* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download phenotype describes a new mutation affecting
History of genetic engineering wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Genetically modified crops wikipedia , lookup
Biology and consumer behaviour wikipedia , lookup
Minimal genome wikipedia , lookup
Preimplantation genetic diagnosis wikipedia , lookup
Ridge (biology) wikipedia , lookup
Genome evolution wikipedia , lookup
Genome (book) wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Mir-92 microRNA precursor family wikipedia , lookup
Microevolution wikipedia , lookup
Gene expression programming wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Genomic imprinting wikipedia , lookup
Journal of Experimental Botany, Vol. 47, No. 299, pp. 755-762, June 1996
Journal of
Experimental
Botany
The rea (red embryonic axis) phenotype describes a new
mutation affecting the response of maize embryos to
abscisic acid and osmotic stress
M. Sturaro1, P. Vernieri2, P. Castiglioni1, G. Binelli1 and G. Gavazzi1'3'4
1
2
3
Dipartimento di Genetica e di Bioiogia dei Microrganismi, Universita di Milano, Milano, Italia
Dipartimento di Bioiogia delle Piante Coltivate, Universita di Pisa, Pisa, Italia
Dipartimento di Fisiologia delle Piante Coltivate e Chimica Agraria, Universita di Milano, Milano, Italia
Received 30 May 1995; Accepted 12 February 1996
Abstract
During a screen for mutants with defective germination, a new phenotype was observed consisting of red
pigmentation of the embryonic axis in the dormant
seed. Segregation ratios, as determined in F2 and
back-crossed progeny, indicate that the phenotype is
due to a recessive single gene mutation that has been
symbolized rea to denote red embryonic axis. A closer
inspection of the rea phenotype revealed that the
mutant is occasionally viviparous, indicating a defect
in abscisic acid (ABA) metabolism. The mutation probably affects ABA sensitivity since no difference in ABA
content was detected in mutant versus normal tissues.
Moreover, when immature mutant and wild-type
embryos were incubated on media containing 10 //M
ABA, only the mutants germinated. ABA-regulated
gene expression in rea embryos differed from that of
embryos of the viviparous mutant vp1 which does not
respond to the inhibitory action of ABA at the level of
immature embryo germination. These results, therefore, indicate that the two genes exert a different role
in the control of embryogenesis.
Key words: Zea mays L, embryo dormancy, ABA.
Introduction
Embryogenesis, encompassing the period between the first
zygotic division and seed maturity, is an important period
of plant development because the principal events
involved in the achievement of the basic structure of the
plant occur during this stage. In maize, where this process
" To whom correspondence should be addressed. Fax: + 39 2 70 630 856.
Oxford University Press 1996
lasts about 50 d, two phases can be distinguished: an
early phase encompassing the first 16 d after pollination
(DAP) where the basic structure of the plant is defined,
and the ensuing maturation phase, from 16 d to seed
maturity, characterized by an increase in size, synthesis
of seed storage products and the acquisition of a dormant
state preventing precocious germination (Sheridan and
Clark, 1987). Abscisic acid (ABA) plays an important
role during the maturation stage of embryogenesis. For
example, it prevents precocious germination and is
involved in the regulation of several genes (Davies and
Jones, 1994).
In maize, mutations affecting ABA metabolism are
recognized because they fail to induce embryo dormancy,
thus resulting in precocious germination, an event referred
to as vivipary (Robertson, 1955). These mutants can be
grouped into two classes: class I represented by vpl,
which have normal levels of endogenous ABA, but are
insensitive to the germination suppressive effect of exogenous ABA, and class II which have a reduced level of the
growth substance, both in the embryo and in the plant,
and are unable to accumulate ABA in response to water
deficit (Robichaud and Sussex, 1986; Neill et al, 1986).
Similarly, in Arabidopsis, mutants affecting ABA metabolism have been classified as ABA-deficient {aba) or ABAinsensitive (abi) (Koorneef et al, 1984). In both species,
the two groups of mutants, respectively, represent defects
in ABA synthesis or responsiveness.
vpl has been studied extensively and its product has
been identified as a transcriptional activator that mediates
the ABA response during seed development (McCarty
et al, 1991). In Arabidopsis, the ABB gene, whose
756
Sturaro et al.
mutation causes germination to be insensitive to abscisic
acid, has been isolated and shown to be structurally
related to the maize vpl gene (Giraudat et al., 1992).
