* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Ectodermal Placodes: Contributions to the
Caridoid escape reaction wikipedia , lookup
Neural coding wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Neuroethology wikipedia , lookup
Artificial neural network wikipedia , lookup
Metastability in the brain wikipedia , lookup
Signal transduction wikipedia , lookup
Microneurography wikipedia , lookup
Synaptogenesis wikipedia , lookup
Types of artificial neural networks wikipedia , lookup
Subventricular zone wikipedia , lookup
Neuroregeneration wikipedia , lookup
Recurrent neural network wikipedia , lookup
Sensory substitution wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Nervous system network models wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Neuroanatomy wikipedia , lookup
Optogenetics wikipedia , lookup
Circumventricular organs wikipedia , lookup
Central pattern generator wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Neural engineering wikipedia , lookup
Channelrhodopsin wikipedia , lookup
AMER. ZOOL., 33:434-447 (1993)
Ectodermal Placodes: Contributions to the Development of the
Vertebrate Head1
JACQUELINE F. WEBB 2 A N D DREW M. NODEN
Department of Anatomy, N. Y.S. College of Veterinary Medicine,
Cornell University, Ithaca, New York 14853
SYNOPSIS. Neurogenic placodes are focal ectodermal thickenings that
give rise to the sensory neurons, and in some cases, the receptor cells of
vertebrate sensory systems. There are no markers for the identification
of undifferentiated placodal epithelia, but derivatives of the nasal placode,
for example, are characterized by unique production of GnRH and olfactory marker protein. Placode morphogenesis occurs by invagination and/
or delamination to form sensory epithelia, sensory neuroblasts and in
some cases, migratory receptor primordia {e.g., lateral line receptors).
Specification of neurogenic placodes and pattern formation of their derivatives has been a subject of study for over eighty years, and is still not
well understood, but, several genes have been implicated in pattern formation in the derivatives of the otic placode. The lateral line system is
unique among placode-derived sensory systems in vertebrates in that it
is only present in anamniotes, it is derived from multiple placodes, has
an extensive migratory component and gives rise to two classes of sensory
receptor organs that mediate two distinct sensory modalities (mechanoreception and electroreception) which share nervous innervation, but project independently to the hindbrain. Nasal and otic placodes, like other
epithelia are capable of inducing skeletogenesis in neural crest and mesodermal mesenchyme and thus via induction contribute to the morphogenesis of the vertebrate skull. The long-standing hypothesis that neuromast receptors induce the formation of the lateral line canals associated
with the dermal bones on the heads of fishes remains untested, but it is
evident that lateral line bones are composed of both dermal bone and
lateral line canal bone and may be subject to two discrete and potentially
conflicting sets of functional demands in the heads of fishes.
code, nasal placode, otic placode and four
epibranchial placodes (contributing to the
trigeminal, facial, glossopharyngeal and
va us
g
ganglia), and multiple lateral line
(dorsolateral) placodes present only in
anamniotes. Each of these placodes forms
in t h e sam
e relative position on the head
and each has a
similar fate in all vertebrate
groups that have been studied. Like the neur a l crest
> placodes produce sensory neurons
and glia, but they do not give rise to the
diversity of cell types derived from neural
1
From the Symposium on Development and Evo- crest (Noden, 1991). In addition, placodes
lution of the Vertebrate Head presented at the Annual are the precursors of Other unique cell types
Meeting of the American Society of Zoologists, 27-30 (egt sensory receptors) that contribute to
INTRODUCTION
Placodes were first described over a century ago by van Wijhe (1883) in shark
embryos. The term "placode" was introduced soon after by von Kupffer (1891)
based on his studies of lamprey embryos
and Platt's (1896) study of the development
of the lateral line system followed five years
later. Five types of placodes are found on
the heads of all vertebrates-the lens pla-
December, at Atlanta, Georgia.
th
Present Address of Jacqueline F. Webb is Department of Biology, Villanova University, Villanova, PA
19085.
nervous system OI most, II
OI the primitive cranial sensory systerns of vertebrates (Fig. 1). In addition to
2
O eriDhera1
m e
Penpnerai
n o t a11
434
n e r v o u s svstem of m o s t
if
435
CONTRIBUTIONS OF ECTODERMAL PLACODES
^^^HET
I
Invagination
MINIMI
I
Delamination
Illlllll
111 111IJ
A
I T
I I 1 1 111 *
Glia cell
Hair cell
cell
^Sensory neuron
^ f a c t o r y receptor
Migrating placodal cell
FIG. 1. Morphogenetic processes and fates of cephalic placodes in vertebrates.
their role as sources of sensory receptors,
associated supporting cells and peripheral
sensory neurons, several neurogenic placodes contribute indirectly to the morphogenesis of connective tissues of the vertebrate head by inducing the formation of
skeletal elements that enclose sensory structures and modify their functional attributes.
The lens placode, a prominent feature of the
heads of all vertebrates is the only welldefined cephalic placode that is not neurogenic. It undergoes invagination to form the
lens vesicle but cells never delaminate from
it and it retains strong epithelial characteristics. Many other ectodermal tissues
undergo early focal thickening {e.g., adenohypophysis, teeth, hair and feathers).
While these regions are frequently called
placodes, this paper will deal only with those
well-defined ectodermal regions that contribute to the morphogenesis of vertebrate
sensory systems.
MORPHOLOGICAL AND BIOCHEMICAL
CHARACTERISTICS OF PLACODES
Placodes are focal ectodermal thickenings
composed of columnar epithelial cells that
are the result of focal changes in the organization of the epithelium. They generally
have well delineated boundaries, although
some neurogenic placodes are diffuse and
multifocal {e.g., ophthalmic (trigeminal)
placode of avian embryos, Hamburger
[1961]). In mammals, the entire surface of
the embryonic head is covered by columnar
epithelium; placodes represent those regions
in which this morphology persists after the
surrounding epithelium has changed to a
squamous configuration (Verwoerd and van
Oostrom, 1979).
