* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 12 Insights into the mechanisms underlying CFTR channel activity
Survey
Document related concepts
Histone acetylation and deacetylation wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Mechanosensitive channels wikipedia , lookup
Magnesium transporter wikipedia , lookup
Protein moonlighting wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Signal transduction wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Protein structure prediction wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Phosphorylation wikipedia , lookup
List of types of proteins wikipedia , lookup
Protein phosphorylation wikipedia , lookup
Transcript
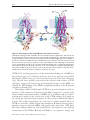
© The Authors Journal compilation © 2011 Biochemical Society Essays Biochem. (2011) 50, 233–248; doi:10.1042/BSE0500233 12 Insights into the mechanisms underlying CFTR channel activity, the molecular basis for cystic fibrosis and strategies for therapy Patrick Kim Chiaw1, Paul D.W. Eckford1 and Christine E. Bear2 Programme in Molecular Structure and Function, Hospital for Sick Children, 555 University Avenue, Toronto, ON, Canada, M5G 1X8 Abstract Mutations in the CFTR (cystic fibrosis transmembrane conductance regulator) cause CF (cystic fibrosis), a fatal genetic disease commonly leading to airway obstruction with recurrent airway inflammation and infection. Pulmonary obstruction in CF has been linked to the loss of CFTR function as a regulated Cl− channel on the lumen‑facing membrane of the epithelium lining the airways. We have learned much about the molecular basis for nucleotideand phosphorylation‑dependent regulation of channel activity of the normal (wild‑type) version of the CFTR protein through electrophysiological studies. The major CF‑causing mutation, F508del‑CFTR, causes the protein to misfold and be retained in the ER (endoplasmic reticulum). Importantly, recent studies in cell culture have shown that retention in the ER can be ‘corrected’ 1These 2To authors are joint primary authors. whom correspondence should be addressed (email [email protected]). 233 234 Essays in Biochemistry volume 50 2011 through the application of certain small‑molecule modulators and, once at the surface, the altered channel function of the major mutant can be ‘potentiated’, pharmacologically. Importantly, two such small molecules, a ‘corrector’ (VX‑809) and a ‘potentiator’ (VX‑770) compound are undergoing clinical trial for the treatment of CF. In this chapter, we describe recent discoveries regarding the wild‑type CFTR and F508del‑CFTR protein, in the context of molecular models based on X‑ray structures of prokaryotic ABC (ATPbinding cassette) proteins. Finally, we discuss the promise of small‑molecule modulators to probe the relationship between structure and function in the wild‑type protein, the molecular defects caused by the most common mutation and the structural changes required to correct these defects. Introduction CF (cystic fibrosis), one of the most common genetic diseases in Caucasian populations, is an autosomal recessive disease caused by mutations in both alleles of the CFTR (cystic fibrosis transmembrane conductance regulator) gene. CFTR is a multi‑spanning membrane protein of the ABC (ATP‑binding cassette) superfamily C subclass (ABCC7), primarily expressed in apical (lumen‑facing) membranes of epithelial tissues such as the airways, intestine, pancreas and sweat ducts, where it controls transepithelial salt and fluid movement [1]. Function and trafficking defects in mutant CFTR lead to reduced secretion of Cl− and fluid in the airway, resulting in viscous airway surface liquid. This increased viscosity prevents the cilia on the luminal membrane from functioning properly, leading to defective mucociliary clearance with bacterial colonization. Whereas CF patients frequently suffer from pancreatic insufficiency (where the pancreas fails to deliver sufficient digestive enzymes into the duodenum to break down food), intestinal obstruction, male sterility and other effects, mortality in CF is primarily caused by chronic lung infection [2]. In this chapter, we discuss the importance of structural information on ABC proteins and homology models of the CFTR protein in enhancing our understanding of ion permeation and gating by the CFTR channel. We also discuss the potential value of small‑molecule ligands as new tools for identify‑ ing the molecular defects caused by disease‑causing mutations. CFTR: a unique ABC protein which functions as a regulated Cl− channel As described in detail throughout this volume, ABC proteins are typically active transporters, dependent on the binding/hydrolysis of ATP at their NBDs (nucleotide‑binding domains) to drive conformational changes in their MSDs (membrane‑spanning domains), also known as TMDs (transmembrane domains), and the transport of substrate up a concentration gradient across the membrane. ABC transporters are thought to bind substrates at a discrete © The Authors Journal compilation © 2011 Biochemical Society P.K. Chiaw, P.D.W. Eckford and C.E. Bear 235 binding site on one side of the membrane, and then bind/hydrolyse nucleotide to induce a shift to an alternative conformation in which access to one side of the membrane is occluded while access to the opposite site is opened [3]. This would occur along with a concomitant change in the binding site affinity for the substrate, and the molecule would be released on the other side of the membrane with a net hydrolysis of an ill‑defined number of ATP molecules. Uniquely, CFTR (Figure 1) functions as a Cl− channel, not as a transporter. In fact, electrophysiological measurements on purified protein nearly 20 years ago [4] showed that CFTR mediates Cl− electrodiffusion along its electrochemical gradient. The unique channel function of CFTR in a family of transporters con‑ tinues to garner considerable attention by membrane protein biophysicists concerned with the structural determinants for channel compared with trans‑ porter function. The ClC family of