* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Immune responses to vaccines involving a combined antigen
Survey
Document related concepts
Lymphopoiesis wikipedia , lookup
Childhood immunizations in the United States wikipedia , lookup
Major histocompatibility complex wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Innate immune system wikipedia , lookup
Immune system wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Hepatitis B wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Molecular mimicry wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Adaptive immune system wikipedia , lookup
Vaccination wikipedia , lookup
DNA vaccination wikipedia , lookup
Immunocontraception wikipedia , lookup
Transcript
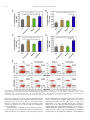
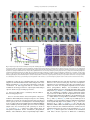
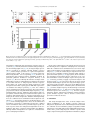
Biomaterials 35 (2014) 6086e6097 Contents lists available at ScienceDirect Biomaterials journal homepage: www.elsevier.com/locate/biomaterials Immune responses to vaccines involving a combined antigenenanoparticle mixture and nanoparticle-encapsulated antigen formulation Weifeng Zhang a, b,1, Lianyan Wang a,1, Yuan Liu a, b, Xiaoming Chen a, b, Qi Liu a, b, Jilei Jia a, b, Tingyuan Yang a, Shaohui Qiu c, Guanghui Ma a, * a National Key Laboratory of Biochemical Engineering, PLA Key Laboratory of Biopharmaceutical Production & Formulation Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, PR China University of Chinese Academy of Sciences, Beijing 100049, PR China c National Institutes for Food and Drug Control, Beijing 100050, PR China b a r t i c l e i n f o a b s t r a c t Article history: Received 24 February 2014 Accepted 6 April 2014 Available online 26 April 2014 Many physicochemical characteristics significantly influence the adjuvant effect of micro/nanoparticles; one critical factor is the kinetics of antigen exposure to the immune system by particle-adjuvanted vaccines. Here, we investigated how various antigenenanoparticle formulations impacted antigen exposure to the immune system and the resultant antigen-specific immune responses. We formulated antigen with poly(lactic-co-glycolic acid) (PLGA) nanoparticles by encapsulating antigen within nanoparticles or by simply mixing soluble antigen with the nanoparticles. Our results indicated that the combined formulation (composed of antigen encapsulated in nanoparticles and antigen mixed with nanoparticles) induced more powerful antigen-specific immune responses than each single-component formulation. Mice immunized with the combined vaccine formulation displayed enhanced induction of antigen-specific IgG antibodies with high avidity, increased cytokine secretion by splenocytes, and improved generation of memory T cell. Enhanced immune responses elicited by the combined vaccine formulation might be attributed to the antigen-depot effect at the injection site, effective provision of both adequate initial antigen exposure and long-term antigen persistence, and efficient induction of dendritic cell (DC) activation and follicular helper T cell differentiation in draining lymph nodes. Understanding the effect of antigenenanoparticle formulations on the resultant immune responses might have significant implications for rational vaccine design. Ó 2014 Elsevier Ltd. All rights reserved. Keywords: Adjuvant Nanoparticles Vaccine formulations Immune response Mechanism of action 1. Introduction Vaccination, considered as one of the most significant achievements in medicine, plays an important role in preventing infectious diseases, and saves more than 3 million people every year [1e3]. However, inadequate immunogenicity and/or safety concerns are still significant obstacles to developing ideal vaccines. While endogenous adjuvants endow traditional vaccines based on attenuated or inactivated pathogens with sufficient immunogenicity, but * Corresponding author. Chinese Academy of Sciences, Institute of Process Engineering, National Key Laboratory of Biochemical Engineering, Bei-Er-Jie No. 1, Zhong-Guan-Cun, Haidian District, Beijing 100190, PR China. Tel./fax: þ86 10 82627072. E-mail address: [email protected] (G. Ma). 1 Both authors contributed equally. http://dx.doi.org/10.1016/j.biomaterials.2014.04.022 0142-9612/Ó 2014 Elsevier Ltd. All rights reserved. the side effects and safety concerns limit the extent to which they can be used against various pathogens, such as HIV and Hepatitis C. Although subunit vaccines based on protein antigens are usually better tolerated and regarded as safer alternatives to traditional vaccines, they are usually poorly immunogenic when used alone and therefore require exogenous adjuvants to augment resultant immune responses [3e5]. Alum is a conventional adjuvant and the only one widely licensed for human use. Despite being used for over 80 years in vaccines [6], alum has some disadvantages, including side effects and safety concerns [7], contributing little to or even suppressing cell-mediated immunity and subsequent CTL responses [8,9], and providing poor adjuvanticity for recombinant protein vaccines [9]. These disadvantages necessitate the development of new adjuvants for subunit vaccines. As one potential alternative, particulate-based adjuvants can act as antigen delivery systems that facilitate the access of antigen W. Zhang et al. / Biomaterials 35 (2014) 6086e6097 to antigen presenting cells (APCs) and regulate the antigen presentation pathway, or as immune potentiators that enhance the subsequent antigen-specific immune responses [3,10,11]. Ever since Kreuter et al. evaluated the adjuvanticity of polymethylmethacrylate nanoparticles for the influenza virus in 1976 [12], biodegradable polymer-based micro/nanoparticles have been extensively investigated as adjuvants for subunit-based vaccines. The efficacy of particle vaccines are significantly influenced by various physicochemical characteristics of micro/nanoparticles (such as particle size [11,13e15], surface charge [14e16], hydrophobicity [17,18]), administration route [19,20], and antigen release kinetics [13,21e23]. Antigen release kinetics affects the efficiency of particulate-based vaccines by regulating antigen exposure to the immune system. For example, Kanchan et al. reported that slow and continuous antigen release from polymerbased particles played a critical role in eliciting memory antibody responses after a single immunization [21]. Demento et al. reported that sustained antigen release from poly(lactic-coglycolic