* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Multiple RNA regulatory elements mediate distinct
Promoter (genetics) wikipedia , lookup
Molecular evolution wikipedia , lookup
Secreted frizzled-related protein 1 wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Alternative splicing wikipedia , lookup
Gene expression profiling wikipedia , lookup
RNA silencing wikipedia , lookup
Biochemical cascade wikipedia , lookup
List of types of proteins wikipedia , lookup
RNA interference wikipedia , lookup
Endogenous retrovirus wikipedia , lookup
Gene regulatory network wikipedia , lookup
Transcriptional regulation wikipedia , lookup
Non-coding RNA wikipedia , lookup
Silencer (genetics) wikipedia , lookup
Polyadenylation wikipedia , lookup
Gene expression wikipedia , lookup
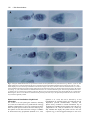
169 Development 119, 169-178 (1993) Printed in Great Britain © The Company of Biologists Limited 1993 Multiple RNA regulatory elements mediate distinct steps in localization of oskar mRNA Jeongsil Kim-Ha*, Philippa J. Webster*, Jeffrey L. Smith and Paul M. Macdonald† Department of Biological Sciences, Stanford University, Stanford, CA 94305, USA *Both authors contributed equally to this work for correspondence †Author SUMMARY Pattern formation in the early development of many organisms relies on localized cytoplasmic proteins, which can be prelocalized as mRNAs. The Drosophila oskar gene, required both for posterior body patterning and germ cell determination, encodes one such mRNA. Localization of oskar mRNA is an elaborate process involving movement of the transcript first into the oocyte from adjacent interconnected nurse cells and then across the length of the oocyte to its posterior pole. We have mapped RNA regulatory elements that direct this localization. Using a hybrid lacZ/oskar mRNA, we identify several elements within the oskar 3 untranslated region that affect different steps in the process: the early movement into the oocyte, accumulation at the anterior margin of the oocyte and finally localization to the posterior pole. This use of multiple cis-acting elements suggests that localization may be orchestrated in a combinatorial fashion, thereby allowing localized mRNAs with ultimately different destinations to employ common mechanisms for shared intermediate steps. INTRODUCTION analysis of the mRNA localization signals. For example, the RNA signals may consist of multiple different elements, each acting independently to program one movement or to target the mRNA to one site. This type of organization might allow the combinatorial use of different RNA elements to program different localization pathways and destinations. Alternatively, the complex RNA signals may be more than just the sum of their parts, and may only be capable of functioning as an intact unit. Drosophila serves as an excellent system in which to study the mechanisms responsible for mRNA localization. Localization of maternal mRNAs figures prominently in Drosophila development as a strategy to deploy molecules controlling patterning decisions. Consequently, extensive genetic screens for mutants defective in embryonic body patterning have provided not only examples of localized maternal mRNAs, but also likely candidates for genes encoding proteins involved in localization of these transcripts [reviewed by St. Johnston and Nüsslein-Volhard (1992)]. The mRNA encoding the anterior body patterning morphogen bicoid (bcd) is positioned at the anterior pole of the egg during oogenesis (Berleth et al., 1988; Stephenson et al., 1988; St. Johnston et al., 1989) and, when translated early in embryogenesis, produces a bcd protein gradient (Driever and Nüsslein-Volhard, 1988). Several patterning mutants are specifically defective in bcd mRNA localization (Stephenson et al., 1988; St. Johnston et al., 1989). One general mechanism for targeting proteins to subcellular locations is mRNA localization. After synthesis in the nucleus, some mRNAs become concentrated at specific sites in the cytoplasm, leading to the subsequent localization of their protein products. Numerous examples of this phenomenon have been recognized in recent years, and it is now apparent that mRNA localization plays an important role in cell function and diversification [reviewed by Gottlieb (1990) and Macdonald (1992b)]. Very little is known about mRNA localization signals, although cis-acting elements necessary for localization have been identified in several systems (Macdonald and Struhl, 1988; Mowry and Melton, 1992; Cheung et al., 1992; Dalby and Glover, 1992; Gavis and Lehmann, 1992). In the few cases where characterization of these elements has progressed beyond their identification, the signals have been found to be large, covering hundreds of nucleotides of RNA (Macdonald and Struhl, 1988; Mowry and Melton, 1992). This complexity may reflect the nature of the mRNA signals examined, as each directs a complex program of localization (St. Johnston et al., 1989; Yisraeli et al., 1990). It seems likely that the RNA signals provide binding sites for proteins involved in localization. Given the large size of the signals, multiple proteins may be expected to bind. Some insight into how such proteins mediate localization may be derived from a detailed Key words: pattern formation, oskar, Drosophila oocyte 170 J. Kim-Ha and others Similarly, the posterior morphogen nanos (nos), which appears in an opposing protein gradient (Smith et al., 1992; Gavis and Lehmann, 1992), is initially localized as an mRNA to the posterior pole of the embryo (Wang and Lehmann, 1991), and mutants defective in posterior body patterning fail to localize nos mRNA (Ephrussi et al., 1991). Numerous additional examples of mRNA localization in Drosophila oogenesis have been discovered in recent years (Aït-Ahmed et al., 1987; Suter et al., 1989; Kim-Ha et al., 1991; Ephrussi et al., 1991; St. Johnston et al., 1991; Golumbeski et al., 1991; Dalby and Glover, 1992; Cheung et al., 1992; Macdonald, 1992a; Barker et al., 1992; Lantz et al., 1992) and should eventually allow comparisons of different and perhaps related mRNA localization signals and mechanisms. Several maternal mRNAs are localized to the posterior pole of the developing Drosophila oocyte. The oskar gene, required both for posterior body patterning and germ cell determination (Lehmann and Nüsslein-Volhard, 1986), encodes one such mRNA (Kim-Ha et al., 1991; Ephrussi et al., 1991). Localization of osk mRNA is an elaborate process involving movement of the transcript first into the oocyte from adjacent interconnected nurse cells, then across the length of the oocyte to its posterior pole. In the experiments described here, we identify the osk mRNA localization