* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Fatty acid amide hydrolase expression in rat choroid plexus
Survey
Document related concepts
Stimulus (physiology) wikipedia , lookup
History of neuroimaging wikipedia , lookup
Neuroinformatics wikipedia , lookup
Neuropsychology wikipedia , lookup
Blood–brain barrier wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
Metastability in the brain wikipedia , lookup
Optogenetics wikipedia , lookup
Subventricular zone wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Haemodynamic response wikipedia , lookup
Neuroanatomy wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Transcript
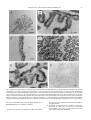
Neuroscience Letters 282 (2000) 13±16 www.elsevier.com/locate/neulet Fatty acid amide hydrolase expression in rat choroid plexus: possible role in regulation of the sleep-inducing action of oleamide Michaela Egertova a, Benjamin F. Cravatt b, Maurice R. Elphick a,* a School of Biological Sciences, Queen Mary and West®eld College, University of London, Mile End Road, London E1 4NS, UK The Skaggs Institute for Chemical Biology and Department of Cell Biology, The Scripps Research Institute, La Jolla, CA 92037, USA b Received 10 November 1999; received in revised form 11 January 2000; accepted 12 January 2000 Abstract The enzyme fatty acid amide hydrolase (FAAH) catalyses hydrolysis of oleamide, a sleep-inducing lipid whose concentration in the cerebrospinal ¯uid (CSF) is elevated in sleep-deprived mammals. Previous studies have reported expression of FAAH by distinct populations of neurons in the rat brain. Here we demonstrate using immunocytochemical methods that FAAH is also expressed by non-neuronal epithelial cells of the rat choroid plexus. The choroid plexus is formed by invaginations of the pia mater into the ventricle cavities of the brain and an important function of the choroidal epithelium is to regulate production and composition of CSF. Therefore, the role of FAAH in epithelial cells of the choroid plexus may be to control the concentration of oleamide in the CSF and as such FAAH may exert an important regulatory role in shaping the duration and magnitude of the sleep-inducing effect of endogenously or exogenously derived oleamide. q 2000 Elsevier Science Ireland Ltd. All rights reserved. Keywords: Fatty acid amide hydrolase; Choroid plexus; Cerebrospinal ¯uid; Oleamide; Sleep; Anandamide; Cannabinoid Fatty acid amide hydrolase (FAAH) is a ,63 kDa membrane-associated enzyme which was originally identi®ed on account of its ability to catalyse hydrolysis of the sleep-inducing lipid oleamide to oleic acid and ammonia [2,3]. In addition, FAAH also catalyses hydrolysis of the putative endocannabinoid anandamide to arachidonic acid and ethanolamine [3,5]. It has been suggested, therefore, that FAAH may participate in the regulation of neural signalling pathways that utilise these and related lipid messenger molecules [3]. Importantly, the distribution of FAAH and CB1-type cannabinoid receptors in rat brain is similar, with FAAH often occurring in neuronal somata that are postsynaptic to CB1-expressing axons [6]. Therefore, the cellular location of FAAH in the rat brain is consistent with a potential role in regulation of endocannabinoids such as anandamide. What is not yet known is how FAAH may participate in regulation of oleamide-based signalling mechanisms. The concentration of oleamide is elevated in the cerebrospinal ¯uid (CSF) of sleep-deprived cats [2] and rats [1]; therefore * Corresponding author. Tel.: 144-207-882-5290; fax: 144-208983-0973. E-mail address: [email protected] (M.R. Elphick) injection of oleamide may mimic a natural rise in the oleamide content of the CSF that occurs with sleep-deprivation and onset of physiological sleep in mammals. Here we have analysed the expression of FAAH in rat brain to investigate a potential role in regulation of the oleamide content of the CSF. Adult Wistar rats were asphyxiated with CO2 and perfused through the heart with 50 ml of 4% paraformaldehyde in phosphate buffered saline (PBS; pH 7.4) as approved by the Queen Mary and West®eld College animal care and use committee and by the Home Of®ce (UK). The brains were removed and post-®xed in Bouin's ®xative before embedding in paraf®n wax. Sets of coronal sections (7 or 10 mm) were collected at 1 mm intervals through the brain and were mounted on glass slides. Dewaxed sections were blocked with 3% normal goat serum in PBS 1 0.2% Triton X-100 (PBST) and then incubated with FAAH antibodies. Two FAAH antibodies raised in rabbits were tested (Fig. 1): (1) af®nity-puri®ed antibodies to a protein comprising amino-acids 38±579 of rat FAAH were tested at a dilution of 1:50 in PBST, as described previously [6] and (2) partially puri®ed antibodies to a peptide comprising aminoacids 328±343 of rat FAAH were tested at a dilution 1:1000 in PBST. The speci®city of immunostaining with FAAH 0304-3940/00/$ - see front matter q 2000 Elsevier Science Ireland Ltd. All rights reserved. PII: S03 04 - 394 0( 0 0) 00 84 1- 7 14 M. Egertova et al. / Neuroscience Letters 282 (2000) 13±16 Fig. 1. Diagram of rat FAAH showing regions of the protein to which the antibodies used in this study were raised. The position of the putative transmembrane domain (TMD) of the protein is also shown. 