Prior to placode formation in avian
embryos the surface ectoderm of the head
undergoes extensive dorso-lateral expansion with the closure of the neural folds and
436
J. F. WEBB AND D. M. NODEN
formation of the rostral and lateral body
folds. This process shifts the precursors of
most of the placodes from a dorsolateral
position to a mid-lateral position where placodal thickening then occurs (D'AmicoMartel and Noden, 1983; Couly and Le
Douarin, 1990). The nasal and otic placodes
are exceptions in that both arise as extensions of the neural fold epithelium (Meier,
1978; Couly and Le Douarin, 1987). Unfortunately, there are no reliable markers for
the identification of undifferentiated placodal epithelia. The locations of undifferentiated placodes, shown in the avian
embryo in Fig. 2 have been determined by
vital staining, extirpation and transplantation analyses in fishes (Landacre, 1910),
amphibians (Stone, 1922) and birds (Hamburger, 1961; D'Amico-Martel and Noden,
1983). Some placodal derivatives, however,
can be identified by unique distributions of
gap junction proteins (Minkoff et al, 1991)
and cell adhesion molecules (Croucher and
Tickle, 1989; Richardson et al, 1987; Levi
et al, 1991). In addition, derivatives of the
nasal placode can be identified by the production of GnRH (gonadotropin releasing
hormone; Schwanzel-Fukuda and Pfaff,
1989,1991; Wray et al, 1989) and olfactory
marker protein (Farbman and Margolis,
1980; Graziadei et al, 1980).
PLACODE INDUCTION AND MORPHOGENESIS
Placodes arise in stereotyped positions on
the head as the result of inductive interactions with different regions of the neural tube
(Jacobson, 1966; Jacobson and Sater, 1988).
The initial morphogenesis of placodes is the
result of two fundamental processes, invagination and delamination, which may occur
alone or in combination to form the receptors and/or neurons of most of the peripheral sensory systems in vertebrates (Fig. 1).
Invagination, a simple infolding of the
surface epithelium produces a closed vesicle
{e.g., lens and otic placodes) or an expanded
cavity (nasal placode). Delamination is the
epithelial-mesenchymal transformation that
produces neuroblasts and in certain situations, glia and non-sensory supporting cells.
Placode-derived mesenchymal cells may differentiate locally to form sensory neurons
(e.g., epibranchial and otic placodes) and
sensory epithelia of complex sense organs
(e.g., olfactory and otic placodes) or may
actively migrate to other parts of the embryo
(e.g., lateral line placodes).
Nasal placode
The nasal placode arises as part of the
rostral margin of the neural tube (Couly and
Le Douarin, 1990). Classical transplantation and tissue recombination experiments
indicate that contacts between prospective
nasal epithelium and underlying endoderm
and chordamesoderm that occur during gastrulation and later interactions of the nasal
epithelium with the telencephalic primordia
all promote nasal placode differentiation
(Jacobson, 1966).
Recent studies have shown that the nasal
placode is the site of origin of a diverse
assemblage of cell types. Invaginated regions
of the nasal placode form the olfactory and
vomeronasal (Jacobson's organ) epithelia.
Olfactory neurons differentiate into primary
sensory receptors in situ without delamination and their axons project to the forebrain as the olfactory nerve (I). In addition,
terminal nerve neurons (nerve 0) also originate in the nasal placode (Northcutt and
Muske, 1991). Based on immunocytochemical studies, Schwanzel-Fukuda and Pfaff
([1989]; see also Wray et al. [1989]) proposed that neurons within the forebrain and
diencephalon that produce GnRH (gonadotropin releasing hormone) arise from the
nasal placode as a result of delamination
and migrate into the brain at or shortly after
olfactory nerve formation. Couly and Le
Douarin (1985) described the presence of
nasal placode-derived cells along the olfactory nerve which they believed represented
primordia of the unique myelin-producing
accessory cells associated with these nerves,
thus suggesting that the nasal placode is the
only placode that forms glia or Schwann
cells.
Otic placode
The otic placode arises adjacent to the
mid-myelencephalon (rhombomeres 5 and
6) shortly after the initiation of somite formation. After placode invagination, the otic
vesicle undergoes regionalized morphogenesis to form specialized epithelial structures
CONTRIBUTIONS OF ECTODERMAL PLACODES
(e.g., semicircular canals, basilar papilla) and
receptors (maculae, organ of Corti). Vestibular and auditory neurons associated with
the Vlllth ganglia arise as the result of
delamination of neuroblasts from the otic
vesicle (Sher, 1971). Transplantation and in
vitro studies have shown that proximity of
the otic placode to the middle region of the
hindbrain is necessary for placode invagination and early otic vesicle morphogenesis
(Stone, 1931; Detwiler, 1951; Noden and
Van der Water, 1986;Yntema, 1937,1939).
Once the otic vesicle has formed, additional
differentiation will occur independently of
further interactions; however, proper spatial organization fails to occur in ectopic
locations unless periotic mesenchyme surrounds the vesicle. Spatial organization of
other ear tissues (e.g., tympanic membrane)
is dependent upon properties inherent within
migrating periotic neural crest cells (Noden,
1983).
Recent analyses have identified the protooncogene int-2, as a necessary component
of the process of ear morphogenesis (Wilkinson et al., 1989; Represa et al., 1991).
However, the full elaboration of the membranous labryrinth, including hair cell
receptors and sensory neurons of the eighth
ganglion requires a series of subsequent
interactions that have yet to be characterized. Genetic analyses have identified two
genes whose expression is necessary for normal otic vesicle differentiation: Pax 3,
(Epstein et al., 1991), and in humans, the
2q37.3 locus (Tassabehji et al., 1992; Baldwin et al., 1992). In addition, transgenic mice
with partial disruption of the Hox 1.6 gene
also show defects in the inner ear and nearby
cranial sensory ganglia (Lufkin et al., 1991;
Chisaka et al., 1992), but its specific role in
ear development has not yet been demonstrated.
Epibranchial placodes
The proximity of the epibranchial placodes to the pharyngeal pouches led to the
hypothesis that these endodermal outpocketings induce the formation of the epibranchial placodes, but this has never been
experimentally substantiated. In addition,
some mandibular-maxillary and all ophthalmic placodal components of the trigem-
437
inal complex are not in proximity to any
pharyngeal pouch, thus raising a question
of the validity of this hypothesis. It has also
been suggested that migrating neural crest
cells initiate or influence placodal development. The presence of placode-derived
cranial sensory ganglia after extirpation of
the precursors of the neural crest contribution to that ganglion suggests that placodal neurons can form in the absence of
the neural crest in that region (Stone, 1923).
However, it is notoriously difficult to exclude
neural crest cells from moving into the region
from which they have been removed.
The four epibranchial placodes contribute to the sensory ganglia of the trigeminal
(V), facial (VII), glossopharyngeal (IX) and
vagus (X) nerves, respectively. In contrast
to the sensory neurons of the "special senses"
(olfaction, vestibulo-auditory, lateral line),
ganglia associated with the trigeminal, facial,
glossopharyngeal and vagus nerves receive
contributions from two distinct embryonic
sources: an epibranchial placode and a portion of the neural crest (Fig. 2; Hamburger,
1961; D'Amico-Martel and Noden, 1983).