signal within the 3′ untranslated region (3′ UTR) of the transcript. We describe a systematic deletion analysis of the osk mRNA localization signal, which has allowed us to identify several elements affecting different steps in the process. This use of multiple cis-acting elements suggests that localization may be orchestrated in a combinatorial fashion, allowing localized mRNAs with ultimately different destinations to employ common mechanisms for shared intermediate steps. MATERIALS AND METHODS Fly strains w1118 flies were used as recipients for P-element-mediated transformation. Mutant strains for analysis were the following: capu2, spir1, stau2, osk2 and osk6 [all mutants are described in Lindsley and Zimm (1992)]. DNA constructions All DNA alterations were introduced by standard cloning procedures into a P-element transformation vector, pCaSpeR (Pirotta, 1988) containing a 9 kb fragment of osk previously shown to rescue the osk mutant body patterning defects in transgenic flies (Kim-Ha et al., 1991). In an initial set of constructs, the complete lacZ-coding sequence was fused to the osk-coding sequence at various positions, followed by the osk 3′ UTR. Unfortunately, transgenic flies bearing these constructs accumulated multiple hybrid osk/lacZ transcripts of different sizes. Northern blot analyses using probes from different regions of the the lacZ gene and osk 3′ UTR revealed that transcripts of less than the expected length were truncated within the lacZ sequences, and thus did not have the osk 3′ UTR sequences (data not shown). To avoid this problem, all subsequent constructs tagged with lacZ carried only the 5′ ~1.3 kb of lacZ-coding sequences, extending from a BamHI site (positioned immediately before codon 9) to the first internal MluI site (olc 3-5) or the first internal SspI site (olc1-2). Both sites, which are separated by only 72 bp, were first modified by addition of NotI linkers. In olc1 and olc5, this lacZ tag was introduced into osk at position 2471 [all nucleotide coordinates are from Kim-Ha et al. (1991)]. The same position defines the right (3′) deletion endpoints of olc2, olc3, olc4 and olc6. Left (5 ′) deletion endpoints are: olc2, 1310; olc3, 510; olc4 and olc6, 347. olc5 and olc7 are deleted for positions 2669-3397. In olc6 and olc8, the 3′-most portion of osk extending from position 3434 and including the polyadenylation signal is replaced with the polyadenylation signal of a Drosophila α-tubulin gene (Macdonald and Struhl, 1988). A series of deletions covering the final 80 bp of the coding region and the 3′ UTR of the osk gene, olc11-17 and olc21-29, were all derived from olc4 using the restriction enzyme sites indicated in Fig. 3. Nucleotide positions of these sites are as follows: StyI, 2471; EcoRI, 2669; DraI, 2793; PvuII, 2914; SphI, 3083; NdeI, 3153; SacII, 3231; PstI, 3342; EcoRI, 3397. NotI linkers were introduced to the right deletion endpoints of olc11 and olc21-24 to allow the fragments to be joined to the NotI site inserted at the end of the lacZ fragment. HindIII linkers were added to both the right and left deletion endpoints of olc12-17 and olc25-29 to join the fragments. Transgenic animals Transgenic fly strains were established by P-element transformation (Rubin and Spradling, 1982; Spradling and Rubin, 1982). Flies carrying constructs to be tested for rescue of the osk mutant were further characterized to identify the chromosome with the transgene. Rescue of the osk− maternal-effect lethality was tested for olc7 and olc8 by crossing into an osk6/osk6 background. Rescue was scored by the ability of the homozygous mutant flies to produce viable offspring. RNA analysis Total ovarian RNA was prepared and RNAse protection assays were performed as previously described (Macdonald et al., 1986), using probes that allow us to compare directly the transcript levels of endogenous osk and each transgene. Two to six independent lines were tested for each construct, with the exception of olc15, for which only one line was recovered. All lines transformed with the same construct were found to express the transgene at comparable levels. One to three lines for each construct were assayed for localization by in situ hybridization. Transcripts were detected by wholemount in situ hybridization to ovaries from flies homozygous for the transgene. The procedure was done according to the method of Tautz and Pfeifle (1989) with modifications previously described in Kim-Ha et al. (1991). The hybridization probe, an in vitro-transcribed antisense RNA complementary to the 1.3 kb lacZ insert, was made with digoxigenin-labeled UTP and hydrolyzed by incubation in 40 mM NaHCO3, 60 mM Na2CO3 at 60°C for 90 minutes. Samples were dehydrated in an ethanol series and mounted in Gary's Magic Mountant (Lawrence et al., 1986) for microscopy. Computer analysis Sequence comparisons and RNA secondary structure analysis were performed using the University of Wisconsin GCG programs, Version 7.1 (Genetics Computer Group, 1991). The nucleotide sequence of the 3′UTR of osk was compared with those of bicoid (Berleth et al., 1988), nanos (Wang and Lehmann, 1991), cyclinB (Whitfield et al., 1990), orb (Lantz et al., 1992), fs(1)K10 (Prost et al., 1988), pumilio (Macdonald, 1992a), yemanucleinα (AïtAhmed et al., 1987), BicaudalD (Wharton and Struhl, 1989), tudor (Golumbeski et al., 1991) and staufen (St. Johnston et al., 1991) using the programs COMPARE and BESTFIT. The secondary structure of the osk 3′UTR was analyzed using the program FOLDRNA to predict the lowest energy structure of the entire region and STEMLOOP to predict smaller regions of potential secondary structure. oskar mRNA localization elements RESULTS The 3 untranslated region of the osk mRNA contains a localization signal Within the Drosophila ovary, individual ovarioles contain a series of progressively older egg chambers. Each egg chamber consists of 15 nurse cells and a single oocyte, interconnected as a consequence of incomplete cytokinesis and surrounded by somatic follicle cells. Localization of osk mRNA occurs in a series of steps during the course of oogenesis (Kim-Ha et al., 1991; Ephrussi et al., 1991). First, osk mRNA is concentrated in the oocyte during early stages of oogenesis, when the oocyte is similar in size to each of the connected nurse cells [stages 1-6; stages are as indicated in King (1970)]. Then, as the oocyte begins to expand relative to the nurse cells, osk mRNA transiently accumulates at the anterior margin of the oocyte (stages 7-9). The anterior accumulation is accompanied by the appearance of osk mRNA at the posterior pole of the oocyte, and, as oogenesis progresses, the anterior localization disappears while posterior