Altered expression of several ABA responsive genes is
observed in different vp mutants: in cultured embryos of
class II mutants there is usually a reduced basal level of
the transcript, but gene expression can be induced by
treating embryos with ABA (Butler and Cuming, 1993;
Paiva and Kriz, 1994; Pla et al., 1991; Thomann et al.,
1992; Williamson and Scandalios, 1992). In the vp]
mutant, on the other hand, some ABA-inducible genes
can not be detected, even following ABA treatment
(Butler and Cuming, 1993; Paiva and Kriz, 1994;
Thomann et al., 1992) while others show vpl independent
expression profiles (Williamson and Scandalios, 1992).
Interestingly, some of the genes requiring a functional
Vpl product for ABA induction are expressed in vpl
mutants in response to an osmotic stress induced by the
osmolyte mannitol (Butler and Cuming, 1993; Thomann
et al, 1992; White and Rivin, 1995) indicating that
different parallel pathways are operating in the perception
of ABA and osmotic stress (Bray, 1993).
In a chemically mutagenized population, a new maize
mutant has been detected, characterized by a strong
pigmentation of the embryonic axis in the dormant seed
and by an incipient vivipary which occasionally leads to
precocious germination and desiccation of the seedling.
The mutant, named rea (red embryonic axis), is unaffected
in the level of endogenous ABA, but exhibits reduced
sensitivity to its presence in the growth medium. These
features make rea a vpl-like mutant, although a linkage
analysis using RFLP markers appears to exclude allelism
between rea and vpl. At the molecular level, the expression of two ABA-responsive genes, as determined in
homozygous rea mutant embryos versus their wild-type
counterparts, is less affected by ABA and is not induced
by osmotic stress.
tion of 0.3 M before autoclaving while ABA (Sigma, mixed
enantiomers) was added at a concentration of 10 /xM or 100 /uM
after autoclaving. Cultures were incubated in a growth chamber
at 26 °C in the dark for 8 d. Germination was determined daily
on the basis of shoot or root emergence. Standard errors of
mean values, when not reported, were less than 5%.
RNA gel blot analysis
RNA was extracted from 1 -3 g of frozen tissue using the
method described by Van Tunen et al. (1988). For Northern
blot analysis, 10 //.g of total RNA for each sample was
electrophoresed through a 1.5% agarose gel containing 20 mM
3-[iV-morpholino] propane sulphonic acid (MOPS), 5 mM
sodium acetate, 0.5 mM EDTA, 18% formaldehyde and blotted
on to nylon filters (Pall Europe, Portsmouth, England). Glbl
and Rabl 7-specific transcripts were detected using 32P-labelled
inserts from the pcGlblS and pMA12 cDNAs, respectively. The
cDNAs were kindly provided by Dr M Pages, Consell Superior
dTnvestigacions Cientifiques, Barcelona, Spain (Rabl 7) and by
Dr AL Kriz, Dekalb Plant Genetics, Discovery Research,
Mistic, CT. 06355, USA (Glbl). Hybridization was carried out
at 65 °C. Filters were washed twice in 2 x SSC, 1% SDS at 65 °C.
ABA determination
The ABA content of embryos and leaves was determined by
radioimmunoassay using DBPA1 monoclonal antibody as
previously described (Bochicchio et al., 1994). Each analysis
was performed in triplicate on pools of six embryos. Since there
is no difference in mutant versus wild-type embryo size, the
results are expressed per unit of fresh weight. ABA accumulation
in stressed plants was determined using the detached leaf test
(Quarrie et al., 1988). For this analysis, 13 mutant and 26 wildtype kernels from a segregating selfed ear were germinated on
filter paper in well-watered conditions. When the developing
seedlings reached the third leaf stage, samples of similar size
were collected from the second leaf and dissected into two
parts, one was immediately frozen to assay the pre-stress ABA
level and one left on dryfilterpaper for 2 h before being frozen,
to evaluate ABA accumulation under water-stress conditions.
To validate the RIA results of this experiment, non-competitive
interference was determined by internal standardization as
described in Bochicchio et al. (1994). Parallelism between the
standard curve and those obtained after adding 10 or 20 ^g of
crude extract to each point confirms the lack of competitive
interference.
Material and methods
RFLP analysis
Genetic stocks
Samples of leaves of each of 93 individuals of a backcrossed
(BC) population and from the two parental lines were collected
and their DNA extracted as described in Dellaporta et al.
(1985). Digestion with restriction endonucleases (EcoRI,
BamHI and Hindlll) was carried out on 10 /xg of genomic
DNA for each sample. The resulting fragments were separated
on 1.5% agarose gels and blotted on nylon membranes. Filters
were hybridized and washed as described for RNA detection.
