* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Amino Acid Metabolism 1 Key Concepts
Clinical neurochemistry wikipedia , lookup
Western blot wikipedia , lookup
Point mutation wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Peptide synthesis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Photosynthesis wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Protein structure prediction wikipedia , lookup
Genetic code wikipedia , lookup
Nitrogen dioxide poisoning wikipedia , lookup
Microbial metabolism wikipedia , lookup
Proteolysis wikipedia , lookup
Plant nutrition wikipedia , lookup
Metalloprotein wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Citric acid cycle wikipedia , lookup
Nitrogen cycle wikipedia , lookup
Biochemistry wikipedia , lookup
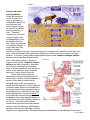
Bioc 460 - Dr. Miesfeld Spring 2008 Amino Acid Metabolism 1 Key Concepts - Nitrogen fixation and assimilation - Protein and amino acid degradation - The Urea cycle - Glucogenic and ketogenic amino acids Key Concept Questions In Amino Acid Metabolism How is atmospheric nitrogen converted into ammonia as a nitrogen source for plants? Which three amino acids serve as the nitrogen donors in urea synthesis? Nitrogen fixation and assimilation by plants and bacteria After carbon, nitrogen is the second most abundant element in the biosphere, and in addition to its presence in amino acids and nucleotides, it is also found in some carbohydrates (glucosamine) and lipids (sphingosine), as well as, the enzyme cofactors thiamine, NAD+, and FAD. Nitrogen in biological compounds ultimately comes from nitrogen gas (N2) which constitutes 80% of our atmosphere. However, N2 must first be reduced to NH3 (the ionized form of ammonia in solution is NH4+) by the process of nitrogen fixation, or oxidized to nitrate (NO3-) by atmospheric lightning, before it can be used by other liver organisms. Nitrogen fixation in nature is carried out by certain types of soil bacteria that live in both the soil and aquatic environments. Rhizobium is an example of a nitrogen-fixing soil bacteria that lives symbiotically with leguminous plants such as beans and alfalfa and has an important role in agriculture by reducing the need for commercial fertilizers. Plants cannot carry out nitrogen fixation on their own, but they can incorporate NH4+ they obtain from the environment into the amino acids glutamate and glutamine through a process called nitrogen assimilation. When animals eat plants, amino acids and nucleotides provide the nitrogen needed to synthesize a variety of biomolecules. Before looking at nitrogen metabolism in a little more detail, we need to answer our four pathway questions about nitrogen fixation and assimilation. 1. What purpose does nitrogen fixation and assimilation serve in the biosphere? • Nitrogen fixation takes place in bacteria and is the primary process by which atmospheric N2 gas is converted to ammonia (NH4+) and nitrogen oxides (NO2- and NO3-) in the biosphere. Two other nitrogen fixation processes are industrial (Haber process) and atmospheric (lightening). • Nitrogen assimilation is the process by which plants and bacteria incorporate NH4+ into organic compounds, most often the NH4+ is incorporated into the amino acids glutamate and glutamine. 2. What are the net reactions of nitrogen fixation and assimilation by plants and bacteria? Nitrogen fixation in bacteria is mediated by the nitrogenase enzyme complex: N2 + 8 H+ 8 e- + 16 ATP + 16 H2O ----> 2 NH3 + H2 + 16 ADP + 16 Pi Nitrogen assimilation in plants using the enzymes glutamine synthetase and glutamate synthase: α-ketoglutarate + NH4+ + ATP + NADPH + H+ --> Glutamate + ADP + Pi + NADP+ 1 of 14 pages Bioc 460 - Dr. Miesfeld Spring 2008 3. What are the key enzymes in nitrogen fixation and assimilation in plants and bacteria? Bacterial nitrogenase complex – is the enzyme that uses redox reactions coupled to ATP hydrolysis to convert N2 gas into 2 NH3. The enzyme has two functional components, dinitrogenase reductase that contains the binding site for ATP and 4Fe-4S redox center, and dinitrogenase which carries out the N2 reduction reaction using Fe-Mo and FeS redox centers. Glutamine synthetase - is found in all organisms and it