acid) (PLGA) nanoparticles favored long-term effectormemory cellular responses [22], and Johansen et al. demonstrated that antigenic stimulation increasing exponentially over days induced more potent CD8þ T cell responses and antiviral immunity than a single dose or multiple doses (equivalent doses, administered daily) [23]. In order to achieve these different antigen-exposure kinetics, most studies fabricated particulate-based vaccines with different antigen-release profiles by regulating particle structure [21], employing different types of particles [22,24], preparing particles with stimuli-responsive polymers [3], and so on. However, relatively little attention was focused on ways to optimally formulate antigens with the particles. Antigen can be formulated with particles through attachment (e.g., conjugation, encapsulation, adsorption) or simple mixing [25]. Although various antigeneparticle formulations significantly affect the kinetics of antigen exposure to the immune system, how various antigeneparticle formulations impact the resultant antigen-specific immune responses remains unknown. We hypothesized that encapsulating antigen in PLGA nanoparticles would increase its antigen persistence in vivo, and combining soluble antigen with the nanoparticle vaccine would improve initial antigen exposure to immune system after vaccination, both together allowing for the generation of stronger and more prolonged adjuvant-induced, antigen-specific immune responses. In the present study, our objectives were therefore to determine the type and strength of the immune responses elicited by different PLGA nanoparticle-based vaccine formulations using ovalbumin (OVA) as the model antigen, and to elucidate the underlying mechanisms of action. 2. Materials and methods 2.1. Mice, reagents, and materials Female Balb/c mice used in this study were purchased from Vital River Laboratories (Beijing, China). All animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals, and were approved by the Experimental Animal Ethics Committee in Beijing. PLGA (75/25, Mw z 13 kDa) was purchased from Lakeshore Biomaterials (Birmingham, AL, USA). Poly(vinyl alcohol) (PVA-217, degree of polymerization 1700, degree of hydrolysis 88.5%) was ordered from Kuraray (Tokyo, Japan). Ovalbumin (OVA) was supplied by SigmaeAldrich (St. Louis, MO, USA). Premix membrane emulsification equipment (FMEM-500M) was provided by the National Engineering Research Center for Biotechnology (Beijing, China). Shirasu porous glass (SPG) membrane was provided by SPG Technology Co. Ltd. (Sadowara, Japan). The medium for splenocytes culture was RPMI 1640 (Gibco, Carlsbad, CA, USA) with 10% (v/v) fetal bovine serum (Gibco, Carlsbad, CA, USA). All mouse cytokines ELISA and fluorochrome-conjugated anti-mouse antibodies for flow cytometric use, were obtained from eBioscience (San Diego, CA, USA), unless otherwise indicated. ELISpotPLUS kits were obtained from Mabtech AB (Nacka Strand, Sweden). All other reagents were of analytical grade. 6087 2.2. Preparation and characterization of PLGA nanoparticles PLGA nanoparticles were prepared using a two-step procedure by combining the solvent extraction method and the premix membrane emulsification technique, as described before with some modifications [26] (Fig. S1). Briefly, 1 mL deionized water containing 100 mg OVA (internal water phase) was added into 12 mL ethyl acetate containing 600 mg PLGA; the primary water in oil (W/O) emulsion was formed by sonication (120 W; Digital Sonifier 450, Branson Ultrasonics Corp., Danbury, CT, USA) in a tube over an ice bath for 1 min (4 s on and 2 s off). To prepare the double emulsion, the resulting primary emulsion was added into 65 mL external water phase containing 1.5% w/v PVA and 0.9% w/v NaCl, and magnetically stirred at 450 rpm for 90 s. Then, the resulting coarse double emulsion was extruded through the SPG membrane under a certain nitrogen pressure for 8 times and nanodroplets with narrow size distribution were obtained. The obtained double emulsion was poured into 800 mL deionized water containing 0.9% w/v NaCl (solidification solution) under magnetic stirring for 4 h to solidify the nanoparticles. The obtained PLGA nanoparticles encapsulating OVA were collected by centrifugation at 15,000 g for 5 min, washed 3 times with deionized water to remove residual PVA, and then lyophilized and stored at 4 C for later use. To prepare blank PLGA nanoparticles, 1 mL of deionized water without OVA was used as the internal water phase. The hydrodynamic size and zeta potential of PLGA nanoparticles were measured by a Nano-ZS Zeta Sizer (Malvern Instruments Ltd., Malvern, UK). Morphology of the PLGA nanoparticles was characterized by scanning electron microscopy (JEM-6700F, JEOL Ltd., Tokyo, Japan). Nano Measurer 1.2 software was employed to measure the size of PLGA nanoparticles according to the scanning electron micrographs. The OVA content of PLGA nanoparticles was determined by incubating 3 mg of lyophilized PLGA nanoparticles in 1 mL of 0.1 M NaOH solution under gentle shaking overnight. Protein concentration in the solution was determined using Micro-BCA protein assay kit (Pierce Biotechnology, Rockford, IL, USA) according to the manufacturer’s instructions. OVA dissolved in 0.1 M NaOH was used to establish a standard curve, and blank PLGA nanoparticles were used as the control. 2.3. Immunization studies As shown in Table S1, 30 mice were randomly divided into 5 groups (n ¼ 6) and intramuscularly immunized with 100 mL (50 mL/hind leg) of different vaccine formulations containing 25 mg of antigen (OVA) (Fig. 1E). Mice were immunized 3 times at 2-week intervals (Fig. S2). Blood samples were collected from the caudal vein before each immunization and 10 days after the third immunization. Sera was separated and stored at 70 C for later analysis. At 10 days after the third immunization, splenocytes were collected for in vitro proliferation, cytokine response, and flow cytometric assays. 