* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Endoderm development in vertebrates: fate mapping
Genome (book) wikipedia , lookup
Long non-coding RNA wikipedia , lookup
X-inactivation wikipedia , lookup
Gene expression programming wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Genomic imprinting wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Epigenetics in stem-cell differentiation wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Gene expression profiling wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Designer baby wikipedia , lookup
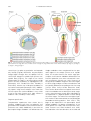
Develop. Growth Differ. (2005) 47, 343– 355 Review Blackwell Publishing, Ltd. Endoderm development in vertebrates: fate mapping, induction and regional specification Kimiko Fukuda1 and Yutaka Kikuchi2,* 1 Department of Biological Sciences, Tokyo Metropolitan University, 1-1 Minamiohsawa, Hachioji, Tokyo 192-0397, Japan and 2Division of Biological Science, Graduate School of Science, Nagoya University, Furo-cho, Chikusa-ku, Nagoya 464-8602, Japan The formation of the vertebrate body plan begins with the differentiation of cells into three germ layers: ectoderm, mesoderm and endoderm. Cells in the endoderm give rise to the epithelial lining of the digestive tract, associated glands and respiratory system. One of the fundamental problems in developmental biology is to elucidate how these three primary germ layers are established from the homologous population of cells in the early blastomere. To address this question, ectoderm and mesoderm development have been extensively analyzed, but study of endoderm development has only begun relatively recently. In this review, we focus on the ‘where’, ‘when’ and ‘how’ of endoderm development in four vertebrate model organisms: the zebrafish, Xenopus, chick and mouse. We discuss the classical fate mapping of the endoderm and the more recent progress in characterizing its induction, segregation and regional specification. Key words: endoderm, fate mapping, induction, regional specification. Introduction Gastrulating vertebrate embryos generate the three germ layers, known as ectoderm, mesoderm and endoderm, during early development. The induction and differentiation of endodermal cells and the formation of organs derived from the gut tube have been poorly analyzed in comparison to ectoderm or mesoderm. However, in the past few years, zebrafish mutants and knockout mice which have disrupted endoderm formation, and the molecular functions of Xenopus genes involved in endoderm formation, have been extensively analyzed. As a result of these studies, our understanding of endoderm development is now fairly advanced in early vertebrate embryos. In particular, these genetic and molecular biological approaches have led to a more detailed understanding of the molecular regulation of the endoderm. In spite of these efforts, however, many mechanisms, such as the fate choice of endodermal cells, the migration of endodermal cells during gastrulation and the regional specification of the endoderm along the anterior–posterior axis, which are essential to fully understanding its differentiation, are not well understood. Here, we attempt to summarize both classical and more recent findings in the study of endoderm development including fate mapping, the molecular pathways involved in its generation leading to the endoderm, its segregation from the ectoderm and mesoderm and its regional specification. Fate maps and the timing of endoderm commitment To analyze ‘how’ the endoderm differentiates, it is essential to know ‘where’ the endoderm is derived and ‘when’ differentiation begins. There have been a number of reports describing fate maps of the early vertebrate embryo that show the origin of the endoderm in various species and in various ways. In addition, by using mainly implantation techniques, many of these studies have revealed the timing of endoderm fate. Xenopus *Author to whom all correspondence should be addressed. Email: [email protected] Received 15 June 2005; accepted 16 June 2005. Many fate maps have been compiled for amphibian embryos over a number of years. Vogt (1929) generated 344 K. Fukuda and Y. Kikuchi a famous fate map of the uropod embryo using vital dyes at the early gastrulation stage. Subsequently, a fate map of the Xenopus embryo at the gastrula stage using a vital dye was described by Keller (1975; 1976). More recently, many researchers have constructed amphibian fate maps using fluorescent dyes (Dale & Slack 1987; Chalmers & Slack 2000). In the amphibian embryo, there is quite a high degree of topographic projection from the very early stages. However, there is also an element of indeterminacy, as endoderm fate is segregated from both the mesodermal and ectodermal fate at a relatively early stage compared to other vertebrates. At the 32-cell stage, the vegetal-most blastomeres (Fig. 1D1– 4 Xenopus) have been shown to contribute to the endoderm (Nakamura & Kishiyama 1971; Nakamura et al. 1978; Dale & Slack 1987) and significant endodermal contributions have also been observed from the dorsal marginal blastomeres (Fig. 1C1–2 Xenopus) (Nakamura et al. 1978; Dale & Slack 1987). Moreover, vegetal blastomeres contribute specifically to endoderm, whereas dorsal marginal blastomeres also contribute to dorsal mesoderm, including the notochord and somites. These observations indicate that whilst there is a general tendency for vegetal blastomeres to differentiate into endoderm, the mesodermal and endoderm fates do overlap partially and fate determination is not complete at the 32-cell stage. Another study of the vegetal pole stage 6 cells (the morula) of Xenopus laevis has shown that they contribute progeny to all three germ layers (Heasman et al. 1984). However, by the midblastula stage (stage 8), the cells are smaller and more confined to the vegetal pole and are observed to contribute exclusively to the endoderm. At early gastrula (stage 10), the prospective lining of the archenteron (the prospective endoderm), the prospective neural area and the prospective epidermal area are represented on the surface, whereas the prospective mesoderm is in the deep layer of the marginal zone (Fig. 1 Xenopus) (Nieuwkoop & Florshtz 1950; Keller 1975; Keller 1976). A thin superficial layer around the vegetal pole, extending relatively further upward from the blastopore pigment line and covering the notochordal, somatic, and lateral plate mesoderm, also contributes to the endoderm (Fig. 1 Xenopus). These superficial cells invaginate as a continuous layer to form the lining of the archenteron during gastrulation (Nieuwkoop & Florshtz 1950; Keller 1975). At the onset of gastrulation, prospective pharyngeal endoderm leads to the involution of the mesoderm through the blastopore (Keller 1976). However, cells in the deep layer of the vegetal pole don’t contribute to the archenteron roof or floor at the gastrula or neurula but will become intestinal endoderm in tadpoles after the elongation of the gut (Chalmers & Slack 2000). These findings reveal that in Xenopus, endoderm fate is segregated prior to gastrulation. After fate mapping was established, some reports investigated whether the restriction of cell fates at midblastula reflected cell determination by implanting labeled vegetal pole cells into the blastocoels of the host embryos and analyzing the tissues, including the labeled progeny. When a single vegetal pole cell from the morula (stage 6) and midblastula was isolated and transplanted into the blastocoel of the late blastula, their progeny were found in all three germ layers (Heasman et al. 1984). When vegetal pole cells from early gastrula were assayed