for Decision-Making? Neuron 47:13-28. Kozmik Z, Daube M, Frei E, Norman B, Kos L, Dishaw LJ, Noll M, Piatigorsky J (2003) Role of Pax Genes in Eye Evolution: A Cnidarian PaxB Gene Uniting Pax2 and Pax6 Functions. Developmental Cell 5:773-785. Kullander K, Klein R (2002) Mechanisms and functions of eph and ephrin signalling. Nat Rev Mol Cell Biol 3:475-486. Ladher RK, Anakwe KU, Gurney AL, Schoenwolf GC, Francis-West PH (2000) Identification of Synergistic Signals Initiating Inner Ear Development. Science 290:1965-1967. Lance-Jones C, Landmesser L (1980) Motoneurone projection patterns in the chick hind limb following early partial reversals of the spinal cord. The Journal of Physiology 302:581-602. Landmesser LT (1980) The Generation of Neuromuscular Specificity. Annual Reviews Neuroscience 3:279-302. Lea R, Papalopulu N, Amaya E, Dorey K (2009) Temporal and spatial expression of FGF ligands and receptors during Xenopus development. Developmental Dynamics 238:1467-1479. 163 Leamey CA, Van Wart A, Sur M (2009) Intrinsic patterning and experience-dependent mechanisms that generate eye-specific projections and binocular circuits in the visual pathway. Current Opinion in Neurobiology 19:181-187. Leperchey F (1979) Embryogeny of facial, mastication, tongue, palate and neck muscles. Rev Stomatol Chir Maxillofac 80:45-67. Levi-Montalcini R (1949) The development to the acoustico-vestibular centers in the chick embryo in the absence of the afferent root fibers and of descending fiber tracts. J Comp Neurol 91:209-241, illust, incl 203 pl. Lewis E, Narins P (1999) The Acoustic Periphery of Amphibians: Anatomy and Physiology. In: Comparative Hearing: Fish and Amphibians, vol. 11 (Fay, R. and Popper, A., eds), pp 101-154: Springer New York. Lewis ER, Leverenz EL, Bialek WS (1985) The vertebrate inner ear. Boca Raton: CRC Press. Liebl DJ, Morris CJ, Henkemeyer M, Parada LF (2003) mRNA expression of ephrins and Eph receptor tyrosine kinases in the neonatal and adult mouse central nervous system. Journal of Neuroscience Research 71:7-22. Lin W, Burgess RW, Dominguez B, Pfaff SL, Sanes JR, Lee KF (2001) Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature 410:1057-1064. Lipovsek M, Im GJ, Franchini LF, Pisciottano F, Katz E, Fuchs PA, Elgoyhen AB (2012) Phylogenetic differences in calcium permeability of the auditory hair cell cholinergic nicotinic receptor. Proceedings of the National Academy of Sciences 109:4308-4313. Liu KS, Gray M, Otto SJ, Fetcho JR, Beattie CE (2003) Mutations in deadly seven/notch1a Reveal Developmental Plasticity in the Escape Response Circuit. The Journal of Neuroscience 23:8159-8166. Lombardo A, Isaccs HV, Slack JM (1998) Expression and functions of FGF-3 in Xenopus development. Int J Dev Biol 42:1101-1117. Lundberg YW, Zhao X, Yamoah EN (2006) Assembly of the otoconia complex to the macular sensory epithelium of the vestibule. Brain research 1091:47-57. Ma Q, Anderson DJ, Fritzsch B (2000) Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. Journal of the Association for Research in Otolaryngology : JARO 1:129-143. Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ (1998) neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron 20:469-482. Mackereth MD, Kwak S-J, Fritz A, Riley BB (2005) Zebrafish pax8 is required for otic placode induction and plays a redundant role with Pax2 genes in the maintenance of the otic placode. Development 132:371-382. 