* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Isolation by Calcium-Dependent Translocation to
SNARE (protein) wikipedia , lookup
Phosphorylation wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Magnesium transporter wikipedia , lookup
Signal transduction wikipedia , lookup
Endomembrane system wikipedia , lookup
Protein folding wikipedia , lookup
Protein phosphorylation wikipedia , lookup
Homology modeling wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Protein moonlighting wikipedia , lookup
List of types of proteins wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Protein structure prediction wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Protein mass spectrometry wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Protein purification wikipedia , lookup
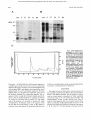
From www.bloodjournal.org by guest on June 16, 2017. For personal use only. Isolation by Calcium-Dependent Translocation to Neutrophil-Specific Granules of a 42-kD Cytosolic Protein, Identified as Being a Fragment of Annexin XI By Carola Sjdlin and Claes Dahlgren Secretion of neutrophil granules is dependent on calcium, but at the same time this process is regulated dflerently for each type of granule. We attempted to find calcium-regulated proteins that selectively translocate from the cytosol to the membranes of the neutrophil granules. An in vitro calcium-dependent translocation assay was designed by mixing cytosol with different neutrophil organelles isolated by subcellular fractionation. lmmunoblotting using an anticytosol antiserum revealed a cytosolic protein of 42 kD that selectively binds to the specific granules of human neutrophils. It was neither associated with the arurophil granules nor with the secretory vesicles/plasma membrane. The pro- T HE NEUTROPHIL granulocyte plays a central role in the defense against microbes. Phagocytosing neutrophils kill and degrade bacteria through the action of toxic oxygen metabolites and proteolytic enzymes.’.2Recognition of the microbes as well as phagolysosome formation, a prerequisite for killing, is dependent on secretion or degranulation of granule constituents. The neutrophil contains four different granule populations (azurophil, specific, and gelatinase granules as well as secretory vesicle^),^ with a content of soluble and membrane-bound proteins that participate in the different cellular events of the activated cell. The secretory process is triggered by stimulating substances that, on coupling to extracellularly exposed receptors, produce signals across the plasma membrane, which gives rise to the intracellular generation of second messengers. Secretion of the neutrophil granules is only partially understood, but a rise in intracellular Ca” is one important ~ i g n a lEven . ~ though the need for calcium is common, secretion of the neutrophil granules is separately regulated. This has been shown using a variety of activating substances, and by manipulating the intracellular Ca” concentration.s Thus, the initial signal has to be diverged inside the cell in order to activate the variety of responses that can be induced in these cells. The mechanism by which Ca” is passing on the secretory signal is not known, but a multitude of calcium binding proteins have been implicated to be the effector molecules.6~’One model for diverged regulation may involve calcium-binding proteins that will affect all types of granules, in concert with other signals and/or cofactors specific for each type of granule. In a second model, the regulating proteins may be unique for a certain type of granule. Hypothesizing either of these models, the granule-regulatoryproteindfactors are expected to be found in the cytosol of the resting cell. Therefore an antiserum against a preparation of neutrophil cytosol was raised in rabbits, and used for identifying Ca*’-dependent cytosolic proteins that are able to associate with neutrophil granules. Among many such proteins, we found one of 42 kD that associates only with the specific granules of the neutrophil. In this work we describe the partial purification of this protein, some biochemical and translocating characteristics,and its identity as a truncated form of annexin XI. Blood, Vol 87, No 11 (June 1). 1996 pp 4817-4823 tein was translocated at a calcium concentration of 100 pmol/L and binding was further increasedby 1 mmol/L calcium. The 42-kD protein was partially purified from neutrophil cytosol by using its afflnity for specific granules and by ion-exchangechromatography. Sodium dodecyl sulfatepolyacrylamidegel electrophoresisof the partly purifiedprotein allowed the 42-kD band to be excised and subjectedto tryptic peptide mapping. Peptides from three peeks were N-terminally sequenced. Searching among known proteins, each one of the amino acid sequences was found to share sequence similarity to annexin XI. 0 1996 by The American Society of Hematology. MATERIALS AND METHODS Isolation of granulocytes. Human polymorphonuclear leukocytes (granulocytes) were isolated from buffy coats obtained from healthy blood bank donors by sedimentation of erythrocytes in dextran, hypotonic lysis, and centrifugation on Ficoll-Hypaque as described by B0yum.’ Granulocytes were washed twice in KrebsRinger medium (120 mmoVL NaCl, 4.9 mmoVL KCl, 1.2 mmoVL MgSO,, 1.7 mmol/L KH2Po4, 8.3 “OIL Na2HP0,, 10 mmoVL glucose, pH 7.3), and thereafter resuspended in saline and treated with diisopropylfluorophosphate (DFF’, 2 p U m L cell suspension, 10 minutes). Isolation of cytosol and organelles. Isolated neutrophils were resuspended in relaxation buffer (100 mmol/L KC1,3 mmom NaCl, 3.5 mmoVL MgClz, 10 mmoVL Pipes, 1 mmollL ATP(Na)2, pH 7.4), supplemented with phenylmethylsulphonyl fluoride (PMSF, 0.5 mmoVL) and Pefabloc (1 mmoVL; Boehringer-Mannheim, Germany), and homogenized by nitrogen cavitation (400 psi, 5 minutes)? The cavitate was collected into EGTA and centrifuged to remove nuclei and debris. The post-nuclear supernatant was layered onto a two-step gradient of Percoll as described.” The a,p, and ybands were collected and identified as azurophil granules (a),specific granules (p), and secretory vesicles/plasma membrane ( y ) , by measuring myeloperoxidase, vitamin B 12-binding protein, and alkaline phosphatase, respectively. The level of purity of the organelle preparations was high, with nearly no cross-contamination of the a- From the Phagocyte Research Laboratory, Department of Medical Microbiology and Immunology, University of Goteborg, Goteborg, Sweden. Submitted November 14, 1995; accepted January 24, 1996. Supported by grants from the Swedish Medical Research Council, King Gustaf the Vth 80-year foundation, the Foundation of Court Judge Emst Colliander and Wqe, the Swedish Society for Medical Research, and the Swedish Society Against Rheumatism. C.S. is the recipient of a PhD grant from the Faculty of Medicine, University of Goteborg. Address reprint requests to Carola Sjolin, BSc, Department of Medical Microbiology and Immunology, Guldhedsgatan 10, S-413 46 Gateborg, Sweden. The publication costs of this article were defrayed in pari by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact. 6 19% by The American Society of Hematology. 0006-4971/96/871I -Oo37$3.O0/0 4817 From www.bloodjournal.org by guest on June 16, 2017. For personal use only. 4818 and y-fraction, while the P-fraction was contaminated with up to 20% of the cell content of azurophil granule^.^^'" Percoll was removed by centrifugation and the organelle fractions were washed once. Organelles were resuspended in relaxation buffer and kept at 4°C until used for translocation experiments. Cytosol for translocation procedures was prepared by cavitating isolated granulocytes as previously described, but the cavitate was collected without EGTA and centrifuged at 100,000g for 1.5 hours (4°C). The pellet was discarded and the supematant stored at 4°C until used as a source of cytosolic proteins. That most of the calciumdependent, membrane-binding proteins remained in the cytosol, despite the lack of EGTA in the medium, was verified by immunoblotting using antibodies to annexin I, 11, 111, IV, V, and VI. Calcium-dependent translocation of cytosolic proteins to isolated organelles. To identify cytosolic proteins that translocate to neutrophil membranes, cytosol and isolated organelles were mixed and incubated at different calcium concentrations as described earlier." The organelles were collected by centrifugation and washed, and thereafter resuspended in relaxation buffer containing EGTA, to release proteins calcium-dependently associated with the organelles. The organelles were collected again and the resulting supematant (EGTA-extract) was stored frozen at -20°C until assayed by immunoblotting. Cytosol antiserum. Human neutrophil cytosol was prepared as described above (EGTA present). Three rabbits were immunized four times at 2-week intervals with 100 fiL of equal parts cytosol preparation (9 mg total proteidml) and Freund's incomplete adjuvant. Animals were bled on four occasions. Sera were kept separate and checked for reactivity against human neutrophil cytosol by immunoblotting of cytosol electrophoresed in sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE). Serum from the fourth bleeding was used as it contained the strongest reactivity against cytosol. SDS-PAGE and immunoblotring. SDS-PAGE was performed according to Laemmli" using 10% (T) homogenous polyacrylamide gels, which were silver stained or electroblotted according to Towbin et all2 onto polyvinylidene difluoride (PVDF) membranes. Membranes