* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download New techniques and the GMO-legislation
Genetic code wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
Gel electrophoresis of nucleic acids wikipedia , lookup
Biochemistry wikipedia , lookup
Genome evolution wikipedia , lookup
Non-coding DNA wikipedia , lookup
Transformation (genetics) wikipedia , lookup
DNA vaccination wikipedia , lookup
Deoxyribozyme wikipedia , lookup
List of types of proteins wikipedia , lookup
Point mutation wikipedia , lookup
Plant breeding wikipedia , lookup
Cre-Lox recombination wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Community fingerprinting wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Genomic library wikipedia , lookup
Molecular evolution wikipedia , lookup
Molecular cloning wikipedia , lookup
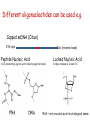
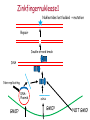
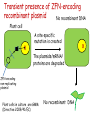
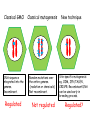
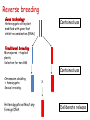
New techniques and the GMO-legislation Mistra Biotech Workshop 2014-05-23 The EU has a common legislation that regulates the use of GMO e.g. Directive 2001/18/EC on the deliberate release into the environment of genetically modified organisms. The Directive regulates: all organisms, except humans both experimental and commercial activities The Directive is process-based, so it is the technique used that decides if a certain product should be regulated or not. The definition and the list of techniques of genetic modification are the same as in Directive 90/220/EEC from 1990 (repealed by 2001/18/EC). Several new techniques has been developed since then and the development continues. It is unclear if certain technique results in a GMO/GMM that should be regulated or not. What defines a GMO? Article 2 (2) in the Directive 2001/18/EC Definition "genetically modified organism" means an organism, with the exception of human beings, in which the genetic material has been altered in a way that does not occur naturally by mating and/or natural recombination. Excludes everything except fertilization! Annex 1A, part 1 Techniques of genetic modification referred to in Article 2(2) are inter alia: 1) recombinant nucleic acid techniques involving the formation of new combinations of genetic material by the insertion of nucleic acid molecules produced by whatever means outside an organism, into any virus, bacterial plasmid or other vector system and their incorporation into a host organism in which they do not naturally occur but in which they are capable of continued propagation. 2) techniques involving the direct introduction into an organism of heritable material prepared outside the organism including micro-injection, macroinjection and micro-encapsulation. 3) cell fusion (including protoplast fusion) or hybridisation techniques where live cells with new combinations of heritable genetic material are formed through the fusion of two or more cells by means of methods that do not occur naturally. Annex 1A, part 2 Techniques referred to in Article 2(2)(b) which are not considered to result in genetic modification…. 1) in vitro fertilisation, 2) natural processes such as: conjugation, transduction, transformation 3) polyploidy induction Annex 1B Techniques/methods of genetic modification yielding organisms to be excluded from the Directive, on the condition that they do not involve the use of recombinant nucleic acid molecules or genetically modified organisms other than those produced by one or more of the techniques/methods listed below are: 1) mutagenesis, 2) cell fusion (including protoplast fusion) of plant cells of organisms which can exchange genetic material through traditional breeding methods. In the GMO:s approved so far a DNA-sequence has been integrated into the genome om the recipient organism -> recombinant DNA. Several of the new techniques results in an organism that do not have any foreign DNA integrated -> no recombinant DNA in the final organism. Working Group on New Techniques Established at the request of the competent authorities in October 2007. Two experts from each Member State nominated. Evaluate a list of eight new techniques. Discussed in the light of: • the definition of a GMO/GMM; • the techniques listed in the Annexes • the most recent available scientific data A report was submitted to the commission in January 2012. Examples of New Techniques Oligonucleotide-Directed Mutagenesis (ODM) Cibus LLC: http://cibus.com/ Site-specific mutation No foreign DNA in the resulting organism. Organisms developed through mutagenesis are excluded from the Directive, on the condition that the technique do not involve the use of recombinant nucleic acid molecules. Key issues discussed Are the synthetic oligonucleotide a recombinant nucleic acid molecule? Are synthetic oligonucleotides heritable