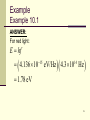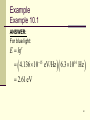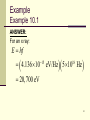* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 10 Notes
James Franck wikipedia , lookup
Franck–Condon principle wikipedia , lookup
Double-slit experiment wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Bremsstrahlung wikipedia , lookup
Particle in a box wikipedia , lookup
Ultrafast laser spectroscopy wikipedia , lookup
Auger electron spectroscopy wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Matter wave wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Atomic orbital wikipedia , lookup
Hydrogen atom wikipedia , lookup
Tight binding wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Wave–particle duality wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Chapter 10 Atomic Physics The quantum hypothesis By the end of the 1800’s, physics had made significant progress. Some physicists feared “that all of their questions might soon be answered.” But some problems defied solution: blackbody radiation, photoelectric effect, and atomic spectra. 2 Blackbody radiation Everything around you is constantly emitting electromagnetic (EM) radiation. A perfectly “black” body would: absorb all light and other EM radiation incident upon it, and be a perfect emitter of EM radiation. Such an object is called a blackbody. The EM radiation emitted by it is called blackbody radiation (BBR). 3 Blackbody radiation, cont’d The characteristics of BBR emitted at a particular frequency can be illustrated with a graph of the radiation intensity versus wavelength. Such a graph is called a blackbody radiation curve. 4 Blackbody radiation, cont’d This graph illustrates two important ways that BBR changes when the temperature of the body is increased. More energy is emitter per second at each wavelength of EM radiation, and 5 Blackbody radiation, cont’d The wavelength at which the most energy is emitted per second shifts to smaller values. In other words, the peak of the BBR curve moves toward smaller wavelengths as the temperature increases. 6 Blackbody radiation, cont’d The principles of electromagnetism explain some of this: A blackbody emits radiation since the atoms and molecules are continually oscillating. Recall that a vibrating electric charge emits EM radiation. But some things could not be answered. An explicit explanation of the two mentioned features could not be resolved. 7 Blackbody radiation, cont’d A “solution” was devised in 1900 by the German physicist Max Planck. He developed a mathematical equation that accurately fit the blackbody radiation curve. This gives the correct formula but no physical insight. He then developed a model that would produce the desired equation. He did not believe the model was physical even though it gave the right answer. 8 Blackbody radiation, cont’d He proposed that an oscillating atom in a blackbody can only exchange certain fixed values of energy. It can have zero energy, or a particular energy E, or 2E, or 3E, …. This means that the energy of each atomic oscillator is quantized. The energy E is called the fundamental quantum of energy for the oscillator. 9 Blackbody radiation, cont’d The idea of quantization can be illustrated with the following figure. On the right, the cat can rest at any height above the floor. On the left, the cat can only rest at certain heights above the floor. 10 Blackbody radiation, cont’d This means that: The left cat’s potential energy can only assume certain values. The right cat’s potential energy can assume any value. 11 Blackbody radiation, cont’d Planck determined that the basic quantum of energy is proportional to the oscillator’s frequency: E hf h 6.63 10 34 J×s The constant h is called Planck’s constant. The allowed energies are then E 0, or E hf , or E 2hf , ... 12 The photoelectric effect The second phenomenon defying classical explanation was the photoelectric effect. The effect occurs when certain EM radiation illuminates a metal then electrons are ejected from the metal. The EM wave gives energy to the electrons and allows them to escape the metal. 13 The photoelectric effect, cont’d Albert Einstein extended Planck’s quantum hypothesis to solve this problem. Planck suggested that light is emitted in discrete bundles of energy. Einstein took this one step farther. He proposed that the light remains in these bundles of energy and is absorbed in this form. 14 The photoelectric effect, cont’d He suggested that the amount of energy in one bundle of energy of frequency f is E hf This allows us to visualize the wave as being composed of individual particles of energy, now called photons. 15 The photoelectric effect, cont’d This allowed all aspects of the photoelectric effect to be understood. Higher-frequency light ejects electrons with more energy because each photon has more energy to impart to the electron. Bright light simply means more photons strike the metal so that more electrons are emitted per second but does not increase their energy. 16 The photoelectric effect, cont’d The energy of a single photon is miniscule. A convenient unit is the electron-Volt (eV). 