The dual origin of the cranial sensory ganglia has been reported in all major vertebrate lineages (Landacre, 1910,1916; Stone,
1922; Knouff, 1927; Yntema, 1937; Hamburger, 1961; Altman and Bayer, 1982; Hiscock and Straznicky, 1986; reviewed in Verwoerd and van Oostrom, 1979). Neural
crest-derived cells are typically located in a
proximal (root) ganglion and the placodederived cells are typically located in a distal
ganglion. Among these there is considerable
variation in the relative contributions of
neural crest and placodal cells, and the
degree of fusion between neighboring proximal ganglia (e.g., proximal VII and VIII in
chick embryos) or between proximal and
distal ganglia of the same system (Fig. 2).
All of the glial cells in these systems are of
neural crest origin (Le Lievre and Le
Douarin, 1975; Noden, 1978a).
There have been many speculations concerning the reasons for the dual origin of the
cranial sensory ganglia, but none have been
satisfactorily demonstrated. The neurons
derived from these two sources are morphologically and biochemically distinct in
the embryo (Stone, 1922; Gaik and Farbman, 1973; Da vies, 1987) and have differ-
438
J. F. WEBB AND D. M. NODEN
NEURAL CREST
PLACODES
Trigeminal G. (V)
ophthalmic lobe
maxillo-mandibular lobe
Geniculate G.
(distal VII)
Root G. (prox. VII)
Vestibulo-cochlear G.
(VIM)
Petrosal G.
(distal IX)
SuperiorJugular G.
(prox. IX-X)
Nodose G.
(distal X)
FIG. 2. Distribution of placodes and neurogenic neural crest and their contribution to the cranial sensory ganglia
in a chick embryo. The lens placode, which is not neurogenic, and the neurogenic nasal placode, which does
not contribute to a cranial sensory ganglion, are indicated for reference.
ent responses to nerve growth factor (Ebendahl and Hedlund, 1975; Davies and
Lumsden, 1983) and brain-derived neurotrophic factor (Lindsay et al, 1986; Davies
et al, 1986), but they are indistinguishable
except by position in adult ganglia. It has
been suggested that neural crest- and placode-derived neurons have different
peripheral projections associated with different (e.g., somatic versus visceral) functional modalities centrally (Landacre, 1910;
see Stone, 1922 for discussion). Recently,
detailed axonal mapping studies in both
embryonic and adult systems have disproved this hypothesis (Noden, 1980a, b;
Covell and Noden, 1989). Alternatively,
Noden (1991) has suggested that vertebrates
retained the population of neurons of placodal origin not because they have a distinct
function in adults, but because placodal
neurons differentiate early establishing
peripheral and central projections before
neural crest cells initiate axonogenesis
(Covell and Noden, 1989) and thus serve
an important role in establishing neuronal
tracks used by neural crest-derived neurons.
Furthermore, in the absence of placodal
neurons, cranial neural crest-derived sensory neurons are unable to establish peripheral projections (Hamburger, 1961; Noden,
1978&) and motor neuron populations fail
to undergo their normal migratory reorganization (Moody and Heaton, 1983). Thus,
placodal neurons serve a necessary but transient role in the assembly of cranial sensory
ganglia; ironically this is the same role filled
by a subpopulation of neural crest-derived
neurons in the trunk.
CONTRIBUTIONS OF ECTODERMAL PLACODES
Lateral line placodes
The auditory and lateral line systems of
fishes and amphibians have traditionally
been considered to be two components of a
single acousticolateralis system. However,
it has been firmly established that these two
systems are developmentally and neuroanatomically distinct arising from separate
placodes, and forming discrete cranial ganglia and nerves that have unique peripheral
innervation and central projection patterns
(Stone, 1922; McCormick, 1983; Song and
Northcutt, 1991*; Northcutt, 1992).
In contrast to the other placode-derived
cranial sensory systems, the lateral line system is derived from multiple placodes (at
least 6, primitively) which generally differentiate into a corresponding number of sensory ganglia (Northcutt, 1992) and migratory receptor precursor cell populations
(Stone, 1922; Landacre, 1927). Receptor
primordia migrate resulting in species-specific patterns of receptor distributions on
the head, trunk and tail. Individual primordia may give rise to both mechanoreceptor neuromasts and ampullary electroreceptors in amphibians (Northcutt, 1992)
which share innervation by individual nerve
branches. Each sensory neuron synapses
with a single receptor cell in either a mechanoreceptor or electroreceptor and has a
discrete central projection based on that
peripheral innervation (medial and dorsal
octavolateralis nuclei, respectively, McCormick, 1983).
The induction and specification of lateral
line placodes in amphibians was the subject
of much interest early in this century (Stone,
1922). In amphibians, the induction of the
lateral line placodes has not been attributed
to the influences of any part of the neural
tube although they arise in stereotyped locations in the pre- and post-otic regions of
ectoderm lateral to the neural tube (Stone,
1922). Efforts to identify inductive influences in the lateral line system (comparable
to the induction of the otic placode, for
example) would be complicated by the fact
that unlike the other placodes which were
fixed in number with the origin of vertebrates, there is considerable variation in the
number of lateral line placodes among the
439
fishes and amphibians in which they have
been identified (Northcutt, 1992).
During embryogenesis in amphibians,
each lateral line placode first acquires a
directional polarity that determines the
identity of its ganglionic and migratory
receptor components (Stone, 1924) and the
lateral line and epibranchial placodes only
become non-equivalent later (Stone, 1928a).
The pre-otic and post-otic lateral line placodes, which will give rise to the lateral line
systems of the head and trunk, respectively,
can still be interchanged if the orientation
of the placode with reference to direction of
the migration of the receptor primordia is
maintained (Stone, 19286). Lateral line
neurons arising from a transplanted graft
will only innervate lateral line receptors
arising from that graft indicating that neurons and receptor precursors may be specified with respect to one another (Stone,
1929a). This could provide an explanation
for the phenomenon of comigration of neurites and receptor precursors documented
in the posterior lateral line system of trunk
of the teleost Brachydanio (Metcalfe, 1985).
