* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download آنفولانزا3
Survey
Document related concepts
Surround optical-fiber immunoassay wikipedia , lookup
Hepatitis C wikipedia , lookup
Ebola virus disease wikipedia , lookup
Diagnosis of HIV/AIDS wikipedia , lookup
Oesophagostomum wikipedia , lookup
Orthohantavirus wikipedia , lookup
Swine influenza wikipedia , lookup
West Nile fever wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Marburg virus disease wikipedia , lookup
Hepatitis B wikipedia , lookup
Henipavirus wikipedia , lookup
Middle East respiratory syndrome wikipedia , lookup
Herpes simplex virus wikipedia , lookup
Transcript
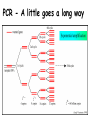
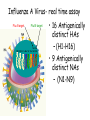
Role of the Laboratory in Diagnosis of Influenza during Seasonal Epidemics Ali rezvani PhD of molecular medicine accurate diagnosis of infection may thus be helpful not only for treatment-related decision making but also for prevention of person-to-person transmission in both hospitals and communities. Influenza Virus Type of nuclear material Neuraminidase Hemagglutinin A/Moscow/21/99 (H3N2) Virus type Geographic origin Strain number Year of isolation Virus subtype سروتایپ های انفوالنزا بر اساس تولید آنتی بادی علیه پروتئین های غشایی هماگلوتینن HAو نوروآمینیداز NAویروس انفوالنزا نوع B ،Aو Cبه سروتیپ های مختلفی تقسیم میشود که در این میان نوع Aدارای 16سروتیپ و نوع Bو Cتنها دارای یک نوع سروتایپ هستند ،سروتیپ های نوع Aشامل : H1N1, H1N2, H2N2, H3N2, H5N1, H7N2 ,H7N3, H9N2, H10N7 ,…. Specimen choice and collection Specimen quality limits test quality Pathogen detection depends on: –Appropriate collection site. –Proper timing of specimen collection. –Effective and timely processing of sufficient specimen Specimen storage and transport Keep specimens at 4ºC If delay >24hrs, freeze at 70ºC or below. Avoid any storage at -20ºC: greater loss in infectivity. In general, diagnostic tests can be grouped into 3 categories.: (1) direct detection, (2) indirect examination (virus isolation) (3) serology. Direct Examination 1. Antigen Detection immunofluorescence, ELISA etc. 2. Electron Microscopy morphology of virus particles immune electron microscopy 3. Light Microscopy histological appearance inclusion bodies 4. Viral Genome Detection hybridization with specific nucleic acid probes polymerase chain reaction (PCR) Indirect Examination 1. Cell Culture cytopathic effect (CPE) haemabsorption immunofluorescence 2. Eggs pocks on CAM haemagglutination inclusion bodies 3. Animals disease or death روش های تشخیص انفوالنزا شماری از روش های آزمایشگاهی برای شناسایی و تشخیص آنفوالنزا وجود دارد که از حساسیت و اختصاصیت های متفاوتی در افتراق آنفوالنزا نوع Aاز Bو Cو یا افتراق زیرگونه های نوع ( Aهمانند افتراق H1N1فصلی از H1N1جدید ) 2009برخوردار هستند این روش ها شامل : -1روش تشخیصی RIDTs 2روش ایمنوفلورسانس مستقیم DFAs3کشت ویروس Viral Culture4-روش ملکولی Real timeRT – PCR RIDTS ( (RAPID INFLUENZA DIAGNOSTIC TEST): این تست ها قابلیت ردیابی نوکلوپروتئین های ویروسی را دارند و از جمله ویژگی های آنها اختصاصیت % 95و اعالم نتیجه در کمترین مدت زمان است. این تست ها را بر اساس قابلیت افتراقی شان به سه دسته زیر تقسیم می کنند : -1تست های که آنفوالنزا نوع Aرا تشخیص می دهند. -2تست های که دو نوع Aو Bرا از یکدیگر تشخیص و افتراق می دهند -3تست های که آنفوالنزا نوع Aو Bرا تشخیص می دهند اما قابلیت افتراق این دو نوع را از یکدیگرندارند. DFAS ) (DIRECT IMMUNOFLUORESCENCE ASSAYS این روش نیز مانند روش RIDTsقابلیت تشخیص آنتی ژن ویروس را دارند و از جمله ویژگی آن اختصاصیت % 96و قابلیت تشخیص و افتراق دو نوع Aو B در مدت زمان 2-4ساعت دارد . معایب این دو روش: .1حساسیت RIDTsو DFAsدر ردیابی H1N1جدید در مقایسه با RT – PCRبترتیب ( )% 10-70و ( -93 )% 47است. .2به دلیل ایجاد پاسخ های منفی کاذب نتایج منفی آنها قابل اعتماد نیستند . .3کلیه نتایج منفی و گاها" نتایج مثبت تست های RIDTsو DFAsبایستی با RT – PCRتایید شوند . Cell Culture Viruses are obligate intracellular organisms – require living cells for virus isolation Advantages: – Relatively sensitive and specific – Can detect many different viruses – Provides a viral isolate for further characterization (serotyping, genotyping, susceptibility) Cell culture –limitations Certain viruses don’t grow or grow slowly Other techniques for detecting viral infection more cost effective Successful culture depends on viability of virus in specimen Serology Detection of rising titres of antibody between acute and convalescent stages of infection, or the detection of IgM in primary infection. Classical Techniques Newer Techniques 1. 2. 3. 4. 5. 1. 2. 3. 4. 5. Complement fixation tests (CFT) Haemagglutination inhibition tests Immunofluorescence techniques (IF) Neutralization tests Counter-immunoelectrophoresis Radioimmunoassay (RIA) Enzyme linked immunosorbent assay (EIA) Particle agglutination Western Blot (WB) RIBA, Line immunoassay Molecular Techniques Molecular techniques for the direct detection of viral genomes in the specimen will play an increasingly important role in the clinical virology laboratory in the 21st century. Why Expanding Role for Diagnostic molecular Lab Results in increasing demand for rapid methods, viral load testing, antiviral susceptibility, genotyping. Increased pool of immunocompromised Increasing antiviral agents Real Time PCR in Diagnosis *Quantitatively measurment of Human Immunodeficiency Virus (HIV). *Detection of Thalassemia, hemophilia,Sickle cell anemia & favism by real time PCR. *Cystic fibrosis. *Phenyl ketonuria. *Use in forensic medicine. *Noninvasive Prenatal Diagnosis by Analysis of Fetal DNA in Maternal Plasma. *Detection and Quantitation of Circulating Plasmodium falciparum DNA. * &… RT – PCR REAL TIME این روش که بر اساس تکثیر اسیدهای نوکلئیک قطعه ای از RNAژنومی استوار است ،.