* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download bioinorganic 1
Lewis acid catalysis wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Geochemistry wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Ligand binding assay wikipedia , lookup
Metallic bonding wikipedia , lookup
List of phenyltropanes wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Western blot wikipedia , lookup
Cation–pi interaction wikipedia , lookup
History of molecular biology wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Biosynthesis wikipedia , lookup
Cooperative binding wikipedia , lookup
Chemical biology wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Coordination complex wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Protein adsorption wikipedia , lookup
Biochemistry wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
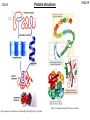
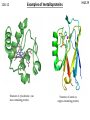
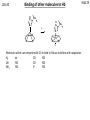
10A-1 What is bioinorganic chemistry? H&S 29 The study of inorganic compounds in biologicial contexts is known as BIOINORGANIC CHEMISTRY. This is a very diverse field, including •basic studies of simple coordination compounds as models for metal in ‘real’ biological systems •studies on metal-containing proteins themselves •studies on naturally occurring metals – their presence and their roles/functions (i.e. answering questions like how do the metals get there? What do they do? How do they do it? •The interaction of metals introduced into biological systems: diagnostics (e.g. MRI using paramagnetic metal ions), therapeutics (drugs, e.g. cisplatin), toxicity. 10A-2 Bioinorganic chemistry H&S 29 BGSV, page 2 10A-3 Elements of biological importance Metals perform a wide variety of functions: Charge/electrolyte balance, electrolytic conductivity (Na, K) Structure control (Ca, Zn, Si, S) Biomineralization (Ca, Mg, Fe, Si, Sr, Cu) Catalysis: Lewis acid-base (Zn, Fe, Ni, mn) Electron transfer (Fe, Cu, Mo) Group Transfer (e.g. Me, O2) (V, Fe, Co, Ni, Cu, Mo, W) Redox (V, Mn, Fe, Co, Ni, Cu, W, S, Se) Energy storage (Fe) H&S 29 10A-4 Transition metals in us H&S 29 10A-5 Overview of protein structure Transition metals in biology are normally found in a specific coordination environment, tightly bound to a protein. This contrasts the environment for Group 1 or 2 metals which are usually loosely bound and/or dissolved in solution. A protein is a polymer comprised of amino acids. R H R H OH H2N * O O H3N * O The amino acid is defined by the identity of the R group (“side chain”). There are twenty naturally occurring amino acids. H&S 29 Natural amino acids 10A-6 R H OH H2 N O http://www.neb.com/nebecomm/tech_reference/general_data/amino_acid_structures.asp H&S 29 H&S 29 The peptide bond 10A-7 Proteins (also known as polypeptides) are sequences of amino acid-based polymers. The connection between two amino acids is a condensation (dehydration) reaction: R1 H OH H2N R2 H + R1 H OH H2N O O - H2O H2N H N O OH O R2 H A dipeptide “monopeptide” = amino acid Proteins are different from most synthetic polymers because normally there is no regular repeating structure in terms of the side chains: R1 H H2N H N O R3 H O R2 H N H O R5 H O R7 H O H H N N N N OH H H O R4 H O R6 H O R8 H H N A full description of a polypeptide requires identification of every one of the side chains. The peptide sequence determines the overall structure, and function, of the protein. 10A-8 Protein structure http://en.wikipedia.org/wiki/Protein_structure http://academic.brooklyn.cuny.edu/biology/bio4fv/page/3d_prot.htm H&S 29 10A-9 Metalloproteins H&S 29 The concentration of “free” (i.e. solvated) transition metal ions in cellular compartments is usually near zero. This contrasts non-transition metal ions (Na, K, Mg) which generally have lower binding constants when found in specific protein binding sites, and are found in relatively high concentrations in living tissues. Transition metals in biology are often strongly associated with proteins. Proteins containing bound metals are called metalloproteins. Many proteins are responsible for catalyzing biochemical reactions; these are known as enzymes. Proteins in which a metal is present and plays a role in the catalysis are often called metalloenzymes. 