* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Student CSE paper
Survey
Document related concepts
Hedgehog signaling pathway wikipedia , lookup
Endomembrane system wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Protein phosphorylation wikipedia , lookup
Protein moonlighting wikipedia , lookup
Bacterial microcompartment wikipedia , lookup
Signal transduction wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
List of types of proteins wikipedia , lookup
Paracrine signalling wikipedia , lookup
Biochemical cascade wikipedia , lookup
Transcript
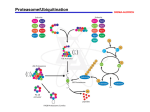
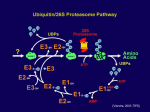
Jennifer Martinez wrote a paper on neurological disorders for a biology class. This paper is excerpted below, with references on the following page. Proteolysis is the breakdown or degradation of proteins and is an important cellular event involving tightly regulated removal of unwanted proteins and retention of those that are essential. The ubiquitin/proteasome pathway plays an important role in the intracellular quality control process by degrading mutated or abnormally folded proteins to prevent their accumulation as intracellular aggregates. Proteolysis by the ubiquitin/proteasome pathway involves two major steps: ubiquitination followed by degradation. A de-ubiquitination step also plays an important role in this pathway 2 . Ubiquitin (Ub) is a small peptide, consisting of 76 amino acids and is abundant in all eukaryotic cells 2. Covalent attachment of ubiquitin to proteins targets them for degradation. Once ubiquitinated, proteins do not accumulate in cells since the Ub/proteasome pathway degrades them. Proteins become ubiquitinated by a series of four enzymatic reactions: the ATP dependent activation of ubiquitin by Ub-activating enzymes (E1), binding of activated ubiquitin to Ub-conjugating enzymes (E2), the covalent conjugation of ubiquitin to the protein substrate by Ub-ligases (E3) and the formation of a polyubiquitin chain by the elongation factor E4. Polyubiquitination acts as a tag for recognition by the 26S proteasome for protein degradation. The 26S proteasome consists of two main particles, the 19S regulatory particle and the 20S catalytic particle. The 19S particle has a lid and base arrangement. The lid contains ATPases and deubiquitinating enzymes, whereas, the base contains polyub-binding subunits. The 20S particle is a cylinder-shaped complex with a catalytic core. The hydrolysis of peptide bonds occurs in this core particle 2,6. Ubiquitin/Proteasome Pathway 12 References 1. De Silva HR, Khan NL, Wood NW. The genetics of Parkinson’s disease. Curr Opin Genet Dev. 2000;10(3):292-298. 2. Figueiredo-Pereira ME, Rockwell P. The ubiquitin/proteasome pathway in neurological disorders. In: Banik NL, Lajtha A, editors. Role of proteases in the pathophysiology of neurodegenerative diseases. New York: Kluwer/Plenum; 2001. 302p. 3. Imai Y, Soda M, Takahashi R. Parkin suppresses unfolded protein stress-induced cell death through its E3 ubiquitin-protein ligase activity. J Biol Chem. 2000;275(46):35661-35664. 4. Parkinson’s disease—hope through research [Internet]. Bethesda (MD): Nat Inst of Neurological Disorders and Stroke; updated 2006 Jun 27 [cited 2006 Jul 6]; [about 20p.]. Available from: http://www.ninds.nih.gov/disorders/ parkinsons_disease/ detail_parkinsons_disease.htm 5. Shimura H, Hattori N, Kubo S, Mizuno Y, Asakawa S, Minoshima S, Shimizu N, Iwai K, Chiba T, Tanaka K, Suzuki T. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet. 2000;25(3):302-305. 6. Wigley WC, Fabunmi RP, Lee MG, Marino CR, Muallem S, Demartino GN, Thomas PJ. Dynamic association of proteasomal machinery with the centrosome. J Cell Biol. 1999;145(3):481-490.