* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download LipidMetabolism
Photosynthesis wikipedia , lookup
Proteolysis wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Metalloprotein wikipedia , lookup
Biochemical cascade wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Butyric acid wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Citric acid cycle wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Biochemistry wikipedia , lookup
Microbial metabolism wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Lipid signaling wikipedia , lookup
Biosynthesis wikipedia , lookup
Biosynthesis of doxorubicin wikipedia , lookup
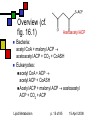
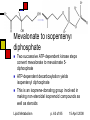
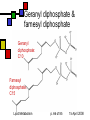
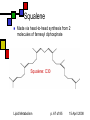
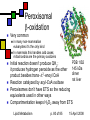
Lipid Metabolism Andy Howard Introductory Biochemistry 15 April 2008 15 April 2008 Making and Breaking Lipids Lipid biosynthesis is a significant route to the creation of energy-storage molecules, membrane components, and hormones; Lipid catabolism is a critical energyproducing pathway, and we also need to understand degradation of functional lipids … but first, a few final slides about plants! Lipid Metabolism p. 2 of 85 15 April 2008 What we’ll discuss End of plant stuff Lipid anabolism Fatty acid synthesis Making fats and phospholipids Eicosanoids Ether lipids Sphingolipids Isoprenoids & steroids Lipid Metabolism Fatty acid oxidation Sequence of reactions for saturated FAs Unsaturations Energetics Phospholipid degradation Steroid and other degradative systems p. 3 of 85 15 April 2008 Crassulacean acid metabolism Leaf cells open to CO2 uptake lose a lot of water during the day (high evaporation rate) Solution: assimilate carbon at night Reactions are as in C4 pathway; cellular specialization and enzyme regulation are different Lipid Metabolism p. 4 of 85 15 April 2008 Stomata and vacuoles Stomata (spaces between cells that can open to allow access for respiration) near mesophylls open only at night, enabling PEP carboxylation to oxalacetate and then reduction to malate Malate stored in central vacuole, then released during the day when the stomata are closed Lipid Metabolism p. 5 of 85 15 April 2008 CAM: day and night University of Newcastle, Plant Physiology program Lipid Metabolism p. 6 of 85 15 April 2008 iClicker quiz question 1 Oxidation of a 2ncarbon fatty acid yields (n-1) QH2, (n-1) NADH, and n acetyl CoA. Initiating the process costs 2 ATPs. Assume we can get 10 ATP per acetyl CoA. How much ATP can we get from oxidizing palmitate? Lipid Metabolism (a) 104 ATP (b) 106 ATP (c ) 108 ATP (d) 112 ATP (e) Undeterminable given the data supplied p. 7 of 85 15 April 2008 Answer to 1st question Palmitate is a C16 carboxylic acid. Therefore in the conditions of the problem, 2n = 16, n = 8, n-1 = 7. Thus we get 7 QH2, 7 NADH, 8 acetyl CoA produced by its oxidation Thus we get 7*2.5 + 7 * 1.5 + 8 * 10 = 17.5 + 10.5 + 80 = 108 ATP produced Starting the process costs 2 ATP, so the net result is 106 ATP gained Lipid Metabolism p. 8 of 85 15 April 2008 iClicker quiz question 2 Why would you not expect to find crassulacean acid metabolism in tropical plants? (a) Tropical plants do not photosynthesize. (b) Tropical plants cannot develop the stomata that close off the chloroplast-containing cavities (c) Water conservation is less critical in areas of high rainfall (d) The waxy coating required to close off the leaves’ access to O2 would dissolve in the high humidity and high temperature of the tropics (e) None of the above Lipid Metabolism p. 9 of 85 15 April 2008 Answer: (c) The primary significance of CAM is conservation of water in regions of low humidity, where evaporation