* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download PDF here
Multielectrode array wikipedia , lookup
Node of Ranvier wikipedia , lookup
Environmental enrichment wikipedia , lookup
Neurotransmitter wikipedia , lookup
End-plate potential wikipedia , lookup
Biochemistry of Alzheimer's disease wikipedia , lookup
Neural coding wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Single-unit recording wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Amyotrophic lateral sclerosis wikipedia , lookup
Caridoid escape reaction wikipedia , lookup
Mirror neuron wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Neuroregeneration wikipedia , lookup
Optogenetics wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Central pattern generator wikipedia , lookup
Development of the nervous system wikipedia , lookup
Neuroanatomy wikipedia , lookup
Embodied language processing wikipedia , lookup
Muscle memory wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Biological neuron model wikipedia , lookup
Axon guidance wikipedia , lookup
Neuromuscular junction wikipedia , lookup
Synaptogenesis wikipedia , lookup
Synaptic gating wikipedia , lookup
Nervous system network models wikipedia , lookup
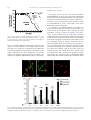
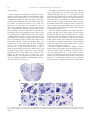
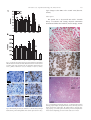
Experimental Neurology 185 (2004) 232 – 240 www.elsevier.com/locate/yexnr Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man Lindsey R. Fischer, a Deborah G. Culver, a Philip Tennant, a Albert A. Davis, a Minsheng Wang, a Amilcar Castellano-Sanchez, b Jaffar Khan, a Meraida A. Polak, a and Jonathan D. Glass a,b,* b a Department of Neurology, Emory University School of Medicine, Atlanta, GA 30322, USA Department of Pathology and Laboratory Medicine, Emory University School of Medicine, Atlanta, GA 30322, USA Received 21 August 2003; revised 12 September 2003; accepted 7 October 2003 Abstract The SOD1 mutant mouse is the most widely used model of human amyotrophic lateral sclerosis (ALS). To determine where and when the pathological changes of motor neuron disease begins, we performed a comprehensive spatiotemporal analysis of disease progression in SOD1G93A mice. Quantitative pathological analysis was performed in the same mice at multiple ages at neuromuscular junctions (NMJ), ventral roots, and spinal cord. In addition, a patient with sporadic ALS who died unexpectedly was examined at autopsy. Mice became clinically weak at 80 days and died at 131 F 5 days. At 47 days, 40% of end-plates were denervated whereas there was no evidence of ventral root or cell body loss. At 80 days, 60% of ventral root axons were lost but there was no loss of motor neurons. Motor neuron loss was well underway by 100 days. Microglial and astrocytic activation around motor neurons was not identified until after the onset of distal axon degeneration. Autopsy of the ALS patient demonstrated denervation and reinnervation changes in muscle but normal appearing motor neurons. We conclude that in this widely studied animal model of human ALS, and in this single human case, motor neuron pathology begins at the distal axon and proceeds in a ‘‘dying back’’ pattern. D 2003 Elsevier Inc. All rights reserved. Keywords: Sclerosis; Axonopathy; Denervation Introduction Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder characterized by progressive weakness leading to paralysis and death. Approximately 2% of cases are due to mutations in the superoxide dismutase (SOD1) gene. Transgenic mice carrying human SOD1 mutations develop progressive weakness similar to patients with ALS, making them useful models for understanding the pathogenesis of disease and testing new therapies. A widely used transgenic mouse model is the high-expressing SOD1G93A mutant that develops clinical disease at 80 – 90 days and dies at about 130 days (Chiu et al., 1995). This model has proven useful for examining the cellular pathology of motor neuron degeneration in ALS, and has provided a tool for developing * Corresponding author. Emory Center for Neurodegenerative Disease, Whitehead Biomedical Research Building, 5th Floor, 615 Michael Street, Mailstop 1941007001, Atlanta GA 30322. Fax: +1-404 727-3728. E-mail address: [email protected] (J.D. Glass). 