in this way, however, their progeny contributed only to gut endoderm (Heasman et al. 1984). These results suggest that vegetal pole cells became committed by the beginning of gastrulation and further analysis of their progenies in more detail showed that this Fig. 1. Comparison of endoderm fate maps of vertebrates. Presumptive endoderm cells develop from common progenitor cells of both the mesoderm and endoderm in zebrafish, chicken and mouse embryos. In Xenopus embryos, some endodermal cells arise from marginal cells which give rise to both endoderm and mesoderm. Endoderm cells are located very close to the node or blastopore (*) at the onset of gastrulation and invaginate into the deep layer. Endoderm development in vertebrates commitment was gradual (Wylie et al. 1987). In addition, single vegetal cells cultured with other vegetal cells became more committed than single cells alone or single cells cultured with other cell types (Wylie et al. 1987). It seems therefore that endoderm commitment is a cell autonomous process, but that cell–cell interaction among vegetal pole cells also contributes to this pathway. Zebrafish Zebrafish fate map studies began in the 1990s and these fate maps showed that both endoderm and mesoderm also originate from a common progenitor, but more extensively than in Xenopus. Both germ layers derive from cells near the blastoderm margin (Kimmel et al. 1990) and both involute into the forming hypoblast (Warga & Kimmel 1990). In 40% epyboly, just prior to gastrulation, endoderm progenitor cells are located in a narrow field of cells along the margin (Fig. 1 zebrafish) (Kimmel et al. 1990; Warga & Nüsslein-Volhard 1999). Furthermore, there is asymmetric distribution of the endoderm progenitor in the margin, and more endoderm progenitor is found dorsally than ventrally (Warga & NüssleinVolhard 1999). In addition, most endoderm progenitors are located within a 2-cell diameter of the blastoderm margin and no progenitors are found more than a 4cell diameter from this margin (Warga & NüssleinVolhard 1999). At the onset of gastrulation, the majority of the endoderm progenitor cells are the earliest deep involuting cells from the blastoderm margin in the newly formed hypoblast (Warga & NüssleinVolhard 1999). Hence, there are mere endoderm progenitors at the marginal zone of the shield stage (gastrulation stage) embryo (Melby et al. 1996; Warga & Nüsslein-Volhard 1999). The fate commitment of the endoderm also occurs just after the onset of gastrulation (Ho & Kimmel 1993; David & Rosa 2001). When marginal cells from late blastulae (30 –40% epiboly) are transplanted into animal blastomeres, they contribute mostly to neuroectodermal tissue, consistent with the fate maps of animal blastomeres, and only a small proportion of transplanted cells contribute to the endoderm (David & Rosa 2001). This contribution to the endoderm dramatically increases, however, when marginal cells from embryos are transplanted at the onset of the gastrulation (50% epiboly) (David & Rosa 2001). Chick Among the higher vertebrates, fate mapping has been most extensively characterized in the chick 345 embryo (Bellairs 1953; Bellairs 1957; Rosenquist 1966; Rosenquist 1971; Fontaine & Le Douarin 1977; Kirby et al. 2003; Lawson & Schoenwolf 2003). In amniotes such as chick, all of the embryonic tissues arise from the epiblast in the early embryo. HNK-1 antibodypositive cells, which are common progenitors of both endoderm and mesoderm (Stern & Canning 1990), are randomly distributed within the epiblast before streak formation occurs (stage XII–XIII) (Canning & Stern 1988). At stage XII−2 (early primitive streak stage), these stained cells are distributed in the more posterior and medial regions. Finally, the primitive streak contains more HNK-positive cells than more remote regions. The chick embryo fate maps have revealed that endoderm progenitors are distributed at the posterior region and form a winged shape, hinged at the posterior end of the midline at stage X (early blastula). This region also gives rise to mesoderm, especially notochord (Hatada & Stern 1994). This prospective endoderm region moves towards the midline and progresses anteriorly during the subsequent stages (Selleck & Stern 1991; Hatada & Stern 1994). After the formation of the primitive streak, endoderm progenitors are located in the deep layer of the anterior primitive streak and in the Hensen’s node (Fig. 1, chick) (Selleck & Stern 1991; Garcia-Martinez & Schoenwolf 1993). These endoderm progenitors are also mesodermal progenitors and disappear from the node immediately following the onset of gastrulation (stage 4) (Selleck & Stern 1991). During gastrulation, endoderm cells invaginate into the lower layer (ventral-most layer) either directly or indirectly after lateral migration in the middle layer (Kimura et al. unpubl. data). When endoderm progenitors in the anterior primitive streak are transplanted into the posterior streak, which contains only mesodermal progenitors, almost all of the grafted cells contribute to the lateral plate mesoderm. After the onset of gastrulation (stage 4), when cells in the middle layer lateral to the Hensen’s node are grafted into the middle layer lateral to the posterior primitive streak, where cells normally contribute to the mesoderm, almost all of the transplanted cells contribute to endoderm. These results show that the fate determination of endoderm cells occurs during gastrulation (Kimura et al. unpubl. data). Mouse In the mouse embryo, the distribution of endoderm progenitor cells is essentially the same as in the chick embryo. In the epiblast, which is the sole source of all embryonic tissue, presumptive endoderm becomes available in the posterior region of 346 K. Fukuda and Y. Kikuchi the prestreak embryo. This region, including the region of presumptive head processes, partially overlaps with the presumptive mesodermal region. After streak formation, the presumptive endoderm area extends anteriorly and this anterior area continues to overlap with the presumptive head process/notochord (Fig. 1, mouse) (Lawson et al. 1986; Lawson & Pedersen 1987). In summary, vertebrate endoderm cells arise from common progenitor cells of both endoderm and mesoderm, at which time fate segregation occurs, following endoderm fate determination. At gastrulation, at least a portion of the endoderm progenitor cells involute from the surface to a deep layer in front of the mesodermal cells. Molecular pathways leading to endoderm induction Zebrafish Our understanding of the molecular mechanisms underlying endoderm induction in the zebrafish originates from the genetic and molecular biological studies of five mutants: one-eyed-pinhead (oep), bonnie and clyde (bon), faust (fau), casanova (cas) and spielohen-grenzen (spg) (Zhang et al. 1998b; Kikuchi et al. 2000; Dickmeis et al. 2001; Kikuchi et al. 2001; Reiter et al. 2001; Sakaguchi et al. 2001; Lunde et al. 2004; Reim et al. 2004). oep encodes a member of the EGF-CFC family of proteins and its protein product acts as cofactor of Nodal (a TGF-β super family protein). The maternal-zygotic oep (MZoep) mutant, which lacks both maternal and zygotic Oep, fails to form any endoderm cells and most mesoderm cells (Zhang et al. 1998b; Gritsman et al. 1999). In the zebrafish blastula embryo, two Nodal genes, cyclops (cyc) and squint (sqt), are expressed throughout the entire marginal domain and the double mutant (cyc; sqt ) of these genes is very similar to the MZoep mutant, in that it is entirely deficient of endoderm cells (Feldman et al. 1998). These results implicate Nodal as an essential signaling molecule for endoderm induction in the zebrafish embryo. bon and fau