incorporates NH4+ into glutamate to form glutamine through an ATP coupled redox reaction. The activity of glutamine synthetase is regulated by allosteric inhibitors (amino acids, AMP), and by covalent modification which is mediated by adenylation. Glutamate synthase - is found in bacteria, plants, and some insects, and it works in concert with glutamine synthetase to replenish glutamate so that the glutamine synthetase reaction is not substrate limited. Glutamate synthase converts α-ketoglutarate and glutamine to 2 glutamate. Glutamate dehydrogenase - is found in all organisms and it interconverts glutamate, NH4+, and αketoglutarate in a redox reaction utilizing either NAD(P)+/NAD(P)H. Under conditions of high NH4+ concentrations in nature, e.g., fertilizer applications in crop fields, glutamate dehydrogenase can assimilate NH4+ into glutamate, however, in animals, glutamate dehydrogenase most often generates NH4+ from glutamate to initiate the process of nitrogen excretion as urea or uric acid. 4. What are examples of nitrogen fixation and assimilation in real life? Natural fertilizers can be used in organic farming to reduce the dependence on industrial sources of nitrogen. The two most common sources of natural fertilizers are manure, if livestock are readily available, and crop rotation practices in which leguminous plants such as soy bean or clover, are planted in alternate seasons with nonleguminous crop plants such as corn and wheat. By plowing under the leguminous plants, the nitrogen Figure 1. contained in the plants is released into the soil and processed by soil bacteria to provide nitrogenous compounds for the corn and wheat plants. Nitrogen fixation In order to obtain nitrogen from the atmosphere for incorporation into biomolecules, the triple bond of N2 must be broken. However, this is not easily done considering that the bond energy of N2 is a staggering 930 kJ/mol. To overcome this high energy barrier, one of three processes are required, 1) biological fixation by bacteria that reduce N2 to NH3 through an ATP-dependent process requiring the multisubunit enzyme complex nitrogenase, 2) industrial fixation by the Haber process in which N2 and H2 gases are heated to ~500ºC under a pressure of ~250 atmospheres (~350 kilopascals) to produce liquid ammonia which is used commercially to make fertilizer (figure 1), or 3) atmospheric fixation as a result of lightning which breaks the N2 triple bond and allows nitrogen to combine with oxygen to form nitrogen oxides that are dissolved in rain and fall to earth. It is estimated that ~90% of the nitrogen incorporated our biosphere comes from biological and industrial fixation, of which almost half of this is ammonia contained in agricultural fertilizers. 2 of 14 pages Bioc 460 - Dr. Miesfeld Spring 2008 Fritz Haber, a German chemist, received the 1918 Nobel Prize in Chemistry for his development of industrial ammonia synthesis which revolutionized agriculture. Biological nitrogen Figure 2. fixation by bacteria requires the activity of nitrogenase, a large protein complex consisting of two functional components. One component is called dinitrogenase reductase (Feprotein) which consists of two identical subunits that each contain a binding site for ATP, and a single 4Fe-4S redox center liganded to cysteine residues in the two subunits (figure 2). The function of the dinitrogenase reductase is to obtain electrons from ferredoxin (or flavodoxin depending on the bacterial species) and pass them onto the second component of the complex called dinitrogenase (MoFe-protein) which catalyzes the reduction of N2 to generate 2 NH3. The nitrogenase reaction requires 16 ATP to overcome the large energy barrier in the N2 triple bond. Bacterial nitrogenase complex N2 + 8 H+ 8 e- + 16 ATP + 16 H2O ----> 2 NH3 + H2 + 16 ADP + 16 Pi The conformational change in dinitrogenase reductase requires the binding and hydrolysis of 2 ATP for each electron transferred. The reduction of N2 to 2 NH3 takes place in the Mo-Fe reaction center Figure 3. of the dinitrogenase component, and as shown in figure 3, requires three discrete steps that each utilize 2 H+ and 2 e, 1) reduction of N2 to form diimine (N2H2), 2) reduction of diimine to form hydrazine (N2H4), and 