0014-4886/$ - see front matter D 2003 Elsevier Inc. All rights reserved. doi:10.1016/j.expneurol.2003.10.004 preclinical data on drugs that may work to slow the course of ALS. There is a general acceptance that weakness and death in the SOD1G93A mutant mouse, and in humans with ALS, occur as a direct consequence of motor neuron death. There are studies, however, that have demonstrated dysfunction and/or degeneration of the neuromuscular junction (NMJ) at times earlier than are reported for the loss of motor neurons. Using immunocytochemical methods, Frey et al. (2000) showed selective loss of fast-firing neuromuscular synapses as early as day 50, and Kennel et al. (1996) reported progressive loss of motor unit numbers by physiologic measures beginning at day 40. Motor neurons were not counted in either of these studies, but reports from other investigators do not suggest a significant loss of motor neurons in the G93A mouse until after 80 – 90 days (Chiu et al., 1995). These data raise the question of where motor neuron dysfunction begins: within the motor neuron cell body, within the motor axon, or even at the level of the neuromuscular junction. Recent reports of abnormalities in retro- L.R. Fischer et al. / Experimental Neurology 185 (2004) 232–240 grade axonal transport leading to motor neuron death support a peripheral localization for factors initiating the onset of this disease (Hafezparast et al., 2003; LaMonte et al., 2002; Puls et al., 2003). Human studies of ALS do not provide an answer to this question, and no data are available correlating weakness or death with loss of spinal motor neurons. In order to better understand the progression of disease in the SOD1G93A mutant mouse, we undertook a systematic pathological study of these animals at multiple time points along the course of disease. In the same animals, we quantified the numbers of spinal motor neurons, axons in the nerve roots, and the degree of denervation at neuromuscular junctions, providing a sequential view of motor neuron pathology in these animals. Our findings demonstrate that before any loss of motor neurons, there is a severe loss of ventral root motor axons and significant denervation at corresponding neuromuscular junctions. Progression of abnormalities was from distal to proximal, indicating a ‘‘dying back’’ pathophysiology. In addition, we had the opportunity to study at autopsy a patient with sporadic ALS who died early and unexpectedly. His pathology also suggested a pattern of disease similar to our findings in the mouse. Materials and methods Animal breeding and behavioral analysis All animal protocols were approved by the Emory University Institutional Animal Care and Use Committee. Animals were housed in microisolator cages on a 12h light – dark cycle and given free access to food and water. Breeding pairs of G93A high-expressing mice were obtained from Jackson Laboratories (Bar Harbor, ME), and identification of mutant mice was by standard PCR analysis on tail snip DNA. Beginning at age 50 days, SOD-1 mutant mice (12 males and 12 females) were tested weekly for their ability to maintain balance on a Rotarod apparatus (Columbus Instruments, Ohio). Two protocols were used, constant velocity at 15 rpm and an accelerating paradigm of 1.4 + 4 rpm/min. Animals were tested three times during each session, and the best performance was recorded. Times were recorded to a maximum of 300 s. Weight was also recorded weekly. Age of death was defined as the time when animals could not right themselves within 30 s of being placed on their backs. Survival was 131 F 5 days; there were no differences in survival between males and females. Neuropathology A separate cohort of transgenic SOD-1 mutants and nontransgenic littermates (controls) were deeply anesthetized and killed by cardiac transection at 28 days (three mutants, 233 three controls), 47 days (three mutants, three controls), 80 days (four mutants, three controls), 100 days (five mutants, three controls), and 120 days (seven mutants, three controls). A group of six SOD-1 mutants was also analyzed at the time of natural death (131 F 5 days). Tissues were harvested and fixed appropriately for evaluation of endplates– neuromuscular junctions (NMJ), ventral roots, and lumbar motor neurons. End-plate –NMJ Medial gastrocnemius, soleus, and tibialis anterior muscles were dissected, pinned in mild stretch, and fixed by immersion for 20 min in 4% paraformaldehyde – PBS (pH 7.4). After rinsing in PBS, muscles were cryoprotected in 20% sucrose – PBS (overnight at 4jC) and flash-frozen in supercooled isopentane. Muscles were sectioned at 40 AM and placed on glass slides for staining. Sections were stained first with rhodomine bungarotoxin (1:40, Molecular Probes) for 30 min. After rinsing and postfixing in ice-cold methanol, sections were labeled with monoclonal antibodies to neurofilament (NF160 1:200, Chemicon) and SV2 (1:30, Iowa Developmental Hybridoma Bank). Secondary antibody was FITC-labeled goat anti-mouse (1:100, Jackson Immunoresearch). Stained sections were examined under a fluorescence microscope. End-plates (red) were scored as ‘‘innervated’’ if there was complete overlap with the axon terminal (green), or ‘‘denervated’’ if the end-plate was not associated with an axon. Some neuromuscular