rates are high and water is scarce. Neither of these conditions pertains in the tropics. Lipid Metabolism p. 10 of 85 15 April 2008 Control of CAM PEP carboxylase inhibited by malate and low pH That prevents activity during daylight, which would lead to futile cycling and competition for CO2 between PEP carboxylase and RuBisCO Lipid Metabolism p. 11 of 85 15 April 2008 Compartmentation in bacteria In photosynthetic bacteria, RuBisCO is concentrated in protein microcompartment called a carboxysome Active carbonic anhydrase there: catalyzes HCO3- OH- + CO2 That tends to keep the CO2 / O2 ratio high Lipid Metabolism p. 12 of 85 15 April 2008 Lipids: What we won’t cover Special Cases Locations for synthesis Regulation by hormones Absorption and mobilization Ketone bodies Lipid Metabolism p. 13 of 85 15 April 2008 Lipid Anabolism Malonyl CoA Generally the starting point for building up lipids are acetyl CoA and malonyl CoA, and their variants acetyl ACP and malonyl ACP Fatty acids Steroids These are energy-requiring reactions: the compounds we’re making are reduced Lipid Metabolism p. 14 of 85 15 April 2008 Overview (cf. fig. 16.1) Acetoacetyl ACP Bacteria: acetyl CoA + malonyl ACP acetoacetyl ACP + CO2 + CoASH Eukaryotes: CoA + ACP acetyl ACP + CoASH Acetyl ACP + malonyl ACP acetoacetyl ACP + CO2 + ACP acetyl Lipid Metabolism p. 15 of 85 15 April 2008 Making malonyl CoA PDB 1w96 (biotin carboxylase domain) 183 kDa trimer yeast Acetyl CoA incorporates an extra methylene via acetyl CoA carboxylase Biotin- and ATPdependent enzyme; similar to pyruvate carboxylase Lipid Metabolism 1uyr (carboxyltransferase domain) 162 kDa dimer; yeast 15 April 2008 p. 16 of 85 Making malonyl ACP Malonyl CoA:ACP transacylase transfers the malonate group from coenzyme A to the acyl carrier protein Ferredoxin-like protein Similar enzyme converts acetyl CoA to acetyl ACP Lipid Metabolism p. 17 of 85 PDB 1NM2 35 kDa monomer Streptomyces coelicolor 15 April 2008 Acyl carrier protein itself Acts as a template on which acyl chain elongation can occur Simple protein: 83 amino acids, mostly helical This is actually an NMR structure Lipid Metabolism PDB 1OR5 9.1 kDa monomer Streptomyces p. 18 of 85 15 April 2008 Initiation reaction We want to start with a four-carbon unit attached to acyl carrier protein We get that by condensing acetyl CoA or acetyl ACP with malonyl ACP with ketoacyl ACP synthase (KAS) to form acetoacetyl ACP Intermediate has KAS covalently attached to both substrates Decarboxylation of enzyme-bound intermediates leads to 4-carbon unit attached to ACP 3+21+4 Lipid Metabolism p. 19 of 85 15 April 2008 Is this typical? Yes! We’ve carboxylated acetyl CoA to make malonyl ACP and then decarboxylated the product of malonyl ACP with acetyl CoA / ACP This provides a favorable freeenergy change (at the expense of ATP) for the overall reaction Similar approach happens in gluconeogenesis (pyruvate oxaloacetate PEP) Lipid Metabolism p. 20 of 85 Ketoacyl ACP synthase PDB 1HNJ 70 kDa dimer; monomer shown E.coli 15 April 2008 Elongations in FA synthesis: overview Acetoacetyl ACP: starting point for elongations Pattern in each elongation is reduction dehydration reduction, resulting in a saturated product Reenter pathway by condensing with malonyl ACP Elongated product plays the same role that acetyl CoA or acetyl ACP plays in the initial -ketoacyl ACP synthase reaction: C2n + C3 -> CO2 + C2n+2 Lipid Metabolism p. 21 of 85 15 April 2008 1st step: reduce ketone sec-alcohol Enzyme:3-ketoacyl ACP reductase Ketone reacts with NADPH + H+ to produce sec-alcohol + NADP D-isomer of sec-alcohol always forms; by contrast, during degradation, L-isomer forms Enzyme is typical NAD(P)-dependent oxidoreductase Lipid Metabolism p. 22 of 85 PDB 2C07 