* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The citric acid cycle is the
Electron transport chain wikipedia , lookup
Biochemical cascade wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Photosynthesis wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Mitochondrion wikipedia , lookup
Butyric acid wikipedia , lookup
Microbial metabolism wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
Basal metabolic rate wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Metalloprotein wikipedia , lookup
Biosynthesis wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Amino acid synthesis wikipedia , lookup
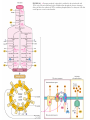
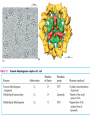
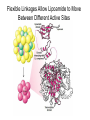
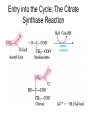
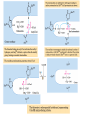
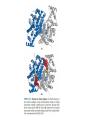
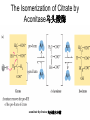
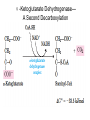
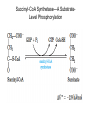
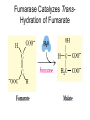
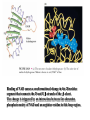
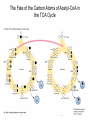
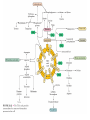
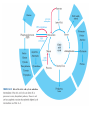
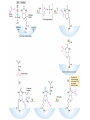
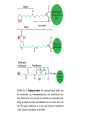
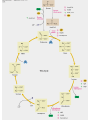
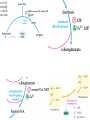
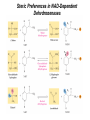
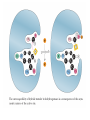
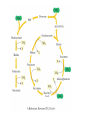
三羧酸循环 The Citric Acid Cycle The tricarboxylic acid (TCA) cycle The TCA Cycle—A Brief Summary • The entry of new carbon units into the cycle is through acetyl-CoA. • This entry metabolite can be formed either from pyruvate (from glycolysis) or from oxidation of fatty acids. • In the process, metabolic energy is captured in the form of ATP, NADH, and enzyme-bound FADH2 (symbolized as [FADH2]). • The citric acid cycle is the central metabolic hub of the cell. • It is the gateway to the aerobic metabolism of any molecule that can be transformed into an acetyl group or dicarboxylic acid. • The cycle is also an important source of precursors, not only for the storage forms of fuels, but also for the building blocks of many other molecules such as amino acids, nucleotide bases, cholesterol, and porphyrin (the organic component of heme). • The citric acid cycle is the final common pathway for the oxidation of fuel molecules— amino acids, fatty acids, and carbohydrates. • Most fuel molecules enter the cycle as acetyl coenzyme A. • citric acid cycle, also called the tricarboxylic acid (TCA) cycle or the Krebs cycle (after its discoverer, Hans Krebs). Under aerobic conditions, the pyruvate generated from glucose is oxidatively decarboxylated to form acetyl CoA. In eukaryotes, the reactions of the citric acid cycle take place inside mitochondria, in contrast with those of glycolysis, which take place in the cytosol. The Bridging Step: Oxidative Decarboxylation of Pyruvate oxidative decarboxylation reaction • pyruvate dehydrogenase complex(PDC) a cluster of enzymes—multiple copies of each of three enzymes— located in the mitochondria of eukaryotic cells and in the cytosol of prokaryotes. • It contains three enzymes: pyruvate dehydrogenase (E1), dihydrolipoyl transacetylase 二氢硫辛酸转乙酰基酶 (E2), and dihydrolipoyl dehydrogenase 二氢硫辛酸脱氢酶 (E3)—each present in multiple copies. • The overall reaction involves a total of five coenzymes: thiamine pyrophosphate焦磷酸 硫胺素, coenzyme A, lipoic acid硫辛酸, NAD, and FAD. 硫辛酸同酶分 子中Lys残基 的ε-NH2以酰 胺键共价结合 Flexible Linkages Allow Lipoamide to Move Between Different Active Sites Thiamine-deficient • As one might predict, mutations in the genes for the subunits of the PDH complex, or a dietary thiamine deficiency, can have severe consequences. • Thiamine-deficient animals are unable to oxidize pyruvate normally. This is of particular importance to the brain, which usually obtains all its energy from the aerobic oxidation of glucose in a pathway that necessarily includes the oxidation of pyruvate. • Beriberi脚气病, a disease that results from thiamine deficiency, is characterized by loss of neural function. This disease occurs primarily in populations that rely on a diet consisting mainly of white (polished) rice, which lacks the hulls in which most of the thiamine of rice is found. • People who habitually consume large amounts of alcohol can also develop thiamine deficiency, because much of their dietary intake consists of the vitamin-free “empty calories” of distilled spirits. • An elevated level of pyruvate in the blood is often an indicator of defects in pyruvate oxidation due to one of these causes. 