were blocked, then incubated with rabbit anti-cytosol antiserum diluted 1/500 in blocking buffer (PBS supplemented with 1% (wthol) dry milk, 1% (wtlvol) bovine serum albumin (BSA), and 0.05% (vol/vol) TWEEN-20). Binding of antibodies was detected by incubation with alkaline phosphatase conjugated swine antirabbit Ig and subsequent development in nitroblue tetrazoliumhromochloro-indolyl-phosphate (NBTISCIP) substrate. Partial purzjkation of the 42-kD protein. An EGTA-extract was prepared (using specific granules, p-fraction) from 4 to 10 buffy coats according to the previous description using a cytosoVgranule ratio of 1:l (in cell equivalents). Instead of relaxation buffer, 2 mmol/L EGTA in 50 mmol/L Na-acetate; pH 5.0 was used in the last step as extracting buffer. The extraction step was repeated once to improve recovery of the 42-kD protein and the second extract was pooled with the first. The pooled sample was either frozen at -70°C or processed immediately in ion-exchange chromatography. An Econo-Pac CM cartridge (BioRad Laboratories, Inc. Hercules, CA) was equilibrated at room temperature (22°C) in 50 mmom Naacetate, 1 mmoln EGTA, 0.05% (vol/vol) Tween-20; pH 5.0 (buffer A). The EGTA-extract was diluted two-fold in buffer A, and Tween20 was added to the previously mentioned final concentration. The extract was applied on the column at a flow rate of 0.7 mL/min, which was then washed with buffer A until free from nonbinding proteins. Bound proteins were eluted by a stepwise gradient of 90, 150, 200, and 1,000 mmol/L NaCl in buffer A at the same flow rate. The 42-kD protein was eluted in the 150 mmol/L NaCl peak. The SJOLlN AND DAHLGREN fractions (1 fractiodminute, 0.7 mL) were analyzed by silver stained SDS-PAGE as well as immunoblotting. The 42-kD protein containing fractions from the CM-EconoPac column were pooled and concentrated in the cold using Ultrafree CL filters (Millipore, Bedford, MA), which were centrifuged at 3,000 rpm (Beckman centrifuge, TH-4 rotor). The buffer of the sample was changed into 20 mmol/L Tris, 1 mmoVL EGTA; pH 8.5 (buffer B) using PDlO columns (Pharmacia, Uppsala, Sweden), thereafter the concentration filters were used again to concentrate the sample to an appropriate volume. A Q-EconoPac column (BioRad Laboratories) was equilibrated at room temperature in buffer B. The concentrated sample containing the 42-kD protein was applied to the column at a flow rate of 0.7 mumin. The 42-kD protein was recovered in the flow-through fraction which was washed off the column by buffer B. Bound proteins were eluted by 1 mol/L NaCl in buffer B. The fractions (1 fraction/ minute, 0.7 mL) were analyzed by silver stained SDS-PAGE as well as immunoblotting. Fractions containing the 42-kD protein were pooled and concentrated in Ultrafree-CL filters. The sample was frozen at -70°C in aliquots until used. In the preparation used for amino acid sequencing, 15 buffy coats were used to prepare the EGTA extract, which contained 4.5 mg of total protein. The pooled and concentrated sample from the CM column, contained 2 mg of protein, which was loaded on the Q column. The yield of partially purified preparation containing the 42-kD protein was 360 pg of total protein (concentration 1 pg/pL). Protein was measured using BCA Protein Assay Reagent. Peptide mapping and internal sequencing of amino acids. The partially purified preparation of 42-kD protein was diluted in reducing Laemmli sample buffer and electrophoresed (33 figllane) in a 15% polyacrylamide gel. The gel was run in the cold at 200 V until the 32.5-kD marker protein of the standard was run out of the gel. Proteins were visualized by staining with Coomassie Brilliant Blue. The 42-kD band from six lanes were excised and digested by treatment with trypsin as described." The digest of the excised protein was chromatographed on a reversed phase HPLC column and the peaks collected and frozen. Peptides from three peaks were N-terminally sequenced using a pulsed-liquid sequencer 473A (Applied Biosystems, New York, NY). Chemicals. Alkaline phosphatase conjugated goat antimouse Ig was from Jackson ImmunoResearch Laboratories, (West Grove, PA). ATP, BCIP, NBT, and PMSF were bought from Sigma Chemical CO, (St Louis, MO). DFP was from Aldrich (Steinheim, Germany). Dextran, Ficoll-Hypaque, and Percoll were products from Pharmacia (Uppsala, Sweden). Pefabloc was from Boehringer-Mannheim (Germany). The PVDF membrane was purchased from Millipore, and the BCA Protein Assay Reagent was from Pierce, (Rockford, IL). RESULTS Calcium-dependent association of cytosolic proteins to neutrophil organelles. To investigate the effect of Ca2+on association of cytosolic proteins with membranes of human neutrophil organelles, isolated a-,B-, and y-fractions (azurophil, specific