* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Protein 4.2 interaction with hereditary spherocytosis mutants of the
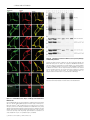
Cell membrane wikipedia , lookup
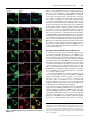
Cytokinesis wikipedia , lookup
Phosphorylation wikipedia , lookup
Endomembrane system wikipedia , lookup
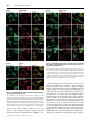
Hedgehog signaling pathway wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Signal transduction wikipedia , lookup
Protein design wikipedia , lookup
Magnesium transporter wikipedia , lookup
Protein domain wikipedia , lookup
Protein folding wikipedia , lookup
Protein moonlighting wikipedia , lookup
Protein phosphorylation wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Protein structure prediction wikipedia , lookup
List of types of proteins wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Protein purification wikipedia , lookup
Proteolysis wikipedia , lookup
Biochem. J. (2011) 433, 313–322 (Printed in Great Britain) 313 doi:10.1042/BJ20101375 Protein 4.2 interaction with hereditary spherocytosis mutants of the cytoplasmic domain of human anion exchanger 1 Susan P. BUSTOS* and Reinhart A. F. REITHMEIER†1 *Department of Biochemistry, University of Toronto, Toronto, ON, Canada, M5S 1A8, and †Department of Medicine, University of Toronto, Toronto, ON, Canada, M5S 1A8 AE1 (anion exchanger 1) and protein 4.2 associate in a protein complex bridging the erythrocyte membrane and cytoskeleton; disruption of the complex results in unstable erythrocytes and HS (hereditary spherocytosis). Three HS mutations (E40K, G130R and P327R) in cdAE1 (the cytoplasmic domain of AE1) occur with deficiencies of protein 4.2. The interaction of wild-type AE1, AE1HS mutants, mdEA1 (the membrane domain of AE1), kAE1 (the kidney isoform of AE1) and AE1SAO (Southeast Asian ovalocytosis AE1) with protein 4.2 was examined in transfected HEK (human embryonic kidney)-293 cells. The HS mutants had wild-type expression levels and plasma-membrane localization. Protein 4.2 expression was not dependent on AE1. Protein 4.2 was localized throughout the cytoplasm and co-localized at the plasma membrane with the HS mutants mdAE1 and kAE1, but at the ER (endoplasmic reticulum) with AE1SAO. Pull-down assays revealed diminished levels of protein 4.2 associated with the HS mutants relative to AE1. The mdAE1 did not bind protein 4.2, whereas kAE1 and AE1SAO bound wild-type amounts of protein 4.2. A protein 4.2 fatty acylation mutant, G2A/C173A, had decreased plasma-membrane localization compared with wildtype protein 4.2, and co-expression with AE1 enhanced its plasmamembrane localization. Subcellular fractionation showed the majority of wild-type and G2A/C173A protein 4.2 was associated with the cytoskeleton of HEK-293 cells. The present study shows that cytoplasmic HS mutants cause impaired binding of protein 4.2 to AE1, leaving protein 4.2 susceptible to loss during erythrocyte development. INTRODUCTION The locations of the three HS mutations are indicated and do not cluster together. We have previously shown that these mutations have little or no effect on the oligomerization, stability or folded structure of the isolated domain, other than a slight destabilization caused by the P327R mutation [7]. Protein 4.2 is a 72 kDa peripheral membrane protein [8] that binds to both AE1 and ankyrin and is believed to strengthen the membrane−cytoskeletal link [9,10]. The 72 kDa isoform of protein 4.2 is referred to as Type II and is the smaller and more predominant of two protein 4.2 splice variants. Type I is the less common larger isoform and has an additional 30 amino acids near the N-terminus [11]. Amino acid numbering of protein 4.2 in the present paper will be according to the Type II isoform. Protein 4.2 is a member of the TG (transglutaminase) family of enzymes, but has no catalytic activity [12]. It is myristoylated at Gly2 [13] and palmitoylated at Cys173 [14], which may allow association with cellular membranes. Protein 4.2 has been shown to associate with the plasma membrane of Sf9 insect cells [15] and Xenopus oocytes [16] in the absence of AE1. In Sf9 cells, myristoylation of Gly2 was required for plasma-membrane localization. In Figure 1 (right-hand panel), a homology model we constructed of protein 4.2 using human TG2 [17] as a template, is placed next to the crystal structure of cdAE1 to show their relative sizes. The N-terminal region of protein 4.2, encompassing residues 1−238, has been shown to represent the cdAE1-binding domain [14]. Residues 157−181 were found to be critical for the interaction and predicted formation of a β-hairpin in the centre of protein 4.2 with Cys173 located at the bend. Another study HS (hereditary spherocytosis) is an inherited human haemolytic anaemia characterized by erythrocytes that become unstable due to weakened interactions between the erythrocyte membrane and cytoskeleton. The AE1 (anion exchanger 1, Band 3) membrane glycoprotein and the cytoskeletal proteins ankyrin, spectrin and protein 4.2 are involved in this interaction, and 20 % of HS cases are caused by mutations in AE1 [1]. AE1 is the major membrane glycoprotein of the erythrocyte and exists as dimers and tetramers. Each monomer comprises two distinct domains: the C-terminal 52 kDa membrane domain (mdAE1) spans the bilayer up to 12 times and carries out the electroneutral exchange of chloride for bicarbonate [2]; the N-terminal 43 kDa cytoplasmic domain (cdAE1) binds glycolytic enzymes, haemoglobin and cytoskeletal proteins, including ankyrin and protein 4.2. Three mis-sense mutations in the cdAE1, E40K (Band 3 Montefiore), G130R (Band 3 Fukuoka) and P327R (Band 3 Tuscaloosa), cause HS while maintaining a normal amount of AE1 in the mature erythrocyte [3−5]. However, a marked deficiency of protein 4.2 is seen, suggesting a destabilization of the protein 4.2−AE1 linkage. The homozygous E40K and G130R mutations result in 88 % and 55 % decreases in protein 4.2 respectively, whereas the heterozygous P327R mutation results in a 29 % decrease in protein 4.2. This deficiency may be due to degradation of unstable protein 4.2 or mis-sorting of protein 4.2 during enucleation of reticulocytes. Figure 1 (left-hand panel) shows the crystal structure of cdAE1 found to exist as a symmetric dimer [6]. Key words: anion exchanger 1 (AE1), erythrocyte cytoskeleton, hereditary spherocytosis (HS), protein 4.2, protein interaction, erythrocyte membrane. Abbreviations used: AE1, anion exchanger 1; AE1SAO, Southeast Asian ovalocytosis AE1; cdAE1, cytoplasmic domain of AE1; CNX, calnexin; Cy3, indocarbocyanine; DMEM, Dulbecco’s modified Eagle’s medium; ER, endoplamic reticulum; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HA, haemagglutinin; HEK, human embryonic kidney; HS, hereditary spherocytosis; kAE1, kidney isoform of AE1; mdEA1, membrane domain of AE1; Ni-NTA, Ni2+ -nitrilotriacetate; PNA, peanut agglutinin; TG, transglutaminase; TM, transmembrane. 