* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 25
Photosynthesis wikipedia , lookup
Plant nutrition wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Butyric acid wikipedia , lookup
Mitogen-activated protein kinase wikipedia , lookup
Point mutation wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Biochemical cascade wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Catalytic triad wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Proteolysis wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Protein structure prediction wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Metalloprotein wikipedia , lookup
Microbial metabolism wikipedia , lookup
Peptide synthesis wikipedia , lookup
Nitrogen cycle wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Genetic code wikipedia , lookup
Citric acid cycle wikipedia , lookup
Biochemistry wikipedia , lookup
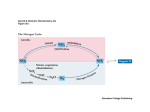
Chapter 25 Nitrogen Acquisition and Amino Acid Metabolism Biochemistry by Reginald Garrett and Charles Grisham 25.1 – Which Metabolic Pathways Allow Organisms to Live on Inorganic Forms of Nitrogen? Nitrogen is cycled between organisms and inanimate enviroment • The principal inorganic forms of N are in an oxidized state – As N2 in the atmosphere – As nitrate (NO3-) in the soils and ocean • All biological compounds contain N in a reduced form (NH4+) Outline 1. Which Metabolic Pathways Allow Organisms to Live on Inorganic Forms of Nitrogen? 2. What Is The Metabolic Fate of Ammonium? 3. What Regulatory Mechanisms Act on Escherichia coli Glutamine Synthetase? 4. How Do Organisms Synthesize Amino Acids? 5. How Does Amino Acid Catabolism Lead into Pathways of Energy Production? • Thus, Nitrogen acquisition must involve 1. The Reduction of the oxidized forms (N2 and NO3-) to NH4+ 2. The incorporation of NH4+ into organic linkage as amino or amido groups • The reduction occurs in microorganisms and green plants. But animals gain N through diet. The reduction of Nitrogen (+3) Nitrogen assimilation and nitrogen fixation 1. Nitrate assimilation occurs in two steps: (+5) – 2e- reduction of nitrate to nitrite – 6e- reduction of nitrite to ammonium (fig 25.1) • Nitrate assimilation accounts for 99% of N acquisition by the biosphere (-3) (+2) (+1) (0) Figure 25.1 The nitrogen cycle. Organic nitrogenous compounds are formed by the incorporation of NH4+ into carbon skeletons. Note that denitrification and nitrogen fixation are anaerobic processes. Nitrate Assimilation • • Nitrate assimilation – the reduction of nitrate to NH4+ in plants, various fungi, and certain bacteria Two steps: 1. Nitrate reductase NO3- + 2 H+ + 2 e- → NO2- + H2O 2. Nitrite reductase NO2- + 8 H+ + 6 e- → NH4+ + 2 H2O • Electrons are transferred from NADH to nitrate 2. Nitrogen fixation involves reduction of N2 in prokaryotes by nitrogenase Nitrate reductase • • • Pathway involves -- SH of enzyme, FAD, cytochrome b557 and Molybdenum cofactor -- all protein-bound Nitrate reductases are cytosolic 210-270 kD dimeric protein MoCo required both for reductase activity and for assembly of enzyme subunits to active dimer NADH NO3[-SH →FAD→cytochrome b557 →MoCo] NADH+ NO2- Nitrite Reductase Light drives reduction of ferredoxins and electrons flow to 4Fe-4S and siroheme and then to nitrite • Nitrite is reduced to ammonium while still bound to siroheme • In higher plants, nitrite reductase is in chloroplasts, but nitrate reductase is cytosolic Figure 25.2 The novel prosthetic groups of nitrate reductase and nitrite reductase. (a) The molybdenum cofactor of nitrate reductase. The molybdenum-free version of this compound is a pterin derivative called