encode a Mix-type homeodomain transcription factor and the transcription factor Gata5, respectively. bon is expressed in both the mesendoderm and mesoderm domains where the panmesoderm marker no tail (ntl; homologue of Xenopus brachyury) is also expressed. In contrast, fau/gata5 expression is restricted to the mesendoderm domain along the animal–vegetal axis in late blastula and early gastrula embryos (Alexander et al. 1999). Both bon and fau/gata5 expression is completely lost in MZoep and cyc; sqt double mutants, demonstrating that their expression is regulated by Nodal signaling (Alexander & Stainier 1999; Rodaway et al. 1999; Reiter et al. 2001). bon and fau/gata5 mutants contain approximately 10% and approximately 60% of the normal number of endoderm cells, respectively, as assessed by the early endoderm marker genes sox17, a high-mobility-group (HMG) transcription factor gene (Alexander & Stainier 1999) and foxA2, a winged helix/forkhead transcription factor gene (formerly known as axial (Strähle et al. 1993)) (Kikuchi et al. 2000; Reiter et al. 2001). These genetic findings indicate that both factors are crucial downstream effectors of Nodal signaling during endoderm induction in the zebrafish. Although the endoderm phenotype in bon or fau/ gata5 disrupted embryos is severe, endoderm cells still remain in bon or fau/gata5 single and double mutants (Kikuchi et al. 2000; Reiter et al. 2001). The partial endoderm induction phenotype in these mutants is likely to be a reflection of the redundant activity of other genes such as Mezzo, which is another Mixtype transcription factor whose expression domain and stage profile overlaps with Bon. In addition, both gain-of-function and loss-of-function experiments using antisense morpholino oligonucleotide (MO) further suggest that Mezzo is a redundant factor of Bon (Poulain & Lepage 2002). In fact, based on overexpression experiments and gene expression analyses using mutant embryos, Bon, Fau/Gata5 and Mezzo are in almost parallel position with each other (Reiter et al. 2001; Poulain & Lepage 2002)(Fig. 2). The cas mutant completely lacks all endodermal cells and organs derived from gut tube. However, mesoderm induction appears to be normal in cas mutants (Alexander et al. 1999). cas encodes a Sox-type transcription factor, and its expression is restricted to endodermal progenitors in the marginal region in the late blastula embryo (Dickmeis et al. 2001; Kikuchi et al. 2001; Sakaguchi et al. 2001). The expression of cas around the marginal domain is absent in MZoep and cyc; sqt mutants and the number of cas-expressing cells is reduced in bon and fau/gata5 mutants, suggesting that cas is a downstream target of Nodal, Bon and Fau/Gata5 (Kikuchi et al. 2001). Cas can also transform a mesodermal fate to an endodermal fate when it is overexpressed, whereas the fate of ectodermal cells in the animal pole region cannot be changed to an endodermal fate by this overexpression (Kikuchi et al. 2001). These data indicate that cas is an essential gene, but not master gene, for endoderm induction and additional factors are necessary for converting ectodermal into endodermal fates. Endoderm development in vertebrates 347 Fig. 2. Molecular pathway leading to endoderm in the zebrafish and Xenopus. In the zebrafish, all hierarchical cascades are based on genetic studies. In contrast, most of the regulatory pathways in Xenopus have been elucidated using animal cap assays and knockdown experiments. (1) Maternal factor (X) upstream of Nodal (Cyc, Sqt) is unknown. (2) squint is a maternal transcript. (3) Tar; Taram-a, type-I TGFβ receptor (Renucci et al. 1996) (4) Xnrs; Xnr1, Xnr2, Xnr4, Xnr5 and Xnr6 (5) Mix-type homeobox proteins; Mixer, Mix.1, Mix.2, Bix.1, Bix.2 (Milk), Bix.3 and Bix.4. Recently, the spg mutant, that was originally identified as a brain mutant, was also shown to have defects in endoderm induction (Lunde et al. 2004; Reim et al. 2004). spg, encoding the transcription factor Pou2/Oct4, is a maternal gene and zygotic spg is expressed ubiquitously prior to gastrulation (Belting et al. 2001; Burgess et al. 2002). To elucidate the function of Spg in endoderm induction, maternal-zygotic spg mutants (MZspg) were generated by injection of spg mRNA into homozygous eggs. In MZspg, expression of the early endoderm markers, sox17 and foxA2, is never initiated and although induction of cas mRNA is detected at the blastula stage, cas expression is not maintained during the gastrula stage (Lunde et al. 2004; Reim et al. 2004). Thus, spg is necessary for the induction of sox17 and foxA2, and for the maintenance of cas expression. Additionally, sox17 promoter–luciferase reporter assays have shown that Cas and Spg synergistically regulate sox17 expression to regulate endoderm induction (Reim et al. 2004). In summary, Figure 2 illustrates the molecular regulatory cascade leading to endoderm induction in the zebrafish. Whereas Gata4, Gata5 and Gata6 are thought to be involved in heart and endoderm development in various model organisms (Charron & Nemer 1999; Molkentin 2000), zebrafish Gata4 and Gata6 function in endoderm induction is not well understood. The maternal factor (X in Fig. 2) upstream of Nodal has not yet been identified and although foxA1 and foxA3 are known to be expressed in endodermal cells and act downstream of Cas, the position of these factors in the regulatory cascade remains unclear. Analyses of five zebrafish mutants have revealed many aspects of the molecular cascade downstream of Nodal signaling. It will be interesting to investigate whether signaling other than Nodal regulates endoderm induction or if other signaling pathways cross-talk with Nodal signaling. Xenopus The Xenopus endoderm originates from the vegetal region, where the endodermal cells are intermingled with yolk cells, in blastula stage embryos. In the Xenopus embryo, the maternal transcript VegT, encoding a T-box transcription factor, is localized in the vegetal hemisphere of the egg and early embryo, and the zygotic VegT transcript is restricted to the equatorial region, where mesoderm cells are formed (Lustig et al. 1996; Stennard et al. 1996; Zhang & King 1996; Horb & Thomsen 1997). Endoderm is never induced, however, and mesoderm is mainly formed from the vegetal region, in VegT-depleted embryos (Zhang et al. 1998a). Moreover, VegT overexpression can ectopically induce the expression of some early endoderm maker genes, indicating that maternal VegT is necessary for endoderm formation and mesoderm patterning in the Xenopus embryo (Zhang et al. 1998a). A recent study using animal cap assays has reported that another maternal factor, Sox7, is also a crucial regulator of endoderm induction and that the ability of VegT to induce endoderm genes, except for sox17 and Xenopus Nodal-related genes (Xnrs; Xnr1, Xnr2 348 K. Fukuda and Y. Kikuchi and Xnr4), appeared to depend upon Sox7 activity (Zhang et al. 2005). Five Nodal-related genes (Xnr1, Xnr2, Xnr4, Xnr5 and Xnr6) are reported to be involved in mesendoderm induction in Xenopus (reviewed by Schier 2003). The Xnr5 and Xnr6 in the vegetal hemisphere are induced by VegT in a cell autonomous manner and are inducers of Xnr1, Xnr2 and Xnr4 (Takahashi et al. 2000). The cleavage mutant, Xnr2, suppresses some endoderm marker expression (Osada & Wright 1999). Additionally, the Nodal-related genes Xnr1, Xnr2 and Xnr4 and the Veg1-like TGF-β family member derrière can restore endoderm gene expression in VegT-depleted embryos (Xanthos et al. 2001). Furthermore, the ability of Sox7 to induce endodermal genes is inhibited by Cerberus, which is an antagonist of Nodal (Zhang et al. 2005). These data demonstrate that Nodal-related genes