1 To whom correspondence should be addressed (email [email protected]). c The Authors Journal compilation c 2011 Biochemical Society 314 Figure 1 S. P. Bustos and R. A. F. Reithmeier Crystal structure of cdAE1 and homology model of protein 4.2 The symmetric dimer of cdAE1 (left-hand panel) has been crystallized and its structure determined by X-ray diffraction (PDB code 1HYN) [6]. The two subunits are coloured in grey and green. Residues Gly130 and Pro327 , the locations of the HS mutations G130R and P327R, are indicated. Residues 1−54 (red dotted line) were not resolved in the structure. The E40K HS mutation is located here and the approximate location of residue Glu40 is indicated on the structure. Residues 1−65 (red) are missing in kAE1. The structure of cdAE1 is shown alongside the homology model of protein 4.2 (right-hand panel). The protein 4.2 structure was created with SWISS-MODEL [45]. The human TG2 structure (PDB code 1KV3) [17] was used as the template instead of sea bream TG, as was used previously [16], since it is a human homologue, is found in erythrocytes, and the mouse TG2 interacts with mouse cdAE1 and protein 4.2 [46]. Our protein 4.2 model is oriented such that the palmitoyl group at Cys173 points upwards towards the conventional placement of the plasma membrane. This orientation matches that of the published crystal structure of cdAE1. The regions in blue and green of the protein 4.2 model represent the 23 kDa N-terminal cdAE1-binding region. Residues 33−45 are coloured in light green. Residues 157−181 are coloured in dark green, and Cys173 is indicated. Structures of cdAE1 and protein 4.2 are on the same scale. aa, amino acids. found that amino acids 33−45 of protein 4.2 contained a cdAE1binding site [18]. Basic residues Arg34 and Arg35 were essential for the interaction and may bind to an acidic region, such as the extreme acidic N-terminus of cdAE1. Since the E40K, G130R and P327R AE1 mutations all affect the levels of protein 4.2 in erythrocytes, we believe that these sites form part of a large binding surface for protein 4.2. We hypothesize that each of these positively charged HS mutations change the surface of cdAE1, thereby weakening protein 4.2 binding. To test this hypothesis, the interaction of protein 4.2 with wild-type AE1 and the three cytoplasmic HS mutants (AE1HS) was studied in transfected HEK (human embryonic kidney)-293 cells. The mdAE1, which is missing the cytoplasmic domain, is unable to bind protein 4.2 [8] and was included in the present studies as a negative control. A truncated kidney isoform of AE1 (kAE1) is missing the first 65 amino acids [19], which are shown in red in Figure 1 (left-hand panel) and include a central β-strand. This isoform exchanges Cl − for HCO3 − at the basolateral membrane of α-intercalated cells in the collecting ducts of the distal nephron [20]. The cytoplasmic domain of kAE1 (cdkAE1) is less stable than erythroid cdAE1 and exists in a more open structure [21]. In addition it does not bind ankyrin or glycolytic enzymes [22,23], most probably due to absence of the acidic N-terminal tail. We included kAE1 in the present studies to see what effect the missing tail and altered structure would have on protein 4.2 binding. Also, protein 4.2 is expressed in kidney cells [24], where it may perform a similar function as in erythrocytes. AE1SAO (Southeast Asian ovalocytosis AE1) is a condition caused by a nine amino acid deletion at the beginning of the first TM (transmembrane) domain [25]. The deletion results in rigid, oval-shaped erythrocytes [26] caused by an increased cytoskeletal attachment [27−29]. We wondered whether this translated into an enhanced interaction with protein 4.2, so we included AE1SAO in c The Authors Journal compilation c 2011 Biochemical Society the present studies. This mutant traffics to the plasma membrane in erythrocyte precursors [30], but is retained in the ER (endoplasmic reticulum) in HEK-293 cells [31], allowing us to determine whether protein 4.2 interaction with AE1 can occur at the ER. The localization and interaction of protein 4.2 with these AE1 variants was examined in HEK-293 cells, as well as the role of fatty acid modifications on protein 4.2 localization. Protein 4.2 had a broad distribution in transfected cells, was predominantly associated with the cytoskeletal fraction and co-localized with AE1 at the plasma membrane. The AE1HS mutants, but neither kAE1 nor AE1SAO, had impaired protein 4.2 binding; this weakened interaction may account for the loss of protein 4.2 during erythrocyte development. EXPERIMENTAL Materials The following is a list of materials used: pcDNA3 vector (Invitrogen); mutagenic primers (ACGT Corporation); QuikChange® site-directed mutagenesis kit (Stratagene); HEK293 cells (A.T.C.C.); DMEM (Dulbecco’s modified Eagle’s medium), calf serum, penicillin and streptomycin (Gibco BRL); LipofectamineTM 2000 (Invitrogen); C12 E8 (Nikko Chemicals); Protein G−Sepharose (Amersham Biosciences); Poly-L-lysine (Sigma−Aldrich); Ni-NTA (Ni2+ -nitrilotriacetate)–agarose resin (Qiagen); mouse anti-AE1 (BRIC 6) antibody, which recognizes an extracellular epitope at the C-terminus of AE1 (Bristol Institute for Transfusion Sciences, Bristol, U.K.); mouse anti-AE1 antibody, which recognizes an intracellular epitope of AE1 (a gift from Dr Michael L. Jennings, University of Arkansas for Medical Sciences, Little Rock, AR, U.S.A.); rabbit anti-Cterminal AE1 antibody raised against the last 16 amino acids of AE1 (SynPep Corporation); mouse anti-HA (haemagglutinin) Interaction of protein 4.2 with HS mutants of AE1 antibody (Covance); rat anti-HA antibody (Roche); rabbit anti-CNX (calnexin) antibody (Stressgen Biotech); lectin PNA (peanut agglutinin) Alexa Fluor® 488 conjugate (Molecular Probes); mouse anti-actin antibody (Chemicon); mouse anti-GAPDH (glyceraldehyde-3-phosphate dehydrogenase) antibody (Millipore); goat horseradish-peroxidase-conjugated anti-rabbit IgG and anti-mouse IgG (New England Biolabs); Cy3 (indocarbocyanine)-conjugated goat anti-mouse, anti-rat and anti-rabbit antibodies (Jackson ImmunoResearch); Alexa Fluor® 488-conjugated goat anti-mouse and anti-rat antibodies (Molecular Probes); and a chemiluminescence kit (Roche). Site-directed mutagenesis The coding sequence for wild-type human AE1 was inserted into the XhoI and BamHI sites of the pcDNA3 vector. The construction of AE1SAO and kAE1 have been described previously in [32] and [33] respectively. The mdAE1 was constructed using PCR on the full-length AE1 protein with a methionine residue engineered at the start of the DNA sequence coding for amino acids Asp369 −Val911 , followed by subcloning into the pcDNA3 vector. Asp369 is the first perfectly conserved residue in the human AE1 family (AE1, AE2 and AE3) defining the beginning of the membrane domain. The AE1HS mutants were created using the QuikChange® mutagenesis kit using complementary mutagenic primers, with wild-type AE1 as template. The coding sequence for wild-type human protein 4.2, Type II, was subcloned from the pGEM-7Zf(+/ − ) vector into the EcoRI site of the pcDNA3 vector. The C-terminal HA tag on protein 4.2 was created by PCR, inserting the tag after Ala691 . The G2A/C173A fatty acylation mutant of protein 4.2 was created using the QuikChange® mutagenesis kit using complementary mutagenic primers, with wild-type HA-tagged protein 4.2 as a template. HAtagged protein 4.2 and the HA-tagged G2A/C173A mutant were used in all experiments, but are referred to as protein 4.2 and G2A/C173A in the text respectively. Sequencing of constructs was performed by ACGT Corporation. Transient transfection and expression of AE1 and protein 4.2 in HEK-293 cells HEK-293 cells were grown in DMEM supplemented with 10 % (v/v) calf serum, 0.5 % penicillin and 0.5 % streptomycin under 5 % CO2 at 37 ◦ C as described previously [34]. Cells were transfected using the LipofectamineTM method [35], with 2 μg of plasmid DNA per well of a six-well plate. SDS/PAGE and immunoblotting Proteins were resolved