* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Where is a Nose with Respect to a Foot? The Left
Neuroanatomy wikipedia , lookup
Neurophilosophy wikipedia , lookup
Neuropsychology wikipedia , lookup
Aging brain wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Affective neuroscience wikipedia , lookup
Cortical cooling wikipedia , lookup
Visual selective attention in dementia wikipedia , lookup
Neuroscience in space wikipedia , lookup
Human brain wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Spatial memory wikipedia , lookup
Metastability in the brain wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Embodied language processing wikipedia , lookup
C1 and P1 (neuroscience) wikipedia , lookup
Emotional lateralization wikipedia , lookup
Time perception wikipedia , lookup
Neuroesthetics wikipedia , lookup
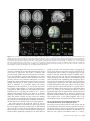
Cerebral Cortex December 2008;18:2879--2890 doi:10.1093/cercor/bhn046 Advance Access publication April 18, 2008 Where is a Nose with Respect to a Foot? The Left Posterior Parietal Cortex Processes Spatial Relationships among Body Parts Corrado Corradi-Dell’Acqua1,2, Maike D. Hesse2,3,4, Raffaella I. Rumiati1 and Gereon R. Fink2,4,5 Neuropsychological studies suggest that patients with left parietal lesions may show impaired localization of parts of either their own or the examiner’s body, despite preserved ability to identify isolated body parts. This deficit, called autotopagnosia, may result from damage to the Body Structural Description (BSD), a representation which codes spatial relationships among body parts. We used functional magnetic resonance imaging to identify the neural mechanisms underlying the BSD. Two human body or building parts (factor: STIMULI) were shown to participants who either identified them or evaluated their distance (factor: TASK). The analysis of the interaction between STIMULI and TASK, which isolates the neural mechanism underlying BSD, revealed an activation of left posterior intraparietal sulcus (IPS) when the distance between body parts was evaluated. The results show that the left IPS processes specifically the information about spatial relationships among body parts and thereby suggest that damage to this area may underlie autotopagnosia. Evidence of such body model is provided, for instance, by studies investigating the perception of complex cutaneous stimuli delivered on the body surface: different scholars showed that these stimuli are processed according not only to the tactile sensitivity of the body segments being stimulated, but also to the spatial orientation of these body segments at the time of the stimulation (Oldfield and Phillips 1983; Parsons and Shimojo 1987; Haggard et al. 2006). Another example providing evidence of a model of one’s own body orientation is given by the rubber hand illusion (Botvinick and Cohen 1998; Tsakiris and Haggard 2005); such illusion occurs when participants see a fake but realistically appearing hand, whereas their real hand is hidden from their sight: when the experimenter brushes simultaneously both the real and the rubber fingers, the majority of participants report to feel the brush strokes where the fake and not the real hand is touched. Moreover, extensive neurophysiological research in the macaque’s brain (Duhamel et al. 1998; Graziano et al. 2000), subsequently confirmed in humans using functional magnetic resonance imaging (fMRI) (Bremmer et al. 2001; Grefkes et al. 2002), showed that neurons in the premotor and parietal cortices respond both to somatosensory stimulation delivered to a given body region and to visual stimulation from the space adjacent to it, independent of the respective body part’s position in space. Thus, multisensory integration about one’s own body does not take into account only information arising from somatosensory and motor maps, but also signals arising from the visual system. This then raises question about how visual information of one’s own body is organized and whether, as for the case of somatosensory and motor modalities, the visual system is also endowed with a specific body representation. The most convincing finding in favor of a visual map of the human body is offered by the observation of patients affected by autotopagnosia (Pick 1922; Odgen 1985; Sirigu et al. 1991; Buxbaum and Coslett 2001; Felician et al. 2003—see CorradiDell’Acqua and Rumiati 2007, as a review). These patients typically fail to point to parts of a body irrespective of whether this is their own, someone else’s, or a line drawing. Interestingly, the errors made by these patients in pointing are more often spatial than categorical, in that they are more likely to indicate by mistake those body parts that are spatially near to the intended target (spatial error: hand / elbow) than those that serve similar functions (categorical error: elbow / knee). Furthermore, they are unable to construct, in a puzzle-like fashion, a full body or a full face by means of tiles depicting isolated parts. However, they also show a spared performance when animals or manmade objects parts are used instead, thus suggesting that their deficit cannot be considered to result Keywords: autotopagnosia, body schema, body structural description, extrastriate body area, intraparietal sulcus Introduction The human cerebral cortex is endowed with multiple maps of the body. These maps vary according to the motor output, the sensory channel from which they extract the information and the reference frame in which this information is recoded. Using electrical stimulation on canine (Walshe 1948) and human (Foerster 1936; Penfield and Rasmussen 1950) cortex, scholars discovered that the primary motor cortex is organized in a somatotopically ordered map, in which each body part is represented approximately proportionally to the number of muscles innervated. Subsequently, studies using microelectrode stimulations (Mitz and Wise 1987; Luppino et al. 1991), anatomical tracing methods (He et al. 1993) and positron emission tomography (Fink et al. 1997) revealed the presence of several nonprimary motor areas, which are also represented somatotopically. Neurophysiological studies also showed the presence of a somatosensory map: Penfield and Rasmussen (1950), for instance, by using cortical electrical stimulation, discovered that the primary somatosensory cortex is organized somatotypically, in that each body part is represented proportionally to the density of cutaneous receptors. The information coded by these multiple maps is integrated in a higher order representation of the body that Head and Holmes (1911) called ‘‘Body Schema,’’ which, in turn, provides the information about one’s own body’s orientation in space. Ó The Author 2008. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: [email protected] 1 Cognitive Neuroscience Sector, Scuola Internazionale Superiore di Studi Avanzati (SISSA-ISAS), 34014 Trieste, Italy, 2 Institute of Neuroscience and Biophysics, Department of Medicine, Research Center Jülich, 52425 Jülich, Germany, 3 Department of Neurology—Cognitive Neurology, University Hospital Aachen, RWTH Aachen, 52074 Aachen, Germany, 4 Brain Imaging Center West, Research Center Jülich, 52425 Jülich, Germany and 5Department of Neurology, University Hospital Cologne, Cologne University, 50924 Cologne, Germany from a generalized impairment of spatial abilities. Moreover, they are able to identify the body parts that they cannot locate, suggesting that their deficit lies neither at the level of visual processing of isolated body parts, nor at the level of the semantic knowledge of the body. Recent accounts postulate that patients affected by autotopagnosia have a damaged visuospatial map which computes the spatial arrangement among parts of a standard body, the socalled ‘‘Body Structural Description’’ (BSD) (Buxbaum and Coslett 2001; Schwoebel and Coslett 2005). Structural description models have 1st been employed in the domain of object recognition (Marr and Nishihara 1978; Biederman 1987) and suggest