硫辛酸 (Thioctic Acid, Lipoic Acid) • 酵母和微生物等必需的生长因子,含硫的 八碳酸,对于动物不能算是一个维生素, 但是一个辅酶,通过氧化还原作用参与生 物体的递氢和传递乙酰基的作用。 • 以硫辛酸(酰胺)为辅酶的酶有:硫辛酸 转乙酰酶、二氢硫辛酸脱氢酶、丙酮酸脱 氢酶、 α-酮戊二酸脱氢酶,与糖代谢关 系密切。 Entry into the Cycle: The Citrate Synthase Reaction Synthases and Synthetases • Synthases合酶 catalyze condensation reactions in which no nucleoside triphosphate (ATP, GTP, and so forth) is required as an energy source. • Synthetases合成酶 catalyze condensations that do use ATP or another nucleoside triphosphate as a source of energy for the synthetic reaction. The Isomerization of Citrate by Aconitase乌头酸酶 aconitate hydratase乌头酸水合酶 The active site of aconitase. The iron-sulfur cluster (red) is coordinated by cysteines (yellow) and isocitrate (white). Fluoroacetate氟乙酸 Blocks the TCA Cycle • trojan horse inhibitor. • Citrate synthase converts fluoroacetate to inhibitory fluorocitrate for its TCA cycle partner, aconitase, blocking the cycle. Isocitrate Dehydrogenase—The First Oxidation in the Cycle There are two different forms of isocitrate dehydrogenase in all cells, one requiring NAD as electron acceptor and the other requiring NADP. In eukaryotic cells, the NADdependent enzyme occurs in the mitochondrial matrix and serves in the citric acid cycle. The main function of the NADP-dependent enzyme, found in both the mitochondrial matrix and the cytosol, may be the generation of NADPH, which is essential for reductive anabolic reactions. Citrate: A Symmetrical Molecule That Reacts Asymmetrically 2 α-Ketoglutarate Dehydrogenase— A Second Decarboxylation • Like the pyruvate dehydrogenase complex, α-ketoglutarate dehydrogenase is a multienzyme complex—consisting of αketoglutarate dehydrogenase, dihydrolipoyl transsuccinylase, and dihydrolipoyl dehydrogenase—that employs five different coenzymes. Succinyl-CoA Synthetase—A SubstrateLevel Phosphorylation • Succinyl-CoA is itself a high-energy intermediate and is utilized in this step of the TCA cycle to drive the phosphorylation of GDP to GTP (in mammals) or ADP to ATP (in plants and bacteria). • The reaction is catalyzed by succinylCoA synthetase, sometimes called succinate Thiokinase琥珀酸硫激酶. Succinate Dehydrogenase—An Oxidation Involving FAD • The oxidation of succinate to fumarate is carried out by succinate dehydrogenase, a membrane-bound enzyme that is located in the innermembrane of mitochondrial . • It is actually part of the electron transport chain. • Succinate dehydrogenase, like aconitase, is an iron–sulfur protein铁硫 蛋白. • Indeed, succinate dehydrogenase contains three different kinds of iron–sulfur clusters, 2Fe-2S (two iron atoms bonded to two inorganic sulfides), 3Fe-4S, and 4Fe-4S. Fumarase Catalyzes TransHydration of Fumarate This enzyme is highly stereospecific; it catalyzes hydration of the trans double bond of fumarate but not the cis double bond of maleate (the cis isomer of fumarate). In the reverse direction (from L-malate to fumarate), fumarase is equally stereospecific: D-malate is not a substrate. 马来酸 Malate Dehydrogenase— Completing the Cycle Binding of NAD causes a conformational change in the 20-residue segment that connects the D and E βstrands of the β-sheet. The change is triggered by an interaction between the adenosine phosphate moiety of NAD and an arginine residue in this loop region. A Summary of the Cycle The Fate of the Carbon Atoms of Acetyl-CoA in the TCA Cycle The TCA Cycle Provides Intermediates for Biosynthetic Pathways • In aerobic organisms, the citric acid cycle is an amphibolic pathway, one that serves in both catabolic and anabolic processes. • Besides its role in the oxidative catabolism of carbohydrates, fatty acids, and amino acids, • the cycle provides precursors for many biosynthetic pathways. malic enzyme Why Is the Oxidation of Acetate So Complicated? Anaplerotic Reactions Pathway 回补途径 • As intermediates of the citric acid cycle are removed to serve as biosynthetic precursors, they are replenished by anaplerotic reactions. Pyruvate carboxylase exists in the mitochondria of animal cells but not in plants. PEP carboxylase occurs in yeast, bacteria, and higher plants, but not