3) reduction of hydrazine to form two molecules of NH3. Besides being energetically expensive, the nitrogenase reaction is inhibited by O2. This means that nitrogen-fixing bacteria need to either perform this reaction under anaerobic conditions, or find a way to reduce O2 levels locally within the cell. The free-living facultative aerobe Klebsiella pneumoniae, for example, only synthesizes the protein components of the nitrogenase complex when it is living in an anaerobic environment and nitrogen fixation is favorable. Another mechanism to increase the efficiency of the nitrogenase reaction is employed by nitrogen-fixing bacteria that live as plant symbionts. One of these bacterial species is Rhizobium meiloti (also known as Sinorhizobium meiloti) which invades the roots of leguminous plants through tubular structures called infection threads (figure 4). Once in side the plant cell, the bacterium loses its cell wall and becomes a bacteroid, containing an inner and outer membrane. The plant provides citrate cycle intermediates such as fumarate and malate to the bacteroids which use them as oxidizable energy sources to generate NADH. The NADH is used to generate ATP by the electron 3 of 14 pages Bioc 460 - Dr. Miesfeld Spring 2008 transport system and oxidative phosphorylation, thereby providing chemical energy to the Rhizobium bacteroid. In turn, the nitrogenase complex of the bacteroid generates NH3 which is used to synthesize amino acids such as glutamate and aspartate that the plant can use as source of nitrogen. Desert plants such as the palo verde tree are leguminous which makes sense because the desert soil is sandy and plants are few and far between (figure 5). Non-leguminous plants obtain nitrogen from the soil as a result of organic decay mediated by bacteria. Of course, agricultural plants get their nitrogen from chemical fertilizers such as ammonium sulfate. Figure 4. Figure 5. Nitrogen assimilation Plants and bacteria, but not animals, use nitrogen assimilation reactions to synthesize nitrogen-containing metabolites, primarily amino acids. Nitrogen assimilation proceeds in one of two ways. First, if NH4+ levels in the soil are high, plants can use the glutamate dehydrogenase reaction to directly incorporate NH4+ into the amino acid glutamate using α-ketoglutarate as the carbon skeleton (figure 6). Animals also contain glutamate dehydrogenase, but because of low levels of free NH4+ in cells (NH4+ is toxic), and correspondingly high levels of glutamate, the reaction is run in the reverse direction as a way to deaminate glutamate and form nitrogenous waste products such as urea and uric acid. A second, and more common way that plants and bacteria incorporate NH4+ into metabolites, is through a two reaction mechanism that functions when NH4+ concentrations are low Figure 6. Glutamate dehydrogenase (most of the time for non-leguminous plants in the wild). In this mechanism, the enzyme glutamine synthetase uses ATP in a coupled reaction to form glutamine from glutamate using NH4+ as the source of nitrogen (figure 7). Next, the glutamine is combined with αketoglutarate in a reaction catalyzed by the enzyme glutamate synthase to form two molecules of glutamate (glutamine contains two nitrogens) as shown in figure 8. While all organisms contain the enzyme glutamine synthetase, only plants and animals, and some insects, have the enzyme glutamate synthase. These three nitrogen assimilation reactions are summarized in Figure 7. Glutamine synthetase 4 of 14 pages Figure 8. Glutamate synthase Bioc 460 - Dr. Miesfeld Spring 2008 figure 9. Importantly, the newly acquired nitrogen in glutamate and glutamine is used to synthesize a variety of other amino acids through aminotransferase enzymes that convert glutamate and α-keto acids into αFigure 9. ketoglutarate and the corresponding αamino acid (figure 10). As shown in figure 11, the Nitrogen Cycle maintains nitrogen balance in our biosphere. Atmospheric nitrogen is converted to NH3 by biological, industrial and atmospheric fixation processes. Bacteria in the soil convert nitrogen to NH3 either as symbionts with leguminous plants (e.g., Rhizobium), or as free-living organisms (e.g., Azotobacter). The NH4+ in the soil derived from