PATTERN FORMATION OF MIGRATORY
RECEPTOR PRIMORDIA
Explanations for the widespread distribution of placode-derived lateral line receptors on the head and trunk of fishes and
amphibians have been sought for over a
century. There is sufficient evidence in elasmobranchs (Johnson, 1917), amphibians
(Stone, 1922; Landacre, 1927), and lungfishes (Pehrson, 1949) to show that receptor
precursor cells originate in the lateral line
placodes on the head and actively migrate
(via "placode elongation") along stereotyped paths depositing small populations of
receptor precursor cells at intervals which
then differentiate thus establishing receptor
distribution on the head and trunk. The
source of patterning information has been
sought in two studies which offer conflicting
explanations. Stone (1938) showed that the
species-specific pattern of neuromast distribution of an amphibian donor is retained
after transplantation of a graft into a host
thus indicating that patterning information
is contained in the migrating primordium.
More recently, Smith et al. (1990) showed
440
J. F. WEBB AND D. M. NODEN
that spatial patterning of neuromast receptors is determined by environmental factors
arising from the migration path taken. Further, it has been suggested that the neural
crest (which migrates much earlier) plays a
role in establishing the pattern of lateral line
migration (Horstadius, 1950; Graveson et
al., 1991).
Lateral line placodes have been identified
in a number of basal (nonteleost) actinopterygian fishes (Lepisosteus, Landacre and
Conger, 1913; Landacre, 1926;Disler, 1971;
Polyodon Bemis and Northcutt, personal
communication). Among teleosts, elongating or migrating primordia have been identified on the head and trunk of Salmo
(Wilson and Mattocks, 1897), Ameiurus
{Ictalurus) (Landacre, 1910) and other
ostariophysans (Lekander, 1949; Disler,
1971). Interestingly, placodes have been
observed only on the trunk but not on the
head of Brachydanio (Metcalfe, 1985) and
Eigenmannia (Vischer, 1989a, b). The
experimental examination of both the placodal origin and eventual fate of putative
lateral line receptor primordia has never
been accomplished in any actinopterygian
fish.
The complex distribution patterns of neuromasts in actinopterygian fishes, and in
teleost fishes in particular (Webb, 1989a)
presents a challenge for understanding how
receptor distributions are determined. Multiple, extensive and diffuse placodal migration paths would have to be demonstrated
to account for the complex distribution patterns of neuromasts found in some teleost
fishes. Alternatively, nerve induction of
receptor differentiation from epithelium can
more easily explain widespread and complex receptor distributions. This model
would require that spatial patterning of
peripheral receptors is directly determined
by spatial patterning of outgrowing lateral
line nerves, but does not preclude the presence of a diffuse population of placodederived stem cells which require neuronal
contact for differentiation. Nerve patterning
would have to be established before receptors differentiate; the mechanism or mechanisms controlling patterning of peripheral
sensory nerves remain unknown.
Descriptive accounts of lateral line devel-
opment in teleosts indicate that neuromasts
may in fact, fall into two classes arising as
a result of placodal differentiation or nerve
induction from epithelium in situ. Both
Lekander (1949) and Disler (1971) recognized that canal neuromasts and their
superficial neuromast homologues in related
taxa ("primary neuromasts") arise from
epithelial primordia that presumably
migrate from the lateral line placodes of the
head (as in amphibia), but that other superficial neuromasts ("secondary neuromasts")
appear to arise in situ during post-larval or
juvenile development without evidence of
a placode-derived primordium, and are presumably under the inductive influence of
neurons of the lateral line nerves. Nerve
induction has been suggested as the mechanism for both neuromast and electroreceptor differentiation in Eigenmannia (Vischer,
1989a) based on the apparent absence of
placodes, the absence of a distinct spatial or
linear sequence of receptor differentiation
(which could presumably indicate the presence of a wave of differentiation accompanying migration) and the fact that nerve outgrowth to the epithelium precedes receptor
differentiation (Vischer et al, 1989).
The direct experimental test of the nerve
induction hypothesis requires the manipulation of nerve patterning during initial
axonal outgrowth that would presumably
alter the location of differentiating neuromasts arising via induction from epithelium. Experimental data on the role of neuronal induction in receptor differentiation
and maintenance stem from the literature
on regeneration of taste buds and other
receptors in post-larval fishes and amphibians (see Bever and Borgen, 1991 for review).
Only a few studies use the lateral line system
to examine these questions. Bailey (1937)
determined that neuromast maintenance
and regeneration in Carassius and Ictalurus
is dependent on the presence of a lateral line
nerve and that deflection of the nerve results
in subsequent neuromast differentiation in
the area of the deflected nerve ending. In
similar experiments, Roth (1986) demonstrated that nerve deflection results in a corresponding displacement of electroreceptor
differentiation in Krytopterus. Interestingly,
Bailey (1937) also showed that in Carassius
441
CONTRIBUTIONS OF ECTODERMAL PLACODES
-Nasal pit
Nasal capsule
Prechordal cartilage-
-Optic vesicle
r
-Lens
ScleraNEURALCREST
MESODERM
Adenohypophysis
Neurocranium
Otic capsule
Parachordal
cartilage
Otic vesicle
Somite
Notochord
FIG. 3. Dorsal view of the components of the chondrocranium of a typical vertebrate A) early during formation
of individual cartilaginous elements and B) later during chondrocranial morphogenesis as cartilaginous elements
get integrated into the chondrocranium. Dark shading represents neural crest and it derivatives, light shading
indicates mesodermal mesenchyme and its derivatives and stippled areas represent skeletal elements or their
anlage.
neuromasts would only regenerate from epithelium lining the canal adjacent to the site
of canal and receptor ablation. This suggests
that the epithelium lining the canal is specialized and may be derived from a continuous migrating primordium. Most recently,
Bever and Borgens (1991) showed that electroreceptors in the catfish Krytopterus can
be induced from epithelium that normally
does not differentiate into electroreceptors
if this epithelium is transplanted into an
electroreceptor-rich region thus supporting
the nerve induction hypothesis. Clearly, the
roles of placode morphogenesis and migration and nerve induction in pattern formation of the mechanoreceptive and electroreceptive lateral line systems need further
study.
CONTRIBUTIONS TO THE VERTEBRATE
SKULL VIA INDUCTIVE INTERACTIONS
Epithelia are involved in the induction of
skeletogenesis in many vertebrate systems
(Hall, 1981; Hall and van Exan, 1982; Pinto
and Hall, 1991). Placodes are essentially
specialized regions of epithelia and also play
a role in skeletogenesis as inducers of elements in the vertebrate skull that are asso-
ciated with placode derived components of
various sensory systems. For instance, after
invagination the nasal and otic placodes
directly induce the differentiation of the the
nasal and otic cartilages that surround the
olfactory organs and inner ears, which subsequently ossify and become incorporated
into the neurocranium (Corsin, 1971; Yntema, 1933;FrenzandVanDeWater, 1991).