granules, and secretory vesicles/plasma membrane, respectively) were mixed with cytosol in the presence of Ca". The cytosol was removed and EGTA was added to the organelles to extract the proteins that were calciumdependently associated with the membranes. The EGTA extracts were assayed by immunoblotting using an antiserum raised against proteins of neutrophil cytosol. As seen in Fig 1, several proteins were Ca'+-dependently associated with the membranes of both azurophil and specific granules as From www.bloodjournal.org by guest on June 16, 2017. For personal use only. 4819 ANNEXIN XI IN HUMAN NEUTROPHILS a 34- B E 6 5 4 3 E 6 5 4 3 EGTA/pC;' Y E 6 5 4 3 - 97 - 66 45- - 45 31- - 31 Fig 1. Calcium-dependent association of cytosolic proteinswith membranesof neutrophil organelles. Isolated azurophil granules (4 specific , granules (PI, and plasma membranelsecretory vesicles ( y ) were mixed, respectively, with cytosol in the presence of 2 mmol/L EGTA (€1 or CaCI2 at 1 pmollL (6). 10 pmol/L (5). 100 pmol/L (4). or 1,000 pmol/L (3). Membranes were collected and washed, then resuspended in 2 mmol/L EGTA to elute Ca2+-dependentproteinsfrom the membranes.The organelles were removed and the supernatants were assayed under reducing conditions in 10% polyacrylamidegels, which were immunoblotted using rabbit antihuman neutrophil cytosol antiserum. (>I indicate the position of the 42-kD protein. well as with the membranes of secretory vesicles/plasma membrane. These proteins were of molecular mass approximately 66, 40, 39, and 35 kD and they have been shown earlier to correspond to annexins.'" Binding to these proteins was to be expected because the cytosol antiserum contains antibodies against annexins I, 11, IV, and VI, as shown by ELISA using annexin monoclonals as catching antibodies (C. Sjolin and C. Dahlgren, unpublished observation). Apart from these proteins commonly translocating to all organelles, a protein of 42-kD, migrating as a double band, was associated with the specific granules, but neither with azurophil granules nor secretory vesicles/plasma membranes. Association with the organelles of this protein was detectable at a calcium concentration of 100 pmol/L and was further intensified by raising the concentration to 1 mmol/L. The 42-kD protein was not detected by antibodies directed against an annexin consensus sequence, which recognizes seven different translocating annexins in human neutrophils.'" Purijcution of the 42-kLl protein. The 42-kD protein a p pears to be a minor protein of the human neutrophil as it can be detected only by immunoblotting of EGTA extracts; silver staining of gels was insufficient for its detection,"' (unless highly concentrated EGTA extracts were used). Since the anticytosol antiserum is directed against a multitude of cytosolic proteins, the 42-kD protein cannot be detected in whole cytosol either. Therefore, the granule-binding capacity of the protein was used as the first step of purification, to enrich the protein sufficiently to be detectable by the anticytosol serum. To maximi= recovery of the protein and to increa5e the efficiency of translocation, a granule/cytosol mixing ratio of 1:l (calculated in cell equivalent..) was used and the calcium concentration was raised to 2 mmoVL. Raising the calcium concentration from 1 to 2 mmoVL did not change the translocation profile (ie, those proteins that translocated); only the quantity of the translocated proteins was altered (increased), as judged by immunoblotting. When the translocated proteins were extracted from the granule membranes, an EGTA-containing Na-acetate buffer of pH 5.0 was used (instead of relaxation buffer), because this procedure wa. found to eliminate a great deal of a major contaminant, annexin I, which migrates close to the 42-kD protein in SDS-PAGE. The EGTA extract waq loaded onto a cation-exchangecolumn, CM-EconoPac, equilibrated in acetate buffer at pH 5.0 (Fig 2). At this pH, the 42-kD protein was bound to the column, and it wa5 eluted by 150 mmoVL NaCI as no 42-kD protein came off the column when raising the NaCl concentration further. For further enrichment, the CM fractions containing the 42-kD protein were pooled, concentrated, and subjected to anion-exchange chromatography at pH 8.5 using a Q-EconoPac column (Fig 3). Examination by immunoblotting (Fig 3A) revealed that a minor part of the 42-kD protein was bound to the column together with only a few contaminants (Fig 3A, lane 39), but the major part of the protein was recovered in the flow through fraction (Fig 3A, lanes 4-10). Several unsuccessful attempts were made to scale up and optimize the conditions for purification of the 42-kD protein. Nevertheless, on inspection of the silverstained gel (Fig 38). it was clear that this purification step still contributed