decomposition, free-living soil bacteria, and man-made fertilizers, is converted to NO2- (nitrite) and NO3- (nitrate) by soil bacteria that carry out the process of nitrification (e.g., Nitrosomonas and Nitrobacter). Plant roots absorb NO2- and NO3- present in the soil and convert them back into NH4+ using nitrite and nitrate reductase enzymes. The assimilation of NH4+ into amino acids by plants provides a source of nitrogen for animals (directly or indirectly). The denitrification process carried out by bacteria that reduce nitrites and nitrates (e.g., Pseudomonas) releases N2 back into the atmosphere, whereas other types of bacteria anaerobically convert NH4+ Figure 10 directly to N2 gas to complete the nitrogen cycle. The fixation and cycling of nitrogen by bacteria and plants, and the dependence of animals on plants as their only source of biological nitrogen, is similar in many ways to the vital role that plants play in providing carbohydrates and O2 for aerobic respiration in non-photosynthetic organisms such as ourselves. 5 of 14 pages Figure 11. Bioc 460 - Dr. Miesfeld Spring 2008 Protein and amino acid degradation Unlike glucose which can be stored in the body as glycogen, or converted to acetyl CoA and stored as fatty acids, nitrogen cannot be stored in a useable form because NH4+ is toxic. Therefore, nitrogen lost as a result of protein and nucleic acid degradation, must be replaced from the diet. When an individual is in nitrogen balance, it means that their daily intake of nitrogen, primarily in the form of protein, equals the amount of nitrogen lost by excretion in the feces and urine. A normal healthy adult needs about 400 grams of protein per day to maintain nitrogen balance. In contrast, young children and pregnant women have a positive nitrogen balance because they accumulate nitrogen in the Figure 12. form of new protein which is needed to support tissue growth. Negative nitrogen balance is a sign of disease or starvation and occurs in individuals with elevated rates of protein breakdown (loss of muscle tissue) or an inability to obtain sufficient amounts of amino acids in their diet. Plants and bacteria have the necessary enzymes to synthesize all 20 amino acids, however, animals depend on protein in their diets to obtain the ten essential amino acids they require for growth and development. Protein digestion in humans takes place in the stomach and the small intestine where proteases cleave the peptide bond to yield amino acids and small oligopeptides. As seen in figure 12, food enters the stomach through the esophagus and protein digestion begins in the stomach as a result of pepsin activation by low pH. After leaving the stomach, duodenumal hormones stimulate protease secretion from the pancreas which leads to protease activation and cleavage of protein fragments into oligopeptides. Aminopeptidases and dipeptidases in the intestinal mucosal cells 6 of 14 pages Bioc 460 - Dr. Miesfeld Spring 2008 degrade the oligopeptides into single amino acids that are then absorbed and transported to the liver. The highly acidic slurry of Figure 13. digested food, called chyme, leaves the stomach by passing through pyloric valve and into the duodenum, resulting in the release of two duodenumal hormones, secretin and cholecystokinin (CCK). The duodenum also secretes enteropeptidase, a protease that specifically activates several protealytic zymogens released from the pancreas. One of these proteases is the pancreatic zymogen trypsinogen which is cleaved to form the endopeptidase trypsin. As shown in figure 13, trypsin cleaves numerous pancreatic zymogens, including chymotrypsinogen, proelastase and procarboxypepetidases A and B, as well as, trypsinogen itself to amplify the protealytic cascade. The combined activity of the pancreatic endopeptidases (trypsin, chymotrypsin, elastase) and exopeptidases (carboxypeptidase A and B), along with aminopeptidases and dipeptidases located on the membrane of intestinal mucosal cells, further digest the protein fragments into individual amino acids that are transported into intestinal epithelial cells and then exported to the blood. The complete degradation of dietary proteins by these digestive proteases results from the distinct substrate