* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Full Text
Survey
Document related concepts
Neuropsychopharmacology wikipedia , lookup
Neural engineering wikipedia , lookup
Convolutional neural network wikipedia , lookup
Subventricular zone wikipedia , lookup
Neuroesthetics wikipedia , lookup
Neuroanatomy wikipedia , lookup
Visual selective attention in dementia wikipedia , lookup
Visual extinction wikipedia , lookup
Neuroanatomy of memory wikipedia , lookup
Neurostimulation wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Microneurography wikipedia , lookup
Neuroregeneration wikipedia , lookup
Transcript
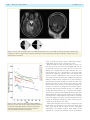
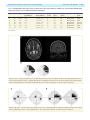
doi:10.1093/brain/awr324 Brain 2012: 135; 534–541 | 534 BRAIN A JOURNAL OF NEUROLOGY The time course of retrograde trans-synaptic degeneration following occipital lobe damage in humans Panitha Jindahra,1,2,3 Aviva Petrie4 and Gordon T. Plant1,2,3 1 2 3 4 The National Hospital for Neurology and Neurosurgery, Queen Square, London, UK Moorfields Eye Hospital, City Road, London, UK St Thomas’ Hospital, Lambeth Palace Road, London, UK Eastman Dental Hospital, Gray’s Inn Road, London, UK Correspondence to: Dr Gordon T. Plant, The National Hospital for Neurology and Neurosurgery, Queen Square, London, WC1N 3BG, UK E-mail: [email protected] Following damage to the human post-geniculate visual pathway retrograde trans-synaptic degeneration of the optic nerve fibres occurs. It has been known for some time from investigations carried out in primates that a decline in the number of retinal ganglion cells follows occipital lobectomy. However, this is not detectable in all species studied and whether this occurs in humans was controversial until recent studies that have shown that following lesions of the occipital lobe, the retinal nerve fibre layer thickness measured by optical coherence tomography is reduced and corresponding shrinkage of the optic tract can be demonstrated by magnetic resonance imaging. The time course of the degeneration in humans is, however, unknown. In the present study, we have used optical coherence tomography to demonstrate for the first time progressive thinning of the retinal nerve fibre layer following occipital lobe/optic radiation damage due to stroke. First, in a group of 38 patients the measurement was taken on a single occasion at a known time interval since the stroke, ranging from 6 days to 67 years. Here, a negative straight line relationship (linear regression r = 0.54, P 5 0.001) was found between nerve fibre layer thickness and elapsed time since injury in log years, giving a rate of decline of 9.08 mm per log year after adjusting for age. This indicates a decelerating rate of loss that differs from the rate of decline found with chronological age in this same group, which shows a steady rate of thinning by 0.4 mm per year (P = 0.006) after adjusting for duration of the disease. In a second study serial measurements were taken following the acute event in a group of seven patients with homonymous hemianopia; here a negative straight line relationship was found between time and nerve fibre layer thickness in micrometres over a period of data collection beginning at a mean of 36.9 days post-stroke (range 5–112) and ending at a mean of 426.6 days post-stroke (range 170–917). Evidence from clinical observation (funduscopy) suggested that retrograde trans-synaptic degeneration occurred in humans only where the damage to the post-geniculate pathway occurred prenatally. The results reported herein add weight to the previous demonstration that this type of degeneration does indeed occur in the human visual system by showing that it can be monitored over time and hence may provide a model for trans-synaptic degeneration in the human central nervous system. Keywords: retrograde trans-synaptic/trans-neuronal degeneration; retinal nerve fibre layer; optical coherence tomography; occipital stroke; post-geniculate lesion Received August 4, 2011. Revised October 5, 2011. Accepted October 16, 2011 ß The Author (2012). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: [email protected] Retrograde trans-synaptic degeneration in the visual pathway Introduction Retrograde trans-synaptic degeneration of neurons, also known as trans-neuronal degeneration, occurs following damage to the human CNS. Evidence from clinical observation had suggested that retrograde trans-synaptic degeneration of neurons of retinal ganglion cells and their axons in the optic nerve and the optic tract occurred only following prenatal (not post-natal) acquired damage to the visual pathway posterior to the lateral geniculate nucleus (Miller et al., 1995). Recent studies, however, have demonstrated thinning of the retinal nerve fibre layer using optical coherence tomography (Jindahra et al., 2009) and of the optic tract using MRI following acquired unilateral occipital damage in humans (Bridge et al., 2011; Cowey et al., 2011). These findings confirm the occurrence of retrograde trans-synaptic degeneration of neurons in the human visual pathway. Degeneration of an axon in the direction of its terminal following direct injury is classified as anterograde degeneration, whereas retrograde degeneration proceeds towards the cell body (Coleman et al., 2005; Saxena et al., 2007). Anterograde degeneration was first described by Augustus Waller (1850) and is also termed Wallerian degeneration. The pathological process in Wallerian degeneration has been the subject of extensive investigation (Danek et al., 1990; Savoiardo et al., 1992; Fitzek et al., 2004). The time course of both Wallerian and retrograde degeneration has been studied, using for example, microglial responses after axotomy in the spinal cord of the rat. Wallerian and retrograde degeneration have been shown to occur simultaneously within days (Koshinaga et al., 1995). In humans, MRI studies have demonstrated both retrograde and Wallerian degeneration at 4 months following infarction of the internal capsule as a short band of increased T2-weighted signal ascending to the precentral gyrus and another longer band descending to the brainstem (Danek et al., 1990), but no imaging studies of earlier changes have been published. Retrograde trans-synaptic degeneration of neurons has been identified histopathologically in the immature human CNS following hypoxic damage (Sakai et al., 1994). Post-mortem histopathological studies of the brains of premature infants revealed retrograde trans-synaptic degeneration of neurons in the olivocerebellar pathway; a cerebellar lesion resulted in neuronal loss in the inferior olive as evidenced by decreased neurofilament staining of axonal fibres, by axonal swelling and by astrogliosis. Axonal swelling is a feature of retrograde trans-synaptic degeneration of neurons shown in studies in human brain. It is associated with axonal transport interruption, which occurs prior to axonal separation (Povlishock et al., 1983; Sakai et al., 1994). In the visual pathway a retrogeniculate lesion may be expected to give rise to retrograde degeneration of the geniculo-cortical neurons damaged directly, which will be followed by retrograde trans-synaptic degeneration of neurons of retinal ganglion cell axons. This has been identified post-mortem in primate retina following occipital lobectomy; the number of retinal ganglion cells in the region of the retina corresponding to the visual field representation of the damaged cortex was found to be massively reduced (Cowey, 1974; Johnson et al., 2000). In addition, anterograde degeneration may be expected of the geniculo-cortical fibres. In Brain 2012: 135; 534–541 | 535 humans, retrograde trans-synaptic degeneration of neurons of the visual pathway has now been identified by post-mortem examination of one case of occipital damage occurring early in life (Beatty et al., 1982), clinical examination (in congenital cases; Hoyt et al., 1972), electroretinography (Porrello et al., 1999), optical coherence tomography (Jindahra et al., 2009), and MRI scan (Bridge et al., 2011; Cowey et al., 2011). Optical coherence tomography has been especially valuable since the thinning of the retinal nerve fibre layer has been shown to correspond to the known trajectories of the crossed and uncrossed projections arising in the nasal and temporal hemiretinae, respectively (Jindahra et al., 2009). Our previous study showed that retinal changes can be detected as early as 3.6 months following damage to the occipital lobe (Jindahra et al., 2009). However, the precise time course of the process is unknown. The present study was carried out to address this issue, which is of fundamental importance to the understanding of the pathological process in humans; of how it might relate to animal studies of retrograde trans-synaptic degeneration of neurons; and for the design of treatment strategies to prevent this class of neurodegeneration occurring following damage to the visual pathway and other regions of the brain. Patients and methods This study was divided into two parts. In the first part (the cross-sectional study), we investigated the relationship between duration of disease and the peripapillary retinal nerve fibre layer thickness. The second part comprised a longitudinal study. Cross-sectional study Thirty-eight