function downstream of Sox7 and VegT and that the Xnrs play important roles in endoderm induction. However, the function of individual Xnrs in endoderm induction remains to be elucidated. The seven Mix-type homeodomain factors (Mixer, Mix.1, Mix.2, Bix1, Bix2/Milk, Bix3 and Bix4) and three Gata factors (Gata4, Gata5 and Gata6) function downstream of VegT and Nodal-related factors (Xanthos et al. 2001) and are thought to be involved in endoderm induction (Weber et al. 2000; Afouda et al. 2005). The Mix-type genes are expressed in the vegetal region and some are also expressed in the marginal domain which gives rise to mesoderm cells. When overexpressed in animal caps, these Mix-type genes exhibit different abilities to activate endoderm gene expression: Mix.1 can activate endoderm gene expression only when co-expressed with the homeobox gene siamois (Lemaire et al. 1998); Bix1 overexpression at low and high levels induces mesoderm and endoderm gene expression, respectively (Tada et al. 1998); Bix2/Milk appears to promote endoderm gene expression at the expense of mesoderm gene expression (Ecochard et al. 1998) and Bix4 can rescue endoderm, but not mesoderm, gene expression in VegT-depleted embryos (Casey et al. 1999). In addition, Mixer is a strong endoderm inducer when overexpressed (Henry & Melton 1998) and MOmediated knockdown data shows that Mixer plays an essential role in controlling the amount of mesoderm induction by the vegetal cells (Kofron et al. 2004). The three zygotic Gata genes (gata4, gata5 and gata6) initiate their expression in vegetal cells fated to form endoderm at the onset of gastrulation. The functional analyses of three these genes demonstrate that Gata5 and Gata6 function as activators of endo- derm genes such as sox17 and HNF1β, whereas the function of Gata4 to induce endoderm genes is dependent upon gata6 induction (Afouda et al. 2005). Additionally, Gata5 enhances the ability of Mixer to induce sox17α expression and acts upstream of Xhex and gata4 in cooperation with Mixer (Xanthos et al. 2001). In summary, Figure 2 illustrates the molecular regulatory cascade leading to endoderm formation in Xenopus embryos. The hierarchical relationship, however, between individual factors in this cascade such as the Mix-type homeobox proteins, the Xnrs and the Gata proteins is still not yet fully clear due to their functional redundancies. Mouse Our understanding of endoderm formation in vertebrates originates mainly from studies of the zebrafish and Xenopus. Although there are several differences between the molecular regulatory cascades in these species, as shown in Figure 2, it seems that the factors involved in endoderm induction and its associated regulatory pathways are conserved between these two vertebrates (Fig. 2). In addition to these species, recent studies have shown that analyses of knockout mice have also contributed to our understanding of endoderm development. In mouse, the hypomorphic allele of Nodal is associated with a complete lack of a definitive endoderm, indicating that Nodal is also an essential factor in the murine embryo, as has been observed in zebrafish and Xenopus (Lowe et al. 2001). Additionally, the Mixtype homeodomain protein, Mixl1, and the Sox-type HMG domain protein, Sox17, are also necessary for definitive endoderm formation in the mouse embryo, as is the case for the zebrafish and Xenopus (Hart et al. 2002; Kanai-Azuma et al. 2002). In contrast to these factors, it is not yet clear whether the Gata family of transcription factors (Gata4, Gata5 and Gata6) function in endoderm induction in the mouse (Molkentin 2000). The knockout mice for each individual Gata factor do not show any defects in endoderm induction, presumably because of the functional redundancy among family members. However, these Gata genes are expressed in extraembryonic tissues, heart and definitive endoderm and transfection of Gata genes into non-endodermal cells can induce specific endoderm gene expression such as IFABP, gastric H+/K+-ATPase and HNF4 (Maeda et al. 1996; Gao et al. 1998; Morrisey et al. 1998). These data suggest that Gata transcription factors are involved in endoderm differentiation in mouse. In addition, although the molecular regulatory pathways Endoderm development in vertebrates leading to endoderm induction in the mouse have not yet been analyzed, it is interesting to note that Nodal, Mix-type, Sox and Gata factors regulate endoderm development in three vertebrates: the zebrafish, Xenopus and mouse. Fate choice between endoderm and mesoderm/ectoderm In the zebrafish embryo, endodermal and mesodermal cells are intermingled along the margin of the blastoderm and in the Xenopus embryo, endodermal and mesodermal cells are generated from the C-tier domain. Furthermore, as observed for zebrafish and Xenopus embryos, mesodermal and definitive endodermal cells arise from the epiblast and migrate through the primitive streak in the mouse. It is unclear, however, how individual mesendoderm cells decide their own fate in vertebrates. On the other hand, specification between endodermal and secondary mesenchymal cells (SMC), which give rise to the majority of the mesodermal cells in the sea urchin embryo, is regulated by Notch signaling (Sherwood & McClay 1999). Notch signaling functions within the presumptive SMC and plays a crucial role in the differential specification of SMC and endoderm in the sea urchin embryo. In the zebrafish, endoderm progenitors arise in the marginal domain in a scattered, not clustered, arrangement, as assessed by cas expression (Kikuchi et al. 2004). Based on the endoderm induction pattern, it seems that Notch signaling may regulate the fate choice of endoderm and mesoderm in the zebrafish. notch1a, deltaC and deltaD are expressed in the marginal domain, which gives rise to cells of either the endoderm or mesoderm (Kikuchi et al. 2004). Moreover, the number of endoderm cells is reduced by the activation of Notch signaling, but is not increased by inhibition of Notch signaling (Kikuchi et al. 2004). These data suggest that an additional signaling mechanism other than Notch is necessary for the segregation of endoderm and mesoderm in the zebrafish, and that the segregation mechanisms in vertebrates are more complicated than in the sea urchin. Recent studies have also shown that maternal B1type Sox transcription factors, which are expressed in animal pole region, regulate germ layer formation in both Xenopus and zebrafish. In Xenopus embryos injected with antibodies against Sox3, the expression levels of endodermal and mesodermal markers are increased and the normal animal–vegetal patterning of mesoderm and endoderm is disrupted (Zhang et al. 2004). Maternal Sox3 can regulate Xnr5 expression and the reduction of Xnr5 generates an abnor- 349 mal gene expression profile and disrupted patterning of the mesendoderm (Zhang et al. 2004) (Fig. 2). These data demonstrate that the maternal B1-type Sox proteins are involved in the fate choices of ectoderm and mesendoderm via the regulation of Nodalrelated gene expression. Regionalization After the endoderm layer is established during gastrulation, it gradually becomes regionalized into anteroposteriorly and dorsolaterally divided organs. Compared with the study of early endoderm differentiation and late organogenesis, however, the molecular pathways that control this process remained largely unknown. Movement of the endoderm In Xenopus, the ventral and margin superficial layer of the blastula, which is presumptive endoderm, becomes invaginated to form the lining of the archenteron. This invagination begins first, and is at its strongest level, in a restricted area just dorsal to the