328±343 antibodies was investigated by pre-absorption of the antibody solution with peptide antigen (20 mm). Bound antibodies were revealed using the ABC peroxidase method (Vector Labs, Burlingham, CA). Images of immunostained sections were captured using a Hamamatsu Digital Camera linked to a Leica DMRD microscope. HiPic 32 (High Performance Image Control System) software was used to import images from the camera and then these were incorporated into a composite ®gure and labelled using Adobe Photoshop 4.0 (Adobe Systems, Inc., Mountain View, CA) with reference to Paxinos and Watson [10]. With both FAAH antibodies tested, FAAH immunoreactivity was localised in the somata of identi®ed neurons in several regions of the brain including cerebellar Purkinje cells, hippocampal pyramidal cells, neocortical pyramidal cells and mitral cells of the olfactory bulbs, as reported previously [6,12]. In addition, FAAH-immunoreactive neurons were also evident in the anterior olfactory nuclei, piriform cortex, amygdala and pontine nuclei (not shown). These observations are consistent with previous analyses of FAAH mRNA and FAAH protein expression in rat brain [11,12]. Importantly, however, FAAH-immunoreactivity was also detected in the choroid plexus which is formed by invaginations of the pia mater into the ventricle cavities of the brain. Here FAAH-immunoreactivity was speci®cally located within cells of the choroidal epithelium but not in the highly vascularised connective tissue that forms the core of the choroid plexus (Fig. 2). As with neuronal expression of FAAH, immunostaining in the choroid plexus was observed with both the FAAH 38±579 antibodies (Fig. 2A±C) and the FAAH 328±343 antibodies (Fig. 2D,E). Moreover, immunostaining with the FAAH 328±343 antibodies was abolished when the antibody solution was preincubated with peptide antigen (Fig. 2F). The pattern of immunostaining observed in the epithelial cells of the choroid plexus with both FAAH antibodies tested was indicative of a cytoplasmic location with no immunostaining evident in nuclei. This is consistent with previous observations of FAAH expression in rat brain neurons and in COS-7 cells transfected with rat FAAH mRNA [6,7]. The detection of FAAH-immunoreactivity in epithelial cells of the choroid plexus is of particular interest because these cells are involved in regulating the composition of the CSF [4]. Underlying the epithelial cell layer of the choroid plexus is a capillary complex which is thought to produce a simple ®ltrate of blood plasma which is then absorbed by the epithelial cells [4]. After further modi®cation of the composition of the absorbed ¯uid, it is then secreted by the epithelial cells into the ventricular cavity [4]. Thus, FAAH is located in cells whose principle role is to regulate the production and composition of the CSF. Therefore, the role of FAAH in epithelial cells of the choroid plexus may be to control the concentration of oleamide and related lipid signalling molecules in the CSF. As such FAAH could exert an important regulatory role in shaping the duration and magnitude of the sleep-inducing effect of endogenously or exogenously derived oleamide. At present it is not known how or where oleamide is synthesised in mammals. Nor is it known how oleamide exerts its powerful sleep-inducing action [1]. Analysis of the effects of oleamide at the cellular level, however, point to a number of potential molecular targets including 5-hydroxytryptamine (5-HT) receptors. For example, oleamide causes potentiation of 5-HT2C and 5-HT2A receptormediated chloride currents in transfected frog oocytes [8]. Interestingly, 5-HT receptors are expressed by choroidal epithelial cells and 5-HT causes a reduction in the secretion of CSF [4]. Therefore the concentration of oleamide in the CSF may in¯uence both the rate of formation and the composition of the CSF. Clearly, further studies are required to investigate these issues. Nevertheless, the detection of FAAH immunoreactivity in the epithelial cells of the choroid plexus provides the ®rst evidence of how the oleamide