junctions were associated with a preterminal axon only, or showed partial overlap between end-plate and terminal. These were labeled as ‘‘intermediate’’. Each muscle was sectioned exhaustively so that all neuromuscular junctions could be evaluated. Mean counts for each group were compared by ANOVA using InStat software (GraphPad, San Diego, CA). Ventral roots Nerve roots were exposed and immersion-fixed in 5% buffered glutaraldehyde (pH 7.4) at 4jC for 48 h. Ventral roots from L-4 were dissected out and stored in 0.1 M phosphate buffer at 4jC. Tissue was treated with 1% osmium tetroxide for 90 min, dehydrated through graded alcohols, and embedded in Epon plastic (EM Sciences, Cincinnati, OH). Cross-sections (720 nm) were stained with toluidine blue, rinsed, and coverslipped. Nerve root sections were imaged at 100 magnification using a Leitz Dialux 22 microscope (Leica Microsystems, Germany), and individual frames were captured using an attached live-feed video camera (DAGE-MTI, Michigan City, IN). Multiple overlapping images were captured so that all axons were counted. Measurements of axon numbers and calibers were made with Image Pro software (Media Cybernetics, Silver Spring, MD) running on a Gateway personal computer (Gateway, San Diego, CA). Axon inte- 234 L.R. Fischer et al. / Experimental Neurology 185 (2004) 232–240 Lumbar motor neurons Fig. 1. Clinical analysis of motor neuron disease in SOD1G93A mice. Animal weight reported as % baseline and Rotarod performance expressed as % survival (note: ‘‘survival’’ here refers to the ability of the animal to remain on the Rotarod for the specified time). riors were visually identified and manually marked as solid objects. The mean diameter of each object was measured. The data were exported to a Microsoft Excel (Microsoft Corporation, Redmond, WA) spreadsheet for analysis. Axon caliber distributions for whole populations and specific subsets were analyzed using histogram plots. Means were compared by ANOVA with Dunnett’s post hoc comparison. The spinal cord was fixed in situ with 4% buffered paraformaldehyde for 48 h. The entire lumbar enlargement was then dissected, embedded in paraffin wax, and exhaustively cross-sectioned at 8 AM, six sections to a slide (123 F 12 slides or 5.90 F 0.58 mm of tissue per specimen). Tissue was deparaffinized in xylene, stained with cresyl violet, differentiated, and coverslipped. A systematic and unbiased sampling procedure was designed using stereologic principles to insure that every motor neuron in the lumbar spinal cord had an equal probability of being counted (Coggeshall and Lekan, 1996). A pilot counting study was done using an 80-day animal to determine the approximate number of sections that would need to be analyzed to count at least 100 –200 motor neurons for each spinal cord specimen. This sample size has been repeatedly shown to produce coefficients of error within reasonable limits for a biological system (West et al., 1991). Based on this pilot study, every 10th slide (a 480Am interval, amounting to 10 –14 slides counted per animal) was chosen for counting, with the first slide chosen by random selection. One ventral horn of one tissue section was analyzed per slide. Sections were imaged in overlapping frames (20 objective) using a Hamamatsu digital camera. Each Nissl-stained neuron in the ventral horn with a Fig. 2. NMJ immunocytochemistry. Motor endplates are identified with a-bungarotoxin (red); axons are identified with neurofilament + SV2 (green). Images depict (A) innervated endplates (yellow indicates overlap), (B) intermediate endplates, (C) denervated endplates, (D) quantitative analysis. Data are shown as % of endplates classified as innervated, denervated, or intermediate. Numbers in parenthesis indicate number of endplates quantified per time point. L.R. Fischer et al. / Experimental Neurology 185 (2004) 232–240 distinct nucleolus and a darkly stained cytoplasm was manually traced using Image Pro software. Area and mean diameter were recorded for each neuron. a-Motor neurons were defined as having cross-sectional area z250 Am2 (Drachman et al., 2002). Data were analyzed as both mean number of a-motor neurons per section and as total number of a-motor neurons per lumbar spinal cord, estimated using the fractionator method (Coggeshall and Lekan, 1996). This method takes into account the fractions used to select the sample to be counted, and then estimates the total population by multiplying back by those fractions. Means were compared by ANOVA with Tukey –Kramer post hoc comparison. 