blastopore which becomes an anterior-most lining (Fig. 3a,b). The suprablastopore region then becomes the archenteron roof, whereas the sub-blastopore region contributes to the archenteron floor (Fig. 3a–c) (Keller 1975). These archenteron cells lining both roof and floor eventually intermingle and contribute to the dorsal gut wall after cell arrangement (Chalmers & Slack 2000). On the other hand, cells in the vegetal pole and the deep layer of the vegetal hemisphere, contribute to the ventral cell layer of the gut wall (Chalmers & Slack 2000). In amniotes, the endoderm first forms a sheet structure which is situated in the most ventral layer of the embryo (Fig. 3d), then folds from the anterior and generates the foregut at the early somite stages (Fig. 3f,g). Later, the endodermal sheet also folds from the posterior to make the hindgut. These two folding events then join together to complete the formation of the gut tube. In the chick, each region of the endoderm along the antroposterior or dorsoventral axis appears as the most ventral layer at different times. At stage 2, the first population of the presumptive endoderm appears as a very limited region in the ventral layer under the rostral primitive streak (Rosenquist 1966; Rosenquist 1971; Rosenquist 1972). These cells contribute only to the mid/hindgut and until gastrulation, the presumptive mid/hindgut region expands laterally and caudally (Kimura et al. unpubl. data). The presumptive dorsal foregut region invaginates into the 350 K. Fukuda and Y. Kikuchi Fig. 3. Cell movement of the endoderm in Xenopus and chicken embryo. (a–c) Xenopus embryo. (a) lateral view of stage 10+. (b) ventral view of stage 10+ and c; medial section of stage 18. (d–g) Ventral view of the chicken embryo. (d) stage 4, (e) stage 5, (f) stage 7, and (g) stage 11. ventral layer just prior to gastrulation and expands only laterally (Fig. 3d). Meanwhile, the presumptive foregut region emerges from the epiblast into the ventral layer through the middle layer (Kimura et al. unpubl. data) (Fig. 3e). The shape of the ventral foregut region at stage 5 is arch-like and the outside part of the arch contributes to hepatogenetic cells (Rosenquist 1971; Fukuda-Taira 1981; Tremblay & Zaret 2005), whereas the inside part contributes to the trachea and thyroid (Rosenquist 1971). Additionally, reports using mouse embryos have shown that the fate map of the murine endoderm is very similar to that of the chick (Lawson et al. 1986; Lawson & Pedersen 1987; Tremblay & Zaret 2005). Regional specification Transplantation experiments have shown that in Xenopus, endoderm cells have no regionality until gastrulation when endoderm fate commitment occurs (Heasman et al. 1984). Additionally, in the chick the presumptive foregut endoderm can differentiate into hindgut endoderm when transplanted into the posterior region of the embryo (Kimura et al. unpubl. data). On the other hand, at the somite stage, presumptive small intestine endoderm differentiates into intestinal epithelium even when cultured with stomach mesenchyme (Yasugi et al. 1991; Hiramatsu & Yasugi 2004). Endoderm fragments from each region of somite stage embryos can be cultured alone and can partially differentiate according to their fate map (Sumiya 1976a; Sumiya 1976b; Matsushita 1999). These results demonstrate that regional commitment within the endoderm occurs during the somite stage. In fact, there have been several genes identified that are expressed in a regional-specific manner during the somite stages. The earliest of these regional specific markers to be characterized are the paired-type homeobox-containing proteins, Pax1 and 9. They begin to be expressed in the presumptive lateral foregut endoderm just before somitogenesis in the chick (Muller et al. 1996) and mouse (Deutsch et al. 1988; Timmons et al. 1994; Neubuser et al. 1995). Pax-9-deficient mice lack derivatives of the third and Endoderm development in vertebrates fourth pharyngeal pouches (Peters et al. 1998). Furthermore, the finding that heterotopical transplanted endoderm fragments from the presumptive lateral foregut endoderm express Pax1 and 9 shows that the expression of these genes in the pharyngeal pouch endoderm is intrinsic (Muller et al. 1996). In the zebrafish, mutants which are deficient the tbx1 gene show defects in the pharyngeal pouches and associated structures (Piotrowski et al. 1996; Piotrowski & Nusslein-Volhard 2000; Piotrowski et al. 2003). In addition, tbx1 is also important for development of the pharyngeal arch in Xenopus (Ataliotis et al. 2005). Taken together with reports that the haploinsufficiency of Tbx1 may be a major determinant of cardiac and craniofacial birth defects associated with DiGeorge syndrome in humans (Lindsay et al. 2001; Yamagishi et al. 2003), it is possible that Tbx1 is important to pharyngeal pouch endoderm differentiation. In the mouse embryo, however, tbx1 is expressed only in the mesoderm in the early stages and expands its expression in the endoderm at a later stage (Chapman et al. 1996; Garg et al. 2001; Yamagishi et al. 2003). The vertebrate Caudal homologue, CdxA and the HMG domain containing the Sox2 gene begin to be expressed in caudal and rostral endoderm at the 8– 10 somite stages (Duprey et al. 1988; Frumkin et al. 1993; Suh et al. 1994; Ishii et al. 1997; Freund et al. 1998; Ishii et al. 1998; Wood & Episkopou 1999; Beck et al. 2003). These genes continue to be expressed in the endoderm and this expression never overlaps. At a later stage, Sox2 is expressed in the endoderm of the esophagus and stomach, whereas CdxA is detected in the endoderm of the small and large intestine. A CdxA-deficient mouse shows malformation of the intestinal epithelium (Suh et al. 1994; Tamai et al. 1999), indicating that this factor is important for intestinal development. The functions of these genes during the somite stages and the pathways that regulate their expression are still unknown however. At the somite stage, there are additional regional marker genes expressed, the paraHox transcription factor gene, Pdx1 and the homeobox gene, Hex1. These genes become active in the presumptive pancreas (Slack 1995; Offield et al. 1996; Kim & Melton 1998; Wang et al. 2001) and liver regions (Yatskievych et al. 1999; Bogue et al. 2000; Zorn & Mason 2001), respectively at the somite stage. The Pdx1-deficient mouse shows significant defects in pancreas development (Slack 1995; Offield et al. 1996) and the Hex1-deficient mouse shows hepatocyte defects (Keng et al. 2000; Martinez Barbera et al. 2000). In summary, the endoderm is regionalized with some potency by each organ in at least the somite 351 stages and each region expresses different transcription factors which may be important to further development. In Drosophila, the regionalization of the endoderm is affected by the mesoderm (Bienz 1994; Bienz 1997). In mouse, the mesoderm is adjacent to the endoderm, which is very important during development. Moreover, when endoderm fragments from gastrulation embryos are cultured with various adjacent tissues, the region specific genes that are expressed are dependent on the adjacent tissues (Wells & Melton 2000). In addition, FGF4, which is expressed in the adjacent mesoderm, can induce the differentiation of endoderm in a concentrationdependent manner. In the zebrafish, mutants which affect bmp signaling show no influence upon the induction of endoderm precursors but produce abnormal gut phenotypes (Tiso et al. 2002). The swirl (bmp2b) mutant shows expansion of the pharyngeal region and reduction of the pancreas and posterior gut region, whereas the chordino (chordin) mutant shows a reduction in the pharyngeal region and expansion