* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Cellular Respiration
Nicotinamide adenine dinucleotide wikipedia , lookup
Magnesium in biology wikipedia , lookup
Metalloprotein wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Biochemical cascade wikipedia , lookup
Signal transduction wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Mitochondrion wikipedia , lookup
Photosynthesis wikipedia , lookup
Electron transport chain wikipedia , lookup
Microbial metabolism wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Citric acid cycle wikipedia , lookup
Biochemistry wikipedia , lookup
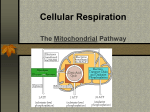
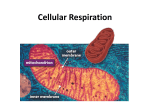
SECTION MENU Cellular Respiration Introduction Mitochondrion Structure Catabolic Pathways Krebs Cycle # Glycolysis Electron Transport Chain Fermentation Oxidative Phosphorylation More Energy With Oxygen Summary of Cellular Respiration Main Menu Visualizing Cell Processes Section Vocabulary Section Quiz (rd Edition) © BioMEDIA ASSOCIATES Introduction Cellular Metabolism Previous Topic Section Menu Main Menu Next Topic Notes: • Photosynthesis is carried out by plants, cyanobacteria and algae. Biochemical pathways in plant chloroplasts convert light energy, H2O and CO2 into stored energy in the form of molecules such as sugars. Oxygen is a waste product. • Cellular respiration releases stored energy by breaking down food molecules such as sugars. Oxygen is consumed. • Photosynthesis and cellular respiration complement each other by cycling CO2 and O2. Photosynthesis produces the O2 and food molecules that cellular respiration uses. Cellular respiration produces CO2 that can be recycled into food by plants. • At night, plants switch from photosynthesis to respiration to meet their energy needs, but they usually burn up only a small portion of their food reserves at this time. Narration Photosynthesis uses light energy to make molecules that fuel the processes of life. During photosynthesis, raw materials—carbon dioxide and water— are converted into energy-rich fuel molecules such as sugars and fatty acids. To harvest the energy stored in these fuel molecules, cells convert them back into carbon dioxide and water. • CO2 is an important atmospheric gas. All organisms generate it, but plants and micro-algae are mainly responsible for re-cycling it. Along with the CO2 from our respiration, humans add large quantities of CO2 to the atmosphere by burning fossil fuels, burning wastes, and so forth. This upsets the natural balance of the CO2 cycle, as we are now observing with global climate change. Obviously, maintaining forests and other long-term plant communities is an important factor for the global balance of CO2. self quiz Visualizing Cell Processes (rd Edition) © BioMEDIA ASSOCIATES Catabolic Pathways Cellular Metabolism Previous Topic Section Menu Main Menu Next Topic Notes: • Fermentation and cellular respiration are the two catabolic (‘breaking - down’) pathways used by organisms to take apart organic molecules and capture energy stored in chemical bonds. The cell’s main energy currency is ATP, adenosine triphosphate, so catabolic pathways are needed to put ATP “in the bank”. • To make ATP, a phosphate group is added to ADP, adenosine diphosphate (an abundant molecule in the cell). energy from ADP + Phosphate + = ATP catabolic reaction • When the cell needs energy, ATP provides it by donating an energy-rich phosphate – re-forming the ADP molecule. energy-rich ATP = + ADP Phosphate Comparison of Catabolic Pathways Energy-Harvesting Processes Uses Oxygen ? Breakdown Products Effeciency of ATP Production Fermentation Cellular Respiration Glycolysis 1 - Glycolysis 2 - Krebs Cycle 3 -Electron Transport Chain No Usually Ethanol or lactic acid H2O and CO2 Low - Only 2 ATP per sugar molecule High - Up to 18 times as much ATP as fermentation • Cells need a constant supply of ATP, so they continually recycle ADP to ATP. For example, an active muscle cell recycles its ATP at a rate of about 10 million molecules per second! • Both fermentation and cellular respiration start with the same process – glycolysis. To understand