* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download ag-ab react
Survey
Document related concepts
Immunoprecipitation wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
DNA vaccination wikipedia , lookup
Adaptive immune system wikipedia , lookup
Complement system wikipedia , lookup
Molecular mimicry wikipedia , lookup
Anti-nuclear antibody wikipedia , lookup
Immunocontraception wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Duffy antigen system wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Transcript
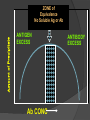
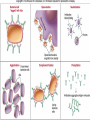
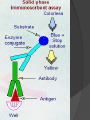
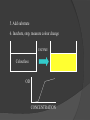
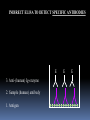
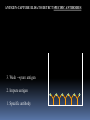
Lock and Key Concept Affinity • Strength of the reaction between a single antigenic determinant and a single Ab combining site High Affinity Low Affinity Ab Ab Ag Ag Affinity = attractive and repulsive forces Avidity • The overall strength of binding between an Ag with many determinants and multivalent Abs Keq = 104 106 1010 Affinity Avidity Avidity Specificity The ability of an individual antibody combining site to react with only one antigenic determinant. The ability of a population of antibody molecules to react with only one antigen. Cross Reactivity • The ability of an individual Ab combining site to react with more than one antigenic determinant. • The ability of a population of Ab molecules to react with more than one Ag Cross reactions Anti-A Ab Anti-A Ab Anti-A Ab Ag A Ag B Ag C Shared epitope Similar epitope Factors Affecting Measurement of Ag/Ab Reactions • Affinity • Avidity Ab excess Ag excess • Ag:Ab ratio • Physical form of Ag Equivalence – Lattice formation Antigen antibody tests Used in both directions Qualitative Quantitative Qualitative/quantitative Qualitative determines antigen or antibody is present or absent Quantitative determines the quantity of the antibody Titer The highest dilution of the specimen usually serum which gives a positive reaction in the test Antigen and antibody reactions in the lab Precipitation tests Agglutination ELISA Radioimmunoassay Immunofluorscence Complement Fixation AGGLUTINATION TESTS Particulate antigen • Slide agglutination • Tube agglutination • Passive agglutination + Slide agglutination test: Passive Agglutination/Hemagglutination Agglutination test done with a soluble antigen coated onto a particle + • Applications – Measurement of Abs to soluble antigens Coombs (Antiglobulin)Tests • Incomplete Ab • Direct Coombs Test – Detects antibodies on erythrocytes + Patient’s RBCs Coombs Reagent (Antiglobulin) Coombs (Antiglobulin)Tests Indirect Coombs Test Detects anti-erythrocyte antibodies in serum Step 1 + Patient’s Serum Target RBCs Step 2 + Coombs Reagent (Antiglobulin) Precipitation tests Soluble antigen reacts with specific antibody in presence of electrolytes at an optimal temp and pH to form an insoluble precipitate Flocculation – precipitate remains suspended as floccules Prozone phenomenon Precipitation takes place when there is optimal proportions of Antigen and antibodies Absence of precipitation in presence of excess of antibodies Such serum should be diluted before doing precipitation tests ZONE of Equivalence No Soluble Ag or Ab ANTIGEN EXCESS Ab CONC ANTIBODY EXCESS Mechanism of precipitation Marrack’s lattice hypothesis Precipitation occurs whenever multivalent antigens combine with bivalent antibodies to from a large lattice Same hypothesis applies for agglutination also Equivalence – Lattice formation Precipitation tests Precipitation techniques Tube precipitation test -Ring test – Ascoli’s thermo precipitation test Flocculation Slide – VDRL for syphilis Tube – Khan test for syphilis Ring test VDRL Radial Immunodiffusion • Method Ab in gel – Ab in gel – Ag in a well Ag Ag Ag Interpretation proportional to the