2.4. Determination of OVA-specific IgG and IgG subclasses OVA-specific IgG, IgG1, and IgG2a in the serum were quantitatively determined by enzyme-linked immunosorbent assay (ELISA) in accordance with a protocol described previously [27]. Briefly, 96-well ELISA plates (Costar, Corning, New York, USA) were coated overnight at 4 C with 2 mg of OVA per well in coating buffer (0.05 M CBS, pH 9.6). Plates were washed with PBST (0.01 M PBS containing 0.05% [m/v] Tween 20, pH 7.4) and blocked by incubating with 2% (m/ v) BSA (Roche, Basel, Switzerland) in PBST for 60 min at 37 C. After washes with PBST, 100 mL per well of appropriate sera dilutions were added to the plates, serially diluted two-fold in dilution buffer (PBST containing 0.1% [m/v] BSA), and incubated for 30 min at 37 C. Plates were then washed and incubated with 100 mL horseradish peroxidase-conjugated goat antibodies against either mouse IgG (SigmaeAldrich, St. Louis, MO, USA), IgG1, or IgG2a (Santa Cruz, CA, USA) (IgG diluted 1:20,000; IgG1 and IgG2a diluted 1:2000) for 30 min at 37 C. Thereafter, the plates were washed again with PBST, and 100 mL of 3,30 ,5,50 -tetramethylbenzidine (TMB) substrate was added to each well and incubated for 20 min at room temperature. After stopping the reaction by adding 50 mL of 2 M H2SO4 to each well, the optical density (OD, 450 nm) was measured by an Infinite M200 Microplate Spectrophotometer (Tecan, Männedorf, Switzerland). Titers were given as the reciprocal sample dilution corresponding to twice higher OD than that of the negative sera. OVA-specific IgG avidity measurement of was carried out by ELISA with a ureaelution step [28]. The antigeneantibody complexes with low-affinity were dissociated by incubating the plates with urea (SigmaeAldrich, St. Louis, MO, USA) at 20 C for 10 min. Avidity index (AI) was calculated in accordance with the following formula: AI ¼ (IgG titer incubated with urea/IgG titer incubated without urea) 100. 2.5. Determination of cytokine levels by ELISA Splenocytes were harvested from vaccinated mice 10 days after the third immunization and restimulated with OVA (50 mg/mL) for 60 h at 37 C in a humid atmosphere with 5% CO2, and the supernatant was collected. IL-4, IL-10, IL-12, and IFN-g levels in the supernatant were measured by Ready-to-use Sandwich ELISA kits (eBioscience, San Diego, CA) according to the manufacturer’s instructions. 6088 W. Zhang et al. / Biomaterials 35 (2014) 6086e6097 Fig. 1. Scanning electron micrographs (A, B) and size distribution (C, D) of PLGA nanoparticles, and schematic illustration of different vaccine formulations (E). The scale bar in (A, B) represents 1 mm. 2.6. ELISpot assays The frequency of IFN-g- and IL-4-secreting cells in splenocytes harvested from vaccinated mice 10 days after the third immunization were measured by ELISpotPLUS kits (Mabtech AB, Nacka Strand, Sweden) according to the manufacturer’s instructions. In brief, 2.5 105 splenocytes were seeded into each well in triplicate and restimulated with 50 mg/mL OVA. The plates were incubated for 36 h in a 37 C humidified incubator with 5% CO2. After emptying and washing the plates, detection antibodies were added, the plates were incubated for 2 h at room temperature, Streptavidin-ALP was added, and the plates were incubated for 1 h at room temperature. Finally, the ready-to-use substrate solution (BCIP/NBT-plus) was added to the plates, and color development was stopped by washing extensively in tap water after distinct spots emerged. Spots were inspected and counted in an ELISpot analysis system (SageCreation, Beijing, China). Results were expressed as the number of antigen-specific spot-forming cells (SFCs) per 106 splenocytes. 2.7. Splenocyte proliferation assay Based on previously described methods [27], the splenocyte proliferation assay was performed to evaluate antigen-specific splenocyte activation. Splenocytes (2.5 106 cells/mL), stimulated with OVA (50 mg/mL) or not, were seeded in triplicate (100 mL/well) in a 96-well plate and incubated at 37 C in a humid atmosphere with 5% CO2. After 72 h, 10 mL of CCK-8 solution (Dojindo, Japan) was added to each well, and the plates were incubated for an additional 4 h. The absorbance at 450 nm (with 620 nm as reference) was measured by an Infinite M200 Microplate Spectrophotometer (Tecan, Männedorf, Switzerland). The results were expressed as the proliferation index (PI), calculated based on the following formula: PI ¼ OD (450 nm) for stimulated cultures/OD (450 nm) for non-stimulated cultures. 2.8. Determination of memory T cell responses by flow cytometry Flow cytometry was performed to measure the percentage of memory T cells in splenocytes collected from immunized mice 10 days after the third immunization. Cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum and restimulated with OVA (50 mg/mL) for 60 h. Cells were then stained with the following set of fluorochrome-conjugated anti-mouse antibodies: eFluor 450eantiCD4, PerCP-Cy5.5eanti-CD8a, PEeanti-CD44, and APCeanti-CD62L (eBioscience). After washing, cell samples were examined by Beckman Coulter CyAnÔ ADP flow cytometer, and data were analyzed by Summit software (version 4.3). 2.9. Antigen persistence at injection sites To monitor antigen persistence at injection sites in vivo, antigen was labeled with the near-infrared dye Cy7 mono-reactive NHS ester (Fanbo Biochemicals, Beijing, China). Balb/c mice (n ¼ 6) were intramuscularly injected in the hind legs with different vaccine formulations containing antigen labeled with the nearinfrared dye Cy7 (25 mg OVA in 100 mL buffer per mouse, half dose at each site). Antigen persistence at the injection sites was documented by Carestream FX PRO in vivo imaging system at the indicated time points (ex: 730 nm; em: 790 nm). Carestream Molecular Imaging Software was used to quantify sum fluorescence intensity at the injection sites. 2.10. Determination of available antigen in draining lymph nodes by immunohistochemical assay The hind legs (n ¼ 4) of Balb/c mice were intramuscularly injected with 50 mL of different vaccine formulations containing 1.25 mg of antigen. At various time points (2 or 7 days after injection), sciatic and popliteal lymph nodes were isolated from the euthanized mice, fixed in 4% paraformaldehyde, embedded into paraffin, and cut to yield 4-mm thick sections. After deparaffinization, sections were incubated with 3% H2O2 for 20 min to inhibit endogenous peroxidase activity. Then, sections were blocked by goat serum and immunostained with antibody against OVA (LifeSpan BioSciences, Inc., Seattle, USA). A secondary antibody from PicTureÔPV6000 kit (Zymed Laboratories Inc., South San Francisco, CA, USA) and diaminobenzidine (ZSGB-Bio, Beijing, China) were used for color development. Specimens were then stained with hematoxylin for cell nucleus identification. Immunohistochemical micrographs were documented by an Olympus BX51 microscope. W. Zhang et al. / Biomaterials 35 (2014) 6086e6097 Table 1 Characteristics of PLGA nanoparticles. Data are shown as mean SEM. Particles Diameter (DLS, nm) PDIa Diameter (SEM, nm) Zeta potential OVA content (mV)b (mg/mg) PLGA NPs 481.2 5.12 0.04 0.01 331.6 3.16 11.93 0.55 e (Blank) PLGA NPs 590.8 4.51 0.17 0.04 341.6 2.90 11.43 0.45 59.65 3.62 (OVA) a b PDI, polydispersity index from dynamic light scattering (DLS). Zeta potential of different nanoparticles was detected in deionized water. 