235 Human neuropathology Autopsy was performed approximately 24 h after death. The entire brain and spinal cord was examined. Microscopic sections were prepared from motor cortex, and from spinal cord and nerve roots from cervical, thoracic, lumbar, and sacral levels. Spinal cord sections were stained with H and E, luxol fast blue for myelin, and with GFAP and HAM-56 for astrocytes and microglia, respectively (Glass et al., 1995). Results Spinal cord immunocytochemistry SOD1 clinical evaluation Spinal cord sections adjacent to those used for counting were stained using standard techniques for astrocytes (GFAP 1:500, DAKO) and microglia (CD11b/Mac1 1:25, Serotec). Sections were developed using the Vectastain ABC kit (Vector Labs, Burlingame, CA), reacted with diaminobenzidine (DAB), and counterstained with hematoxylin. The first indication of clinical disease was decreased performance on the accelerating Rotarod at day 78. Performance on the constant Rotarod began to decline at day 85. Weight was not a sensitive measure of disease onset, and did not deviate from baseline until after 120 days (Fig. 1). Fig. 3. (A) Light microscope images of L4 ventral roots from SOD1G93A mice at the time points indicated. (B) Number of ventral root axons reported as % control. Number of animals examined at each time point in parentheses. (C) Small versus large axons from 80 days to death. Note relative increase in proportion of small caliber axons, indicating regeneration. Data are means F SEM. Numbers in parentheses indicate number of animals examined (** indicates P < 0.01 vs. control). 236 L.R. Fischer et al. / Experimental Neurology 185 (2004) 232–240 Neuropathology Initial evaluation of end-plates and ventral roots in 80day-old presymptomatic animals showed significant abnormalities in both, prompting us to look for the initial neuropathologic changes at earlier time points. At 28 days, virtually 100% of end-plates were innervated, and there was no evidence of axonal degeneration in the ventral root. Denervation of end-plates was significant by day 47 (40%) and continued to progress up to the time of death (Fig. 2). Neuromuscular junctions showing terminal axons but no end-plate overlap (‘‘intermediate’’) represented approximately 10% of the population between days 47 and 120, and dropped to minimal numbers at the time of death. Pathological changes in the ventral root were not seen until 80 days (Fig. 3). By day 80, the density of intact motor axons was markedly reduced, with the larger caliber axons preferentially affected. Actively degenerating fibers were recognized at all time points after 80 days, constituting 14.71 F 6.01% of total axons at day 80, 11.25 F 1.35% at day 100, 7.29 F 1.61% at day 120, and 6.95 F 1.60% at death. The total number of ventral root fibers, however, did not decrease after 80 days. In fact, there was a statistically significant increase in axon numbers at 120 days as compared to 80 and 100 days (Fig. 3B). There was also a marked increase in the proportion of small caliber axons (Fig. 3C), suggesting regeneration. Regenerating ‘‘clusters’’ of axons, however, were not seen. Vacuolation of large motor neuron cell bodies, indicating active degeneration, was first observed at day 80 and continued through death (Fig. 4). The numbers of large motor neurons did not decrease, however, until the 100-day time point. Quantitative analysis of a-motor neurons was performed both as mean number of a-motor neurons per section, and as an estimate of total neuron number using the fractionator method. Both methods yielded a similar outcome, showing a loss of approximately 40% of neurons with area z250 Am2 between days 80 and 100, when compared to littermate controls (Fig. 5). There was an apparent increase in the number of a-motor neurons with age in the control groups. Post hoc analysis demonstrated that the mean cross-sectional area of motor neurons increased from 181.82 Am2 at day 28 to 254.67 Am2 at day 120. Since size-exclusion (z250 Am2) was used to define a-motor neurons, this explains the increase with age in the number of neurons in this category. The total number of neurons counted in the control groups, however, did not change over time (data not shown). In order to indirectly examine the ‘‘health’’ of lumbar motor neurons at time points before frank degeneration, we stained the spinal cords with markers for activated astrocytes and microglia (Hall et al., 1998). Reactive astrocytosis at a level different from littermate controls was barely evident at 47 days and increased in intensity to the time of death (Fig. 6). Microglial activation was less prominent than astrocytosis at all stages and did not precede patho- Fig. 4. (A) Macroscopic view of lumbar spinal cord. Boxed area is region defined as ventral horn for motor neuron counting. (B) Representative images from the ventral horn of SOD1G93A animals at the ages indicated. Twenty-eight-day micrograph depicts outlining of neuron as seen with Image Pro analysis software. L.R. Fischer