of the pancreatic and posterior gut regions (Tiso et al. 2002). When the activity of the enzyme responsible for early embryonic retinoic acid synthesis, retinaldehyde dehydrogenase 2 (RALDH2/ALDH1a2) is inhibited in mouse embryos, the pharyngeal endoderm develops rudimentary and pouch-derived organs which fail to establish. Raldh2 expression is restricted to the posterior-most pharyngeal mesoderm and RA target genes (Hoxa1, Hoxb1) are downregulated in both the pharyngeal endoderm and mesoderm of these mutant embryos. Thus, RA is one of the diffusible mesodermal signals that patterns the pharyngeal endoderm (Niederreither et al. 2003). In summary, endoderm regionalization is controlled by soluble factors which are provided by the adjacent germ layers. Conclusion We describe the recent advances made in our understanding of endoderm specification and differentiation during early vertebrate development. The genetic, biological and molecular approaches using vertebrate model organisms such as the zebrafish, Xenopus, chick and mouse have greatly contributed to the elucidation of endoderm formation. However, many questions still remain and a great number of experiments will be necessary in the future to address these. We believe that the elucidation of the molecular mechanisms underlying endoderm development improves not only our knowledge of basic developmental biology, but also our understanding of the regeneration of endodermal organs such as 352 K. Fukuda and Y. Kikuchi the lung, liver, pancreas and intestine. In particular, we contend that the elucidation of the pathways that control the segregation of the three germ layers and regulate the regional specification of endoderm will be useful in future studies that seek to understand the differentiation of embryonic stem cells to various types of endodermal organs. Acknowledgements Y. K. is supported by the Ministry of Education, Culture, Sports, Science and Technology and the Mitsubishi Foundation and K. F. is supported by the Ministry of Education, Culture, Sports, Science and Technology. References Afouda, B. A., Ciau-Uitz, A. & Patient, R. 2005. GATA4, 5 and 6 mediate TGFβ maintenance of endodermal gene expression in Xenopus embryos. Development 132, 763 –774. Alexander, J., Rothenberg, M., Henry, G. L. & Stainier, D. Y. 1999. casanova plays an early and essential role in endoderm formation in zebrafish. Dev. Biol. 215, 343 –357. Alexander, J. & Stainier, D. Y. 1999. A molecular pathway leading to endoderm formation in zebrafish. Curr. Biol. 9, 1147–1157. Ataliotis, P., Ivins, S., Mohun, T. J. & Scambler, P. J. 2005. XTbx1 is a transcriptional activator involved in head and pharyngeal arch development in Xenopus laevis. Dev. Dyn. 232, 979–991. Beck, F., Chawengsaksophak, K., Luckett, J. et al. 2003. A study of regional gut endoderm potency by analysis of Cdx2 null mutant chimaeric mice. Dev. Biol. 255, 399 – 406. Bellairs, R. 1953. Studies on the development of the foregut in the chick blastoderm. 1. The presumptive foregut area. J. Embryol. Exp. Morph. 1, 115 –124. Bellairs, R. 1957. Studies on the development of the foregut in the chick embryo. 4. Mesodermal induction and mitosis. J. Embryol. Exp. Morph. 5, 340 –350. Belting, H. G., Hauptmann, G., Meyer, D. et al. 2001. spiel ohne grenzen/pou2 is required during establishment of the zebrafish midbrain–hindbrain boundary organizer. Development 128, 4165–4176. Bienz, M. 1994. Homeotic genes and positional signalling in the Drosophila viscera. Trends Genet. 10, 22–26. Bienz, M. 1997. Endoderm induction in Drosophila: the nuclear targets of the inducing signals. Curr. Opin Genet. Dev. 7, 683–688. Bogue, C. W., Ganea, G. R., Sturm, E., Ianucci, R. & Jacobs, H. C. 2000. Hex expression suggests a role in the development and function of organs derived from foregut endoderm. Dev. Dyn. 219, 84 – 89. Burgess, S., Reim, G., Chen, W., Hopkins, N. & Brand, M. 2002. The zebrafish spiel-ohne-grenzen (spg) gene encodes the POU domain protein Pou2 related to mammalian Oct4 and is essential for formation of the midbrain and hindbrain, and for pre-gastrula morphogenesis. Development 129, 905 – 916. Canning, D. R. & Stern, C. D. 1988. Changes in the expression of the carbohydrate epitope HNK-1 associated with mesoderm induction in the chick embryo. Development 104, 643 – 655. Casey, E. S., Tada, M., Fairclough, L., Wylie, C. C., Heasman, J. & Smith, J. C. 1999. Bix4 is activated directly by VegT and mediates endoderm formation in Xenopus development. Development 126, 4193 – 4200. Chalmers, A. D. & Slack, J. M. 2000. The Xenopus tadpole gut: fate maps and morphogenetic movements. Development 127, 381–392. Chapman, D. L., Garvey, N., Hancock, S. et al. 1996. Expression of the T-box family genes, Tbx1-Tbx5, during early mouse development. Dev. Dyn. 206, 379 –390. Charron, F. & Nemer, M. 1999. GATA transcription factors and cardiac development. Semin. Cell Dev. Biol. 10, 85 –91. Dale, L. & Slack, J. M. 1987. Fate map for the 32-cell stage of Xenopus laevis. Development 99, 527– 551. David, N. B. & Rosa, F. M. 2001. Cell autonomous commitment to an endodermal fate and behaviour by activation of Nodal signalling. Development 128, 3937–3947. Deutsch, U., Dressler, G. R. & Gruss, P. 1988. Pax 1, a member of a paired box homologous murine gene family, is expressed in segmented structures during development. Cell 53, 617– 625. Dickmeis, T., Mourrain, P., Saint-Etienne, L.et al. 2001. A crucial component of the endoderm formation pathway, CASANOVA, is encoded by a novel sox-related gene. Genes Dev. 15, 1487–1492. Duprey, P., Chowdhury, K., Dressler, G. R. et al. 1988. A mouse gene homologous to the Drosophila gene caudal is expressed in epithelial cells from the embryonic intestine. Genes Dev. 2, 1647–1654. Ecochard, V., Cayrol, C., Rey, S. et al. 1998. A novel Xenopus Mix-like gene milk involved in the control of the endomesodermal fates. Development 125, 2577–2585. Feldman, B., Gates, M. A., Egan, E. S. et al. 1998. Zebrafish organizer development and germ-layer formation require nodal-related signals. Nature 395, 181–185. Fontaine, J. & Le Douarin, N. M. 1977. Analysis of endoderm formation in the avian blastoderm by the use of quail-chick chimaeras. The problem of the neurectodermal origin of the cells of the APUD series. J. Embryol. Exp. Morph. 41, 209–222. Freund, J. N., Domon-Dell, C., Kedinger, M. & Duluc, I. 1998. The Cdx-1 and Cdx-2 homeobox genes in the intestine. Biochem. Cell Biol. 76, 957–969. Frumkin, A., Haffner, R., Shapira, E., Tarcic, N., Gruenbaum, Y. & Fainsod, A. 1993. The chicken CdxA homeobox gene and axial positioning during gastrulation. Development 118, 553 –562. Fukuda-Taira, S. 1981. Location of pre-hepatic cells in the early developmental stages of quail embryo. J. Embryol. Exp. Morph. 