localization increases (stages 9-10). During stage 10, osk mRNA also accumulates in the nurse cells; this mRNA is apparently transferred into the oocyte together with the rest of the nurse cell contents in stage 11. Localized mRNAs typically have localization signals 171 within their transcripts. To define the osk localization signal, we generated transgenic fly strains expressing modified forms of the osk gene. In the first, olc1 (osk localization construct 1), the osk gene is ‘tagged’ by the addition of a foreign DNA sequence (~1.3 kb of the coding region of the E.coli lacZ gene). The tag allows us to follow transcripts of the transgene by in situ hybridization without simultaneous hybridization to the endogenous osk message. Localization of the olc1 mRNA is identical to that of the endogenous osk mRNA, both in oogenesis (data not shown; pattern is the same as that of olc4, Fig. 1B) and in embryogenesis (data not shown). To confirm that the normal mechanisms are being used to localize the olc1 mRNA, we also monitored its distribution in mutants defective in osk mRNA localization. In ovaries of flies homozygous for cappuccino, spire, staufen or osk mutations, olc1 transcripts behaved like those of the endogenous osk gene (data not shown). Therefore, addition of the foreign sequence tag does not significantly alter localization of the transgenic osk mRNA. Additional osk transgenes from the initial series map the mRNA localization signal to the 3′ UTR. olc2-olc6 are derived from olc1 by deleting portions of osk sequences (Fig. 1A, left panel); each was tested for mRNA localization by in situ hybridization. Progressive removal of almost all of the osk-coding region in olc2, olc3 and olc4 has no Fig. 1. Sequences necessary for osk mRNA localization are located in the 3′ UTR. (A) Constructs used to create transgenic fly strains expressing altered forms of the osk gene are shown in schematic form. 2,124 bp of the coding region and 925 bp of the 3′UTR were progressively removed. The transcribed portion of osk is shown as a heavy line, deletions are indicated by interruptions in the lines and the heterologous polyadenylation signal from tubulin is represented by a hatched box. Foreign DNA used to tag the mRNA is ~1.3 kb of the E. coli lacZ gene, shown as an inverted triangle at the site of insertion, near the 3′ end of the osk-coding region. Localization properties of the encoded transcripts during oogenesis are indicated by a plus or a minus; localization was monitored by in situ hybridization for olc1olc6, and by rescue of osk− mutant flies for olc7 and olc8. Failure to detect localized transcripts is not due to lack of expression, as shown in the right panel. Total ovarian RNA from olc1, olc5 or w1118 control flies carrying no transgene was probed for osk transcripts by an RNAase protection assay. The probe includes both lacZ and osk sequences, allowing the endogenous and transgene mRNAs to be distinguished and compared directly; the upper band corresponds to the transgenes and does not appear in the w1118 control, while the lower band corresponds to osk and appears in all samples. olc1 and olc5 mRNAs are present at similar levels. (B,C) Detection of transgenic mRNA distribution patterns in ovaries by in situ hybridization. Early stage egg chambers are at left, with egg chambers of increasing age displayed rightwards. Panel B shows the distribution of olc4 mRNA. Just as for the endogenous osk mRNA, the tagged olc4 mRNA is concentrated in the oocyte during early stages of oogenesis (left), transiently accumulates at the anterior margin of the oocyte as it begins to expand during mid oogenesis (center) and then becomes localized to the posterior pole of the oocyte during later stages (right). Similar results were obtained for olc1, olc2, olc3 and olc6 mRNAs in oogenesis (data not shown). Panel C shows the results of in situ hybridization to olc5 mRNA. No localized transcripts are detectable. Rarely, there is some weak transcript concentration within early stage oocytes. 172 J. Kim-Ha and others effect on osk localization up to at least stage 10B of oogenesis, as shown for the most extreme example, olc4, in Fig. 1B. Similarly, replacement of the extreme 3′ portion of the osk mRNA in olc6 with a heterologous polyadenylation signal also has no noticeable effect on localization. In contrast, olc5, which lacks a 728 bp portion of the 1,043 bp osk 3′ UTR, is severely impaired (Fig. 1C). The only vestige of localization is weak and inconsistent concentration in early stage oocytes; olc5 mRNA is never detected at the posterior pole of the oocyte. This effect is not due to altered levels of transgene expression, as olc1 and olc5 transcript levels are similar (Fig. 1A, right panel). Complementary results were obtained with two osk derivatives in which only the 3′ UTR was altered. The olc7 and olc8 transgenes retain the complete osk-coding region; function was tested by their ability to rescue the body patterning defects of an osk mutant. Again, replacement of the extreme 3′ portion of the osk mRNA with a heterologous polyadenylation signal has no noticeable effect on osk function (olc8), while deletion of a large portion of the 3′ UTR eliminates the ability to rescue (olc7). Although these functional assays provide no indication of the nature of the defect, the olc7 result is consistent with a defect in mRNA localization. Distributions of the tagged transgene mRNAs for olc1, olc4 and olc5 were also monitored in early stage embryos (data not shown). Transcripts of olc1 are restricted to the posterior pole, as is wild-type osk mRNA (Kim-Ha et al., 1991; Ephrussi et al., 1991), while olc5 transcripts remain unlocalized in embryos, as they are in ovaries. Curiously, however, olc4 transcripts are no longer found at the posterior, despite being localized normally in late stages of oogenesis. This result may be related to the low yet consistent levels of olc4 expression (Fig. 2), and is considered in more detail in the Discussion; however, as the loss of detectable localized olc4 message apparently occurs quite late in oogenesis, it does not affect our analysis of the cisacting elements controlling the initial localization of osk mRNA to the posterior pole of the oocyte. Mapping functional localization elements within the osk 3 UTR Additional transgenic deletion mutants were constructed to map more precisely the mRNA localization elements within the osk 3′ UTR (Fig. 3). All are derived from olc4, which is expressed at low levels (Fig. 2) but displays wild-type mRNA localization until the very late stages of oogenesis (Fig. 1B). The low level of expression may reveal modest defects in localization more readily than would the transgenes expressed at