164 Magrassi L, Graziadei PPC (1985) Interaction of the transplanted olfactory placode with the optic stalk and the diencephalon inXenopus laevis embryos. Neuroscience 15:903-921. Maklad A, Fritzsch B (1999) Incomplete segregation of endorgan-specific vestibular ganglion cells in mice and rats. J Vestib Res 9:387-399. Maklad A, Fritzsch B (2002) The developmental segregation of posterior crista and saccular vestibular fibers in mice: a carbocyanine tracer study using confocal microscopy. Developmental Brain Research 135:1-17. Maklad A, Fritzsch B (2003) Development of vestibular afferent projections into the hindbrain and their central targets. Brain Res Bull 60:497-510. Maklad A, Kamel S, Wong E, Fritzsch B (2010) Development and organization of polarity-specific segregation of primary vestibular afferent fibers in mice. Cell and tissue research 340:303-321. Mansouri A, Goudreau G, Gruss P (1999) Pax Genes and Their Role in Organogenesis. Cancer Research 59:1707s-1710s. Mao CA, Wang SW, Pan P, Klein WH (2008) Rewiring the retinal ganglion cell gene regulatory network: Neurod1 promotes retinal ganglion cell fate in the absence of Math5. Development 135:3379-3388. Mao Y-T, Hua T-M, Pallas SL (2011) Competition and convergence between auditory and cross-modal visual inputs to primary auditory cortical areas. Journal of Neurophysiology 105:1558-1573. Markl H (1974) The perception of gravity and of angular acceleration in invertebrates. In: Handbook of Sensory Physiology, vol. VI/1 Vestibular System (Kornhuber, H. H., ed) Berlin: Springer Verlag. Meredith GE (1988) Comparative view of the central organization of afferent and efferent circuitry for the inner ear. Acta biologica Hungarica 39:229-249. Meyer R (1982) Tetrodotoxin blocks the formation of ocular dominance columns in goldfish. Science 218:589-591. Mikheeva IB, Tsaplina NI, Grigor'eva EE, Bezgina EN, Shtanchaev RS, Mikahailova GZ, Tiras NR, Moshkov DA (2011) Ultrastructure of Mauthner neurons after optokinetic stimulation and eye enucleation. Morfologiia 139:30-35. Mishima T, Mizuguchi Y, Kawahigashi Y, Takizawa T, Takizawa T (2007) RT-PCRbased analysis of microRNA (miR-1 and -124) expression in mouse CNS. Brain research 1131:37-43. Miyake N, Chilton J, Psatha M, Cheng L, Andrews C, Chan W-M, Law K, Crosier M, Lindsay S, Cheung M, Allen J, Gutowski NJ, Ellard S, Young E, Iannaccone A, Appukuttan B, Stout JT, Christiansen S, Ciccarelli ML, Baldi A, Campioni M, Zenteno JC, Davenport D, Mariani LE, Sahin M, Guthrie S, Engle EC (2008) Human CHN1 Mutations Hyperactivate α2-Chimaerin and Cause Duane's Retraction Syndrome. Science 321:839-843. 165 Morrison E, Graziadei PC (1996) An ultrastructural study of glomeruli associated with vomeronasal organs transplanted into the rat CNS. Anatomy and Embryology 193:331-339. Morsli H, Choo D, Ryan A, Johnson R, Wu DK (1998) Development of the mouse inner ear and origin of its sensory organs. J Neurosci 18:3327-3335. Mostafapour SP, Cochran SL, Del Puerto NM, Rubel EW (2000) Patterns of cell death in mouse anteroventral cochlear nucleus neurons after unilateral cochlea removal. The Journal of comparative neurology 426:561-571. Mower GD, Christen WG, Caplan CJ (1984) Absence of ocular dominance columns in binocularly deprived cats. Investigative Opthalmology and Visual Science 25 (Suppl.):214 (ARVO abstr.). Murakami Y, Pasqualetti M, Takio Y, Hirano S, Rijli FM, Kuratani S (2004) Segmental development of reticulospinal and branchiomotor neurons in lamprey: insights into the evolution of the vertebrate hindbrain. Development 131:983-995. Newlands SD, Perachio AA (2003) Central projections of the vestibular nerve: a review and single fiber study in the Mongolian gerbil. Brain Research Bulletin 60:475495. Newlands SD, Vrabec JT, Purcell IM, Stewart CM, Zimmerman BE, Perachio AA (2003) Central projections of the saccular and utricular nerves in macaques. The Journal of comparative neurology 466:31-47. Nieber