molybdopterin. (b) Siroheme, a uroporphyrin derivative, is a member of the isobacteriochlorin class of hemes, a group of porphyrins in which adjacent pyrrole rings are reduced. Siroheme is novel in having eight carboxylate-containing side chains. These carboxylate groups may act as H+ donors during the reduction of NO2- to NH4+. Nitrogen fixation • • • • Figure 25.3 Domain organization within the enzymes of nitrate assimilation. The numbers denote residue number along the amino acid sequence of the proteins. N2 + 10 H+ + 8 e- → 2 NH4+ + H2 Only occurs in certain prokaryotes Rhizobia fix nitrogen in symbiotic association with leguminous plants Rhizobia fix N for the plant and plant provides Rhizobia with carbon substrates Fundamental requirements: 1. 2. 3. 4. Nitrogenase A strong reductant (reduced ferredoxin) ATP O-free conditions Nitrogenase Complex Figure 25.4 The triple bond in N2 must be broken during nitrogen fixation. Two metalloprotein components: nitrogenase reductase and nitrogenase • Nitrogenase reductase – Fe-protein – is a 60 kD homodimer with a single 4Fe-4S cluster • N2 reduction to ammonia is thermodynamically favorable • However, the activation barrier for breaking the N-N triple bond is enormous • 16 ATP provide the needed activation energy • Very oxygen-sensitive • Binds MgATP and hydrolyzes 2 ATPs per electron transferred • Reduction of N2 to 2NH3 + H2 requires 4 pairs of electrons, so 16 ATP are consumed per N2 Nitrogenase • MoFe-protein • A 220 kD α2β2 heterotetramer • Each molecule of enzyme contains 2 Mo, 32 Fe, 30 equivalents of acid-labile sulfide (FeS clusters, etc) • Four 4Fe-4S clusters plus two FeMoCo, an iron-molybdenum cofactor • Nitrogenase is slow enzyme – 12 e- pairs per second, i.e., only three molecules of N2 per second – As much as 5% of cellular protein may be nitrogenase Figure 25.5 Structures of the two types of metal clusters found in nitrogenase. (a) The P-cluster consists of two Fe4S3 clusters that share an S atom. (b) The FeMo-cofactor contains 1 Mo, 7Fe, and 9S atoms. Homocitrate provides two oxo ligands to the Mo atom. The regulation of nitrogen Fixation • 1. 2. • Two regulatory controls ADP inhibits the activity of nitrogease NH4+ represses the expression of nif genes Some organism, ADP-ribosylation of nitrogenase reductase Figure 25.6 The nitrogenase reaction. Depending on the bacterium, electrons for N2 reduction may come from light, NADH, hydrogen gas, or pyruvate. The primary e- donor for the nitrogenase system is reduced ferredoxin. Figure 25.8 Regulation of nitrogen fixation. (a) ADP inhibits nitrogenase activity. (b) NH4+ represses nif gene expression. (c) In some organisms, the nitrogenase complex is regulated by covalent modification. ADPribosylation of nitrogenase reductase leads to its inactivation. Nitrogenase reductase is a distant relative of the signal-transducing Gprotein superfamily. 25.2 – What Is The Metabolic Fate of Ammonium? Ammonium enters organic linkage via three major reactions in all cells 1. 2. 3. • Carbamoyl-phosphate synthetase (CPS) Glutamate dehydrogenase (GDH) Glutamine synthetase (GS) Asparagine synthetase (some microorganisms) Carbamoyl-phosphate synthetase I (CPS-I) Glutamate dehydrogenase Ammonium is converted to carbamoyl-P NH4+ + α-ketoglutarate + NADPH + 2 H+ → glutamate + NADP+ + H2O NH4+ + HCO3- + 2 ATP → carbamoyl phosphate + 2 ADP + Pi + 2 H+ (H2N-COO-PO32-) • • Reductive amination of α-ketoglutarate to form glutamate Two ATP required – one to activate bicarbonate – one to phosphorylate carbamate • This reaction is an early step in the urea cycle Glutamine synthetase NH4+ + glutamate + ATP → glutamine + ADP + Pi • ATP-dependent