Clones for RFLP analysis were obtained from Dr D Grand
(Pioneer Hi-Bred Int.), Dr B Burr (Brookhaven National
Laboratory) and Dr D Hoisington (University of Missoury,
Columbia). The fragments recovered after PstI digestion were
labelled by random primed extension (Prime-a-Gene kit,
Promega). For RFLP analysis, 12 markers located on chromosome 3 (2 on 3S and 10 on 3L arms) were used to detect
polymorphism between the two parental lines. The six markers
which showed polymorphism (php20558, umc26, bnl5.37,
bnl6.16, umc!7, php 10080) were subsequently used to test
The rea phenotype first appeared in the progeny of a sample of
seeds of the W22 inbred line mutagenized with the alkylating
agent ethyl methane sulphonate (Gavazzi et al., 1993). The
mutant stock was outcrossed to the inbred line W64A and then
selfed for two cycles to obtain a homozygous stock. The
viviparous vp] mutant introduced in the W22 background was
obtained by Dr DS Robertson, Iowa State University, Ames,
IA 50011.
Embryo culture
Ears were harvested 28 d after pollination (DAP) and seeds
were surface-sterilized with 5% sodium hypochlorite for 15 min
and then rinsed in sterile distilled water. Embryos were removed
aseptically and transferred to Murashige and Skoog (MS)
medium, pH 5.6, containing 3% sucrose and solidified with
0.8% agar. When required, mannitol was added at a concentra-
rea phenotype of maize embryos
association with the rea locus in the BC population. Linkage
analysis was performed by multipoint analysis of data using
the MAPMAKER/EXP program (Lander et al, 1987).
Results
Mutant description and genetic analysis
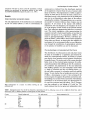
The red pigmentation of the embryonic axis conditioned
by the rea mutant (Plate 1) is due to accumulation of
757
anthocyanin as deduced from the absorbance spectrum
of the pigments extracted from the axis of mature seeds
(data not shown). This pigmentation is visible at approximately 25 DAP and by the time the seed is mature it is
distributed evenly in the outermost layer of the embryonic
axis, but is not detectable in other parts of the embryo,
including the scutellum. This pigmentation was a convenient marker for mutant embryos when vivipary was not
apparent. In all crosses of the mutant with different
inbred lines, the trait disappears, but reappears in the F 2
or in the progeny of back crosses to the parental mutant
line. Thus, embryonic pigmentation behaves as a recessive
trait. The mutant segregation, while approximating the
expected one-half in backcrossed families, is less than the
expected one-quarter in F 2 populations (Table 1). The
extent is genotype-dependent. However, individual progenies of selfed Fj plants differ in their mutant segregation
values (data not shown), an observation that suggests the
involvement of an environmental effect on rea expression,
even though the contribution of gametophytic selection
or the presence of modifiers can not be discounted.
The rea phenotype is not associated with the R locus
Plate 1. Enlargement of a mature rea embryo showing the red
embryonic axis.
The distribution of anthocyanin in seed and plant tissues
of maize is controlled by a regulatory gene denoted R,
which is a transcriptional activator. R consists of a small
gene family whose members control pigment distribution
in specific tissues. The stocks used in the crosses described
below carry all the structural genes necessary for anthocyanin biosynthesis while differing in their R constitution.
R-r conditions red pigmentation of the seed (aleurone)
and seedling (primary root and coleoptile) tissues while
r-r leads to colourless seed but red seedling tissues, r-g,
the null allele, accounts for colourless seed and seedling
tissues. To test whether the red embryonic axis trait is an
R effect, a homozygous rea and r-r line carrying all the
structural genes for pigment biosynthesis (colourless seed,
red seedling) was crossed with a second line with identical
constitution in the structural genes, but homozygous for
R-r and Rea (coloured seed and plant). The F 2 progeny
was then analysed to test linkage of R with rea. The
results indicate that the two genes are independent, since
Table 1. Segregation ratios (%) of the rea mutation as determined in the F2, following crosses of rea to different inbred lines, and in
backcrosses (BC) of Rea/rea females to homozygous rea male parents
Crosses
BC
Genetic background
No. seeds scored
Mutant
segregation (%)
Mul
±SE
W22
W64A
B73
A188
K6
W22
B73
ACR line
2554
1226
918
803
1297
287
878
537
19.3
8.9
21.0
15.1
8.1
47.0
65.0
49.3
3.2
2.6
3.9
1.9
2.6
1.0
7.0
3.1
758 Sturaro et al.
the pigmented embryonic axis can also be recovered in
the R-r background, thus excluding the hypothesis that
this pigmentation is an effect of the r allele present in the
rea line. The progeny of selfed heterozygous r-g Rea/r-r
rea plants (r-g: colourless seed and seedling) was also
analysed without recovering any rea non-red seedlings
(corresponding genotype: r-g/r-g rea/rea expected, in the
F 2 population with a frequency of 1/16th). This indicates
that a functional r allele must be present in the plant
tissues in order for the rea mutation to be manifested in
the dormant embryo.