high levels. [Alternatively, it could be posited that transgenes expressed at high levels would overload the localization machinery and thereby reveal subtle defects in localization. However, in flies with four extra copies of the wild-type osk gene, obvious defects in mRNA localization are only rarely observed (Smith et al., 1992).] Among the mutants derived from olc4, olc11 through olc17 include adjacent and non-overlapping deletions that collectively eliminate most of the osk 3′ UTR. Mutants olc21 through olc29 have larger deletions that extend various distances from either end of the above region. All the deletion mutants retain the olc4 lacZ tag, allowing Fig. 2. The olc4 transgene is expressed at a low but constant level. Total ovarian RNA from flies carrying the indicated transgenes was subjected to an RNAase protection assay. The probe contains both lacZ and osk sequences, allowing a direct comparison of transcript levels. Transgene mRNAs are detected as two bands, as indicated, while the endogenous osk mRNA is represented by a single band. Each independent olc4 transgene is expressed at levels significantly and consistently lower than the endogenous osk gene and the other transgenes from the initial set, including olc3, which is most closely related. Notably, olc4 transcripts are localized correctly in ovaries. All of the later series of constructs are derived from olc4 and are expressed at similar low levels (data not shown). the distribution of hybrid transcripts to be followed by in situ hybridization. The expression levels of the transgenic constructs were determined by RNAse protection and all were found to be similar to that of the parent construct olc4 (data not shown). Analysis of the various mutants provides a striking result; different regions of the osk 3′ UTR are required for different phases of the localization process. In the sections below, we describe each type of defective localization pattern, progressing chronologically through the normal sequence of events. Mutants defective in the concentration of osk mRNA in the oocyte Among the smaller deletion mutants, olc15 and olc16 display the earliest defects in localization, lacking the strong concentration of osk mRNA in the oocyte during stages 16. Although some transcript accumulation in the oocyte can be detected, it is consistently less than for olc4. Moreover, unlike wild-type osk and olc4, transcripts of both mutants accumulate noticeably in all nurse cells at early stages (Fig. 4A). olc15 and olc16 therefore define a localization element or elements required for efficient movement of the transcript from the nurse cells to the oocyte. However, these two mutants do have somewhat different effects. At later stages of oogenesis, olc15 shows normal posterior localization of oskar mRNA localization elements 173 Fig. 3. Mutants for mapping of the osk localization signal and properties of their transcripts. All mutants derive from olc4 (Fig. 1A) and differ only in the region shown, which includes the final 80 bp of coding region (at left) and the 3′ UTR of the osk gene. Each transcript is represented by a heavy line, interrupted for deletions. Three patterns in osk localization are indicated; the first and last are normal events, with wild-type scored as plus. Minus indicates that the mutant is significantly impaired, although the step may not be completely eliminated. The middle step, prolonged anterior accumulation, does not occur in wild-type, where it is scored as minus. Plus indicates that this abnormal step occurs. Note that early defects may obscure later events. the modest level of transcripts which do enter the oocyte; in contrast, olc16 transcripts cannot be detected at the posterior pole (Fig. 4A, right). It is not clear if the late defect in olc16 localization is simply a consequence of severely impaired transport to the oocyte, or if olc16 is also specifically defective in localization to the posterior pole of the oocyte (see Discussion). Larger deletions that lack the olc16 region, olc25-29, also display an early defect in localization to the oocyte and do not support normal posterior localization (Fig. 3). Mutants defective in osk mRNA movement through the oocyte Two small deletion mutants are impaired in a normal intermediate step in localization, the accumulation of osk mRNA at the anterior margin of mid-stage oocytes. This pattern is typically very transient and is no longer detected in stage 10 egg chambers (see Fig. 1B). In contrast, the anterior accumulation of olc17 transcripts is both enhanced and prolonged (Fig. 4B). The defect is itself transient and olc17 mRNAs eventually become localized to the posterior pole (Fig. 4B). A similar but less pronounced effect is observed for olc13 (Fig. 3). The localization phenotype of both these mutant transcripts closely resembles that of wild-type osk mRNA in certain BicaudalD (BicD) mutants (Kim-Ha et al., 1991; Ephrussi et al., 1991), suggesting that localization elements disrupted by the olc17 and olc13 deletions may mediate a step in which BicD acts. Mutants defective in posterior localization of osk mRNA Mutants olc11 and olc12 do not strongly affect mRNA localization (Figs 3, 4C). However, when both regions are absent, in olc21, posterior localization at mid and late stages is greatly reduced. Some transcript accumulation at the anterior margin of the oocyte is present, but posteriorly localized transcripts can be detected only by extensive overdevelopment of the in situ hybridization detection reaction, and then only inconsistently. Despite this defect, movement of olc21 mRNA into the oocyte at early stages remains apparently normal (Fig. 4D). Thus, elements present within the deleted regions must play a major role in posterior localization within the oocyte, but are not required for either transport into the oocyte or the transient accumulation at its anterior margin in stages 8-9. Mutants lacking multiple localization elements Most of the mutants with larger deletions lack multiple localization elements involved in different steps. For each of the three general examples provided by our mutants, the results are as would be expected from the normal progression of the different steps. The first is the combination of deletions impairing movement to the oocyte (olc 15 and olc16) and displaying enhanced anterior accumulation of the transcripts (olc17 and olc13). Mutant olc25, which is effectively a combination of olc16 and olc17, behaves like the olc16 mutant alone; transcripts are not concentrated in the oocyte. Anterior accumulation is not observed, presumably because of the low level of transcripts within the oocyte. Additional larger mutations encompassing both elements (olc26-olc29) display similar phenotypes (Fig. 3). The mutants olc22 and olc23 have deletions of a pair of elements, which individually cause prolonged anterior accumulation (olc13) and impair posterior localization of the message within the oocyte (olc21). Transcripts of these mutants display the initial defect, strong anterior accumulation in mid-stage oocytes. In olc13 mutants, this defect is transient and transcripts eventually move to the posterior pole of the oocyte. In the larger olc22 and olc23 mutants, the anterior accumulation of transcripts remains transient, but there is no accompanying localization to the posterior pole. Finally, one large mutant, olc24, lacks localization elements involved in each of the three identified steps. Not surprisingly, olc24 transcripts are not localized at any stage (Fig. 3). 