amidation of γ-carboxyl of glutamate to glutamine • Glutamine is a major N donor in the biosynthesis of many organic N compounds, therefore GS activity is tightly regulated Figure 25.10 (a) The enzymatic reaction catalyzed by glutamine synthetase. (b) The reaction proceeds by (a) activation of the γcarboxyl group of Glu by ATP, followed by (b) amidation by NH4+. The major pathways of Ammonium Assimilation Two principal pathways 1. Principal route: GDH/GS in organisms rich in N – See Figures 25.12 and 25.13 2. Secondary route: GS/GOGAT in organisms confronting N limitation – – GOGAT is glutamate synthase or glutamate:oxo-glutarate amino transferase See Figures 25.12 and 25.13 Figure 25.11 The GDH/GS pathway of ammonium assimilation. The sum of these reactions is the conversion of 1 αketoglutarate to 1 glutamine at the expense of 2 NH4+, 1 ATP, and 1 NADPH. Figure 25.11 The GDH/GS pathway of ammonium assimilation. Figure 25.12 The glutamate synthase (GOGAT)reaction, showing the reductants exploited by different organisms in this reductive amination reaction. Figure 25.13 The GS/GOGAT pathway of ammonium assimilation. The sum of these reactions results in the conversion of 1 α-ketoglutarate to 1 glutamine at the expense of 2 ATP and 1 NADPH. 25.3 – What Regulatory Mechanisms Act on Glutamine Synthetase • GS in E. coli is regulated in three ways: 1. Feedback inhibition 2. Covalent modification (interconverts between inactive and active forms) 3. Regulation of gene expression and protein synthesis control the amount of GS in cells • But no such regulation occurs in eukaryotic versions of GS Allosteric Regulation of Glutamine Synthetase • Nine different feedback inhibitors: Gly, Ala, Ser, His, Trp, CTP, AMP, carbamoyl-P and glucosamine6-P • Gly, Ala, Ser are indicators of amino acid metabolism in cells • Other six are end products of a biochemical pathway • AMP competes with ATP of binding at the ATP substrate site • This effectively controls glutamine’s contributions to metabolism Figure 25.14 The subunit organization of bacterial glutamine synthetase. (a) Diagram showing its dodecameric structure as a stack of two hexagons. (b) Molecular structure of glutamine synthetase from Salmonella typhimurium (a close relative of E.coli), as revealed by X-ray crystallographic analysis. (From Almassy, R. J., Janson, C. A., Hamlin, R., Xuong, N.-H., and Eisenberg, D., 1986. Novel subunitsubunit interactions in the structure of glutamine synthetase.Nature 323:304. Photos courtesy of S.-H. Liaw and D. Eisenberg.) Figure 25.15 The allosteric regulation of glutamine synthetase activity by feedback inhibition. Covalent Modification of Glutamine Synthetase • Each subunit is adenylylated at Tyr-397 • Adenylylation inactivates GS by adenylyl transferase • Adenylyl transferase catalyzes both the adenylylation and deadenylylation – PII (regulatory protein) controls these • AT:PIIA catalyzes adenylylation • AT:PIID (PII-UMP) catalyzes deadenylylation • α-Ketoglutarate and Gln also affect Figure 25.16 Covalent modification of GS: Adenylylation of Tyr397 in the glutamine synthetase polypeptide via an ATP-dependent reaction catalyzed by the converter enzyme adenylyl transferase (AT). From 1 through 12 GS monomers in the GS holoenzyme can be modified, with progressive inactivation as the ratio of [modified]/[unmodified] GS subunits increases. Figure 25.17 The cyclic cascade system regulating the covalent modification of GS. Gene Expression regulates GS • Gene GlnA is actively transcribed only if transcriptional enhancer NRI is in its phosphorylated form, NRI-P • NRI is phosphorylated by NRII, a protein kinase • If NRII is complexed with PIIA