F, Pieler T, Henningfeld KA (2009) Comparative expression analysis of the neurogenins in Xenopus tropicalis and Xenopus laevis. Developmental Dynamics 238:451-458. Nieuwkoop P, Faber J (1994) Normal table of Xenopus laevis (Daudin): a systematical and chronological survey of the development from the fertilized egg till the end of metamorphosis. New York: Garland Publishing, Inc. Nikundiwe AM, Nieuwenhuys R (1983) The cell masses in the brainstem of the South African clawed frog Xenopus laevis: a topographical and topological analysis. J Comp Neurol 213:199-219. Noden DM, Francis-West P (2006) The differentiation and morphogenesis of craniofacial muscles. Dev Dyn 235:1194-1218. Ohyama T, Groves AK, Martin K (2007) The first steps towards hearing: mechanisms of otic placode induction. Int J Dev Biol 51:463-472. Ohyama T, Mohamed OA, Taketo MM, Dufort D, Groves AK (2006) Wnt signals mediate a fate decision between otic placode and epidermis. Development 133:865-875. Osman AA, Schrader AD, Hawkes AJ, Akil O, Bergeron A, Lustig LR, Simmons DD (2008) Muscle-like nicotinic receptor accessory molecules in sensory hair cells of the inner ear. Mol Cell Neurosci 38:153-169. 166 Pan N, Kopecky B, Jahan I, Fritzsch B (2012) Understanding the evolution and development of neurosensory transcription factors of the ear to enhance therapeutic translation. Cell and tissue research 349:415-432. Park B-Y, Saint-Jeannet J-P (2008) Hindbrain-derived Wnt and Fgf signals cooperate to specify the otic placode in Xenopus. Developmental Biology 324:108-121. Parks TN (1979) Afferent influences on the development of the brain stem auditory nuclei of the chicken: otocycst ablation. J Comp Neurol 183:665-678. Parks TN (1981) Changes in the length and organization of nucleus laminaris dendrites after unilateral otocyst ablation in chick embryos. J Comp Neurol 202:45-57. Pasic TR, Rubel EW (1989) Rapid changes in cochlear nucleus cell size following blockade of auditory nerve electrical activity in gerbils. The Journal of comparative neurology 283:474-480. Paterson NF (1949) The development of the inner ear of Xenopus laevis. Proceedings of the Zoological Society of London 119:269-291. Patten SA, Sihra RK, Dhami KS, Coutts CA, Ali DW (2007) Differential expression of PKC isoforms in developing zebrafish. International Journal of Developmental Neuroscience 25:155-164. Pauley S, Wright TJ, Pirvola U, Ornitz D, Beisel K, Fritzsch B (2003) Expression and function of FGF10 in mammalian inner ear development. Dev Dyn 227:203-215. Pfeffer PL, Gerster T, Lun K, Brand M, Busslinger M (1998) Characterization of three novel members of the zebrafish Pax2/5/8 family: dependency of Pax5 and Pax8 expression on the Pax2.1 (noi) function. Development 125:3063-3074. Piatt J (1969) The influence of VIIth and VIIIth cranial nerve roots upon the differentiation of Mauthner's cell in Ambystoma. Developmental Biology 19:608616. Pieper M, Eagleson GW, Wosniok W, Schlosser G (2011) Origin and segregation of cranial placodes in Xenopus laevis. Developmental Biology 360:257-275. Pierce ML, Weston MD, Fritzsch B, Gabel HW, Ruvkun G, Soukup GA (2008) MicroRNA-183 family conservation and ciliated neurosensory organ expression. Evol Dev 10:106-113. Porter JD, Baker RS (1997) Absence of oculomotor and trochlear motoneurons leads to altered extraocular muscle development in the Wnt-1 null mutant mouse. Developmental Brain Research 100:121-126. Qian Y, Fritzsch B, Shirasawa S, Chen C-L, Choi Y, Ma Q (2001) Formation of brainstem (nor)adrenergic centers and first-order relay visceral sensory neurons is dependent on homeodomain protein Rnx/Tlx3. Genes & development 15:25332545. Quick QA, Serrano EE (2005) Inner ear formation during the early larval development of Xenopus laevis. Developmental Dynamics 234:791-801. 