patients with acquired homonymous hemianopia were recruited. All subjects had thorough neurological and ophthalmic examinations including intraocular pressure measurement. In each case it was confirmed that the hemianopia was the consequence of a single retrogeniculate lesion, which was identified by appropriate neuro-imaging. There was no imaging evidence of optic tract damage in any of these patients. Duration of the visual field defect ranged from 6 days to 67 years (mean 7.71 years). Age in the hemianopic group ranged from 17–83 years (mean 59.92 years). Twentythree control subjects were recruited. Age in the control group ranged from 23–61 years (mean 43 years). Exclusion criteria included evidence of more than one occipital lesion and evidence of any ophthalmic disorder such as retinopathy, glaucoma and high myopia ( 6.00 diopters or more). The peripapillary retinal nerve fibre layer thickness was measured by optical coherence tomography (Stratus OCT, software version 4.0.1; Carl Zeiss Meditec, Inc.) on a single occasion. A 3.4 mm diameter circular fast retinal nerve fibre layer thickness protocol was employed. The strength of signal was at least 7. We evaluated the retinal nerve fibre layer thickness in both eyes of each subject and took the mean for further statistical analysis. The aim of this part of the study was to ascertain the relationship between retinal nerve fibre layer thickness and the time elapsed since the occipital damage. Longitudinal study In the second part of the study, 11 patients were recruited, seven with homonymous hemianopia and four with smaller homonymous visual 536 | Brain 2012: 135; 534–541 field defects. All cases had occipital cerebral infarction as the cause of the post-geniculate damage except for Case 4 (optic radiation haemorrhage) and Case 7 (lateral geniculate nucleus infarction). All subjects had thorough neurological and ophthalmic examinations including intraocular pressure measurement. Exclusion criteria were as for the first (cross-sectional) part of the study. Peripapillary retinal nerve fibre layer thickness was measured by optical coherence tomography in each case as soon as possible after the onset of stroke and on several occasions thereafter. We evaluated the retinal nerve fibre layer thickness in both eyes of each subject and took the mean for further statistical analysis. A minimum of three measurements was made. The initial measurement was taken at a time interval ranging from 5–112 days post-stroke and the last at an interval ranging from 170–917 days post-stroke. The aim of this part of the study was to determine the time course of retinal nerve fibre layer thinning in individual cases. The retinal nerve fibre layer measurements were carried out by a single operator (P.J.) who has been shown to have a high reproducibility of measurements (data not shown). All participants gave their informed consent and the study was approved by the ethics committee of the National Hospital for Neurology and Neurosurgery. Statistical analysis In the first part, multivariable linear regression analysis was performed to evaluate the relationship between the mean peripapillary retinal nerve fibre layer thickness (in mm) of both eyes and log elapsed time in years, with linear age in years as a covariate since it is known that the retinal nerve fibre layer thickness declines with age. The logarithm of elapsed time was shown to provide a better fit to the data than a linear or square root relationship. In the second part, univariable linear regression analysis was used to investigate the relationship between retinal nerve fibre layer thickness (mm) and time elapsed in days after the onset of the stroke. This was carried out separately for each individual in each of the two groups, those with large and those with small visual field defects. A multilevel random effects analysis was used to estimate the overall slope for each group. The assumptions underlying the univariable and multivariable regression analyses were checked by a study of the residuals and found to be satisfied. A Mann–Whitney U-test was used to compare the distribution of the slopes in the two groups of cases as there were only three patients in one group. A significance level of 0.05 was used throughout. IBM SPSS v 18.0 (www.spss.com) and Stata version 11 (StataCorp LP, www.stata.com/products) were used to analyse the data. Results P. Jindahra et al. Figure 1 Multiple linear regression analysis between the retinal nerve fibre layer thickness, duration and age in patients with homonymous hemianopia. The 3D plot shows the negative linear relationships between retinal nerve fibre layer