that an object is seen as the collection of its parts, each of which is coded independently from the others. Thus, the overall representation of the item can be seen as an organized model which combines the spatial positions of parts into a whole (see also Peissig and Tarr 2007, for a review). This notion has been adopted by Buxbaum, Coslett, and Schwoebel (Buxbaum and Coslett 2001; Schwoebel and Coslett 2005) to describe the impaired process of the body representation observed in autotopagnosic patients: even though these patients are able to process isolated body parts, they have trouble in coding their spatial relations. Recently, Schwoebel and Coslett (2005) had 70 stroke patients to perform a task requiring mental spatio-motor transformations of one’s own body (a hand laterality task—Parsons 1987), a task in which patients’ real hand movements were compared with their imagined movements in order to assess their ability to simulate actions, and a pointing task similar to the one used to assess autotopagnosia. Moreover, patients choose from a set of body part pictures either 1) the 1 that was closer to a target part in a standard body, or 2) the 1 that served the same function of a target part. Two patients were impaired in the pointing task as well as in matching body parts for distance, but performed flawlessly in all the other tasks. In contrast, 3 patients were found selectively impaired at matching body parts by function, whereas thirteen were impaired in either the hand laterality or real/imagined hand movement task despite being still able to perform normally on all other tasks. Thus, the coding of the spatial relations among 2 visually presented body parts can be dissociated from both the semantic knowledge about those parts as well as the coding of one’s online posture. Schwoebel and Coslett (2005), accordingly, advocated the psychological validity of the BSD. It remains, however, unclear how the BSD relates to the brain. Localization based on neuropsychological studies of patients has proved difficult due to lesion sizes; nevertheless, an analysis of the lesions of patients with autotopagnosia indicates that the left posterior parietal cortex is the area responsible for this deficit (Odgen 1985; Semenza 1988; Denes et al. 2000; Schwoebel et al. 2001; Guariglia et al. 2002; Felician et al. 2003), even though a recent group study suggested that the left middle temporal cortex might be involved as well (Schwoebel and Coslett 2005). Moreover, fMRI studies associated both the middle temporal cortex and the posterior parietal cortex of the left hemisphere with tasks which may tap the BSD. For instance, Downing et al. (2001) observed the posterior part of the middle temporal gyrus bilaterally more active when subjects perceived body parts relative to other kinds of stimuli, and called this region extrastriate body area (EBA). EBA’s sensitivity was also found to be larger when the body parts were shown from an allocentric (e.g., looking at others’ parts) 2880 Neural Correlates of Body Structural Description d Corradi-Dell’Acqua et al. rather than an egocentric (e.g., looking at one’s own toes) view (Chan et al. 2004; Saxe et al. 2006), thus suggesting that this region may be involved in a visual model of the body. With regards to the left posterior parietal cortex, Felician et al. (2004) engaged healthy participants in a pointing task similar to the one used to diagnose autotopagnposia, and found an activation in both the left intraparietal sulcus (IPS) and the left superior parietal cortex (SPC). The same regions were documented by Le Clec’H et al. (2000) who asked participants to code the spatial relationship among parts of the human body with respect to non-bodily control stimuli (e.g., for the body parts ‘‘is this body part above or below a shoulder in a standing body?’’, for the numbers ‘‘is this number greater or smaller that 5?’’). These neuropsychological and imaging findings suggest that the neural basis of the BSD may lie either in the middle temporal cortex (presumably corresponding to EBA) or in the left posterior parietal cortex (over or around the left posterior IPS). In the present study we aimed at directly specifying the neural mechanisms underlying the BSD. We accordingly designed an fMRI study in which twenty participants performed the following task: 2 images of body parts, vertically aligned at various distances to each other, were presented on a screen for 750 ms, followed by a mask and the display of 2 vertical lines. One of these lines represented the distance between the midpoints of the 2 body parts on the screen. Participants were asked to indicate the perceived distance by means of a forced choice between these 2 lines (see Fig. 1). As a nonspatial control task, vertically oriented names of body parts replaced the lines. Participants indicated which body part had been presented as an image (irrespective of their distance) by means of a forced choice between the 2 names. Thus, the neural correlates of the BSD can be identified by disentangling those neural networks associated with coding body parts’ spatial relations from those associated with coding body parts per se. Moreover, such effect needs to be body-part specific, that is, the neural correlates of BSD should not be found significantly associated with coding the spatial relations of 2 nonbody parts, relative to coding the nonbody parts per se. Thus, as a nonbody control condition, the same tasks were performed using building parts instead of body parts as stimuli. This constitutes a 2 factorial design with the factors TASK (spatial judgment vs. identification task) and STIMULI (human body parts vs. building parts). The critical analysis relies on the interaction, in which increases of neural activity associated with judging the distance between body parts (relative to the identification thereof) are controlled for task differences per se. Experimental Procedures Participants Twenty subjects (11 males and 9 females, aged 20--34, average age 23.7) participated in the study. None of the participants had any history of neurological or psychiatric illness. Informed consent was obtained from all subjects, who were naı̈ve to the purpose of the experiment. The study was approved by the local ethics committee and conducted in accordance with the Declaration of Helsinki. One participant was excluded from further analysis due to motion artifacts ( >4 mm) during the fMRI scanning, thus reducing the number of participants included in the analysis to nineteen. Figure 1. Example trial sequences for both kinds of task. Participant were presented for 750 ms with either 2 human body or building parts that were vertically aligned, followed by a flickering masking stimulus of 250 ms. In the Spatial Judgment task participants had to evaluate the distance between the images perceived, and to indicate which of the 2 subsequently presented lines depicted this distance. In the Identification task participants were asked to identify the body/buildings parts and to indicate the name of 1 of these. Stimuli On each trial, 2 photographs were presented on a computer screen as endpoints of a virtual vertical line of 3 different lengths (6.14, 6.89, and 7.64 cm—corresponding to, for a viewing distance of 29 cm, visual angles of 12.09°, 13.55°, and 15.01°, respectively). Images depicted either parts of the human body (i.e., nose, ear, belly-button, foot) or parts of a building (i.e., chimney pot, roof tiles, window, door). Upper parts of the body or building were always presented on the screen above lower parts (e.g., the ear [or the chimney, respectively] appeared always above the belly-button [or the door, respectively]). All images had a vertical axis of 2.40 cm (corresponding to a visual angle of 4.74°), were taken from a frontal orientation, and presented in black and white on a white background. Seven pairs of photographs were chosen for both body and building parts; these pairs were matched according to their allocentric distance, that is, according to