1 electronvolt 1.6 10 19 1 eV 1.6 10 19 coulomb 1 volt J One electron-volt is the potential energy of each electron in a 1-volt battery. 17 Example Example 10.1 Compare the energies associated with a quantum of each of the following types of EM radiation. 14 f 4.3 10 Hz red light: 14 f 6.3 10 Hz blue light: 18 f 5 10 Hz x ray: 18 Example Example 10.1 ANSWER: For red light: E hf 4.136 10 15 eV/Hz 4.3 10 Hz 14 1.78 eV 19 Example Example 10.1 ANSWER: For blue light: E hf 4.136 10 15 eV/Hz 6.3 10 Hz 14 2.61 eV 20 Example Example 10.1 ANSWER: For an x ray: E hf 4.136 10 15 eV/Hz 5 10 Hz 18 20, 700 eV 21 Example Example 10.1 DISCUSSION: Notice the significant increase in the energy of the x ray compared to the visible light. This explains why high doses of x rays can do serious damage to living cells. 22 Applications of the photoelectric effect The photoelectric effect is the key to “interfacing” light with electricity. The figure shows a schematic of a device that can detect light. 23 Applications of the photoelectric effect, cont’d When light strikes the metal plate, electrons are emitted. The electrons are pushed across the tube because of the potential difference. 24 Applications of the photoelectric effect, cont’d The flow of electrons “closes” the circuit. The ammeter then measures the current flow due to the electrons. Since there is a current, there is light. 25 Applications of the photoelectric effect, cont’d A similar approach works for photocopiers. 26 Atomic spectra The third problem that classical physics could not resolve is the emission spectra of the elements. Imagine shining the light from a heated filament through a prism. The light is separated into a range of colors. This spectrum is called a continuous spectrum since it is a continuous band of colors. 27 Atomic spectra, cont’d Now imagine heating a gas-filled tube. The gas will emit some EM radiation. After this light passes through a prism, only certain lines of color appear. 28 Atomic spectra, cont’d This type of spectrum is called an emission- line spectrum. Because it is due to the light emitted by the gas and it is not continuous. 29 Atomic spectra, cont’d Here are some emission spectra for various elements. Notice that each has its own distinct sets of lines. 30 Bohr model of the atom Bohr constructed a model of the atom called the Bohr model: The atom forms a miniature “solar system.” The electron orbits are quantized. the nucleus is at the center and the electrons move about the nucleus in well-defined orbits. the electrons can only be in certain orbits about a given atomic nucleus. Electrons may “jump” from one orbit to another. 31 Bohr model of the atom, cont’d Here is a figure that illustrates the Bohr model. The electron orbits the nucleus. The electron can only orbit in specific orbits. 32 Bohr model of the atom, cont’d Transitions from one orbit to another involve discrete amounts of energy. The energy to change levels is the difference in the two energy levels. 33 Bohr model of the atom, cont’d Let’s consider hydrogen. One electron and one proton. Orbit 1 is the innermost orbit and corresponds to the lowest energy state of the electron. The amount of energy required to just remove an electron from the proton is the ionization energy. The electron is no longer bound to the nucleus. The atom is ionized because there is no longer the same number of electrons and protons. 34 Bohr model of the atom, cont’d Imagine an electron that is in the sixth allowed orbit. So it has energy E6. Let the electron make a transition to the second orbit. So it has energy E2. The electron must lose energy in the amount E E6 E2 35 Bohr model of the atom, cont’d This is called a radiative transition because the electron loses energy by emitting a photon of the appropriate energy. The change in energy of the electron must equal the photon energy: E E6 E2 hf This gives a formula for the frequency of the emitted light according to which orbits are involved in the transition. 36 Bohr model of the atom, cont’d The frequency of the emitted light is proportional to the energy of the electron orbits involved in the transition. A downward electron transition can also occur during a collision with another particle. A collisional transition. 37 Bohr model of the atom, cont’d An atom can also absorb a photon. The electron can gain energy from the incoming photon. This increase in the electron’s energy causes it to transition to a higher energy orbit. 38 Bohr model of the atom, cont’d If broad-spectrum light is passed through a material, the light will cause transitions to higher energy orbits. This reduces the number of photons of the corresponding energy. 39 Bohr model of the atom, cont’d The spectrum emerging from the material has dark bands at certain frequencies. This type of spectrum is called an absorption spectrum. 40 Bohr model of the atom, cont’d One unexplained result of the Bohr model was that the angular momentum of the electron in its orbit is quantized. Mathematically, this means the allowed angular momentum can only have the values: h h h , or 2 , or 3 , ... 