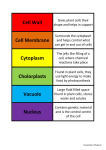
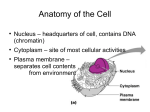
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Ooplasmic donation in humans The potential for epigenic
Whole genome sequencing wikipedia , lookup
Epigenetics of depression wikipedia , lookup
Behavioural genetics wikipedia , lookup
Cell-free fetal DNA wikipedia , lookup
Fetal origins hypothesis wikipedia , lookup
Epigenetics of diabetes Type 2 wikipedia , lookup
Heritability of IQ wikipedia , lookup
Human–animal hybrid wikipedia , lookup
Cancer epigenetics wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Minimal genome wikipedia , lookup
Mitochondrial DNA wikipedia , lookup
Pathogenomics wikipedia , lookup
Human genetic variation wikipedia , lookup
Oncogenomics wikipedia , lookup
Birth defect wikipedia , lookup
Public health genomics wikipedia , lookup
Human Genome Project wikipedia , lookup
Human genome wikipedia , lookup
Microevolution wikipedia , lookup
History of genetic engineering wikipedia , lookup
Genome (book) wikipedia , lookup
Genome evolution wikipedia , lookup
Epigenetics wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Designer baby wikipedia , lookup
Epigenetic clock wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Genomic imprinting wikipedia , lookup
Behavioral epigenetics wikipedia , lookup
Human Reproduction Vol.17, No.4 pp. 850–852, 2002 DEBATE Ooplasmic donation in humans The potential for epigenic modifications Susan M.Hawes1,5, Carmen Sapienza2,3 and Keith E.Latham2,4 1Centre for Early Human Development, Institute of Reproduction and Development, Monash University, Melbourne, Victoria 3168, Australia, 2The Fels Institute for Cancer Research and Molecular Biology, 3Department of Pathology and 4Department of Biochemistry, Temple University School of Medicine, 3307 North Broad Street, Philadelphia, PA 19140, USA 5To whom correspondence should be addressed. E-mail: [email protected] Ooplasm donation, wherein ooplasm is transferred from a donor oocyte to a recipient oocyte in an effort to increase embryo viability, has been applied in the human, with resulting pregnancies and births. Neither the safety nor efficacy of this method has been adequately investigated. Mitochondrial heteroplasmy in the blood of children conceived using ooplasm donation has recently been described. A follow-up study of children born following the use of this technique primarily focused on the presence of mitochondria from the donor oocyte highlighting possible problems due to mitochondrial heteroplasmy. Other effects related to epigenetic events may also arise, but have not been addressed. Studies using inbred mouse strains reveal that genetically diverse ooplasms can impose diverse epigenetic modifications on parental genomes. Incompatibilities produced by combining maternal genome and ooplasm from different genotypes leads to defects in gene expression and development. Such defects can be heritable and observed in the next generation. Given the potential for epigenetic modifications to arise following ooplasm donation, the safety and efficacy of this method need to be evaluated in a suitable animal model. Key words: assisted reproduction/mitochondrial heteroplasmy/ooplasm donation Introduction Cytoplasmic transfer between human oocytes (ooplasm donation) and germinal vesicle transfer, which represents a complete cytoplasmic exchange, have been performed recently as a means to try to improve the outcome of assisted reproduction methods (Cohen et al., 1997, 1998; Zhang et al., 1999). These procedures have been performed essentially in the absence of any basic research to evaluate either the efficacy or the potential risk of the methods. Traditionally, the adaptation of novel scientific techniques for application to human IVF methods involves a ‘try-it-and-see’ approach. Initially, development of culture media for human oocyte and embryo culture involved applying the experience of mouse studies to the human (Bavister et al., 1995; review). This strategy was successful for limited endpoints, such as successful fertilization, embryo cleavage and, ultimately, pregnancy and live birth following embryo transfer. In contrast to this strategy, ICSI, developed for the alleviation of male infertility, had not been investigated in an animal model prior to its use for human IVF (Van Steirteghem et al., 1993). Cohen and colleagues first reported the use of ooplasm donation in humans as a means of obtaining pregnancy in a group of women they categorize as having ‘recurrent implantation failure’ (Cohen et al., 1997, 1998). As pregnancies were obtained after this procedure, an ad hoc explanation of the 850 biological basis has been that a small amount of injected cytoplasm restores some unknown property lacking in the recipient oocyte. Mitochondria, present in cytoplasm and responsible for respiratory processes, provide one possible basis for this effect (Barritt et al., 2001a). Cohen’s group has recently reported that two out of 15 children born as a result of this technique still have traces of donor mitochondrial DNA in their blood cells at 1 year of age (Barritt et al., 2001a). A potential for adverse effects of cytoplasm transfer may exist, based on studies undertaken in