167 Reh T, Constantine-Paton M (1985) Eye-specific segregation requires neural activity in three-eyed Rana pipiens. The Journal of Neuroscience 5:1132-1143. Retzius G (1881) Das Gehörorgan der Wirbeltiere. vol. 1 Stockholm: Samson and Wallin. Riley BB, Phillips BT (2003) Ringing in the new ear: resolution of cell interactions in otic development. Dev Biol 261:289-312. Rinkwitz S, Bober EVA, Baker R (2001) Development of the Vertebrate Inner Ear. Annals of the New York Academy of Sciences 942:1-14. Roberts BL, Meredith GE (1992) The efferent innervation of the ear: Variations on an enigma. In: The evolutionary biology of hearing(Webster, D. B. et al., eds), pp li, 859 p. New York: Springer-Verlag. Ronca AE, Fritzsch B, Alberts JR, Bruce LL (2000) Effects of microgravity on vestibular development and function in rats: Genetics and environment. Korean Journal of Biological Sciences 4:215-221. Rössert C, Moore LE, Straka H, Glasauer S (2011) Cellular and Network Contributions to Vestibular Signal Processing: Impact of Ion Conductances, Synaptic Inhibition, and Noise. The Journal of Neuroscience 31:8359-8372. Rubel EW, Fritzsch B (2002) Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci 25:51-101. Ryugo DK, Parks TN (2003) Primary innervation of the avian and mammalian cochlear nucleus. Brain Research Bulletin 60:435-456. Sanes JR, Apel ED, Burgess RW, Emerson RB, Feng G, Gautam M, Glass D, Grady RM, Krejci E, Lichtman JW, Lu JT, Massoulie J, Miner JH, Moscoso LM, Nguyen Q, Nichol M, Noakes PG, Patton BL, Son YJ, Yancopoulos GD, Zhou H (1998) Development of the neuromuscular junction: genetic analysis in mice. J Physiol Paris 92:167-172. Schimmang T (2007) Expression and functions of FGF ligands during early otic development. Int J Dev Biol 51:473-481. Schlosser G (2002) Development and evolution of lateral line placodes in amphibians I. Development. Zoology 105:119-146. Schlosser G (2005) Evolutionary origins of vertebrate placodes: Insights from developmental studies and from comparisons with other deuterostomes. J Exp Zool 301B:347-399. Schlosser G (2010) Chapter Four - Making Senses: Development of Vertebrate Cranial Placodes. In: International Review of Cell and Molecular Biology, vol. Volume 283 (Kwang, J., ed), pp 129-234: Academic Press. Schlosser G, Ahrens K (2004) Molecular anatomy of placode development in Xenopus laevis. Developmental Biology 271:439-466. 168 Schmidt JT, Tieman SB (1985) Eye-specific segregation of optic afferents in mammals, fish, and frogs: The role of activity. Cellular and Molecular Neurobiology 5:5-34. Seipel K, Yanze N, Schmid V (2004) Developmental and evolutionary aspects of the basic helix-loop-helix transcription factors Atonal-like 1 and Achaete-scute homolog 2 in the jellyfish. Developmental Biology 269:331-345. Sgard F, Charpantier E, Bertrand S, Walker N, Caput D, Graham D, Bertrand D, Besnard F (2002) A Novel Human Nicotinic Receptor Subunit, α10, That Confers Functionality to the α9-Subunit. Molecular Pharmacology 61:150-159. Sholl DA (1953) Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat 87:387-406. Siddiqui SA, Cramer KS (2005) Differential expression of Eph receptors and ephrins in the cochlear ganglion and eighth cranial nerve of the chick embryo. The Journal of comparative neurology 482:309-319. Sie KCY, Rubel EW (1992) Rapid changes in protein synthesis and cell size in the cochlear nucleus following eighth nerve activity blockade or cochlea ablation. The Journal of comparative neurology 320:501-508. Sienkiewicz W, Dudek A (2010) Sources of the