it acts as a phosphatase, not a kinase Figure 25.18 Transcriptional regulation of GlnA expression through the reversible phosphorylation of NRI, as controlled by NRII and its association with PIIA. (kinase) 25.4 – Amino Acid Biosynthesis • Plants and microorganisms can make all 20 amino acids and all other needed N metabolites • In these organisms, glutamate is the source of N, via transamination (aminotransferase) reactions • Mammals can make only 10 of the 20 AAs • The others are classed as "essential" amino acids and must be obtained in the diet (phosphatase) Amino acids are formed from αketo acids by transamination Amino acid1 + α-keto acid2 → α-keto acid1 + Amino acid2 • Transamination (aminotransferase) reactions • Named according their amino acid substrate – Glutamate-asparate aminotransferase Figure 25.19 Glutamate-dependent transamination of α-keto acid carbon skeletons is a primary mechanism for amino acid synthesis. Amino Acid Biosynthesis can be organized into families • According to the intermediates that they are made from The Mechanism of the Aminotransferase (Transamination) Reaction The α-Ketoglutarate Family Glu, Gln, Pro, Arg, and sometimes Lys • The routes for Glu and Gln synthesis were described when we considered pathways of ammonia assimilation – Transamination of α-Ketoglutarate gives glutamate – Amidation of glutamate gives glutamine • Proline is derived from glutamate • Ornithine is also derived from glutamate – the similarity to the proline pathway • Arginine are part of the urea cycle (1) N-acetylglutamate synthase (2) N-acetylglutamate kinase (3) N-acetylglutamate-5semialdehyde dehydrogenase (4) N-acetylornithine δ-aminotransferase Figure 25.20 The pathway of proline biosynthesis from glutamate. The enzymes are (1) γ-glutamyl kinase, (2) glutamate-5-semialdehyde dehydrogenase, and (4) Δ1-pyrroline-5-carboxylate reductase; reaction (3) occurs nonenzymatically. (5) N-acetylornithine deacetylase Carbamoyl-phosphate synthetase I • Ornithine has three metabolic roles – – – • To serve as precursor to arginine To function as an intermediate in the urea cycle To act as an intermediate in arginine degradation δ-NH3+ of ornithine is carbamoylated by onithine transcarbamoylase in urea cycle • Carbamoyl-phosphate synthetase I (CPS-I) – NH3-dependent mitochondrial CPS isozyme 1. HCO3- is activated via an ATP-dependent phosphorylation 2. Ammonia attacks the carbonyl carbon of carbonyl-P, displacing Pi to form carbamate 3. Carbamate is phosphorylated via a second ATP to give carbamoyl-P • CPS-I represents the committed step in urea cycle • Activated by N-acetylglutamate – Figure 25.22 The mechanism of action of CPS-I, the NH3-dependent mitochondrial CPS isozyme. (1) HCO3- is activated via an ATP-dependent phosphorylation. (2) Ammonia attacks the carbonyl carbon of carbonyl-P, displacing Pi to form carbamate. (3) Carbamate is phosphorylated via a second ATP to give carbamoyl-P. The Urea Cycle • The carbon skeleton of arginine is derived from α-ketoglutarate • N and C in the guanidino group of Arg come from NH4+, HCO3- (carbamoyl-P), and the α-NH2 of Glu and Asp • Breakdown of Arg in the urea cycle releases two N and one C as urea • Important N excretion mechanism in livers of terrestrial vertebrates • Urea cycle is linked to TCA by fumarate 1. 2. 3. 