membrane proteins resembles the ABC family as it too comprises Cl− channels and transporters (recently reviewed in [5]). Within the family of ClC proteins, very subtle structural differences (substitution of a single residue in the case of the Escherichia coli ClC) are suf‑ ficient to convert a ClC transporter into a channel. It remains to be determined whether this is the case for ABC proteins. Although the high‑resolution structure of the full‑length CFTR pro‑ tein has not been solved, structures have been determined for NBD1 of Figure 1. Schematic diagram of CFTR showing its domain organization As a member of the ABC superfamily, CFTR (N‑terminal half is shown schematically in teal and the C‑terminal half in pink) possesses two MSDs (MSD1 and MSD2, darker teal and pink respec‑ tively), two NBDs (NBD1 and NBD2, lighter teal and pink respectively) and the unique regula‑ tory R domain (orange) that is phosphorylated (green Ps) in the active protein. The MSDs are linked to the NBDs via ICLs. Coupling helices in the ICLs are thought to interact with the NBDs and are shown as cylinders that contact the NBDs. Phe508 (F508) is shown in yellow. Domain structures are coloured to match the colouring of the models in Figure 2. © The Authors Journal compilation © 2011 Biochemical Society 236 Essays in Biochemistry volume 50 2011 Figure 2. Homology models of CFTR open and closed structures Structural homology models of CFTR in the closed (left) and open (right) states, with the N‑ and C‑terminal halves of the protein shown in teal and pink respectively. The R domain is shown in orange and Phe508, which is located at the interface between NBD1 (light teal) and the TMDs, is shown as a yellow space‑filling structure. ADP bound to the structures (one for the closed state and two for the open state) is shown as a blue space‑filling structure. Opening of the channel occurs with binding of ATP to the canonical nucleotide‑binding site as suggested diagrammati‑ cally (forward direction), allowing movement of Cl− ions through the channel, while hydrolysis and release of ADP and Pi from the site (reverse direction) closes the channel to the flow of Cl−. Models were rendered from the co‑ordinates of Mornon et al. [11]. CFTR [6,7], revealing properties of the nucleotide‑binding site of NBD1 at the atomic level. Low‑resolution structures have been generated of purified full‑length CFTR using cryo‑electron microscopy by Ford and colleagues [8,9]. Overall, these models, generated in the presence and absence of ATP analogues, suggest that long‑range conformational changes in the MSDs are induced by ATP binding to the NBDs and phosphorylation of the R domain (regulatory domain) [10]. Homology models of full‑length CFTR have been developed recently on the basis of the structures of intact bacterial ABC transporters, together with high‑resolution structural models of NBD1 [6,7]. Callebaut and co‑workers constructed a model of the closed state of the CFTR channel [11] based on the closed‑apo (inward‑facing) structure of Vibrio cholerae MsbA [12] (Figure 2, left). The weakly homologous N‑ (teal) and C‑ (pink) terminal halves of CFTR are joined by a linker region that contains an R domain (orange) that is thought to interact with the NBDs and regulate their interaction. ATP (dark blue space‑filling model) has been modelled into the degenerate site (lacking conservation of the Walker B motif and Walker C or ‘signature’ motif), and © The Authors Journal compilation © 2011 Biochemical Society P.K. Chiaw, P.D.W. Eckford and C.E. Bear 237 the clinically relevant Phe508 residue is shown in yellow space‑filling struc‑ ture to highlight its proximity to the NBD–MSD interface. Upon binding of ATP to the canonical (possessing conserved Walker motifs) ATP‑binding site, a rigid‑body movement occurs in NBD2 to bring the NBDs together and probably opens the pore across the membrane. The open‑channel struc‑ ture of CFTR (Figure 2, right) was modelled by Callebaut and colleagues [11] and by Riordan and colleagues [13] on the outward‑facing structure of the Staphylococcus aureus Sav1866 exporter [14] and shows the interaction of both nucleotide‑binding sites with ATP and the concomitant reorganization of the MSDs in the protein upon binding of ATP to the canonical site. Upon hydrolysis of ATP in the canonical site, the protein is expected to revert to the closed state (Figure 2, left). The first full‑length mammalian ABC protein structure, that of Pgp (P‑glycoprotein) (ABCB1), was recently solved [15], and is in good agreement with the structures of bacterial ABC proteins such as MsbA. Whereas the physiological relevance of the wide‑open inward‑facing Pgp structure remains in question, the overall domain architecture is otherwise very much as anticipated for both prokaryotic and eukaryotic ABC proteins and indirectly supports the suitability of constructing CFTR models based on prokaryotic protein structures. The role of the NBD in CFTR channel gating Typically, the two NBDs of ABC proteins interact in a head‑to‑tail arrangement to form two nucleotide‑binding sites at their interface [16]. Although no structural data for a CFTR NBD1–NBD2 dimer exists, data from NBD1–NBD1 homodimeric crystal structures do suggest that the CFTR NBDs associate in the head‑to‑tail manner anticipated for all ABC proteins [17]. For most ABC members (including Pgp), ATP hydrolysis occurs at two symmetrical catalytic sites formed at the NBD dimer interface in an alternating fashion. On the other hand, for CFTR and other members of the C subfamily of ABC proteins [including MRP1 (multidrug‑resistance protein 1)], hydrolysis occurs only at one of the two ATP‑binding sites, the ‘canonical’ catalytic site [18,19]. The other, the ‘degenerate’ ATP‑binding site, is defective in ATP hydrolysis and is thought to be stably occupied by nucleotide. This difference probably accounts for the much lower ATPase activity by CFTR (~70 nmol/mg of protein per min) [20] relative to the activity of Pgp (5000 nmol/mg of protein per min) [21]. Furthermore, using purified and