whether they were in reality 1) highly distant to each other (e.g., the pairs ‘‘ear / foot’’, that is the ear displayed above the foot as in the example provided in Figure 1, and ‘‘nose / foot’’ for human body parts, and ‘‘chimney / door’’ and ‘‘roof / door’’ for building parts), or 2) intermediately distant to each other (e.g., ‘‘ear / bellybutton’’, ‘‘nose / belly-button’’ and ‘‘belly-button / foot’’ for human body parts, and ‘‘chimney / window’’, ‘‘roof / window’’ and ‘‘window / door’’ for the building parts), or 3) close to each other (e.g., the pairs ‘‘ear / nose’’ and ‘‘nose / ear’’ for human body parts, and ‘‘chimney / roof’’ and ‘‘roof / chimney’’, which were shown in either of the 2 possible combinations—that is, ear or nose on top of the other). Experimental Set-Up Participants lay supine with their head fixated by firm foam pads and each hand placed on a button-box for manual responses. Stimuli were presented using Presentation 9.0 (Neurobehavioral Systems, CA) and projected on a screen from a distance of 29 cm through a mirror fixed to the head coil. Each experimental trial consisted of the following events (see Fig. 1): the 2 body/building parts appeared on the screen for 750 ms, followed by a flickering checkerboard presented Cerebral Cortex December 2008, V 18 N 12 2881 for 250 ms, thereby masking the image positions. Subsequently, 2 lines appeared 2.25 cm (corresponding to a visual angle of 4.44°) to the right and left of the center of the screen for 1000 ms (spatial judgment task), followed by a 500 ms white screen. In the control conditions, 2 words were presented vertically in the same positions as the lines (identification task). Each trial lasted 2500 ms. In the spatial judgment task, the length of 1 line corresponded to the distance between the 2 body/building parts previously seen, whereas that of the other line was either 2.4 cm (corresponding to a visual angle of 4.74°) longer or shorter (50% of the trials each). Subjects were asked to indicate via button-press with the right or left hand (50% of the trials each) which of the 2 lines was consistent with the distance between the body/building images seen earlier. In the identification task, the words were the German names of 2 of the 4 body parts used as visual stimuli (i.e., Ohr [Ear, Lexical Frequency: 292], Nase [Nose, Lex. Freq.: 211], Bauchnabel [Belly-Button, Lex. Freq.: 4], and Fuß [Foot, Lex. Freq.: 591]) or 2 of the 4 building parts used as visual stimuli (i.e., Kamin [Chimney, Lex. Freq.: 52], Dach [Roof, Lex. Freq.: 205], Fenster [Window, Lex. Freq.: 534], and Tür [Door, Lex. Freq.: 68].—Lexical Frequency values were obtained by the CELEX Lexical Database [Baayan et al. 1993]). One of the 2 words referred to either of the 2 body/building parts previously seen; the other referred to a part which was not shown (see Fig. 1). Subjects were asked to indicate via button-press with the right or left hand (50% of the trials each) if the name of 1 of the parts previously seen was on the right (50% of the trials) or on the left side (50% of the trials) of the screen. This constitutes a 2 3 2 factorial design with the factors STIMULI (human body vs. building parts) and TASK (spatial judgment [SJ] versus identification [I]) with 4 conditions: 1) BodySJ, participants performed the spatial judgment task with body parts; 2) BodyI, identification task with body parts; 3) BuildingSJ, spatial judgment task with building parts; and 4) BuildingI, identification task with building parts. Within each condition, 84 trials (7 pairs 3 3 distances on the screen 3 4 repetitions) were presented grouped into 12 blocks, each consisting of 7 trials. The blocks were introduced by short instructions informing the subjects about the upcoming task (1500 ms) and separated by a blank screen interval of 17.5 s (implicit baseline). The overall experiment consisted of 48 blocks (12 blocks per condition 3 4 conditions). Both block order and trial order within each block were randomized. Image Acquisition A Siemens Sonata 1.5-T whole-body MRI scanner was used to acquire both T1-weighted anatomical images and gradient-echo planar T2-weighted images with blood oxygenation level-dependent (BOLD) contrast. The scanning sequence was a trajectory-based reconstruction sequence with a repetition time (TR) of 3020 ms and an echo time (TE) of 66 ms. Each volume comprised 30 axial slices with an in-plane resolution of 3.125 3 3.125 mm, a slice thickness of 4 mm, and 0.4 mm interval between slices. For each subject, 586 volumes were acquired during the whole experimental session (approximately 28 min). For the anatomical images the following parameters were used: TR = 2.2 ms, TE = 3.93 ms, inversion time = 1200 ms, field of view = 256 mm, number of sagittal slices = 128, slice thickness = 1 mm, interslice gap 0.5 mm, flip angle = 15°. 2882 Neural Correlates of Body Structural Description d Corradi-Dell’Acqua et al. Image Processing Statistical analysis was carried out using the general linear model framework (Friston et al. 1995) implemented in the SPM2 software package (Wellcome Department of Imaging Neuroscience, London, UK). For each subject, the 1st 6 volumes were discarded. To correct for subject motion, the functional images were realigned to a 1st functional image, normalized to a template based on 152 brains from the Montreal Neurological Institute (MNI), and then smoothed by convolution with an 8-mm full-width at half-maximum (FWHM) Gaussian kernel. A high-pass filter (using a cut-off of 128 s) was finally applied. On the 1st level, for each individual subject, we fitted a linear regression model (general linear model) to the data, by modeling each block condition of the 2 3 2 design with a boxcar function convoluted with a canonical hemodynamic response function. We also included the 6 differential realignment parameters as regressors to control for movementrelated variance. Additionally, in order to single-out artifacts due to difficulty differences across task conditions, participants average reaction times (RTs) and Accuracy for each block were entered as additional regressors of no interest. Specific effects were tested by applying appropriate contrasts to the parameters estimates for each condition. On the 2nd level, 1-sample t-tests were performed on the above contrast images of all subjects to give random-effects statistical parametric maps (SPMs). Voxels were identified as significant only if they passed a height threshold of t = 3.61 using an uncorrected voxel-level threshold of P < 0.001 and a minimum cluster size of at least 140 activated voxels, corresponding to a P < 0.05 corrected at the cluster level. Additionally, with regards to the interaction term (BodySJ – BodyI) – (BuildingSJ – BuildingI), we restricted our analysis to those voxels which exhibited the simple effect BodySJ – BodyI greater than t = 1.73 (corresponding to an uncorrected voxellevel threshold of P < 0.05). Additionally, voxels that the MNI tissue probability maps (Evans et al. 1994) reported to be more probably associated to the white matter/cerebrospinal fluid than to the gray matter were removed by the mask. Thus the cluster threshold for the interaction term was of 85 activated voxels, corresponding to a P < 0.05 corrected for the mask. The localization of the functional activations with respect to cytoarchitectonic areas was analyzed based on probabilistic cytoarchitectonic maps derived from the analysis of cortical areas in a sample of 10 human post-mortem brains, which were subsequently normalized to the MNI reference space. The significant results of the random-effects analysis were compared with the cytoarchitectonic maps using the SPM Anatomy toolbox (Eickhoff et al. 2005). Results Behavioral Results For each subject, and for each condition, the median value of RTs of all correct trials and the Accuracies were calculated and used in a 2 (TASK: spatial judgment and identification task) x 2 (STIMULI: human body and building parts) x 3 (SCREEN DISTANCE: 6.14, 6.89, and 7.14 cm) x 3 (ALLOCENRIC DISTANCE: close, intermediate, distant—see Methods section for a more detailed description) repeated measures ANOVA. Because SCREEN DISTANCE and ALLOCENTRIC DISTANCE had more than 2 levels, polynomial contrasts were used in order to assess linear effects across all factor levels. Statistical analysis was carried out using SPSS 14.0 software (SPSS Inc, Chertsey, UK). Overall conditions participants were 91.03% accurate (mean squares [MSE] 566.83, F1,18 = 8604.56, P < 0.001). A main effect of STIMULI was found to be significant (MSE = 0.87, F1,18 = 7.09, P < 0.05), with building parts (89.91 ± 1.06%) eliciting more errors than body parts (92.16 ± 1.03%). Moreover, a main effect of SCREEN DISTANCE was found to be significant (MSE = 0.52, F2,36 = 4.14, P < 0.01): participants were more accurate when the parts were 6.89 cm apart (92.51 ± 1.04%), then when the distance was 6.14 cm (91.09 ± 1.16%) or 7.64 cm (89.50 ± 1.25%). Finally, a main effect of ALLOCENTRIC DISTANCE was found to be significant (MSE = 0.75, F2,36 = 6.51, P < 0.01): participants were more accurate when the parts chosen were close to each other in the real body/building (92.93 ± 0.85%—e.g., ‘‘ear’’ / ‘‘nose’’ or ‘‘chimney’’ / ‘‘tiles’’) and made more errors if these had an intermediate (90.43 ± 1.02%—e.g., ‘‘ear’’ / ‘‘belly-button’’ or ‘‘chimney’’ / ‘‘windows’’) or large (89.74 ± 1.41%—e.g., ‘‘ear’’ / ‘‘foot’’ or ‘‘chimney’’ / ‘‘door’’) distance in the real body/building. Polynomial contrasts insured that the Accuracy Rates changed consistently with a linear trend across all the levels of ALLOCENTRIC DISTANCE (MSE = 0.12, F1,18 = 10.56, P < 0.01). Figure 2A,B depicts Accuracy Rates plotted across the 3 levels of allocentric distance for each kind of task and stimulus: when human body parts were shown, the Accuracy decreased linearly with the allocentric distance irrespectively of the task employed (Fig. 2A), whereas when building parts were shown the Accuracy decreases linearly with the allocentric distance only when the identification task was employed (Fig. 2B). No other main effects or interactions were found to be significant. With regards the RTs, over all conditions participants took 737.21 ms on average for delivering a correct response (MSE = 371741577.90, F1,18 = 2242.81, P < 0.001). A main effect of TASK was found to be significant (MSE = 1933560.02, F1,18 = 50.95, P < 0.001), with participants being slower in the identification (790.38 ± 15.03 ms) than in the spatial judgment task (684.04 ± 19.23 ms). A main effect of STIMULI was also found to be significant (MSE = 19669.38, F1,18 = 6.79, P < 0.05), with participants being faster with body parts (731.85 ± 15.92 ms) than building parts (742.57 ± 15.84 ms). Finally, the interaction STIMULI * TASK was found to be significant (MSE = 2402.84, F1,18 = 5.81, P < 0.05), in that during the identification (but not during the spatial judgment) task, RTs to building parts were longer than those to body parts (see Table 1). Neural Activations Those clusters which survived a threshold of P < 0.05 corrected for multiple comparisons are reported in Table 2. Main Effect of TASK Consistent with other studies (Fink et al. 2003), the spatial judgment task, relative to the identification task [i.e., (BodySJ + BuildingSJ) – (BodyI + BuildingI)], led to differential activation of a predominantly right-hemisphere network comprising the parietal cortex (extending from the postcentral gyrus to the IPS and the supramarginal gyrus), the middle and the inferior frontal gyri (extending into Brodmann area 44), the middle occipital gyrus and the inferior temporal gyrus. Moreover, 2 additional clusters of neural activation were found in the left postcentral and supramarginal gyri (see also Fig. 3A). Consistent with Cohen et al. (2003), the identification task, relative to the spatial judgment task [i.e., (BodyI + BuildingI) – (BodySJ + BuildingSJ)], led to differential activations in the lingual gyrus bilaterally, the inferior occipital gyrus (x = –44, y = –84, z = –14) and extending anteriorly to about y = –42 (see Fig. 3B), thus including the Visual Word Form Area (a left hemisphere brain region which is supposed to hold a representation of abstract letter identities invariant of parameters such as spatial position, size, or font). Additional clusters of activation were also observed in the left thalamus, the left inferior frontal gyrus (extending into areas 44 and 45), in the middle and superior frontal gyri (extending into areas 6 and 4) and the left middle temporal gyrus. Main Effect of STIMULI No differential activation was observed (when applying a threshold of P < 0.05, corrected for multiple comparisons for the whole brain) when body parts were presented, relative to buildings parts [i.e., (BodySJ + BodyI) – (BuildingSJ + BuildingI)]. Following Downing et al. (2001), we had hypothesized that EBA should be activated in our experiment. Accordingly, a region of interest analysis was conducted based on the coordinates reported by Downing et al. (2001) (MNI coordinates: x = ±51, y = –71, z = 1) using spherical regions with a radius of 8 mm (corresponding to the FWHM Gaussian kernel used to smooth functional images). This volume of interest analysis revealed a significant increase in the BOLD-signal in the left EBA [x = –51, y = –74, z = –2; t(18) = 4.15, Ke = 10, P < 0.05 small volume correction—see Fig. 3C], but not in the right EBA. The opposite contrast [building parts relative to body parts—that is, (BuildingSJ + BuildingI) – (BodySJ + BodyI)] led to significant differential activations bilaterally of the parahippocampal and fusiform gyri (see Fig. 3D), consistent with previous studies which described the parahippocampal gyrus as responding strongly to a wide variety of stimuli depicting places and spatial layouts (both outdoor and indoor scenes) compared with various nonplace control stimuli (Aguirre et al. 1998; Epstein and Kanwisher 1998). Three additional clusters of activation were observed: in the middle occipital gyrus bilaterally and in the left superior occipital gyrus. Interactions In order to identify those regions specific for the BSD, we tested the interaction term (BodySJ – BodyI) – (BuildingSJ – BuildingI). Moreover, we used a mask which included in the analysis only those voxels which exhibited a simple effect BodySJ – BodyI greater than t = 1.73 (corresponding to an uncorrected voxel-level threshold of P < 0.05). This procedure allowed us to roule out those brain regions which exhibit the same BOLD response in the 2 tasks when body parts are used, thus reflecting uniquely an effect of identifying (but not judging the distance among) building parts, which is not central to the main purpose of the study. We found a significant increase of the BOLD-signal in the caudal portion of the SPC (x = –22, y = –74, z = 46; Ke = 88, P < 0.05 corrected for the mask), extending vertically from z = 60 to z = 40, thus including to the caudal part of the IPS (see Fig. 4A,B) as well as the dorsal portion of a region in the left superior occipital gyrus which has been associated with the main effect of building parts (see Figs 3D and 4C). A percentage signal change analysis showed that this interaction resulted from an increased neural activity when judging body parts’ distances (relative to the Cerebral Cortex December 2008, V 18 N 12 2883 Figure 2. Behavioral results. Accuracies (A, B) and RTs (C, D) with standard error of the mean (SEM) bars plotted against the 3 levels of the Allocentric distance. Black circles depict the Spatial Judgment Task, whereas white triangles over dashed lines depict the Identification Task. The left column (A, C) represents those conditions in which body parts are shown, whereas the right column (B, D) represents those conditions in which building parts are shown. Table 1 Mean RTs for correct responses and mean accuracies as a function of TASK and STIMULUS (values in parentheses are standard errors) RTs (ms) Spatial judgment Identification Accuracy (%) Human body Building Human