174 J. Kim-Ha and others Fig. 4. Transgenic mRNA distributions of representative mutants. In situ hybridizations are presented as in Fig. 1B and C. (A) olc16. This mutant is defective in early movement into the oocyte, and does not show subsequent localization to the posterior pole. (B) olc17. This mutant displays excessive transcript accumulation at the anterior margin of the oocyte. Similar results were obtained for olc13, as well as for larger deletion mutants which remove either element but not the elements necessary for efficient transport from the nurse cells into the oocyte (Fig. 3). (C,D) A demonstration of redundant elements in the olc11 and olc12 deleted regions. The transcript distribution for olc11 (C) and also for olc12 (not shown) is similar to wild-type. However, the olc21 mutant transcript (D), missing both the olc11 and the olc12 deleted regions, shows a severe defect in localization to the posterior pole of the oocyte; the prior transport from the nurse cells into the oocyte remains apparently normal. Apical nurse cell localization of hybrid osk transcripts For several of our osk hybrid gene constructs, including olc1, olc26, olc27 and olc28, a very small fraction of the egg chambers examined have a novel site of transcript accumulation, at apical regions of each of the nurse cells (Fig. 5A). This pattern was not observed for the wild-type osk mRNA in whole-mount ovary preparations (Kim-Ha et al., 1991; Ephrussi et al., 1991), but can be detected by in situ hybridizations to sectioned ovaries (N. Pokrywka and E. Stephenson, personal communication). Curiously, this pattern closely resembles a normal intermediate step in localization of bcd mRNA to the anterior pole of the oocyte [(Stephenson et al., 1988; St. Johnston et al., 1989); Fig. 5B]. Mutants that display this pattern all have the lacZ sequence tag, yet not all tagged transcripts are seen to be oskar mRNA localization elements Fig. 5. Apical localization of olc27 (A) and bcd (B) transcripts in nurse cells. Arrowheads indicate some of the apical regions where the transcripts are concentrated. Note that, with extended development of the in situ hybridization detection reaction, a very low level of posteriorly localized olc27 mRNA can be detected (see Discussion). apically localized. However, given the low frequency at which we detect this pattern, it is possible that all of our constructs behave similarly, but that the transient nature of this localization does not make it readily detectable. Perhaps osk mRNA normally passes very rapidly through the apical zones of the nurse cells in the course of localization and thus is not observed in whole-mount staining, whereas the movement of the hybrid transcripts is somewhat slowed down and thus can occasionally be detected. DISCUSSION Organization of mRNA localization elements A variety of localized mRNAs have been identified in Drosophila as well as in other systems. For example, actin mRNA is preferentially concentrated at the growing margins of cultured fibroblasts, a relatively simple localization pattern (Lawrence and Singer, 1986). More elaborate patterns of localization are seen for the localized maternal mRNAs of Xenopus and Drosophila (e.g. St. Johnston et al., 1989; Yisraeli et al., 1990; Kim-Ha et al., 1991; Ephrussi et al., 1991). Although patterns of localization range from simple to quite complex, all such mRNAs appear to contain cis-acting localization signals; presumably these signals are recognized and bound by proteins. Currently, the most extensively characterized cis-acting localization signals are from mRNAs with fairly elaborate localization patterns; all cis-acting elements examined thus far occupy large segments of RNA (Macdonald and Struhl, 1988; Mowry and Melton, 1992), suggesting that multiple proteins bind to the 175 signals. Because very little is known about fine structure within the large localization signals, we know little about how the multiple steps of localization are directed. One possibility is that the localization signal acts as a single indivisible unit to specify the entire process. Alternatively, complex localization signals might consist of multiple separate elements, each directing a particular step in the process. Such a modular organization could allow the combinatorial assembly of localization signals, with which different mRNAs would be directed along common pathways, but to different ultimate destinations. Our analysis of the osk localization signal provides strong support for the latter model. We find multiple distinct elements within the osk localization signal, with different elements responsible for different steps in the process. Furthermore, our results clearly demonstrate that at least some localization elements act independently. This feature supports the notion that the localization signal is indeed a composite of protein-binding sites, which need not function within a larger unit. Steps directed by any individual element may be comparable to the entire localization process for mRNAs with simple localization profiles. Consequently, what is learned about individual localization elements from complex localization signals may apply to the cis-acting signals of mRNAs with simple localization patterns, and vice versa. RNAs frequently have functionally significant secondary structure. While computer programs (see Materials and Methods) predict many possible stem-loop structures throughout the osk mRNA 3′UTR, we do not know which, if any, of these structures actually form. Our data do not support the idea of a single large secondary structure that is necessary for the proper presentation of the multiple binding sites, as we are able to delete large portions of the 3′ UTR (for example, olc21-23) and still retain some steps in the localization pathway. oskar localization elements and candidate binding factors Genes involved in osk mRNA localization