reconstituted CFTR protein, we provide direct evidence that the ATPase activity of phosphorylated CFTR is diminished by mutations in either the conserved Walker A or B motifs comprising the canonical catalytic site [20]. ATP binding and hydrolysis at this canonical catalytic site is associated with gating of the CFTR channel [18,19]. Addition of MgATP to membrane patches harbouring PKA (protein kinase A)‑phosphorylated CFTR leads to burst‑like channel opening [22]. ATP hydrolysis at this catalytic site has been linked to closure after the open channel burst in electrophysiological studies © The Authors Journal compilation © 2011 Biochemical Society 238 Essays in Biochemistry volume 50 2011 comparing gating kinetics in the normal protein and mutants lacking the key residues in the canonical catalytic site [22]. There are several excellent reviews of the putative mechanisms underlying ATP‑dependent gating and, recently, a kinetic scheme supporting a strict coupling of ATP binding and hydrolysis to gating has been proposed [19,23–25]. The role of the coupling helices in gating As modelled by Riordan and co‑workers [13] and by Callebaut and co‑workers [11] (Figure 2), the cytosolic connections between the transmembrane segments in the membrane may be structured as lengthy helical segments extending from the membrane domain, perpendicular to the plane of the lipid bilayer. Shorter helical segments at the foot of these long extensions and lying parallel to the surface of the NBD heterodimer have been called the ‘coupling helices’. These coupling helices are thought to dock on to hydrophobic patches on the NBDs. In the transition from ‘closed’ to ‘open’ conformations in the models of Callebaut and co‑workers, there is a sliding of NBDs relative to one another upon ATP binding and/or hydrolysis at the catalytic site [11]. These authors suggest that the coupling helices provide a pivot point above which twisting along the long axis of the helical extension and the transmembrane helices occurs. An ‘iris‑like’ opening mechanism has been described in computational studies for other channels including the CorA Mg2+ channel [26]. Recent studies by Kirk and colleagues lend support to the idea that changes in the relative orientation of the long helical segments are instrumental for channel gating and may occur subsequent to ATP binding and/or hydrolysis by the NBDs [27]. The molecular basis for permeation through the CFTR channel Once the gate (or gates) are opened, anions (predominantly Cl− or HCO3−) are conducted via intramembrane helices in the MSDs of CFTR [28]. To date, although the identity of all of the helical segments which comprise the pore is unknown, electrophysiological (patch‑clamp) and mutagenesis studies have confirmed a critical role for the sixth transmembrane segment in contributing to the conduction path [29–31]. Patch‑clamp studies of anion conduction also support the idea that the pore in the membrane is asymmetric with a relatively large cytosolic vestibule. Net positive charge on the surface of the inner [comprising the cytosolic face of TMHs (transmembrane helices) 1, 5, 6 and 12] and the outer (comprising the external face of TMH6 and the extracellular loops between TMH1–TMH2 and TMH11–TMH12) vestibules appears to ensure efficient anion entry into the pore [32]. Using cysteine‑scanning mutagenesis and thiol chemistry together with electrophysiological studies and molecular dynamics simulations, Dawson and colleagues confirmed the apparent fidelity of the Sav1866 model in predicting © The Authors Journal compilation © 2011 Biochemical Society P.K. Chiaw, P.D.W. Eckford and C.E. Bear 239 which residues in TMH6 face the aqueous pore of CFTR [33]. These findings support the presence of a structural constriction of the pore close to the extra‑ cellular surface of the protein in the vicinity of Thr338 and Thr339 as described previously by Linsdell [28]. Interestingly, accessibility of substituted cysteine residues in TMH6 to thiol‑group‑specific reagents depends on ATP binding to the NBDs and phosphorylation of the R domain [34], consistent with the notion that the structure of the pore may be dynamic and regulated. The role of the R domain in channel gating In addition to the structural features shared with other ABC proteins, CFTR also contains a unique 241‑residue R domain in the linker region that joins the two halves of the protein [35] and is essential for its activity [36]. The highly charged R domain possesses multiple phosphorylation sites for PKA and other kinases including PKC (protein kinase C) and AMPK (AMP‑activated protein kinase) [37–39]. As a result of elegant biochemical, structural and computational studies, we now understand that the R domain is a flexible disordered region of the protein which undergoes dynamic interactions with other CFTR domains [40,41]. Although this domain is disordered, it possesses regions with the propensity to form α‑helices in the non‑phosphorylated state and PKA phosphorylation reduces this propensity. These findings are consistent with other biophysical and computational studies showing that the isolated R domain may become less compact with phosphorylation [42]. In the context of the full‑length CFTR protein, phosphorylation of the R domain probably alters multiple interactions with other domains, with certain interactions becoming weaker [41] and others acquiring higher affin‑ ity [39]. For example, phosphorylation may weaken interactions between the R domain and patches on NBD1 (possibly including the regulatory insertion and the regulatory extension) which, in the resting state, prevents heterodimeri‑ zation of NBD1 and NBD2, an important trigger for ATP‑dependent channel opening [7,19,41,43]. On the other hand, phosphorylation may promote the direct interactions between specific regions in the R domain and structure(s) in the membrane domains, the N‑ and/or the C‑terminus. There appears to be an interaction between regions of the R domain and acidic residues near the N‑terminal domain that affect PKA‑dependent CFTR