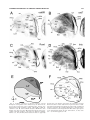
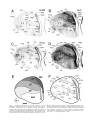
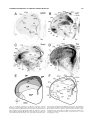
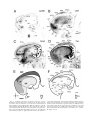
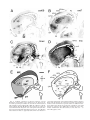
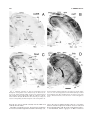
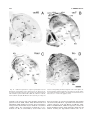
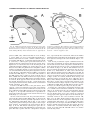
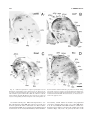
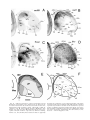
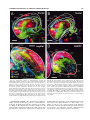
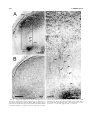
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Cadherin Expression by Embryonic Divisions and
Neuroplasticity wikipedia , lookup
Brain morphometry wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Synaptic gating wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Neuroeconomics wikipedia , lookup
Metastability in the brain wikipedia , lookup
Optogenetics wikipedia , lookup
Neuroanatomy wikipedia , lookup
Neurogenomics wikipedia , lookup
Subventricular zone wikipedia , lookup
Anatomy of the cerebellum wikipedia , lookup
Gene expression programming wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Aging brain wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Development of the nervous system wikipedia , lookup
Sexually dimorphic nucleus wikipedia , lookup
Hypothalamus wikipedia , lookup
THE JOURNAL OF COMPARATIVE NEUROLOGY 438:253–285 (2001) Cadherin Expression by Embryonic Divisions and Derived Gray Matter Structures in the Telencephalon of the Chicken CHRISTOPH REDIES,1* LORETA MEDINA,2 AND LUIS PUELLES2 Institute of Anatomy, University of Essen, School of Medicine, D-45122 Essen, Germany 2 Department of Morphological Sciences, University of Murcia, Murcia 30100, Spain 1 ABSTRACT The expression of three cadherins (cadherin-6B, cadherin-7, and R-cadherin) was studied by immunohistochemistry in the telencephalon of chicken embryos at intermediate stages of development (11 and 15 days of incubation). Expression patterns were related to cytoarchitecture and to previously published data on functional connections and on the expression of gene regulatory proteins. Our results indicate that, like in other regions of the embryonic chicken brain, the expression of each cadherin is restricted to parts of embryonic divisions as well as to particular nuclei, areas or their subdivisions. The expression patterns are largely complementary with partial overlap. The regional expression of the cadherins respects the boundary between the pallium and the subpallium as well as between various pallial and subpallial subdivisions. Novel subdivisions were found in several telencephalic areas. For example, subjacent to the hyperstriatum, the neostriatum contains multiple islands of cells with a profile of cadherin expression that differs from the surrounding matrix (“island fields”). Moreover, the expression of each cadherin is apparently associated with parts of intratelencephalic neural circuits and of thalamopallial and basal ganglia pathways. These results support a role for cadherins in the aggregation and differentiation of gray matter structures within embryonic brain divisions. The cadherin immunostaining patterns are interpreted in the context of a recently proposed divisional scheme of the avian pallium that postulates medial, dorsal, lateral, and ventral divisions as complete radial histogenetic units (Puelles et al. [2000]). J. Comp. Neurol. 438:253–285, 2001. © 2001 Wiley-Liss, Inc. Indexing terms: cell adhesion molecules; avian pallium; forebrain; basal ganglia; thalamopallial circuits Cadherins are a large family of calcium-dependent cell surface glycoproteins (for review, see Nollet et al., 2000) that mediate cell– cell adhesion and, thereby, regulate tissue morphogenesis in the vertebrate brain and many other organs (for review, see Takeichi, 1995; Gumbiner, 1996). In general, cells expressing the same cadherin subtype tend to aggregate, whereas cells expressing different cadherin subtypes tend to segregate (for review, see Takeichi, 1995; for exceptions, see Shimoyama et al., 2000). This preferentially homotypic binding of cadherinexpressing cell populations has been proposed to mediate the selective association of neural cells as well as their connections (for review, see Redies, 1995, 2000). In particular, cadherin-mediated adhesive specificity was postulated to be involved in the formation, maintenance, or both, of divisional borders in the embryonic brain (Gänzler © 2001 WILEY-LISS, INC. and Redies, 1995; Espeseth et al., 1998), in the formation of gray matter structures, such as brain nuclei (Redies et al., 1993; Gänzler and Redies, 1995; Yoon et al., 2000); in the outgrowth, pathfinding, and fasciculation of neurites (Matsunaga et al., 1988; Bixby and Zhang, 1990; Redies et Grant sponsor: DFG; Grant number: Re 616/4-1; Grant sponsor: Seneca Foundation; Grant number: PB/25/FS/99; Grant sponsor: MCYT; Grant number: BFI2000-1359-C02-02; Grant sponsor: CICYT; Grant number: PB98-0397; Grant sponsor: Spanish