have been identified by maternal effect mutants defective in body patterning. Mutations in the genes cappuccino, spire, staufen, BicD and even osk itself arrest or otherwise affect localization at different stages of the process (Kim-Ha et al., 1991; Ephrussi et al., 1991; Suter and Steward, 1991). Similarly, we have found that the deletion of different fragments of the osk 3′UTR affects different steps of localization. Mutations in a gene whose product interacts with one of the cis-localization elements identified here might be expected to affect localization of the endogenous osk mRNA in a way that mimics the loss of the element. Below, we describe the different localization elements that we have defined, and discuss possible factors that might interact with each one. The localization element(s) defined by transgenes olc15 and olc16 is defective in efficient transport of the transcript from the nurse cells into the oocyte. For one of these mutants, olc15, the defect is largely limited to the early stages of oogenesis. There is substantial olc15 transcript accumulation within later stage oocytes, such that some posteriorly localized mRNA can be detected. Several explanations are possible for the different behaviors of olc15 and olc16 176 J. Kim-Ha and others mRNAs. First, olc15 may simply be less severe than olc16. Secondly, there may be different requirements for transport from the nurse cells to the oocyte at different stages of oogenesis. Finally, olc16 may be deficient in two processes - transport into the oocyte and posterior localization within the oocyte - while olc15 is defective in only the first. What factors might interact with the olc15/olc16 elements? Mutants of egalitarian and certain BicD alleles do in fact block the early concentration of osk mRNA in the oocyte (Ephrussi et al., 1991; Suter and Steward, 1991). However, these mutations also cause an arrest of oogenesis at a very early stage (Mohler and Wieschaus, 1986; Schüpbach and Wieschaus, 1991), and such a general defect makes it difficult to determine if specific interactions between the encoded proteins and osk mRNA are likely. A second type of localization element is defined by the olc13 and olc17 transgenes. Deletion of either region results in a prolonged accumulation of the transcript at the anterior margin of the oocyte, prior to movement to the posterior pole. This anterior accumulation is normally a very transient intermediate in osk mRNA localization. Notably, a very similar phenotype of osk localization is associated with particular mutations in the BicD gene (Kim-Ha et al., 1991; Ephrussi et al., 1991). BicD encodes a putative coiled-coil myosin-related protein (Suter et al., 1989; Wharton and Struhl, 1989), but we do not know how BicD acts in the localization process. The BicD protein could contact osk mRNA directly in the regions defined by olc13 and/or olc17. Alternatively, BicD might act indirectly, perhaps by facilitating the transition between two types of movement: from the nurse cells to oocyte, and within the oocyte from the anterior site of entry to the final destination at the posterior pole. In any event, our results suggest that mutant BicD proteins that display this phenotype are simply not performing their normal function as well as they should. Previously, these BicD mutations have been thought to confer a novel activity on the encoded protein (Mohler and Wieschaus, 1986). Curiously, a number of genes can be mutated to exhibit a bicaudal phenotype and the appearance of this phenotype is frequently sensitive to the genetic background (Bull, 1966; Nüsslein-Volhard, 1977; Mohler and Wieschaus, 1986; Tearle and Nüsslein-Volhard, 1987). We suggest that in some or all of these cases the phenotype arises through modest and possibly transient defects in osk mRNA localization, which allow osk protein to accumulate in the anterior of the oocyte at levels sufficient to override normal anterior development. Mutants olc13 and olc17 produce a similar phenotype of enhanced anterior accumulation, yet have deletions distant from one another. This behavior might arise if both regions were involved in a shared secondary structure. Computer analysis predicts a double-stranded ‘stem’ which could form between bases 2901-2910 and 3373-3382, contained within the sequence elements defined by olc13 and olc17, respectively; the validity of this prediction in vivo is unknown. Alternatively, the olc13 and olc17 regions may contain separate binding sites for the same localization protein, presumably a protein that must interact with multiple sites for full function. A third localization element is defined by olc21, which is specifically defective in posterior localization within the oocyte. A trans-acting factor that might mediate the role of this region is stau (St. Johnston et al., 1991). Both stau mutants and olc21 result in an accumulation of mRNA at the anterior margin of the oocyte, although the stau effect is much more prominent (Kim-Ha et al., 1991; Ephrussi et al., 1991). In both cases, the accumulated mRNA eventually disperses, but does not continue to the posterior pole of the oocyte (as it does in olc13, olc17 or BicD mutants). The olc21 region clearly contains more than one localization element, as it can be deleted in two parts with no significant effect on localization of the transgenic mRNAs (olc11 and olc12). Redundancy can be invoked to explain this observation; redundant binding sites for the same localization protein may lie in each mRNA segment, or redundant mechanisms may employ different binding sites in adjacent regions of the mRNA. Wild-type osk mRNA must be actively maintained at the posterior pole of the oocyte during late stages of oogenesis. Such maintenance depends upon osk protein; in certain osk mutants, osk mRNA diffuses away from the posterior pole during late oogenesis and no localized transcripts can be detected in the embryo (Kim-Ha et al., 1991; Ephrussi et al., 1991). As described in the Results, transcripts of olc4 (upon which all other deletion constructs are based) are not detected at the posterior pole of early embryos. It is possible that olc4 lacks some of the sequences that mediate the osk protein-dependent maintenance of the mRNA at the posterior pole; if so, it cannot be lacking all of the elements required for this maintenance, as olc4 transcripts remain localized until at least stage 10B of oogenesis while, in the most extreme osk mutant, localization is lost by stage 9 (Kim-Ha et al., 1991; Ephrussi et al., 1991). Alternatively, little is known about the turnover rates and relative levels of localized osk message throughout oogenesis and early embryogenesis, and it is possible that the low starting levels