thickness (RNFL; mm; mean of both eyes; z-axis) and both elapsed time since the stroke (log years; x-axis), and chronological age at the time the measurement was taken (years; y-axis) in patients with homonymous hemianopia. There is a straight line relationship with the log of the number of years since the stroke and with a linear plot of chronological age. The relationship is described by the equation: Retinal nerve fibre layer thickness (mm) = 110.3 (9.08) (elapsed time in log years) (0.4) (age at measurement in years). n = 38, r = 0.54, P 5 0.001. the stroke, with mean retinal nerve fibre layer thickness decreasing on average by 0.40 mm as age increased by 1 year (95% CI 0.12 to 0.68; P = 0.006) (Fig. 1 and Table 1). For the control group, a linear regression was calculated with age as an independent factor and the mean of retinal nerve fibre layer thickness of both eyes as a dependent variable. The mean retinal nerve fibre layer thickness declined by 0.11 mm (95% CI 0.45–0.2) as age increased by 1 year in the control group (P = 0.5) (Fig. 2 and Table 1). Our control group had insufficient sample size to reach statistical significance but the trend was of a similar order to results obtained in other studies of the relationship between age and retinal nerve fibre layer thickness, which has been shown to decrease in healthy adults by 0.2–0.3 mm with each year of age (Budenz et al., 2007; Barboni et al., 2011). Cross-sectional study Longitudinal study To investigate the effect of elapsed time since the injury on the thickness after adjusting for age of the patient, a multivariable linear regression analysis was performed with linear age and log elapsed time as covariates. This showed that, after adjusting for the effect of age, elapsed time in log years had a significant effect on mean thickness with the mean thickness decreasing on average by 9.08 mm per log year [95% confidence interval (CI) 5.00– 13.16; P 5 0.001]. Chronological age also had a significant effect on mean thickness, after adjusting for elapsed time since A total of 82 measurements from 11 patients were analysed using a univariable linear regression analysis for each patient, with mean retinal nerve fibre layer thickness of both eyes (mm) as the outcome variable and elapsed time (days after stroke) as the explanatory variable. The results are shown for two groups: those with large visual field defects (Figs 3 and 4; Table 2) and those with small visual field defects (Figs 5–7; Table 3). All hemianopia cases showed a decreasing trend during the period of measurement. All cases with larger visual field defects had a negative slope, Retrograde trans-synaptic degeneration in the visual pathway | 537 Brain 2012: 135; 534–541 Table 1 Model summary of multivariable linear regression analysis on the retinal nerve fibre layer thickness (mm; mean of both eyes of each subject) in patients with homonymous hemianopia and controls Group Covariates n Slope (95% CI) r P-value Occipital damage Time elapsed since stroke in log years Age (yrs) Age (yrs) 38 38 23 9.0 ( 13.2 to 5) 0.4 ( 0.7 to 0.1) 0.11 ( 0.45 to 0.2) 0.54 0.66 0.16 50.001 0.006 0.5 Controls 0.063, minimum 0.009) for the homonymous hemianopia group and 0.002 (maximum 0.008, minimum 0.003) for the small visual field defect group. The median slope of the homonymous hemianopia group was significantly different from zero (P 5 0.001) whereas the median slope of the small visual field defect group was not significantly different from zero (P = 0.2). Furthermore, a Mann–Whitney U-test indicated that there was a significant difference in the distribution of the slopes (mm/day post-stroke) between large and small post-geniculate lesions (P = 0.016). The patient with lateral geniculate nucleus infarction was excluded in this last analysis. Discussion Figure 2 Relationship between the mean retinal nerve fibre layer thickness of the two eyes and age in normal subjects. Blue line = mean of all data. Dashed lines = 95% CI of each individual plot, n = 23. although only four out of the seven cases had slopes that were statistically significantly different from zero. In the four cases with significant regression coefficients, the initial measurements were made within a month of stroke onset and none showed macular sparing. The third case, who did not exhibit a significant linear relationship, had macular sparing and an improving visual field. The range of the rate of reduction of the retinal nerve fibre layer thickness was from 0.9–6.3 mm for every 100 days of elapsed time. None of the cases with small visual field defects exhibited a relationship between retinal nerve fibre layer thickness and