* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download L10v02b_-_citric_acid_cycle.stamped_doc
Survey
Document related concepts
Fatty acid metabolism wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Radical (chemistry) wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Photosynthesis wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Metalloprotein wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Electron transport chain wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Microbial metabolism wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Transcript
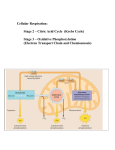
L10v02b_-_citric_acid_cycle [00:00:00.00] [00:00:00.69] SPEAKER 1: Hi there. In this video clip, we're going to continue our discussion on central metabolism. And we left off with acetyl CoA being produced from either sugar or fatty acids being present in the inner mitochondrial matrix and being just about ready to integrate into the citric acid cycle. [00:00:23.61] And here is the two carbon molecule of acetyl CoA being condensed with a four carbon molecule, oxaloacetate, forming a 6 carbon molecule of citric acid or citrate. And that's why this is called the citric acid cycle. It's also called, as I mentioned, the TCA cycle for tricarboxylic acid cycle or the Krebs cycle. Once again, we will not be memorizing structures for this course. But if you're taking the MCATS, you will need to memorize them, which includes the molecules not even written out here, but alpha-keto glutarate, succinate, fumarate, malate, and then finally, getting converted into oxaloacetate. [00:01:15.70] There are two steps where we lose carbon as carbon dioxide and five energy generating steps when we produce either NADH, or FADH2, or GTP, which although not as common as a ATP, is still used very often in energy requiring and regulatory processes. Now NADH and FADH2 both function as electron carriers. NADH has higher energy electrons. [00:01:53.74] There's not enough energy being produced between steps five and six to produce another molecule of NADH. And rather than throwing that energy away, cells are able to save that energy in the form of a less energetic molecule, FADH2. Although, FADH2 will not make as much ATP is NADH will. [00:02:20.12] Now like acetyl CoA, you will not need to memorize either of these two structures, NAD or FADH2. But I'd like to show it to you. This is the end part, the nicotinamide group. The adenine diphosphate group is, again, similar to what you saw for acetyl CoA, or it's similar to ATP. [00:02:43.67] And when you carry electrons, you're going to add a hydride ion here at this carbon. And the fact that you've added an extra electron in the form of hydride ion rather than hydrogen atom is manifested in the fact that the positively charged nitrogen here is now neutral and will stay so until a hydride ion is again lost when we do oxidative phosphorylation. You don't need to remember these structures. [00:03:14.14] Here's FADH2. In this case, we still have the ADP basic units and then a flavin ring. This is the F in the acronym. And we're going to add, in this case, two hydrogen across this unit and two electrons. So this is the reduced form. And this is the oxidized form. This is the more energetic form which can then donate electrons into the oxidative phosphorylation chain. [00:03:51.32] So here we have an overview of what we've covered today. Glycolysis out in the cytosol producing pyruvate from glucose molecules being transported into the inner mitochondrial matrix where pyruvate is converted to acetyl CoA. It's co-opted into the citric acid cycle in which the two carbon molecules present in acetyl CoA will be released as two molecules of carbon dioxide. [00:04:22.83] In the meantime, as we're doing that, we're making molecules of NADH and FADH2. NADH will introduce its higher energy electrons at the beginning of the electron transport chain. FADH, which is less energetic, will introduce its electrons further along the way. We'll get three molecules of a ATP for each NADH and approximately two ATP molecules for each molecule of FADH2. [00:04:54.86] And these electrons that are going to be present in the electron transport chain are finally going to end up on the molecule oxygen producing water-- this being, again, the reason that we need to breeze. This is why we die when we don't have oxygen is because the electrons get backed up in this chain, and we are not able to make sufficient ATP to meet basic metabolic needs. [00:05:21.84] These are the enzymes involved in this step. Cytochrome C oxidase is the protein to which cyanide binds, and that's why cyanide is a poison. So essentially, oxygen is a molecule we need as a place to dump electrons when we're done with them when they're not very energetic. [00:05:43.71] So in the last two slides, I want to go back to the process of anaerobic respiration, which does not involve the citric acid cycle or oxidative phosphorylation because you don't have oxygen present to accept the electrons. And as you recall when we're talking about glycolysis is NAD plus is a required molecule to put in as a reactant for the process to go from glucose to pyruvate. And if you don't have oxygen present, the only way you're going to get ATP is if you continue to break it down. So that means you need a constant supply of NAD plus. [00:06:32.53] The problem is every time you break down a molecule of glucose, you use up your supply of NAD plus, and you're making a lot of NADH, which you have in vast excess, and which is not that useful if there's not oxygen around. So what's going to happen in fermentation is you're going to take this pyruvate that you've made. You're going to take the electrons from NADH. [00:06:59.13] And you're going to produce lactic acid. And when this builds up in the muscles, that's when you get cramps. But since we've gotten rid of the electrons from NADH, we've regenerated the NAD plus, which in the absence of the oxygen is the valuable molecule of these two. [00:07:18.87] Now later on after the run is over and you have plenty of oxygen, the electrons that you've dumped onto lactic acid can be put back on NAD plus reforming an NADH. So you haven't lost it. And then NADH can go ahead and produce lots of ATP using oxidative phosphorylation. So in that sense, lactic acid becomes a temporary electron parking spot. [00:07:48.05] And bacteria have evolved a different strategy for the same problem of regenerating NAD plus for which we're all probably pretty grateful. Pyruvate gets converted to acetaldehyde, and then it accepts electrons from NADH producing ethanol. And this is the process which produces beers, wines, and other alcoholic beverages. [00:08:11.99] But the important point for-- and the ethanol is excreted because in high concentrations, ethanol is toxic to these organisms. But the good thing from their point of view is that you've re-generated NAD plus, allowing you to continue to break glycolysis-- glucose down to pyruvate producing the only ATP you're going to make in the absence of oxygen. OK, thanks.