specificities of the enzymes. Another source of free amino acids in the body comes from Figure 14. degradation of cellular proteins which occurs continuously in all cells. Most eukaryotic cellular proteins are degraded by one of two pathways, 1) an ATP-independent process that degrades proteins inside cellular vesicles called lysosomes, and 2) an ATPdependent pathway that targets specific proteins for degradation in proteasomes if they contain a polymer of ubiquitin protein covalently attached to lysine residues. In order for proteins to be degraded by the proteasome, they must first be "tagged" on lysine residues by covalent linkage of ubiquitin (figure 14). Ubiquitin is a highly conserved 76 amino acid protein found in all eukaryotic cells that is specifically attached to proteins by ubiquitin ligating enzymes. The signal for 7 of 14 pages Bioc 460 - Dr. Miesfeld Spring 2008 Figure 15. ubiquination can be specific residues within the target protein, or a physical or chemical property of the protein, such as a phosphorylated residue or abnormal conformation, that are recognized by the ubiquitin ligase. Ubiquinated proteins enter the proteasome one at a time where the ubiquitin is removed and recycled, and then polypeptide is cleaved into small oligopeptides (6-10 amino acids long) that are released into the cytosol and degraded into individual amino acids. As shown in figure 15, the proteasome consists of a 20S protealytic core, so named because of its sedimentation properties in a density gradient (S is Svedberg units), and two 19S regulatory complexes that serve as caps to regulate protein entry into the protealytic core. The 19S complexes contain binding sites for ubiquitinated proteins and encode ATP hydrolyzing enzymes that function in the protein unfolding process required before the polypeptide can enter the internal chamber and be degraded. The intact Figure 16. proteasome is 26S and has often been called the "garbage disposer" of the cell. Since cells cannot store amino acids that accumulate as a result of protein degradation, they must either be recycled for protein synthesis, or deaminated in order to salvage their carbon skeletons for use in other pathways. As shown in figure 16, deamination of amino acids results in generation of NH4+ which is used in the synthesis of other nitrogen-containing compounds, or excreted in the form of urea or uric acid in terrestrial animals, whereas, aquatic animals can excrete NH4+ directly. 8 of 14 pages Bioc 460 - Dr. Miesfeld Spring 2008 The urea cycle removes toxic ammonia from the body As described earlier, glutamate and glutamine function as the primary nitrogen carriers in most organisms. In mammals, this nitrogen ends up in the liver where it is converted to urea. As illustrated in figure 17, the two nitrogens in urea are derived from the NH4+ released when glutamate or glutamine are Figure 17. deaminated, and from aspartate which is formed when oxaloacetate is transaminated by aspartate aminotransferase. The carbon atom in urea comes from CO2 (HCO3-) that is produced in the mitochondrial matrix by the citrate cycle (the oxygen atom is derived from H2O in the final reaction of the cycle). Before we examine urea synthesis in detail, let's answer the four metabolic questions that pertain to the urea cycle. Note that this is the last major metabolic pathway we examine in this course. 1. What does the urea cycle accomplish for the organism? Urea synthesis provides an efficient mechanism for land animals to remove excess nitrogen from the body. Urea is synthesized in the liver and exported to the kidneys where it enters the bladder. 2. What is the net reaction of the urea cycle? NH4+ + HCO3- + aspartate + 3 ATP ---> urea + fumarate + 2 ADP + 2 Pi + AMP + PPi 3. What is the key regulated enzyme in urea synthesis? Carbamoyl phosphate synthetase I – catalyzes the commitment step in the urea cycle; the activity of this mitochondrial enzyme is activated by N-acetylglutamate in response to elevated levels of glutamate and arginine. 