time elapsed that reached statistical significance, with two cases having positive associations and two having negative associations. One case in this group did exhibit a greater rate of change than the other cases (2.1 mm/100 days). This case had a lesion involving the lateral geniculate nucleus (Fig. 5) whereas the others had lesions of the occipital lobe (Table 2) and it was impossible to exclude damage to the optic tract, which would give rise to direct retrograde degeneration. However, even this was a trend that did not reach statistical significance. A multilevel random effects linear regression analysis of retinal nerve fibre layer thickness on elapsed time (days) estimated the median slope as 0.017 (maximum A negative linear relationship between the retinal nerve fibre layer thickness and elapsed time since the stroke in log years was established in the present cross-sectional study for patients with complete or near complete homonymous hemianopia. Thinning of the retinal nerve fibre layer of all cases with homonymous hemianopia can be seen to have developed in the first few months following injury to the post-geniculate visual pathway. The degeneration was progressive in the first few years, becoming relatively stable or evolving slowly in later years. An effect of age on the retinal nerve fibre layer thickness is well established in the normal population; therefore, a multivariable linear regression was performed for two factors, namely, elapsed time in log years and age at the time of measurement. An equation was derived from the relationship as follows: Mean retinal nerve fibre layer thickness ðmmÞ ¼ 110:3 ð9:08Þ (elapsed time in log years) ð0:4Þ (age at the time of measurement) ð1Þ After adjusting for age, the retinal nerve fibre layer change was 9.08 mm for each log year. For instance, the mean retinal nerve fibre layer thickness of the two eyes would be 110.3 (9.08) (1) (0.4) (60) = 77.2 mm at the end of 10 years in a patient whose stroke occurred at age 50. After a further 10 years, the mean retinal nerve fibre layer thickness would be 110.3 (9.08) (1.3) (0.4) (70) = 70.5 mm. Figure 8 shows further examples. It is noteworthy that the baseline retinal nerve fibre layer thickness is diverse in the normal population: there are confounding factors apart from age such as refractive error and ethnicity (Budenz et al., 2007). However, the equation has given an overall picture of the degeneration and has shown in this example that the rate of retinal nerve fibre layer thinning is strikingly high in the first few years after the stroke before becoming relatively slow. The change 538 | Brain 2012: 135; 534–541 P. Jindahra et al. Figure 3 Case 8. Axial T2-weighted and coronal fluid attenuated inversion recovery MRI scans (A) revealed right occipital infarct, resulting in an almost complete left homonymous hemianopia shown on Humphrey perimetry 24-2 (B). This is typical of the cases shown in Fig. 6 and Table 2. Figure 4 Mean retinal nerve fibre layer thickness (RNFL) of both eyes over time after stroke in patients with homonymous hemianopia. Relationships between retinal nerve fibre layer thickness and time elapsed since the stroke is shown for each patient with homonymous hemianopia. in the second decade after the stroke is 0.67 mm/year, which is slightly higher than the effect of increasing age alone. When measuring the retinal nerve fibre layer on several occasions after the stroke over time in the longitudinal study, the rate of degeneration seemed to be high in the first 1–2 years. The findings were compatible with the results obtained in the crosssectional study. The rate of degeneration for each patient with homonymous hemianopia appeared linear with varying slope and the mean slope would indicate a loss of 4.4 mm in the first year, which is 10 times the attrition rate resulting from ageing and within the confidence limits of Equation (1). In the homonymous hemianopia group, patients showing less retinal nerve fibre layer thinning in the first few years after the stroke tended to show greater visual recovery. The rate of retrograde trans-synaptic degeneration of neurons in our study had a trend that is similar to results from adult old world monkeys: Cowey et al. (1999) have recently demonstrated that the rate of retrograde trans-synaptic degeneration of neurons developed within the first few years after unilateral striatal decortication, becoming stable 4 years thereafter. The authors have recently published a larger data set comparing both retinal ganglion cell numbers and optic tract thickness in 26 monkeys at survival times ranging from 0.28 to 14.29 years postoccipital