body Building 684.50 (19.39) 779.19 (16.28) 683.58 (19.84) 801.56 (14.53) 91.45 (1.73) 92.87 (1.10) 90.48 (1.62) 89.34 (1.57) identification thereof), whereas with building parts no significant difference between the 2 tasks was visible (see Fig. 4D). The inverse interaction, that is, spatial judgment task (relative to the identification task) in the building parts condition, contrasted with the spatial judgment task (relative to the identification task) in the body parts condition [i.e., (BuildingL – BuildingN) – (BodyL – BodyN)], did not reveal any significant differential increase of brain activity. Discussion Coding Space between Screen-Parts, Object-Parts, and Body-Parts Our behavioral results suggest a perceptual grouping effect, in that participants’ assessment of both the identity and the spatial relations of body parts stimuli was easier when these belong to body portions which are close to one another (e.g., an ear and a nose), and become progressively harder the more distant the body parts were (e.g., an ear and a foot—see Fig. 2), even if such information is irrelevant for the main purpose of the task. Perceptual grouping effects have been frequently documented in experimental psychology. For instance, Baylis and Driver (1993) had participants assessing the spatial relationship 2884 Neural Correlates of Body Structural Description d Corradi-Dell’Acqua et al. between object parts on a video display, and found that participants’ response times were faster at judging the distance between parts of the same object than parts of 2 different objects. Likewise, Behrmann et al. (1998) and Zemel et al. (2002) had participants performing a same-different task based on shape properties of object parts, and found that comparisons among parts of the same object were easier than comparisons among parts of different objects. Moreover, Vecera et al. (2001) had participants reporting attributes that appeared on the same part or on different parts of a single multipart object and found them being more accurate in reporting the attributes on the same part than attributes on different parts. All the studies reviewed above show how attentional selection does not rely only on scene-based properties of the display, but also on the representation of the object structure. Also in our experiment, the images adopted as stimuli are not processed only according to their position within the scene, but also within the human body structure. Conceptually there may be at least 3 kinds of regions involved in our study: those involved in coding the spatial relation between parts of the video display (screen-parts); those involved in coding the position that the parts used as stimuli have with respect to the whole object (object-parts); and those involved in coding the spatial relations between object-parts, but only when this object is a human body (bodyparts). In our study we can disentangle between regions coding the relations between body-parts from regions coding the relations between screen-parts and object-parts. Indeed, whereas the last 2 sets of regions can be associated with by the main effect of TASK (spatial judgment vs. identification task), the former is tested by the interaction term, which verifies whether the effect of TASK is limited to the case in which the stimuli are body parts. We found that several regions in the Table 2 Significant clusters showing an increase in activation during 1) the spatial judgment task relative to the identification task, 2) the identification task relative to the spatial judgment task, 3) perception of building parts relative to human body parts, and 4) the interaction STIMULI * TASK Region Cytoarchitectonic probabilistic maps Side Coordinates Ke x y z 1) Spatial judgment task versus identification task: (BodySJ þ BuildingSJ) (BodyI þ BuildingI) Postcentral gyrus Area 1 [80--100%] R Supramarginal gyrus Area 2 [10--60%] Midd. frontal gyrus R Inf. frontal gyrus (p. orb.) Inf. frontal gyrus (p. orb.) Area 44 [40--80%] R Inf. temporal gyrus R Postcentral gyrus Area 2 [0--50%] L Midd. occipital gyrus R Postcentral gyrus Area 1 [30--80%] L Supramarginal gyrus Area 2 [0--60%] 48 60 44 46 56 64 62 44 44 40 36 28 46 42 10 52 26 82 40 40 64 46 12 2 16 12 44 22 64 50 2) Identification task versus spatial judgment task: (BodyI þ BuildingI) (BodySJ þ BuildingSJ) Lingual gyrus Area 18 [0--10%] R Inf. occipital gyrus L Putamen L Thalamus Inf. frontal gyrus Area 44 [30--60%] L Inf. frontal gyrus (p. tri.) Area 45 [30--50%] Sup. frontal gyrus Area 6 [40--80%] L Middle temporal gyrus L Precentral gyrus Area 6 [10--20%] L Area 4a [10--30%] Caudate nucleus (head) R 16 44 22 20 46 46 6 52 50 42 12 50 84 0 28 16 28 2 42 4 12 2 2 14 66 0 18 28 66 12 50 46 22 3) Building versus human body parts: (BuildingSJ þ BuildingI) (BodySJ þ BodyI) Parahippocampal gyrus Fusiform gyrus Parahippocampal gyrus Fusiform gyrus Midd. occipital gyrus Midd. occipital gyrus Area 18 [0--20%] Precuneus Sup. occipital gyrus 26 28 30 26 40 26 16 20 40 32 48 28 84 94 64 74 20 22 20 28 14 12 42 34 22 74 46 4) Interaction: (BodySJ BodyI) (BuildingSJ BuildingI) Sup. parietal cortex R L R L L L 2921§ 1327§ 594§ 313§ 299§ 276{ 224{ 19 354§ 2101§ 1880§ 938§ 750§ 661§ 171|| 1105§ 1018§ 526§ 417§ 308§ 88** Note: All clusters survive a threshold of P \ 0.05 corrected for multiple comparisons at the cluster level for the whole brain or for the mask, respectively. Coordinates (in standard MNI space) refer to maximally activated foci as indicated by the highest T score within an area of activation: x 5 distance (mm) to right (þ) or left () of the midsagittal line; y 5 distance anterior (þ) or posterior () to the vertical plane through the anterior commissure (AC); z 5 distance above (þ) or below () the intercommissural (AC--PC) line. L and R refer to left and right hemispheres, respectively. §P \ 0.001, { P \ 0.01, ||P \ 0.05, and **P \ 0.05 corr. for the mask. right hemisphere, including the postcentral gyrus, the supramarginal and the intraparietal cortex were activated by spatial processing of stimuli irrespective of their identity, whereas the left posterior SPC extending to the IPS was found active only when the stimuli were body parts. The involvement of regions in the right hemisphere for scene-based spatial judgments is consistent with previous fMRI studies which had participants carrying out perceptual tasks on egocentric (rather than allocentric) spatial localizations (Vallar et al. 1999; Galati et al. 2000; Committeri et al. 2004; Neggers et al. 2006), and with patient-studies which reported that lesions in the right posterior parietal cortex, as well as in the right premotor cortex, may induce unilateral neglect which, in most cases, involves a disruption of egocentric representations of space (Vallar and Perani 1986; Leibovitch et al. 1998; Halligan et al. 2003). Right hemisphere regions were also associated with object-based spatial judgments. For instance, studies using the mental rotation paradigm (Shepard and Metzler 1971) found the right SPC involved in object-based judgments of oblique stimuli irrespective of whether these were human bodies (Zacks et al. 2003) or alphabetic stimuli (Harris and Miniussi 2003; see also Parsons 2003). Moreover, Behrmann et al. (2006) reported the case of a patient with integrative agnosia and a lesion of the right temporal cortex, who after being trained to identify abstract objects (each of which was composed of subparts), was unable to report the correct arrangement of the parts in the target object, despite being still able to identify them when presented together with other stimuli. The Neural Basis of the BSD Structural description models (Marr and Nishihara 1978; Biederman 1987) imply that object processing occurs in 2 stages: 1) processing the image features and 2) coding their spatial relationships. Recent clinical observations suggest that a human shape may undergo the same 2-stage-processing, only the latter of which seems to be damaged in autotopagnosic patients (Sirigu et al. 1991; Buxbaum