6089 2.13. Statistical analysis All values in the present study were expressed as the mean standard error of the mean (SEM). Statistical analysis was performed using GraphPad Prism 5.0 software (San Diego, CA, USA). Differences between two groups were tested using an unpaired, two-tailed Student’s t-test. Differences among multiple groups were tested by one-way ANOVA followed by Tukey’s multiple comparison. Significant differences between the groups were expressed as follows: *p < 0.05, **p < 0.01, and ***p < 0.001. 3. Results 3.1. Preparation and characterization of PLGA nanoparticles 2.11. Expression of MHC and co-stimulatory molecules on dendritic cells in draining lymph nodes Balb/c mice (n ¼ 3) were intramuscularly vaccinated with different vaccine formulations (25 mg antigen in 100 mL buffer per mouse, half dose at each site), and mice were euthanized at 1, 2, and 7 days post-immunization. Popliteal lymph nodes were harvested and processed into single cell suspension. Cells were stained with a mixture of anti-mouse antibodies (eFluor 450eanti-CD11c, APCeanti-MHC I, FITCe anti-MHC II, and PEeanti-CD86; all from eBioscience). Expression of MHC I, MHC II, and CD86 on CD11cþ DCs was determined by CyAnÔ ADP flow cytometer (Beckman Coulter, California, USA) and analyzed using Summit software (version 4.3.02). 2.12. Determination of follicular helper CD4þ T cells in draining lymph nodes Balb/c mice (n ¼ 3) were intramuscularly vaccinated with different vaccine formulations (25 mg antigen in 100 mL buffer per mouse, half dose at each site), and mice were euthanized 9 days after immunization. Popliteal lymph nodes were harvested and processed into single cell suspension. Cells were stained with a mixture of anti-mouse antibodies (eFluor 450eanti-CD4, APCeanti-CXCR5, and PEe anti-PD-1; all from eBioscience). The percentage of follicular helper CD4þ T cells (CD4þCXCR5hiPD-1hi) was determined by CyAnÔ ADP flow cytometer (Beckman Coulter, California, USA) and analyzed using Summit software (version 4.3.02). To generate different PLGA nanoparticle-adjuvanted vaccine formulations, PLGA nanoparticles were prepared by emulsificationesolvent extraction method combined with the premix membrane emulsification technique [26]. The double emulsion was prepared by simply magnetic stirring which has little harm on antigen structure (Fig. S8), compared to ultrasonication or homogenization. Scanning electron micrographs revealed that both blank PLGA nanoparticles and PLGA nanoparticles encapsulating OVA were spherical particles with narrow size distributions (Fig. 1A and B), and their respective diameters were 481.2 5.12 nm and 590.8 4.51 nm (Table 1, Fig. 1C and D). Thus, similar sizes were acquired for blank and antigen-encapsulated PLGA nanoparticles. The zeta potential of the blank and antigen-encapsulated particles were also similar at 11.93 0.55 mV and 11.43 0.45 mV, respectively. The antigen content in the antigen-encapsulated particles was 59.65 3.62 mg/mg, as determined by Micro-BCA kit (Table 1). As shown in Fig. S3, the in vitro release profile revealed Fig. 2. Antigen-specific IgG antibody responses in Balb/c mice immunized with different vaccine formulations. Mice (n ¼ 6) were intramuscularly vaccinated on day 0, 14, and 28 as described in the Methods section. (A) IgG titers in the serum at the indicated time points after first immunization. (B) Antigen-specific IgG1 and IgG2a levels and (C) IgG2a/IgG1 ratios in the serum of immunized mice 10 days after the third immunization (serum diluted 1:200). (D) Avidity index of anti-OVA IgG in the serum of immunized mice 10 days after the third immunization. Data are expressed as the mean SEM (n ¼ 6). *p < 0.05; **p < 0.01; ***p < 0.001. 6090 W. Zhang et al. / Biomaterials 35 (2014) 6086e6097 Fig. 3. Cytokine secretion by splenocytes. Mice (n ¼ 6) were immunized three times as described in the Methods section. Splenocytes were harvested 10 days after the third immunization. For ELISA assay, splenocytes were restimulated with OVA (50 mg/mL) in culture for 60 h ex vivo. IFN-g (A), IL-12 (B), IL-4 (C), and IL-10 (D) levels in the supernatant were measured by ELISA. For evaluating the frequency of cytokine-secreting splenocytes, the frequency of (E) IFN-g- and (F) IL-4-secreting cells were analyzed by ELISpot. SFC ¼ spot-forming cells. Data are expressed as the mean SEM (n ¼ 6). *p < 0.05; **p < 0.01; ***p < 0.001. that the antigen encapsulated within PLGA nanoparticles was released in a sustained manner for at least the 15-day evaluation period. 3.2. Systemic antibody responses in vaccinated mice To investigate the effect of various antigenenanoparticle formulations on antibody response, serum was collected over time from mice intramuscularly immunized with soluble antigen alone (Ag), antigen encapsulated within particles (Encapsulation), soluble antigen mixed with blank particles (Mixture), and a combined vaccine formulation (antigen encapsulated within particles þ soluble antigen mixed with blank particles, Combination). Serum IgG, IgG1, and IgG2a titers were then evaluated by ELISA. As shown in Fig. 2A, the combined vaccine formulation induced significantly higher antigen-specific IgG titers than soluble antigen alone and antigen mixed with PLGA nanoparticles on day 14 after primary immunization, and significantly higher titers than all other formulations on day 28 and 38. On day 28 after primary immunization, serum IgG titers elicited by antigen encapsulated within PLGA nanoparticles were significantly higher than that elicited by soluble antigen alone or antigen mixed with particles. On W. Zhang et al. / Biomaterials 35 (2014) 6086e6097 6091 levels were produced by splenocytes from mice immunized with antigen encapsulated in particles and the combined vaccine formulation, which were significantly higher than those for soluble antigen mixed with blank particles or soluble antigen alone. Results of ELISpot assay revealed that the frequencies of IL-4- and IFN-gsecreting splenocytes in mice immunized with Encapsulation and Combination formulations were significantly higher than those for Mixture formulation and soluble antigen alone (Fig. 3E and F, and Fig. S4). On the whole, the Encapsulation formulation and the Combination formulation induced higher levels of both Th1 and Th2 cytokines secretion by splenocytes, indicating stronger immune responses. 