* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Use of rabies virus as a transneuronal tracer of neuronal
Caridoid escape reaction wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Nervous system network models wikipedia , lookup
Neuromuscular junction wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Synaptic gating wikipedia , lookup
Axon guidance wikipedia , lookup
Neuroscience in space wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Optogenetics wikipedia , lookup
Central pattern generator wikipedia , lookup
Development of the nervous system wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Chemical synapse wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Circumventricular organs wikipedia , lookup
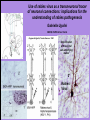
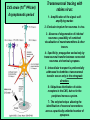
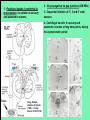
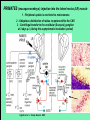
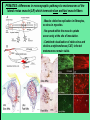
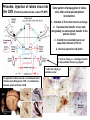
Use of rabies virus as a transneuronal tracer of neuronal connections: implications for the understanding of rabies pathogenesis Gabriella Ugolini NBCM, CNRS Gif-sur-Yvette Kuypers & Ugolini, Trends Neurosci. 1990 Amplification of the signal: self-amplifying marker 3° Rabies Virus CVS strain (1010 PFU/ml) Asymptomatic period Transneuronal tracing with rabies virus: 1 - Amplification of the signal: selfamplifying marker. 2 - Exclusive tropism for neurones in vivo. 3 - Absence of degeneration of infected neurones: possibility of combined visualisation of neurotransmitters & other tracers. 4 - Specificity: propagation exclusively by transneuronal transfer between connected neurones at chemical synapses. 5 - Intracellular transport is preferentially addressed to dendrites: transneuronal transfer occurs only in the retrograde direction. 6 - Ubiquitous distribution of rabies receptors in the CNS, but not in the peripheral nervous system. 7 - The only technique allowing the identification of neuronal connections across a practically unlimited number of synapses. 1 - Peripheral uptake is restricted to motoneurons: no uptake via sensory and autonomic neurons 2 - No propagation via gap junctions (DM MNs) 3 - Sequential infection of 2°, 3 and 4° order neurons 4 - Centrifugal transfer to sensory and autonomic neurons at long time points, during the asymptomatic period RAT Tang, Rampin, Giuliano & Ugolini (1999) J. Comp. Neurol. 414:167-192. PRIMATES (macaque monkeys): injection into the lateral rectus (LR) muscle 1 - Peripheral uptake is restricted to motoneurons 2 - Ubiquitous distribution of rabies receptors within the CNS 3 - Centrifugal transfer to the vestibular (Scarpa’s) ganglion at 3 days p.i. (during the asymptomatic incubation period) Ugolini et al. J. Comp. Neurol. 2006 PRIMATES: differences in monosynaptic pathways to motoneurons of the lateral rectus muscle (LR) which innervate slow and fast muscle fibers - Muscle: defective replication in fibrocytes, no virus in myocites. - No spread within the muscle: uptake occurs only at the site of inoculation. - Combined visualisation of rabies virus and choline acetyltransferase (CAT): infected motoneurons remain viable. Primates: Injection of rabies virus into the CNS (Posterior parietal cortex, areas VIP, MIP) Same pattern of propagation of rabies virus after central and peripheral inoculations: 1 - Infection of first-order neurons at 2 days. 2 - Transneuronal transfer occurs only retrogradely (no anterograde transfer to the pontine nuclei). 3 - Transfer to connected neurons at sequential intervals of 12 hrs. 4 - No local spread or cell death. 3° order at 3 days p.i.: centrifugal transfer to the vestibular (Scarpa’s) ganglion 2° order at 2.5 days p.i.: vestibular nuclei Co-injection of rabies virus & a conventional tracer (Cholera toxin B fragment, CTB) = no interference between uptake of rabies & CTB 3° Scarpa’s ganglion Rabies pathogenesis: 1. In primates & rodents (rats, guinea pigs), peripheral uptake is restricted to motor endplates/axons: in keeping with the presynaptic location of NCAM and with a role of Ach nicotinic receptors (despite their mainly post-synaptic location). 2. Rabies virus does not spread within the muscle: uptake occurs only at the site of inoculation = importance of complete wound infiltration with rabies antibodies as soon as possible, to prevent virus entry! 3. Motoneurons are the only gateway for propagation of rabies virus to the CNS. 4. Rabies virus propagates exclusively by retrograde transneuronal transfer at chemical synapses - not via gap junctions or local spread. 5. Transneuronal transfer occurs only retrogradely – due to the fact that, after replication, centrifugal intracellular transport of rabies targets only dendrites, and not axons. 6. Retrograde transport and transneuronal transfer occur at high speed, by active axonal transport (P/LC8 interactions and microtubulebased transport) 7. Ubiquitous distribution of rabies receptor(s) within the CNS: transneuronal transfer involves all known populations connected to first-order neurons, regardless of their transmitters. 8. Extensive propagation of rabies virus within the CNS during the asymptomatic period! Each successive step of transfer (to 2°, 3°, 4° order neurons) requires only 12 hrs, regardless of the distance. 9. Infection of sensory and autonomic neurons requires longer incubation times because it reflects centrifugal propagation from the CNS to the periphery. Acknowledgments Supported by: EU grants BIO4-CT98-0546 (TransVirus) & QLK6-CT-2002-0015 (EUROKINESIS)