The rea mutant is not affected in ABA synthesis
Endogenous ABA levels in developing maize embryos
increase from 14 DAP and reach a peak at about 35
DAP (Bochicchio et al., 1994). However, the ABA content of class II vp mutants at 35 DAP is less than 25% of
the wild type (Paiva and Kriz, 1994). To determine if the
viviparous phenotype of the rea mutant was due to a
defect in ABA biosynthesis, the level of this compound
was assayed in immature (35 and 40 DAP) embryos
segregating from a +/rea selfed ear. The evaluation of
the endogenous ABA level in immature embryos does
not reveal any significant difference in ABA content of
mutant versus normal embryos (Table 2). Similarly, the
ABA content of mutant seedlings grown under normal
conditions or after 2 h of water stress is not different
from wild-type siblings (Fig. 1). Taken together, these
results seem to rule out the involvement of the rea gene
in ABA synthesis.
Table 2. ABA content of immature rea and wild-type F2 embryos
Mutant rea embryos are insensitive to exogenous ABA and
mannitol
Homozygous vp] embryos are much less inhibited in
germination than their wild-type counterparts when
grown in the presence of ABA (10 /xM) or mannitol
(0.3 M) (Robichaud and Sussex, 1986; Neill et al., 1987).
To assay whether the rea mutation confers a similar
reduction in ABA and osmotic stress sensitivity, immature
embryos were exposed to these conditions and their effect
was quantified on the basis of germination efficiencies
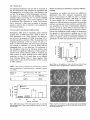
(Fig. 2), growth in culture (Plate 2) and time-course of
germination (Fig. 3). Homozygous vpl embryos of identical age were included as a positive control to demonstrate
that growth of ABA-insensitive seedlings was not disrupted (Fig. 4), while immature wild-type embryos were
used as a negative control to demonstrate inhibition of
germination by ABA and mannitol. The results reported
|
0Wt
• rea
#
100
.2
8
»
60
°
I «
20
0
I
MS
ABA
ABA
100(]M
Mannitol
0.3M
Growth media
Fig. 2. Effect of the addition of ABA (10 /xM and 100 /^M) and
mannitol (0.3 M) on the germination of immature rea embryos.
ABA was determined by radioimmunoassay as described in Quarrie
etal. (1988).
ABA content (ng g" 1 FW +SE)
Embryo age
(DAP)
35
40
-
1600
UJ
c i", 1200
o i
800
m \
400
o
< a>
<
rea
Wild type
173 + 8
116+15
160 + 1
119 + 7
0 Control
[H water stress
1
Ol
c
0->
rea
rea
wt
Growth conditions
wt
Fig. 1. ABA content (ng g ' DW ± S E ) in leaves of F 2 rea and wildtype seedlings grown in well-watered conditions (control) or after 2 h
of water stress.
Plate 2. Growth of immature rea and normal F 2 embryos on MS basic
medium (A) or in the presence of 100 jiM ABA (B), 10 ^M ABA (C),
and 0.3 M mannitol (D).
rea phenotype of maize embryos
to wild-type embryos, even when placed on medium
lacking ABA (Plate 2). However, when compared to vpl,
the rea embryos were more severely inhibited in their
growth when cultured on media supplemented with 10 /nM
ABA or 0.3 M mannitol, and their shoot elongation in
the presence of 100 ^M ABA was totally prevented. In
contrast, in vpl embryos only shoot length was affected
(Fig. 4).
A
MS
100
•
60
20
0
B
ABAIOpM
rea and vpl are not allelic
100 •
——
60
ma
/ -
20
t
>
C
|
,
,
,
4wt
1
»
Mannitol 0.3 M
^____— rea
100
r
60
•z.
20
00
759
1
__
L—
mml
2
3
4
—T
5
wt
T
6
7
8
Days of culture
Fig. 3. Germination time-course of immature (28 DAP) rea versus
normal seedlings on different media1 (A) MS basic medium, (B) basic
medium with 10 ^.M ABA and (C) basic medium with 0.3 M mannitol.