64, 73 – 85. Gao, X., Sedgwick, T., Shi, Y. B. & Evans, T. 1998. Distinct functions are implicated for the GATA-4, -5, and -6 transcription factors in the regulation of intestine epithelial cell differentiation. Mol. Cell Biol. 18, 2901–2911. Garcia-Martinez, V. & Schoenwolf, G. C. 1993. Primitive-streak origin of the cardiovascular system in avian embryos. Dev. Biol. 159, 706 –719. Garg, V., Yamagishi, C., Hu, T., Kathiriya, I. S., Yamagishi, H. & Srivastava, D. 2001. Tbx1, a DiGeorge syndrome candidate gene, is regulated by sonic hedgehog during pharyngeal arch development. Dev. Biol. 235, 62–73. Gritsman, K., Zhang, J., Cheng, S., Heckscher, E., Talbot, W. S. & Schier, A. F. 1999. The EGF-CFC protein one-eyed pinhead is essential for nodal signaling. Cell 97, 121–132. Endoderm development in vertebrates Hart, A. H., Hartley, L., Sourris, K. et al. 2002. Mixl1 is required for axial mesendoderm morphogenesis and patterning in the murine embryo. Development 129, 3597–3608. Hatada, Y. & Stern, C. D. 1994. A fate map of the epiblast of the early chick embryo. Development 120, 2879 –2889. Heasman, J., Wylie, C. C., Hausen, P. & Smith, J. C. 1984. Fates and states of determination of single vegetal pole blastomeres of X. laevis. Cell 37, 185 –194. Henry, G. L. & Melton, D. A. 1998. Mixer, a homeobox gene required for endoderm development. Science 281, 91– 96. Hiramatsu, H. & Yasugi, S. 2004. Molecular analysis of the determination of developmental fate in the small intestinal epithelium in the chicken embryo. Int. J. Dev. Biol. 48, 1141–1148. Ho, R. K. & Kimmel, C. B. 1993. Commitment of cell fate in the early zebrafish embryo. Science 261, 109 –111. Horb, M. E. & Thomsen, G. H. 1997. A vegetally localized T-box transcription factor in Xenopus eggs specifies mesoderm and endoderm and is essential for embryonic mesoderm formation. Development 124, 1689 –1698. Ishii, Y., Fukuda, K., Saiga, H., Matsushita, S. & Yasugi, S. 1997. Early specification of intestinal epithelium in the chicken embryo: a study on the localization and regulation of CdxA expression. Dev. Growth Differ. 39, 643 – 653. Ishii, Y., Rex, M., Scotting, P. J. & Yasugi, S. 1998. Regionspecific expression of chicken Sox2 in the developing gut and lung epithelium: regulation by epithelial–mesenchymal interactions. Dev. Dyn. 213, 464 – 475. Kanai-Azuma, M., Kanai, Y., Gad, J. M. et al. 2002. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development 129, 2367–2379. Keller, R. E. 1975. Vital dye mapping of the gastrula and neurula of Xenopus laevis. I. Prospective areas and morphogenetic movements of the superficial layer. Dev. Biol. 42, 222–241. Keller, R. E. 1976. Vital dye mapping of the gastrula and neurula of Xenopus laevis. II. Prospective areas and morphogenetic movements of the deep layer. Dev. Biol. 51, 118 –137. Keng, V. W., Yagi, H., Ikawa, M. et al. 2000. Homeobox gene Hex is essential for onset of mouse embryonic liver development and differentiation of the monocyte lineage. Biochem. Biophys. Res. Commun. 276, 1155 –1161. Kikuchi, Y., Agathon, A., Alexander, J. et al. 2001. Casanova encodes a novel Sox-related protein necessary and sufficient for early endoderm formation in zebrafish. Genes Dev. 15, 1493 –1505. Kikuchi, Y., Trinh, L. A., Reiter, J. F., Alexander, J., Yelon, D. & Stainier, D. Y. 2000. The zebrafish bonnie and clyde gene encodes a Mix family homeodomain protein that regulates the generation of endodermal precursors. Genes Dev. 14, 1279–1289. Kikuchi, Y., Verkade, H., Reiter, J. F. et al. 2004. Notch signaling can regulate endoderm formation in zebrafish. Dev. Dyn. 229, 756–762. Kim, S. K. & Melton, D. A. 1998. Pancreas development is promoted by cyclopamine, a hedgehog signaling inhibitor. Proc. Natl Acad. Sci. USA 95, 13036 –13041. Kimmel, C. B., Warga, R. M. & Schilling, T. F. 1990. Origin and organization of the zebrafish fate map. Development 108, 581–594. Kirby, M. L., Lawson, A., Stadt, H. A. et al. 2003. Hensen’s node gives rise to the ventral midline of the foregut: implications for organizing head and heart development. Dev. Biol. 253, 175–188. Kofron, M., Wylie, C. & Heasman, J. 2004. The role of Mixer in patterning the early Xenopus embryo. Development 131, 2431–2441. 353 Lawson, K. A., Meneses, J. J. & Pedersen, R. A. 1986. Cell fate and cell lineage in the endoderm of the presomite mouse embryo, studied with an intracellular tracer. Dev. Biol. 115, 325 –339. Lawson, K. A. & Pedersen, R. A. 1987. Cell fate, morphogenetic movement and population kinetics of embryonic endoderm at the time of germ layer formation in the mouse. Development 101, 627– 652. Lawson, A. & Schoenwolf, G. C. 2003. Epiblast and primitivestreak origins of the endoderm in the gastrulating chick embryo. Development 130, 3491–3501. Lemaire, P., Darras, S., Caillol, D. & Kodjabachian, L. 1998. A role for the vegetally expressed Xenopus gene Mix.1 in endoderm formation and in the restriction of mesoderm to the marginal zone. Development 125, 2371–2380. Lindsay, E. A., Vitelli, F., Su, H. et al. 2001. Tbx1 haploinsufficieny in the DiGeorge syndrome region causes aortic arch defects in mice. Nature 410, 97–101. Lowe, L. A., Yamada, S. & Kuehn, M. R. 2001. Genetic dissection of nodal function in patterning the mouse embryo. Development 128, 1831–1843. Lunde, K., Belting, H. G. & Driever, W. 2004. Zebrafish pou5f1/ pou2, homolog of mammalian Oct4, functions in the endoderm specification cascade. Curr. Biol. 14, 48 –55. Lustig, K. D., Kroll, K. L., Sun, E. E. & Kirschner, M. W. 1996. Expression cloning of a Xenopus T-related gene (Xombi ) involved in mesodermal patterning and blastopore lip formation. Development 122, 4001– 4012. Maeda, M., Kubo, K., Nishi, T. & Futai, M. 1996. Roles of gastric GATA DNA-binding proteins. J. Exp. Biol. 199, 513 –520. Martinez Barbera, J. P., Clements, M. et al. 2000. The homeobox gene Hex is required in definitive endodermal tissues for normal forebrain, liver and thyroid formation. Development 127, 2433 –2445. Matsushita, S. 1999. Fate mapping study of the endoderm in the posterior part of the 1.5-day-old chick embryo. Dev. Growth Differ. 41, 313 –319. Melby, A. E., Warga, R. M. & Kimmel, C. B. 1996. Specification of cell fates at the dorsal margin of the zebrafish gastrula. Development 122, 2225 –2237. Molkentin, J. D. 2000. The zinc finger-containing transcription factors GATA-4 -5, and – 6. Ubiquitously expressed regulators of tissue-specific gene expression. J. Biol. Chem. 275, 38949 –38952. Morrisey, E. E., Tang, Z., Sigrist, K., Lu, M. M., Jiang, F., Ip, H. S. & Parmacek, M. S. 1998. GATA6 regulates HNF4 and is required for differentiation of visceral endoderm in the mouse embryo. Genes Dev. 12, 3579 –3590. Muller, T. S., Ebensperger, C., Neubuser, A. et al. 1996. Expression of avian Pax1 and Pax9 is intrinsically regulated in the pharyngeal endoderm, but depends on environmental influences in the paraxial mesoderm. Dev. Biol. 178, 403 – 417. Nakamura, O. & Kishiyama, H. 1971. Prospective fates of blastomeres at the 32 cell stage of xenopus laevis embryo. Proc. Japan Acad. 47, 407– 412. Nakamura, O., Takasaki, H. & Nagata, A. 1978. Further studies on the prospective fates of blastomeres at the 32 cell stage of Xenopus laevis embryos. Med. Biol. 56, 355 –360. Neubuser, A., Koseki, H. & Balling, R. 1995. Characterization and developmental expression of Pax9, a paired-boxcontaining gene related to Pax1. Dev. Biol. 170, 701–716. Niederreither, K., Vermot, J., Le Roux, I., Schuhbaur, B., Chambon, P. & Dolle, P. 2003. The regional pattern of retinoic acid synthesis by RALDH2 is essential for the development of 354 K. Fukuda and Y. Kikuchi posterior pharyngeal arches and the enteric nervous system. Development 130, 2525 –2534. Nieuwkoop, P. & Florshtz, P. 1950. Quelques caracteres speciaux de le gastrulation et de la neurulation de loeuf de Xenopus laevis, Daud. et de quelques autes Anoures. Arch. Biol. (Liege) 61, 113 –150. Offield, M. F., Jetton, T. L., Labosky, P. A. et al. 1996. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development 122, 983 –995. Osada, S. I. & Wright, C. V. 1999. Xenopus nodal-related signaling is essential for mesendodermal patterning during early embryogenesis. Development 126, 3229 –3240. Peters, H., Neubuser, A., Kratochwil, K. & Balling, R. 1998. Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities. Genes Dev. 12, 2735 –2747. Piotrowski, T., Ahn, D. G., Schilling, T. F. et al. 2003. The zebrafish van gogh mutation disrupts tbx1, which is involved in the DiGeorge deletion syndrome in humans. Development 130, 5043 –5052. Piotrowski, T. & Nusslein-Volhard, C. 2000. The endoderm plays an important role in patterning the segmented pharyngeal region in zebrafish (Danio rerio). Dev. Biol. 225, 339 –356. Piotrowski, T., Schilling, T. F., Brand, M. et al. 1996. Jaw and branchial arch mutants in zebrafish II: anterior arches and cartilage differentiation. Development 123, 345 –356. Poulain, M. & Lepage, T. 2002. Mezzo, a paired-like homeobox protein is an immediate target of Nodal signalling and regulates endoderm specification in zebrafish. Development 129, 4901– 4914. Reim, G., Mizoguchi, T., Stainier, D. Y., Kikuchi, Y. & Brand, M. 2004. The POU domain protein spg (pou2/Oct4) is essential for endoderm formation in cooperation with the HMG domain protein casanova. Dev. Cell 6, 91–101. Reiter, J. F., Kikuchi, Y. & Stainier, D. Y. 2001. Multiple roles for Gata5 in zebrafish endoderm formation. Development 128, 125–135. Renucci, A., Lemarchandel, V. & Rosa, F. 1996. An activated form of type I serine/threonine kinase receptor TARAM-A reveals a specific signalling pathway involved in fish head organiser formation. Development 122, 3735 –3743. Rodaway, A., Takeda, H., Koshida, S. et al. 1999. Induction of the mesendoderm in the zebrafish germ ring by yolk cell-derived TGF-β family signals and discrimination of mesoderm and endoderm by FGF. Development 126, 3067–3078. Rosenquist, G. C. 1966. A radioautographic study of labelled grafts in the chick blastoderm. Development from primitivestreak stages to stage 12. Contrib. Embryol. Carnegie Inst. Wash. 38, 71–110. Rosenquist, G. C. 1971. The location of the pregut endoderm in the chick embryo at the primitive streak stage as determined by radioautographic mapping. Dev. Biol. 26, 323 – 335. Rosenquist, G. C. 1972. Endoderm movements in the chick embryo between the early short streak and head process stages. J. Exp. Zool. 180, 95 –103. Sakaguchi, T., Kuroiwa, A. & Takeda, H. 2001. A novel sox gene, 226D7, acts downstream of Nodal signaling to specify endoderm precursors in zebrafish. Mech. Dev. 107, 25 –38. Schier, A. F. 2003. Nodal signaling in vertebrate development. Annu. Rev. Cell Dev. Biol. 19, 589 – 621. Selleck, M. A. & Stern, C. D. 1991. Fate mapping and cell lineage analysis of Hensen’s node in the chick embryo. Development 112, 615 – 626. Sherwood, D. R. & McClay, D. R. 1999. LvNotch signaling mediates secondary mesenchyme specification in the sea urchin embryo. Development 126, 1703 –1713. Slack, J. M. 1995. Developmental biology of the pancreas. Development 121, 1569 –1580. Stennard, F., Carnac, G. & Gurdon, J. B. 1996. The Xenopus Tbox gene, Antipodean, encodes a vegetally localised maternal mRNA and can trigger mesoderm formation. Development 122, 4179 – 4188. Stern, C. D. & Canning, D. R. 1990. Origin of cells giving rise to mesoderm and endoderm in chick embryo. Nature 343, 273 –275. Strähle, U., Blader, P., Henrique, D. & Ingham, P. W. 1993. Axial, a zebrafish gene expressed along the developing body axis, shows altered expression in cyclops mutant embryos. Genes Dev. 7, 1436 –1446. Suh, E., Chen, L., Taylor, J. & Traber, P. G. 1994. A homeodomain protein related to caudal regulates intestine-specific gene transcription. Mol. Cell Biol. 14, 7340 –7351. Sumiya, M. 1976a. Localization of autodifferentiation potencies in the early endoderm of the chick embryo. Jour. Fac. of Sci., Univ. Tokyo IV (13), 363 – 381. Sumiya, M. 1976b. Differentiation of the digestive tract epithelium of the chicken embryo cultured in vitro. Enveloped in a fragment of the vitelline membrane, in the absent of mesenchyme. Roux’s Arch. Dev. Biol. 179, 1–17. Tada, M., Casey, E. S., Fairclough, L. & Smith, J. C. 1998. Bix1, a direct target of Xenopus T-box genes, causes formation of ventral mesoderm and endoderm. Development 125, 3997– 4006. Takahashi, S., Yokota, C., Takano, K. et al. 2000. Two novel nodal-related genes initiate early inductive events in IXenopus Nieuwkoop center. Development 127, 5319 – 5329. Tamai, Y., Nakajima, R., Ishikawa, T., Takaku, K., Seldin, M. F. & Taketo, M. M. 1999. Colonic hamartoma development by anomalous duplication in Cdx2 knockout mice. Cancer Res. 59, 2965 –2970. Timmons, P. M., Wallin, J., Rigby, P. W. & Balling, R. 1994. Expression and function of Pax 1 during development of the pectoral girdle. Development 120, 2773 –2785. Tiso, N., Filippi, A., Pauls, S., Bortolussi, M. & Argenton, F. 2002. BMP signalling regulates anteroposterior endoderm patterning in zebrafish. Mech. Dev. 118, 29 – 37. Tremblay, K. D. & Zaret, K. S. 2005. Distinct populations of endoderm cells converge to generate the embryonic liver bud and ventral foregut tissues. Dev. Biol. 280, 87–99. Vogt, W. 1929. Gestaltungsanalyse am Amphibienkeim mit oertlicher Vitalfaurbung. II. Gastrulation und Mesodermbildung bie Urodelen und Anuren. Roux' Archiv fur Entwicklungs Mechanik der Organismen 120, 384 –706. Wang, H., Maechler, P., Ritz-Laser, B. et al. 2001. Pdx1 level defines pancreatic gene expression pattern and cell lineage differentiation. J. Biol. Chem. 276, 25279 –25286. Warga, R. M. & Kimmel, C. B. 1990. Cell movements during epiboly and gastrulation in zebrafish. Development 108, 569 –580. Warga, R. M. & Nüsslein-Volhard, C. 1999. Origin and development of the zebrafish endoderm. Development 126, 827– 838. Weber, H., Symes, C. E., Walmsley, M. E., Rodaway, A. R. & Patient, R. K. 2000. A role for GATA5 in Xenopus endoderm specification. Development 127, 4345 – 4360. Wells, J. M. & Melton, D. A. 2000. Early mouse endoderm is patterned by soluble factors from adjacent germ layers. Development 127, 1563 –1572. Endoderm development in vertebrates Wood, H. B. & Episkopou, V. 1999. Comparative expression of the mouse Sox1, Sox2 and Sox3 genes from pre-gastrulation to early somite stages. Mech. Dev. 86, 197– 201. Wylie, C. C., Snape, A., Heasman, J. & Smith, J. C. 1987. Vegetal pole cells and commitment to form endoderm in Xenopus laevis. Dev. Biol. 119, 496 –502. Xanthos, J. B., Kofron, M., Wylie, C. & Heasman, J. 2001. Maternal VegT is the initiator of a molecular network specifying endoderm in Xenopus laevis. Development 128, 167–180. Yamagishi, H., Maeda, J., Hu, T. et al. 2003. Tbx1 is regulated by tissue-specific forkhead proteins through a common Sonic hedgehog-responsive enhancer. Genes Dev. 17, 269 –281. Yasugi, S., Takeda, H. & Fukuda, K. 1991. Early determination of developmental fate in presumptive intestinal enoderm of the chicken embryo. Dev. Growth Differ. 33, 235 –241. Yatskievych, T. A., Pascoe, S. & Antin, P. B. 1999. Expression of the homebox gene Hex during early stages of chick embryo development. Mech. Dev. 80, 107–109. Zhang, C., Basta, T., Fawcett, S. R. & Klymkowsky, M. W. 2005. SOX7 is an immediate-early target of VegT and regulates 355 Nodal-related gene expression in Xenopus. Dev. Biol. 278, 526 – 541. Zhang, C., Basta, T., Hernandez-Lagunas, L. et al. 2004. Repression of nodal expression by maternal B1-type SOXs regulates germ layer formation in Xenopus and zebrafish. Dev. Biol. 273, 23 –37. Zhang, J., Houston, D. W., King, M. L., Payne, C., Wylie, C. & Heasman, J. 1998a. The role of maternal VegT in establishing the primary germ layers in Xenopus embryos. Cell 94, 515 – 524. Zhang, J. & King, M. L. 1996. Xenopus VegT RNA is localized to the vegetal cortex during oogenesis and encodes a novel T-box transcription factor involved in mesodermal patterning. Development 122, 4119 – 4129. Zhang, J., Talbot, W. S. & Schier, A. F. 1998b. Positional cloning identifies zebrafish one-eyed pinhead as a permissive EGF-related ligand required during gastrulation. Cell 92, 241–251. Zorn, A. M. & Mason, J. 2001. Gene expression in the embryonic Xenopus liver. Mech. Dev. 103, 153 –157.