* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Document
Survey
Document related concepts
Cell membrane wikipedia , lookup
Cell growth wikipedia , lookup
Cytokinesis wikipedia , lookup
Tissue engineering wikipedia , lookup
Cell culture wikipedia , lookup
Extracellular matrix wikipedia , lookup
Signal transduction wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cellular differentiation wikipedia , lookup
Endomembrane system wikipedia , lookup
Transcript
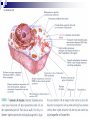
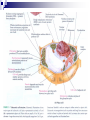
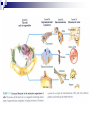
Laying the Ground Work: Dynamic Cell, Chemical Foundation, Protein Structure and Function; Dynamic cell growth, division and movement are hallmarks of life and are essential for the formation of an organism, yet our understanding of the molecular basis of these processes is far from complete The Cell is the fundamental unit of life • Microscopic examination of any organism reveals that it is composed of membrane-enclosed structure called cells • The enclosing membrane is called the cell membrane or the plasma membrane • Cells vary enormously in size and shape but • Even the largest cells would to be much larger to be visible to the naked eye • Within this tiny object thousands of chemical reaction are taking place - all regulated, all designed to serve specific function • Collectively these reactions serve the function of maintaining the cell and permitting it to replicate when time is right • Perhaps the most amazing thing about the living cell is that so much organized activity takes place in such a small space • Following figures show prototypical animal and plant cells along with some common shapes and sizes of bacteria Fig 1.7 Nelson and Cox 5th Ed – 2008 • The evolutionary tree is bisected into a lower prokaryotic domain and upper eukaryotic domain • The term prokaryote and eukaryote refer to the most basic division between cell types • The fundamental difference is that eukaryotic cells contain a membrane-bounded nucleus, whereas prokaryotes do not • The cells of prokaryote usually lack most of the other membranebounded organelles as well • Plants, fungi and animals are eukaryotes and bacteria are prokaryotes • The biochemical functions associated with organelles are frequently present in bacteria, but they are usually located on the inner plasma membrane Fig 1.1 Voet • Cells are organized in variety of ways in different living forms • Prokaryotes of given type produce cells that are very similar in appearance • A bacterial cell replicates by a process in which two identical daughter cells arise from an identical parent cell • Simple eukaryotes can also exist as single non-associating cells • Eukaryotes of increasing complexity can contain many cells with specialized structure and functions • For example, humans contain about 1014 cells of more than a 100 different types. • Although all cells in multi-cellular organisms have common constituents and functions – specialized cells types have unique chemical composition, structure and biochemical reactions – establish and maintain their specialized functions • Such cells arise during embryonic development by the complex processes of cell proliferation and cell differentiation • Except germ cells – all cell types contain the same genetic information – faithfully replicated to daughter cells • Cell differentiation is a process where by some of this genetic information is activated in some cells – synthesis of certain proteins and not other proteins • Thus specialized cells come to have different complements of enzymes and metabolic capacities Fig 1.2 Zubay Fig 1.14 Voet Cells are Composed of Small Molecules, Macromolecules and Organelles: • There are a structural hierarchy in cellular organization Fig 1.11 Nelson and Cox 5th Ed P 54, Boyer Table 2.1 Boyer 3rd Ed Table 3.1 ? • Except for water, most of the molecules, found in the cells are Lipids or macro-molecules, and can be classified into four categories: Lipids • Carbohydrates Proteins and Nucleic acids Each type of macromolecule possesses distinct chemical properties that suit it for the functions it serves in the cell Fig 1.6, Boyer 3rd Ed, 2005 Fig 1.3, Zubay Fig 1.4, Zubay Fig 1.5, Zubay Fig 1.6, Zubay Macromolecules Fold into Complex Three-Dimensional Structures: • • The complex folding of bio-macromolecules rarely entails making or breaking covalent linkages Rather the folding process is dedicated by: o Primary structure and o The way in which different elements of the macromolecule interact with each other and with water • The forces that determine folding are non-covalent in character Water is a Primary Factor in Determining the Type of Structures that Form: • Water, as we have seen, is the major component of living systems • and interacts with many bio-molecules • Some are water-loving or hydrophilic • Others are water abhorring or hydrophobic • and still others are amphiphatic or in between • What properties of a molecules make it hydrophilic or hydrophobic? • First, consider the molecular properties of H2O and • how water interacts with itself? • An individual water molecules has a significant dipole that is due to greater electro negativity of the oxygen atom over the hydrogen atom • This dipole leads to strong interaction between water molecules, in the form of hydrogen bond • A hydrogen bond is a non-covalent interaction between polar molecules, one of which is an unshielded proton Fig 1.7 Zubay In solid water or ice, the polar forces hold the individual molecules together in a regular three-dimensional lattice Fig 1.8 Zubay Most of the hydrogen bond present in ice are also present in liquid water Hence water is a highly hydrogen-bonded structure, not too different from ice, but with somewhat less regular structure in which the individual molecules have greater mobility P 58 Boyer 1st Ed The dipolar properties of water molecules affect the interaction between water and other moles. That dissolve in water For example, a favorable interaction accounts for the high solubility of sodium chloride in water Fig 3.10 Brum Fig 1.9 Zubay The kinds of ion-dipole interactions that take place between H2O and simple ions such as Na+ and Cl- are also important in interaction between the charged or polar, groups on bio-molecules and water • Thus bio-molecules that contain charged residues, H-bond forming substituents or other kinds of polar groups are hydrophilic • In the form of small molecules such groups tend to be very soluble in water • When attached to biopolymers they determine which parts of the molecule will be oriented on the exposed surface, where they can make contact with water • Apolar groups such as neutral hydrocarbon side chains do not contain significant dipoles or the capacity for forming hydrogen bonds • Consequently, they have nothing to gain by interacting with water, as evidence by their poor solubility in water • Where such hydrophobic molecules are present in water, the water forms a rigid clathrate (cage like) structure around them Fig 1.10 Zubay Fig 1.11 Zubay Fig 1.12 Zubay Fig 1.13 Zubay • Enormous structural diversity of proteins begins with the amino acid sequence of polypeptide chains • Each protein consists of one or more unique polypeptide chains and • Each of these polypeptide chains is folded into a three dimensional structure • This final folded arrangement is called its conformation • Folded arrangement of proteins can be defined in four levels i.e. hierarchy of structural organization Fig 4.14 Zubay Fig 4.15 Brum • Most proteins exist in unique conformations exquisitely suited to their function • It is the availability of a wide variety of conformations that permits proteins as a group to perform a broader range of functions than any other class of biomolecules Table 4.4 Boyer 2nd Ed / Table 3.4 Boyer 3rd Ed x--------------------------x----------------------------x------------------------------x