lobectomy (Cowey et al., 2011). Patients with small field defects from the occipital lesions had stable results. The explanation for the stable result in the small defect group may be that the optical coherence tomography methodology is not sensitive enough to detect changes of this magnitude. It is possible that thinning could be demonstrated Retrograde trans-synaptic degeneration in the visual pathway Brain 2012: 135; 534–541 | 539 Table 2 Relationship between the means of retinal nerve fibre layer thickness of both eyes (mm) and time following the stroke onset (days) in cases with homonymous hemianopia Case r P-value Thickness change (mm/100 days) 95% CI of the change/100 days Number of visits First visit (days) Macular sparing Lesion Followed up VF 1 3 4 6 8 10 11 0.7 0.9 0.5 0.7 0.5 0.8 0.7 0.04 50.001 0.2 0.03 0.3 0.04 0.1 1.1 2.7 0.9 1.7 1 2.7 6.3 2.2 to 0.1 3.6 to 1.7 2 to 0.4 3.2 to 0.3 3.2 to 1.2 5.1 to 0.2 14.3 to 1.7 10 10 10 9 6 7 7 21 13 112 21 26 30 96 No No No No Yes Yes No Occipital lobe Occipital lobe Optic radiation Occipital lobe Occipital lobe Occipital lobe Occipital lobe Improved Stable Stable Improved Improved Stable Stable VF = visual field. Figure 5 Case 7. Axial T2-weighted and coronal fluid attenuated inversion recovery MRI scans (A) revealed high signal intensity over the left lateral geniculate nucleus, compatible with infarction. Humphrey perimetry 24-2 for the left and right eye were demonstrated in (B), showing right superior homonymous quadrantanopia. The results for this patient are shown in Fig. 7 and Table 3. Figure 6 (A) Case 2. Left homonymous paracentral scotoma from a right lingual infarction. (B) Case 9. Right homonymous incomplete superior quardrantanopia from a left occipital infarction. The results for these patients are shown in Fig. 7 and Table 3. 540 | Brain 2012: 135; 534–541 P. Jindahra et al. using a technique that could measure the retinal ganglion cell layer thickness across the retina, rather than the projection of the retinal ganglion cell axons in the peripapillary retinal nerve fibre layer, as it would be possible to compare the affected and unaffected regions of the retina directly. An alternative and scientifically more intriguing possibility is that healthy neurons subserving surviving regions of the visual field at one or more level in the visual pathway may provide a protective mechanism. The results do indicate that the size of the lesion plays an important role in determining the magnitude of the retrograde trans-synaptic degeneration of neurons. However, the patient who had a small field defect from a lateral geniculate nucleus lesion, showed the most retinal nerve fibre layer regression among patients in the small defect group. The slope of the regression line was comparable to that of the homonymous hemianopia group but did not reach the level of statistical significance. This implies that a strategic lesion can have an important influence on the retrograde trans-synaptic degeneration of neurons outcome independently of the lesion size. Figure 7 Mean peripapillary retinal nerve fibre layer thickness (RNFL) of both eyes over time after stroke in patients with small visual field defects. Plot shows the relationships between the retinal nerve fibre layer thickness and elapsed time since the stroke in patients with minor field defects. Conclusion It is now well established that retrograde trans-synaptic degeneration occurs in the human visual system following damage to the Table 3 Relationship between the means of retinal nerve fibre layer thickness of both eyes (mm) and time following the stroke onset (days) in patients with smaller field defects Case r P-value Thickness change (mm/100 days) 95% CI of the change/100 days Number of visits First visit (days) Lesion Followed up VF 2 5 7 9 0.2 0.97 0.7 0.3 0.8 0.15 0.06 0.6 0.2 0.8 2.1 0.3 2 to 2.4 1.5 to 3.1 4.2 to 0.1 1.6 to 0.9 5 3 8 7 36 21 25 5 LGN Occipital lobe LGN Occipital lobe Stable Stable Stable Improved LGN = lateral geniculate nucleus; VF = visual field. Figure 8 Prediction of mean retinal nerve fibre layer thickness (RNFL) of both eyes in a patient with homonymous hemianopia from unilateral occipital stroke at age 50 years. The graph was derived from calculations using Equation (1). Retrograde trans-synaptic degeneration in the visual pathway occipital lobe; it has been demonstrated in an autopsy study (Beatty et al., 1992) and in life by optical coherence tomography imaging of the retina and MRI of the optic tract. In this study, the time course of the degeneration has been demonstrated using optical coherence tomography measurements of the retinal nerve fibre layer