125 kDa tetramer; Monomer shown Plasmodium falciparum 15 April 2008 2nd step: alcohol to enoyl ACP 3-hydroxyacyl ACP dehydratase Eliminates water at beta, alpha postions to produce PDB 1DCI 182 kDa hexamer trans-2-enoyl ACP: trimer shown R–CHOH–CH2-CO-S-ACP Rat mitochondria R–CH=CH–CO-S-ACP + H2O Note that this is a derivative of a trans-fatty acid; but it’s complexed to ACP! This form is primarily helical; there is an alternative found in Aeromonas that is an alpha-beta roll structure Lipid Metabolism p. 23 of 85 15 April 2008 3rd step: enoyl CoA to saturated ACP PDB 2Z6I Enzyme: enoyl-ACP reductase 73 kDa dimer Leaves behind fully saturated FA Streptococcus complexed to acyl carrier protein: pneumoniae R–CH=CH–CO-S-ACP R–CH2CH2CO-S-ACP This can then condense with malonyl ACP with decarboxylation to form longer beta-ketoacyl ACP: Rn-ACP + malonyl-CoA -keto-Rn+2-ACP + CO2 + CoASH Enzyme is FMN-dependent Lipid Metabolism p. 24 of 85 15 April 2008 How does this end? Generally starts at C4 and goes to C16 or C18. Condensing enzyme won’t fit longer FAs Completed fatty acid is cleaved from ACP by action of a thioesterase with a 3-layer Rossmann fold Lipid Metabolism p. 25 of 85 Palmitoyl thioesterase I PDB 1EI9 31 kDa monomer bovine 15 April 2008 The overall reaction Acetyl CoA + 7 Malonyl CoA + 14NADPH + 14 H+ 14 NADP + Palmitate + 7CO2 + 8HS-CoA + 6H2O In bacteria we have separate enzymes: a type II fatty acid synthesis system In animals we have a type I FA synthesis system: a large, multi-functional enzyme including the phosphopantatheine group by which the ACP attaches Lipid Metabolism p. 26 of 85 15 April 2008 iClicker question What advantage, if any, might be associated with type I fatty acid synthesis systems? (a) None (b) Reactants remain associated with the enzymatic complex, reducing diffusive inefficiencies (c) Lowered probability of undesirable reductions of metabolites (d) Lowered probability of undesirable oxidations of metabolites (e) improved solubility of products Lipid Metabolism p. 27 of 85 15 April 2008 Answer: (b) If the enzyme doesn’t have to find the substrate at the beginning of each reaction, things will proceed more readily. Lipid Metabolism p. 28 of 85 15 April 2008 Activating fatty acids Activate stearate or palmitate via acyl CoA synthetase: R–COO- + CoASH + ATP PDB 1BS0 R–CO–SCoA + AMP + PPi 42 kDa monomer As usual, PPi hydrolysis drives E.coli the reaction to the right PLP-dependent reaction Bacteria have one acyl CoA synthetase Mammals: four isozymes for different FA lengths (small, medium, long, very long) Lipid Metabolism p. 29 of 85 15 April 2008 Extending and unsaturating fatty acids There are applications for FAs with more than 18 carbons and FAs with >=1 cis double bonds Elongases and desaturases exist to handle these needs (fig. 16.7) Desaturase adds a cis-double bond; if the FA already has unsaturations, the new one is added three carbons closer to the carboxyl Elongases condense FA with malonyl CoA; decarboxylation means we add two carbons Lipid Metabolism p. 30 of 85 15 April 2008 Bacterial Desaturases Acyl ACP desaturases in bacteria simply add a cis double bond in place of the normal trans double bond at the second phase of elongation; the cis double bond thus created remains during subsequent rounds Ferritin-like structure Lipid Metabolism PDB 1ZA0; 30 kDa monomer Mycobacterium tuberculosis p. 31 of 85 15 April 2008 Eukaryotic Desaturases Desaturases like stearoyl ACP desaturase in eukaryotes act on the completed saturated fatty acyl CoA species Enzyme is ferritin-like or RNRlike Mammals can’t synthesize linoleate and they need it, so it has to be part of the diet Lipid Metabolism PDB 1OQ9 80 kDa dimer monomer shown castor bean p. 32 of 85 15 April 2008 Making