and Coslett 2001; Schwoebel and Coslett 2005). Our study extends these clinical findings by distinguishing between those areas associated with processing human body parts, and activated in both the spatial judgment and the identification tasks, and those areas associated with coding their spatial relations, and activated only in the spatial judgment, but not in the identification, task. We found that left EBA was active during visual processing of body parts irrespective of the task performed (see also Urgesi et al. 2007, for a similar argument). This result is consistent with previous imaging studies which described EBA responding to visual stimuli depicting human bodies or body parts in Cerebral Cortex December 2008, V 18 N 12 2885 Figure 3. Cortical activations associated with the TASK (A, B) and STIMULI (C, D) main effects: (A) glass-brain images and surface rendering of functional contrasts testing the spatial judgment task relative to the identification task; (B) surface rendering of functional contrasts testing the identification task relative to the spatial judgment task; (C) surface rendering, axial (z 5 2) and sagittal (x 5 53) sections of the functional contrast testing human body parts relative to building parts; (D) glass-brain images and surface rendering of functional contrasts testing building parts relative to human body parts. a category selective fashion (Downing et al. 2001; Downing, Chan, et al. 2006). Moreover, repetitive transcranial magnetic stimulation over EBA has been shown to impair identification of human body parts (but not face or object parts—Urgesi et al. 2004), thus showing that EBA is not only correlated with, but also causally involved in visual processing of isolated body parts, which is a necessary prerequisite for building a structural description of a human shape. However, the functional pattern exhibited by EBA has been shown to go beyond the identification of a specific category of visual stimuli: indeed, 2886 Neural Correlates of Body Structural Description d Corradi-Dell’Acqua et al. this region was found to be involved also in processing either biological motion (Grossman and Blake 2002; Peelen et al. 2006) or static pictures depicting human bodies in action (Downing, Peelen, et al. 2006), as well as in blind execution (either real or imaginary) of simple visually guided movements (Astafiev et al. 2004). We also found the posterior portion of the left SPC and IPS being more active while assessing the spatial relationships among body parts rather during the identification thereof. This activation was body parts specific, in that no task difference Figure 4. Cortical activations associated with the interaction STIMULI * TASK. (A, B) Sagittal (x 5 22) and axial (z 5 40, 46, 52, 58) sections reporting an activation on the caudal portion of the superior parietal lobe. The activated area extends vertically from z 5 40 to z 5 60, and involves the caudal part of the left SPC as well as the caudal part of the intraparietal sulcus. (C) Surface rendering of the functional contrasts testing the interaction term (depicted in red) and the main effect building relative to human body parts (green). (D) The percentage signal changes associated with 2 overlapping regions found significant for the interaction term (left graph—local maxima: 22, 74, 46) and for the main effect of STIMULI (right graph—local maxima: 16, 64, 42) are also displayed together with standard error of the mean (SEM) bars: turquoise lines refer to human body parts visual stimuli, whereas red dashed lines refer to building parts. (E) Differential percentage signal changes describing, for each region and for each kind of stimulus, the Spatial Judgment task minus the Identification task, are displayed together with SEM bars. was found when nonbody parts were used, and, therefore, it cannot be considered due to coding the spatial relations among screen-parts or object-parts. This result is consistent with previous clinical studies which document patients with lesion in the left hemisphere (Odgen 1985; Buxbaum and Coslett 2001; Schwoebel et al. 2001) and, more specifically, in the left posterior parietal cortex (Semenza 1988; Denes et al. 2000; Guariglia et al. 2002), suffering from autotopagnosia. One of the striking characteristics of these patients is their preserved ability to reproduce, in a puzzle-like fashion, the correct arrangement of sub-components of any kind of objects but faces (Odgen 1985; Guariglia et al. 2002) and human bodies (Guariglia et al. 2002). Similarly, the left posterior IPS has been implicated also in a pointing task similar to the 1 used to diagnose autotopagnosia (Felician et al. 2004), or in mentally browsing a visual model of the human body (Le Clec’H et al. 2000). Our results converge, and also extend, the findings reviewed above by specifying, both anatomically and functionally, a region over and around the left caudal IPS as involved in the BSD and which, if damaged, may lead to autotopagnosia. One could argue that the functional pattern of the left posterior SPC and IPS is inconsistent with these regions being involved in spatial assessment of body parts, in that building parts never elicit a weaker response than human body parts. As shown in Figure 4C, a region in the left superior occipital gyrus, which has been significantly associated to the main effect of STIMULI, extends to the caudal IPS partially overlapping the region which has been associated with the interaction term. The functional pattern of these 2 overlapping regions is quite similar in that they both respond more to building than to human body parts (see Fig. 4D), thus suggesting the neural activity measured in the SPC and IPS to be confounded by the activity measured in the neighboring (and overlapping) region. However, the left caudal SPC and IPS (but not the left superior occipital gyrus) responds also more when assessing the spatial relations among human body parts relative to their identification (see Fig. 4E, in which confounds due to a general effect of STIMULI are hidden); such signal increase is significantly larger than 0 (as insured by the masking procedure) as well as significantly larger than the signal increase associated with task differences using building parts as stimuli (as insured by the significant interaction effect). Thus, the left caudal SPC and IPS alone respond more to the spatial judgment than to the identification task, but only when the stimuli used are body parts. Do the Left Posterior SPC and IPS Respond to the Attentional Demands of the Employed Tasks? The regions in the posterior portion of the left SPC and IPS found significantly associated with the interaction term have also been described to activate proportionally to the attentional demands of visuospatial tasks (Corbetta et al. 2000; Hahn et al. 2006—see also Corbetta and Shulman 2002, as a review). Cerebral Cortex December 2008, V 18 N 12 2887 Moreover, regions in the IPS have been documented active when participants performed nonspatial, selective attention tasks (Coull and Frith 1998; Wojciulik and Kanwisher 1999; Marois et al. 2000—see also Husain and Nachev 2007, as a review). One could argue that the sensitivity of these regions to the interaction term may reflect differential attentional demands in the 4 conditions. However, this interpretation seems unlikely. First of all, the design matrix in the 1st level analysis included participants average RTs and accuracy rates (which are our only available predictors for testing this alternative hypothesis) as additional regressors of no interest; thus, each brain region whose activity was modulated uniquely by task difficulty should be explained by these regressors and, therefore, not be revealed by the analysis of the factors STIMULI and TASK. Second, the functional pattern of the left posterior SPC and IPS (described in Fig. 4D), is inconsistent with task difficulty of the 4 block conditions (described by Table 1). In particular, the Identification Task delays participants significantly