2 2 2 41 Quantum mechanics Even with its shortcomings, the Bohr model indicated that new physics was needed to describe the atom. Louis de Broglie proposed that electrons have wavelike properties. We know that light has wave-like properties. diffraction, refraction, etc. We also know light has particle-like properties. blackbody radiation, photoelectric effect, etc. 42 Quantum mechanics, cont’d He suggested that the wavelength of a particle depends on its momentum. Recall that momentum is the product of mass and velocity. h mv So the higher the momentum, the shorter the wavelength. That means the higher the frequency. 43 Example Example 10.2 What is the de Broglie wavelength of an electron with speed 2.19×106 m/s? (This is the approximate speed of an electron in the smallest orbit in hydrogen.) The electron mass is 9.11×10-31 kg. 44 Example Example 10.2 ANSWER: The problem gives us: m 9.1110 31 kg v 2.19 10 m/s 6 The de Broglie wavelength is then: 34 h 6.63 10 J-s 31 6 mv 9.1110 kg 2.19 10 m/s 3.3 10 10 m 0.332 nm. 45 Example Example 10.2 DISCUSSION: This wavelength is on the same length scale as the diameter of atoms. Thus electrons are useful for probing the structure of atoms. 46 Quantum mechanics, cont’d Experiments were performed by shooting electrons and x-rays through a solid. The same diffraction pattern was obtained. 47 Quantum mechanics, cont’d de Broglie’s hypothesis was also able to explain Bohr’s quantized orbits. Since the electron acts like a wave, the wave must fit along the circumference of the electron’s orbit. This means that only orbits with wholenumbered multiples of the wavelength are valid. 48 Quantum mechanics, cont’d Since the circumference must equal some multiple of the wavelength: 2 r or 2 or ... This means h h 2 r mrv mv 2 This supports the Bohr model. 49 Example Example 10.3 Using the results of Example 10.2, find the radius of the smallest orbit in the hydrogen atom. 0.332 nm 50 Example Example 10.3 ANSWER: The radius is: 0.332 nm r 2 2 3.14 0.0529 nm. 51 Quantum mechanics, cont’d These developments introduce an entirely new branch of physics called quantum mechanics. It deals with physical systems that are quantized. A pivotal contribution is called the Heisenberg uncertainty principle. It is based on the observation that atoms are actually composed of objects that have wavelike properties. 52 Quantum mechanics, cont’d The difference between a wave and a particle is physical extent. We can imagine a particle as a small ball with a precise position. A wave must be spread out and does not have a precise position. So our particles are more like fuzzy cotton balls rather than shrunken marbles. This means you cannot precisely state their position. 53 Quantum mechanics, cont’d Heisenberg was able to develop the formula: x mv h x is the uncertainty of the particle’s position, mv is the uncertainty of the particle’s momentum, and h is Planck’s constant. This means if you have high certainty of where the particle is, you have little knowledge of what its momentum is. So the resolution of our experiments is limited by nature — not because of our ignorance. 54 Quantum mechanics, cont’d Schrödinger established a model in which the hydrogen atom is described by a wave function. The wave function describes the probability of finding the atom in a particular configuration. 55 Atomic structure We now know that the Bohr model is not accurate. The atom is actually pictured as a tiny nucleus surrounded by an “electron cloud.” The density of the cloud indicates the probability of the finding the electron at some point. 56 Atomic structure, cont’d Since the exact position of the electron is not known, it is more useful to describe the electron’s orbit by its energy. The electrons are described as being in certain allowed energy states called energy levels. The lowest energy level is called the ground state. The higher energy states are referred to as excited states. 57 Atomic structure, cont’d These energy states are represented by an energy-level diagram. Each energy level is labeled with a quantum number, n. The ground state has n = 1. 58 Atomic structure, cont’d The difference in energy between adjacent states decreases as n increases. The highest state is labeled as n = . This energy is the ionization energy. The numbers on the left of the energy-level diagram indicate the energy of each state. 59 Atomic structure, cont’d The negative values indicate that the electron is bound to the nucleus. The change in electron energy as a result of an “energy-level transition” is found by comparing the energies of the two states. 60 Atomic structure, cont’d The photon energy for a transition between the ground state (n = 1) and the first excited state (n = 2) is: E E2 E1 3.4 eV 13.6 eV 10.2 eV. Referring to Figure 10.7, this is a photon of UV light. The frequency is 2.5×1015 Hz. 61 Atomic structure, cont’d Here is a diagram of several downward transitions. For each transition, the wavelength is given in nanometers. 