3.4. Splenocyte proliferation assay Fig. 4. Proliferative responses of splenocytes responding to antigen ex vivo. Mice (n ¼ 6) were immunized three times as described in the Methods section. Splenocytes were harvested 10 days after the third immunization and restimulated ex vivo with antigen. Splenocyte proliferation was measured using CCK-8 kit, and the proliferation index was calculated. Data are expressed as the mean SEM (n ¼ 6). *p < 0.05. day 38, IgG response induced by antigen entrapped within particles was more potent than that by soluble antigen alone. IgG2a antibody production reveals a Th1-polarized immune response, and the ratio of IgG2a/IgG1 is indicative of Th1-biased immune response [35]. Both Encapsulation and Combination vaccine formulations induced stronger IgG1 and IgG2a responses than soluble antigen (p < 0.05, Fig. 2B). However, soluble antigen mixed with blank particles induced stronger IgG2a response than soluble antigen, but comparable IgG1 response (Fig. 2B). All PLGA nanoparticles-adjuvanted vaccine formulations generated similar IgG2a/ IgG1 ratios, which were significantly larger than that for antigen alone (Fig. 2C). To evaluate the quality of the antibody response, the avidity between antigen and IgG antibody in the serum was determined by ELISA combined with a urea-elution step. As shown in Fig. 2D, the avidity of the IgG antibody induced by these PLGA nanoparticlesbased vaccines was higher than that for antigen alone. Immunization with the Mixture formulation elicited IgG responses with significantly higher avidity than the encapsulation formulation. The combined vaccine formulation elicited IgG antibody with significantly higher avidity than that for antigen alone. Taken together, all nanoparticles-adjuvanted vaccine formulations (Encapsulation, Mixture, and Combination) induced more potent antibody responses than the soluble antigen. Furthermore, compared to Encapsulation and Mixture formulations, mice immunized with the combined vaccine formulation developed more potent antigen-specific IgG antibody responses, with higher titers and relatively higher avidity. 3.3. Cytokine levels secreted by ex vivo restimulated splenocytes Since we observed that PLGA nanoparticles-adjuvanted vaccine formulations induced a Th1-biased antibody response in mice compared to soluble antigen alone, we next asked whether these formulations could also alter the cytokine profiles in the mice toward a Th1 bias. Splenocytes harvested from vaccinated mice were restimulated ex vivo with OVA, and Th1 (IFN-g, IL-12) and Th2 (IL-4, IL-10) cytokines in the supernatant were determined by ELISA. As shown in Fig. 3AeD, similar IFN-g, IL-12, IL-4, and IL-10 cytokine An ex vivo splenocyte proliferation assay was performed to assess the influence of various antigenenanoparticle formulations on splenocyte proliferative responses. As shown in Fig. 4, under the stimulation of OVA, splenocytes collected from mice immunized with the combined vaccine formulation proliferated more efficiently than those collected from mice immunized with soluble antigen mixed with nanoparticles or soluble antigen alone (p < 0.05). Hence, we believed that the combined vaccine formulation developed more potent antigen-specific immune responses than other formulations. 3.5. Memory T cell responses The ultimate goal of vaccination is to generate immune memory that can rapidly respond to pathogens upon reinfection, and memory T cells are important components of these memory immune responses. Since CD44hiCD62Llow and CD44hiCD62Lhi were regarded as markers for effector-memory and central-memory T cells, respectively [29], we evaluated their frequency by flow cytometry. The frequency of CD44hiCD62Lhi central-memory CD4þ (Fig. 5A) and CD8þ (Fig. 5C) T cells was similar between splenocytes harvested from mice immunized with the Encapsulation formulation and the Combination formulation, both of which were significantly higher than that for soluble antigen mixed with blank particles or soluble antigen alone. With respect to CD44hiCD62Llow effector-memory T cells, the frequency of memory CD4þ (Fig. 5B) and CD8þ (Fig. 5D) T cells was significantly higher for mice immunized with the combined vaccine formulation, as compared to mice immunized with soluble antigen mixed with blank particles or soluble antigen alone (p < 0.05). Representative FACS plots of the mean percentages are shown in Fig. 5E. In summary, the combined vaccine formulation induced the strongest memory T cell responses among the formulations tested, indicating that this formulation might provide better protection against reinfection. 3.6. Antigen persistence at injection sites and antigen transport into draining lymph nodes To uncover the mechanisms underlying the enhanced immune responses elicited by the combined vaccine formulation, we first determined antigen persistence at the injection sites as well as the amount of available antigen in draining lymph nodes by in vivo imaging and immunohistochemistry, respectively. To track and visualize the various formulations by in vivo imaging, we injected formulations containing fluorescently labeled antigen. As shown in Fig. 6A and B, none of the initial fluorescence remained at the injection sites 6 h after injection in animals immunized with the formulations containing soluble antigen (soluble antigen alone or soluble antigen mixed with blank particles), and there was no significant difference between their decreasing rates of 6092 W. Zhang et al. / Biomaterials 35 (2014) 6086e6097 Fig. 5. Frequency of central (CD44hiCD62Lhi)/effector (CD44hiCD62Llow) memory CD4þ and CD8þ T cells. Mice (n ¼ 6) were immunized three times as described in the Methods section. Splenocytes were harvested 10 days after the third immunization and restimulated ex vivo with antigen for 60 h. The frequency of CD44hiCD62Lhi CD4þ T cells (A), CD44hiCD62Llow CD4þ T cells (B), CD44hiCD62Lhi CD8þ T cells (C), and CD44hiCD62Llow CD8þ T cells (D) were measured by flow cytometry. FACS plots in (E) are representative of the mean percentages of 6 mice in each group. Data in (A), (B), (C), and (D) are expressed as the mean SEM (n ¼ 6). *p < 0.05; **p < 0.01. fluorescence intensity. In contrast, antigen encapsulated