more than the Spatial Judgment Task, especially when building parts are shown; this is inconsistent with the functional pattern of the left posterior SPC and IPS in which the Spatial Judgment task is associated with a higher neural activity than the Identification task when body parts (but not building parts) are shown. BSD and One’s Own Body The functional pattern of the left posterior IPS contrasts noticeably with previous imaging studies in which regions in both the anterior and ventral IPS (either bilaterally or in the left hemisphere only) were found active when participants experienced the rubber hand illusion (Ehrsson et al. 2004, 2005; Tsakiris et al. 2007), as well as when they carried out tasks which required them to process inputs arising from multiple sensory modalities (Bremmer et al. 2001; Grefkes et al. 2002). Taken together these results suggest that, whereas regions in the anterior and ventral IPS bilaterally take part in the integration of multimodal information about one’s own body and body posture (i.e., the body schema), regions in the left posterior IPS are involved in processing the spatial relations among parts of an abstract body (i.e., the BSD). Lesions in the left hemisphere of right-handed patients may induce ideomotor apraxia, which results in the inability to imitate meaningless gestures even by using the nonparetic limb ispilateral to the lesion (De Renzi et al. 1980; De Renzi and Faglioni 1999; Goldenberg and Strauss 2002). Goldenberg and Karnath (2006) have recently found a double dissociation between patients who are unable to imitate hand postures (which show a lesion over the left temporo-parietal junction extending to the inferior parietal lobe) and patients who are unable to imitate finger postures (which show a lesion over the left inferior frontal gyrus). They suggested that hands (but not finger) postures can be coded as simple spatial relationships between a limited set of discrete body parts (e.g. the position of the hand with respect to eyes, chin, shoulders, etc.—Goldenberg 2002), and that such spatial information may be stored in the left parietal cortex. Our results converge with this theory by identifying a region in the left caudal IPS in which spatial relations among visually presented body parts are specifically processed. It is, therefore, possible that, during the perception of a meaningless gesture, the visual information about body parts spatial relations may be coded by this region, and that imitation may result in applying this spatial information over 2888 Neural Correlates of Body Structural Description d Corradi-Dell’Acqua et al. portions of one’s own body which are homologous to the ones seen. Funding Deutsche Forschungsgemeinschaft (KFO 112, TP1) to G.F.R. Notes We are grateful to out colleagues of the Institute of Neuroscience and Biophysics, Department of Medicine, Research Center Jülich. Conflict of Interest : None declared. Address correspondence to Dr. Corrado Corradi-Dell’Acqua. Cognitive Neuroscience Sector, Scuola Internazionale Superiore di Studi Avanzati (SISSA/ISAS), via Beirut 2-4, 34014 Trieste, Italy. Email: [email protected]. References Aguirre GK, Zarahn E, D’Esposito M. 1998. An area within human ventral cortex sensitive to ‘‘building’’ stimuli: evidence and implications. Neuron. 21:373--383. Astafiev SV, Stanley CM, Shulman GL, Corbetta M. 2004. Extrastriate body area in human occipital cortex responds to the performance of motor actions. Nat Neurosci. 7:542--548. Baayan RH, Piepenbrock R, Gulikers L. 1993. The CELEX lexical database (CD-ROM). Philadelphia (PA): Linguistic Data Consortium, University of Pennsylvania. Baylis GC, Driver J. 1993. Visual attention and objects: evidence for hierarchical coding of location. J Exp Psychol Hum Percept Perform. 19:451--470. Behrmann M, Peterson MA, Moscovitch M, Suzuki S. 2006. Independent representation of parts and the relations between them: evidence from integrative agnosia. J Exp Psychol Hum Percept Perform. 32:1169--1184. Behrmann M, Zemel RS, Mozer MC. 1998. Object-based attention and occlusion: evidence from normal participants and a computational model. J Exp Psychol Hum Percept Perform. 24:1011--1036. Biederman I. 1987. Recognition-by-components: a theory of human image understanding. Psychol Rev. 94:115--147. Botvinick M, Cohen J. 1998. Rubber hands ‘feel’ touch that eyes see. Nature. 391:756. Bremmer F, Schlack A, Shah NJ, Zafiris O, Kubischik M, Hoffmann K, Zilles K, Fink GR. 2001. Polymodal motion processing in posterior parietal and premotor cortex: a human fMRI study strongly implies equivalencies between humans and monkeys. Neuron. 29:287--296. Buxbaum LJ, Coslett HB. 2001. Specialised structural description of human body parts: Evidence from autotopagnosia. Cogn Neuropsychol. 14:289--306. Chan AW, Peelen MV, Downing PE. 2004. The effect of viewpoint on body representation in the extrastriate body area. Neuroreport. 15:2407--2410. Cohen L, Martinaud O, Lemer C, Lehéricy S, Samson Y, Obadia M, Slachevsky A, Dehaene S. 2003. Visual word recognition in the left and right hemispheres: anatomical and functional correlates of peripheral alexias. Cereb Cortex. 13:1313--1333. Committeri G, Galati G, Paradis A, Pizzamiglio L, Berthoz A, LeBihan D. 2004. Reference frames for spatial cognition: different brain areas are involved in viewer-, object-, and landmark-centered judgments about object location. J Cogn Neurosci. 16:1517--1535. Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. 2000. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci. 3:292--297. Corbetta M, Shulman GL. 2002. Control of goal-directed and stimulusdriven attention in the brain. Nat Rev Neurosci. 3:201--215. Corradi-Dell’Acqua C, Rumiati RI. 2007. What the brain knows about the body: evidence for dissociable representations. Brain development in learning environments: embodied and perceptual advancements. Newcastle (UK): Cambridge Scholars Publishing. p. 50--64. Coull JT, Frith CD. 1998. Differential activation of right superior parietal cortex and intraparietal sulcus by spatial and nonspatial attention. Neuroimage. 8:176--187. De Renzi E, Faglioni P. 1999. Apraxia. In: Denes G, Pizzamiglio L, editors. Handbook of clinical and experimental neuropsychology. Hove (UK): Psychology Press. p. 421--440. De Renzi E, Motti F, Nichelli P. 1980. Imitating gestures. A quantitative approach to ideomotor apraxia. Arch Neurol. 37:6--10. Denes G, Cappelletti JY, Zilli T, Dalla Porta F, Gallana A. 2000. A category-specific deficit of spatial representation: the case of autotopagnosia. Neuropsychologia. 38:345--350. Downing PE, Chan AW, Peelen MV, Dodds CM, Kanwisher N. 2006. Domain specificity in visual cortex. Cereb Cortex. 16:1453--1461. Downing PE, Jiang Y, Shuman M, Kanwisher N. 2001. A cortical area selective for visual processing of the human body. Science. 293:2470--2473. Downing PE, Peelen MV, Wiggett AJ, Tew BD. 2006. The role of the extrastriate body area in action perception. Soc Neurosci. 1:52--62. Duhamel JR, Colby CL, Goldberg ME. 1998. Ventral intraparietal area of the macaque: congruent visual and somatic response properties. J Neurophysiol. 79:126--136. Ehrsson HH, Holmes NP, Passingham RE. 2005. Touching a rubber hand: feeling of body ownership is associated with activity in multisensory brain areas. J Neurosci. 25:10564--10573. Ehrsson HH, Spence C, Passingham RE. 2004. That’s my hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science. 305:875--877. Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. 2005. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 25:1325--1335. Epstein R, Kanwisher N. 1998. A cortical representation of the local visual environment. Nature. 392:598--601. Evans AC, Kamber M, Collins DL, MacDonald D. 1994. An MRI based probabilistic atlas of neuroanatomy. In: Shorvon S, Fish D, Andermann F, Byder GM, editors. Magnetic resonance scanning and epilepsy. New York: Plenum. p. 263--274. Felician O, Ceccaldi M, Didic M, Thinus-Blanc C, Poncet M. 2003. Pointing to body parts: a double dissociation study. Neuropsychologia. 