One possible explanation for the loss of ABA sensitivity
in the mutant is allelism between rea and vpl. Vpl besides
cotrolling embryo dormancy, regulates anthocyanin synthesis in the aleurone layer of the seed. Therefore, the
vpl mutant in the presence of a full set of active genes
for anthocyanin production has a non-coloured aleurone
(Robertson, 1955). To test the hypothesis of allelism
heterozygous +/vpl females were crossed with homozygous rea male parents; the resulting seeds, carrying a full
set of genes for anthocyanin production, had dormant
non-coloured embryos but a fully coloured aleurone. This
result indicates complementation between rea and vpl.
The association of the rea phenotype with six RFLP
markers of chromosome 3 (see methods) was then
checked, all but one of which {phpl0080) were linked to
the vpl locus. None of these markers appears to be linked
to rea (data not shown). The hypothesis of independence
is supported by an LOD score of 2.77 versus the most
likely position for association.
Gene expression in response to ABA and osmotic stress
MS
ABAIOuM
ABA100uM Mann.0.3M
Growth media
Fig. 4. Effect of the addition of ABA (10 MM and 100 ^M ) and
mannitol (0.3 M) to MS basic medium on rea (A) and vpl (B) shoot
elongation. Measurements were taken after 8 d of culture.
in Fig. 2 and Plate 2 show that rea mutants were altered
in both ABA and stress response in terms of germination
and shoot growth. Besides showing a higher germination
capacity in the presence of 10 /xM ABA or 0.3 M mannitol, they also exhibited more rapid germination compared
The vpl mutation, besides conferring ABA insensitivity,
exerts a negative effect on the induction of several ABA
responsive genes. A similar, but not identical, effect is
observed if the mutant is exposed to high osmotic conditions. Glbl transcription is not activated by ABA or
osmotic stress in vpl embryos (Paiva and Kriz, 1994;
Rivin and Grudt, 1991), and neither is rabl7 as can be
inferred from translational studies (Butler and Cuming,
1993). However, application of mannitol before the onset
of vivipary triggers rabl 7 expression in vpl mutants.
To assay how the transcription of these two genes is
affected in rea mutants, Northern analysis was performed
of Glbl and rabl 7 expression in immature embryos cultured for 8 d on media supplemented with 10 [xM and
100 /xM ABA or 0.3 M mannitol. Wild-type embryos
from the same segregating ear (F 2 ) or homozygous + / +
ears (F 3 ) were placed on the same Petri plates to evaluate
the effectiveness of the various treatments in inducing
Glbl and rabl 7 gene expression. Homozygous vpl
embryos of the same age (28 DAP) were used as a
positive control. Both mutant and normal embryos show,
at the time of their excision, Glbl and rabl 7 transcripts
(data not shown) that are no longer detectable after 8 d
760 Sturaro et al.
of culture on the basic MS medium (Plate 3). Addition
of ABA to the medium induced Glbl and rabl 7 expression
in rea embryos, although to a lesser extent than in wildtype embryos, while mannitol does not have any effect.
Thus, while in the vpl mutant the regulation of these
genes by ABA and by mannitol is impaired, in rea only
the regulation by mannitol is affected.
Discussion
Genetic dissection by means of single-gene mutants is a
powerful tool for the elucidation of different aspects of
plant morphogenesis and development. Embryo defective
mutants have been isolated in several plant species including Arabidopsis (Meinke, 1991) and maize (Sheridan and
Neuffer, 1981). Recent work suggests that a complex
network of genetically defined events regulates the onset
of embryo dormancy involving on the one hand vegetative
growth suppression, and on the other an induction of the
synthesis of specific embryo proteins. Abscisic acid has a
primary role in the control of embryo dormancy although
other ABA-independent pathways might exist.
In maize, vivipary is a diagnostic phenotype for the
involvement of ABA since all known vp mutants are
either ABA-deficient or ABA-insensitive. To establish
Mutant
MS
Mb
ABA
1OOuM
ABA
1Q M
ManrV
a 3 M
m wt m wt Mm wt I \m wt
A Rab17
rea
vpl
t
BGIbi
rea
vpl
Plate 3. Effect of ABA and mannitol on the accumulation of rabl 7 and
Glbl transcripts in immature (28 DAP) rea and vpl embryos, determined
after 8 d of culture on basic MS medium or with ABA (10 /*M) and
mannitol (0.3 M) added. Total RNA was isolated and analysed by
RNA blot hybridization with probes complementary to Rabl7 (A) and
Glbl (B). RNA from normal sibling embryos was included as a control.