channel activity [44]. According to recent electron microscopy studies, the whole CFTR protein becomes more compact after phosphorylation, supporting the idea that this modification may enhance certain intramolecular interactions mediated by the R domain [9]. Phosphorylation of the R domain modulates the activity of the protein in a complex manner. It is well known that phosphorylation by PKA is absolutely required for channel function [38,45,46] and occurs on at least nine sites in the R domain and one in NBD1 [36,47]. Our biochemical studies show that the apparent ATP affinity for the intrinsic ATPase activity of CFTR is also modu‑ lated by PKA‑dependent phosphorylation [48]. These biochemical findings are © The Authors Journal compilation © 2011 Biochemical Society 240 Essays in Biochemistry volume 50 2011 consistent with the role of phosphorylation in permitting heterodimerization of NBD1 and NBD2, ATP binding to the catalytic site and ATP‑dependent channel opening [43]. Ostedgaard et al. [40] were the first to present a model of the R domain wherein its ‘disordered’ structure was critical in conferring its biological activity. In this model, multiple phosphoserine residues in the disordered R domain could function interchangeably to mediate the intramo‑ lecular interactions necessary for channel opening. Furthermore, these authors suggested that, although no one phosphoserine residue is required, different phosphoserine residues could have quantitatively different effects. In general terms, this model is consistent with experimental data obtained by patch‑clamp studies of the CFTR channel activity [36,43,46,49], as well as structural stud‑ ies of the isolated domain [41]. However, the relative role of each individual PKA phosphosite remains unclear, with certain sites thought to be stimulatory, whereas others are thought to be inhibitory to channel opening [49]. Therefore, although there has been significant progress to date, the molecular basis for phosphorylation‑dependent activation remains unclear. Studies of disease‑causing mutations provide insight into the role for domain–domain interactions underlying protein folding To date, over 1800 mutations have been detected in CF patients, with the most prevalent mutation being the deletion of Phe 508 (F508del‑CFTR) (http://www.genet.sickkids.on.ca/cftr/app). This mutation occurs on at least one allele in ~70% of CF patients [50] and results in misfolding and retention of the mutant protein in the ER (endoplasmic reticulum) [51] (Figure 3). In the ER, the mutant protein is targeted for degradation via the ERAD (ER‑associated degradation) pathway [52]. The molecular basis for the misfolding of F508del‑CFTR remains poorly understood. X‑ray crystallography studies by Lewis et al. [6] on the isolated mutant form of the human NBD1 (hNBD1‑F508del) suggests that deletion of Phe 508 does not impede the fold of the independent domain, but rather may alter the surface topology of NBD1 in the immediate vicinity of the mutation site and hence may alter domain–domain assembly. However, as many solubility‑enhancing mutations were introduced in order to obtain the crystal structures of human NBD1, the impact of deleting Phe 508 on this individual domain requires further study. In full‑length CFTR, these solubility‑enhancing mutations improve the processing and channel gating of F508del‑CFTR [53]. Homology models of CFTR based on the bacterial ABC transporter Sav1866 illustrate a putative consequence of the Phe508 deletion in the context of the full‑length protein (see Figures 1 and 2). According to both of these models, Phe508 contributes to a hydrophobic groove on the surface of NBD1 with which the ‘coupling helix’ of ICL4 (fourth intracellular loop) normally engages [11,13]. These models prompted the hypothesis that this particular interaction may be perturbed in the case of the major mutant F508del‑CFTR. © The Authors Journal compilation © 2011 Biochemical Society P.K. Chiaw, P.D.W. Eckford and C.E. Bear 241 Figure 3. Aberrant exposure of trafficking motifs leads to retention of F508del‑CFTR in the ER Left: cartoon showing the positive role of an ER forward trafficking motif (intense green sphere and green arrow) in mediating ER exit of wild‑type CFTR. Retrograde trafficking of wild‑type CFTR back to the ER from the Golgi may be minimized due to masking of an ER‑retention motif (red outlined arrow and pale red sphere). Right: cartoon of F508del‑CFTR mistrafficking. The majority of the mutant protein is misfolded and thus remains in the ER. Misfolding leads to dysfunction of an ER forward trafficking motif (pale green sphere and green outlined arrow) and enhanced retrograde trafficking via an exposed ER retention motif (intense red arrow and intense red sphere). Using a cysteine cross‑linking approach (on the background of the full‑length protein lacking its endogenous cysteine residues), the research groups of Riordan [13] and Clarke [54] showed that this interface was disrupted in the F508del‑CFTR protein. Furthermore, it appears likely that additional domain–domain interactions are modified in this mutant, perhaps second‑ ary to the loss of the specific interface described above. Limited proteolysis studies performed by Du et al. [55] have also revealed that the F508del‑CFTR mutation in NBD1 alters the assembly of the second half of the CFTR protein and enhances protease‑susceptibility of both NBD2 and MSD2 in the context of the full‑length mutant protein. Despite this progress, our understanding of CFTR folding remains incomplete. For example, it still remains unclear whether each domain folds independently of the others and then assembles or requires association with disparate distant regions for a stable fold of each domain to be achieved. Importantly, we have only looked at regions that can be readily monitored. Hence, the role of the N‑ and C‑termini, the R domain and the intracellular loops in folding, or misfolding in the case of F508del‑CFTR, have not been examined in a systematic way. As a consequence of protein misfolding