41:1307--1316. Felician O, Romaiguère P, Anton J, Nazarian B, Roth M, Poncet M, Roll J. 2004. The role of human left superior parietal lobule in body part localization. Ann Neurol. 55:749--751. Fink GR, Frackowiak RS, Pietrzyk U, Passingham RE. 1997. Multiple nonprimary motor areas in the human cortex. J Neurophysiol. 77:2164--2174. Fink GR, Marshall JC, Weiss PH, Stephan T, Grefkes C, Shah NJ, Zilles K, Dieterich M. 2003. Performing allocentric visuospatial judgments with induced distortion of the egocentric reference frame: an fMRI study with clinical implications. Neuroimage. 20:1505--1517. Foerster O. 1936. Motorische Felder und Bahnen. Berlin: Springer. Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R. 1995. Analysis of fMRI time-series revisited. Neuroimage. 2:45--53. Galati G, Lobel E, Vallar G, Berthoz A, Pizzamiglio L, Le Bihan D. 2000. The neural basis of egocentric and allocentric coding of space in humans: a functional magnetic resonance study. Exp Brain Res. 133:156--164. Goldenberg G. 2002. Body perception disorders. In: Ramachandran VS, editor. Encyclopedia of the human brain. San Diego (CA): Academic. p. 443--458. Goldenberg G, Karnath H. 2006. The neural basis of imitation is body part specific. J Neurosci. 26:6282--6287. Goldenberg G, Strauss S. 2002. Hemisphere asymmetries for imitation of novel gestures. Neurology. 59:893--897. Graziano MS, Cooke DF, Taylor CS. 2000. Coding the location of the arm by sight. Science. 290:1782--1786. Grefkes C, Weiss PH, Zilles K, Fink GR. 2002. Crossmodal processing of object features in human anterior intraparietal cortex: an fMRI study implies equivalencies between humans and monkeys. Neuron. 35:173--184. Grossman ED, Blake R. 2002. Brain areas active during visual perception of biological motion. Neuron. 35:1167--1175. Guariglia C, Piccardi L, Puglisi Allegra MC, Traballesi M. 2002. Is autotopoagnosia real? EC says yes. A case study. Neuropsychologia. 40:1744--1749. Haggard P, Kitadono K, Press C, Taylor-Clarke M. 2006. The brain’s fingers and hands. Exp Brain Res. 172:94--102. Hahn B, Ross TJ, Stein EA. 2006. Neuroanatomical dissociation between bottom-up and top-down processes of visuospatial selective attention. Neuroimage. 32:842--853. Halligan PW, Fink GR, Marshall JC, Vallar G. 2003. Spatial cognition: evidence from visual neglect. Trends Cogn Sci. 7:125--133. Harris IM, Miniussi C. 2003. Parietal lobe contribution to mental rotation demonstrated with rTMS. J Cogn Neurosci. 15:315--323. He SQ, Dum RP, Strick PL. 1993. Topographic organization of corticospinal projections from the frontal lobe: motor areas on the lateral surface of the hemisphere. J Neurosci. 13:952--980. Head H, Holmes G. 1911. Sensory disturbances from cerebral lesions. Brain. 34:102--254. Husain M, Nachev P. 2007. Space and the parietal cortex. Trends Cogn Sci. 11:30--36. Le Clec’H G, Dehaene S, Cohen L, Mehler J, Dupoux E, Poline JB, Lehéricy S, van de Moortele PF, Le Bihan D. 2000. Distinct cortical areas for names of numbers and body parts independent of language and input modality. Neuroimage. 12:381--391. Leibovitch FS, Black SE, Caldwell CB, Ebert PL, Ehrlich LE, Szalai JP. 1998. Brain-behavior correlations in hemispatial neglect using CT and SPECT: the Sunnybrook Stroke Study. Neurology. 50:901--908. Luppino G, Matelli M, Camarda RM, Gallese V, Rizzolatti G. 1991. Multiple representations of body movements in mesial area 6 and the adjacent cingulate cortex: an intracortical microstimulation study in the macaque monkey. J Comp Neurol. 311:463--482. Marois R, Chun MM, Gore JC. 2000. Neural correlates of the attentional blink. Neuron. 28:299--308. Marr D, Nishihara HK. 1978. Representation and recognition of the spatial organization of three-dimensional shapes. Proc R Soc Lond B Biol Sci. 200:269--294. Mitz AR, Wise SP. 1987. The somatotopic organization of the supplementary motor area: intracortical microstimulation mapping. J Neurosci. 7:1010--1021. Neggers SFW, Van der Lubbe RHJ, Ramsey NF, Postma A. 2006. Interactions between ego- and allocentric neuronal representations of space. Neuroimage. 31:320--331. Odgen J. 1985. Autotopagnosia: occurrence in a patent without nominal aphasia and with intact ability to point to parts of animals and objects. Brain. 108:1009--1022. Oldfield SR, Phillips JR. 1983. The spatial characteristics of tactile form perception. Perception. 12:615--626. Parsons LM. 1987. Imagined spatial transformations of one’s hands and feet. Cognit Psychol. 19:178--241. Parsons LM. 2003. Superior parietal cortices and varieties of mental rotation. Trends Cogn Sci. 7:515--517. Parsons LM, Shimojo S. 1987. Perceived spatial organization of cutaneous patterns on surfaces of the human body in various positions. J Exp Psychol Hum Percept Perform. 13:488--504. Peelen MV, Wiggett AJ, Downing PE. 2006. Patterns of fMRI activity dissociate overlapping functional brain areas that respond to biological motion. Neuron. 49:815--822. Peissig JJ, Tarr MJ. 2007. Visual object recognition: do we know more now than we did 20 years ago? Annu Rev Psychol. 58:75--96. Penfield W, Rasmussen T. 1950. The cerebral cortex of man. A clinical study of localization of function. New York: Macmillan. Pick A. 1922. Störung der Orientierung am eigenen Körper. Beitrag zur Lehre von Bewußtsein des eigenen Körpers. Psychol Forsch. 2:303--318. Saxe R, Jamal N, Powell L. 2006. My body or yours? The effect of visual perspective on cortical body representations. Cereb Cortex. 16:178--182. Schwoebel J, Coslett HB. 2005. Evidence for multiple, distinct representations of the human body. J Cogn Neurosci. 17:543--553. Schwoebel J, Coslett HB, Buxbaum LJ. 2001. Compensatory coding of body part location in autotopagnosia: evidence for extrinsic egocentric coding. Cogn Neuropsychol. 18:363--381. Cerebral Cortex December 2008, V 18 N 12 2889 Semenza C. 1988. Impairment in localization of body parts following brain damage. Cortex. 24:443--449. Shepard RN, Metzler J. 1971. Mental rotation of three-dimensional objects. Science. 171:701--703. Sirigu A, Grafman J, Bressler K, Sunderland T. 1991. Multiple representations contribute to body knowledge processing. Evidence from a case of autotopagnosia. Brain. 114(Pt 1B):629--642. Tsakiris M, Haggard P. 2005. The rubber hand illusion revisited: visuotactile integration and self-attribution. J Exp Psychol Hum Percept Perform. 31:80--91. Tsakiris M, Hesse MD, Boy C, Haggard P, Fink GR. 2007. Neural signatures of body ownership: a sensory network for bodily selfconsciousness. Cereb Cortex. 17:2235--2244. Urgesi C, Berlucchi G, Aglioti SM. 2004. Magnetic stimulation of extrastriate body area impairs visual processing of nonfacial body parts. Curr Biol. 14:2130--2134. Urgesi C, Calvo-Merino B, Haggard P, Aglioti SM. 2007. Transcranial magnetic stimulation reveals two cortical pathways for visual body processing. J Neurosci. 27:8023--8030. 2890 Neural Correlates of Body Structural Description d Corradi-Dell’Acqua et al. Vallar G, Lobel E, Galati G, Berthoz A, Pizzamiglio L, Le Bihan D. 1999. A fronto-parietal system for computing the egocentric spatial frame of reference in humans. Exp Brain Res. 124:281--286. Vallar G, Perani D. 1986. The anatomy of unilateral neglect after righthemisphere stroke lesions. A clinical/CT-scan correlation study in man. Neuropsychologia. 24:609--622. Vecera SP, Behrmann M, Filapek JC. 2001. Attending to the parts of a single object: part-based selection limitations. Percept Psychophys. 63:308--321. Walshe FMR. 1948. Critical studies in neurology. London: E. and S. Livingstone, Ltd. Wojciulik E, Kanwisher N. 1999. The generality of parietal involvement in visual attention. Neuron. 23:747--764. Zacks JM, Gilliam F, Ojemann JG. 2003. Selective disturbance of mental rotation by cortical stimulation. Neuropsychologia. 41: 1659--1667. Zemel RS, Behrmann M, Mozer MC, Bavelier D. 2002. Experiencedependent perceptual grouping and object-based attention. J Exp Psychol Hum Percept Perform. 28:202--217.