4. What is an example of the urea cycle in real life? A deficiency in the enzyme argininosuccinase inhibits flux through the urea cycle and causes hyperammonemia and neurological symptoms. This metabolic disease can be treated with a low protein diet that is supplemented with arginine, thereby resulting in argininosuccinate excretion a substitute for urea. As shown in figure 18, amino acids are transported to the liver where the nitrogen is removed and used for urea synthesis. There are three sources of these amino acids 1) amino acids derived from the digestion of dietary proteins, 2) the amino acid glutamine, which is generated from glutamate and NH4+ in peripheral tissues by glutamine synthetase, and 3) the amino acid alanine, which is formed by the alanine aminotransferase reaction as a way to remove excess nitrogen from exercising (or starving) skeletal muscle. Dietary amino acids in the blood are 9 of 14 pages Bioc 460 - Dr. Miesfeld Spring 2008 taken up by the liver Figure 18. where aminotransferase enzymes transfer the amino group to αketoglutarate to form glutamate. Amino acids derived from the degradation of cellular proteins are also deaminated to generate glutamate. The glutamate is imported into the mitochondrial matrix where it is metabolized by the enzyme glutamate dehydrogenase to produce NH4+ which is used to make the urea cycle precursor carbamoyl phosphate. In addition, some of the glutamate is converted to aspartate by the aspartate aminotransferase reaction and fed into the urea cycle as the second source of nitrogen. Glutamine, which carries excess two nitrogen atoms to the liver from peripheral tissues, is deaminated by the enzyme glutaminase to generate NH4+ and glutamate. The NH4+ is used to make carbamoyl phosphate directly, and the glutamate, is deaminated by glutamate dehydrogenase to liberate a second molecule of NH4+ for carbamoyl synthesis. Urea is synthesized in the liver and transported through the blood to the kidneys where it is concentrated and excreted in urine. As shown in figure 19, five enzymatic reactions are required for urea synthesis, two of which occur inside mitochondria and three others in the cytosol. It can be seen that two of the reactions require ATP hydrolysis (reactions 1 and 3), resulting in the expenditure of four high energy phosphate bonds for every mole of urea produced. The urea cycle was discovered in 1932 by Hans Krebs and a medical student who worked in his lab, Kurt Henseleit. Krebs is the same biochemist who later described the citrate cycle and was co-recipient of the 1953 Noble Prize in Physiology or Medicine. In fact, elucidation of the urea cycle gave Krebs insights into how cyclic pathways work and he exploited this knowledge to unravel the complexities of the citrate cycle just five years later in 1937. The starting point for urea synthesis is the formation of carbamoyl phosphate from NH4+ and HCO3- in a reaction catalyzed by the enzyme carbamoyl phosphate synthetase I. This 10 of 14 pages Bioc 460 - Dr. Miesfeld Spring 2008 Figure 19. mitochondrial enzyme is distinct from carbamoyl phosphate synthetase II which is a cytosolic enzyme involved in pyrimidine biosynthesis. The three step carbamoyl phosphate synthetase I reaction represents the commitment step in urea synthesis and requires the hydrolysis of 2 ATP. Carbamoyl phosphate is then combined with ornithine to form citrulline in a mitochondrial reaction catalyzed by the enzyme ornithine transcarbamoylase. The citrulline is then exported to the cytosol where it is first activated by AMP before being converted to arginosuccinate when aspartate displaces the AMP. This reaction is catalyzed by the cytosolic enzyme argininosuccinate synthetase and results in the incorporation of a second nitrogen atom. Note that cleavage of PPi by pyrophosphatase means that this reaction consumes two high energy phosphate bonds. Argininosuccinate is cleaved in the next reaction by the enzyme argininosuccinase to yield fumarate and arginine which contains both nitrogens. Lastly, the enzyme arginase converts arginine to urea and ornithine to complete the cycle. Ornithine has the same role in the urea cycle as oxaloacetate does in the citrate cycle, namely, as both the product of the last reaction and the substrate of the first reaction. By including the pyrophosphatase reaction, it can be seen that four high energy phosphate bonds are required (4 ATP equivalents) for every molecule of urea that is synthesized, and moreover, that the C4 carbon backbone of aspartate gives rise to fumarate: NH4+ + CO2 + aspartate + 3 ATP ---> urea + fumarate + 2 ADP + AMP + 4 Pi Since fumarate is an intermediate in the citrate cycle that can be used to form oxaloacetate, and transamination of oxaloacetate using glutamate generates aspartate (via the aspartate aminotransferase reaction), the urea cycle and citrate cycle are metabolically linked through shared intermediates. 