to the purification of the 42-kD protein. For attaining the aim of this study, namely to identify the 42-kD protein, the level of purity obtained by the procedure described was sufficient to perform tryptic peptide mapping and subsequent internal N-terminal sequencing of peptides derived from the protein. For this purpose, the flowthrough fractions from the Q column were pooled, concentrated, and run in SDS-PAGE. Determination qf sequences of internal peptides of the 42- From www.bloodjournal.org by guest on June 16, 2017. For personal use only. SJbLIN AND DAHLGREN 4820 A ext 6 37 54 71 84 ext 6 37 54 71 84 (kDa) 9 7 - 66 - I 45 - * - D \*- r, 31 - ml.0 0.8 0.6 0.4 02 10 20 30 40 50 a0 Fraction number kD protein. In SDS-PAGE, the 42-kD protein migrated as a double band, and the lower of the two was excised and digested with trypsin. The digest was chromatographed using reversed phase HPLC and peptides from three peaks (out of approximately 15) were N-terminally sequenced. The three peptide peaks gave altogether four sequences, since one of the fractions contained two sequencable peptides. The sequences (Table 1) could all be found in human annexin XI. In fact, they all matched perfectly to sequences in annexin XI, except for one amino acid in position 275. The amino acid in this position is L (Leucine) in annexin XI, which corresponded to E (Glutamic acid) in our sequence. From the fact that the 42 kD protein is some 12 kD smaller in molecular mass than annexin XI (predicted molecular mass 70 80 90 5 sr Fig 2. Cation-exchange chromatography of EGTA extract from specific granules. An EGTA extract was chromatographed on a CM-EconoPac column at pH 5.0. The flowthrough fractions were collected and the column was eluted with a stepwise gradient of NaCl (-4. (A) The loaded material lextl, the flowthrough fraction (61, and the peak fractions (numbers 37, 54, 71, 84) of the material eluted were analyzed in immunoblotting using the anti-cytosol antiserum. (D)indicates the position of the 42-kD protein. (6)Silver staining of the same samples as in (A). IC) Representative chromatogram of a separation on a CM-EconoPac column. 54 kD), we conclude that the specific granule-bindingprotein recovered is a truncated form of annexin XI. DISCUSSION We sought to find and characterize cytosolic proteins of the neutrophil, which have the ability to bind calcium-dependently to the different granules. For this purpose an antiserum was raised against a preparation of whole neutrophil cytosol. By using an in vitro translocation procedure and immunoblotting, several cytosolic proteins were found to bind all of the organelles that were isolated. Some of these (those of molecular weight approximately 66 and 35 to 40 kD) have previously been found to be identical with annexin I, 11, IV, and VI."' No other proteins associated with the From www.bloodjournal.org by guest on June 16, 2017. For personal use only. 482 1 ANNEXIN XI IN HUMAN NEUTROPHILS with the nucleus.*' In neutrophils, the presence of annexin XI has not been reported before, but both human," rabbit," and bovine2' forms of this protein have been cloned and the amino acid sequence deduced. The predicted molecular mass of human annexin XI is 54 kD, but the protein isolated after translocation to specific granules had a molecular mass of approximately 42 kD. However, as the 42-kD protein exhibits a calcium-dependent translocating behavior and as the protocol used for its isolation is similar to the procedures used by others to isolate annexin XI," it is beyond doubt that the molecule we have isolated is derived from annexin XI. The reason why the antibodies, directed against the annexin consensus sequence,'" failed to recognize the 42-kD protein is most likely because of the fact that the sequence similarity between the consensus peptide (used for raising the antibodies; C-M-K-G-L-G-T-D-E-D-T-L-I) and annexin XI, at the most, only amounted to four amino acids in a row. The molecular mass of the isolated protein indicates that it is a truncated form of annexin XI. It has been reported that there exists alternative splicing of pre-mRNA of bovine annexin XI," which gives rise to two types of annexin XI, differing in the N-terminal tail. This may provide an explanation for the origin of the 42-kD protein. However, we favor the hypothesis that the 42-kD form of annexin XI is a cleavage product of the full-length annexin XI. In consequence, full size annexin XI is expected to be found in the EGTA extracts of specific granules and, in retrospect, there are indications of its presence. Some of our preparations, but not all, did indeed contain proteins in the 54-kD region, which were immunostained with the anti-cytosol antiserum. The blot shown in this study contains only a very faint band in this