by SDS/PAGE (8 % or 10 % gels) [36] and transferred to nitrocellulose membrane [37]. AE1 was detected using a mouse monoclonal anti-AE1 antibody [38]. HA-tagged protein 4.2 was detected using a mouse monoclonal anti-HA antibody. Actin was detected using a mouse anti-actin antibody, and GAPDH was detected using a mouse anti-GAPDH antibody. Goat horseradish-peroxidase-conjugated anti-mouse IgG was then added, followed by detection by chemiluminescence and film exposure or by a VersaDoc Imaging System Model 5000. Band intensities of immunoblots in the linear range of intensity were determined using ImageJ 1.41o software. Immunofluorescence and confocal microscopy HEK-293 cells transfected with pcDNA3 plasmids were grown on glass coverslips. In some cases, coverslips were coated with PolyL-lysine, but this made no difference to the growth or adherence 315 of the cells. Cells were fixed with 3.8 % (w/v) paraformaldehyde for 15 min and washed once with 100 mM glycine. Cells were either non-permeabilized or permeabilized and incubated with antibodies. Non-permeabilized cells were blocked with 0.2 % BSA for 30 min, followed by incubation with 1:100 diluted mouse anti-AE1 (BRIC 6) antibody or 1:100 diluted PNA Alexa Fluor® 488-conjugated antibody in 0.2 % BSA for 30 min. These cells were then permeabilized with 0.2 % Triton X-100 for 5 min and blocked with 0.2 % BSA for 30 min. Next, 1:250 diluted rat antiHA antibody was added in 0.2 % BSA for 30 min. Cells that were permeabilized at the beginning were permeabilized with 0.2 % Triton X-100 for 5 min and blocked with 0.2 % BSA for 30 min. These cells were then incubated with 1:500 diluted mouse antiAE1 antibody, 1:100 diluted mouse anti-AE1 (BRIC 6) antibody, 1:250 diluted rat anti-HA antibody or 1:250 diluted rabbit antiCNX antibody in 0.2 % BSA for 30 min. Following several washes, samples were incubated with a 1:1000 dilution of Alexa Fluor® 488-conjugated goat anti-mouse antibody, Alexa Fluor® 488-conjugated goat anti-rat antibody, Cy3-conjugated goat antimouse antibody, Cy3-conjugated donkey anti-rat antibody or Cy3conjugated donkey anti-rabbit antibody for 30 min. A Zeiss laser confocal microscope LSM 510 was used to observe the samples. Ni-NTA pull-down HEK-293 cells transiently transfected with His6 -tagged AE1 and HA-tagged protein 4.2 constructs, were harvested with lysis buffer (1 % C12 E8 , 300 mM NaCl and 10 mM imidazole with protease inhibitors in PBS). Cell lysates were centrifuged at 14 000 g for 30 min at 4 ◦ C to remove insoluble material. The supernatants were added to 50 μl of a 50 % slurry of Ni-NTA–agarose in binding buffer (0.1 % C12 E8 , 300 mM NaCl and 10 mM imidazole with protease inhibitors in PBS) and incubated for 2 h at 4 ◦ C. Resin was washed with 0.3 ml of wash buffer (0.2 % C12 E8 , 300 mM NaCl and 30 mM imidazole with protease inhibitors in PBS) three times. Bound proteins were eluted with elution buffer (0.5 % C12 E8 , 300 mM NaCl and 500 mM imidazole in PBS) and solubilized in 2 × SDS sample buffer. Samples were analysed by SDS/PAGE (8 % gels) and immunoblotting was performed as described above. Band intensities were determined using ImageJ 1.41o software. The amount of protein 4.2 associated with the amount of AE1 eluted from the resin was calculated from immunoblots from eight separate transfection experiments and normalized to the total amount of protein 4.2 expressed in the cells. Each value was reported as relative to that of AE1, which was set to 100 %, to give a value of protein 4.2 relative binding. Results for protein 4.2 binding are given as means + − S.D. Mean values were considered to be significantly different (P < 0.05) when the Student’s t test was used. Co-immunoprecipitation HEK-293 cells transiently transfected with AE1 and HA-tagged protein 4.2 constructs were harvested with lysis buffer (1 % C12 E8 with protease inhibitors in PBS). Cell lysates were centrifuged at 14 000 g for 30 min at 4 ◦ C to remove insoluble material. AE1 was immunoprecipitated from supernatants with 4 μl of rabbit anti-C-terminal AE1 antibody followed by 100 μl of Protein G−Sepharose. Proteins were eluted with 25 μl of 0.1 M glycine (pH 2.5) on ice for 20 min. Then, 2 μl of 1 M Tris (pH 9.0) was added to the effluent and proteins were solubilized in 2 × SDS sample buffer. Samples were analysed by SDS/PAGE (8 % gels) and immunoblotting was performed as described above. Band intensities were determined using ImageJ 1.41o software from various blot exposures, ensuring that the band intensities were c The Authors Journal compilation c 2011 Biochemical Society 316 S. P. Bustos and R. A. F. Reithmeier in the linear range. Linear dilutions of protein were included on the blots to ensure linearity. The amount of protein 4.2 associated with the amount of AE1 eluted from the resin was calculated from immunoblots from eight separate transfection experiments and normalized to the total amount of protein 4.2 expressed in the cells. Each value was reported as relative to that of AE1, which was set to 100 %, to give a value of protein 4.2 relative binding. Results for protein 4.2 binding are given as means + − S.D. Mean values were considered to be significantly different (P < 0.05) when the Student’s t test was used. Subcellular fractionation of HEK-293 cells expressing wild-type or G2A/C173A protein 4.2 HEK-293 cells transiently transfected with HA-tagged wildtype protein 4.2 or the G2A/C173A protein 4.2 mutant were suspended in PBS with protease inhibitors, followed by sonication at 50 % duty for 20 pulses on ice to lyse cells. Cell lysates were centrifuged at 1500 g for 10 min at 4 ◦ C to remove cell debris and unbroken cells. The supernatant, containing the membrane, cytoskeletal and cytoplasmic fractions (labelled the total fraction) was collected and centrifuged at 50 000 rev./min for 1 h at 4 ◦ C. The resulting supernatant contained the soluble cytosolic fraction and was labelled S1. The pellet was washed with 1 ml of PBS with protease inhibitors and centrifuged at 50 000 rev./min for 1 h at 4 ◦ C and the wash fraction was labelled Sw. The pellet, containing the membrane and cytoskeletal fractions, was resuspended in lysis buffer (1 % C12 E8 with protease inhibitors in PBS) and was labelled P1. Samples were slowly rotated for 1 h at 4 ◦ C to solubilize the membrane proteins in the detergent. Samples were then centrifuged at 50 000 rev./min for 1 h at 4 ◦ C. The resulting supernatant contained detergent-soluble membrane protein and was labelled S2. The pellet contained the cytoskeletal fraction and was labelled P2. Samples were solubilized in 2 × SDS sample buffer and analysed by SDS/PAGE (10 % gels). Immunoblotting was performed as described above and band intensities were determined using ImageJ 1.41o software. The total amount of wild-type and G2A/C173A protein 4.2 was set to 100 %, with the cellular fractions reported as relative percentages of the total. Results for the amount of protein 4.2 in each fraction are given as means + − S.D. Detection of GAPDH was used as a marker for soluble cytosolic proteins. Actin was used as marker for soluble (G-actin) and cytoskeletal (F-actin) proteins. Figure 2 Expression of AE1 proteins and protein 4.2 in HEK-293 cells Immunoblot analysis was performed on whole-cell detergent extracts from HEK-293 cells expressing AE1 proteins and protein 4.2. A mouse monoclonal anti-AE1 antibody was used to detect AE1 proteins (A) and a mouse monoclonal anti-HA antibody was used to detect HA-tagged protein 4.2 (B). Incubation with horseradish-peroxidase-conjugated anti-mouse IgG and detection by chemiluminescence was then performed (see the Materials and methods section). The molecular mass in kDa is indicated on the left-hand side. RESULTS the non-transfected control cells (results not shown). In multiple transfection experiments, there was no statistically significant difference in the amount of protein 4.2 expressed in the absence or presence of AE1, AE1HS mutants, mdAE1, kAE1 or AE1SAO. These results show that the AE1HS mutants had stabilities similar to those of AE1, and that co-expression with AE1 was not necessary for the expression of protein 4.2 in transfected HEK293 cells. Expression of protein 4.2 and AE1 proteins in transfected HEK-293 cells Co-localization of protein 4.2 and AE1 in HEK-293 cells Protein 4.2 was expressed alone or co-expressed with AE1, the HS mutants (E40K, G130R and P327R), as well as mdAE1, kAE1 and AE1SAO in transiently transfected HEK-293 cells. Figure 2 shows a representative immunoblot of total cell extracts. AE1 and AE1HS proteins were detected as approx. 