Ministerio de Relaciones Exteriores and DAAD; Grant number: HA1996-0149; Grant sponsor: EC-BIOTECH Program; Grant number: BIO4-CT96-0042. *Correspondence to: Christoph Redies, Institute of Anatomy, University Hospital Essen, Hufelandstrasse 55, D-45122 Essen, Germany. E-mail: [email protected] Received 11 May 2000; Revised 8 February 2001; Accepted 20 April 2001 254 C. REDIES ET AL. al., 1992, 1993; Iwai et al., 1997; for review, see Redies, 1997); in the formation of specific neural circuits (Redies et al., 1993; Arndt and Redies, 1996); and in synaptogenesis (Fannon and Colman, 1996; Uchida et al., 1996; for review, see Redies, 2000). By mapping the expression of four cadherin subtypes, we have previously studied the developing diencephalon of the chicken, delineated its embryonic divisions, and determined their gray matter derivatives (Redies et al., 2000; Yoon et al., 2000). The cadherin mapping results were interpreted in relation to previous data on brain development, anatomic organization, and connectivity. Together, these studies indicated that the primary divisional pattern of the diencephalon is translated into a complex Abbreviations Aa Acc Ai Aid Aii Aim Aiv al Am Ap APH APHcl APHi APHl APHm Bas BSTa BSTp c ca CA cad6B cad7 CDL cpa CPA CPi CPP d DLP DP dss DT DVR E Ec E11 E15 EIF EmT f HA HD HDs HDp HIS HISp Hp HV HVC HVCp HVF HVFs HVI HVd HVds HVv HVvs HVn HVp INP is l anterior archistriatum nucleus accumbens intermediate archistriatum intermediate archistriatum, dorsal part intermediate archistriatum, intermediate part intermediate archistriatum, medial portion of intermediate part intermediate archistriatum, ventral part ansa lenticularis medial archistriatum posterior archistriatum parahippocampal area parahippocampal area, caudolateral part parahippocampal area, intermediate part parahippocampal area, lateral part parahippocampal area, medial part basal nucleus of neostriatum bed nucleus of the stria terminalis, anterior part bed nucleus of the stria terminalis, posterior part caudal anterior commissure nucleus of the anterior commissure cadherin-6B cadherin-7 dorsolateral corticoid area pallial commissure nucleus of the pallial commissure piriform cortex prepiriform cortex dorsal dorsolateral posterior nucleus of the dorsal thalamus dorsal pallium dorsal suprapallial sulcus dorsal thalamus dorsal ventricular ridge ectostriatum ectostriatal core embryonic day 11 embryonic day 15 ectostriatal island field eminentia thalami fimbria fibers accessory hyperstriatum dorsal hyperstriatum dorsal hyperstriatum, superficial part dorsal hyperstriatum, periventricular part supreme intercalate hyperstriatum supreme intercalate hyperstriatum, periventricular part hippocampus ventral hyperstriatum ventral hyperstriatum, caudal portion ventral hyperstriatum, caudal portion, periventricular part ventral hyperstriatum, frontal portion ventral hyperstriatum, frontal portion, superficial part ventral hyperstriatum, intermediate portion ventral hyperstriatum, intermediate portion, dorsal area ventral hyperstriatum, intermediate portion, dorsal area, superficial part ventral hyperstriatum, intermediate portion, ventral area ventral hyperstriatum, intermediate portion, ventral area, superficial part nucleus of the ventral hyperstriatum ventral hyperstriatum, periventricular part intrapeduncular nucleus neostriatal islands lateral L L1 L2 L3 LA lad lfb lfs lfsm lh lmd LOT LP lsa lv m mE Mes MP mz N NC Ncad NCL NCLp NCp NF NFp NI NIF NIp NL OA OB olf om p5 PAd PAm PAv pch pE POM r Rcad rE rlis S SbP SI SL SM sme st ST STl STm TeO thio Tn TO TPO tsm v va VP vss field L field L, area 1 (ventral) field L, area 2 (intermediate) field L, area 3 (dorsal) lateroanterior nucleus of the ventral thalamus dorsal archistriatal lamina lateral forebrain bundle superior frontal lamina supreme frontal lamina hyperstriatal lamina dorsal medullary lamina nucleus of the lateral olfactory tract lateral pallium lateral striatal artery lateral ventricle medial medial area of periectostriatal belt mesencephalon medial pallium neostriatal marginal zone neostriatum caudal neostriatum N-cadherin caudal neostriatum, lateral part caudal neostriatum, lateral part, periventricular portion caudal neostriatum, periventricular part frontal neostriatum frontal neostriatum, periventricular part intermediate neostriatum neostriatal island field intermediate neostriatum, periventricular part lateral neostriatum anterior olfactory nucleus olfactory bulb olfactory tract occipito-mesencephalic tract prosomere 5 dorsal pallidum medial pallidum ventral pallidum choroid plexus periectostriatal belt medial (main) portion of preoptic nucleus rostral R-cadherin periectostriatal belt, retroectostriatal portion rostrolateral island of the ectostriatal island field septum subpallium innominate substance lateral septum medial septum stria medullaris stria terminalis striatum striatum, lateral part striatum, medial part optic tectum thionine staining nucleus