within nanoparticles and antigen in the combined formulation persisted at the injection sites for as long as 168 h (7 days) after injection. Moreover, compared to the combined vaccine formulation, more antigen was detained at sites injected with the Encapsulation vaccine formulation. Considering that significantly different antigen persistence profiles at injection sites existed between various formulations, we wondered whether these differences affected antigen transport into draining lymph nodes. We therefore determined the available antigen contained in the draining lymph nodes over time by immunohistochemistry. At the early stage (2 days postimmunization), the most abundant antigen was detected in lymph nodes of mice immunized with the Mixture formulation (soluble antigen mixed with blank nanoparticles), followed by mice immunized with the combined vaccine formulation (Fig. 6C, upper panel). At the later stage (7 days post-immunization), immunization with either the Encapsulation formulation or the Combination W. Zhang et al. / Biomaterials 35 (2014) 6086e6097 6093 Fig. 6. Antigen depot at injection sites and antigen transport into draining lymph nodes. (AeB) Antigen persistence at the sites of injection. Balb/c mice (n ¼ 6) were intramuscularly injected in the hind legs with different vaccine formulations containing antigen labeled with the near-infrared dye Cy7. Antigen persistence at injection sites was evaluated and documented by an in vivo imaging system at the indicated time points. Carestream Molecular Imaging Software was employed to quantify the sum fluorescence intensity at the injection sites. (A) Representative fluorescence images and (B) quantitative fluorescence intensity of antigen persisting at injection sites. (C) Antigen level in draining lymph nodes determined by immunohistochemical assay. Balb/c mice were intramuscularly injected with 50 mL of different vaccine formulations containing 12.5 mg of antigen. At 2 days (upper panels) or 7 days (lower panels) after injection, draining lymph nodes were isolated from euthanized mice, embedded in paraffin, and cut into sections. Sections were stained with antibody against antigen (OVA) and hematoxylin for coloration of antigen and the cell nucleus, respectively. Data in (A) and (C) are representative of the 4 mice in each group. Yellow areas indicated by arrows in (C) represent antigen. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.) formulation resulted in more abundant antigen in the draining lymph nodes than immunization with soluble antigen alone or the Mixture formulation (Fig. 6C, lower panel). Taking these results into consideration, our data suggested that the combined vaccine formulation effectively provided not only adequate initial antigen exposure but also long-term antigen persistence. 3.7. Expression of MHC and the co-stimulatory molecule CD86 on DCs in draining lymph nodes Since we observed that the various nanoparticleeantigen formulations differentially affected antigen transport to the draining lymph nodes over time, we next wondered how they each affected vaccine potency in terms of activating DCs in the draining lymph nodes. To address this question, MHC molecules (MHC I and MHC II) and co-stimulatory molecule (CD86) expression on DCs in draining lymph nodes were measured by flow cytometry. As shown in Fig. 7AeF and Fig. S57, compared to soluble antigen alone, all nanoparticles-adjuvanted vaccine formulations (Encapsulation, Mixture, and Combination) induced significantly higher MHC I, MHC II, and CD86 expression 24 h after vaccination; no significant differences were observed among any of the three nanoparticleadjuvanted vaccine formulations. After 48 h, the following observations were made: the nanoparticle-adjuvanted vaccine formulations (Encapsulation, Mixture, and Combination) induced comparable MHC II expression, all significantly higher than soluble antigen alone (Fig. 7D and Fig. S6). The Mixture vaccine formulation and the Combination formulation elicited significantly higher expression levels of MHC I than the Encapsulation vaccine formulation and soluble antigen alone (Mixture vs Ag, Combination vs Ag: p < 0.001; Mixture vs Encapsulation, Combination vs Encapsulation: p < 0.05; Fig. 7B and Fig. S5). With respect to CD86 expression, the Mixture vaccine formulation and the Combination formulation elicited significantly higher levels (Mixture vs Ag: p < 0.01; Combination vs Ag: p < 0.05; Fig. 7F and Fig. S7). At 7 days after immunization, MHC I, MHC II, and CD86 expression was significantly higher on DCs from mice immunized with the combined vaccine formulation than all of the other vaccine formulations (Fig. 7AeF and Fig. S5e7). Taken together, the combined vaccine formulation could induce effective expression of MHC and co-stimulatory 6094 W. Zhang et al. / Biomaterials 35 (2014) 6086e6097 Fig. 7. Expression of MHC I, MHC II, and co-stimulatory molecule CD86 on DCs in draining lymph nodes of immunized mice. Balb/c mice (n ¼ 3) were intramuscularly vaccinated with different vaccine formulations. At the indicated time points (1, 2, or 7 days post-immunization), mice were euthanized, and popliteal lymph nodes were isolated. The percentage and MFI of MHC I (A, B), MHC II (C, D), and CD86 (E, F) expression on CD11cþ DCs was determined by flow cytometry. Data are expressed as the mean SEM (n ¼ 3). *p < 0.05; **p < 0.01; ***p < 0.001. molecules on DCs in the draining lymph node, both at the initial stage and in the later stages after immunization. 3.8. Frequency of follicular helper CD4þ T cells in draining lymph nodes Follicular helper CD4þ T cells play an important role in helping B cells produce high-affinity antibody responses [30]. It has been reported that follicular helper T cell differentiation requires continuous antigen presentation [31]. In order to elucidate whether the combined vaccine formulation might have favored the generation of antibody responses by improving follicular helper T cell activation, we determined the frequency of follicular helper CD4þ T cells in the draining lymph nodes of immunized mice. Indeed, the frequency of CD4þCXCR5hiPD-1hi follicular helper T cells was significantly higher in the draining lymph nodes of mice immunized with the combined vaccine formulation, as compared to those for other vaccine formulations (p < 0.05, Fig. 8A and B). 