size-range, but it is possible that the double band around 49 kD is also a truncated form of annexin XI. This suggestion gains support by the fact that this protein follows exactly the biochemical behavior of the 42-kD protein throughout the isolation procedure. Analysis of the sequence data allows the conclusion to be made, that it is the Nterminal rather than the C-terminal part of annexin XI that has been cleaved. Two observations speak for this, namely azurophil granules, as could be identified with the cytosol antiserum, whereas both specific granules and the secretory vesicles/plasma membrane showed capacity to bind (calcium-dependently) a number of other proteins as well. Selective association of cytosolic proteins has been shown to occur with the plasma membrane, eg, gran~alcin,.'.'~ p47 phox and p67 phox,I5 but not in the case of specific granules. We therefore took interest in a protein of approximately 42 kD, translocating to the specific granules at a calcium concentration of 100 pmol/L. Peptide antibodies directed against an annexin consensus sequence were unable to identify this protein as an annexin. The protein could neither be found associated with azurophil granules nor with secretory vesicles/plasma membrane, and was therefore interesting as a putative specific participator in the pathway that regulates exocytosis of specific granules. It may be argued that the unphysiologic calcium concentration needed for translocation in vitro implies that the phenomenon has no biological significance. It is, however, interesting to note that the intracellular calcium concentration can rise to the millimolar range locally in an activated cell.'.'' It has also been shown that annexin I11 accumulates around the phagosome during phagocytosis" and around intracellular inclusions of Chlamydiae," even though annexin 111 needs the same high calcium concentration for translocation to neutrophil organelles in our in vitro system (C. Sjolin and C. Dahlgren, unpublished observations). The reason for this could be that the calcium-dependency and binding-capacity of the organelles is altered during the isolation procedure. However, if this is the case, then the fact that both annexin IV and VI binds equally well to all the different sets of organelles" indicates that the different organelles are equally affected by the isolation procedure. After enrichment of the 42-kD protein, four internal amino acid sequences could be determined, all of which matched with sequences of human annexin XI. Annexin XI, also known as calcyclin associated protein (CAP-50)" and human autoantigen 56K." is an annexin that has been found in the cytosol of several different cells as well as in association A B Q 4 7 10 13 39 Q 4 7 10 13 39 ma)97Fig 3. Enrichment of the 42-kD protein by anionexchange chromatography. The 42-kD protein containing fractions from the CM column were pooled, concentrated, and loaded onto a Q-EconoPaccolumn at pH 8.5. (A) The sample applied on the Q column (01,the flowthrough fractions (4, 7, 10, 131, and the peak fraction of the material eluted with 1 mol/L NaCl (39) were analyzed by immunoblotting using the anti-cytosol antiserum. (D)indicatesthe position of the 42-kD protein. (SISilver staining of the same samples as in (A). 66-~ 45 D 31 - - . m - -=0 - From www.bloodjournal.org by guest on June 16, 2017. For personal use only. 4822 S J ~ L I NAND DAHLGREN Table 1. Alignment of Sequences From Peptides With Sequences of Human Annexin XI Source of Sequence Annexin XI Sequence in One-Letter Code 'y Initial Yield 160 ~ t Fraction 1* (sequence 1) AnnexinXI Fraction I* (sequence 2) Annexin XI Y P G Cl P P I / V ( P ) ( L ) ( P I s t 2F- I n 279 T P V E FDI/V Y * 'FA ** 5 5-10pmol /I 2 Dmol 393 A ~ Fraction 2 A X L V A 1 pmol t. t, Annexin XI Fraction 3 S L Y 3 pmol The lower band of the doublet of the 42 kD protein was excised from SDS-PAGE gels and subjected to tryptic peptide mapping using reversed phase HPLC. Peptides from three peaks were sequenced by automated amino acid sequencing. The yielded sequences were searched for in a database and found to match with human annexin XI. The figure shows the peptide sequences aligned to the corresponding sequences of annexin XI as reported by Misaki et a1.201nitial yield refers to the amount of amino acid chopped off in the first cycle of Edman degradation. Underlined letters (K or R) are cleavage sites indicate a peak in the for trypsin. Amino acids in parentheses ( chromatogram less than one picomole. * Fraction 1 proved to contain a strong (no 1) as well as a weaker sequence (no 2). which were sequenced simultaneously. t Tyrosin precedes the strong peptide of fraction 1 (sequence 1). No cleavage site for trypsin. *There was a single peak of prolin in the second Edman-cycle, which matches with both the strong and the weak sequence. 