concentration Quantitative Diameter2 Diameter of ring is Ig levels Ag Concentration Ag Immunoelectrophoresis Method Ags are separated by electrophoresis – Ab is placed in trough cut in the agar + Ag Ag Ab Ag Ab • Interpretation – Precipitin arc represent individual antigens Countercurrent electrophoresis Method Ag and Ab migrate toward each other by electrophoresis Used only when Ag and Ab have opposite charges - + Ag • Qualitative –Rapid Ab Complement fixation test (CFT) Complement plays an imp role in Ag-Ab reaction. In the presence of appropriate Abs: Complement lyses erythrocytes Kills bacteria, Immobilizes motile organisms Promotes phagocytosis Contributes to tissue damage in hypersensitivity reactions Principle of CFT: Ability of Ag-Ab complexes to fix complement Very sensitive test, can detect minute quantities of Ag & Ab All reagents have to be precisely standardised Eg: Wasserman reaction for sero-diagnosis of syphilis Complement Fixation assay (positive test) Complement Fixation Assay (negative test) Interpretation of result: Lysis of erythrocytes: Negative CFT Absence of Erythrocyte lysis: Positive CFT Indirect CFT: For testing sera that do not fix guinea pig complement: Hemolysis indicates a positive result CONGLUTINATING COMPLEMENT ABSORPTION TEST: Uses horse complement which is non-hemolytic Indicator system is sheep RBC mixed with bovine serum which contains a globulin called Conglutinin, which acts as Ab to the complement Other complement dependent serological test: 1. Immune adherence test 2. Immobilisation test: T.pallidum 3. Bacterial cytolytic test NEUTRALIZATION TEST Virus neutralization tests 2. Bacteriophage neutralization test 3. Toxin neutralization test (exotoxin) 4. Schick test: tests the ability of circulating antitoxin to neutralize diphtheria toxin injected ID 5. ASO test: 6. Nagler’s reaction: alpha toxin of Cl.perfringens 1. IMMUNOASSAYS RIA ELISA IMMUNOCHROMATOGRAPHY CHEMILUMINSCENCE ASSAY IMMUNOBLOTTING Radioimmunoassay Radioisotopes and enzymes are conjugated to Ag /Ab BINDER LIGAND ASSAY Ag whose concentration is to be determined is called Ligand (analyte) RIA is a competitive binding assay in which fixed amounts of Ab and radiolabelled Ag react in the presence of unlabelled Ag Labelled and unlabelled Ags compete for the limited binding sites on the Ab Applications of RIA: Quantitation of hormones. Drugs, tumor markers, IgE, and viral antigens Immunoflourescence Immunofluorescence is an antigen-antibody reaction where the Abs are tagged (labelled) with a fluorescent dye and the antigen-antibody complex is Immunofluorescence • Direct – Ab to tissue Ag is labeled with fluorochrome Fluorochrome Labeled Ab Ag Tissue Section ELISA (types): Direct indirect Sandwich Competetive Casette BASIC FORMAT Solid phase = 96 / 384-well microplate 1. Coat solid phase with antigen when analysing antibody antibody when analysing antigen Analyte = antibody Analyte = antigen Incubate, wash 2. Block free binding sites. Incubate. Wash. Analyte = antibody Analyte = antigen 3. Add sample. Incubate. Wash Analyte = antibody Analyte = antigen 4. Add conjugate. Incubate. Wash. E E Analyte = antibody E E Analyte = antigen 5. Add substrate 6. Incubate, stop, measure colour change ENZYME Colourless OD CONCENTRATION AMPLIFICATION E Directly conjugated developing antibody may give weak signal amplify with E E unlabelled (rabbit) anti-(human) Ig followed by anti-(rabbit) Ig-enzyme or E S E-S B S-E Biotin-labelled anti-Ig followed by streptavidin-enzyme SUBSTRATES See Sigma catalogue for list of conjugates and substrates Orthophenylene diamine hydrochloride (OPD) Tetramethyl benzidine (TMP) Horse radish peroxidase (HRP) Orange, 490 nm Yellow, 450 nm Spectrophotometer Paranitrophenyl phosphate (PNP) Methyl umbelliferol phosphate Alkaline phosphatase Yellow, 405 nm Spectrophotometer Methyl umbelliferone 365 nm 445 nm Fluorimeter INDIRECT ELISA TO