95 kDa bands (Figure 2A, lanes 2, 4, 5 and 6). No immunoreactive band was seen in the empty-vector-transfected control lane (Figure 2A, lane 1). All three AE1HS proteins had similar expression levels compared with the wild-type protein. The immunodetection of mdAE1 (Figure 2A, lane 3) was variable and often lower than AE1 owing to the presence of multiple bands. Expression (results not shown) of kAE1 was comparable with AE1, whereas AE1SAO was lower, as reported previously [31]. HA-tagged protein 4.2 expressed in HEK-293 cells in the absence or presence of AE1 proteins was readily detected as an approx. 72 kDa immunoreactive band (Figure 2B, lanes 1−6). No HA-immunoreactive band was seen in Localization of wild-type and mutant AE1 proteins expressed in HEK-293 cells was determined by immunofluorescence and confocal microscopy (Supplementary Figure S1 at http://www.BiochemJ.org/bj/433/bj4330313add.htm). AE1 was readily detected at the cell surface, indicating that it had trafficked to the plasma membrane, as seen in previous studies [39]. The three AE1HS mutants, kAE1 and mdAE1 were also detected at the cell surface, at similar intensities, indicating their ability to traffic to the plasma membrane. In contrast, AE1SAO was not detected at the cell surface, but only at the ER, indicating its inability to traffic from the ER to the plasma membrane in transfected HEK-293 cells, as was reported previously [31]. HA-tagged protein 4.2 was expressed alone or with AE1, AE1HS, mdAE1, kAE1 and AE1SAO proteins and visualized by immunofluorescence and confocal microscopy to determine its intracellular localization in HEK-293 cells. The first row of c The Authors Journal compilation c 2011 Biochemical Society Interaction of protein 4.2 with HS mutants of AE1 317 Figure 3 shows immunofluorescence of protein 4.2 expressed alone in permeabilized cells and CNX, an ER-resident protein. Protein 4.2 (green) displayed a wide distribution throughout the cell and also co-localized with CNX (blue) as seen by the cyan colour in the merged image, suggesting that some protein 4.2 localizes at the ER. In the first column of the remaining rows, protein 4.2 displayed a similar wide distribution when co-expressed with all AE1 proteins, similar to when expressed alone. The second column shows the immunofluorescence of AE1 proteins in permeabilized cells. The proteins were predominantly localized at the cell surface, with the exception of AE1SAO, which was intracellular, similar to when the proteins were expressed in the absence of protein 4.2 (Supplementary Figure S1). In the merged images in the third column, the yellow colour indicates that a fraction of protein 4.2 co-localized to the plasma membrane with AE1, AE1HS, mdAE1 and kAE1 proteins. Its co-localization with mdAE1 is interesting, since mdAE1 lacks the cytoplasmic domain which is needed for protein 4.2 interaction [8]. Protein 4.2 also co-localized with AE1SAO, indicating its localization at the ER. The green staining in the merged images indicates localization of protein 4.2 in other cellular compartments. Thus protein 4.2 has a wide intracellular distribution in transfected HEK-293 cells, localizing to the ER and other intracellular compartments, as well as to the plasma membrane. Interaction of protein 4.2 with AE1 proteins in HEK-293 cells To examine the interaction of protein 4.2 with the AE1 proteins we conducted Ni-NTA pull-down assays on HEK-293 cells transiently expressing His6 -tagged AE1 proteins and HA-tagged protein 4.2. Figure 4 shows representative immunoblots of protein 4.2 associated with AE1 (top panel), AE1 pulled down (middle panel) and total protein 4.2 in the HEK-293 lysate (bottom panel). The amount of protein 4.2 bound to AE1 proteins was calculated as described in the Materials and methods section. The control experiment with protein 4.2 expressed in the absence of AE1 (lane 1) showed background amounts of bound protein 4.2. The AE1HS mutants consistently bound less protein 4.2 at levels of 54 + − 14 % (E40K), 43 + − 14 % (G130R) and 65 + − 29 % (P327R), n = 8, relative to wild-type AE1. As expected, the mdAE1 was unable to pull-down protein 4.2 above background levels (lane 6). The kAE1 and AE1SAO proteins bound similar amounts of protein 4.2 at levels of 110 + − 33 %, n = 8, relative to wild-type AE1 − 21 % and 99 + respectively. These small differences were not statistically significant, indicating that the loss of the first 65 amino acids for kAE1 or deletion of nine amino acids at the first TM domain in AE1SAO does not diminish protein 4.2 binding. To confirm the protein 4.2 binding impairment by the AE1HS mutants seen in the Ni-NTA pull-down assays, we conducted coimmunoprecipitation experiments on HEK-293 cells transiently expressing AE1HS proteins and protein 4.2. Figure 5(A) shows representative immunoblots of protein 4.2 associated with AE1 (top panel), AE1 bound to the resin (middle panel) and total protein 4.2 in the HEK-293 lysate (bottom panel). The amount of protein 4.2 bound to AE1HS proteins relative to wild-type Figure 3 Immunofluorescence images of protein 4.2 and AE1 proteins in HEK-293 cells Permeabilized cells expressing HA-tagged protein 4.2 alone or co-expressed with wild-type or mutant AE1 proteins were incubated with rat anti-HA and rabbit anti-CNX or mouse monoclonal anti-AE1 antibodies. Incubation of samples with fluorescently labelled secondary antibodies and confocal microscopy was performed (see the Materials and methods section). In the merged image of the top row, cyan indicates co-localization of protein 4.2 (green) and CNX (blue). In the remaining merged images, yellow indicates co-localization of protein 4.2 (green) and AE1 (red). perm., permeabilized. c The Authors Journal compilation c 2011 Biochemical Society 318 Figure 4 S. P. Bustos and R. A. F. Reithmeier Ni-NTA pull-down of wild-type and mutant His6 -tagged AE1 with protein 4.2 in HEK-293 cells His6 -tagged AE1 in whole-cell extracts was prepared with the detergent C12 E8 and pulled-down using Ni-NTA agarose. Total and bound fractions were resolved by SDS/PAGE, and immunoblots were probed with mouse anti-AE1 antibody to detect AE1 and mouse anti-HA antibody to detect HA-tagged protein 4.2 (see the Materials and methods section). Immunoblot band intensities were measured from eight independent experiments. The amount of protein 4.2 bound to the P327R HS mutant appears high