arachidonate We can convert dietary linoleate to archidonyl CoA via desaturation and elongations (fig. 16.7) The fact that the new double bonds start 3 carbons away from the previous one means they’re not conjugated Lipid Metabolism p. 33 of 85 15 April 2008 Phosphatidates Phosphatidates are intermediates in making triacylglycerol & glycerophospholipids Fatty acyl groups esterifying 1 and 2 positions of glycerol, phosphate esterifying 3 position Lipid Metabolism p. 34 of 85 15 April 2008 Making phosphatidates Glycerol-3-phosphate acyltransferase transfers acyl CoA to 1 position of glycerol3-phosphate; prefers saturated chains 1-acylglycerol-3-phosphate acyl transferase transfers acyl CoA to 2 position of resulting molecule; prefers unsaturated chains Lipid Metabolism p. 35 of 85 PDB 1IUQ 40 kDa monomer Cucurbita 15 April 2008 Making triacylglycerols Phosphatidate phosphatase gets rid of the phosphate at the 3 position by hydrolysis to make 1,2-diacylglycerol A bit counterintuitive in making phospholipids: why get rid of the phosphate when you’re going to put a phosphorylated compound back at 3 position? But the groups you add already have phosphate on them Lipid Metabolism p. 36 of 85 15 April 2008 Further steps in making triacylglycerols Diacylglycerol acyltransferase catalyzes reaction between 1,2-diacylglycerol and acyl CoA to form triacylglycerol See fig. 16.9, left-hand side Lipid Metabolism p. 37 of 85 15 April 2008 Making phospholipids from 1,2-diacylglycerol 1,2-diacylglycerol reacts with CDPcholine to form phosphatidylcholine with liberation of cytidine monophosphate 1,2-diacylglycerol reacts with CDPethanolamine to form phosphatidylethanolamine this can be methylated 3 times to make phosphatidylcholine S-adenosylmethionine is the methyl donor in that case Lipid Metabolism p. 38 of 85 15 April 2008 CDPethanolamine How do we get CDP-alcohols? Easy: CTP + alcohol phosphate CDP-alcohol + PPi As usual, reaction is driven to the right by hydrolysis of PPi Enzymes are CTP:phosphoethanolamine cytidylyltransferase and CTP:phosphocholine cytidylyltransferase Lipid Metabolism p. 39 of 85 15 April 2008 Making acidic phospholipids Phosphatidate activated to CDPdiacylglycerol as catalyzed by CTP:phosphatidate cytidylyltransferase with release of PPi (see previous reactions) This can react with serine or inositol to form the relevant phospholipids; see fig. 16.10. This route to phosphatidylserine is found only in bacteria Lipid Metabolism p. 40 of 85 15 April 2008 Phosphatidylserine Lipid Metabolism p. 41 of 85 15 April 2008 Phosphatidylinositol Phosphatidylinositol is made by this CDPdiacylglycerol pathway in bacteria and eukaryotes Lipid Metabolism p. 42 of 85 15 April 2008 Making phosphatidylserine Alternative approach to phosphatidylserine found in eukaryotes: make phosphatidylethanolamine, then phosphatidylethanolamine:serine transferase swaps serine for ethanolamine When we do it that way, we can recover phosphatidylethanolamine back by a decarboxylation (or another exchange) Ethanolamine is just serine without COO- ! Lipid Metabolism p. 43 of 85 15 April 2008 Where does this happen? Mostly in the endoplasmic reticulum in eukaryotes Biosynthesis enzymes are membrane bound but have their active sites facing the cytosol so they can pick up the watersoluble metabolites from which they can build up phospholipids and other lipids Lipid Metabolism p. 44 of 85 15 April 2008 Making eicosanoids Classes of eicosanoids: Prostaglandins and thromboxanes Leukotrienes Remember that we make arachidonate from linoleoyl CoA; eiconsanoids made from arachidonate Reactions involve formation of oxygencontaining rings; thus the enzymes are cyclooxygenases Lipid Metabolism p. 45 of 85 15 April 2008 What eiconsanoids do They’re