11 of 14 pages Bioc 460 - Dr. Miesfeld Spring 2008 As shown in figure 20, the aspartate-argininosuccinate shunt converts fumarate, produced in the cytosol by the urea cycle, into malate that is used to make oxaloacetate in the citrate cycle. Oxaloacetate combines with glutamate to generate aspartate and α-ketoglutarate, Figure 20. and then the aspartate is transported back into the cytosol where it is used as a substrate in the argininosuccinate synthetase reaction of the urea cycle. In the simplest version of this bypass reaction, sometimes called the "Krebs bicycle" pathway, fumarate is converted to malate in the cytosol by an isozyme of fumarase. The malate can then be transported into the mitochondrial matrix using the malate-aspartate shuttle and converted to oxaloacetate by malate dehydrogenase. The resulting oxaloacetate is used as a substrate in the aspartate aminotransferase reaction to generate aspartate which is transported to the cytosol where it serves as a urea cycle substrate. Besides recycling fumarate to generate oxaloacetate for the aspartate aminotransferase reaction, the aspartate-argininosuccinate shunt also produces an NADH in the malate dehydrogenase reaction that can be used by the electron transport system to generate 2.5 ATP. This net yield of ATP helps offset the energetic cost of the urea cycle (4 ATP equivalents). Inherited defects in many of the urea cycle enzymes have been observed clinically. While complete loss of a urea cycle enzyme causes death shortly after birth, deficiencies in urea cycle enzymes results in hyperammonemia (elevated ammonia levels in the blood). Most urea cycle disorders also lead to a build-up of glutamine and glutamate which function as osmolites that can cause brain swelling and associated neurological symptoms. Fortunately, it is possible to treat some of the urea cycle disorders by restricting dietary protein as a means to limit nitrogen intake. In addition, by providing metabolic substrates that increase the biosynthesis of nitrogen containing compounds that can be excreted, it is often possible to decrease the severity of hyperammonemia. For example, argininosuccinase deficiency can be treated effectively by putting patients on a protein-depleted diet that is supplemented with high doses of L-arginine. As shown in figure 21, this regimen increases flux through a "short-circuited" urea cycle by converting arginine to ornithine, which combines with carbamoyl phosphate and aspartate to generate argininosuccinate. Since argininosuccinate is soluble and can be excreted in the urine, it functions as a metabolic 12 of 14 pages Bioc 460 - Dr. Miesfeld Spring 2008 replacement for urea. Note that supplementing the diet with ornithine would also give the same result, but it is more feasible to use arginine. Figure 21. Degradation of glucogenic and ketogenic amino acids The carbon backbones of eleven of the twenty standard amino acids can be converted into pyruvate or acetyl-CoA, which can then be used for energy conversion by the citrate cycle and oxidative phosphorylation reactions. The other nine amino acids are converted to the citrate cycle intermediates α− ketoglutarate, fumarate, succinyl-CoA, and oxaloacetate, which can be used for glucose synthesis by conversion of oxaloacetate to phosphoenolypyruvate. Under normal conditions, amino acid degradation accounts for ~10-15% of the metabolic fuel for animals, more so for animals with high protein diets or during starvation when muscle protein is degraded. Alternatively, when the energy charge of the cell is high, or metabolic fuel needs to be exported from the liver to other parts of the body, amino Figure 22. acid degradation can be used to provide precursors for glucose and fatty acid synthesis. Amino