in this blot. However, after correcting for bound AE1 and total protein 4.2, the amount of protein 4.2 bound to P327R HS mutant was less than that bound to AE1. The molecular mass in kDa is indicated on the left-hand side. AE1 was calculated as described in the Materials and methods section. The control experiment with protein 4.2 expressed in the absence of AE1 (Figure 5A, lane 1) showed no bound protein 4.2. The AE1HS proteins (Figure 5A, lanes 3−5) consistently co-purified less protein 4.2 relative to AE1 (Figure 5A, lane 2). In Figure 5(B) we show the results for AE1 (Figure 5B, lane 1) and mdAE1 (Figure 5B, lane 2) where the amount of immunoreactive mdAE1 expressed was comparable with AE1. As seen in lane 2 (Figure 5B), there was no bound protein 4.2. In agreement with the Ni-NTA pull-down results, the three AE1HS mutants consistently bound less protein 4.2 at levels of 75 + − 16 % (E40K), 68 + − 16 % (G130R) and 79 + − 17 % (P327R), n = 8, relative to wild-type AE1. Clearly, protein 4.2 could still interact with all three AE1HS mutants; however, at a statistically significant lower level compared with wild-type AE1. Thus the HS mutations in the cdAE1 result in impaired binding of protein 4.2 in HEK-293 cells. Co-localization of wild-type and G2A/C173A protein 4.2 and AE1 in HEK-293 cells The ability of protein 4.2 to co-localize with mdAE1 at the plasma membrane of HEK cells despite a lack of interaction was puzzling. The requirement of protein 4.2 Gly2 myristoylation for plasmamembrane localization in Sf9 cells led us to believe its acylation state may allow for similar localization in HEK-293 cells in the absence of cdAE1. To confirm this, we constructed a double mutant removing sites of myristoylation and palmitoylation (G2A/C173A) and observed its co-localization with AE1 proteins. Figure 6 shows the immunofluorescence of HA-tagged protein 4.2 and the G2A/C173A mutant expressed with AE1, mdAE1 and AE1SAO. In the first row, wild-type protein 4.2 is widely distributed throughout the cell and co-localizes with AE1 at the plasma membrane, as seen by the yellow colour in the merged image. In the second row, the G2A/C173A mutant has c The Authors Journal compilation c 2011 Biochemical Society a similar cellular distribution and co-localizes to the plasma membrane with AE1. In the third row, the co-localization of protein 4.2 and mdAE1 is again seen by the yellow colour at the plasma membrane. However, in the fourth row, when the G2A/C173A mutant is expressed with mdAE1, there is very little yellow staining at the plasma membrane, indicating poor plasma-membrane localization. This suggests that the fatty acid moieties promote protein 4.2 targeting to the plasma membrane. This also suggests that the presence of wild-type AE1 allows the G2A/C173A mutant to localize to the plasma membrane, as seen in the second row, consistent with an interaction between these proteins. The fifth row shows, as before, that wild-type protein 4.2 also co-localizes with AE1SAO at the ER. In the sixth row, the G2A/C173A mutant is also seen to co-localize with AE1SAO at the ER. Co-localization of protein 4.2 and the G2A/C173A mutant with AE1SAO is not complete, as seen by distinct green and red staining in the merged image. This indicates that both wild-type and G2A/C173A protein 4.2 are associated with other intracellular compartments in addition to the ER. Co-localization of wild-type and G2A/C173A protein 4.2 and cell-surface glycans in the absence or presence of AE1 in HEK-293 cells In order to further support the idea that the G2A/C173A mutant displays impaired plasma-membrane targeting, HAtagged protein 4.2 and the G2A/C173A mutant were expressed either alone or with AE1, and their localization to the plasma membrane using PNA binding was determined. PNA is a lectin that binds to the disaccharide galactose-β1,3-Nacetylgalactosamine found in oligosaccharides of glycoproteins [40] serving as a marker of the outer surface of the plasma membrane. In the first row of Figure 7, protein 4.2 expressed alone is widely distributed within the cell (green) and the red Interaction of protein 4.2 with HS mutants of AE1 Figure 5 319 Co-immunoprecipitation of wild-type and mutant AE1 with protein 4.2 in HEK-293 cells (A) AE1 in whole-cell extracts prepared with the detergent C12 E8 was immunoprecipitated with rabbit anti-C-terminal AE1 antibody followed by Protein G−Sepharose binding. Total and bound fractions were resolved by SDS/PAGE and immunoblots probed with mouse anti-AE1 antibody to detect AE1 and mouse anti-HA antibody to detect any co-purifying HA-tagged protein 4.2 (see the Materials and methods section). Immunoblot band intensities were measured from eight independent experiments. The mdAE1 lanes were excluded from these blots due to variable and low expression levels, and the remaining lanes on the same blots were spliced together for clarity (splice points are denoted by vertical black lines). (B) Immunoblots following co-immunoprecipitation of AE1 and mdAE1 and associated protein 4.2 from an experiment with comparable amounts of bound AE1 and mdAE1 proteins. The molecular mass in kDa is indicated on the left-hand side. stain, representing bound PNA, marks the outside surface of the cell. Even though PNA is on the outside of the bilayer and protein 4.2 on the inner side, we see an overlap of fluorescence seen by the yellow colour in the merged image since the confocal microscope cannot resolve the thickness of the membrane. A distinctive green/yellow/red pattern (going from the inside of the cell outward) is seen in the merged image, indicating that protein 4.2 associates with the plasma membrane in the absence of AE1. The same pattern is seen in the second row when protein 4.2 is expressed with AE1. In the third row when the acylation mutant is expressed alone, there is no yellow colour in the merged image, indicating its inability to associate with the plasma membrane. In the fourth row when the acylation mutant is co-expressed with AE1, the yellow colour is seen in the merged image, indicating that AE1 can localize the acylation mutant of protein 4.2 to the plasma membrane. Subcellular fractionation of HEK-293 cells expressing wild-type or G2A/C173A protein 4.2 In order to confirm that impaired plasma-membrane localization was not indirectly caused by impaired cytoskeletal attachment by the double mutant, we performed subcellular fractionation of HEK-293 cells expressing HA-tagged wild-type and G2A/C173A mutant protein 4.2 (Supplementary Figure S2 at http://www.BiochemJ.org/bj/433/bj4330313add.htm). After centrifugation at 50 000 rev./min the majority of protein 4.2 (73 + − 3 %, n = 4) was associated with the membrane/cytoskeleton fraction compared with the cytosolic fraction. Similar results (71 + − 6 %, n = 4) were seen with the acylation mutant. Detergent extraction of the membrane/cytoskeletal fraction revealed that most (87 %) of the membrane-associated protein 4.2 was in the cytoskeletal fraction, with a small amount in the membrane fraction. Similar results (92 %) were seen with the acylation mutant, although the amount solubilized by detergent was decreased relative to wild-type protein 4.2 (8 % compared with 13 %). These observations support a previous report [15] where both myristoylated and non-myristoylated (G2A) protein 4.2 expressed in Sf9 cells were associated with the particulate fraction. These results indicate that fatty acylation does not affect protein 4.2 cytoskeleton association. The majority