and/or misassembly caused by the Phe508 deletion, the presentation of both di‑acidic ER exit motifs and di‑arginine ER retention/retrieval signals are altered, thus leading to ER reten‑ tion of the major mutant (Figure 3). Studies by Balch and co‑workers suggested © The Authors Journal compilation © 2011 Biochemical Society 242 Essays in Biochemistry volume 50 2011 that proper presentation of the ER exit motif, the di‑acidic motif D565AD, is lacking, or masked, in F508del‑CFTR [56]. Hypothetically, masking of the D565AD motif would, in turn, prevent association of the mutant protein with the Sec24 protein and exit from the ER via COPII (coatamer protein II)‑coated vesicles [56]. To further compound this defect in trafficking out of the ER, any F508del‑CFTR protein which does manage to reach the Golgi is thought to be sent back to the ER via retrograde trafficking by virtue of the aberrant expo‑ sure of di‑arginine (RXR) retention/retrieval motifs [57]. One such di‑arginine motif (R553AR) appears to play a predominant role, as secondary site suppres‑ sor mutations (in yeast expression studies) have been identified which dis‑ rupt this motif and lead to partial rescue of the primary trafficking defect in F508del‑CFTR [58]. Furthermore, in our own work, we showed that delivery of a synthetic peptide bearing this motif (R553AR), via Tat (transactivator of transcription)‑mediated transduction, partially rescued the F508del‑CFTR bio‑ synthetic defect, probably by competing with the retrograde trafficking machin‑ ery (Figure 3) [59]. Together, these studies support the idea that deletion of Phe508 causes abnormal assembly of NBD1 with the rest of the protein leading to a relative burial of D565AD and exposure of R553AR. As both of these motifs reside in the helical subdomain of NBD1, we suggest that this subdomain may exhibit an altered structure in the context of the whole molecule. In turn, this putative defect may contribute to misassembly of NBD1 with other domains of F508del‑CFTR or aberrant interactions with different proteins. Our studies of the ATPase activity of reconstituted F508del‑CFTR revealed that it retains par‑ tial catalytic activity, arguing that any structural defect in the helical subdomain (comprising part of the catalytic site) may be subtle. Small‑molecule ligands identified through high‑throughput chemical screens provide powerful new tools in studies of the molecular defects caused by disease‑associated mutations The first evidence that the trafficking defect of the Phe508 deletion could be modified to allow partial escape from the ER and functional expression on the cell surface was published by Welsh and colleagues almost 20 years ago [60]. These researchers showed that low‑temperature incubation (e.g. 27°C) of cells expressing the major mutant was effective in causing its expression on the cell surface [60]. Likewise, several small‑molecule modulators, identified in high‑throughput screens of chemical libraries, have been found to ‘rescue’ the trafficking defect [61–63]. High‑throughput screens also led to the identification of ‘potentiator’ compounds which enhance the channel activity of F508del‑CFTR once rescued to reach the cell surface. Clinical trials of one corrector (VX‑809) and one potentiator (VX‑770) compound are currently underway, and these trials are generating considerable excitement in the field. To date, however, the molecular target(s) for these compounds remains unknown, limiting future discovery of novel compounds. © The Authors Journal compilation © 2011 Biochemical Society P.K. Chiaw, P.D.W. Eckford and C.E. Bear 243 It has been hypothesized that certain small‑molecule correctors act by binding directly to the F508del‑CFTR protein to induce an ER-exit‑competent fold [64]. Using the cysteine cross‑linking approach described above, Clarke and colleagues have shown that the addition of the small‑molecule correctors VRT‑325 and Corrector 4a restores normal interactions within the transmem‑ brane domains [64]. Such studies support the notion that these compounds may promote ER exit by inducing a more compact fold in F508del‑CFTR. Recently, we showed that addition of the corrector compound VRT‑325 (at concentrations known to mediate biosynthetic rescue) to proteoliposomes bearing partially purified F508del‑CFTR led to significant inhibition of its apparent affinity for ATP and intrinsic ATPase activity [65]. At the same concentration, acute treat‑ ment of ‘temperature‑corrected’ cells with VRT‑325 exerted a modest inhibi‑ tory effect on channel activation. Using current models of CFTR structure and function, we speculate that direct binding of VRT‑325 leads to a series of allos‑ teric transitions which significantly alter the dynamic conformational changes required for continuous cycles of ATP binding and hydrolysis and, consequent‑ ly, inhibit ATP‑dependent channel gating. We speculate that this alteration of conformational dynamics contributes to its activity as a ‘corrector’. As mentioned above, small molecules with potentiator activity are capable of increasing the channel activity of biosynthetically rescued F508del‑CFTR [62,66,67]. Direct binding of the potentiator VRT‑532 to F508del‑CFTR was supported in our activity assays of partially purified and functionally reconsti‑ tuted F508del‑CFTR [62,66,67]. We observed that VRT‑532 induced a more modest effect than the corrector compound on the ATPase activity of partially purified F508del‑CFTR. VRT‑532 binding did not significantly affect the apparent ATP affinity, rather it lowered the rate of ATP turnover, an effect predicted to stabilize the channel open state and consistent with the potentia‑ tor activity of VRT‑532. Clearly, future studies revealing the binding sites for these small molecules on CFTR or F508del‑CFTR are essential for our under‑ standing of their mechanism of action and will provide insight into the mol‑ ecular basis for disease caused by the most common mutation. Future directions and challenges The goal of solving the molecular mechanisms of action of CFTR