Thus, neurogenic placodes are capable of
inducing the differentiation of cartilage from
both neural crest cells (nasal capsule) and
from mesoderm (otic capsule) (Fig. 3).
The lateral line canals of the head and
trunk of bony fishes are also thought to differentiate as a result of induction by putative placode derivatives (Devillers, 1947;
Lekander, 1949; Disler, 1971; see above),
the canal neuromasts of the mechanoreceptive lateral line system. Neuromasts differentiate in fish larvae as superficial neuromasts in stereotyped patterns in the
epithelium of the head. A subset of these
neuromasts are located in stereotyped positions relative to dermal bones and are designated as presumptive canal neuromasts
(Webb, 19896). Canal formation begins as
a set of parallel ridges rise on either side of
442
J. F. WEBB AND D. M. NODEN
,il.il.il
B
0 m )
0 m )
0 w )
0"
a
•iLiliil
0
IN
FIG. 4. Pattern of lateral line canal formation in actinopterygian fishes. A) Neuromasts arise as superficial
neuromasts in the epithelium, B) canal enclosure occurs first around individual neuromasts, C) Neighboring
canal segments fuse forming a common pore between neuromasts, D) fusion of canal segments continues until
a complete canal is formed. These canals ossify independently of the dermal bones beneath them (not pictured)
with which they typically fuse.
each neuromast which fuse thus enclosing
each neuromast under an epithelial bridge.
An intramembranous ossification may form
within each bridge forming a bony lateral
line ossicle; a linear series of ossicles may
fuse to form a pored lateral line canal (Fig.
4). Finally, lateral line ossicles may secondarily fuse with underlying dermal bones to
form composite lateral line bones of the head
(Allis, 1889; Holmgren and Pehrson, 1949;
Kapoor, 1970) (Fig. 5). The degree of lateral
line canal formation and the degree of canal
fusion with underlying dermal bones
accounts for much of the variation in lateral
line canal morphology among fishes (Webb,
1989a).
The observation that canal formation and
ossification is initiated around individual
neuromasts in teleosts (Allis, 1889; Webb,
19896) resulting in a pore-neuromast-pore
pattern (Fig. 4) suggests that canal neuromasts may actively induce the morphogenesis of the lateral line canals. This hypothesis has been experimentally tested only once
by Devillers (1947) who ablated neuromasts
of the supraorbital canal in salmon and noted
the abnormal morphogenesis of the frontal
bone. Among non-actinopterygian fishes, the
story may be different. In elasmobranchiomorph and sarcopterygian fishes in
which dermal bone is normally reduced or
totally absent, multiple neuromasts are
located between canal pores (Webb and
Northcutt, in preparation). This fundamental difference in neuromast distribution
within canals suggests that the pattern (and
possibly the process of lateral line canal formation) is fundamentally different in actinopterygian fishes and in sarcopterygian and
elasmobranchiomorph fishes. Mechanisms
of neuromast pattern formation, morphogenetic mechanisms and inductive relationships in the formation of lateral line canals
and the relationship of these processes to
pattern formation of the dermal bones must
be established. Only then can we begin to
understand how the lateral line system is
organized and how it was established as
CONTRIBUTIONS OF ECTODERMAL PLACODES
443
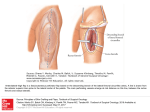
B
FIG. 5. Lateral line bones are composite structures composed of a dermal element and a lateral line canal. A)
Pre-opercular bone with lateral line canal (c) with large pores (p) from the blind side of the head of an adult
flatfish, Glyptocephalus zachirus. Bar = 5 mm. B) Cross section through the post-otic canal in the supracleithrum
of the butterflyfish, Chaetodon octofasciatus showing a neuromast with overlying cupula (n) in the lateral line
canal deeply embedded in bone (b). Bar = 10 nm.
structural components of the head (and
trunk) of all anamniotes.
Lateral line canals are an essential component of the mechanosensory lateral line
system of most fishes and a prominent, but
often overlooked functional component of
fish skulls. Recent work indicates that the
lateral line canals act as filters for vibrational stimuli (van Netten and Kroese, 1989;
Denton and Gray, 1989) and that variation
in canal morphology among teleost fishes
(Webb, 1989a) accounts for a significant
amount of functional versatility in the system (Denton and Gray, 1989). Some of the
lateral line canals are contained in motile
elements of the fish skull, notably the preoperculum and the mandible which are thus
composite structures which function in
mechanoreception as well as in mediating
movements responsible for the generation
of water flow during feeding and gill ventilation. The morphology of these composite structures needs to be considered experimentally as the result of potentially
conflicting functional demands, as a component of both sensory and motor systems.
CONCLUSIONS
1. Ectodermal placodes are a morphologically homogeneous and evolutionarily
conservative series of features of the vertebrate head. Their derivatives, however,
are remarkably diverse and contribute to all
of the sensory systems in vertebrates.
2. Like the neural crest, some placodederived mesenchymal cells are capable of
extensive migrations (lateral line receptor
primordia), but the vast majority of placodal cells remain on the head and differentiate into sensory neurons which may
innervate receptors on the head and on the
trunk, and sensory epithelia of the cranial
sensory systems in all vertebrates.
3. Placodes are capable of inducing skeletal differentiation from neural crest (nasal
capsule) and mesodermal mesenchyme (otic
capsule) and may also be responsible for the
formation of the lateral line canals of fishes.
Thus, placodes contribute not only to the
444
J. F. WEBB AND D. M. NODEN
of extracellular matrix and cell surface molecules
during development of the nasal placode. Development 106:493-509.
D'Amico-Martel, A. and D. M. Noden. 1983. Contributions of placodal and neural crest cells to avian
cranial peripheral ganglia. Am. J. Anat. 166:445468.
Davies, A. M. 1987. Molecular and cellular aspects
of patterning of sensory neurone connections in
ACKNOWLEDGMENTS
the vertebrate nervous system. Development 101:
185-205.
This work was supported by NIH NRSA
Davies, A. M. and A. G. S. Lumsden. 1983. Influence
NS08283 to J.F.W. and NIH DE06632 to
of NGF on developing dorsomedial and ventroD.M.N.
lateral neurons in chick and mouse trigeminal ganglia. Int. J. Neurosci. 1:171-177.
Davies, A. M., H. Thoenen, and Y-A. Barde. 1986.