of the actin (75 %) was seen in the cytosolic fraction compared with the remainder in the membrane/cytoskeletal fraction (Supplementary Figure S2), in agreement with a previous study in HEK-293 cells [41]. Virtually all of the GAPDH, a soluble protein, was detected in the cytosol, with a negligible amount detected in the membrane/cytoskeleton fraction. These results support our assignment of cellular compartments to the various fractions, allowing us to localize the majority of wild-type and G2A/C173A protein 4.2 to the cytoskeletal compartment with confidence. DISCUSSION In the present study, we were able to express full-length HAtagged protein 4.2 with AE1 proteins in HEK-293 cells. The AE1HS proteins expressed to similar levels as AE1 and could traffic to the plasma membrane of the cells. These findings are consistent with the observation that the levels of these HS mutants in patient’s erythrocytes are normal [3−5]. This is also consistent with our observation that the mutations do not have a major effect on the structure or stability of the isolated cytoplasmic domain [7]. We also demonstrated that protein 4.2 was expressed to similar levels in HEK-293 cells in the absence or presence of AE1 proteins. Thus protein 4.2 association with AE1 is not required for its expression in HEK-293 cells. When co-expressed in HEK-293 cells, protein 4.2 and AE1 co-localized at the plasma membrane. Similar observations were made with the AE1HS mutants and kAE1, but also with mdAE1, which does not interact with protein 4.2. In contrast, protein 4.2 co-localized at the ER with AE1SAO. Furthermore, protein 4.2 could target to the plasma membrane in the absence of AE1, as seen by its co-localization with PNA. This result agrees with previous reports of protein 4.2 localization at the plasma membrane of Xenopus oocytes [16] and Sf9 cells [15] in the absence of AE1. We also found protein 4.2 widely distributed among intracellular compartments, including the ER, as seen by its co-localization with CNX. These results show that protein 4.2 c The Authors Journal compilation c 2011 Biochemical Society 320 S. P. Bustos and R. A. F. Reithmeier Figure 7 Immunofluorescence images of cell-surface glycans using PNA and wild-type and G2A/C173A protein 4.2 expressed in the absence or presence of AE1 in HEK-293 cells Non-permeabilized HEK-293 cells expressing wild-type or mutant protein 4.2 proteins in the absence or presence of AE1 were incubated with PNA Alexa Fluor® 488 conjugate to detect the outer membrane. Cells were then permeabilized and incubated with rat anti-HA antibody to detect HA-tagged protein 4.2 or G2A/C173A. Incubation of samples with fluorescently labelled anti-rat secondary antibody and confocal microscopy was performed as described in the Materials and methods section. In the merged image, yellow indicates the co-localization of protein 4.2 (intracellular) and PNA (extracellular) at the level of the plasma membrane. GC, G2A/C173A mutant; perm., permeabilized; non-perm., non-permeabilized. Figure 6 Immunofluorescence images of wild-type and G2A/C173A protein 4.2 and AE1 proteins in HEK-293 cells Non-permeabilized cells expressing wild-type or mutant protein 4.2 and wild-type or mdAE1 proteins (first four rows) were incubated with the mouse monoclonal anti-AE1 antibody (BRIC 6) against an external epitope. Cells were then permeabilized and incubated with a rat anti-HA antibody. In the last two rows, permeabilized cells expressing wild-type or mutant protein 4.2 and AE1SAO were incubated with mouse anti-AE1 and rat anti-HA antibodies. Incubation of samples with fluorescently labelled secondary antibodies and confocal microscopy was performed (see the Materials and methods section). In the merged images, yellow indicates co-localization of protein 4.2 and AE1. GC, G2A/C173A mutant; perm., permeabilized; non-perm., non-permeabilized. associates with various membranes within the cell in the absence of AE1, including the plasma membrane, the ER membrane and other intracellular membranes in HEK-293 cells. c The Authors Journal compilation c 2011 Biochemical Society Pull-down experiments revealed that protein 4.2 can associate with AE1, AE1HS, kAE1 and AE1SAO, but not mdAE1, in whole-cell detergent extracts. Protein 4.2 binding to AE1HS mutants was diminished relative to AE1. The binding impairment is likely to be due to a change in the protein 4.2-binding surface on cdAE1, since purified cdAE1 proteins with these mutations have been shown not to cause any gross structural change in the domain [7]. Introduction of a positive charge by the E40K, G130R or P327R mutants may serve to disrupt the acidic protein 4.2-binding surface on cdAE1. As shown in the model of cdAE1 (Figure 1), these mutations are not localized to a ‘hot spot’, but are widely distributed on one surface of the protein. Residues 40 and 130 on one monomer occur on the same side of the domain as residue 327 from the other monomer, perhaps requiring dimer formation to create the binding surface for protein 4.2. The cdAE1-binding regions shown in the model of protein 4.2 are predicted to form a binding surface that could be large enough to interact with all three AE1HS mutation sites. It is not surprising, then, that alterations of residues at these distant sites in cdAE1 affect protein 4.2 binding. Interaction of protein 4.2 with HS mutants of AE1 The binding of protein 4.2 to kAE1 as measured in Ni-NTA pulldowns was not significantly different from that of AE1. This result may seem puzzling when we consider that the kidney isoform is missing the first 65 residues of the N-terminus, whereas the E40K HS mutant which has a single point mutation in this region shows impaired binding to protein 4.2. However, this mutation replaces an acidic with a basic residue. This introduction of a positive charge into a potential binding spot may compromise protein 4.2 binding in a way that absence of the region does not. As was mentioned above, it is believed that the Arg−Arg motif of protein 4.2 (residues 34 and 35) is necessary for the cdAE1 interaction and that this motif has an electrostatic interaction with an acidic region of cdAE1, possibly at the N-terminus. If this acidic region were to become more basic, the electrostatic repulsion could result in the binding impairment we saw with E40K. The cytoplasmic domain of kAE1 has been shown to be a more open structure than AE1 [21], and it is not known how this would affect protein binding. It is possible that this open structure compensates for the missing central β-strand. Wild-type binding levels of AE1SAO to protein 4.2 were not surprising as the cytoplasmic domain of this mutant is unchanged, deficiencies of protein 4.2 in AE1SAO erythrocytes have not been reported, and AE1SAO exhibits increased binding to the cytoskeleton in erythrocytes [28]. The co-localization of protein 4.2 with mdAE1 at the plasma membrane, despite a lack of interaction, turned our attention to the possible role of fatty acid modifications in plasmamembrane localization. We found that G2A/C173A protein 4.2 had diminished plasma-membrane localization when expressed alone or with mdAE1. This suggests that protein 4.2 is able to associate with the plasma membrane via its lipid anchors, as suggested in previous reports [15,42]. This is at least the case in HEK-293 cells where AE1 is not needed for protein 4.2 stabilization or plasma-membrane localization. Subcellular fractionation studies showed that the majority of wild-type and G2A/C173A