how cells get energy from food, it is first essential to understand glycolysis. • Fermentation occurs in the absence of oxygen. It only uses one energy-harvesting process, glycolysis. Only a small portion of the energy available in chemical bonds is collected. Cellular respiration combines glycolysis with two extra processes: the Krebs cycle and the electron transport chain. These three processes together extract much more energy than glycolysis alone. self quiz Visualizing Cell Processes (rd Edition) © BioMEDIA ASSOCIATES Glycolysis/ Cellular Metabolism Previous Topic Section Menu Main Menu Next Topic Notes: • Fermentation and celluar respiration both start with the same energy-harvesting process – glycolysis. During glycolysis, a sugar molecule, glucose, breaks down into two molecules of pyruvate (the ion form of pyruvic acid). • All cells use glycolysis to break down food molecules. It is one of the most ancient biochemical pathways - it must have evolved very early in protocells leading to the three domains of life. • Glycolysis doesn’t need a special region of the cell or organelle – it takes place right in the cell’s cytosol. Again, this indicates a primitive biochemical pathway that evolved in prokaryotes. Narration This module examines glycolysis, the first stage in the breakdown of a sugar, a simple molecule, into two molecules of pyruvic acid. Glycolysis occurs in the cytosol, not in mitochondria where the final steps of aerobic metabolism take place. • After glycolysis has converted sugars (and other food molecules) to pyruvate, two things can happen to the pyruvate. 1) In most anaerobic bacteria, and other cells lacking oxygen (such as our muscle cells during heavy exercise), the pyruvate is converted to a simple 2- or 3- carbon compound that is excreted out of the cell such as ethanol or lactic acid. This is fermentation. 2) The pyruvate can enter mitochondria, and, in the presence of oxygen, be broken down further into H 2O and CO 2, through two more processes – the Krebs cycle linked to the electron transport chain . This is cellular respiration. self quiz Visualizing Cell Processes (rd Edition) © BioMEDIA ASSOCIATES Glycolysis Poster Glycolysis/ Cellular Metabolism Previous Topic Section Menu Main Menu Next Topic Notes: • In glycolysis, a glucose molecule entering the cell is acted on by a new enzyme in each of nine steps. Step one transfers a phosphate group from ATP to the sugar, a process called phosphorylation. The phosphorylated sugar has an electrical charge preventing it from leaving the cell, due the impermeability of the cell membrane to ions. In step three another molecule of ATP is used up, and another phosphate group is added to the sugar. In step four the molecule is split into two three-carbon sugars. These sugars are isomers of each other, but only one, glyceraldehyde phosphate, P-GAL, can be used in the steps that follow. Therefore, the second sugar is converted to P-GAL by an enzyme called isomerase . These first four steps are called the energy-investment phase. Narration The process (Glycolysis) occurs in a series of nine steps mediated by enzymes, depicted here by cylinders. In steps one through four, two ATPs are used to split a glucose molecule into two molecules of glyceraldehyde phosphate— P-GAL. O C H OH H C C H H O P Glyceraldehyde phosphate Extensions: • Step One: conversion of glucose to glucose-6-phosphate. This phosphorylation is accomplished by the enzyme hexokinase. • Step Two: glucose-6-phosphate is rearranged into its isomer, fructose-6-phosphate, by the enzyme phosphoglucoisomerase. • Step Three: the enzyme phosphofructokinase adds a phosphate group from a second ATP to create fructose 1,6-biphosphate. • Step Four: cleavage of fructose 1,6-biphosphate into two isomer sugars, dihydroxyacetone phosphate and glyceraldehyde phosphate (P-GAL). The enzyme aldolase cleaves them, and the enzyme isomerase changes all the sugars to the P-GAL form. self quiz Visualizing Cell Processes (rd Edition) © BioMEDIA ASSOCIATES Glycolysis Poster Glycolysis/ Cellular Metabolism Previous Topic Section Menu Main Menu Next Topic Notes: • In step five, the