REFERENCES
The response of chick sensory neurons to brainderived neurotrophic factor. J. Neurosci. 6:1897Allis, E. P. 1889. The anatomy and development of
1904.
the lateral line system in Amia. J. Morph. 2:463566.
de Beer, G. R. 1924. Note on placodes and the ophAltman, J. and S. A. Bayer. 1982. Development of
thalmic nerves. Quart. J. Microsc. Sci. 68:661665.
the cranial nerve ganglia and related nuclei in the
rat. Adv. Anat. Embryol. Cell Biol. 74:1-89.
Denton, E. J. and J. A. B. Gray. 1989. Some observations on the forces acting on neuromasts in fish
Baldwin, C. T., C. F. Hoth, J. A. Amos, E. O. da Silva,
lateral line canals. In S. Coombs, P. Gorner, and
and A. Milunskyu. 1992. An exonic mutation in
H. Munz (eds.), The mechanosensory lateral line—
the HuP2 paired domain gene causes Waardenneurobiology and evolution. New York, Springerburg's Syndrome. Nature 355:637-638.
Verlag.
Bailey, S. W. 1937. An experimental study of the
origin of lateral-line structures in embryonic and Detwiler, S. R. 1951. Structural and functional
adult teleosts. J. Exp. Zool. 76:187-233.
adjustments following reversal of the embryonic
medulla in Amblystoma. J. Exp. Zool. 116:431Bever, M. M. and R. B. Borgens. 1991. The regen446.
eration of electroreceptors in Kryptopterus. J.
Devillers, C. 1947. Recherches dur le crane dermique
Comp. Neurol. 309:200-217.
des teleosteens. Annales de palaeontology, Paris.
Chisaka, O., T. S. Musci, and M. R. Capecchi. 1992.
33:1-94.
Developmental defects of the ear, cranial nerves
Disler, N. N. 1971. Lateral line sense organs and their
and hindbrain resulting from targeted disruption
importance in fish behavior. Translated from Rusof the mouse homeobox gene Hox-1.6. Nature
sian, Israel Program for Scientific Translations,
355:516-520.
Jerusalem.
Corsin, J. 1971. Influence des placodes olfactives et
des ebauches optiques sur la morphogenese du Ebendahl, T. and K. O. Hedlund. 1975. Effects of
nerve growth factor on the chick embryo. ZOON
squelette cranien chez Pleurodeles waltlii Michah.
Annales d'Embryologie et de Morphogenese. 1(1):
3:33-47.
41-48.
Epstein, D. J., M. Vekemans, and P. Gros. 1991.
splotch (Sp2H), a mutation affecting development
Couly, G. F. and N. M. Le Douarin. 1985. Mapping
of the mouse neural tube, shows a deletion within
of the early neural primordium in quail-chick chithe paired homeodomain of Pax-3. Cell 67:767maeras. I. Developmental relationships between
774.
placodes, facial ectoderm and prosencephalon.
Devel. Biol. 110:422-439.
Farbman, A. I. and F. L. Margolis. 1980. Olfactory
marker protein during ontogeny: ImmunohistoCouly, G.. F. and N. M. Le Douarin. 1987. Mapping
chemical localization. Devel. Biol. 74:205-215.
of the early neural primordium in quail-chick chimaeras. II. The presencephalic neural plate and Frenz,D.A.andT.R.VandeWater. 1991. Epithelial
neural folds: Implications for the genesis of cephalic
control of periotic mesenchyme chondrogeneisis.
human congenital abnormalities. Dev. Biol. 120:
Devel. Biol. 144:38-46.
198-214.
Gaik, G. C. and A. I. Farbman. 1973. The chicken
trigeminal ganglion. II. Fine structure of the neuCouly, G. F. and N. M. Le Douarin. 1990. Head
rons during development. J. Morphol. 141:57-76.
morphogenesis in embryonic avian chimeras: Evidence for a segmental pattern in the ectoderm cor- Grainger, R. M., J. J. Henry, and R. A. Henderson.
responding to the neuromeres. Development 108:
1988. Reinvestigation of the role of the optic ves543-558.
icle in embryonic lens induction. Development
102:517-526.
Covell, D. A. and D. M. Noden. 1989. Development
of the avian embryonic trigeminal sensory-motor Graveson, A., S. Smith, and B. K. Hall. 1991. Neural
crest affects lateral-line neuromast deposition in
complex. J. Comp. Neurol. 286:488-503.
the axolotl. Amer. Zool. 31:82A.
Croucher, S. J. and C. Tickle. 1989. Characterization
of epithelial domains in the nasal passages of chick
Graziadei, G. A. M., R. S. Stanley, and P. P. C. Graembryos: Spatial and temporal mapping of a range
ziadei. 1980. The olfactory marker protein in the
receptors of the peripheral nervous system
and neurons of the peripheral and central
nervous systems (GnRH neurons from nasal
placode), but through inductive interactions
they contribute to the formation of structural components of the vertebrate skull.
CONTRIBUTIONS OF ECTODERMAL PLACODES
olfactory system of the mouse during development. Neuroscience 5:1239-1252.
Hall,B. K. 1981. The induction of neural crest-derived
cartilage and bone by embryonic epithelia: An
analysis of the mode of action of an epithelialmesenchymal interaction. J. Embryol. Exp. Morphol. 64:305-320.
Hall, B. K. and R. J. van Exan. 1982. Induction of
bone by epithelial cell products. J. Embryol. Exp.
Morph. 69:37^16.
Hamburger, V. 1961. Experimental analysis of the
dual origin of the trigeminal ganglion in the chick
embryo. J. Exper. Zool. 148:91-123.
Hiscock, J. A. and C. Straznicky. 1986. The development of the neurons of the IXth and Xth sensory
ganglia in chick embryos. Histol. Histopath. 1:129137.
Holmgren, N. and T. Pehrson. 1949. Some remarks
on the ontogenetical development of the sensory
lines on the cheek in fishes and amphibians. Acta
Zool. 30:1-66.
Horstadius, S. 1950. The neural crest. Oxford University Press, London.
Jacobson, A. G. 1966. Inductive processes in embryonic development. Science 152:25-34.
Jacobson, A. G. and A. K. Sater. 1988. Features of
embryonic induction. Development 104:341-359.
Johnson, S. E. 1917. Structure and development of
the sense organs of the lateral line canal system of
selachians (Mustelis canis and Squalus acanthias).