and the consequences of disease‑causing mutations continues to be a daunting task. Important novel research tools are being developed, such as high‑resolution structures of other ABC family members (in different ligand‑bound states) that lead to the generation of models which predict their molecular mechanism of action. Such models have been informative with respect to our understanding of the assembly of the multiple domains of CFTR. However, in our opinion, the potential of these models in revealing the molecular mechanism of CFTR as a Cl− channel will only be fully realized when novel biophysical methods for studying conformational dynamics by the full‑length CFTR protein directly are developed to complement single‑channel gating studies. Future progress in this field will © The Authors Journal compilation © 2011 Biochemical Society 244 Essays in Biochemistry volume 50 2011 be enhanced due to the identification of chemical ligands capable of promoting specific channel gating transitions. Molecular mechanisms underlying normal CFTR gating and the perturbations arising from disease‑causing mutations may be revealed once the docking sites for small molecules are defined. Summary • • • • • • • CFTR is an ABC‑type protein that is a unique channel‑forming member of this transporter family. A variety of CFTR mutations result in defective membrane traffick‑ ing and/or channel activity and lead to CF, a chronic and fatal genetic disease that leads to chronic lung obstruction and infection. Our understanding of the structure and function of CFTR has increased through the elucidation of the crystal structures of the NBDs of the protein, the three‑dimensional structure of the intact protein at low resolution and molecular homology modelling that has suggested structural changes likely to occur during the normal function of the protein. Electrophysiological measurements have shed light on the normal func‑ tion of CFTR, its regulation by phosphorylation and nucleotide bind‑ ing, and the effects of CFTR mutations. F508del‑CFTR is the major CF mutation, which causes the protein to misfold and be retained in the ER. ER retention can be ‘corrected’ by small‑molecule modulators, which increase the amount of mutant CFTR at the cell surface, whereas ‘potentiator’ molecules increase the channel activity at that location and are useful tools to further probe the function of CFTR. The corrector VX‑809 and the potentiator VX‑770 are undergoing clinical trial for the treatment of CF, with the potential for pharmaco‑ logical treatment to improve both the lifespan and the quality of life of those suffering from CF. The studies conducted in the Bear laboratory were supported by Operating Grants to C.E.B. from the Canadian Institutes of Health Research (CIHR) [grant numbers GPG‑102171 and MOP 97954], Cystic Fibrosis Canada (CFC) and the U.S. Cystic Fibrosis Foundation (CFF). P.K.C. was supported by a Studentship Award provided by the CIHR (training programme in the structure of membrane proteins) and P.D.W.E. was supported by a Fellowship awarded through CFC. References 1. 2. Rowe, S.M., Miller, S. and Sorscher, E.J. (2005) Cystic fibrosis. N. Engl. J. Med. 352, 1992–2001 Boucher, R.C. (2007) Cystic fibrosis: a disease of vulnerability to airway surface dehydration. Trends Mol. Med. 13, 231–240 © The Authors Journal compilation © 2011 Biochemical Society P.K. Chiaw, P.D.W. Eckford and C.E. Bear 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 245 Dawson, R.J., Hollenstein, K. and Locher, K.P. (2007) Uptake or extrusion: crystal structures of full ABC transporters suggest a common mechanism. Mol. Microbiol. 65, 250–257 Bear, C.E., Li, C.H., Kartner, N., Bridges, R.J., Jensen, T.J., Ramjeesingh, M. and Riordan, J.R. (1992) Purification and functional reconstitution of the cystic fibrosis transmembrane conduct‑ ance regulator (CFTR). Cell 68, 809–818 Accardi, A. and Picollo, A. (2010) CLC channels and transporters: proteins with borderline per‑ sonalities. Biochim. Biophys. Acta 1798, 1457–1464 Lewis, H.A., Zhao, X., Wang, C., Sauder, J.M., Rooney, I., Noland, B.W., Lorimer, D., Kearins, M.C., Conners, K., Condon, B. et al. (2005) Impact of the ΔF508 mutation in first nucleotide‑ binding domain of human cystic fibrosis transmembrane conductance regulator on domain folding and structure. J. Biol. Chem. 280, 1346–1353 Lewis, H.A., Buchanan, S.G., Burley, S.K., Conners, K., Dickey, M., Dorwart, M., Fowler, R., Gao, X., Guggino, W.B., Hendrickson, W.A. et al. (2004) Structure of nucleotide‑binding domain 1 of the cystic fibrosis transmembrane conductance regulator. EMBO J. 23, 282–293 Rosenberg, M.F., Kamis, A.B., Aleksandrov, L.A., Ford, R.C. and Riordan, J.R. (2004) Purification and crystallization of the cystic fibrosis transmembrane conductance regulator (CFTR). J. Biol. Chem. 279, 39051–39057 Zhang, L., Aleksandrov, L.A., Zhao, Z., Birtley, J.R., Riordan, J.R. and Ford, R.C. (2009) Architecture of the cystic fibrosis transmembrane conductance regulator protein and structural changes associated with phosphorylation and nucleotide binding. J. Struct. Biol. 167, 242–251 Awayn, N.H., Rosenberg, M.F., Kamis, A.B., Aleksandrov, L.A., Riordan, J.R. and Ford, R.C. (2005) Crystallographic and single‑particle analyses of native‑ and nucleotide‑bound forms of the cystic fibrosis transmembrane conductance regulator (CFTR) protein. Biochem. Soc. Trans. 