sugar intermediate is oxidized. Electrons transfer to NAD (in the presence of H+) forming NADH, one for each of the two P-GAL’s. In step six glycolysis starts to produce some ATP, one for each of the two P-GAL’s, so the net energy is now at zero. • The final step produces 2 ATP. Therefore, for every single glucose molecule entering gylcolysis, 2 ATP and 2 NADH result. The last five steps of glycolysis are called the energy-yielding phase. Extensions: • Step Five: P-GAL molecules are oxidized by triose phosphate dehydrogenase into 1,3-diphosphoglycerate. Two NADH (one from each P-GAL) are formed. Narration: In the next steps (steps 5-9 out of 9), electrons from P-GAL are transferred to molecules of NAD+, converting them into molecules of NADH. And two molecules of ATP are produced, paying back the original investment of two ATPs. The final reaction of glycolysis generates two more ATPs and two molecules of pyruvic acid— an important fuel molecule for mitochondria. So the nine steps of glycolysis generate a net gain of two ATPs and two NADHs— energy for cell use. • Step Six: 1,3-diphosphoglycerate becomes 3-phosphoglycerate through the action of the enzyme phosphoglycerokinase. Two ATP (one from each P-GAL product) are harvested. • Step Seven: 3-phosphoglycerate becomes 2-phosphoglycerate through the action of phosphoglyceromutase. • Step Eight: the enzyme enolase forms a double bond in the substrate 2-phosphoglycerate, releasing water and phosphenolpyruvate. • Step Nine: pyruvate kinase catalyses the production of pyruvate, and creates two molecules of ATP at the same time. self quiz Visualizing Cell Processes (rd Edition) © BioMEDIA ASSOCIATES Glycolysis Poster Fermentation Cellular Metabolism Previous Topic Section Menu Main Menu Next Topic Notes: • Fermentation (like cellular respiration) uses glycolysis to produce pyruvate plus 2 ATP and 2 NADH in the cytosol of the cell. However, in fermentation, no more ATP is produced because there is no oxygen present. Without oxygen, the energy still stored in the chemical bonds of pyruvate is not available to the cell. • Fermentation includes glycolysis plus reactions that transfer electrons from NADH to pyruvate or a molecule derived from pyruvate. The two main types of fermentation are: alcohol fermentation and lactic acid fermentation. • Alcohol fermentation converts pyruvate to ethanol (ethyl alcohol) in two steps. These release CO2 and regenerate NAD+ (from NADH, as shown in the poster image and video clip). Alcohol fermentation by yeasts is used in brewing and wine making. Narration In microbes that carry out fermentation exclusively all of their energy carriers are generated by glycolysis. Fermentation is an ancient method of anaerobic metabolism evolving long before oxygen appeared in the atmosphere. • Lactic acid fermentation converts pyruvate to lactate with no release of CO2. Lactic acid fermentation by bacteria or fungi is used to make yogurt and cheese. Humans experience lactic acid fermentation in muscle cells when oxygen is in low supply. The accumulation of lactic acid as a waste product in our muscles may cause cramps. self quiz Visualizing Cell Processes (rd Edition) © BioMEDIA ASSOCIATES More Energy With Oxygen/' Cellular Metabolism Previous Topic Section Menu Main Menu Next Topic Notes: • In the anaerobic environment of early Earth (a reducing atmosphere), oxygen gas was extremely reactive. When the first cyanobacteria began releasing oxygen, they created habitats toxic to the leagues of archea and bacteria that had evolved without oxygen. • Natural selection favored bacteria with mutations that could deal with the oxygen, including those that could USE oxygen to harvest energy from food molecules. The advantages were huge. As much as 18 times more ATP could be produced by catabolic reactions when oxygen is present . • Oxygen is highly electronegative – it has a strong affinity for electrons. This chemical power is harnessed by cellular respiration and used to extract the energy remaining in pyruvate formed by glycolysis. Pyruvate is broken down (oxidized) all the way to CO 2 and H2O. Narration When cyanobacteria began