4. Discussion Vaccination plays an important role in protecting individuals against pathogens, as well as in preventing and controlling infectious diseases. With the development of modern vaccines, the development of more efficacious adjuvants is imperative for the successful induction of sufficient immune responses. Among various potential adjuvants, polymeric micro/nanoparticles represent a kind of promising vaccine adjuvant, as they can simultaneously augment both humoral and cellular immune responses. Many physicochemical characteristics significantly affect the adjuvant effect of micro-/nano-particles, and one critical factor is the kinetics of antigen exposure to the immune system. In this study, we investigated how various antigenenanoparticle formulations impacted antigen exposure to the immune system and evaluated the resultant antigen-specific immune responses. Our findings indicated that the combined vaccine formulation composed of antigen encapsulated in nanoparticles plus soluble antigen mixed with blank nanoparticles induced the most powerful antigen-specific immune responses among any of the other formulations tested. Indeed, mice immunized with the combined vaccine formulation displayed enhanced induction of antigenspecific IgG antibodies with high avidity, increased cytokine secretion by splenocytes, and increased generation of memory T cells. Overall, the results presented here suggest that combining antigen encapsulated within nanoparticles with soluble antigen mixed with blank nanoparticles improved the efficacy of nanoparticle-based vaccine. By monitoring antigen persistence at the injection site and detecting antigen levels in draining lymph nodes over time, we found that encapsulating antigen into W. Zhang et al. / Biomaterials 35 (2014) 6086e6097 6095 Fig. 8. The frequency of follicular helper CD4þ T cells in the draining lymph nodes of immunized mice. Balb/c mice (n ¼ 3) were intramuscularly vaccinated with different vaccine formulations. Mice were euthanized 9 days later, and popliteal lymph nodes were isolated. The frequency of follicular helper CD4þ T cells (CD4þCXCR5hiPD-1hi) was determined by flow cytometry. (A) Representative flow cytometry plots and (B) percentage of follicular helper CD4þ T cells (CD4þCXCR5hiPD-1hi) in CD4þ T cells. Data are expressed as the mean SEM (n ¼ 3). *p < 0.05. nanoparticles significantly prolonged antigen retention at the injection site (Fig. 6A and B) and achieved sustained antigen transport into the draining lymph node (Fig. 6C). Prolonged antigen presentation is propitious to inducing powerful immune responses [32,33]. Indeed, antigen persistence is required throughout the expansion phase of CD4þ T cell responses [34], and is required for DC licensing and CD8þ T cell cross-priming [35,36]. Demento et al. demonstrated that sustained antigen availability mediated by particulate platforms facilitated a long-term memory T cell response [22]. Moreover, although the duration of antigen availability plays a critical role, initial antigen presentation is also pivotal to successful induction of antigen-specific immune responses. Indeed, Blair et al. showed that the initial engagement of TCR through interaction with cognate peptideeMHC was required for T cell activation and antigen specificity [33]. Here, the combined vaccine formulation effectively provided not only long-term antigen persistence but also adequate initial antigen exposure (Fig. 6C). Following antigen exposure, maturation and activation of DCs is a prerequisite of effective antigen presentation and subsequent T cell activation. And follicular helper T cells play an important role in helping B cells activate and produce antibodies with high avidity. Here, the combined vaccine formulation induced higher DC activation (Fig. 7) and a higher frequency of follicular helper T cells (Fig. 8) in the draining lymph nodes. Thus, the aforementioned mechanisms could help to explain why and how the combined vaccine formulation elicited more powerful antigen-specific immune responses than other nanoparticle-adjuvanted vaccine formulations with single component (Encapsulation and Mixture), although further investigations are required to understand the precise underlying mechanisms. Based on the results in the present study, we therefore proposed the following model to explain the underlying mode of action by which the combined vaccine formulation acted as a vaccine adjuvant to promote antigen-specific immune responses toward a given antigen (Fig. 9). First, the soluble antigen component in the combined vaccine formulation provided adequate initial antigen to prime the immune system (Fig. 6C, upper panel). At the same time, the nanoparticle-encapsulated antigen component in the combined formulation created an antigen depot at the injection site (Fig. 6A and B), providing a supply of persistent antigen to the immune system (Fig. 6C, lower panel). Effective provision of both adequate initial antigen exposure and long-term antigen persistence likely resulted in higher expression of both MHC molecules (MHC I and MHC II) and the co-stimulatory molecule CD86 on DCs (Fig. 7) as well as a higher frequency of follicular helper T cells in the draining lymph nodes (Fig. 8). Then, more potent antigen-specific immune responses were elicited (Figs. 2e5). Therefore, by optimizing the antigenenanoparticle formulation, enhanced antigenspecific immune responses could be elicited to achieve better prevention of infectious diseases. 5. Conclusions This study investigated the effect of various antigenenanoparticle formulations on the immune responses elicited by nanoparticle-adjuvanted vaccines. Our findings demonstrated that the combined vaccine formulation (antigen encapsulated within nanoparticles, and soluble antigen mixed with blank nanoparticles) elicited more potent antigen-specific immune responses than single-component nanoparticle-adjuvanted formulations (antigen 6096 W. Zhang et al. / Biomaterials 35 (2014) 6086e6097 Fig. 9. Schematic illustration of the proposed mode of action of the combined vaccine formulation composed of PLGA nanoparticles-encapsulated antigen and soluble antigen mixed with blank nanoparticles. encapsulated within nanoparticles, or soluble antigen mixed with blank nanoparticles). The enhanced immune responses elicited by the combined vaccine formulation might be attributed to the antigen-depot effect at the injection site, effective provision of both adequate initial antigen exposure and long-term antigen persistence, and efficient induction of DC activation and follicular helper T cell differentiation in draining lymph nodes. Understanding