33, 996–999 Mornon, J.P., Lehn, P. and Callebaut, I. (2009) Molecular models of the open and closed states of the whole human CFTR protein. Cell. Mol. Life Sci. 66, 3469–3486 Ward, A., Reyes, C.L., Yu, J., Roth, C.B. and Chang, G. (2007) Flexibility in the ABC transporter MsbA: alternating access with a twist. Proc. Natl. Acad. Sci. U.S.A. 104, 19005–19010 Serohijos, A.W., Hegedus, T., Aleksandrov, A.A., He, L., Cui, L., Dokholyan, N.V. and Riordan, J.R. (2008) Phenylalanine‑508 mediates a cytoplasmic‑membrane domain contact in the CFTR 3D structure crucial to assembly and channel function. Proc. Natl. Acad. Sci. U.S.A. 105, 3256–3261 Dawson, R.J. and Locher, K.P. (2006) Structure of a bacterial multidrug ABC transporter. Nature 443, 180–185 Aller, S.G., Yu, J., Ward, A., Weng, Y., Chittaboina, S., Zhuo, R., Harrell, P.M., Trinh, Y.T., Zhang, Q., Urbatsch, I.L. and Chang, G. (2009) Structure of P‑glycoprotein reveals a molecular basis for poly‑specific drug binding. Science 323, 1718–1722 Karpowich, N., Martsinkevich, O., Millen, L., Yuan, Y.R., Dai, P.L., MacVey, K., Thomas, P.J. and Hunt, J.F. (2001) Crystal structures of the MJ1267 ATP binding cassette reveal an induced‑fit effect at the ATPase active site of an ABC transporter. Structure 9, 571–586 Atwell, S., Brouillette, C.G., Conners, K., Emtage, S., Gheyi, T., Guggino, W.B., Hendle, J., Hunt, J.F., Lewis, H.A., Lu, F. et al. (2010) Structures of a minimal human CFTR first nucleotide‑binding domain as a monomer, head‑to‑tail homodimer, and pathogenic mutant. Protein Eng. Des. Sel. 23, 375–384 Gadsby, D.C., Vergani, P. and Csanady, L. (2006) The ABC protein turned chloride channel whose failure causes cystic fibrosis. Nature 440, 477–483 Hwang, T.C. and Sheppard, D.N. (2009) Gating of the CFTR Cl− channel by ATP‑driven nucleotide‑binding domain dimerisation. J. Physiol. 587, 2151–2161 Ramjeesingh, M., Ugwu, F., Stratford, F.L., Huan, L.J., Li, C. and Bear, C.E. (2008) The intact CFTR protein mediates ATPase rather than adenylate kinase activity. Biochem. J. 412, 315–321 Beaudet, L. and Gros, P. (1998) Mutational analysis of P‑glycoprotein in yeast Saccharomyces cerevisiae. Methods Enzymol. 292, 414–427 Zeltwanger, S., Wang, F., Wang, G.T., Gillis, K.D. and Hwang, T.C. (1999) Gating of cystic fibrosis transmembrane conductance regulator chloride channels by adenosine triphosphate hydrolysis: quantitative analysis of a cyclic gating scheme. J. Gen. Physiol. 113, 541–554 © The Authors Journal compilation © 2011 Biochemical Society 246 Essays in Biochemistry volume 50 2011 23. Aleksandrov, A.A., Cui, L. and Riordan, J.R. (2009) Relationship between nucleotide binding and ion channel gating in cystic fibrosis transmembrane conductance regulator. J. Physiol. 587, 2875–2886 24. Csanady, L., Vergani, P. and Gadsby, D.C. (2010) Strict coupling between CFTR’s catalytic cycle and gating of its Cl− ion pore revealed by distributions of open channel burst durations. Proc. Natl. Acad. Sci. U.S.A. 107, 1241–1246 25. Muallem, D. and Vergani, P. (2009) ATP hydrolysis‑driven gating in cystic fibrosis transmembrane conductance regulator. Philos. Trans. R. Soc. London Ser. B 364, 247–255 26. Chakrabarti, N., Neale, C., Payandeh, J., Pai, E.F. and Pomes, R. (2010) An iris‑like mechanism of pore dilation in the CorA magnesium transport system. Biophys. J. 98, 784–792 27. Wang, W., Wu, J., Bernard, K., Li, G., Wang, G., Bevensee, M.O. and Kirk, K.L. (2010) ATP‑independent CFTR channel gating and allosteric modulation by phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 107, 3888–3893 28. Linsdell, P. (2006) Mechanism of chloride permeation in the cystic fibrosis transmembrane con‑ ductance regulator chloride channel. Exp. Physiol. 91, 123–129 29. Sheppard, D.N. and Welsh, M.J. (1999) Structure and function of the CFTR chloride channel. Physiol. Rev. 79, S23–S45 30. McCarty, N.A. (2000) Permeation through the CFTR chloride channel. J. Exp. Biol. 203, 1947–1962 31. Linsdell, P., Tabcharani, J.A., Rommens, J.M., Hou, Y.X., Chang, X.B., Tsui, L.C., Riordan, J.R. and Hanrahan, J.W. (1997) Permeability of wild‑type and mutant cystic fibrosis transmembrane con‑ ductance regulator chloride channels to polyatomic anions. J. Gen. Physiol. 110, 355–364 32. Zhou, J.J., Li, M.S., Qi, J. and Linsdell, P. (2010) Regulation of conductance by the number of fixed positive charges in the intracellular vestibule of the CFTR chloride channel pore. J. Gen. Physiol. 135, 229–245 33. Alexander, C., Ivetac, A., Liu, X., Norimatsu, Y., Serrano, J.R., Landstrom, A., Sansom, M. and Dawson, D.C. (2009) Cystic fibrosis transmembrane conductance regulator: using differential reactivity toward channel‑permeant and channel‑impermeant thiol‑reactive probes to test a mol‑ ecular model for the pore. Biochemistry 48, 10078–10088 34. Fatehi, M. and Linsdell, P. (2008) State‑dependent access of anions to the cystic fibrosis trans‑ membrane conductance regulator chloride channel pore. J. Biol. Chem. 283, 6102–6109 35. Riordan, J.R., Rommens, J.M., Kerem, B., Alon, N., Rozmahel, R., Grzelczak, Z., Zielenski, J., Lok, S., Plavsic, N., Chou, J.L. et al. (1989) Identification of the cystic fibrosis gene: cloning and charac‑ terization of complementary DNA. Science 245, 1066–1073 36. Cheng, S.H., Rich, D.P., Marshall, J., Gregory, R.J., Welsh, M.J. and Smith, A.E. (1991) Phosphorylation of the R domain by cAMP‑dependent protein kinase regulates the CFTR chloride channel. Cell 66, 1027–1036 37. Kongsuphol, P., Hieke, B., Ousingsawat, J., Almaca, J., Viollet, B., Schreiber, R. and Kunzelmann, K. (2009) Regulation of Cl− secretion by AMPK in vivo. Pflügers Arch. 457, 1071–1078 38. Tabcharani, J.A., Chang, X.B., Riordan, J.R. and Hanrahan, J.W. (1991) Phosphorylation‑regulated Cl− channel in CHO cells stably expressing the cystic fibrosis gene. Nature 352, 628–631 39. Chappe, V., Irvine, T., Liao, J., Evagelidis, A. and Hanrahan, J.W. (2005) Phosphorylation of CFTR by PKA promotes binding of the regulatory domain. EMBO J. 24, 2730–2740 40. Ostedgaard, L.S., Baldursson, O., Vermeer, D.W., Welsh, M.J. and Robertson, A.D. (2000) A functional R domain from cystic fibrosis transmembrane conductance regulator is predominantly unstructured in solution. Proc. Natl. Acad. Sci. U.S.A. 97, 5657–5662 41. Baker, J.M., Hudson, R.P., Kanelis, V., Choy, W.Y., Thibodeau, P.H., Thomas, P.J. and Forman‑Kay, J.D. (2007) CFTR regulatory region interacts with NBD1 predominantly via multiple transient helices. Nat. Struct. Mol. Biol. 14, 738–745 42. Hegedus, T., Serohijos, A.W., Dokholyan, N.V., He, L. and Riordan, J.R. (2008) Computational studies reveal phosphorylation‑dependent changes in the unstructured R domain of CFTR. J. Mol. Biol. 378, 1052–1063 