liberating oxygen into the atmosphere, around two billion years ago they drastically changed the evolutionary course of all life to follow. Oxygen’s powerful attraction for electrons made it possible to break down the end product of glycolysis— pyruvic acid— to carbon dioxide and water. Extensions: • Cyanobacteria flourished in the early environment because they made food from sunlight, water and CO2. Today, cyanobacteria are still abundant in: lakes, ponds and rivers; surface waters of the ocean; scums that form on wet surfaces; in wet soil, as symbiotic organisms in lichens, sponges, corals, etc.; and in other habitats. self quiz Visualizing Cell Processes (rd Edition) © BioMEDIA ASSOCIATES More Energy With Oxygen/ Cellular Metabolism Previous Topic Section Menu Main Menu Next Topic Notes: • The number of ATP molecules produced by cellular respiration is up to 38 total. This is a vast improvement over the 2 molecules produced by fermentation . The chemistry behind this increase results from the power of oxygen to attract electrons, also known as the electromotive power, or electronegativity. • Only 2 of the potential 32-38 ATPs produced during cellular respiration actually come from glycolysis. Extensions: • Glycolysis is the only metabolic pathway common to all living organisms, suggesting that glycolysis evolved at the beginning of life, some 3.5+ billion years ago – a time when oxygen was not available on earth. Narration Aerobic respiration is an efficient way to extract energy from fuel molecules. Without oxygen, a molecule of glucose produces two ATPs through glycolysis. The same molecule produces up to 38 ATPs when metabolized using oxygen. As aerobic respiration became established in protist-like microbes and oxygen became more abundant, the pace of evolution picked up, leading to multicellular organisms and the evolutionary explosion of the major animal phyla. • Early cells on earth (3.5 b.y.a) were probably bathed in their food – a rich organic soup: - continuous secretion of organic acids lowered the pH . Proteins that acted as proton pumps to keep the pH inside the cell neutral were favored. ATP synthase may have its roots here. - the supply of ATP was limited. Membrane bound proteins that could transport H+ using electron transport between molecules of different redox potential without spending ATP were favored - some prokaryotes evolved electron transport chains that harnessed more energy than they needed to maintain internal pH. Those cells that could manufacture their own ATP must have had a tremendous competitive advantage. self quiz Visualizing Cell Processes (rd Edition) © BioMEDIA ASSOCIATES Mitochondrion Structure Cellular Metabolism Previous Topic Section Menu Main Menu Next Topic Notes: • The mitochondrion is made up of two membranes, the inner and the outer membrane. The outer membrane is smooth but the inner membrane is convoluted with infoldings called cristae that increase surface area. • In the matrix, many different enzymes are concentrated, and this is where the Krebs cycle takes place. Proteins, such as the enzyme that makes ATP, ATP synthase, are built into the inner membrane. Narration Most of a eukaryotic cell’s ATP synthesis occurs in mitochondria. A mitochondrion is a sack within a sack. The inner sack is folded, increasing surface area for ATP synthesis. Between the inner and outer membranes is the inter-membrane space— a reservoir for hydrogen ions used for synthesizing ATP from ADP. The inner chamber, known as the matrix, is a soup of enzymes that dismantle fuel molecules in the Krebs cycle. The knobs are where ATP is synthesized. Simply put, a mitochondrion is an energy transformer where energy and fuel molecules, such as pyruvic acid, are transferred to ATP. Carbon dioxide, the carbon end product of cellular respiration, diffuses out leaving the cell through the plasma membrane. Extensions: • According to the endosymbiotic theory, mitochondria represent ingested cells that evolved over time to become endosymbionts which evolved further to become integrated into the eukaryotic host. One of the many pieces of evidence supporting this is that the