the effect of the antigenenanoparticle formulations on the resultant immune responses might have significant implications for rational vaccine design. Acknowledgments This work was financially supported by the 973 Program (Grant No. 2013CB531500), Special Fund for Agro-scientific Research in the Public Interest (Grant No. 201303046), and the 863 Program (Grant No. 2012AA02A406). Appendix A. Supplementary data Supplementary data related to this article can be found at http:// dx.doi.org/10.1016/j.biomaterials.2014.04.022. References [1] Ulmer JB, Valley U, Rappuoli R. Vaccine manufacturing: challenges and solutions. Nat Biotech 2006;24:1377e83. [2] Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat Immunol 2011;131:509e17. [3] Broaders KE, Cohen JA, Beaudette TT, Bachelder EM, Fréchet JMJ. Acetalated dextran is a chemically and biologically tunable material for particulate immunotherapy. Proc Natl Acad Sci USA 2009;106:5497e502. [4] Baumgartner CK, Malherbe LP. Regulation of CD4 T-cell receptor diversity by vaccine adjuvants. Immunology 2010;130:16e22. [5] Sokolova V, Knuschke T, Kovtun A, Buer J, Epple M, Westendorf AM. The use of calcium phosphate nanoparticles encapsulating toll-like receptor ligands and the antigen hemagglutinin to induce dendritic cell maturation and T cell activation. Biomaterials 2010;31:5627e33. [6] Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol 2009;9:287e93. [7] Zaharoff DA, Rogers CJ, Hance KW, Schlom J, Greiner JW. Chitosan solution enhances both humoral and cell-mediated immune responses to subcutaneous vaccination. Vaccine 2007;25:2085e94. [8] Chen X, Kim P, Farinelli B, Doukas A, Yun S-H, Gelfand JA, et al. A novel laser vaccine adjuvant increases the motility of antigen presenting cells. PLoS One 2010;5:e13776. [9] Singh M, O’Hagan DT. Recent advances in vaccine adjuvants. Pharm Res 2002;19:715e28. [10] De Temmerman ML, Rejman J, Demeester J, Irvine DJ, Gander B, De Smedt SC. Particulate vaccines: on the quest for optimal delivery and immune response. Drug Discov Today 2011;16:569e82. [11] Oyewumi MO, Kumar A, Cui ZR. Nano-microparticles as immune adjuvants: correlating particle sizes and the resultant immune responses. Expert Rev Vaccines 2010;9:1095e107. [12] Kreuter J, Speiser PP. New adjuvants on a polymethylmethacrylate base. Infect Immun 1976;13:204e10. [13] Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol 2010;10:787e96. [14] Foged C, Brodin B, Frokjaer S, Sundblad A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int J Pharm 2005;298:315e22. [15] Kohli AK, Alpar HO. Potential use of nanoparticles for transcutaneous vaccine delivery: effect of particle size and charge. Int J Pharm 2004;275:13e7. W. Zhang et al. / Biomaterials 35 (2014) 6086e6097 [16] Gomez JMM, Csaba N, Fischer S, Sichelstiel A, Kundig TM, Gander B, et al. Surface coating of PLGA microparticles with protamine enhances their immunological performance through facilitated phagocytosis. J Control Release 2008;130:161e7. [17] Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol 2004;4:469e78. [18] Moyano DF, Goldsmith M, Solfiell DJ, Landesman-Milo D, Miranda OR, Peer D, et al. Nanoparticle hydrophobicity dictates immune response. J Am Chem Soc 2012;134:3965e7. [19] Mohanan D, Slutter B, Henriksen-Lacey M, Jiskoot W, Bouwstra JA, Perrie Y, et al. Administration routes affect the quality of immune responses: a crosssectional evaluation of particulate antigen-delivery systems. J Control Release 2010;147:342e9. [20] Lesterhuis WJ, de Vries IJM, Schreibelt G, Lambeck AJA, Aarntzen EHJG, Jacobs JFM, et al. Route of administration modulates the induction of dendritic cell vaccine-induced antigen-specific t cells in advanced melanoma patients. Clin Cancer Res 2011;17:5725e35. [21] Kanchan V, Katare YK, Panda AK. Memory antibody response from antigen loaded polymer particles and the effect of antigen release kinetics. Biomaterials 2009;30:4763e76. [22] Demento SL, Cui WG, Criscione JM, Stern E, Tulipan J, Kaech SM, et al. Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomaterials 2012;33:4957e64. [23] Johansen P, Storni T, Rettig L, Qiu ZY, Der-Sarkissian A, Smith KA, et al. Antigen kinetics determines immune reactivity. Proc Natl Acad Sci USA 2008;105: 5189e94. [24] Rizwan SB, McBurney WT, Young K, Hanley T, Boyd BJ, Rades T, et al. Cubosomes containing the adjuvants imiquimod and monophosphoryl lipid A stimulate robust cellular and humoral immune responses. J Control Release 2013;165:16e21. [25] Zhao L, Seth A, Wibowo N, Zhao CX, Mitter N, Yu CZ, et al. Nanoparticle vaccines. Vaccine 2014;32:327e37. 6097 [26] Zhang W, Wang L, Liu Y, Chen X, Li J, Yang T, et al. Comparison of PLA microparticles and alum as adjuvants for H5N1 influenza split vaccine: adjuvanticity evaluation and preliminary action mode analysis. Pharm Res 2014;31:1015e31. [27] Yuan L, Wu LH, Chen JA, Wu QA, Hu SH. Paclitaxel acts as an adjuvant to promote both Th1 and Th2 immune responses induced by ovalbumin in mice. Vaccine 2010;28:4402e10. [28] Moon JJ, Suh H, Li AV, Ockenhouse CF, Yadava A, Irvine DJ. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc Natl Acad Sci USA 2012;109:1080e5. [29] Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol 2002;2:251e62. [30] Yu D, Vinuesa CG. The elusive identity of T follicular helper cells. Trends Immunol 2010;31:377e83. [31] Deenick EK, Chan AN, Ma CS, Gatto D, Schwartzberg PL, Brink R, et al. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity 2010;33:241e53. [32] Obst R, van Santen HM, Melamed R, Kamphorst A, Benoist C, Mathis D. Sustained antigen presentation can promote an immunogenic T cell response, like dendritic cell activation. Proc Natl Acad Sci USA 2007;104:15460e5. [33] Blair DA, Turner DL, Bose TO, Pham QM, Bouchard KR, Williams KJ, et al. Duration of antigen availability influences the expansion and memory differentiation of T cells. J Immunol 2011;187:2310e21. [34] Obst R, van Santen HM, Mathis D, Benoist I. Antigen persistence is required throughout the expansion phase of a CD4þ T cell response. J Exp Med 2005;201:1555e65. [35] Jusforgues-Saklani H, Uhl M, Blachere N, Lemaitre F, Lantz O, Bousso P, et al. Antigen persistence is required for dendritic cell licensing and CD8þ T cell cross-priming. J Immunol 2008;181:3067e76. [36] Cockburn IA, Chen YC, Overstreet MG, Lees JR, van Rooijen N, Farber DL, et al. Prolonged antigen presentation is required for optimal CD8þ T cell responses against malaria liver stage parasites. PLoS Pathog 2010;6:e1000877.