4. Because N-acetylglutamate is a precursor to orinithine synthesis and essential to the operation of the urea cycle → amino acid catabolism ↑ → glutamate level (N-acetylglutamate) ↑ → Stimulate CPS-I → Raise overall Urea cycle activity Ornithine transcarbamoylase (OTCase) Argininosuccinate synthetase Argininosuccinase Arginase The Urea Cycle Lysine Biosynthesis • Two pathways: 1. α-aminoadipate pathway 2. diaminopimelate pathway (Asp) • Lysine derived from α-ketoglutarate – – • • • • Reactions 1 through 4 are reminiscent of the first four reactions in the citric acid cycle α-ketooadipate Transamination gives α-aminoadipate Adenylylation activates the δ-COOH for reduction Reductive amination give saccharopine Oxidative cleavage yields lysine Figure 25.24 Lysine biosynthesis in certain fungi and Euglena: the α-aminoadipic acid pathway. Reactions 1 through 4 are reminiscent of the first four reactions in the citric acid cycle, except that the product α-ketoadipate has an additional ⎯CH2⎯ unit. Reaction 5 is catalyzed by a glutamate-dependent aminotransferase; reaction 6 is the adenylylation of the δ-carboxyl of αaminoadipate to give the 6-adenylyl derivative. Reductive deadenylylation by an NADPH-dependent dehydrogenase in reaction 7 gives αaminoadipic-6-semialdehyde, which in reaction 8 is coupled with glutamate via its amino group by a second NADPH-dependent dehydrogenase. Oxidative removal of the αketoglutarate moiety by NAD+dependent saccharopine dehydrogenase in reaction 9 leaves this amino group as the ε-NH3+ of lysine. The Aspartate Family Asp, Asn, Lys, Met, Thr, Ile • Transamination of Oxaloaceate gives Aspartate (aspartate aminotransferase) • Amidation of Asp gives Asparagine ( asparagine synthetase) • Met, Thr and Lys are made from Aspartate • β-Aspartyl semialdehyde and homoserine are branch points • Isoleucine, four of its six carbons derived from Asp (via Thr) and two come from pyruvate Figure 25.25 Aspartate biosynthesis via transamination of oxaloacetate by glutamate. Figure 25.27 Biosynthesis of threonine, methionine, and lysine, members of the aspartate family of amino acids. β-Aspartyl-semialdehyde is a common precursor to all three. Figure 25.26 Asparagine biosynthesis from Asp, Gln, and ATP by asparagine synthetase. β-Aspartyladenylate is an enzymebond intermediate. It is formed by aspartokinase (reaction 1) and β-aspartylsemialdehyde dehydrogenase (reaction 2). Figure 25.27 Biosynthesis of threonine, methionine, and lysine, members of the aspartate family of amino acids. Figure 25.27 Biosynthesis of threonine, methionine, and lysine, members of the aspartate family of amino acids. β-Aspartyl-semialdehyde Figure 25.27 Biosynthesis of threonine, methionine, and lysine, members of the aspartate family of amino acids. • In E. coli – Three isozymes of aspartokinase – Uniquely controlled by one of the three endproducts • Role of methionine – in methylations via S-adenosylmethionine (SAM; S-AdoMet) – polyamine biosynthesis The Pyruvate Family Ala, Val, Leu, and Ile • Transamination of pyruvate gives Alanine • Valine is derived from pyruvate • Ile synthesis from Thr mimics Val synthesis from pyruvate (Fig. 25.29) – Threonine deaminase (also called threonine dehydratase or serine dehydratase) is sensitive to Ile – Ile and val pathway employ the same set of enzymes Figure 25.28 The synthesis of Sadenosylmethionine (SAM) • Leu synthesis begins with an α-keto isovalerate – Isopropylmalate synthase is sensitive to Leu Figure 25.29 Biosynthesis of valine and isoleucine. Threonine deaminase isopropylmalate synthase isopropylmalate dehydratase Acetohydroxy acid synthase Acetohydroxy acid isomeroreductase isopropylmalate dehydrogenase Dihydroxy acid dehydratase Glutamate-dependent aminotransferase 3-Phosphoglycerate Family Ser, Gly, Cys leucine aminotransferase Figure 25.30 Biosynthesis of leucine. • Serine hydroxymethylase (PLP-dependent) transfers the β-carbon of Ser to THF to make glycine 1. 