J. Comp. Neurol. 28:1-74.
Kapoor, A. S. 1970. Development of dermal bones
related to sensory canals of the head in the fishes
Ophicephalus punctatus Bloch (Ophicephalidae)
and Wallago attu B. & Schn. (Siluridae). Zool. J.
Linn. Soc. 49:69-97.
Knouff, R. A. 1927. The origin of the cranial ganglia
of Rana. Joum. Comp. Neurol. 44:259-361.
Landacre, F. L. 1910. The origin of the cranial ganglia
in Ameiurus. J. Comp. Neurol. 20:309-411.
Landacre, F. L. 1916. The cerebral ganglia and early
nerves of Squalus acanthias. J. Comp. Neurol. 27:
19-67.
Landacre, F. L. 1926. The primitive lines ofAmblystoma jeffersonianum. J. Comp. Neurol. 40:471495.
Landacre, F. L. 1927. The differentiation of the
preauditory and postauditory primitive lines into
preauditory and postauditory placodes, lateralis
ganglia and migratory lateral-line placodes in
Amblystomajeffersonianum. J. Comp. Neurol. 44:
29-59.
Landacre, F. L. and A. C. Conger. 1913. The origin
of the lateral line primoridia in Lepidosteus osseus.
J. Comp. Neurol. 23:575-633.
Lekander, B. 1949. The sensory line system and the
canal bones in the head of some Osteriophysi. Acta
Zool. 30:1-131.
Le Lievre, C. S. and N. M. Le Douarin. 1975. Mesenchymal derivatives of the neural crest: Analysis
of chimeric quail and chick embryos. J. Embryol.
Exp. Morphol. 34:125-154.
Levi, G., B. Gumbiner, and J. P. Thiery. 1991. The
distribution of E-cadherin during Xenopus laevis
development. Development 111:159-169.
445
Lindsay, R. M., H. Thoenen, and Y. A. Barde. 1986.
Placode and neural crest-derived neurons are
responsive to brain-derived neurotrophic factor.
Devel. Biol. 112:319-328.
Lufkin, T., A. Dierich, M. LeMeur, M. Mark, and P.
Chambon. 1991. Disruption of the Hox-1.6
homeobox gene results in defects in a region corresponding to its rostral domain of expression.
Cell 66:1105-1119.
McCormick, C. A. 1983. Organization and evolution
of the octavolateralis area of fishes. In R. G.
Northcutt and R. E. Davis (eds.), Fish neurobiology, Vol. 1, pp. 179-213. University of Michigan
Press, Ann Arbor.
Meier, S. 1978. Development of the embryonic chick
otic placode. II. Electron microscopic analysis.
Anat. Rec. 191:459-478.
Metcalfe, W. K. 1985. Sensory neuron growth cones
comigrate with posterior lateral line primordial
cells in zebrafish. J. Comp. Neurol. 238:218-224.
Minkoff, R., S. B. Parker, and E. L. Hertzberg. 1991.
Analysis of distribution patterns of gap junctions
during development of embryonic chick facial primordia and brain. Development 111:509-522.
Moody, S. A. and M. B. Heaton. 1983. Developmental relationships between trigeminal motoneurons in chick embryos. J. Comp. Neurol. 213:
327-364.
Noden, D. M. 1978a. The control of avian cephalic
neural crest cytodifferentiation. I. Skeletal and
connective tissues. Devel. Biol. 67:296-312.
Noden, D. M. 19786. The control of avian cephalic
neural crest cytodifferentiation. II. Neural tissues.
Devel. Biol. 67:313-329.
Noden, D. M. 1980a. Somatotopic and functional
organization of the avian trigeminal ganglion: An
HRP analysis in the hatchling chick. J. Comp.
Neurol. 190:405^128.
Noden, D. M. 19806. The migration and cytodifferentiation of cranial neural crest. In R. M. Pratt
and R. L. Christiansen (eds.), Current research
trends in prenatal craniofacial development, pp. 3 25. Elsevier North Holland, New York.
Noden, D. M. 1983. The role of the neural crest in
patterning ofavian cranial skeletal, connective and
muscle tissues. Devel. Biol. 96:144-165.
Noden, D. M. 1991. Vertebrate craniofacial development: The relation between ontogenetic process
and morphological outcome. Brain, Behav. Evol.
38:190-225.
Noden, D. M. and T. R. Van De Water. 1986. The
developing ear: Tissue origins and interactions. In
Ruben, R. J., T. R. Van De Water, and E. W.
Rubel (eds.), The biology of change in otolaryngology, pp. 15-46. Elsevier Sci. Pub. (Biomed.
Division).
Northcutt, R. G. 1992. The phytogeny of octavolateralis ontogenies: A reaffirmation of Garstang's
phylogenetic hypothesis. In D. B. Webster, R. R.
Fay, and A. N. Popper (eds.), The evolutionary
biology ofhearing, pp. 21-48. Springer-Verlag, New
York.
Northcutt, R. G. and L. E. Muske. 1991. Experimental embryological evidence of the placodal origin of GnRH and FMRF-amide neurons of the
446
J. F. WEBB AND D. M. NODEN
terminal nerve and preoptic area in salamanders.
Soc. Neurosci. Abstr. 1991:321.
Pehrson, T. 1949. The ontogeny of the lateral line
system in the head of dipnoans. Acta Zool. 30:
153-182.
Pinto, C. B. and B. K. Hall. 1991. Toward an understanding of the epithelial requirement for osteogenesis in scleral mesenchyme of the embryonic
chick. J. Exp. Zool. 259:92-108.
Platt,J. 1896. Ontogenetic differentiation of the ectoderm in Necturus. Study II. Development of the
peripheral nervous system. Quart. J. Microsc. Sci.
38:485-547.
Represa, J. Y. Leon, C. Minor, and F. Giraldez. 1991.
The int-2 proto-oncogene is responsible for induction of the inner ear. Nature 353:561-563.
Roth, A. 1986. Afferent nerve fibers induce electroreception in the skin of fish. Naturwissenschaften
73:264-265.
Richardson, G. P., K. L. Crossin, C. M. Chuong, and
G. M. Edelman. 1987. Expression of cell adhesion molecules during embryonic induction. III.
Development of the otic placode. Devel. Biol. 119:
217-230.
Schwanzel-Fukuda, M.. and D. W. Pfaff. 1989. Origin of luteinizing hormone-releasing hormone
neurons. Nature 338:161-164.
Schwanzel-Fukuda, M. and D.W.Pfaff. 1991. Migration of LHRH-immunoreactive neurons from the
olfactory placode rationalizes olfacto-hormonal
relationships. J. Steroid Biochem. Molec. Biol. 39:
565-572.