et al. / Experimental Neurology 185 (2004) 232–240 237 logic changes at the NMJ or the ventral roots (data not shown). Case report The patient was a 58-year-old man with a 6-month history of weakness and wasting. Physical examination demonstrated diffuse fasciculations, muscle atrophy, normal Fig. 5. (A) Number of a-motor neurons per section. Numbers in parentheses indicate number of animals examined. (B) Total number of a-motor neurons in lumbar spinal cord, estimated using the fractionator method. Data are means F SEM (** indicates P < 0.01, *** indicates P < 0.001 vs. control). Fig. 6. GFAP staining for astrocytes in SOD1G93A animals 28 days through death. Staining is first distinguishable from control spinal cord at 47 days. Fig. 7. Neuropathology from human ALS case. (A) H and E stained section showing grouped atrophy and angulated fibers (insert), indicating acute and chronic denervation – reinnervation. (B) ATPase (pH 4.2) showing fibertype grouping. (C) Lumbar spinal cord showing a normal complement of motor neurons. Morphology of motor neurons better seen in insert. 238 L.R. Fischer et al. / Experimental Neurology 185 (2004) 232–240 to brisk reflexes, and normal sensation. Forced vital capacity was 2.7 l (57% of predicted). Electromyographic examination showed changes consistent with acute and chronic denervation in upper and lower extremities, and in thoracic paraspinal muscles. Two weeks after this examination, he died unexpectedly during a minor surgical procedure. Neuropathological analysis (Fig. 7) demonstrated grouped atrophy and fiber-type grouping in skeletal muscles from the legs and thorax. Ventral roots at all levels showed postmortem autolytic changes but little axonal degeneration, and all levels of the spinal cord appeared normal. Astrocytic and microglial activations were not prominent at any level. Corticospinal tract degeneration was not noted, and motor cortex appeared normal. Discussion We examined the cell body, axon, and NMJ at multiple time points to illustrate the spatiotemporal progression of motor neuron pathology in the high-expressing SOD1G93A mutant mouse and related these findings to the clinical onset of weakness in these animals. As in previous studies, we first recognized signs of clinical disease at about 80 days, but there were significant pathological changes at much earlier time points. Quantitative analysis demonstrated denervation at the NMJ by day 47, followed by severe loss of motor axons from the ventral root between days 47 and 80, and loss of a-motor neuron cell bodies from the lumbar spinal cord after day 80. This pattern implies that motor neuron disease in the SOD1G93A mouse is actually a ‘‘dying back’’ motor neuropathy where distal axonal degeneration occurs early during the disease, before neuronal degeneration, and onset of symptoms. The human case, though anecdotal and non-quantified, showed a similar pattern of disease with prominent evidence of motor neuron degeneration only in muscle. The published neuropathology of human ALS is restricted to postmortem analysis of late stage disease (Bradley et al., 1983; Sobue et al., 1983). These types of analyses cannot be expected to identify the earliest morphological changes in the motor unit. Our quantitative analysis of the SOD1G93A suggested partial ‘‘recovery’’ at 120 days in the ventral roots and at end-plates. We believe the transient increase in innervated end-plates represents a phase of compensatory reinnervation, as was documented previously in the SOD1 mutant mouse as well as in humans with ALS (Bjornskov et al., 1984; Frey et al., 2000). In the ventral roots, the relative increase in the number of axons indicates regeneration. This finding is accentuated by the presence of actively degenerating axons at all stages between 80 days and death. Without regeneration, active and continuous axonal degeneration should result in reduction of axon numbers. In addition, the shift in axon diameters toward most small axons likely reflects the presence of regenerates. Early pathological changes in the periphery are perhaps a surprising finding in what has long been considered classic motor neuron disease. However, ‘‘dying back’’ or slowly evolving distal to proximal axonal degeneration is a common pattern seen in a wide variety of degenerative and toxic conditions of the central and peripheral nervous systems (Glass, 2002). The pathophysiology of distal axonal degeneration is poorly understood, but the longest and largest nerve fibers with the highest metabolic demand seem to be the most susceptible to ‘‘dying back’’. Thus, it has been suggested that distal axonal degeneration represents a sizedependent ‘‘undernourishment’’ of the most distal region of the axon (Cavanagh, 1964; Spencer and Schaumburg, 1976). Several