taeniae olfactory tubercle temporo-parieto-occipital area septo-mesencephalic tract ventral vallecula ventral pallium ventral suprapallial sulcus CADHERIN EXPRESSION IN CHICKEN TELENCEPHALON framework of potentially adhesive cues. This framework is reflected, in part, by the regional and differential expression of cadherins. Each cadherin-defined diencephalic division is secondarily transformed to give rise to a fully developed domain of gray matter in the mature diencephalon, which extends radially from the ventricular surface to the pial surface. Within each domain, additional regionalization in the cadherin expression patterns takes place, as individual brain nuclei or cell layers are formed (Redies et al., 2000; Yoon et al., 2000). In some cases, differential cadherin expression reflects an evolving functional differentiation within gray matter structures. Examples are the restricted expression of cadherins in subdivisions of the thalamic nucleus rotundus in the chicken (Redies et al., 2000) and, in the cerebellar cortex, the parasagittal stripes of cadherin expression (Arndt et al., 1998). In the current work, we mapped in the telencephalon the expression of four cadherin subtypes (cadherin-6B, cadherin-7, R-cadherin, and N-cadherin), which were analyzed previously in the diencephalon of the chicken (Redies et al., 2000). The analysis was carried out at an intermediate stage of telencephalic development when most gray matter structures have already been formed and assume their final topologic positions. At this time, the cadherin expression profile is still relatively distinct in the embryonic divisions (11 and 15 days of incubation; E11–E15). Preliminary results showed that cadherin expression was very similar at the two stages examined. The cadherin immunostaining patterns were compared with the cytoarchitecture of the telencephalon, as well as to published data on its development, neurochemistry, or connections, and to other data available in the literature on the avian brain. The present findings were interpreted within established schemes of telencephalic gray matter divisions. Particular findings were also related to the novel scheme of postulated avian pallial subdivisions that were recently proposed by Puelles et al. (1999, 2000). As observed in the chicken diencephalon (Redies et al., 2000), the cadherin expression patterns can be related not only to some of the major telencephalic divisions, but also they can be related to the differentiating gray matter structures that derive from these divisions. Within some divisions, several additional cell groups or areas were identified that were not noticed previously by using other criteria. Some of these results suggest new aspects on the connections and function of the avian telencephalon to consider in future studies in a comparative context. MATERIAL AND METHODS For the current study, the same set of immunostained sections was used that was prepared and analyzed for our previous study on the chicken diencephalon (Redies et al., 2000). The materials and methods used in the current work have been described in the publications by Yoon et al. (2000) and Redies et al. (2000). Antibodies and immunohistochemistry In brief, fertilized eggs from domestic chicken (Gallus domesticus) were incubated at 38°C and 65% humidity in a forced-draft incubator (Ehret, Emmendingen, Germany). Before decapitation of the embryos, eggs were cooled on ice to induce deep anesthesia, in accordance with national and institutional guidelines on the use of animals in research (“Tierschutzgesetz”). Embryos were fixed at embry- 255 onic day 11 (E11, stage 37 according to Hamburger and Hamilton, 1951) and at E15 (stage 41). For each embryonic stage and for each cadherin subtype, several series of consecutive sections were obtained (E11: three transverse series, one horizontal series, and one sagittal series; E15: one transverse series, one horizontal series, and one sagittal series). The series of sections were incubated with primary monoclonal antibodies against chicken N-cadherin (NCD-2; Hatta and Takeichi, 1986), against chicken R-cadherin (RCD-2; Redies et al., 1992; Arndt and Redies, 1996), and against chicken cadherin-6B and cadherin-7 (CC6B-1 and CC7-1, respectively; Nakagawa and Takeichi, 1998), as described previously (Yoon et al., 2000). The antibodies were a kind gift of Dr. M. Takeichi and Dr. S. Nakagawa. Data analysis and terminology All sections stained were visualized with Axiophot or Ultraphot microscopes (Zeiss, Oberkochen, Germany). Photographic images of the sections, which were selected for the figures, were scanned by using a computer-based image processing system, enhanced in contrast, if required, and labelled with the Freehand software (Macromedia, San Francisco, CA) and the Photoshop software (Adobe Systems, Mountain View, CA). To display simultaneously the immunostaining results for three cadherins (Fig. 16), photographic images were enhanced in