The blot was also hybridized with Tub, a 1 kb cDNA clone of maize
tubulin (Dolfini el al.. 1993), to verify equal RNA loading (data
not shown).
whether rea belongs to one of these two classes of
mutants, endogenous ABA levels and sensitivity to the
hormone was analysed. In normal embryos, the ABA
level increases reaching a peak at about 35 DAP
(Bochicchio et al, 1994); at the same time, the embryo
becomes less sensitive to ABA while progressing towards
embryo dormancy.
ABA-deficient mutant embryos of class II have ABA
endogenous levels that are less than 25% of wild-type
levels (Paiva and Kriz, 1994). Seedlings rescued from
viviparous kernels contain less ABA than normal siblings
and the level does not increase in response to water deficit
(Neill et al., 1986). On the contrary, immature rea
embryos have a normal amount of endogenous ABA.
Similarly, in the seedling, both the basal level and the
ABA accumulation in response to water stress are about
the same as in non-mutant siblings. These results rule out
the involvement of rea in ABA biosynthesis.
The mutants responsiveness to ABA during embryogenesis was assayed by placing immature embryos on a
culture medium supplemented with ABA. At a concentration of 10 fxM this growth regulator normally prevents
germination of wild-type embryos, vpl and some class II
vp mutant embryos, however, show a decreased sensitivity
to ABA, with vpl being the least sensitive of all
(Robichaud et al, 1980). Similarly, it was found that
immature rea embryos germinate in the presence of 10 /nM
ABA while their non-mutant siblings almost totally fail
to germinate. These results, namely reduced ABA sensitivity and precocious germination on basal medium, indicate
that the rea mutant does not enter proper developmental
arrest during the maturation stage of embryogenesis
because of a reduced response to the ABA inhibitory
signal. It would be interesting to test whether other rea
mutants exhibit reduced ABA sensitivity as a means to
establish whether rea mutant represents a leaky or a null
mutation. Three new rea alleles have recently been identified that will allow us to address this question. As with
exogenous ABA, osmotic stress caused by osmolytes such
as mannitol added to the culture medium prevents precocious embryo germination while triggering ABA biosynthesis. The inhibitory effect on germination, however, is
not merely a consequence of ABA induction (Neill et al,
1987). Mutant vpl embryos are less affected than wildtype siblings by osmotic stress. Similarly, rea embryos
germinate on media supplemented with 0.3 M mannitol,
a condition that prevents growth of normal embryos.
Both ABA and osmotic stress exert positive regulation
on the transcription of a number of genes that seem to
be part of a general programme of response to stress and
that are normally active during the later stages of embryogenesis (Galau et al, 1987; Morris et al, 1990). The
product of one of these, rabl7, accumulates during the
maturation phase of embryogenesis and disappears after
the onset of germination. ABA treatments and water
rea phenotype of maize embryos
stress induce its transcription in embryonic and seedling
tissues (Goday et al, 1988; Pla et al, 1989). Another
ABA inducible gene, GIbl, is only expressed during
embryogenesis starting from 20 DAP (Belanger and Kritz,
1989). However, germination in the presence of ABA
maintains Glbl expression at high levels (Kriz et al.,
1990). In mutant vpl embryos, ABA does not induce the
transcription of some of the ABA-inducible genes, including rabll and Glbl (our data and Paiva and Kriz, 1994),
although others are induced normally. These results suggest that there is more than one ABA response pathway,
and that the Vpl gene product participates in one of
these, whilst others are Vpl independent. The response
to osmotic stress in terms of ABA-inducible gene expression in homozygous vpl embryos does not always correlate with the ABA response; for some genes, only one of
these two effectors is capable of promoting transcription.
This suggests the existence of discrete parallel pathways
mediating ABA and stress responses (Thomann et al,
1992; Butler and Cuming, 1993).
When assayed in the rea mutant, the expression of
both rabl7 and Glbl is promoted by ABA, though to a
lesser extent than in normal embryos, while mannitol has
no such effect. These results, and those related to germination in the presence of applied ABA and mannitol, can
be explained by assuming that rea is mutated in a gene
involved in the response to both ABA and osmotic stress.