motor and somatic sensory innervation of the trapezius muscle in the rat. Vet Med Czech 55:242-252. Sillar KT (2009) Mauthner cells. Current Biology 19:R353-R355. Simmons D, Duncan J, Caprona DC, Fritzsch B (2011) Development of the Inner Ear Efferent System. In: Auditory and Vestibular Efferents, vol. 38, pp 187-216 New York: Springer Simmons DD (2002) Development of the inner ear efferent system across vertebrate species. J Neurobiol 53:228-250. Skowronska-Krawczyk D, Chiodini F, Ebeling M, Alliod C, Kundzewicz A, Castro D, Ballivet M, Guillemot F, Matter-Sadzinski L, Matter J-M (2009) Conserved regulatory sequences in Atoh7 mediate non-conserved regulatory responses in retina ontogenesis. Development 136:3767-3777. Solomon KS, Kwak S-J, Fritz A (2004) Genetic interactions underlying otic placode induction and formation. Developmental Dynamics 230:419-433. Springer AD, Cohen SM (1981) Optic fiber segregation in goldfish with two eyes innervating one tectal lobe. Brain research 225:23-36. Steward O, Rubel EW (1985) Afferent influences on brain stem auditory nuclei of the chicken: Cessation of amino acid incorporation as an antecedent to age-dependent transneuronal degeneration. The Journal of comparative neurology 231:385-395. Straka H (2010) Ontogenetic rules and constraints of vestibulo-ocular reflex development. Current Opinion in Neurobiology 20:689-695. Straka H, Holler S, Goto F (2002) Patterns of Canal and Otolith Afferent Input Convergence in Frog Second-Order Vestibular Neurons. Journal of Neurophysiology 88:2287-2301. 169 Straka H, Lambert FM, Pfanzelt S, Beraneck M (2009) Vestibulo-ocular Signal Transformation in Frequency-Tuned Channels. Annals of the New York Academy of Sciences 1164:37-44. Sugai T, Yano J, Sugitani M, Ooyama H (1992) Actions of cholinergic agonists and antagonists on the efferent synapse in the frog sacculus. Hearing Research 61:5664. Sun S-K, Dee CT, Tripathi VB, Rengifo A, Hirst CS, Scotting PJ (2007) Epibranchial and otic placodes are induced by a common Fgf signal, but their subsequent development is independent. Developmental Biology 303:675-686. Suneja SK, Potashner SJ (2002) TrkB levels in the cochlear nucleus after unilateral cochlear ablation: correlations with post-lesion plasticity. Brain research 957:366368. Swindale NV (1981) Absence of ocular dominance patches in dark reared cats. Nature 290:332-333. Swindale NV, Cynader MS (1983) Physiological segregation of geniculocortocal afferents in the visual cortex of dark reared cats. Neuroscience Abstracts 9:24. Takano-Maruyama M, Chen Y, Gaufo GO (2010) Placodal sensory ganglia coordinate the formation of the cranial visceral motor pathway. Developmental Dynamics 239:1155-1161. Tierney TS, Russell FA, Moore DR (1997) Susceptibility of developing cochlear nucleus neurons to deafferentation-induced death abruptly ends just before the onset of hearing. The Journal of comparative neurology 378:295-306. Tonniges J, Hansen M, Duncan J, Bassett MJ, Fritzsch B, Gray BD, Easwaran A, Nichols MG (2010) Photo- and bio-physical characterization of novel violet and nearinfrared lipophilic fluorophores for neuronal tracing. Journal of Microscopy 9999. Torres M, Giráldez F (1998) The development of the vertebrate inner ear. Mechanisms of Development 71:5-21. Torres M, Gomez-Pardo E, Gruss P (1996) Pax2 contributes to inner ear patterning and optic nerve trajectory. Development 122:3381-3391. Tsuchida T, Ensini M, Morton SB, Baldassare M, Edlund T, Jessell TM, Pfaff SL (1994) Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell 79:957-970. Tsunoyama K, Gojobori T (1998) Evolution of nicotinic acetylcholine receptor subunits. Molecular Biology and Evolution 15:518-527. Uchino Y, Kushiro K (2011) Differences between otolith- and semicircular canalactivated neural circuitry in the vestibular system. Neuroscience Research 71:315327. 