43. Mense, M., Vergani, P., White, D.M., Altberg, G., Nairn, A.C. and Gadsby, D.C. (2006) In vivo phosphorylation of CFTR promotes formation of a nucleotide‑binding domain heterodimer. EMBO J. 25, 4728–4739 © The Authors Journal compilation © 2011 Biochemical Society P.K. Chiaw, P.D.W. Eckford and C.E. Bear 247 44. Naren, A.P., Cormet‑Boyaka, E., Fu, J., Villain, M., Blalock, J.E., Quick, M.W. and Kirk, K.L. (1999) CFTR chloride channel regulation by an interdomain interaction. Science 286, 544–548 45. Seibert, F.S., Chang, X.B., Aleksandrov, A.A., Clarke, D.M., Hanrahan, J.W. and Riordan, J.R. (1999) Influence of phosphorylation by protein kinase A on CFTR at the cell surface and endo‑ plasmic reticulum. Biochim. Biophys. Acta 1461, 275–283 46. Chang, X.B., Tabcharani, J.A., Hou, Y.X., Jensen, T.J., Kartner, N., Alon, N., Hanrahan, J.W. and Riordan, J.R. (1993) Protein kinase A (PKA) still activates CFTR chloride channel after mutagen‑ esis of all 10 PKA consensus phosphorylation sites. J. Biol. Chem. 268, 11304–11311 47. Gadsby, D.C. and Nairn, A.C. (1999) Control of CFTR channel gating by phosphorylation and nucleotide hydrolysis. Physiol. Rev. 79, S77–S107 48. Li, C., Ramjeesingh, M., Wang, W., Garami, E., Hewryk, M., Lee, D., Rommens, J.M., Galley, K. and Bear, C.E. (1996) ATPase activity of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 271, 28463–28468 49. Wilkinson, D.J., Strong, T.V., Mansoura, M.K., Wood, D.L., Smith, S.S., Collins, F.S. and Dawson, D.C. (1997) CFTR activation: additive effects of stimulatory and inhibitory phosphorylation sites in the R domain. Am. J. Physiol. 273, L127–L133 50. Kerem, B., Rommens, J.M., Buchanan, J.A., Markiewicz, D., Cox, T.K., Chakravarti, A., Buchwald, M. and Tsui, L.C. (1989) Identification of the cystic fibrosis gene: genetic analysis. Science 245, 1073–1080 51. Cheng, S.H., Gregory, R.J., Marshall, J., Paul, S., Souza, D.W., White, G.A., O’Riordan, C.R. and Smith, A.E. (1990) Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell 63, 827–834 52. Younger, J.M., Chen, L., Ren, H.Y., Rosser, M.F., Turnbull, E.L., Fan, C.Y., Patterson, C. and Cyr, D.M. (2006) Sequential quality‑control checkpoints triage misfolded cystic fibrosis transmembrane conductance regulator. Cell 126, 571–582 53. Pissarra, L.S., Farinha, C.M., Xu, Z., Schmidt, A., Thibodeau, P.H., Cai, Z., Thomas, P.J., Sheppard, D.N. and Amaral, M.D. (2008) Solubilizing mutations used to crystallize one CFTR domain attenu‑ ate the trafficking and channel defects caused by the major cystic fibrosis mutation. Chem. Biol. 15, 62–69 54. Chen, E.Y., Bartlett, M.C., Loo, T.W. and Clarke, D.M. (2004) The ΔF508 mutation disrupts pack‑ ing of the transmembrane segments of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 279, 39620–39627 55. Du, K., Sharma, M. and Lukacs, G.L. (2005) The ΔF508 cystic fibrosis mutation impairs domain– domain interactions and arrests post‑translational folding of CFTR. Nat. Struct. Mol. Biol. 12, 17–25 56. Wang, X., Matteson, J., An, Y., Moyer, B., Yoo, J.S., Bannykh, S., Wilson, I.A., Riordan, J.R. and Balch, W.E. (2004) COPII‑dependent export of cystic fibrosis transmembrane conductance regu‑ lator from the ER uses a di‑acidic exit code. J. Cell Biol. 167, 65–74 57. Chang, X.B., Cui, L., Hou, Y.X., Jensen, T.J., Aleksandrov, A.A., Mengos, A. and Riordan, J.R. (1999) Removal of multiple arginine‑framed trafficking signals overcomes misprocessing of ΔF508 CFTR present in most patients with cystic fibrosis. Mol. Cell 4, 137–142 58. Teem, J.L., Berger, H.A., Ostedgaard, L.S., Rich, D.P., Tsui, L.C. and Welsh, M.J. (1993) Identification of revertants for the cystic fibrosis ΔF508 mutation using STE6‑CFTR chimeras in yeast. Cell 73, 335–346 59. Kim Chiaw, P., Huan, L.J., Gagnon, S., Ly, D., Sweezey, N., Rotin, D., Deber, C.M. and Bear, C.E. (2009) Functional rescue of ΔF508‑CFTR by peptides designed to mimic sorting motifs. Chem. Biol. 16, 520–530 60. Denning, G.M., Anderson, M.P., Amara, J.F., Marshall, J., Smith, A.E. and Welsh, M.J. (1992) Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature‑ sensitive. Nature 358, 761–764 61. Robert, R., Carlile, G.W., Liao, J., Balghi, H., Lesimple, P., Liu, N., Kus, B., Rotin, D., Wilke, M., de Jonge, H.R. et al. (2010) Correction of the ΔPhe508 cystic fibrosis transmembrane conductance regulator trafficking defect by the bioavailable compound glafenine. Mol. Pharmacol. 77, 922–930 © The Authors Journal compilation © 2011 Biochemical Society 248 Essays in Biochemistry volume 50 2011 62. Van Goor, F., Straley, K.S., Cao, D., Gonzalez, J., Hadida, S., Hazlewood, A., Joubran, J., Knapp, T., Makings, L.R., Miller, M. et al. (2006) Rescue of ΔF508‑CFTR trafficking and gating in human cystic fibrosis airway primary cultures by small molecules. Am. J. Physiol. Lung Cell. Mol. Physiol. 290, L1117–L1130 63. Pedemonte, N., Lukacs, G.L., Du, K., Caci, E., Zegarra‑Moran, O., Galietta, L.J. and Verkman, A.S. (2005) Small‑molecule correctors of defective ΔF508‑CFTR cellular processing identified by high‑throughput screening. J. Clin. Invest. 115, 2564–2571 64. Wang, Y., Loo, T.W., Bartlett, M.C. and Clarke, D.M. (2007) Correctors promote maturation of cystic fibrosis transmembrane conductance regulator (CFTR)‑processing mutants by binding to the protein. J. Biol. Chem. 282, 33247–33251 65. Kim Chiaw, P., Wellhauser, L., Huan, L.J., Ramjeesingh, M. and Bear, C. (2010) A chemical correc‑ tor modifies the channel function of F508del‑CFTR. Mol. Pharmacol. 78, 411–418 66. Van Goor, F., Hadida, S., Grootenhuis, P.D., Burton, B., Cao, D., Neuberger, T., Turnbull, A., Singh, A., Joubran, J., Hazlewood, A. et al. (2009) Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX‑770. Proc. Natl. Acad. Sci. U.S.A. 106, 18825–18830 67. Wellhauser, L., Kim Chiaw, P., Pasyk, S., Li, C., Ramjeesingh, M. and Bear, C.E. (2009) A small‑molecule modulator interacts directly with ΔPhe508‑CFTR to modify its ATPase activity and conformational stability. Mol. Pharmacol. 75, 1430–1438 © The Authors Journal compilation © 2011 Biochemical Society