3-Phosphoglycerate dehydrogenase diverts 3-PG from glycolysis to amino acid synthesis pathways (3phosphohydroxypyruvate) 2. Transamination by Glu gives 3phosphoserine (3-phosphoserine aminotransferase) 3. Phosphoserine phosphatase yields serine Figure 25.32 Biosynthesis of glycine from serine (a) via serine hydroxymethyltransferase and (b) via glycine oxidase. • A PLP-dependent enzyme makes Cys ATP sulfurylase Some bacteria Adenosine-5'-phosphosulfate-3'-phosphokinase. most microorganism and plants O-acetylserine sulfhydrylase serine acetyltransferase Figure 25.33 Cysteine biosynthesis. (a) Direct sulfhydrylation of serine by H2S. (b) H2S-dependent sulfhydrylation of O-acetylserine. Figure 25.34 Sulfate assimilation and the generation of sulfide for synthesis of organic S compounds. Aromatic Amino Acids Phe, Tyr, Trp, His • Shikimate pathway yields chorismate, thence Phe, Tyr, Trp • Chorismate as a branch point in this pathway (Figs. 25.35) • Chorismate is synthesized from PEP and erythrose-4-P – Via shikimate pathway – The side chain of chorismate is derived from a second PEP Figure 25.35 Some of the aromatic compounds derived from chorismate. (1) 2-keto-3-deoxy-D-arabino-heptulosonate-7-P synthase (2) dehydroquinate synthase (3) 5-dehydroquinate dehydratase (4) shikimate dehydrogenase The biosynthesis of phenylalanine, tyrosine, and tryptophan • At chorismate, the pathway separates into three branches, each leading to one of the aromatic amino acids • Mammals can synthesize tyrosine from phenylalanine by phenylalanine hydroxylase (Phenylalanine-4monooxygenase) (5) shikimate kinase (6) 3-enolpyruvyl-shikimate-5-phosphate synthase (7) chorismate synthase. Figure 25.37 The biosynthesis of phenylalanine, tyrosine, and tryptophan from chorismate. (1) chorismate mutase (2) prephenate dehydratase (3) phenylalanine aminotransferase (4) prephenate dehydrogenase (5) tyrosine aminotransferase (6) anthranilate synthase (7) anthranilate-phosphoribosyl transferase (8) N-(5'-phosphoribosyl)anthranilate isomerase (9) indole-3-glycerol phosphate synthase (10) tryptophan synthase (a-subunit) (11) tryptophan synthase (bsubunit). Figure 25.38 The formation of tyrosine from phenylalanine. Hitidine biosynthesis • His synthesis, like that of Trp, shares metabolic intermediates (PRPP) with purine biosynthetic pathway • His operon • Begin from PRPP and ATP • The intermediate 5-aminoimidazole-4carboxamide ribonucleotide (AICAR) is a purine precursor (replenish ATP; Ch 26) Figure 25.40 The pathway of histidine biosynthesis. (1) ATP-phosphoribosyl transferase (2) pyrophosphohydrolase (3) phosphoribosyl-AMP cyclohydrolase (4) phosphoribosylformimino-5aminoimidazole carboxamide ribonucleotide isomerase (5) glutamine amidotransferase (6) imidazole glycerol-P dehydratase (7) L-histidinol phosphate aminotransferase (8) histidinol phosphate phosphatase (9) histidinol dehydrogenase. Amino Acid Biosynthesis Inhibitors as Herbicides • A variety of herbicides have been developed as inhibitors of plant enzymes that synthesize “essential” amino acids • These substances show no effect on animals • For example, glyphosate, sold as RoundUp, is a PEP analog that acts as an uncompetitive inhibitor of 3-enolpyruvylshikimate-5-P synthase. Amino acid synthesis inhibitors as herbicides (inhibitor of 3-enolpyruvyl-shikimate-5phosphate synthase) (fig 25.36) (inhibitor of acetohydroxy acid synthase in biosynthesis of valine and isoleucine) (fig 25.29) (inhibitor of imidazol glycerol-P dehydrtase in biosynthesis of histidine) (fig 25.40) (inhibitor of glutamine synthetase) 25.5 – Degradation of Amino Acids The 20 amino acids are degraded to produce (mostly) TCA intermediates • Energy requirement – 90% from oxidation of