of olc4 transcript are simply insufficient to be detected by early embryogenesis. It is notable that the assay used here to monitor localization, detection of a tagged transgenic mRNA by in situ hybridization, does not readily allow us to detect subtle differences in the levels of localized transcripts. Consequently, modest defects in localization will have been missed; for example, it is possible that both olc11 and olc12 do in fact have slight defects, but it is only when both deletions are combined in olc21 that we are able to detect a phenotype. We also note that the possibilities for redundancies in localization elements or mechanisms are substantial. In many of the deletion mutants that are defective in either transport into the oocyte or posterior localization, very low levels of correctly localized mRNAs can be detected by increasing the sensitivity of the in situ hybridization reaction. For example, a very low level of posteriorly localized olc27 mRNA, which lacks both olc15 and olc16 regions, can be detected with extended development of the in situ hybridization detection reaction (Fig. 5A). No striking repeated sequences, which might be expected for redundant binding sites, were found within the osk 3′UTR. However, RNA-binding proteins frequently recognize both structural and sequence elements and multiple copies of a binding site for one such protein might not be obvious from simple sequence comparisons. oskar mRNA localization elements Conservation of localization elements and localization mechanisms In Drosophila, a number of maternal mRNAs are known to be localized during oogenesis. How similar are the mechanisms used to localize these mRNAs? For osk mRNA, we find that at least some cis-acting elements can act independently of the entire localization signal, suggesting that they could also be used in partially localizing other mRNAs. Many localized transcripts, including osk, share a similar first step in localization, involving specific early movement into the oocyte from the nurse cells (Whitfield et al., 1989; Raff et al., 1990; St. Johnston et al., 1991; Golumbeski et al., 1991; Suter and Steward, 1991; Lantz et al., 1992; Cheung et al., 1992; Aït-Ahmed et al., 1992). Although there are differences in the timing of movement into the oocyte, some of these mRNAs might also use the localization element identified by olc15 and olc16. Similarly, the osk, fs(1)K10, and yemanucleinα mRNAs, which are all transiently concentrated at the anterior margin of the oocyte in a process requiring capu and spir, might share a localization element. Computer comparisons of the sequences of various localized mRNAs (see Materials and Methods) do not reveal any striking homologies. However, the sequences mediating localization of these other mRNAs are currently at best only roughly mapped (Cheung et al., 1992), and it is difficult to ask if cis-acting elements are in fact conserved. Our results identify regions of approximately 100-200 bases in the osk 3′UTR which contain RNA localization elements; if the elements themselves are small and/or contain variable nucleotides at some positions, we will need to know much more about the specific nature of the putative factor-binding sites before we can detect them in other RNAs using sequence comparisons. In addition to the possible conservation of cis-acting localization elements, there are also suggestions that basic mechanisms may be conserved. In particular, hybrid osk/lacZ transcripts from many of our transgenes transiently accumulate at apical regions of the nurse cells. As mentioned previously, these are sites where bcd mRNA normally accumulates during its movement to the anterior pole of the oocyte (Stephenson et al., 1988; St. Johnston et al., 1989). It may be that both osk and bcd mRNAs traffic through the same location, but that osk normally does so without interruption while bcd has a programmed pause. As this step in the localization of bcd mRNA requires microtubules (Pokrywka and Stephenson, 1991), which are themselves concentrated at the apical regions of the nurse cells (Theurkauf et al., 1992), movement of osk mRNA from the nurse cells to the oocyte may also rely on microtubules. Characterization of the cis-acting osk localization signal has revealed multiple elements that independently direct steps in the posterior localization of osk mRNA. Mapping the fine structure of these elements and identifying factors that bind them may ultimately allow us to elucidate mechanisms underlying not only the movement of osk, but also of many other localized mRNAs. We thank Trudi Schüpbach and Christianne Nüsslein-Volhard for fly stocks, Vince Pirotta for the CaSpeR transformation vector, and Andrew Leask, Robin Wharton and Joan Wilson for comments on the manuscript. Supported by a David and Lucile Packard Fel- 177 lowship (P. M. M.) and an NIH training grant #HD07249-11 (P. J. W.). P. M. M. is a PEW Scholar in the Biomedical Sciences. REFERENCES Aït-Ahmed, O., Bellon, B., Capri, M., Joblet, C. and Thomas-Delaage, M. (1992). The yemanuclein- : a new Drosophila DNA binding protein specific for the oocyte nucleus. Mech. Dev. 37, 69-80. Aït-Ahmed, O., Thomas-Cavallin, M. and Rosset, R. (1987). Isolation and characterization of a region of the Drosophila genome which contains a cluster of differentially expressed maternal genes (yema gene region). Dev. Biol. 122, 153-162. Barker, D. D., Wang, C., Moore, J., Dickinson, L. K. and Lehmann, R. (1992). Pumilio is essential for function but not for distribution of the Drosophila abdominal determinant nanos. Genes Dev. 6, 2312-2326. Berleth, T., Burri, M., Thoma, G., Bopp, D., Richstein, S., Frigerio, G., Noll, M. and Nüsslein-Volhard, C. (1988). The role of localization of bicoid RNA in organizing the anterior pattern of the Drosophila embryo. EMBO J. 7, 1749-1756. Bull, A. L. (1966). Bicaudal, a genetic factor which affects the polarity of the embryo in Drosophila melanogaster. J. Exp. Zool. 161, 221-242. Cheung, H.-K., Serano, T. L. and Cohen, R. S. (1992). Evidence for a highly selective RNA transport system and its role in establishing the dorsoventral axis of the Drosophila egg. Development 114, 653-661. Dalby, B. and Glover, D. M. (1992). 