rodent models. Surprisingly, these studies have received little or no attention in the context of human oocyte cytoplasm transplantation. In fact, the debate concerning the safety of the method has centred on the possible effect of mitochondrial heteroplasmy (Cummins, 2001). However, possible epigenetic effects exerted upon maternal or paternal genomes by ‘foreign’ cytoplasm must also be taken into account. Numerous studies have revealed that different genotypes of mice modify maternal and paternal genomes differently (Baldacci et al., 1992; Reik et al., 1993; Renard et al., 1994; Pardo-Manuel de Villena et al., 1997, 2000; Roemer et al., 1997; Latham and Sapienza, 1998; Pickard et al., 2001) either during oogenesis (in the case of the maternal genome) or during the period immediately following fertilization. These differences have been revealed either through simple breeding studies (wherein a striking incidence © European Society of Human Reproduction and Embryology Ooplasm donation of embryo lethality is observed), through methylation effects on inherited transgenes, or through microsurgical techniques similar in outcome (i.e. maternal pronuclear exchange between oocytes of different strains) to the cytoplasm transfer procedure. The latter procedures have resulted in abnormalities in gene expression, morphology and physiology, and most disturbingly, these defects can be transmitted to the next generation (Roemer et al., 1997). The genes responsible for these effects are being mapped and may soon be isolated. Other studies have revealed genetic incompatibilities that affect blastomere integrity in the early embryo (Hawes et al., 2001), creating the possibility that cytoplasm transfer could possibly have immediate adverse effects on embryo viability beyond the epigenetic defects described above. A number of these studies have clearly shown this effect(s) being mediated by oocyte cytoplasm action upon pronuclei or embryonic nuclei. For example, transfer of ooplasm from the inbred mouse DDK strain or oocyte RNA to non-DDK oocytes converts the oocyte to a DDK phenotype and causes postzygotic lethality (Renard et al., 1994). The recent paper by Pickard et al. reveals an interesting co-operation between ooplasmic factors and factors expressed post-zygotically to affect transgene function (Pickard et al., 2001). Pronuclear transfers with DBA/2 and C57BL/6 strains reveal that the ooplasm strain-specifically modifies paternal pronuclei postfertilization (Latham and Solter, 1991). Such effects may reflect the fact that much of the epigenetic information displayed in fetal and adult mammals is not completely elaborated in the genome, but rather is acquired ontogenetically (Latham et al., 1995; Latham, 1999). Extrapolating the published observations of these inbred mouse studies to what effects may occur in children born through the use of similar techniques is difficult. Doing so will require knowledge of gene identities, human homologues and allele frequencies in the population. Nevertheless, taking into account the striking observations seen in mouse studies is important when debating possible future implications in the adaptation of this technique in the human, particularly as epigenetic modifications may affect future generations. Moreover, the potential effects of cytoplasm transfer need not be limited to those abnormalities observed in mice. There is abundant evidence to indicate that epigenetic effects leading to aberrant regulation of imprinted genes in humans is associated with serious disease (Falls et al., 1999). The possibility that effects on imprinted genes could arise as a consequence of cytoplasm transfer, and a subsequent imprinting modification of one or both parental genomes must be considered. Although proponents of the ooplasm donation and germinal vesicle transfer methods may opine that it is safe and effective, there is no significant evidence that either opinion is justified. There are no objective criteria to determine whether repeated failure in IVF or ICSI is due to a nuclear or cytoplasmic defect. There are no objective criteria by which individuals are diagnosed with ‘bad’ ooplasm and conversely no objective criteria by which a donor oocyte is diagnosed to have ‘good’ ooplasm. Furthermore, there is no evidence that all oocytes from a given individual are of the same quality. Even in the event that such descriptive terms could be correctly applied, there is no evidence that a case of ‘bad’ ooplasm can be cured by the addition of a dollop of ‘good’ ooplasm. Indeed the whole approach may be akin to trying to improve a bottle of spoiled milk by adding a cup of fresh. The results obtained to date in the clinical setting have been obtained in the absence of suitable experimental controls, and thus it cannot be judged that, when a pregnancy is achieved following the cytoplasm transfer, the transfer in fact contributed to that success. Little consideration has been given to the possibility that some individuals may harbour genetic or epigenetic defects (related to age, for example) that affect chromosome segregation during meiosis and possibly mitosis. If such a defect exists in an individual, and is somehow rescued by cytoplasm transfer, this