protein 4.2 expressed in HEK-293 cells was associated with the cytoskeleton. This demonstrates that the G2A/C173A mutation does not affect cytoskeletal binding. A portion (<30 %) of wild-type and G2A/C173A protein 4.2 was seen in the cytosolic fraction, which agrees with the immunofluorescence localization results where the protein was broadly localized throughout the cell. The present study shows that AE1 is not required for the expression and plasma-membrane localization of protein 4.2 in HEK-293 cells. Also, the three AE1HS mutants, E40K, G130R and P327R, have impaired binding to protein 4.2, which contributes to the molecular basis of HS in patients with these mutations. How this impaired binding translates into protein 4.2 deficiency in erythrocytes is yet to be determined. Protein 4.2 and AE1 have been found to appear simultaneously during erythropoiesis and interact with each other as soon as they are expressed [43]. Indeed, protein 4.2 may associate with AE1 at the ER during their biosynthesis and traffic together to the cell surface. The fate of protein 4.2 that is unable to fully associate with AE1HS mutants during differentiation is not known. Protein association with the cytoskeleton can determine protein sorting during enucleation of the differentiating erythrocyte [44], where an increased cytoskeletal association causes retention in the reticulocyte. It is possible that the portion of protein 4.2 unable to bind to AE1HS never makes it to the membrane, or does not localize to a region of the membrane where protein 4.2 can bind to the cytoskeleton. This would prevent its retention in the reticulocyte during enucleation and explain its deficiency in erythrocytes of patients with these three cytoplasmic AE1HS mutations. Studies of protein 4.2 sorting during enucleation of erythrocyte precursors from healthy individuals and HS patients, 321 or relevant HS mouse models, would help to determine the mechanism of protein 4.2 loss in HS. AUTHOR CONTRIBUTION All experiments were carried out by Susan Bustos, who wrote the manuscript and prepared the Figures. Reinhart Reithmeier developed and supervised the research project, and edited the manuscript. ACKNOWLEDGEMENTS We thank Jing Li (University of Toronto, Toronto, ON, Canada) for her assistance in carrying out replicates of some experiments and Dr Michael Jennings (University of Arkansas for Medical Sciences, Little Rock, AR, U.S.A.) for the mouse monoclonal anti-AE1 antibody. FUNDING This work was supported by the Canadian Institutes of Health Research [FRN15266/MOP102493]; and a Graduate Student Fellowship (to S.P.B.) from the Canadian Blood Services. REFERENCES 1 Lux, S. E. and Palek, J. (1995) Disorders of the red cell membrane. In Blood: Principles and Practice of Hematology (Handin, R. I., Lux, S. E. and Stossel, T. P., eds), p. 1701, Lippincott, Philadelphia, PA 2 Jennings, M. L. (1989) Structure and function of the red blood cell anion transport protein. Annu. Rev. Biophys. Biophys. Chem. 18, 397–430 3 Rybicki, A. C., Qiu, J. J., Musto, S., Rosen, N. L., Nagel, R. L. and Schwartz, R. S. (1993) Human erythrocyte protein 42 deficiency associated with hemolytic anemia and a homozygous 40glutamic acid→lysine substitution in the cytoplasmic domain of band 3 (band 3 Montefiore). Blood 81, 2155–2165 4 Inoue, T., Kanzaki, A., Kaku, M., Yawata, A., Takezono, M., Okamoto, N., Wada, H., Sugihara, T., Yamada, O., Katayama, Y. et al. (1998) Homozygous missense mutation (band 3 Fukuoka: G130R): a mild form of hereditary spherocytosis with near-normal band 3 content and minimal changes of membrane ultrastructure despite moderate protein 4.2 deficiency. Br. J. Haematol. 102, 932–939 5 Jarolim, P., Palek, J., Rubin, H. L., Prchal, J. T., Korsgren, C. and Cohen, C. M. (1992) Band 3 Tuscaloosa: Pro327 →Arg327 substitution in the cytoplasmic domain of erythrocyte band 3 protein associated with spherocytic hemolytic anemia and partial deficiency of protein 4.2. Blood 80, 523–529 6 Zhang, D., Kiyatkin, A., Bolin, J. T. and Low, P. S. (2000) Crystallographic structure and functional interpretation of the cytoplasmic domain of erythrocyte membrane band 3. Blood 96, 2925–2933 7 Bustos, S. P. and Reithmeier, R. A. (2006) Structure and stability of hereditary spherocytosis mutants of the cytosolic domain of the erythrocyte anion exchanger 1 protein. Biochemistry 45, 1026–1034 8 Korsgren, C. and Cohen, C. M. (1986) Purification and properties of human erythrocyte band 4.2. Association with the cytoplasmic domain of band 3. J. Biol. Chem. 261, 5536–5543 9 Korsgren, C. and Cohen, C. M. (1988) Associations of human erythrocyte band 4.2. Binding to ankyrin and to the cytoplasmic domain of band 3. J. Biol. Chem. 263, 10212–10218 10 Rybicki, A. C., Schwartz, R. S., Hustedt, E. J. and Cobb, C. E. (1996) Increased rotational mobility and extractability of band 3 from protein 4.2-deficient erythrocyte membranes: evidence of a role for protein 42 in strengthening the band 3-cytoskeleton linkage. Blood 88, 2745–2753 11 Cohen, C. M., Dotimas, E. and Korsgren, C. (1993) Human erythrocyte membrane protein band 4.2 (pallidin). Semin. Hematol. 30, 119–137 12 Korsgren, C., Lawler, J., Lambert, S., Speicher, D. and Cohen, C. M. (1990) Complete amino acid sequence and homologies of human erythrocyte membrane protein band 4.2. Proc. Natl. Acad. Sci. U.S.A. 87, 613–617 13 Risinger, M. A., Dotimas, E. M. and Cohen, C. M. (1992) Human erythrocyte protein 4.2, a high copy number membrane protein, is N-myristylated. J. Biol. Chem. 267, 5680–5685 14 Bhattacharyya, R., Das, A. K., Moitra, P. K., Pal, B., Mandal, I. and Basu, J. (1999) Mapping of a palmitoylatable band 3-binding domain of human erythrocyte membrane protein 4.2. Biochem. J. 340, 505–512 15 Risinger, M. A., Korsgren, C. and Cohen, C. M. (1996) Role of N-myristylation in targeting of band 4.2 (pallidin) in nonerythroid cells. Exp. Cell Res. 229, 421–431 c The Authors Journal compilation c 2011 Biochemical Society 322 S. P. Bustos and R. A. F. Reithmeier 16 Toye, A. M., Ghosh, S., Young, M. T., Jones, G. K., Sessions, R. B., Ramauge, M., Leclerc, P., Basu, J., Delaunay, J. and Tanner, M. J. (2005) Protein-4.2 association with band 3 (AE1, SLCA4) in Xenopus oocytes: effects of three natural protein-4.2 mutations associated with hemolytic anemia. Blood 105, 4088–4095 17 Liu, S., Cerione, R. A. and Clardy, J. (2002) Structural basis for the guanine nucleotide-binding activity of tissue transglutaminase and its regulation of transamidation activity. Proc. Natl. Acad. Sci. U.S.A. 99, 2743–2747 18 Rybicki, A. C., Musto, S. and Schwartz, R. S. (1995) Identification of a band-3 binding site near the N-terminus of erythrocyte membrane protein 4.2. Biochem. J. 309, 677–681 19 Kollert-Jons, A., Wagner, S., Hubner, S., Appelhans, H. and Drenckhahn, D. (1993) Anion exchanger 1 in human kidney and oncocytoma differs from erythroid AE1 in its NH2 terminus. Am. J. Physiol. 265, F813–F821 20 Wagner, S., Vogel, R., Lietzke, R., Koob, R. and Drenckhahn, D. (1987) Immunochemical characterization of a band 3-like anion exchanger in collecting duct of human kidney. Am. J. Physiol. 