3′ non-translated sequences in Drosophila cyclin B transcripts direct posterior pole accumulation late in oogenesis and peri-nuclear association in syncytial embryos. Development 115, 989-997. Driever, W. and Nüsslein-Volhard, C. (1988). A gradient of bicoid protein in Drosophila embryos. Cell 54, 83-93. Ephrussi, A., Dickinson, L. K. and Lehmann, R. (1991). oskar organizes the germ plasm and directs localization of the posterior determinant nanos. Cell 66, 37-50. Gavis, E. R. and Lehmann, R. (1992). Localization of nanos RNA controls embryonic polarity. Cell 71, 301-313. Genetics Computer Group (1991). Program Manual for the GCG Package, Version 7. Golumbeski, G. S., Bardsley, A., Tax, F. and Boswell, R. E. (1991). tudor, a posterior-group gene of Drosophila melanogaster, encodes a novel protein and an mRNA localized during mid-oogenesis. Genes Dev. 5, 2060-2070. Gottlieb, E. (1990). Messenger RNA transport and localization. Current Opinion in Cell Biol. 2, 1080-1086. Kim-Ha, J., Smith, J. L. and Macdonald, P. M. (1991). oskar mRNA is localized to the posterior pole of the Drosophila ooctye. Cell 66, 23-35. King, R. C. (1970). Ovarian Development in Drosophila melanogaster. New York: Academic Press. Lantz, V., Ambrosio, L. and Schedl, P. (1992). The Drosophila orbgene is predicted to encode sex-specific germline RNA-binding proteins and has localized transcripts in ovaries and early embryos. Development 115, 7588. Lawrence, P. A., Johnston, P. and Morata, G. (1986) Methods of marking cells. In Drosophila: A Practical Approach (ed. D. B. Roberts), pp. 229242. Oxford: IRL Press. Lawrence, J. B. and Singer, R. H. (1986). Intracellular localization of messenger RNAs for cytoskeletal proteins. Cell 45, 407-415. Lehmann, R. and Nüsslein-Volhard, C. (1986). Abdominal segmentation, pole cell formation, and embryonic polarity require the localized activity of oskar, a maternal gene in Drosophila. Cell 47, 141-152. Lindsley, D. L. and Zimm, G. G. (1992). The Genome of Drosophila melanogaster. San Diego: Academic Press, Inc. Macdonald, P. M. (1992a). The Drosophila pumilio gene: an unusually long transcription unit and an unusual protein. Development 114, 221232. Macdonald, P. M. (1992b). The means to the ends: localization of maternal messenger RNAs. Sem. Dev. Biol. 3, 413-424. Macdonald, P. M., Ingham, P. and Struhl, G. (1986). Isolation, structure and expression of even-skipped: A second pair-rule gene of Drosophila containing a homeo box. Cell 47, 721-734. Macdonald, P. M. and Struhl, G. (1988). Cis-acting sequences responsible for anterior localization of bicoid mRNA in Drosophila embryos. Nature 336, 595-598. 178 J. Kim-Ha and others Mohler, J. and Wieschaus, E. F. (1986). Dominant maternal-effect mutations of Drosophila melanogaster causing the production of double abdomen embryos. Genetics 112, 803-822. Mowry, K. L. and Melton, D. A. (1992). Vegetal messenger RNA localization directed by a 340-nt RNA sequence element in Xenopus oocytes. Science 255, 991-994. Nüsslein-Volhard, C. (1977). Genetic analysis of pattern formation in the embryo of Drosophila melanogaster. Characterization of the maternaleffect mutant bicaudal. Roux' Arch. Dev. Biol. 183, 249-268. Pirotta, V. (1988). Vectors for P-mediated transformation in Drosophila. In Vectors: A Survey of Molecular Cloning Vectors and their Uses (ed. R. L. Rodriguez and D. T. Denhardt), pp. 437-456. Boston: Butterworths. Pokrywka, N. and Stephenson, E. C. (1991). Microtubules mediate the localization of bicoid mRNA during Drosophila oogenesis. Development 113, 55-66. Prost, E., Deryckere, F., Roos, C., Haenlin, M., Pantesco, V. and Mohier, E. (1988). Role of the oocyte nucleus in determination of the dorsoventral polarity of Drosophila as revealed by molecular analysis of the K10 gene. Genes Dev. 2, 891-900. Raff, J. W., Whitfield, W. G. F. and Glover, D. M. (1990). Two distinct mechanisms localise cyclin B transcripts in syncytial Drosophila embryos. Development 110, 1249-1261. Rubin, G. M. and Spradling, A. C. (1982). Genetic transformation of Drosophila with transposable element vectors. Science 218, 348-353. Schüpbach, T. and Wieschaus, E. (1991). Female sterile mutations on the second chromosome of Drosophila melanogaster. II. Mutations blocking oogenesis or altering egg morphology. Genetics 129, 1119-1136. Smith, J. L., Wilson, J. E. and Macdonald, P. M. (1992). Overexpression of oskar directs ectopic activaton of nanos and presumptive pole cell formation in Drosophila embryos. Cell 70, 849-859. Spradling, A. C. and Rubin, G. M. (1982). Transposition of cloned P elements into Drosophila germ line chromosomes. Science 218, 341347. St. Johnston, D., Beuchle, D. and Nüsslein-Volhard, C. (1991). staufen, a gene required to localize maternal RNAs in the Drosophila egg. Cell 66, 51-63. St. Johnston, D., Driever, W., Berleth, T., Richstein, S. and NüssleinVolhard, C. (1989). Multiple steps in the localization of bicoid RNA to the anterior pole of the Drosophila oocyte. Development 107 Supplement, 13-19. St. Johnston, D. and Nüsslein-Volhard, C. (1992). The origin of pattern and polarity in the Drosophila embryo. Cell 68, 201-219. Stephenson, E. C., Chao, Y. and Fackenthal, J. D. (1988). Molecular analysis of the swallow gene of Drosophila melanogaster. Genes Dev. 2, 1655-1665. Suter, B., Romberg, L. M. and Steward, R. (1989). Bicaudal-D, a Drosophila gene involved in developmental asymmetry: localized transcript accumulation in ovaries and sequence similarity to myosin heavy chain tail domains. Genes Dev. 3, 1957-1968. Suter, B. and Steward, R. (1991). Requirement for phosphorylation and localization of the bicaudal-D protein in Drosophila oocyte differentiation. Cell 67, 917-926. Tautz, D. and Pfeifle, C. (1989). A non radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals a translational control of the segementation gene hunchback. Chromosoma 98, 81-85. Tearle, R. and Nüsslein-Volhard, C. (1987). Tubingen mutants and stocklist. Dros. Inf. Ser. 66, 209-269. Theurkauf, W. E., Smiley, S., Wong, M. L. and Alberts, B. M. (1992). Reorganization of the cytoskeleton during Drosophila oogenesis: implications for axis specifications and intercellular transport. Development 115, 923-936. Wang, C. and Lehmann, R. (1991). Nanos is the localized posterior determinant in Drosophila. Cell 66, 637-647. Wharton, R. P. and Struhl, G. (1989). Structure of the Drosophila BicaudalD protein and its role in localizing the posterior determinant nanos. Cell 59, 881-892. Whitfield, W. G., Gonzalez, C., Maldanado-Codina, G. and Glover, D. M. (1990). The a- and b-type cyclins of Drosophila are accumulated and destroyed in temporally distinct events that define separable phases of the G2-M transition. EMBO J 9, 2563-2572. Whitfield, W. G. F., Gonzalez, C., Sanchez-Herrero, E. and Glover, D. M. (1989). Transcripts of one of two Drosophila cyclin genes become localised in pole cells during embryogenesis. Nature 338, 337-340. Yisraeli, J. K., Sokol, S. and Melton, D. A. (1990). A two-step model for the localization of maternal mRNA in Xenopus oocytes: Involvement of microtubules and microfilaments in the translocation and anchoring of Vg1 mRNA. Development 108, 289-298. (Accepted 28 May 1993)