like hormones, but they act very locally: within µm of the cell in which they’re produced Involved in platelet aggregation, blood clots, constriction of smooth muscles Mediate pain sensitivity, inflammation, swelling Therefore enzymes that interconvert them are significant drug targets! Lipid Metabolism p. 46 of 85 15 April 2008 Prostaglandin H2 Synthesizing prostaglandins PDB 2OYU Prostaglandin H synthase (PGHS) 132 kDa dimer binds on inner surface of ER Monomer shown sheep Cyclooxygenase activity makes a hydroperoxide; this is converted to PGH2 PGH2 gets converted to other prostaglandins, prostacyclin, thromboxane A2 (fig. 16.12) Lipid Metabolism p. 47 of 85 15 April 2008 How aspirin works Aspirin blocks irreversibly inhibits the COX activity of PGHS by transferring an acetyl group to an active-site Ser That blocks eiconsanoid production, which reduces swelling and pain But there are side effects because some PGHS isozymes are necessary Lipid Metabolism p. 48 of 85 15 April 2008 Cyclooxygenase inhibition Cox-1 is constitutive and regulates secretion of mucin in the stomach Cox-2 is inducible and promotes inflammation, pain, fever Aspirin inhibits both: the mucin-secretion inhibition means that causes bleeding or ulcers in the stomach lining Other nonsteroidal anti-inflammatories (NSAIDs) besides aspirin compete with arachidonate rather than binding covalently to COX-1 and COX-2 Lipid Metabolism p. 49 of 85 15 April 2008 Could we find a COX-2 inhibitor? This would eliminate the stomach irritation that aspirin causes Some structure-based inhibitors have been developed They work as expected; but They also increase risk of cardiovascular disease Prof. Prancan (Rush U) discussed these issues in his February 2007 colloquium Lipid Metabolism p. 50 of 85 15 April 2008 Leukotrienes Lipoxygenases convert arachidonate to these compounds, which contain 3 conjugated double bonds These compounds interact with GPCRs Involved in inflammatory and allergic reactions Also involved in the pathophysiology of asthma Lipid Metabolism Leukotriene B4 p. 51 of 85 PDB 2P0M 146 kDa dimer rabbit 15 April 2008 Synthesis of ether lipids Remember: these are lipids with ether linkages instead of acyl linkages Begins with dihydroxyacetone phosphate Acyltransferase acylates DHAP C-1 1-alkyl-DHAP synthase swaps an alcohol for the acyl group at C-1 Keto group at C2 of DHAP is reduced to an alcohol (NADPH-dependent reaction) Lipid Metabolism p. 52 of 85 15 April 2008 Ether lipids, continued 1-alkylglycerophosphate acyltransferase adds another acyl group at C-2 Dephosphorylated at C-3 (as with phospholipids … take the P off, put it back on …) Phosphocholine or other phosphate-based ligand added at C-3 Plasmalogens earn a double bond between the two carbons adjacent to the ether oxygen on C-1 Lipid Metabolism p. 53 of 85 15 April 2008 ceramide Sphingolipid synthesis These are based formally on sphingosine, a C18 unsaturated amino alcohol (fig.16.14) Condense serine with palmitoyl CoA to make 3ketosphinganine and CO2 NADPH-reduce this to sphinganine Acetylate the amine group to make Nacylsphinganine Beta-unsaturate the palmitoyl group to make ceramide, the basis for all other sphingolipids Lipid Metabolism p. 54 of 85 15 April 2008 ceramide Sphingolipid synthesis These are based formally on sphingosine, a C18 unsaturated amino alcohol (fig.16.14) Condense serine with palmitoyl CoA to make 3ketosphinganine and CO2 NADPH-reduce this to sphinganine Acetylate the amine group to make Nacylsphinganine Beta-unsaturate the palmitoyl group to make ceramide, the basis for all other sphingolipids Lipid Metabolism p. 55 of 85 15 April 2008 ceramide Sphingolipid synthesis These are based formally on sphingosine, a C18 unsaturated amino alcohol (fig.16.14) Condense serine with palmitoyl CoA to make 3ketosphinganine and CO2 NADPH-reduce this to sphinganine Acetylate the amine group to make Nacylsphinganine Beta-unsaturate the palmitoyl group to make ceramide, the basis for all other sphingolipids Lipid Metabolism p. 56 of 85 15 April 2008 spingomyelin Other sphingolipids React ceramide with phosphatidylcholine; products are sphingomyelin and 1,2diacylglycerol React ceramide with UDP-galactose to form a galactocerebroside Additional UDP-sugars or CMP-N-acetylneuraminic acid can be added Lipid Metabolism p. 57 of 85 15 April 2008 Steroid synthesis: overview Cholesterol is important on its own & as a precursor of steroid hormones, bile salts Derived formally from isoprene Isoprenoid synthesis based on mevalonate & isopentenyl diphosphate Lipid Metabolism p. 58 of 85 15 April 2008 Making HMG-CoA Condense 3 molecules of acetyl CoA: 2 acetyl CoA acetoacetyl CoA + CoASH; catalyzed by acetoacetyl CoA synthase Acetoacetyl CoA + acetyl CoA + H2O 3-hydroxy-3-methylglutaryl CoA + CoASH + H+ catalyzed by HMG CoA synthase These are important intermediates: precursor to steroids and ketone bodies Not the committed step toward isoprenoids because we can also make ketone bodies from HMG-CoA Lipid Metabolism p. 59 of 85 15 April 2008 iClicker quiz question Creation of new C-C bonds requires energy. Where is it coming from in these condensations? (a) enzymatic catalysis (b) hydrolysis of ATP (c) hydrolysis of thioether bonds (d) hydrolysis of thioester bonds (e) none of the above Lipid Metabolism p. 60 of 85 15 April 2008 Answer: (d) (a) no. Enzymatic catalysis doesn’t change thermodynamics: it changes kinetics (b) no. There’s no ATP involved. (c) no. These acyl CoA molecules contain thioester linkages (d) yes. Hydrolysis of thioester linkages yields substantial amounts of free energy Lipid Metabolism p. 61 of 85 15 April 2008 HMGCoA to mevalonate PDB 1DQA PDB 1DQA 205 kDa tetramer 205 kDa Human tetramer human HMGCoA reductase is the first committed step on pathway toward isoprenoids HMGCoA + 2NADPH + 2H+ mevalonate + 2NADP+ + CoASH Many drug-discovery projects involve inhibition of this enzyme Lipid Metabolism p. 62 of 85 15 April 2008 Mevalonate to isopentenyl diphosphate Two successive ATP-dependent kinase steps convert mevalonate to mevalonate 5diphosphate ATP-dependent decarboxylation yields isopentenyl diphosphate This is an isoprene-donating group involved in making non-steroidal isoprenoid compounds as well as steroids Lipid Metabolism p. 63 of 85 15 April 2008 Mevalonate kinase Converts mevalonate to mevalonate 5-phosphate Secondary control point in isoprenoid synthetic pathway Human diseases associated with abnormalities Mevalonic aciduria Hyperimmunoglobulinemia (Periodic fever syndrome) Lipid Metabolism p. 64 of 85 PDB 2HFS 73 kDa dimer; monomer shown Leishmania major 15 April 2008 Isopentenyl diphosphate to squalene Isomerized to dimethylallyl diphosphate That condenses with another molecule of IPDP to make geranyl diphosphate (C10) Another condensation with IPDP (with the same enzyme) makes farnesyl diphosphate (C15) Two farnesyl diP fuse head-to-head to make squalene (C30, no heteroatoms) Lipid Metabolism p. 65 of 85 15 April 2008 Geranyl diphosphate & farnesyl diphosphate Geranyl diphosphate: C10 Farnesyl diphosphate: C15 Lipid Metabolism p. 66 of 85 15 April 2008 Squalene Made via head-to-head synthesis from 2 molecules of farnesyl diphosphate Squalene: C30 Lipid Metabolism p. 67 of 85 15 April 2008 lanosterol Squalene to cholesterol Several messy steps move the double bonds around replace double bonds with ring closures lanosterol Eliminate 3 methyls, move one double bond, remove another double bond, and voila: cholesterol Lipid Metabolism lanosterol