What is still unclear is the relationship between the
reduced ABA and stress sensitivity of the rea mutant and
the induction of anthocyanin biosynthesis in the embryonic axis. Pigmentation could merely be the result of a
general precocious activation of some post-germination
specific biosynthesis caused by a limited or improper
onset of embryo dormancy in the rea mutant or, alternatively, there could be a direct effect of ABA perception on
the regulation of anthocyanin biosynthesis. It is noteworthy to observe that Vpl, besides modulating ABA
responses in the embryo, exerts a positive regulation upon
the Cl gene, which itself controls genes involved in
anthocyanin biosynthesis (McCarty et al, 1991). In this
context the Rea gene would prevent the pigmentation of
the embryonic axis. A specific interrelationship between
anthocyanin synthesis and seed maturation processes has
also been demonstrated in Arabidopsis. In this species
mutants have been identified, like led (Meinke et al,
1994) and/wsJ (Baumlein et al, 1994) that appear to be
desiccation-intolerant, viviparous, accumulate anthocyanin, and contain reduced amounts of storage proteins.
These mutants together with the pleiotropic viviparous
mutants of maize may identify intrinsic factors that couple
the maturation pathway to embryo morphogenesis.
Summarizing, Vpl can be envisaged as a member of a
network of genes involved, through separate pathways,
in the control of critical events associated with embryogenesis. According to this model, Vpl mediates the expres-
761
sion of some but not all ABA regulated genes while rea
exerts its effect on ABA perception, normally leading to
the suppression of precocious germination. Other ABAassociated effects could be mediated by genes still uncharacterized. Another possibility is to assume that Vpl and
rea exert independent control on embryogenesis while
sharing some functions such as ABA sensitivity or osmotic
stress induction of specific genes. Indeed, the recent
discovery of Lee genes in Arabidopsis (Meinke et al,
1994) demonstrates that normal perception of ABA by
the Abi3 gene is necessary, but not sufficient, for many
important events associated with embryogenesis, the two
genes operating through different pathways to ensure that
the developing seed is prepared for desiccation and
dormancy.
Acknowledgements
This work was supported by a grant to G Gavazzi from
Ministero delle Risorse Agricole Alimentari e Forestali. We are
grateful to M Pages and AL Kritz for their generous provision
of probes and to C Bowler for critical reading of the manuscript.
References
Baumlein H, Miser a S, Luerben H, Kolle K, Horstmann C,
Wobus U, Muller AJ. 1994. The FUS3 gene of Arabidopsis
thaliana is a regulator of gene expression during late
embryogenesis. The Plant Journal 6, 379-87.
Belanger FC, Kritz AL. 1989. Molecular characterization of the
major maize embryo globulin encoded by Glbl gene. Plant
Physiology 91, 636-43.
Bochicchio A, Vernieri P, Puliga S, Balducci F, Vazzana C.
1994. Acquisition of desiccation tolerance by isolated maize
embryos exposed to different conditions: the questionable
role of endogenous abscisic acid. Physiologia Plantarum
91, 615-22.
Bray EA. 1993. Molecular responses to water deficit. Plant
Physiology 103, 1035-40.
Butler WM, Cuming AC. 1993. Differential molecular responses
to abscisic acid and osmotic stress in viviparous maize
embryos. Planta 189, 47-54.
Davies WJ, Jones HG. (eds) 1994. Abscisic acid: physiology and
biochemistry. Oxford: BIOS Scientific Publishers.
Dellaporta SL, Wood J, Hicks JB. 1985. A plant DNA
minipreparation: version 2. Plant Molecular Biology Reporter
1, 19-22.
Dolfini S, Consonni G, Mereghetti M, Tonelli C. 1993.
Antiparallel expression of the sense and antisense transcripts
of maize tubulin genes. Molecular and General Genetics
241, 161-9.
Galau GA, Bijaisoradat N, Hughes DW. 1987. Accumulation
kinetics of cotton Late-Embryogenesis-Abundant mRNA and
storage protein mRNA: co-ordinate regulation during
embryogenesis and the role of abscisic acid. Development
Biology 123, 198-212.
Gavazzi G, Dolfini S, Galbiati M. Helentjaris T, Landoni M,
Pelucchi N, Todesco G. 1993. Mutants affecting germination
and early seedling development in maize. Maydica 38, 265-74.
Giraudat J, Hauge BM, Valon C, Smalle J, Parcy F, Goodman
762 Sturaro et al.
HM. 1992. Isolation of the Arctbidopsis ABI3 gene by
positional cloning. The Plant Cell A, 1251-61.
Goday A, Sanchez-Martinez D, Gomez J, Puigdomenech P,
Pages M. 1988. Gene expression in developing Zea mays
embryos: regulation by abscisic acid of a highly phosphorylated 23 to 25 kDa group of proteins. Plant Physiology
88, 564-9.