thickness. In cases of homonymous hemianopia the thinning of the retinal nerve fibre layer occurs at a rate that is maximal in the first few years after the occipital injury, followed by a much slower rate in ensuing years. This time course is similar to the pattern that has been demonstrated in primates following occipital lobectomy. In cases of smaller visual field defects no thinning of the retinal nerve fibre layer could be demonstrated except in a case in which damage to the optic tract (and therefore direct retrograde degeneration) is likely to have occurred. Whether the failure to detect thinning in such cases is due to lack of sensitivity of the technique or to a protective effect of the surviving regions of the visual field is not known. Funding The work described in this paper is supported by the University College London Comprehensive Biomedical Research Centre and the Moorfields Biomedical Research Centre. References Barboni P, Savini G, Parisi V, Carbonelli M, La Morgia C, Maresca A, et al. Retinal nerve fiber layer thickness in dominant optic atrophy measurements by optical coherence tomography and correlation with age. Ophthalmology 2011; 118: 2076–80. Beatty RM, Sadun AA, Smith L, Vonsattel JP, Richardson EP Jr. Direct demonstration of transsynaptic degeneration in the human visual system: a comparison of retrograde and anterograde changes. J Neurol Neurosurg Psychiatry 1982; 45: 143–6. Bridge H, Jindahra P, Barbur J, Plant GT. Imaging reveals optic tract degeneration in hemianopia. Invest Ophthalmol Vis Sci 2011; 52: 382–8. Budenz DL, Anderson DR, Varma R, Schuman J, Cantor L, Savell J, et al. Determinants of normal retinal nerve fiber layer thickness measured by Stratus OCT. Ophthalmology 2007; 114: 1046–52. Coleman M. Axon degeneration mechanisms: commonality amid diversity. Nat Rev Neurosci 2005; 6: 889–98. Brain 2012: 135; 534–541 | 541 Cowey A. Atrophy of retinal ganglion cells after removal of striate cortex in a rhesus monkey. Perception 1974; 3: 257–60. Cowey A, Alexander I, Stoerig P. Transneuronal retrograde degeneration of retinal ganglion cells and optic tract in hemianopic monkeys and humans. Brain 2011; 134: 2149–57. Cowey A, Stoerig P, Williams C. Variance in transneuronal retrograde ganglion cell degeneration in monkeys after removal of striate cortex: effects of size of the cortical leisons. Vision Res 1999; 39: 3642–52. Danek A, Bauer M, Fries W. Tracing neuronal connections in the human brain by magnetic resonance imaging in vivo. Eur J Neurosci 1990; 2: 112–5. Fitzek C, Fitzek S, Stoeter P. Bilateral Wallerian degeneration of the medial cerebellar peduncles after ponto-mesencepahalic infarction. Eur J Radiol 2004; 49: 198–203. Hoyt WF, Rios-Montenegro EN, Behrens MM, Eckelhoff RJ. Homonymous hemioptic hypoplasia. Fundoscopic features in standard and red-free illumination in three patients with congenital hemiplegia. Br J Ophthalmol 1972; 56: 537–45. Jindahra P, Petrie A, Plant GT. Retrograde trans-synaptic retinal ganglion cell loss identified by optical coherence tomography. Brain 2009; 132: 628–34. Johnson H, Cowey A. Transneuronal retrograde degeneration of retinal ganglion cells following restricted lesions of striate cortex in the monkey. Exp Brain Res 2000; 132: 269–75. Koshinaga M, Whittemore SR. The temporal and spatial activation of microglia in fibre tracts undergoing anterograde and retrograde degeneration following spinal cord lesion. J Neurotrauma 1995; 12: 209–22. Miller NR, Newman NJ. Walsh and Hoyt’s clinical neuro-ophthalmology. 5th edn. Baltimore: The Williams & Wilkin Company; 1995. Porrello G, Falsini B. Retinal ganglion cell dysfunction in humans following post-geniculate lesions: specific spatio-temporal losses revealed by pattern ERG. Vision Res 1999; 39: 1739–45. Povlishock JT, Becker DP, Cheng CL, Vaughan GW. Axonal change in minor head injury. J Neuropathol Exp Neurol 1983; 42: 225–42. Sakai T, Matsuda H, Watanabe N, Kamei A, Takashima S. Olivocerebellar retrograde transsynaptic degeneration from the lateral cerebellar hemisphere to the medial inferior olivary nucleus in an infant. Brain Dev 1994; 16: 229–32. Savoiardo M, Pareyson D, Grisoli M, Forester M, D’Incerti L, Farina L. The effects of Wallerian degeneration of the optic radiations demonstrated by MRI. Neuroradiology 1992; 34: 323–5. Saxena S, Caroni P. Mechanisms of axon degeneration: from development to disease. Prog Neurobiol 2007; 83: 174–91. Waller A. Experiments on the sections of glossopharyngeal and hypoglossal nerves of the frog and observations of the alterations produced thereby in the structure of their primitive fibres. Phil Trans R Soc Lond 1850; 140: 423–9.