acid degradation pathways are somewhat complex, and therefore, it is convenient to think about amino acid degradation pathways in terms of the metabolites they produce, and whether these metabolites are precursors to glucose or ketone bodies. As shown in figure 22, amino acids that give rise to pyruvate, or any of the citrate cycle intermediates, are called glucogenic amino acids because pyruvate and oxaloacetate are precursors in the gluconeogenic pathway (αketoglutarate, succinyl-CoA and fumarate can be converted to oxaloacetate). In contrast, amino acids that are converted into acetyl-CoA or acetoacetyl-CoA are called ketogenic amino 13 of 14 pages Bioc 460 - Dr. Miesfeld Spring 2008 acids because they can give rise to ketone bodies. Notice in figure 22 that eight of the amino acids (aspartate, isoleucine, leucine, lysine, phenylalanine, threonine, tryptophan, tyrosine), are converted to more than one metabolite. ANSWERS TO KEY CONCEPT QUESTIONS IN AMINO ACID METABOLISM: Atmospheric nitrogen (N2) is converted into ammonia (NH3) as a nitrogen source for plants by three distinct processes; 1) biological fixation by soil bacteria, 2) industrial fixation by the Haber process, and 3) atmospheric fixation as a result of lightning. All organisms require nitrogen for the synthesis of numerous biomolecules, the most abundant of which are amino acids and nucleotides. However, since the N=N bond is so strong (930 kJ/mol), a large amount of energy is required to overcome the energy barrier needed for this reduction reaction to go forward. Biological fixation is carried out by two types of soil bacteria, free-living bacteria and symbiotic bacteria, both of which use an ATP-dependent reaction mechanism catalyzed by the enzyme nitrogenase to reduce N2 to NH3 (which becomes ammonium ion, NH4+). Nitrogenase consists of two functional components, dinitrogenase reductase which accepts electrons from ferredoxin to be used in the reduction reaction, and dinitrogenase which catalyzes the reduction reaction. ATP binding and hydrolysis provides energy for conformational changes in the nitrogenase complex that are needed to convert dinitrogenase reductase from an electron acceptor to an electron donor. Since the nitrogenase reaction is inhibited by O2, nitrogen-fixing bacteria have evolved various mechanisms to reduce O2 levels, one of which involves high local concentrations of the hemecontaining protein leghemoglobin that is produced by leguminous plants. Importantly, since much of the NH4+ present in the soil is oxidized to nitrate (NO3-) and nitrite (NO2-) by nitrifying bacteria, plants have nitrate and nitrite reductase enzymes to convert these compounds back into NH4+. Urea is a waste product synthesized in terrestrial vertebrates (and some invertebrates) from the NH4+ released from glutamine and glutamate, the nitrogen atom of aspartate, and CO2 produced by the citrate cycle in the mitochondrial matrix. Glutamine serves as a nitrogen carrier in the body that transports NH4+ from the peripheral tissues to the liver where it is deaminated by the enzyme glutaminase in the mitochondrial matrix to release NH4+ and regenerate glutamate. Another source of NH4+ comes from amino acids in the blood (derived from digestion or cellular degradation of proteins) which are taken up by the liver and used as substrates in aminotransferase reactions that transfer the -amino group to -ketoglutarate to form glutamate. The enzyme glutamate dehydrogenase has the important job in the liver of releasing the NH4+ from glutamate and producing -ketoglutarate. The free NH4+ is then combined with CO2 to form the urea cycle substrate carbamoyl phosphate in a mitochondrial reaction catalyzed by the enzyme carbamoyl phosphate synthetase I. The second nitrogen in urea comes from aspartate in a cytosolic reaction in which citrulline is converted to argininosuccinate by the addition of aspartate. Since aspartate is formed from oxaloacetate by the aspartate aminotransferase reaction in mitochondria, and urea cycle product fumarate can be converted to oxaloacetate by the citrate cycle, the urea cycle and citrate cycle are metabolically linked through their shared intermediates aspartate and fumarate (Krebs bicycle). 14 of 14 pages