Sher, A. E. 1971. The embryonic and postnatal development of the inner ear of the mouse. Acta Otolaryngol. 285:3-77.
Smith, S. C , M. J. Lannoo, and J. B. Armstrong. 1990.
Development of the mechanoreceptive lateral-line
system in the axolotl: Placode specification, guidance of migration, and the origin of neuromast
polarity. Anat. Embryol. 182:171-180.
Song, J. and R. G. Northcutt. 1991a. Morphology,
distribution and innervation of the lateral-line
receptors of the Florida gar, Lepisosteus platyrhincus. Brain, Behav. Evol. 37:10-37.
Song, J. and R. G. Northcutt. 19916. The primary
projections of the lateral-line nerves of the Florida
gar, Lepisosteus platyrhincus. Brain, Behav. and
Evol. 37:38-63.
Stone, L. S. 1922. Experiments on the development
of the cranial ganglia and the lateral line sense
organs in Amblystoma punctatum. J. Exp. Zool.
35:421-496.
Stone, L. S. 1924. Experiments on the transplantation
of placodes of the cranial ganglia in the amphibian
embryo. I. Heterotopic transplantations of the
ophthalmic placode upon the head of Amblystoma
punctatum. J. Comp. Neurol. 38(1):73—105.
Stone, L. S. 1928a. Experiments on the transplantation of placodes of the cranial ganglia in the
amphibian embryo. II. Heterotopic transplantations of the ophthalmic placode upon the head
and body of Amblystoma punctatum. J. Comp.
Neurol. 47(1 ):91-116.
Stone, L. S. 19286. Experiments on the transplan-
tation of placodes of the cranial ganglia in the
amphibian embryo. III. Preauditory and postauditory placodal material interchanged. J. Comp.
Neurol. 47:117-154.
Stone, L. S. 1928c. Primitive lines in Amblystoma
and their relation to the migratory lateral-line primordia. J. Comp. Neurol. 45(1): 169-190.
Stone, L. S. 1929a. Experiments on the transplantation of placodes of the cranial ganglia in the
amphibian embryo. IV. Heterotopic transplantations of the postauditory placodal material upon
the head and body of Amblystoma punctatum. J.
Comp. Neurol. 48:311-330.
Stone, L. S. 1931. Induction of the ear by the medulla
and its relation to experiments on the lateralis system in Amphibia. Science 74:577.
Stone, L. S. 1938. Further experimental studies of
the development of lateral-line sense organs in
amphibians observed in living preparations. J.
Comp. Neurol. 68:83-119.
Szekely, G. 1959. Functional specificity of cranial
sensory neuroblasts in urodela. Acta Biol. Acad.
Sci. Hung. 10:107-115.
Tassabehji, M., A. P. Read, V. E. Newton, R. Harris,
R. Balling, P. Gruss, and T. Strachan. 1992.
Waardenburg's syndrome patients have mutations
in the human homologue of the Pax-3 paired box
gene. Nature. 355:635-636.
Van Der Water, T. R. 1988. Tissue interactions and
cell differentiation: Neurone-sensory cell interaction during otic development. Development
103(Suppl.):185-193.
VanNetten,S.M.andA.B.A.Kroese. 1989. Dynamic
behavior and micromechanical properties of the
cupula. In S. Coombs, P. Gorner, and H. Munz.
The mechanosensory lateral line— neurobiology and
evolution, pp. 247-264. Springer-Verlag, New
York.
van Wijhe, J. W. 1883. Uber die Mesodermsegmente
und die Entwicklung der Nerven des Selachierkopfes. Verh. Acad. Setensch. (Amsterdam) 22(E):
1-50.
Verwoerd, C. D. A. and C. G. van Oostrom. 1979.
Cephalic neural crest and placodes. Adv. Anat.
Embryo, and Cell Biol. 58:1-75.
Vischer, H. A. 1989a. The development of lateralline receptors in Eigenmannia (Teleostei, Gymnotiformes). II. The mechanoreceptive lateral-line
system. Brain, Behav. and Evol. 33:205-222.
Vischer, H. A. 19896. The development of lateralline receptors in Eigenmannia (Teleostei, Gymnotiformes). II. The electroreceptive lateral-line
system. Brain, Behav. and Evol. 33:223-236.
Vischer, H. A., M. J. Lannoo, and W. Heiligenberg.
1989. Development of the electrosensory nervous
system in Eigenmannia (Gymnotiformes): I. The
peripheral nervous system. J. Comp. Neurol. 290:
16-40.
Von Kupffer, C. 1891. The development of the cranial nerves of vertebrates. J. Comp. Neurol. 1:246—
332.
Webb, J. F. 1989a. Gross morphology and evolution
of the mechanoreceptive lateral line system in teleost fishes. Brain, Behav. Evol. 33:34-53.
CONTRIBUTIONS OF ECTODERMAL PLACODES
Webb, J. F. 19896. Neuromast morphology and lateral line trunk canal ontogeny in two species of
cichlids: An SEM study. J. Morph. 202:53-68.
Wilkinson, D. G., S. Bhatt, and A. P. McMahon. 1989.
Expression pattern of the FGF related proto-oncogene int-2 suggests multiple roles in fetal development. Development 105:131-136.
Wilson, H. V. and J. E. Mattocks. 1897. The lateral
sensory anlage in the salmon. Anat. Anz. 13:658660.
Wray, S., P. Grant and H. Gamer. 1989. Evidence
that cells expressing LHRH messenger RNA in
the mouse are derived from progenitor cells in the
447
olfactory placode. Proc. Nat. Acad. Sci. U.S.A. 86:
8132-8136.
Yntema, C. L. 1933. Experiments on the determination of the ear ectoderm in the embryo of
Amblystoma punctatum. J. Exp. Zool. 317-357.
Yntema, C. L. 1937. An experimental study of the
origin of the cells which constitute the Vllth and
VHIth cranial ganglia and nerves in the embryo
ofAmblystoma punctatum. Journ. Exper. Zool. 75:
75-101.
Yntema, C. L. 1939. Self-differentiation of heterotopic ear ectoderm in the embryo of Ambytstoma
punctatum. J. Exp. Zool. 80:1-17.





















![[SENSORY LANGUAGE WRITING TOOL]](http://s1.studyres.com/store/data/014348242_1-6458abd974b03da267bcaa1c7b2177cc-150x150.png)