* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Intact PTH
Survey
Document related concepts
Monoclonal antibody wikipedia , lookup
SNP genotyping wikipedia , lookup
Point mutation wikipedia , lookup
Pharmacometabolomics wikipedia , lookup
Surround optical-fiber immunoassay wikipedia , lookup
Peptide synthesis wikipedia , lookup
Community fingerprinting wikipedia , lookup
Proteolysis wikipedia , lookup
Western blot wikipedia , lookup
Ligand binding assay wikipedia , lookup
Genetic code wikipedia , lookup
Biochemistry wikipedia , lookup
Transcript
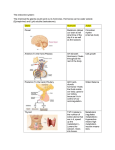
0195BH1013D-1.qxp_Layout 1 2/11/15 6:52 AM Page 1 Technical Data Sheet Intact PTH For In Vitro Diagnostic Use The MicroVue PTH EIA measures the amount of intact Parathyroid Hormone (PTH) in experimental samples. PTH (Parathyroid hormone, Parathormone, Parathyrin) is biosynthesized in the parathyroid gland as a pre-proparathyroid hormone, a larger molecular precursor consisting of 115 amino acids. Following sequential intracellular cleavage of a 25 amino acid sequence, pre-proparathyroid hormone is converted to an intermediate, a 90 amino acid polypeptide, proparathyroid hormone. With additional proteolytic modification, proparathyroid hormone is then converted to parathyroid hormone, an 84 amino acid polypeptide. In healthy individuals, regulation of parathyroid hormone secretion normally occurs via a negative feedback action of serum calcium on the parathyroid glands. Intact PTH is biologically active and clears very rapidly from the circulation with a half-life of less than four minutes. Intact PTH assays are important for the differentiation of primary hyperparathyroidism from the other (nonparathyroid-mediated) forms of hypercalcemia, such as malignancy, sarcoidosis and thyrotoxicosis. The measurement of parathyroid hormone is the most specific way of making the diagnosis of primary hyperparathyroidism. In the presence of hypercalcemia, an elevated level of parathyroid hormone virtually establishes the diagnosis. In over 90% of patients with primary hyperparathyroidism, the parathyroid hormone will be elevated. Format ELISA 96-well microplate with reagents sufficient to test 40 samples in duplicate Sample type: Serum, plasma or other experimental samples Controls included Species Reactivity Human Specimen Samples collected to avoid hemolysis Assay Steps Dilute Wash Buffer; reconstitute Standard and Controls Pipette 25 µL of Standards, Control and samples into assay wells Add 50 µL of Biotin Labeled Antibody and 50 µL of Enzyme Labeled Antibody to each well Incubate 3 hours ± 30 minutes at 22°C to 28°C with shaking Wash the assay wells five times Pipette 150 µL Substrate Solution Incubate 30 ± 5 minutes at 22°C to 28°C with shaking Add 100 µL of Stop Solution to each assay well Measure absorbance at 450 nm and again at 405 nm Assay Performance Method: ELISA Analyte: Intact PTH Specimen Volume: 25 µL Limit of Detection: 1.57 pg/mL Assay Range: 0-700 pg/mL Precision (inter-assay): 2.8%-3.6% Precision (intra-assay): 3.7%-6.1% Assay Time: Approx. 4 hours Quidel | Specialty Products | Research to Rapids® | quidel.com | 800.874.1517 | 858.552.1100 SL0205 | 0195BH1013D-1 (02/15) MicroVue Intact PTH EIA – Cat. #8044