mitochondrion has two membranes – an inner one corresponding to the endosymbiont’s original membrane, and an outer membrane corresponding to the host’s food vacuole membrane. Another element of evidence is that mitochondria have their own DNA, totally separate from the cell DNA. This is likely vestigial DNA left over from the endosymbiont. A mitochondrialike symbiont would have conferred a great advantage to a host cell, providing a method to rid the cell of toxic O 2 and sharing the high yield of energy it harvested through aerobic respiration. self quiz Visualizing Cell Processes Web Link RE 01 Web Link RE 04 (rd Edition) © BioMEDIA ASSOCIATES Krebs Cycle/ Cellular Metabolism Previous Topic Section Menu Main Menu Next Topic Notes: • The Krebs cycle, also called the citric acid cycle , is the second phase in cellular respiration . It occurs in the matrix of the mitochondria, and requires oxygen to proceed. The Krebs cycle oxidizes (and breaks down) the product of glycolysis: pyruvate . • Pyruvate enters the matrix through transport proteins in the mitochondrial membranes. Inside, it reacts in a three-step process. 1) Pyruvate releases CO 2 and becomes acetate , 2) NAD+ is reduced to NADH and 3) coenzyme A (CoA) combines with the acetate, creating acetyl coenzyme A ( acetyl CoA). Narration In eukaryotic cells, pyruvic acid enters the mitochondrion where in the matrix, it reacts with coenzyme A to form an important intermediate: acetyl coenzyme A. Acetyl Co A feeds a two-carbon group into a series of reactions called the Krebs cycle in which the carbon backbones are broken down producing more energy-carrier molecules. • The acetyl CoA is now ready to feed acetate into the Krebs cycle to be further oxidized , meaning that the Krebs cycle intermediates loose electrons. These electrons are first donated to energy-carrier molecules , NADH and FADH 2 which, in turn, pass the electrons to the electron transport chain . Extensions: • The Krebs cycle shows a net transfer of carbon atoms of zero. Two carbons enter in the form of acetate and two different carbons leave in the oxidized form of CO 2, as a waste product. • The Krebs cycle depends on the regeneration of a compound called oxaloacetate , which, when combined with acetyl CoA, begins the cycle all over again. self quiz Visualizing Cell Processes Krebs Cycle Poster (rd Edition) © BioMEDIA ASSOCIATES Krebs Cycle/( Cellular Metabolism Previous Topic Section Menu Main Menu Next Topic Notes: • There are eight steps in the Krebs cycle. • In the first step, acetyl CoA adds it’s acetate (2-carbon) to oxaloacetate (4-carbon) producing citrate (6-Carbon). The coenzyme, CoA, is once again available to be primed with an acetate group (from pyruvate) or to be used in step four. • In step two, water is added to citrate to produce isocitrate (6carbon). • In step three, CO 2 is released producing alpha-ketoglutarate (5-carbon). At this point one NAD+ is reduced (gains electrons) to NADH. Narration Acetyl co A feeds a two-carbon group into a series of reactions called the Krebs cycle in which the carbon backbones are broken down producing more energy-carrier molecules. Beginning the cycle, a two-carbon group from acetyl co A, joins a four-carbon molecule creating a six-carbon intermediate. This molecule then reacts to give up carbon dioxide creating one NADH energy carrier. The next reaction yields another molecule of carbon dioxide and provides enough energy to charge another NADH and produce one ATP. • In step four, another CO2 is lost and another NAD+ is reduced (gains electrons) to NADH. Also, CoA attaches and forms succinyl CoA (the succinyl group is 4-carbon). self quiz Visualizing Cell Processes Krebs Cycle Poster (rd Edition) © BioMEDIA ASSOCIATES Krebs Cycle/ Cellular Metabolism Previous Topic Section Menu Main Menu Next Topic Notes: • Steps 5-8 (out of eight) of the Krebs cycle. • In step five, the CoA of succinyl CoA is replaced by a phosphate group, which is transferred immediately to GDP to make guanosine triphosphate (GTP) and the compound succinate (4-carbon). If GTP donates it’s phosphate group to ADP, ATP will result. • In step