content of the CSF may be regulated physiologically. Also of relevance here is the compound 2-octyl gbromoacetoacetate which was originally isolated from human CSF as a sleep-inducing hormone [13]. This molecule is structurally related to oleamide suggesting that it may also interact with FAAH. This idea has been examined and indeed 2-octyl g-bromoacetoacetate is a potent but reversible inhibitor of FAAH [9]. If 2-octyl g-bromoacetoacetate is a natural constituent of the CSF, it may in¯uence FAAH activity in epithelial cells of the choroid plexus. Therefore, the sleep-inducing properties of 2-octyl gbromoacetoacetate may be mediated indirectly through inhibition of FAAH activity in epithelial cells of the choroid plexus and a consequent elevation of oleamide in the CSF. This work was supported by grants to M.R.E. from the Leverhulme Trust (F476U) and the Wellcome Trust (057058). We are particularly grateful to Professor John Priestley's research group (Biomedical Sciences, QMW) for use of the digital photomicroscope and for advice in M. Egertova et al. / Neuroscience Letters 282 (2000) 13±16 15 Fig. 2. FAAH-immunoreactivity in the choroid plexus with FAAH 38±579 antibodies (A±C) and FAAH 328±343 antibodies (D,E). (A) Low magni®cation micrograph showing immunoreactivity in the choroid plexus (CPl) of the dorsal third ventricle (D3V). (B) Detail of immunoreactivity in choroidal epithelial cells (arrow) of the dorsal third ventricle. Note the absence of staining in the vascularised core of the choroid plexus (*). (C) Immunoreactivity in the choroid plexus of the lateral ventricle. (D) Immunoreactivity in the choroid plexus of the fourth ventricle (4V). (E) Detail of immunoreactivity in choroidal epithelial cells (arrow) of the fourth ventricle. Note the absence of staining in the vascularised core of the choroid plexus (*). (F) Pre-incubation of FAAH 328±343 antibodies with peptide antigen abolishes immunostaining in a section adjacent to that shown in (E). Scale bars: (A) 100 mm; (B,E,F) 25 mm; (C) 25 mm; (D) 50 mm. the use of Adobe Photoshop software. Many thanks also to Richard Melarange for technical assistance. [1] Basile, A.S., Hanus, L. and Mendelson, W.B., Characteriza- tion of the hypnotic properties of oleamide. NeuroReport, 10 (1999) 947±951. [2] Cravatt, B.F., Prospero-Garcia, O., Siuzdak, G., Gilula, N.B., Henriksen, S.J., Boger, D.L. and Lerner, R.A., Chemical characterization of a family of brain lipids that induce sleep. Science, 268 (1995) 1506±1509. 16 M. Egertova et al. / Neuroscience Letters 282 (2000) 13±16 [3] Cravatt, B.F., Giang, D.K., May®eld, S.P., Boger, D.L., Lerner, R.A. and Gilula, N.B., Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature, 384 (1996) 83±87. [4] Davson, H. and Segal, M.B., Physiology of the CSF and Blood±Brain Barriers, CRC Press, Boca Raton, 1996, 822 pp. [5] Devane, W.A., Hanus, L., Breuer, A., Pertwee, R.G., Stevenson, L.A., Grif®n, G., Gibson, D., Mandelbaum, D., Etinger, A. and Mechoulam, R., Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science, 258 (1992) 1946±1949. [6] EgertovaÂ, M., Giang, D.K., Cravatt, B.F. and Elphick, M.R., A new perspective on cannabinoid signalling: complementary localization of fatty acid amide hydrolase and the CB1 receptor in rat brain. Proc. R. Soc. Lond. B, 265 (1998) 2081± 2085. [7] Giang, D.K. and Cravatt, B.F., Molecular characterization of human and mouse fatty acid amide hydrolases. Proc. Natl. Acad. Sci. USA, 94 (1997) 2238±2242. [8] Huidobro-Toro, J.P. and Harris, R.A., Brain lipids that induce sleep are novel modulators of 5-hydroxytrypt- [9] [10] [11] [12] [13] amine receptors. Proc. Natl. Acad. Sci. USA, 93 (1996) 8078±8082. Patricelli, M.P., Patterson, J.E., Boger, D.L. and Cravatt, B.F., An endogenous sleep-inducing compound is a novel competitive inhibitor of fatty acid amide hydrolase. Bioorg. Med. Chem. Lett., 8 (1998) 613±618. Paxinos, G. and Watson, C., The Rat Brain in Stereotaxic Coordinates, 2nd edn., Academic Press, London, 1986, 145 pp. Thomas, E.A., Cravatt, B.F., Danielson, P.E., Gilula, N.B. and Sutcliffe, J.G., Fatty acid amide hydrolase, the degradative enzyme for anandamide and oleamide, has selective distribution in neurons within the rat central nervous system. J. Neurosci. Res., 50 (1997) 1047±1052. Tsou, K., Nogueron, M.I., Muthian, S., SanÄudo-PenÄa, M.C., Hillard, C.J., Deutsch, D.G. and Walker, J.M., Fatty acid amide hydrolase is located preferentially in large neurons in the rat central nervous system as revealed by immunohistochemistry. Neurosci. Lett., 254 (1998) 137±140. Yanagisawa, I. and Yoshikawa, H.A., A bromine compound isolated from human cerebrospinal ¯uid. Biochim. Biophys. Acta, 329 (1973) 283±294.