animal models of motor neuron disease have been characterized as ‘‘dying back’’ neuropathies. In the mnd (motoneuron degeneration) and the pmn (progressive motor neuronopathy) mouse models, morphological analysis of the NMJ showed synaptic weakening and ‘‘dying back’’ before clinical manifestations of disease (Frey et al., 2000). Pathological analysis of the Smn mouse model of spinal muscular atrophy indicated NMJ abnormalities and a massive (78%) loss of ventral root axons at a time when the spinal cord showed only a 30% loss of motor neurons (Cifuentes-Dias et al., 2002). Analysis of the NMJ in dogs with hereditary canine spinal muscular atrophy (HCSMA) also revealed presymptomatic motor terminal dysfunction (Rich et al., 2002). Early synaptic changes were linked to deficits in quantal release of ACh before the actual degeneration of the motor terminal and the distal axon in this model. There are many possible explanations for the ‘‘dying back’’ pattern seen in the SOD1 mouse. As suspected for sensory neuropathies, this pattern might be due to a sublethal insult to the cell body that results in undernourishment of the distal axon. Accumulation of insoluble complexes of mutant SOD1 protein (Johnston et al., 2000) or chronic glutamate toxicity (Rothstein et al., 1993) could be responsible for insufficient maintenance of the distal axon, leading to early denervation at the NMJ while the cell body remains structurally intact. We investigated this issue by looking for markers of early astrocyte and microglial activation, hypothesizing that glial activation might be an indirect marker of abnormalities at the level of the spinal cord. We found some increased staining for GFAP at 47 days, but little evidence of microglial activation. Other investigators showed no increase in either microglial or astrocytic markers until 80 days in this model (Hall et al., 1998), long after NMJ and ventral roots are abnormal. Also, it is not clear that glial activation indicates local abnormalities in the motor neuron cell bodies. Peripheral axotomy clearly results in astrocytic and microglial activation around motor neurons (Graeber et al., 1988; Tetzlaff et al., 1986). There is a growing literature describing mechanisms of motor neuron degeneration that primarily involve defects in axonal structure or function. Accumulation of abnormal neurofilaments leading to slowing of anterograde axonal transport has been shown to be an early event in SOD1 mice (Williamson and Cleveland, 1999; Zhang et al., 1997). Disrupted anterograde transport may prevent components L.R. Fischer et al. / Experimental Neurology 185 (2004) 232–240 necessary for synaptic activity or trophic support of the axon from reaching the nerve terminal, potentially resulting in distal axonal degeneration (Griffin and Watson, 1988). More intriguing is recent evidence of a critical role for retrograde axonal transport in the maintenance of motor neurons. Transgenic mice overexpressing the dynamitin subunit of dynactin, a protein that regulates dynein activity, develop late-onset, progressive motor neuron disease due to the inhibition of retrograde transport (LaMonte et al., 2002). Furthermore, two mouse mutants generated by random mutagenesis and showing progressive motor neuron disease were found to have missense mutations in the cytoplasmic dynein heavy chain (Hafezparast et al., 2003). These mutations selectively inhibited fast retrograde transport in amotor neurons, resulting in neuronal loss and perinuclear inclusions positive for ubiquitin, SOD1, CDK5, and neurofilaments. In humans, a point mutation in the gene encoding the p150 subunit of dynactin has been identified in a family with a slowly progressive, autosomal dominant form of motor neuron disease (Puls et al., 2003). Therefore, in both mice and humans, axonal abnormalities that inhibit retrograde transport confer selective damage to motor neurons, possibly by preventing the delivery of target-derived neurotrophic factors back to the cell body. Neuronal and axonal degeneration are not necessarily linked, and in some situations, may occur by separate and independent mechanisms (Coleman and Perry, 2002). For example, Finn et al. (2000) showed that withdrawal of NGF from cultured neurons causes death of both the cell body and the axon, but only the cell body dies via caspase-mediated apoptosis. The Wlds (slow-Wallerian degeneration) mouse also provides several examples of how axonal degeneration (or survival) can occur in isolation of events at the neuronal cell body. When deprived of NGF, cultured Wlds sympathetic neurons exhibit neuronal apoptosis that is indistinguishable from wild-type neurons, while degeneration of neurites is selectively delayed (Deckwerth and Johnson, 1994). Experimental findings in the pmn model