six, electrons from succinate oxidize FAD to form FADH 2.This forms fumarate (4-carbon). Like NADH, the FADH2 energy-carrier molecule will donate it’s electrons to the electron transport chain. • In step seven, water is added (hydrolysis) – fumarate becomes malate (4-carbon) . Narration The four-carbon intermediate has sufficient energetic electrons to charge two more energy-carrier molecules reforming the original four-carbon molecule that will react with an incoming acetyl Co A to complete a full cycle. • In step eight, the final step, malate is oxidized producing another molecule of NADH and regenerating oxaloacetate (4-carbon). The cycle is ready to begin again. self quiz Visualizing Cell Processes Krebs Cycle Poster (rd Edition) © BioMEDIA ASSOCIATES Krebs Cycle ) Summary Cellular Metabolism (Energy carriers produced for each Acetyl)CoA) Previous Topic Section Menu Main Menu Next Topic Notes: • In sum, this is what the Krebs cycle produces from one sugar: 2 ATP 6 NADH 2 FADH2 Since 2 ATP is the same amount of ATP produced by glycolysis, it is very clear that the energy-carrier molecules, NADH and FADH2 are needed to charge up more ADP to make ATP, before they donate their energized electrons to oxygen. • This is exactly what happens in the third process of cellular respiration involving the electron transport chain. In this process, the energy carrier molecules work with proteins embedded in the mitochondrial inner-membrane to make more ATPs. Narration So each acetyl Co A entering the Krebs cycle produces enough energetic electrons to charge several energy carriers. Extensions: • When ATP is produced in the first two stages of cellular respiration, (glycolysis and the Kreb’s cycle) the reactions are linked to active, phosphorylated intermediates, or ‘substrates’. This type of reaction is called substrate level phosphorylation . • In the final stage of cellular respiration, the elctron transport chain, coupled with ATP synthase, produces 34 ATP molecules in reactions where oxygen forms water.This process of ATP synthesis is called oxidative phosphorylation. self quiz Visualizing Cell Processes Krebs Cycle Poster (rd Edition) © BioMEDIA ASSOCIATES Electron Transport Chain Cellular Metabolism Previous Topic Section Menu Main Menu Next Topic Notes: • The electron transport chain is a series of molecules embedded in the inner mitochondrial membrane. The cristae provide space for thousands of copies of the electron transport chain in each mitochondrion. The chain is made up of proteins with some non-protein prosthetic groups attached that are necessary for the catalytic functions of the enzymes. During electron transfer, these prosthetic groups alter between a reduced and an oxidized state as they accept and donate electrons. • The function of the electron transport chain is to gradually release the energy produced by glycolysis and the Krebs cycle in manageable amounts. The overall energy drop for electrons travelling from NADH to oxygen is 53 kcal/mol. However this system makes it possible to successfully utilize the energy for ATP synthesis. Narration The Krebs cycle produces the energy carriers that transfer energy to proteins embedded in the inner membrane of a mitochondrion. These electron transport proteins pull hydrogen ions out of the matrix, literally jamming them into the intermembrane space setting up conditions for ATP synthesis. Extensions: • Electrons transferred from food during glycolysis and the Krebs cycle are transferred by NADH to the first molecule of the electron transport chain, flavoprotein. In the next redox reaction, the electron passes to an iron-sulfur protein. Next, the electron passes to ubiquinone. This electron carrier is a lipid. All the carrier molecules below this step are called cytochromes and they contain a heme group. The last cytochrome of the group, cyt a3 passes the electron to molecular oxygen (O 2). The oxygen also picks up a pair of hydrogen (H +) ions from the aqueous solution to form water. self quiz Visualizing Cell Processes Web Link RE 02 Web Link RE 03 (rd Edition) © BioMEDIA ASSOCIATES Oxidative Phosphorylation Cellular Metabolism Previous