از اختصاصیت و حساسیت باالی برخورداراست و قابلیت تشخیص و افتراق انواع آنفوالنزا و زیر گونه های آن را از یکدیگر دارد . Why use a molecular test to diagnose an infectious disease? Need an accurate and timely diagnosis Important for initiating the proper treatment Important for preventing the spread of a contagious disease What are the advantages of using a molecular test? High sensitivity Can theoretically detect the presence of a single organism High specificity Can detect specific genotypes Can determine drug resistance Can predict virulence What are the advantages of using a molecular test? Speed Quicker than traditional culturing for certain organisms Simplicity Some assays are now automated Nucleic Acid Detection Short length of viral genome makes them ideal candidate for nucleic-acid based diagnosis PCR –conventional PCR – agarose gel detection of product –Real-time PCR- products detected using probes or intercalating dyes within the reaction. –Microarrays – chip or bead based. Reverse transcription polymerase chain reaction (RTPCR) • RT-PCR is a process whereby RNA is first converted to complementary DNA (cDNA) and a section of the genome is then amplified through the use of primers that bind specifically to this target area. • amplification of small amounts of nucleic acid which enables highly sensitive detection of minute amounts of viral genome. PCR - A little goes a long way Conventional PCR assay for subtyping influenza virus • • • • Based on amplification of HA target gene using specific primers Amplicon can then be used for sequence analysis for strain identification Requires gel analysis about about 2 days Controls Specimens HA1 HA3 Infl B Taqman technology for real-time PCR Very sensitive, very specific Turn around time about 6-8 hours from sample receipt Current Influenza A Taqman Assay From 10-2 to 10 -8 Cycle 97% Efficiency Y-intercept 35.4 R2 .995 Influenza A Virus- real time assay Flu A target Flu B target M1 NS1 PB1 PB2 PA HA NP NA M2 NS2 • 16 Antigenically distinct HAs – (H1-H16) • 9 Antigenically distinct NAs – (N1-N9) Factors Influencing Results of Molecular Assays • Influenza viral shedding in the upper respiratory tract generally declines substantially after 4 days • Patients with lower respiratory tract disease may have prolonged replication. • influenza viral Factors Influencing Results of Molecular Assays • Immunosuppressed patients and persons receiving systemic corticosteroids can also have prolonged influenza viral replication • Molecular tests can detect influenza viral RNA (positive results) for a longer duration than other influenza testing Factors Influencing Results of Molecular Assays o o Time from illness onset to collection of respiratory specimens for testing specimens should ideally be collected as early as possible (ideally less than 4 days after illness onset when influenza viral shedding is highest) Source of respiratory specimens The best upper respiratory tract specimens to detect influenza viral RNA by RT-PCR are nasopharyngeal swabs, washes or aspirates; other acceptable specimens are a nasal and/or throat swab. Respiratory specimens • Nasal washes and nasopharyngeal aspirates tend to be more sensitive than pharyngeal swabs. • NP wash or aspirate is generally considered the ‘gold standard’ for virus isolation. Swabs • swabs with a synthetic tip (e.g., polyester or Dacron®) and an aluminum or plastic shaft. • • Swabs with cotton tips and wooden shafts are not recommended. Respiratory specimens • in order to increase the potential for virus detection, multiple respiratory specimens from different sites should be obtained from the same patient on at least two consecutive days. Interpretation of Testing Results • even with RT-PCR, false negative results can occur due to improper or poor clinical specimen collection or from poor handling of a specimen after collection and before testing. • • A negative result can also occur by testing a specimen that was collected when the patient is no longer shedding detectable influenza virus. • False positive results, although rare, can occur (e.g., due to lab contamination or other factors). Negative result o For hospitalized patients, especially for patients with lower respiratory tract disease, if no other etiology is identified and influenza is still clinically suspected, additional specimens should be collected and tested,. Positive result o o o does not necessarily mean viable virus is present. a person who recently received intranasal administration of live attenuated influenza virus vaccine (LAIV) may indicate detection of vaccine virus. . Advantages of Molecular Assays likelihood of a false positive or false negative result is low and therefore, the The interpretation of the result is less impacted by the level of influenza activity in the community