5 The amino acid chromatogram of this cycle showed a low yield of I and V. After matching of the sequence to annexin XI, it is seen that the V probably comes from sequence 1 of fraction 1. I matches with sequence 2. 1The chromatogram showed a very small peak at the prolin position, which could be confirmed after alignment to the annexin XI sequence. 11 The prolin-peak of the chromatogram higher than in the previous cycle. ** E in the sequenced peptide, but L in the annexin XI sequence. tt No peak in the second cycle, which is consistent with the knowledge that histidine is often hard to detect in Edman degradation procedures. that there is no trypsin cleavage site N-terminally of the strong sequence of fraction 1 (sequence 1 in Table 1). That such a peptide would be sequencable at all, could be explained if the sequence corresponds to the N-terminal of the 42-kD protein. In other words, it is likely that the N-terminal of the 42-kD protein corresponds to amino acid 152 of annexin XI. The calculated molecular weight of such a protein would be approximately 39 kD, which is not too far from our estimations. Furthermore, the calculated PI would be 8.14, which also is in agreement with the biochemical behavior of the 42-kD protein. The second argument is that if the C-terminal were chopped off to give a protein with a molecular mass of 42 kD, then the SLY sequence in position 480-482 would not have been present in the truncated protein; a truncation of the protein C-terminally of the SLY sequence would render approximately 95% of annexin XI intact, which in turn would give a molecule of about 51 kD. Members of the annexin family are characterized by the ability to bind biological membranes in a calcium-dependent manner. Even though the precise mechanisms whereby annexin molecules bind to membranes is as yet not fully elucidated, it is known that the N-terminal sequences of annexins are unique to each type of annexin, and that structural alterations in this part of the molecule act to regulate specific functions of the annexin. The importance of the Nterminal part is clearly illustrated by the effects induced by an N-terminal truncation of annexin I, which can be mediated by a membrane-bound protease that specifically cleaves the annexin I m ~ l e c u l e . ~Truncation ~ ~ * ~ of annexin I has been shown to change the biological properties of the protein in that the ability to aggregate granules is altered:' as is the selectivity with respect to granular binding,26and in that less calcium is required for membrane binding." This work has not been directly concerned with elucidating the mechanisms and relevance of the truncation of annexin XI. However, when taking into consideration that N-terminal modifications in the form of phosphorylations (which has indeed been shown for annexin XIz9),truncations, and co-protein binding (calcyclin3','') are thought to be the means by which regulation of cellular processes occur, it is obvious that these matters are of importance and need to be investigated in more detail. Degradation of annexin XI as well as the binding characteristics of the full length protein compared with the truncated form@) will be studied as soon as we have access to specific annexin XI antibodies. ACKNOWLEDGMENT Many thanks to Anders Blomberg and Joakim Norbeck for performing peptide mapping and amino acid sequencing. REFERENCES I . Klebanoff SJ: Oxygen metabolites from phagocytes, in Gallin JI, Goldstein IM, Snyderman R (eds): Inflammation: Basic Principles and Clinical Correlates, 2nd Ed. New York, NY Raven Press, 1992, p 541 2. Elsbach P, Weiss J: Oxygen-independent antimicrobial systems o f phagocytes, in Gallin JI, Goldstein IM, Snyderman R (eds): Inflammation: Basic Principles and Clinical Correlates, 2nd Ed. New York, NY, Raven Press, 1992, p 603 3. Borregaard N, Lollike K, Kjeldsen L, SengelW H, Bastholm L, Nielsen MH, Bainton DF: Human neutrophil granules and secretory vesicles. Eur J Haematol 51:187, 1993 4. Almers, W: Exocytosis. Annu Rev Physiol 52:607, 1990 5. Lew PD, Monod A, Waldvogel FA, Dewald B, Baggiolini M, Pozzan T: Quantitative analysis o f the cytosolic free calcium dependency o f exocytosis from three subcellular compartments in intact human neutrophils. J Cell Biol 102:2197, 1986 6. Creutz CE: The annexins and exocytosis. Science 258:924, 1992 7. Burgoyne RD, Morgan A: Regulated exocytosis. Biochem J 293:305, 1993 From www.bloodjournal.org by guest on June 16, 2017. For personal use only. ANNEXIN XI IN HUMAN NEUTROPHILS 8. Boyum A: Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Invest 97:77, 1968 9. Borregaard N,Heiple JM, Simons ER, Clark RA: Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: Translocation during activation. J Cell Biol 9752, 1983 10. Sjolin C, Stendahl 0, Dahlgren C: Calcium-induced translocation of annexins to subcellular granules of human neutrophils. Biochem J 300:325, 1994 11. Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680, 1970 12. Towbin H, Staehelin T, Gordon J: Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc Natl Acad Sci USA 76:4350, 1979 13. Norbeck J, Blomberg A: Gene linkage of two-dimensional polyacrylamide gel electrophoresis resolved proteins from isogene families in Saccharomyces cerevisiae by microsequencing of in-gel trypsin generated peptides. Electrophoresis 16:149, 1995 14. Borregaard N, Kjeldsen L, Lollike K, sen gel^ H: CaZCdependent translocation of cytosolic proteins to isolated granule subpopulations and plasma membrane from human neutrophils. FEBS Lett 304:195, 1992 15. Nauseef WM: Cytosolic oxidase factors in the NADPH-dependent oxidase of human neutrophils. Eur J Haematol51:301, 1993 16. Cheek TR: Calcium regulation and homeostasis. Current Biology 3:199, 1991 17. Emst JD: Annexin 111 translocates to the periphagosomal region when neutrophils ingest opsonized yeast. J Immunol 146:3110, 1991 18. Majeed M, Emst JD, Magnusson K-E, Kihlstrom E, Stendahl 0: Selective translocation of annexins during intracellular redistribution of Chlamydia truchomatis in HeLa and McCoy cells. Infect Immunol 62:126, 1994 19. Tokumitsu H, Mizutani A, Minami H, Kobayashi R, Hidaka H: A calcyclin-associated protein is a newly identified member of the Ca”/phospholipid-binding proteins, annexin family. J Biol Chem 267:8919, 1992 20. Misaki Y, Pruijn GJM, van der Kemp AWCM, van Venrooij WJ: The 56K autoantigen is identical to human annexin XI. J Biol Chem 269:4240, 1994 21. Mizutani A, Usuda N, Tokumitsu H, Minami H, Yasui K, Kobayashi R, Hidaka H: CAP-50, a newly identified annexin, localizes in nuclei of cultured fibroblast 3Y1 Cells. J Biol Chem 267:13498, 1992 22. Tokumitsu H, Mizutani A, Muramatsu M, Yokota T, Arai K, Hidaka H: Molecular cloning of rabbit CAP-50, a calcyclin-associated annexin protein. Biochem Biophys Res Commun 186:1227, 1992 23. Towle CA, Treadwell BV: Identification of a novel mammalian annexin. J Biol Chem 2675416, 1992 24. Towle CA, Weissbach L, Treadwell BV: Alternatively spliced annexin XI transcripts encode proteins that differ near the aminoterminus. Biochim Biophys Acta 1131:223, 1992 25. Chuah SY, Pallen CJ: Calcium-dependent and phosphorylation-stimulated proteolysis of lipocortin I by an endogenous A43 1 cell membrane protease. J Biol Chem 264:21160, 1989 26. SjBlin C, Dahlgren C: Diverse effects of different neutrophil organelles on truncation and membrane-binding characteristics of annexin I. Biochim Biophys Acta 1996 (in press) 27. Wang W, Creutz C E Role of the amino-terminal domain in regulating interactions of annexin I with membranes: Effects of amino-terminal truncation and mutagenesis of the phosphorylation sites. Biochemistry 33:275, 1994 28. Ando Y, Imamura S, Hong Y-M, Owada MK, Kakunaga T, Kannagi R: Enhancement of calcium sensitivity of lipocortin I in phospholipid binding induced by limited proteolysis and phosphorylation at the amino terminus as analyzed by phospholipid affinity column chromatography. J Biol Chem 264:6948, 1989 29. Mizutani A, Tokumitsu H, Kobayashi R, Hidaka H: Phosphorylation of annexin XI (CAP-50) in SR-3YI cells. J Biol Chem 268:15517, 1993 30. Tokumitsu H, Mizutani A, Hidaka H. Calcyclin-binding site located on the NHZ-terminaldomain of rabbit CAP-50 (annexin XI): Functional expression of CAP-50 in Escherichia coli. Arch Biochem Biophys 303:302, 1993 31. Watanabe M, Ando Y, Tokumitsu H, Hidaka H: Binding site of annexin XI on the calcyclin molecule. Biochem Biophys Res Commun 196:1376, 1993 From www.bloodjournal.org by guest on June 16, 2017. For personal use only. 1996 87: 4817-4823 Isolation by calcium-dependent translation to neutrophil-specific granules of a 42-kD cytosolic protein, identified as being a fragment of annexin XI C Sjolin and C Dahlgren Updated information and services can be found at: http://www.bloodjournal.org/content/87/11/4817.full.html Articles on similar topics can be found in the following Blood collections Information about reproducing this article in parts or in its entirety may be found online at: http://www.bloodjournal.org/site/misc/rights.xhtml#repub_requests Information about ordering reprints may be found online at: http://www.bloodjournal.org/site/misc/rights.xhtml#reprints Information about subscriptions and ASH membership may be found online at: http://www.bloodjournal.org/site/subscriptions/index.xhtml Blood (print ISSN 0006-4971, online ISSN 1528-0020), is published weekly by the American Society of Hematology, 2021 L St, NW, Suite 900, Washington DC 20036. Copyright 2011 by The American Society of Hematology; all rights reserved.