10A-10 Metalloproteins H&S 29 The metal is found in a specific place/site with a set coordination environment. The binding constants in these systems are usually very high: Metals are bound to proteins by amino acid side chains. This requires that the side chains be able to function as a ligand. There is often a correlation between hardness/softness of a donor atom and the metals (hard vs soft) to which the ligand preferentially binds. For example many of the O donors bind to Ca, Mg, K whereas the softer donors (e.g. S based) bind to Fe, Cu, etc. 10A-11 Amino acids as ligands H&S 29 Neutral ligands Anionic ligands (acidic H noted) http://www.neb.com/nebecomm/tech_reference/general_data/amino_acid_structures.asp 10A-12 Examples of metalloproteins Structure of cytochrome c (an iron-containing protein) Structure of azurin (a copper-containing protein) H&S 29 10A-13 The biochemistry of iron H&S 29 Iron is the fifth most abundant element in our solar system and the most abundant element on earth So it shouldn’t be surprising that iron is perhaps the most common and versatile transition metal found in living systems. Oxygen transport and storage Dioxygen (O2) is one of the most important substrates for sustaining life. Its main use is in respiration (intake, transport, storage of O2 as a prelude to its use in chemical reactions). O2 is one of the most important “chemical fuels” (outside of photosynthesis). O2 needs to be continuously supplied to cells. This is accomplished by metal-containing enzymes which take up O2 from the external environment (air), transport O2 to efficient storage sites where it can be utilized. 10A-14 Hemoglobin and myoglobin H&S 29 Myoglobin (Mb) is an iron-containing protein which stores O2. The molecular weight of Mb is ~ 17,000 (153 amino acid residues and one heme unit) Hemoglobin (also spelled haemoglobin) (Hb) is a closely related iron-containing protein which transports O2. M.Wt. ~ 64,500 (see picture)- it’s a tetramer of Mb. Hemoglobin is found in red blood cells (and in fact give blood its colour). H&S 29 Heme 10A-15 The active sites for both Hb and Mb is the iron centres, whose coordination environment is defined NH N by a porphyrin. A heme unit is an iron-porphyrin. Porphyrins normally carry a -2 charge when bound N HN to a metal. The specific porphyrin found in Hb and Mb is called a porphyrin ring protoporphyrin IX. O N N N N HN OH Fe N N OH O protoporphryn IX 1. Draw a square of 4 N atoms. N NH N 2. Connect Ns with three-carbon chains. 3. Connect the C’s adjacent to Ns with two-carbon chains. 3. Put protons on two opposite Ns. 4. Put double bonds in one of the NH-containing 5 membered rings. 5. Complete alternating single/double bonds around the ring. H&S 29 O2 binding to Hb 10A-16 Hemoglobin’s active sites (all 4 of them) without the O2 is known as deoxyhemoglobin. The active site contains five-coordinate, pseudo-square planar Fe(+2). The fifth donor site is an imidazole ring from a histidine residue in the polypeptide chain. In deoxyhemoglobin the iron lies above the protoporphyrin IX plane, probably because the size of the Fe(+2) (high-spin) ion is a little too large to fit in the middle of the porphyrin ring. When O2 binds, the Fe falls into the plane of the porphyrin and becomes six coordinate. The iron is oxidized from Fe(+2) to Fe(+3) and becomes low spin (S = ½). The dioxygen is reduced to superoxide, O2(1-). N N Fe N CO2H = N N N Fe N N CO2H H N His H N N O2 N Fe N N N N N N FeIII O deoxyhemoglobin His O N N 10A-17 O2 binding to Hb Binding of O2 to a metal site can be expressed as: The pressure at which half of the metal sites are oxygen-bound (i.e. [M] = [MO2] can be expressed as follows: The fraction of metal binding sites occupied by oxygen, (theta), can be expressed by the following: H&S 29 10A-18 O2 binding to Hb H&S 29 Qualitatively, binding to a single metal site should exhibit rapid increase in theta at low pressures and then saturation at higher pressures (depending on p1/2(O2). Curves depend ultimately on Kp. What is observed is quite different…. H&S 29 O2 binding to Hb 10A-19 Hemoglobin has four heme binding sites. If there were no cooperativity between them, each would have the same equilibrium binding constants. However this is not the case: Hb + O2 HbO2 HbO2 + O2 Hb(O2)2 Hb(O2)2 + O2 Hb(O2)3 Hb(O2)3 + O2 Hb(O2)4 K1 = 5-60 K4 = 3000-6000 Binding of one O2 increases the binding affinity for the next one! Hemoglobin takes oxygen to muscle tissues where the O2 is taken up by myoglobin. Because Mb has only one heme site it cannot exhibit cooperative binding effects like hemoglobin does. However it must bind O2 much better than Hb in order to favour transfer of O2: Hb(O2)n + Mb Hb + nMbO2 K>> 1 10A-20 Binding of other molecules to Hb H N H&S 29 His H N N His L N Fe N N N N N N FeIII N N L Molecules which can compete with O2 to bind to Hb can interfere with respiration. N2 no CO YES CNYES SO YES NO2 YES S2YES H&S 29 Hb/Mb Model compound studies 10A-21 One of the central thrusts in bioinorganic chemistry is to design simple complexes which mimic the structure and function of naturally occurring metalloenzymes. “function follows structure” is the general ideology. It seems logical that a “simple” five coordinate Fe(II) porphyrin complex would be an excellent structural model for Hb and Mb. However the reaction of “simple” (synthetic) Heme structures with O2 does not follow the same path as Hemoglobin. Ph N Ph O2 N Fe N O2 Ph N N N Me N Me N N Me Me N N FeIII N N FeIII N H2O N N Ph N N N N N N Me N Me N N FeIII O N N N N N FeIII N N N N Me H&S 29 Hb/Mb Model compound studies 10A-22 This clearly cannot happen in Hb or Mb because the heme units are not free to associate like this. This is a good example of a critical feature of many metal-containing proteins: they exhibit unusual chemistry in vivo because of the structurally constrained environment in which they exist. Better model compounds have been designed which provide a more constrained environment for the incoming O2. One of the best known examples is the so-called ‘picket fence’ porphyrin which binds O2 to give an Fe(+3)-superoxide adduct which strongly resembles HbO2. O N H O NH NH N N N N HN FeII N HN O H N O N Me N N N O2 N O O N FeII N N N Me H&S 29 Iron-sulfur proteins 10A-23 A large number of inorganic-containing proteins contain Fe and S assembled into clusters in which the S atoms (either sulfide, S2-, or as the deprotonated form of cysteine, -SCys) bridge Fe(2+) or Fe(3+) centres. Cys S Cys S Cys Cys S S Cys S Fe S Fe S S S Cys Fe Cys S His S Cys N Rubredoxin His N cysteinate Cys Fe Fe S S S Cys sulfide (S2-) 2Fe-2S ferredoxins S S Cys S Fe Fe S Cys S S Fe S Cys S Cys S Fe Fe S S S S Fe Fe S S Cys 4Fe-4S ferredoxins Cys 3Fe-4S ferredoxins Cys 10A-24 Iron-sulfur proteins H&S 29 Electron transfer normally involves movement of one electron at a time (the ultimate use is usually coupled to bond making-breaking). Few naturally occurring organic substrates can do this. Transition metals are excellent for electron transfer because they can adopt more than one oxidation state. Iron is particularly adept at this because the redox potential can be controlled by its environment (coordination sphere). e.g. Rubredoxin: Fe(SR)42- has Fe in +2 state. One electron oxidation gives Fe(SR)4- which has Fe in +3 oxidation state. In the Fe/S clusters the iron centers are close enough so that the individual iron centres interact with one another (through the bridging S atoms). In many cases the cluster is better thought of as a single redox-active entity. 