Koorneef M, Reuling G, Karssen CM. 1984. The isolation and
characterization of abscisic acid-insensitive mutants of
Arabidopsis thaliana. Physiologia Plantarum 61, 377-83.
Kriz AR, Wallace MS, Paiva R. 1990. Globulin gene expression
in embryos of maize viviparous mutants. Plant Physiology
92, 538-42.
Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ,
Lincoln SE, Newberg L. 1987. MAPMAKER: an interactive
computer package for constucting primary linkage maps of
experimental and natural populations. Genomics 1, 174-81.
McCarty DR, Hartori T, Carson CB, Vasil V, Lazar M, Vasil
IK. 1991. The Viviparous-1 developmental gene of maize
encodes a novel transcriptional activator. Cell 66, 895-905.
Meinke DW. 1991. Perspectives on genetic analysis of plant
embryogenesis. The Plant Cell 3, 857-66.
Meinke DW, Franzmann LH, Nickle TC, Yeung EC. 1994.
Leafy cotyledon mutants of Arabidopsis. The Plant Cell
6, 1049-64.
Morris PC, Kumar A, Bowles DJ, Cuming AC. 1990. Osmotic
stress and ABA regulate expression of the wheat Em genes.
European Journal of Biochemistry 190, 625-30.
Neill SJ, Horgan R, Parry AD. 1986. The carotenoid and
abscisic acid content of viviparous kernels and seedlings of
Zea mays L. Planta 169, 87-96.
Neill SJ, Horgan R, Rees AF. 1987. Seed development and
vivipary in Zea mays L. Planta 171, 358-64.
Paiva R, Kriz A. 1994. Effect of abscisic acid on embryo-specific
gene expression during normal and precocious germination
in normal and viviparous maize {Zea mays) embryos. Planta
192, 332-9.
Pla M, Goday A, Vilardell J, Gomez J, Pages M. 1989.
Differential regulation of ABA-induced 23-25 kDa proteins
in embryo and vegetative tissue of the viviparous mutants of
maize. Plant Molecular Biology 13, 385-94.
Pla M, Gomez J, Goday A, Pages M. 1991. Regulation of the
abscisic acid-responsive gene rab28 in maize viviparous
mutants. Molecular and General Genetics 230, 394-400.
Quarrie SA, Whitford PN, Wang TL, Cook SH, Henson IE,
Loveys BR. 1988. A monoclonal antibody to (S)-abscisic
acid: its characterization and use in a radioimmunoassay for
measuring abscisic acid in crude extracts of cereal and lupin
leaves. Planta 173, 330-9.
Rivin CJ, Grudt T. 1991. Abscisic acid and the developmental
regulation of embryo storage proteins in maize. Plant
Physiology 95, 358-65.
Robertson DS. 1955. The genetics of vivipary in maize. Genetics
40, 745-60.
Robichaud CS, Sussex IM. 1986. The response of viviparous-1
and wild type embryos of Zea mays to culture in the presence
of abscisic acid. Journal of Plant Physiology 126, 235-42.
Robichaud CS, Wong J, Sussex IM. 1980. Control of in vitro
growth of viviparous embryo mutants of maize by abscisic
acid. Developmental Genetics 1, 325-30.
Sheridan WF, Neuffer MG. 1981. Maize mutants altered in
embryo development. In: Subtelney S, Abbot U, eds. Levels
of genetic control in development. New York: Alan R.
Liss, 137-56.
Sheridan WF, Clark JK. 1987. Maize embryogeny: a promising
experimental system. Trends in Genetics 3, 3-6.
Thomann EB, SoUinger J, White C, Rivin CJ. 1992.
Accumulation of group 3 late embryogenesis abundant
proteins in Zea mays embryos. Plant Physiology 99, 607-14.
Van Tunen AJ, Koes RE, Spelt CE, van der Krol AR, Stuitje
AR, Mol NM. 1988. Cloning of two chalcone flavanone
isomerase genes from Petunia hybrida: co-ordinate, lightregulated and differential expression of flavonoid genes.
EMBO Journall, 1257-63.
White CN, Rivin CJ. 1995. Sequence and regulation of a late
embryogenesis abundant group 3 protein of maize. Plant
Physiology 108, 1337-8.
Williamson JD, Scandalios J. 1992. Differential response of
maize catalases to abscisic acid: Vpl transcriptional activator
is not required for abscisic acid-regulated Cat! expression.
Proceedings of the Natural Academy of Science USA 89,
8842-6.