synthase; converts 2,3oxidosqualene to lanosterol PDB 1W6K 81 kDa monomer human p. 68 of 85 15 April 2008 What happens to cholesterol? Inserted into membranes Assembled into lipoproteins Derivatized to make bile salts Modified into hormones Lipid Metabolism p. 69 of 85 15 April 2008 Other isoprenoids Generally made from isopentenyl pyrophosphate Pathways to isopentenyl pyroP are ancient: used in bacteria Pathways to steroids comparatively recent Cholesterol essential in animal membranes; plants have other sterols like campesterol (24-methyl-cholesterol) Lipid Metabolism p. 70 of 85 15 April 2008 Lipid catabolism We’ve been focusing on making lipids Now we’ll look at how they’re broken down As energy sources In recycling lipid components that have functional or structural significance Lipid Metabolism p. 71 of 85 15 April 2008 Fatty acid (beta) oxidation Degradation proceeds 2 C at a time somewhat like synthesis Called -oxidation because in each round the form that gets shortened is a -ketoacyl CoA Activated form is acyl CoA, not acyl ACP Product is n molecules of acetyl CoA from a 2n-carbon fatty acid Yields n-1 NADH and n-1 QH2 Occurs in the mitochondrion or peroxisome, whereas synthesis occurs in the cytosol Lipid Metabolism p. 72 of 85 15 April 2008 Reactions in -oxidation Proceeds through cycle n times for a 2ncarbon fatty acid Diagram courtesy Richard Paselk, Humboldt State U. Lipid Metabolism p. 73 of 85 15 April 2008 Acyl-CoA dehydrogenase Converts fatty acid saturated at C2,3 to trans-2-Enoyl CoA: —CH2—CH2—COSCoA Several isozymes for various sizes of FAs Lipid Metabolism medium-chain acyl CoA dehydrogenase PDB 3MDE 169kDa tetramer; dimer shown Pig liver p. 74 of 85 15 April 2008 ElectronTransferring Flavoprotein (ETF) Here, it converts FADH2 created by acyl-CoA dehydrogenase back to FAD via Fe-S protein Rossmann-fold protein Plays role in other redox reactions Ultimate acceptor is Q, which can be re-oxidized in the ETS Lipid Metabolism p. 75 of 85 PDB 1EFV 63 kDa heterodimer human 15 April 2008 Hydration step Enzyme is 2-enoyl CoA dehydratase - roll protein onverts enoyl CoA to L-3hydroxyacyl CoA Remember this is the opposite stereochemistry relative to synthetic intermediate Lipid Metabolism p. 76 of 85 PDB structure 1S9C: dehydratase domain of human multifunctional enzyme 15 April 2008 Second oxidative step Enzyme is L-3-hydroxyacylCoA dehydrogenase NADH is reduced product NADH can be used in biosynthesis (via shuttles) or oxidized in the ETS Rossmann-fold protein Lipid Metabolism dehydrogenase domain of multifunctional enzyme PDB 1E6W 114 kDa tetramer rat p. 77 of 85 15 April 2008 Thiolysis HS-CoA attacks C3carbonyl and cleaves off acetyl CoA, resulting in shortening by two carbons Enzyme is 3-ketoacyl-CoA thiolase Similar to acetoacyl-CoA thiolase found in isopentenyl diP pathway Substrate can go through another round Lipid Metabolism p. 78 of 85 PDB 1QFL 171 kDa tetramer Zooglea 15 April 2008 Formal similarity … between FA oxidation steps 1-3 and middle reactions of TCA cycle: –CH2CH2– oxidized to trans-CH=CH—: like succinate to fumarate Trans-ene hydrated to L-CHOH-CH2—: like fumarate to L-malate Alcohol oxidized to ketone: like L-malate to oxalacetate Lipid Metabolism p. 79 of 85 15 April 2008 Peroxisomal -oxidation Very common in many non-mammalian eukaryotes it’s the only kind In mammals this handles odd cases; mitochondria are the primary oxidizers PDB 1IS2 Initial reaction doesn’t produce QH2: 145 kDa it produces hydrogen peroxide as the other dimer product besides trans-2-enoyl CoA rat liver Reaction catalyzed by acyl-CoA oxidase Peroxisomes don’t have ETS so the reducing equivalents used in other ways Compartmentation keeps H2O2 away from ETS Lipid Metabolism p. 80 of 85 15 April 2008