62 Atomic structure, cont’d From this figure, some conclusions may be inferred. Transitions to the ground state (n = 1) result in the emission of ultraviolet photons. This series of emission lines is referred to as the Lyman series. Transitions from higher energy levels to the first excited state (n = 2) result in the emission of visible photons. This series is referred to as the Balmer series. Transitions from higher levels to n = 3 result in the emission of infrared photons. This series is referred to as the Paschen series. 63 Example Example 10.4 Find the frequency and wavelength of the photon emitted when a hydrogen atom goes from the n = 3 state to the n = 2 state. 64 Example Example 10.4 ANSWER: From the figure, we find: E2 3.4 eV E3 1.51 eV So the energy is: E E3 E2 1.51 eV 3.4 eV 1.89 eV. 65 Example Example 10.4 ANSWER: The frequency is: E 1.89 eV f 15 h 4.136 10 eV/Hz 4.57 10 Hz. 14 66 Example Example 10.4 ANSWER: Which gives a wavelength of: c 3 10 m/s 14 f 4.57 10 Hz 8 7 6.57 10 m 657 nm. 67 Example Example 10.4 DISCUSSION: This wavelength corresponds to a photon of visible light — red. 68 Atomic structure, cont’d An electron does not have to make a direct “jump” to a certain energy level. For an electron in the n = 4 level, it can transition to n = 2 and then to the n = 1 level. Such a process is called a cascade. 69 Atomic structure, cont’d The energy-level diagram helps illustrate what happens when hydrogen gas in a tube is heated to high temperatures or excited by passing an electric current through it. The atoms will be excited into higher energy levels. The excited atoms will undergo transitions to lower energies, emitting photons. Although several transitions are possible for a given temperature, some are more favored. Such a system produces the emission spectrum of hydrogen. 70 Atomic structure, cont’d Here are two examples of emission spectra for excited gases. A neon sign and the aurora borealis. 71 Atomic structure, cont’d So far, we’ve only discussed hydrogen. It is the simplest element since it only has a single electron. As you examine elements of higher atomic number, the number of electrons increases. For the neutral atom, the atomic number indicates the number of electrons. By examining the emission spectra of various elements, Wolfgang Pauli discovered that certain transitions to lower states did not occur when the lower states were already occupied. 72 Atomic structure, cont’d This led him to develop the Pauli exclusion principle: Two electrons cannot occupy precisely the same quantum state at the same time. This applies to an entire class of elementary particles, known as fermions. The electron is just one species of this class. For each energy level, there exist a set number of quantum states available to the electron. 73 Atomic structure, cont’d Once all the quantum states are occupied for a given energy level, additional electrons must occupy other energy levels that have vacancies. The number of electrons available for each energy level are: n = 1 can have no more than 2 electrons. n = 2 can have no more than 8 electrons. n = 3 can have no more than 18 electrons. The general rule is 2n2 electrons for energy level n. 74 Atomic structure, cont’d Here are some examples. 75 Atomic structure, cont’d The structure of the periodic table of elements reflects the idea behind the Pauli exclusion principle. 76 Laser The word laser is an acronym derived from the phrase “Light Amplification by Stimulated Emission of Radiation.” Imagine an electron in an atom has been excited to some higher energy level. This might be due to a collision or by absorption of a photon. The electron will remain in this state for only a short time. 77 Laser, cont’d If this decay to a lower energy level occurs spontaneously, a photon will be emitted in some random direction. There is an alternative process through which the electron can return to its original energy level: stimulated emission. 78 Laser, cont’d This involves a second photon of the same energy as the original photon. Consider a group of atoms, having their electrons in this same excited state, that is “bathed” in light consisting of photons with energy E. The atoms will be stimulated to decay by emitting additional photons of energy E. This increases the intensity of the light. 79 Laser, cont’d The stimulating radiation and the additional emitted radiation are in phase. The crests and troughs of the EM waves at a given point all match up. Such radiation is said to be coherent. Ordinary light is incoherent. 80 Laser, cont’d To achieve this light amplification requires some ingenuity. Most excited states decay very quickly. Some states are called metastable because they do not decay for a much longer time. Perhaps 0.000 1 s instead of 0.000 000 000 1 s. Atoms in this state do not decay during the time it takes to excite more than half of the other atoms. This process is called pumping. 81 Laser, cont’d With such a majority of atoms in the upper energy level, such a situation is called population inversion. Here is an energy-level diagram indicating the population inversion. 82 Laser, cont’d Here is a schematic of a ruby laser system. 83