Topic • Section Menu Main Menu Next Topic Notes: ATP synthase uses an ion gradient to drive oxidative phosphorylation. In this case the ion gradient is a proton or hydrogen ion (H+) gradient. The energy for ATP synthesis comes from the difference in concentration of H+ on opposite sides of the inner mitochondrial membrane. This can also be considered a difference in pH since pH is a measure of H+ concentration. • A flow of electrons through the molecules of the electron transport chain is used to pump H+ (protons) across the inner membrane from the matrix to the intermembrane space. The H+ will diffuse back across the membrane, but only through the ATP synthase proteins. Narration Electrochemical activities within a mitochondrion synthesize most of a cell’s ATP. During the process electron transport proteins pump hydrogen ions into the intermembrane space, creating an imbalance in concentration and in electrical charge. The imbalance initiates a backflow of hydrogen ions through ATP synthase— the knobs on the mitochondrion’s inner membrane. The enzyme uses energy from the backflow of hydrogen ions to synthesize most of a cell’s ATP. • The flow of H+ through the ATP synthase is used to drive the phosphorylation of ADP. Therefore, ATP production depends upon the redox reactions that occur on the electron transport chain, which creates the H+ gradient. This coupling is called chemiosmosis. Chemiosmosis differs from osmosis because protons are being pushed across the membrane, while water molecules freely diffuse in osmosis . Extensions: • The mechanism for creating a H+ gradient from the electron transport chain has been elucidated by scientists. In essence, only some of the carrier molecules in the chain can accept or release H + along with electrons. Therefore at certain stages of the chain, electron transfers cause H+ to be collected and deposited into the intermembrane space. The gradient that results is called a protonmotive force , and has the ability to do work. The mechanism for ATP synthase to harness this down hill current of H+ has not yet been discovered. self quiz Visualizing Cell Processes (rd Edition) © BioMEDIA ASSOCIATES Summary of Cellular Respiration Cellular Metabolism Previous Topic Section Menu Main Menu Next Topic Summary: • Cellular Respiration involves three processes: 1) glycolysis, 2) Krebs cycle (citric acid cycle), and 3) electron transport. • In the cytosol, glycolysis breaks down (oxidizes) sugars to pyruvate in nine steps. In this process 2 ATP molecules and 2 NADH energy carrier molecules are created for each sugar. • Pyruvate travels into the mitochondrion, where it will enter a cycle of reactions called the Krebs cycle. In the Krebs cycle, carbons are conserved – a 2-carbon reactant (acetyl-) enters the cycle, and two oxidized carbons (2 CO 2) leave the cycle. The eight steps of the Krebs cycle produce 2 ATP, 6 NADH, and 2 FADH 2 for every sugar broken down. • • • • • Summary of Cellular Respiration Glycolysis creates pyruvic acid which enters the mitochondrion. The Krebs cycle produces energy carrier molecules that drive an electron transport chain (ETC). The ETC pumps protons into the intermembrane space. Energy from the back-flow of protons through ATP synthase converts ADP to ATP. ATP exits the mitochondrion to the cytosol where it energizes the chemical reactions of the cell. • The NADH and FADH 2 energy-carrier molecules travel to the inner membrane of the mitochondrion where they donate electrons to a series of embedded energy-carrier molecules called the electron transport chain.The electrons are ultimately donated to oxygen in the presence of hydrogen ions, and water is formed. • Using energy from activated electrons transferred from the Krebs cycle by NADH and FADH 2 , the ETC proteins (and one lipid) pump hydrogen ions into the intermembrane space. A special transport protein, ATP synthase, harvests energy as the proteins return to the matrix, to create new ATP molecules from ADP and phosphate. About 30-34 ATP are formed from one original sugar in this oxidative phosphorylation. Visualizing Cell Processes (rd Edition) © BioMEDIA ASSOCIATES