material? Different oligonucleotides can be used e.g. Capped ssDNA (Cibus) CY3-dye Peptide Nucleic Acid Idc (reverse base) N-(2-aminoethyl)-glycine units linked by peptide bonds. Locked Nucleic Acid bridge between 2‘-O and 4‘-C PNA – not a nucleic acid in a biological sense Conclusions from the Working Group All experts ODM results in changes that can be obtained with other forms of mutagenesis. ODM is expected to generate fewer unintentional changes than traditional mutagenesis. The majority of the experts Oligonucleotides in this technique cannot be considered as recombinant nucleic acids or heritable material and that they are not capable of continued propagation. ODM is captured by Annex IB -> excluded Herbicide tolerant rapeseed developed using ODM Commercially available in the US where the authorites consider it to be a non-GM. Recently approved in Canada (March 2014). Field trials in the UK where the authorites consider it to be a non-GM. Cibus asked the competent authority in Sweden if they need to apply for field trials according to Directive 2001/18/EC. The answer was no. Zincfingernuklease 1 (ZFN1) Protein complex consists of: DNA-binding domain: Zincfingers (designed to bind to a specific site in the genome) DNA-cleaving domain: Nuklease Zincfingers Nuklease Zinkfingernuklease1 Nukleotides lost/added -> mutation Repair Double-strand break DNA Non-replicating DNAPlasmid GMO? mRNA GMO? NOT GMO! Transient presence of ZFN-encoding recombinant plasmid No recombinant DNA Plant cell X A site-specific mutation is created The plasmids/mRNA/ proteins are degraded ZFN-encoding non-replicating plasmid Plant cells in culture are GMM (Directive 2009/41/EC) No recombinant DNA X Conclusions from the Working Group All experts Results in changes that can be obtained with other forms of mutagenesis. Is expected to generate fewer unintentional changes than traditional mutagenesis. If ZFN-proteins are introduced directly the technique is fully captured by annex 1b -> excluded If plasmids/mRNA is used it should be excluded. Two different views: Already excluded/inclusion in Annex 1B needed. Classical GMO Classical mutagenesis DNA-sequence integrated into the genome. Recombinant. Random mutations over the entire genome. (radiation or chemicals) Not recombinant. Regulated Not regulated New technique Site-specific mutagenesis eg. ODM, ZFN,TALEN, CRISPR. Recombinant DNA can be used early in breeding process. Regulated? Cisgenesis DNA from the same species or a species´ that the recipient organism can hybridize with through conventional breeding techniques. The Working Groups conclusions All experts Captured by Directive 2001/18/EC -> regulated May in some cases meet the criteria of self-cloning in Directive 2009/41/EC on contained use of GMM -> excluded. Animal and plant cells in culture = GMM Reverse breeding Gene technology Contained use Heterozygote elite plant modified with gene that inhibit recombination (RNAi) Traditional breeding Microspores -> haploid plants. Selection for non-GM. Contained use Chromosom doubling -> homozygote. Sexual crossing. Heterozygote without any foreign DNA. X Deliberate release Conclusions from the Working Group The resulting plants and their offspring may be considered as not within the scope of the Directives based on: The resulting organisms have never contained any inserted foreign DNA. The genetic composition of the offspring is the same as the original plant material. The resulting organisms can be obtained by traditional breeding techniques. Other activities at the EU-level The European Food Safety Authoritys GMO-panel Determine whether there is a need for new guidance or whether the existing guidance on risk assessment should to be updated or further elaborated. What are the risks in terms of impact on humans, animals and the environment that the techniques could pose, irrespective of whether or not they fall under the GMO legislation? After the evaluation of two of the techniques, the Commission announced that the panel do not have to continue their work with the other techniques. DG Joint Research Center (2011) New plant breeding techniques State-of-the-art and prospects for commercial development Some conclusions Companies and research institutes based in the EU play a prominent role in research and development. Commercialization likely within 2-3 years (i.e. now!). The main constraints for the adoption of the techniques are the regulatory uncertainty and the potentially high costs for risk assessment and registration (if the crops derived by these techniques are classified as GMOs). The result of several of the techniques cannot be distinguished from those produced by conventional techniques or by natural variation. A detection method is a regulatory requirement. GMO or not GMO? Excluded or not excluded? These are the questions. Thanks for listening! Marie Nyman www.genteknik.se