* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download intracellular protein synthesis, post
SNARE (protein) wikipedia , lookup
Phosphorylation wikipedia , lookup
Cytokinesis wikipedia , lookup
Cell membrane wikipedia , lookup
Magnesium transporter wikipedia , lookup
Protein (nutrient) wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Protein structure prediction wikipedia , lookup
Endomembrane system wikipedia , lookup
Protein phosphorylation wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Protein moonlighting wikipedia , lookup
Signal transduction wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Chemical biology wikipedia , lookup
Protein mass spectrometry wikipedia , lookup
Protein–protein interaction wikipedia , lookup
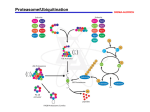
628s Biochemical SocietyTransactions( 1 996) 24 6 1 FUNCTION OF THE PROTEASOME IN PROTEIN TURNOVER AND ANTIGEN PRESENTATION. Alfred L. G o l d b u . Dept. of Cell Biology, Harvard Medical School, Boston, MA 02115, U.S.A. 63 INTRACELLULAR PROTEIN The proteasome is the primary site in cells for the complete degradation of cell proteins and for production of most antigenic peptides presented to the immune system on MHC-class I molecules. In this process, intracellular proteins are degraded to 8-9 residue fragments, are then transported into the ER, and become associated with class I molecules. 20s and 2 6 s proteasomes degrade proteins processively to oligopeptides of 7-11 residues in length. Selective inhibitors of proteasome function (peptide aldehyde or lactacystin) inhibit the degradation of short-lived and long-lived cell proteins and prevent the appearance of antigenic peptides on the cell surface. Mutations that block or promote ubiquitin conjugation prevent or stimulate antigen presentation. y-Interferon (y-IFN) induces new proteasome subunits, LMP2 and LMP7. Their incorporation in place of constitutive subunits increases cleavage of model peptides after basic and hydrophobic residues, and decreases after acidic residues. Transfections with LMP2 or LMP7 genes cause similar changes in peptidase activities. These changes should enhance production of peptides preferentially transported into the ER and bound to class I molecules and enhance immune surveillance. Brian F.C. Clark & Suresh I.S. Rattan Laboratory of Cellular Ageing Institute of Molecular and Structural Biology Aarhus University, 8000 Aarhus C, Denmark 62 BACTERIAL TOXINS AND CELL WEWBRANETRAFFIC Cesare MONTECUCCO Dipartimento d i Scienze Biomediche, U n i v e r s i t a d i Padova, V i a Trieste 75, PADOVA, ITALY C e l l membrane t r a f f i c i s a h i g h l y c o o r d i n a t e d phenomenon e s s e n t i a l for t h e d e l i v e r y and uptake of p r o t e i n s , l i p i d s and smaller molecules. B a c t e r i a produce a v a r i e t y of protein toxins t h a t interfere w i t h v i t a l a s p e c t s of c e l l physiology. It i s now emerging t h a t bacteria produce t o x i n s t h a t e n t e r s i n t o c e l l s and a f f e c t a selected s t e p of c e l l membrane t r a f f i c k i n g . Two examples w i l l be provided h e r e . 1. Some C l o s t r i d i a produce n e u r o t o x i n s , which are m e t a l l o p r o t e i n a s e s able to e n t e r i n t h e neuron c y t o s o l and r e c o g n i z e s p e c i f i c a l l y t h e t h r e e SNARE p r o t e i n s . These f i n d i n g s i d e n t i f y VAMP, SNAP-25 and s y n t a x i n as e s s e n t i a l components of t h e e x o c y t o s i s machinery and i n d i c a t e t h a t n e u r o t o x i n s are new t o o l s i n t h e s t u d y of e x o c y t o s i s . Examples of t h e u s e of t h e s e t o x i n s i n c e l l b i o l o g y and i n medicine w i l l be p r e s e n t e d . 2 . Toxigenic s t r a i n s of Helicobacter p y l o r i cause gastrointestinal ulcers and are a s s o c i a t e d to stomach adenocarcinomas. They produce vacA, a c y t o t o x i n t h a t induces t h e formation and growth of c e l l u l a r vacuoles t h a t f i l l t h e c e l l volume and t h e c e l l d i e s . W e have found t h a t t h e v a c u o l a r ATPase proton pump i s p r e s e n t on v a c u o l e s a n d . a c i d i f i e s t h e i r lumen. Evidence w i l l be p r e s e n t e d t h a t vacuoles o r i g i n a t e f r o m a rab7 c o n t r o l l e d homotypic fusion of late endosomal compartments, which t h e n s w e l l f o l l o w i n g t h e accumulation of ammonium i o n s , produced by h y d r o l y s i s of u r e a by t h e powerful H . p y l o r i urease. SYNTHESIS, POST-TRANSLATIONAL MODIFICATIONS AND AGEING Changes in the functioning of proteins during ageing can be due to inefficient and inaccurate protein synthesis, altered pattern of post-translational modifications, and defective pathways of protein turnover. Slowing-down of bulk protein synthesis is a widely recognized biochemical change with age. Elongation factors appear to play a crucial role in the regulation of protein synthesis during ageing. One of the reasons for the accumulation of abnormal proteins during ageing is post-synthetic modifications including oxidation of amino acids, deamidation, racemization, and spontaneous changes in protein conformation. The relation between protein synthesis, modifications and turnover, and ageing remains to be elucidated.