170 Urness LD, Paxton CN, Wang X, Schoenwolf GC, Mansour SL (2010) FGF signaling regulates otic placode induction and refinement by controlling both ectodermal target genes and hindbrain Wnt8a. Developmental Biology 340:595-604. Vargas-Lizardi P, Lyser KM (1974) Time of origin of Mauthner's neuron in Xenopus laevis embryos. Developmental Biology 38:220-228. Vendrell V, Carnicero E, Giraldez F, Alonso MT, Schimmang T (2000) Induction of inner ear fate by FGF3. Development 127:2011-2019. Vernino S, Hopkins S, Wang Z (2009) Autonomic ganglia, acetylcholine receptor antibodies, and autoimmune ganglionopathy. Autonomic Neuroscience 146:3-7. Vetter DE, Katz E, Maison SF, Taranda J, Turcan S, Ballestero J, Liberman MC, Elgoyhen AB, Boulter J (2007) The α10 nicotinic acetylcholine receptor subunit is required for normal synaptic function and integrity of the olivocochlear system. Proceedings of the National Academy of Sciences 104:20594-20599. Vetter DE, Liberman MC, Mann J, Barhanin J, Boulter J, Brown MC, Saffiote-Kolman J, Heinemann SF, Elgoyhen AB (1999) Role of alpha9 nicotinic ACh receptor subunits in the development and function of cochlear efferent innervation. Neuron 23:93-103. Wada H, Saiga H, Satoh N, Holland PW (1998) Tripartite organization of the ancestral chordate brain and the antiquity of placodes: insights from ascidian Pax-2/5/8, Hox and Otx genes. Development 125:1113-1122. Waldman EH, Castillo A, Collazo A (2007) Ablation studies on the developing inner ear reveal a propensity for mirror duplications. Developmental Dynamics 236:12371248. Weston MD, Pierce ML, Rocha-Sanchez S, Beisel KW, Soukup GA (2006) MicroRNA gene expression in the mouse inner ear. Brain research 1111:95-104. Wiesel TN (1982) The postnatal development of the visual cortex and the influence of environment. Bioscience Reports 2:351-377. Will U (1982) Efferent neurons of the lateral-line system and the VIII cranial nerve in the brainstem of anurans. Cell and tissue research 225:673-685. Winklbauer R, Hausen P (1983) Development of the lateral line system in Xenopus laevis: I. Normal development and cell movement in the supraorbital system. Journal of Embryology and Experimental Morphology 76:265-281. Woolf NK, Ryan AF (1984) The development of auditory function in the cochlea of the mongolian gerbil. Hearing Research 13:277-283. Woolf NK, Ryan AF (1985) Ontogeny of neural discharge patterns in the ventral cochlear nucleus of the Mongolian gerbil. Developmental Brain Research 17:131-147. Wright TJ, Mansour SL (2003) Fgf3 and Fgf10 are required for mouse otic placode induction. Development 130:3379-3390. 171 Yang T, Kersigo J, Jahan I, Pan N, Fritzsch B (2011) The molecular basis of making spiral ganglion neurons and connecting them to hair cells of the organ of Corti. Hearing Research 278:21-33. Yntema CL (1950) An analysis of induction of the ear from foreign ectoderm in the salamander embryo. Journal of Experimental Zoology 113:211-243. Zhang X, Tang N, Hadden TJ, Rishi AK (2011) Akt, FoxO and regulation of apoptosis. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1813:19781986. Zottoli SJ, Hordes AR, Faber DS (1987) Localization of optic tectal input to the ventral dendrite of the goldfish Mauthner cell. Brain research 401:113-121. Zuo J, Treadaway J, Buckner TW, Fritzsch B (1999) Visualization of alpha9 acetylcholine receptor expression in hair cells of transgenic mice containing a modified bacterial artificial chromosome. Proc Natl Acad Sci U S A 96:1410014105.