clearly show that primary axonal pathology may lead to a motor neuron disease phenotype. Pmn is due to a mutation in a tubulinspecific chaperone protein, affecting microtubule assembly in axons (Bommel et al., 2002; Martin et al., 2003). Crossing of the pmn mouse with the anti-apoptotic Bcl-2 overexpressing mouse rescued motor neuron cell bodies, but did not prevent axonal degeneration or prolong survival (Sagot et al., 1997). Conversely, crossing the pmn mouse with the Wlds mouse prevented axonal degeneration and resulted in extended survival from 40.5 to 62 days. The Wlds gene also delayed both retrograde transport deficits and synaptic pathology (Ferri et al., 2003). Moreover, the prevention of axonal degeneration in pmn by the Wlds gene resulted in prolonged survival of motor neurons, indicating that in some circumstances, loss of innervation may result in motor neuron death. These are perhaps the most compelling data supporting the importance of axonal degeneration in the pathogenesis of motor neuron diseases, as well as the idea 239 that axonal degeneration may somehow initiate the cell death program in the parent neuron. Treatments to rescue motor neurons according to the cell death (or ‘‘dying-forward’’) model of motor neuron pathology in fALS have shown only limited success in SOD1 and other mouse models (Sagot et al., 1997) as well as in humans (Carter et al., 2003). By separating the motor neuron from its target muscle, axonal degeneration may be the more important contributor to the progressive deterioration of motor function in fALS, therefore representing a very important and neglected therapeutic target (Coleman and Perry, 2002). Furthermore, if cell body degeneration is relatively late compared to axonal degeneration, early intervention at the first sign of motor symptoms could potentially prevent the subsequent irreversible loss of motor neurons. Acknowledgments This work was supported by NIH grant P01 NS40405(JDG) and by a grant from the Robert Packard Center for ALS Research at Johns Hopkins. We thank Dr. Mark Rich for helpful discussion and Raphael James, Karen Carney, and Dayna McDermott for technical assistance. References Bjornskov, E.K., Norris Jr., F.H., Mower-Kuby, J., 1984. Quantitative axon terminal and end-plate morphology in amyotrophic lateral sclerosis. Arch. Neurol. 41, 527 – 530. Bommel, H., Xie, G., Rossoll, W., Wiese, S., Jablonka, S., Boehm, T., Sendtner, M., 2002. Missense mutation in the tubulin-specific chaperone E (Tbce) gene in the mouse mutant progressive motor neuronopathy, a model of human motoneuron disease. J. Cell Biol. 159, 563 – 569. Bradley, W.G., Good, P., Rasool, C.G., Adelman, L.S., 1983. Morphometric and biochemical studies of peripheral nerves in amyotrophic lateral sclerosis. Ann. Neurol. 14, 267 – 277. Carter, G.T., Krivickas, L.S., Weydt, P., Weiss, M.D., Miller, R.G., 2003. Drug therapy for amyotrophic lateral sclerosis: where are we now? Investig. Drugs J. 6, 147 – 153. Cavanagh, J.B., 1964. The significance of the ‘‘dying back’’ process in human and experimental neurological diseases. Int. Rev. Exp. Pathol. 3, 219 – 267. Chiu, A.Y., Zhai, P., Dal Canto, M.C., Peters, T.M., Kwon, Y.W., Prattis, S.M., Gurney, M.E., 1995. Age-dependent penetrance of disease in a transgenic mouse model of familial amyotrophic lateral sclerosis. Mol. Cell. Neurosci. 6, 349 – 362. Cifuentes-Dias, C., Nicole, S., Velasco, M.E., Borra-Cebrian, C., Panazzo, C., Frugier, T., Millet, G., Roblot, N., Joshi, V., Melki, J., 2002. Neurofilament accumulation at the motor endplate and lack of axonal sprouting in a spinal muscular atrophy mouse model. Hum. Mol. Genet. 11, 1439 – 1447. Coggeshall, R.E., Lekan, H.A., 1996. Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J. Comp. Neurol. 364, 6 – 15. Coleman, M.P., Perry, V.H., 2002. Axon pathology in neurological disease: a neglected therapeutic target. Trends Neurosci. 25, 532 – 537. Deckwerth, T.L., Johnson Jr., E.M., 1994. Neurites can remain viable after destruction of the neuronal soma by programmed cell death (apoptosis). Dev. Biol. 165, 63 – 72. 240 L.R. Fischer et al. / Experimental Neurology 185 (2004) 232–240 Drachman, D.B., Frank, K., Dykes-Hoberg, M., Teismann, P., Almer, G., Przedborski, S., Rothstein, J.D., 2002. Cyclooxygenase 2 inhibition protects motor neurons and prolongs survival in a transgenic mouse model of ALS. Ann. Neurol. 52, 771 – 778. Ferri, A., Sanes, J.R., Coleman, M.P., Cunningham, J.M., Kato, A.C., 2003. Inhibiting axon degeneration and synapse loss attenuates apoptosis and disease progression in a mouse model of motoneuron disease. Curr. Biol. 13, 669 – 673. Finn, J.T., Weil, M., Archer, F., Siman, R., Srinivasan, A., Raff, M.C., 2000. Evidence that Wallerian degeneration and localized axon degeneration induced by local neurotrophin deprivation do not involve caspases. J. Neurosci. 