carbohydrates and fats – 10% from oxidation of amino acids • The primary physiological purpose of amino acids is to serve as building blocks for protein synthesis • The classifications of amino acids in Fig. 25.41 • Glucogenic and ketogenic C-3 family (pyruvate): Ala, Ser, Cys, Gly, Thr, Trp C-4 family (oxaloaceate & fumarate): Oxaloaceate: Asp, Asn Fumarate: Asp, Phe, Tyr Figure 25.41 Metabolic degradation of the common amino acids. Glucogenic amino acids are shown in pink, ketogenic in blue. Those that give rise to precursors for glucose synthesis, such as α-ketoglutarate, succinyl-CoA, fumarate, oxaloacetate, and pyruvate, are termed glucogenic (shown in pink). Those degraded to acetyl-CoA or acetoacetate are called ketogenic (shown in blue) because they can be converted to fatty acids or ketone bodies. Some amino acids are both glucogenic and ketogenic. C-3 family: Ala, Ser, Cys, Gly, Thr, Trp C-5 family (α-ketoglutarate): Glu, Gln, Arg, Pro, His Succinyl-CoA: Ile, Met, Val Acetyl-CoA & acetoacetate Ile, Leu, Thr, Trp Leu, Lys, Phe, Tyr Figure 25.42 Formation of pyruvate from alanine, serine, cysteine, glycine, tryptophan, or threonine. Figure 25.44 Valine, isoleucine, and methionine are converted via propionyl-CoA to succinyl-CoA for entry into the citric acid cycle. The shaded carbon atoms of the three amino acids give rise to propionyl-CoA. All three amino acids lose their α-carboxyl group as CO2. Figure 25.43 The degradation of the C-5 family of amino acids leads to α-ketoglutarate via glutamate. The histidine carbons, numbered 1 through 5, become carbons 1 through 5 of glutamate, as indicated. Leucine is Degraded to Acetyl-CoA and Acetoacetate Figure 25.45 Leucine is one of only two purely ketogenic amino acids; the other is lysine. Deamination of leucine via a transamination reaction yields αketoisocaproate, which is oxidatively decarboxylated to isovaleryl-CoA. Subsequent reactions give β-hydroxy-β-methylglutaryl-CoA, which is then cleaved to yield acetyl-CoA and acetoacetate, a ketone body. Methionine first becomes Sadenosylmethionine, then homocysteine (see Figure 25.28). The terminal two carbons of isoleucine become acetyl-CoA. The Predominant Pathway of Lysine Degradation is the Saccharopine Pathway Figure 25.46 Lysine is degraded through saccharopine and α-aminoadipate to αketoadipate. Oxidative decarboxylation yields glutaryl-CoA, which can be transformed into acetoacetyl-CoA and then acetoacetate. Phenylalanine and Tyrosine Are Degraded to Acetoacetate and Fumarate • The first reaction in phenylalanine degradation is the hydroxylation reaction of tyrosine biosynthesis • Both these amino acids thus share a common degradative pathway • Transamination of tyrosine yields phydroxyphenylpyruvate • A vitamin C-dependent dioxygenase then produces homogentisate • Ring opening and isomerization gives 4-fumarylacetoacetate, which is hydrolyzed to acetoacetate and fumarate Hereditary defects Maple syrup urine disease – After the initial step (deamination) to produce αketo acids – The defect in oxidative decarboxylation of Ile, Leu, and Val (25.44) Phenylketonuria – The defect in phenylalanine hydoxylase (25.38) – Accumulation of phenylpyruvate Alkaptouria – Homogentisate dioxygenase (25.47) Figure 25.47 Phenylalanine and tyrosine degradation. (1) Transamination of Tyr gives p-hydroxyphenylpyruvate (2) p-hydroxy-phenylpyruvate dioxygenase (vitamin C-dependent) (3) homogentisate dioxygenase (4) 4-Maleylacetoacetate isomerase (5) is hydrolyzed by fumarylacetoacetase. Nitrogen excretion Ammonotelic: – Ammonia – Aquatic animals Ureotelic: – Urea – Terrestrial vetebrates Uricotelic: – Uric acid – Birds and reptiles