253, F213–F221 21 Pang, A. J., Bustos, S. P. and Reithmeier, R. A. (2008) Structural characterization of the cytosolic domain of kidney chloride/bicarbonate anion exchanger 1 (kAE1). Biochemistry 47, 4510–4517 22 Ding, Y., Casey, J. R. and Kopito, R. R. (1994) The major kidney AE1 isoform does not bind ankyrin (Ank1) in vitro . An essential role for the 79 NH2 -terminal amino acid residues of band 3. J. Biol. Chem. 269, 32201–32208 23 Wang, C. C., Moriyama, R., Lombardo, C. R. and Low, P. S. (1995) Partial characterization of the cytoplasmic domain of human kidney band 3. J. Biol. Chem. 270, 17892–17897 24 Friedrichs, B., Koob, R., Kraemer, D. and Drenckhahn, D. (1989) Demonstration of immunoreactive forms of erythrocyte protein 4.2 in nonerythroid cells and tissues. Eur. J. Cell Biol. 48, 121–127 25 Jarolim, P., Palek, J., Amato, D., Hassan, K., Sapak, P., Nurse, G. T., Rubin, H. L., Zhai, S., Sahr, K. E. and Liu, S. C. (1991) Deletion in erythrocyte band 3 gene in malaria-resistant Southeast Asian ovalocytosis. Proc. Natl. Acad. Sci. U.S.A. 88, 11022–11026 26 Mohandas, N., Lie-Injo, L. E., Friedman, M. and Mak, J. W. (1984) Rigid membranes of Malayan ovalocytes: a likely genetic barrier against malaria. Blood 63, 1385–1392 27 Liu, S. C., Zhai, S., Palek, J., Golan, D. E., Amato, D., Hassan, K., Nurse, G. T., Babona, D., Coetzer, T., Jarolim, P. et al. (1990) Molecular defect of the band 3 protein in Southeast Asian ovalocytosis. N. Engl. J. Med. 323, 1530–1538 28 Liu, S. C., Palek, J., Yi, S. J., Nichols, P. E., Derick, L. H., Chiou, S. S., Amato, D., Corbett, J. D., Cho, M. R. and Golan, D. E. (1995) Molecular basis of altered red blood cell membrane properties in Southeast Asian ovalocytosis: role of the mutant band 3 protein in band 3 oligomerization and retention by the membrane skeleton. Blood 86, 349–358 29 Sarabia, V. E., Casey, J. R. and Reithmeier, R. A. (1993) Molecular characterization of the band 3 protein from Southeast Asian ovalocytes. J. Biol. Chem. 268, 10676–10680 30 Schofield, A. E., Reardon, D. M. and Tanner, M. J. (1992) Defective anion transport activity of the abnormal band 3 in hereditary ovalocytic red blood cells. Nature 355, 836–838 Received 25 August 2010/28 October 2010; accepted 1 November 2010 Published as BJ Immediate Publication 1 November 2010, doi:10.1042/BJ20101375 c The Authors Journal compilation c 2011 Biochemical Society 31 Cheung, J. C., Cordat, E. and Reithmeier, R. A. (2005) Trafficking defects of the Southeast Asian ovalocytosis deletion mutant of anion exchanger 1 membrane proteins. Biochem. J. 392, 425–434 32 Cheung, J. C. and Reithmeier, R. A. (2005) Membrane integration and topology of the first transmembrane segment in normal and Southeast Asian ovalocytosis human erythrocyte anion exchanger 1. Mol. Membr. Biol. 22, 203–214 33 Quilty, J. A., Li, J. and Reithmeier, R. A. (2002) Impaired trafficking of distal renal tubular acidosis mutants of the human kidney anion exchanger kAE1. Am. J. Physiol. Renal Physiol. 282, F810–F820 34 Popov, M., Li, J. and Reithmeier, R. A. (1999) Transmembrane folding of the human erythrocyte anion exchanger (AE1, Band 3) determined by scanning and insertional N-glycosylation mutagenesis. Biochem. J. 339, 269–279 35 Sells, M. A., Li, J. and Chernoff, J. (1995) Delivery of protein into cells using polycationic liposomes. Biotechniques 19, 72–76 36 Laemmli, U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 37 Towbin, H., Staehelin, T. and Gordon, J. (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U.S.A. 76, 4350–4354 38 Jennings, M. L., Anderson, M. P. and Monaghan, R. (1986) Monoclonal antibodies against human erythrocyte band 3 protein. Localization of proteolytic cleavage sites and stilbenedisulfonate-binding lysine residues. J. Biol. Chem. 261, 9002–9010 39 Tang, X. B., Fujinaga, J., Kopito, R. and Casey, J. R. (1998) Topology of the region surrounding Glu681 of human AE1 protein, the erythrocyte anion exchanger. J. Biol. Chem. 273, 22545–22553 40 Slupsky, J. R., Duggan-Keen, M., Booth, L. A., Karpas, A., Rhodes, E. G., Cawley, J. C. and Zuzel, M. (1993) The peanut-agglutinin (PNA)-binding surface components of malignant plasma cells. Br. J. Haematol. 83, 567–573 41 Oprea, G. E., Krober, S., McWhorter, M. L., Rossoll, W., Muller, S., Krawczak, M., Bassell, G. J., Beattie, C. E. and Wirth, B. (2008) Plastin 3 is a protective modifier of autosomal recessive spinal muscular atrophy. Science 320, 524–527 42 Das, A. K., Bhattacharya, R., Kundu, M., Chakrabarti, P. and Basu, J. (1994) Human erythrocyte membrane protein 4.2 is palmitoylated. Eur. J. Biochem. 224, 575–580 43 van den Akker, E., Satchwell, T. J., Pellegrin, S., Flatt, J. F., Maigre, M., Daniels, G., Delaunay, J., Bruce, L. J. and Toye, A. M. (2010) Investigating the key membrane protein changes during in vitro erythropoiesis of protein 4.2− cells (mutations Chartres 1 and 2). Haematologica 95, 1278–1286 44 Lee, J. C., Gimm, J. A., Lo, A. J., Koury, M. J., Krauss, S. W., Mohandas, N. and Chasis, J. A. (2004) Mechanism of protein sorting during erythroblast enucleation: role of cytoskeletal connectivity. Blood 103, 1912–1919 45 Arnold, K., Bordoli, L., Kopp, J. and Schwede, T. (2006) The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22, 195–201 46 Gutierrez, E. and Sung, L. A. (2007) Interactions of recombinant mouse erythrocyte transglutaminase with membrane skeletal proteins. J. Membr. Biol. 219, 93–104 Biochem. J. (2011) 433, 313–322 (Printed in Great Britain) doi:10.1042/BJ20101375 SUPPLEMENTARY ONLINE DATA Protein 4.2 interaction with hereditary spherocytosis mutants of the cytoplasmic domain of human anion exchanger 1 Susan P. BUSTOS* and Reinhart A. F. REITHMEIER†1 *Department of Biochemistry, University of Toronto, Toronto, ON, Canada, M5S 1A8, and †Department of Medicine, University of Toronto, Toronto, ON, Canada, M5S 1A8 See the page that follow for Supplementary Figures S1 and S2. 1 To whom correspondence should be addressed (email [email protected]). c The Authors Journal compilation c 2011 Biochemical Society S. P. Bustos and R. A. F. Reithmeier Figure S2 Subcellular fractionation of HEK-293 cells expressing wild-type or G2A/C173A protein 4.2 Following subcellular fractionation of HEK-293 cells expressing HA-tagged wild-type and G2A/C173A mutant protein 4.2, fractions were subjected to SDS/PAGE and transferred to nitrocellulose. Blots were probed with mouse anti-HA antibody, mouse anti-actin antibody and mouse anti-GAPDH antibody to detect protein 4.2, actin and GAPDH respectively (see the Materials and methods in the main text). Immunoblot band intensities were measured from four independent experiments. The molecular mass in kDa is indicated on the left-hand side. S1, cytosolic fraction; Sw, wash fraction; P1, membrane/cytoskeletal fraction; S2, detergent-solubilized membrane fraction; P2, cytoskeletal fraction; WT, wild-type. Received 25 August 2010/28 October 2010; accepted 1 November 2010 Published as BJ Immediate Publication 1 November 2010, doi:10.1042/BJ20101375 Figure S1 Immunofluorescence images of wild-type and mutant AE1 in HEK-293 cells Non-permeabilized HEK-293 cells expressing wild-type or mutant AE1 proteins were incubated with mouse anti-AE1 andtibody (BRIC 6) against an external epitope, followed by incubation with Alexa Fluor® 488-conjugated goat anti-mouse antibody to detect cell-surface AE1 (green). Cells were then washed, permeabilized and incubated with the same primary antibody followed by incubation with Cy3-conjugated goat anti-mouse antibody to detect total AE1 (red). Confocal microscopy was then performed. An enlarged region of the cell is shown in the inset for each image. In the merged image, yellow indicates the co-localization of cell surface and total AE1. non-perm., non-permeabilized; perm., permeabilized. c The Authors Journal compilation c 2011 Biochemical Society