10A-25 Iron-sulfur proteins e.g. 2Fe-2S ferredoxins: [Fe2S2(SR)4]4[Fe2S2(SR)4]3[Fe2S2(SR)4]2- Each Fe is +2 Each Fe is +3 e.g. 4Fe-4S ferredoxins: [Fe4S4(SR)4]4[Fe4S4(SR)4]3[Fe4S4(SR)4]2[Fe4S4(SR)4]1[Fe4S4(SR)4]0 Each Fe is +2 Each Fe is +3 (not known) H&S 29 10A-26 Identifying charge state of Fe/S clusters H&S 29 The iron ions are tetrahedral: as such the individual ions are high spin configuration. The electronic spectroscopy of Fe(2+) and Fe(3+) are distinct: the (3+) ions have extremely weak d-d transitions (Spin forbidden) while the (+2) ions have detectable d-d transitions in the visible. The overall spin state of an Fe/S cluster results from magnetic interactions between the individual Fe ions. BUT the way in which the individual spins interact can be different if two metals have different oxidation states. Cys Cys S S Cys H&S 29 Fe2S2 clusters: magnetism 10A-27 S Fe Fe S =Fe (+2) = S S Cys =Fe (+3) Antiferromagnetic interactions between individual Fe ions predominates. S=0 S=0 10A-28 Fe2S2 clusters: magnetism H&S 29 S =1/2 In the “mixed valent” state where one Fe is +3 and one is +2, the interactions between ions remains antiferromagentic if the ions are localized. Delocalization of charge can lead to ferromagnetic interactions: The two Fe ions are indistinguishable. S =9/2 Conceptually this can be described as the ‘odd’ electron being shared between the two ions (see below). The spin being shared must have the opposite spin to all the others. 10A-29 H&S 29 Fe2S2 clusters: magnetism Antiferromagnetic interactions in a delocalized cluster are not possible: S =1/2?? not a ground state configuration for Fe(+3) In mixed valent Fe2S2 the Fe ions are localized and the S=1/2 is the correct description of the magnetic state. S =1/2 Fe4S4 clusters: magnetism 10A-30 S S Cys S Fe S Cys S Cys Fe S Fe S Fe S Cys Fe4S4(SR)4]4- S=0 Fe4S4(SR)4]3- S = 1/2 (9/2 -2 -2) Fe4S4(SR)4]2- S=0 (9/2 -9/2) Fe4S4(SR)4]-2- S = 1/2 (9/2 -5/2 -5/2) H&S 29 H&S 29 Nitrogen 10A-31 Nitrogen is a vital element in biology. R1 H H2N H N O R3 H O R2 H N H O R5 H O R7 H O H H N N N N OH H H O R4 H O R6 H O R8 H H N Amino acids/proteins O N N OH Fe N N OH Nucleic acids (DNA, RNA) O Tetrapyrroles (pophyrins and related) H&S 29 Nitrogen fixation 10A-32 In virtually all nitrogen-containing biomolecules the nitrogen is in its fully reduced form i.e. in the same oxidation state as ammonia (NH3) or ammonium cation (NH4+). The source of the nitrogen (and the principle form of the element on Earth) is dinitrogen, N2. So living systems need to convert dinitrogen to a reduced form (NH3) to make it available for incorporation into essential biomolecules which contain N. This process is known as nitrogen fixation and is the first part of the overall Nitrogen cycle. O2 NH3 nitrification NH2OH Nitrogen fixation NO2- N2 NO3denitrification N2O Nitrate assimilation NO NO2NO2- 10A-33 Chemical approaches to nitrogen fixation The Haber (Haber – Bosch) process: industrial synthesis of ammonia (109 t/yr) N2 + 3H2 Ni H2O + CH4 (natural gas) 800-1000°C Fe 400°C H2 + CO2 2 NH3 H2O, K2CO3 KHCO3 H&S 29 Nitrogen fixation: challenges 10A-34 N H&S 29 N The NN bond in dinitrogen is one of the strongest of all bonds (BDE ~ 945 kJ/mol) And the bond is non-polar. There are also large kinetic barriers to reducing N2 because of the need to form intermediates (e.g. HN=NH, H2NNH2) en route to making ammonia. These factors create special challenges in reducing N2 to NH3 Some bacteria contain enzymes which can catalyze the transformation of dinitrogen to ammonia – these are called nitrogenases. The “simple” stoichiometry for N2 reduction is exothermic: But the activation energy is estimated to be 420 kJ/mol. Hence the need for a catalyst. 10A-35 Structure of nitrogenase H&S 29 10A-36 H&S 29 Structure of metal clusters in FeMo protein His FeMo cofactor Cys Cys S P cluster cofactor S Cys S Fe S Fe Fe S Fe S S S Fe Fe Fe Fe S Cys Cys S S Cys S Cys 10A-37 Nitrogenase mechanism H&S 29 Mechanism of dinitrogen reduction is unknown! Neither the Fe protein nor the FeMo protein bind N2 separately. Together they do bind N2 but subsequent reduction occurs too rapidly to allow detection of intermediates. Nitrogenase is strongly inhibited by (a) CO and (b) O2. There is consensus opinion that N2 initially binds somewhere at the FeMo cluster. The P cluster acts as an electron transfer relay (in accord with its resemblance to more common ferredoxintype clusters), though it is not yet known at what stage of catalysis this cluster is involved. Theoretically predicted binding modes of N2 to the core of the ntirogenase FeMo cluster. See Chem. Rev. 2004, p387.