20, 1333 – 1341. Frey, D., Schneider, C., Xu, L., Borg, J., Spooren, W., Caroni, P., 2000. Early and selective loss of neuromuscular synapse subtypes with low sprouting competence in motoneuron diseases. J. Neurosci. 20, 2534 – 2542. Glass, J.D., 2002. Pathophysiology of nerve and root disorders. In: McArthur, J. (Ed.), Diseases of the Nervous System: Clinical Neuroscience and Therapeutic Principles, vol. II. Cambridge Univ. Press, Cambridge, pp. 1075 – 1091. Glass, J.D., Fedor, H., Wesselingh, S.L., McArthur, J.C., 1995. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann. Neurol. 38, 755 – 762. Graeber, M.B., Streit, W.J., Kreutzberg, G.W., 1988. Axotomy of the rat facial nerve leads to increased CR3 complement receptor expression by activated microglial cells. J. Neurosci. Res. 21, 18 – 24. Griffin, J.W., Watson, D.F., 1988. Axonal transport in peripheral neuropathies. Ann. Neurol. 23, 3 – 13. Hafezparast, M., Klocke, R., Ruhrberg, C., Marquardt, A., Ahmad-Annuar, A., Bowen, S., Lalli, G., Witherden, A.S., Hummerich, H., Nicholson, S., Morgan, P.J., Oozageer, R., Priestley, J.V., Averill, S., King, V.R., Ball, S., Peters, J., Toda, T., Yamamoto, A., Hiraoka, Y., Augustin, M., Korthaus, D., Wattler, S., Wabnitz, P., Dickneite, C., Lampel, S., Boehme, F., Peraus, G., Popp, A., Rudelius, M., Schlegel, J., Fuchs, H., Hrabe de Angelis, M., Schiavo, G., Shima, D.T., Russ, A.P., Stumm, G., Martin, J.E., Fisher, E.M.C., 2003. Mutations in dynein link motor neuron degeneration to defects in retrograde transport. Science 300, 808 – 812. Hall, E.D., Oostveen, J.A., Gurney, M.E., 1998. Relationship of microglial and astrocytic activation to disease onset and progression in a transgenic model of familial ALS. Glia 23, 249 – 256. Johnston, J.A., Dalton, M.J., Gurney, M.E., Kopito, R.R., 2000. Formation of high molecular weight complexes of mutant Cu,Zn-superoxide dismutase in a mouse model for familial amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. U. S. A. 97, 12571 – 12576. Kennel, P.F., Finiels, F., Revah, F., Mallet, J., 1996. Neuromuscular func- tion impairment is not caused by motor neuron loss in FALS mice: an electromyographic study. NeuroReport 7, 1427 – 1431. LaMonte, B., Wallace, K.E., Holloway, B.A., Shelly, S.S., Ascano, J., Tokito, M., Van Winkle, T., Howland, D.S., Holzbaur, E.L.F., 2002. Disruption of dynein/dynactin inhibits axonal transport in motor neurons causing late-onset progressive deterioration. Neuron 34, 715 – 727. Martin, N., Jaubert, J., Gounon, P., Salido, E., Haase, G., Szatanik, M., Guenet, J.-L., 2003. A missense mutation in Tbce causes progressive motor neuronopathy in mice. Nat. Genet. 32, 443 – 447. Puls, I., Jonnakuty, C., LaMonte, B., Holzbaur, E.L.F., Tokito, M., Mann, E., Floeter, M.K., Bidus, K., Drayna, D., Oh, S.J., Brown, J., Robert, H., Ludlow, C.L., Fischbeck, K.H., 2003. Mutant dynactin in motor neuron disease. Nat. Genet. 33, 455 – 456. Rich, M.M., Wang, X., Cope, T.C., Pinter, M.J., 2002. Reduced neuromuscular quantal content with normal synaptic release time course and depression in canine motor neuron disease. J. Neurophysiol. 88, 3305 – 3314. Rothstein, J.D., Jin, L., Dykes-Hoberg, M., Kuncl, R.W., 1993. Chronic inhibition of glutamate uptake produces a model of slow neurotoxicity. Proc. Natl. Acad. Sci. U. S. A. 90, 6591 – 6595. Sagot, Y., Vejsada, R., Kato, A., 1997. Clinical and molecular aspects of motoneurone diseases: animal models, neurotrophic factors and Bcl-2 oncoprotein. Trends Pharmacol. Sci. 18, 330 – 337. Sobue, G., Hashizume, Y., Sahashi, K., Takahashi, A., Mukai, E., Matsuoka, Y., Mukoyama, M., 1983. Amyotrophic lateral sclerosis: lack of central chromatolytic response of motor neurocytons corresponding to active axonal degeneration. Arch. Neurol. 40, 306 – 309. Spencer, P.S., Schaumburg, H.H., 1976. Central – peripheral distal axonopathy—The pathogenesis of dying-back polyneuropathies. In: Zimmerman, H. (Ed.), Prog. Neuropathol., vol. 3. Grune & Stratton, New York, pp. 253 – 295. Tetzlaff, W., Graeger, M.B., Kreutzberg, G.W., 1986. Reaction of motoneurons and their microenvironment to axotomy. Exp. Brain Res. (Supp. 13), 3 – 8. West, M.J., Slomianka, L., Gundersen, H.J.G., 1991. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat. Rec. 231, 482 – 497. Williamson, T.L., Cleveland, D.W., 1999. Slowing of axonal transport is a very early event in the toxicity of ALS-linked SOD1 mutants to motor neurons. Nat. Neurosci. 2, 50 – 56. Zhang, B., Tu, P., Abtahian, F., Trojanowski, J.Q., Lee, V.M., 1997. Neurofilaments and orthograde transport are reduced in ventral root axons of transgenic mice that express human SOD1 with a G93A mutation. J. Cell Biol. 139, 1307 – 1315.