DETECT SPECIFIC ANTIBODIES E 3. Anti-(human) Ig-enzyme 2. Sample (human) antibody 1. Antigen E E ANTIGEN-CAPTURE ELISA TO DETECT SPECIFIC ANTIBODIES Useful when pure antigen not available or antigen coats poorly 1. Specific antibody ANTIGEN-CAPTURE ELISA TO DETECT SPECIFIC ANTIBODIES 2. Impure antigen eg tissue homogenate 1. Specific antibody ANTIGEN-CAPTURE ELISA TO DETECT SPECIFIC ANTIBODIES 3. Wash pure antigen 2. Impure antigen 1. Specific antibody Types of immunodetection systems 2. Indirect immunodetection 1. Direct immunodetection Secondary antibody conjugated with enzyme system Primary antibody conjugated with enzyme system HRP HRP HRP HRP Ag Ag antigen Ag Ag Ag horseradish peroxidase ConsultantHRP General Surgeon 3. Sandwich indirect 4. Indirect immunodetection (A00692Y) immunodetection streptavidin with biotin linkers Antigen applied in soluble form Biotinylated primary antibodies HRP HRP HRP HRP HRP HRP HRP HRP HRP Ag Ag Ag Ag Streptavidin Competitive ELISA Solid Phase Non-Competitive RIA/ELISA Ab detection Labeled Anti-Ig Immobilize Ag Incubate with sample Add labeled anti-Ig Amount of labeled Ab bound is proportional to amount of Ab in the sample • Quantitative Ab in Patient’s sample Immobilized Ag Solid Phase Solid Phase Non-Competitive RIA/ELISA Ag detection Labeled Ab Immobilize Ab Incubate with sample Add labeled antibody Amount of labeled Ab bound is proportional to the amount of Ag in the sample • Quantitative Ag in Patient’s sample Ag Immobilized Solid Phase Cassette ELISA Few sample can be tetsed Specific antigens are coated on nitroclellulose membrane These strips are incubated with patients serum positive samples develop a colred spot or band Immunochromatography Chemiluminescent Immunoassays The process of chemiluminescence occurs when energy in the form of light is released from matter during a chemical reaction. Chemiluminescent Immunoassays Can be used for heterogeneous or homogeneous assays. Can attach label to antigen or antibody. Heterogeneous assays use competitive and sandwich assay. Competitive assays used to measure smaller analytes. Sandwich assays are used to measure larger analytes. Chemiluminescent Immunoassay Many applications. Can measure antigen or antibody. Add chemiluminescently tagged analyte. Measure light which is emitted which is directly related to concentration although competitive binding assays are available. t HIV-1 Western Blot Lane1: Positive Control Lane 2: Negative Control Sample A: Negative Sample B: Indeterminate Sample C: Positive TESTS FOR ANTIGEN-ANTIBODY REACTIONS Flow Cytometry Flow cytometry is commonly used in the clinical laboratory to identify and enumerate cells bearing a particular antigen. Cells in suspension are labeled with a fluorescent tag by either direct or indirect immunofluorescence. The cells are then analyzed on the flow cytometer. The figure at right illustrates the principle of flow cytometry. In a flow cytometer, the cells exit a flow cell and are illuminated with a laser beam. The amount of laser light that is scattered off the cells as they passes through the laser can be measured, which gives information concerning the size of the cells. In addition, the laser can excite the fluorochrome on the cells and the fluorescent light emitted by the cells can be measured by one or more detectors. TESTS FOR ANTIGEN-ANTIBODY REACTIONS The type of data that is obtained from the flow cytometer is shown below. In a one parameter histogram, increasing amount of fluorescence (e.g. green fluorescence) is plotted on the x axis and the number of cells exhibiting that amount of fluorescence is plotted on the y axis. The fraction of cells that are fluorescent can be determined by integrating the area under the curve. In a two parameter histogram, the x axis is one parameter (e.g. red fluorescence) and the y axis is the second parameter (e.g. green fluorescence). The number of cells is