in animals. Malic enzyme苹果酸酶 is found in the cytosol or mitochondria of many animal and plant cells. • The pyruvate carboxylase reaction requires the vitamin biotin生物素 , which is the prosthetic group of the enzyme. • Biotin plays a key role in many carboxylation reactions. It is a specialized carrier of one-carbon groups in their most oxidized form: CO2. Regulation of the Citric Acid Cycle Regulation of the Citric Acid Cycle • The flow of carbon atoms from pyruvate into and through the citric acid cycle is under tight regulation at four sites: – The conversion of pyruvate to acetyl-CoA, the starting material for the cycle (the pyruvate dehydrogenase complex reaction). – The entry of acetyl-CoA into the cycle (the citrate synthase reaction). – The isocitrate dehydrogenase reaction. –α-ketoglutarate dehydrogenase reactions. Regulation of Pyruvate Dehydrogenase Complex • Pyruvate Dehydrogenase Complex is allosterically inhibited by ATP and by acetyl-CoA and NADH. • The allosteric inhibition of pyruvate oxidation is greatly enhanced when long-chain fatty acids are available. • AMP, CoA, and NAD+ allosterically activate the Pyruvate Dehydrogenase Complex(PDC). • Acetyl-CoA specifically blocks dihydrolipoyl transacetylase, and NADH acts on dihydrolipoyl dehydrogenase. Mg2+ is needed The mammalian pyruvate dehydrogenase is also regulated by covalent modifications. Hans Krebs and the Discovery of the TCA Cycle • 1932 Krebs was studying the rates of oxidation of succinate, fumarate, acetate, malate, and citrate by kidney and liver tissue. • In 1935 in Hungary, Albert SzentGyörgyi was studying the oxidation of similar organic substrates by pigeon breast muscle. • he observed that addition of any of three four-carbon dicarboxylic acids— fumarate, succinate, or malate—caused the consumption of much more oxygen than was required for the oxidation of the added substance itself. • Carl Martius and Franz Knoop found that citric acid could be converted to isocitrate and then to αketoglutarate. • This finding was significant because it was already known that α-ketoglutarate could be enzymatically oxidized to succinate. • In 1937 Krebs found that citrate could be formed in muscle suspensions if oxaloacetate and either pyruvate or acetate were added. • It is a cycle. Steric Preferences in NAD-Dependent Dehydrogenases Fool’s Gold and the Reductive Citric Acid Cycle—The First Metabolic Pathway? • How did life arise on the planet Earth? It was once supposed that a reducing atmosphere, together with random synthesis of organic compounds, gave rise to a prebiotic “soup,” in which the first living things appeared. However, certain key compounds, such as arginine, lysine, and histidine, the straightchain fatty acids, porphyrins, and essential coenzymes, have not been convincingly synthesized under simulated prebiotic conditions. • This and other problems have led researchers to consider other models for the evolution of life. • One of these alternate models, postulated by Günter Wächtershäuser, involves an archaic version of the TCA cycle running in the reverse (reductive) direction. • Reversal of the TCA cycle results in assimilation of CO2 and fixation of carbon as shown. • For each turn of the reversed cycle, two carbons are fixed in the formation of isocitrate and two more are fixed in the reductive transformation of acetyl-CoA to oxaloacetate. Thus, for every succinate that enters the reversed cycle, two succinates are returned, making the cycle highly autocatalytic. • Because TCA cycle intermediates are involved in many biosynthetic pathways, a reversed TCA cycle would be a bountifuland broad source of metabolic substrates. A reversed, reductive TCA cycle would require energy input to drive it. What might have been the thermodynamic driving force for such a cycle? Wächtershäuser hypothesizes that the anaerobic reaction of FeS and H2S to form insoluble FeS2 (pyrite黄铁矿,also known as fool’s gold) in the prebiotic milieu could have been the driving reaction: Substrate Channeling through Multienzyme Complexes May Occur in the Citric Acid Cycle • Although the enzymes of the citric acid cycle are usually described as soluble components of the mitochondrial matrix (except for succinate dehydrogenase, which is membrane-bound), growing evidence suggests that within the mitochondrion these enzymes exist as multienzyme complexes.