may leave the potential for chromosome missegregation during mitotic divisions, particularly given the non-disjunction-prone nature of early cleavage divisions (Bean et al., 2001). Such concerns are relevant, given that two of 15 pregnancies have resulted in abortion or miscarriage of fetuses with Turner’s syndrome (Barritt et al., 2001b). It is an admirable goal to try to help infertile couples produce children. The urge to do so, however, must be balanced by a certain degree of caution, regardless of the nobility of this incentive. Pioneering methods such as cytoplasm transfer or germinal vesicle transfer may prove to be valuable treatments for infertility, but should be evaluated in a suitable animal model for both efficacy and safety. Efforts need to be taken to identify genes that may affect epigenetic processes following such procedures, so that appropriate screens can be applied before the method is used. Additional efforts should be taken to understand the genetic basis for infertility in the relevant cases, which could lead to a more refined approach than the currently available microsurgical methods. References Baldacci, P.A., Richoux, V., Renard, J.P., Guenet, J.L. and Babinet, C. (1992) The locus Om, responsible for the DDK syndrome, maps close to Sigje on mouse chromosome 11. Mamm. Genome, 100–105. Barritt, J.A., Brenner, C.A., Malter, H.E. and Cohen, J. (2001a) Mitochondria in human offspring derived from ooplasmic transplantation. Hum. Reprod., 16, 513–516. Barritt, J.A., Brenner, C.A., Malter, H.E. and Cohen, J. (2001b) RBM online. Vol. 3, No. 1. Bavister B.D. (1995) Culture of preimplantation embryos: facts and artifacts. Hum. Reprod. Update, 1, 91–148. Bean, C.J., Hunat, P.A., Millie, E.A. and Hassold, T.J. (2001) Analysis of malsegregating mouse Y chromosome: evidence that the earliest cleavage divisions of the mammalian embryo are non-disjunction-prone. Hum. Mol. Genet., 10, 963–972. Cohen, J., Scott, R., Schimmel, T., Levron, J. and Willadsen, S. (1997) Birth of infant after transfer of anucleate donor oocyte cytoplasm into recipient eggs. Lancet, 350, 186–187. Cohen, J., Scott, R., Alikani, M., Schimmel, T., Munne, S., Levron, J., Wu, L., Brenner, C., Warner, C. and Willadsen, S. (1998) Ooplasmic transfer in mature human oocytes. Mol. Hum. Reprod., 4, 269–280. Cummins, J.M. (2001) Mitochondria: potential roles in embryogenesis and nucleocytoplasmic transfer. Hum. Reprod. Update, 7, 217–228. Falls, J.G., Pulford, D.J., Wylie, A.A. and Jirtle, R.L. (1999) Genomic imprinting: implications for human disease. Am. J. Pathol., 154, 635–647. Hawes, S.M., Chung, Y.G. and Latham, K.E. (2001) Genetic and epigenetic factors affecting blastomere fragmentation in two cell stage mouse embryos. Biol. Reprod., 65, 1050–1056. Latham, K.E. (1999) Epigenetic modification and imprinting of the mammalian genome during development. Curr. Topics Dev. Biol., 43, 1–49. 851 S.M.Hawes, C.Sapienza and K.E.Latham Latham, K.E. and Solter, D.(1991) Effect of egg composition on the developmental capacity of androgenetic mouse embryos. Development, 113, 561–568. Latham, K.E., McGrath, J. and Solter, D. (1995) Mechanistic and developmental aspects of genetic imprinting in mammals. Int. Rev. Cytol., 169, 53–98. Latham, K.E. and Sapienza, C. (1998) Localization of genes encoding egg modifiers of paternal genome function to mouse chromosomes one and two. Development, 125, 929–935. Pardo-Manuel de Villena, F., Naumova, A.K., Verner, A.E., Jin, W.H. and Sapienza, C. (1997) Confirmation of maternal transmission ratio distortion at Om and direct evidence that the maternal and paternal ‘DDK syndrome’ genes are linked. Mamm. Genome, 8, 642–646. Pardo-Manuel de Villena, F., de La Casa-Esperon, E., Williams, J.W., Malette, J.M., Rosa, M. and Sapienza, C. (2000) Heritability of the maternal meiotic drive system linked to Om and high-resolution mapping of the Responder locus in mouse. Genetics, 155, 283–289. Pickard, B., Dean, W., Engemann, S., Bergmann, K., Fuermann, M., Jung, 852 M., Reis, A., Allen, N., Reik, W. and Walter, J. (2001) Epigenetic targeting in the mouse zygote marks DNA for later methylation: a mechanism for maternal effects in development. Mech. Dev., 103, 35–47. Reik, W., Römer, I., Barton, S.C., Surani, M.A., Howlett, S.K. and Klose, J. (1993). Adult phenotype in the mouse can be affected by epigenetic events in the early embryo. Development, 119, 933–942. Renard, J.P., Baldacci, P., Richoux-Duranthon, V., Pournin, S. and Babinet, C. (1994) A maternal factor affecting mouse blastocyst formation. Development, 120, 797–802. Roemer, I., Reik, W., Dean, W. and Klose, J. (1997) Epigenetic inheritance in the mouse. Curr. Biol., 7, 277–280. Van Steirteghem, A.C., Liu, J., Joris, H., Nagy, Z., Janssenswillen, C., Tournaye, H., Derde, M.P., Van Assche, E. and Devroey, P. (1993) Higher success rate by intracytoplasmic sperm injection than by subzonal insemination. Report of a second series of 300 consecutive treatment cycles. Hum. Reprod., 8, 1055–1060. Zhang, J., Wang, C.W., Krey, L., Liu, H., Meng, L., Blaszczyk, A., Adler, A. and Grifo, J. (1999) In vitro maturation of human preovulatory oocytes reconstructed by germinal vesicle transfer. Fertil. Steril., 71, 726–731.