contrast, color coded, and superimposed by using the Photoshop software. For identification of telencephalic gray matter structures, the terminology used in the atlas of the chicken brain by Kuenzel and Masson (1988) and in the atlas of the pigeon brain by Karten and Hodos (1967) was followed generally. However, based on recent suggestions by a committee revising the terminology of the avian forebrain (in alphabetical order: A. Csillag, W. Kuenzel, L. Medina, L. Puelles, A. Reiner, G. Striedter, M. Wild, unpublished data), the following widely approved modern terms were used for subpallial structures (traditional terms are given in parenthesis): lateral striatum (paleostriatum augmentatum), medial striatum (rostral and caudodorsal part of the parolfactory lobe), dorsal pallidum (paleostriatum primitivum), and ventral pallidum (caudoventral pallidal area interstitial to the medial forebrain bundle). In the Results section, we keep mostly within the nomenclature used in the above-mentioned atlases for the telencephalic pallium. The data are schematically represented and interpreted also in the context of a hypothetical divisional scheme that emphasizes possible homologies with the mammalian pallial divisions, as proposed on the basis of gene expression data (see Discussion; Puelles et al., 1999, 2000). RESULTS Cadherin-6B (cad6B), cadherin-7 (cad7), and R-cadherin (Rcad) each show a distinct regional immunoreactivity pattern in the telencephalon of the embryonic chicken. The patterns are largely complementary but partial overlap occurs in some regions, especially between cad6B and cad7, as noted previously in other brain areas (Arndt et al., 1998; Wöhrn et al., 1998, 1999; Redies et al., 2000). N-cadherin (Ncad) immunostaining is generally weaker and relatively uniform in the telencephalon. This uniformity may be partially due to the widespread expression of 256 C. REDIES ET AL. Ncad by radial glia (Inuzuka et al., 1991; Redies et al., 1993). In contrast to the diencephalon, no regions of very high or especially distinct Ncad immunostaining were found in the telencephalon at the stages examined. The expression of the other cadherins more clearly relates to the cytoarchitecture of the avian telencephalon, including many traditional neuroanatomic divisions and specific cell groups (nuclei, laminae, bands, or areas). Immunostaining results for cad6B, cad7, and Rcad are shown in Figures 1– 6 for representative levels from a series of transverse sections at E11. In addition, correlative results are shown in Figures 7–15 for selected levels from a representative series of parasagittal sections through the telencephalon of an E15 chicken embryo. In each figure, adjacent sections immunostained for either cad6B, cad7, or Rcad are shown in panels A, B, and C, respectively. An adjacent section stained for thionine is shown in panels D to demonstrate cytoarchitecture. In the Discussion section, the immunostaining results will be related to a recently proposed model of telencephalic pallial subdivisions (Puelles et al., 1999, 2000). For reference, schematic diagrams of the major telencephalic divisions of this model are shown in panels E and F, respectively, of Figures 1– 6 and 15 (see Discussion section). Similar diagrams for the sections shown in Figures 7, 9, 11, and 13 are displayed in Figures 8, 10, 12, and 14, respectively. Results for Ncad immunostaining are described in the text only. Table 1 lists the immunoreactivity of cad6B, cad7, Rcad, and Ncad for all anatomic structures that were defined in the current study. With the few exceptions noted in the text, cadherin immunoreactivity did not change from E11 to E15 in these structures. In addition to cadherin expression by cell groups, numerous axonal fibers expressing specific cadherin subtypes are observed (see Table 1). A detailed description of these fiber tracts and the gray matter areas, which they connect, will be the subject of a separate report (L. Medina, L. Puelles, C. Redies, unpublished data). Subpallium The subpallium is located ventral to the dorsal medullary lamina (lmd), a cell-poor glial palisade that separates the two major telencephalic divisions, i.e., the pallial and subpallial territories (Källén, 1962; Striedter and Beydler, 1997; Puelles et al., 1999, 2000). The lmd coincides with an abrupt change in cad7 expression from relatively low in the subpallium to high in the pallium (Figs. 2–5, 9 –14). In contrast, expression of cad6B is relatively low in the pallium and becomes moderate or high in some parts of the subpallium. Rcad is also relatively low in the subpallium and moderate to high in parts of the pallium (see same figures as above). The subpallium includes the basal ganglia (striatum and pallidum), the major part of the septum, and other basal forebrain areas like the bed nucleus of the stria terminalis, the intrapeduncular nucleus, the innominate substance, and the nucleus of the diagonal band (Karten and Dubbeldam, 1973; Reiner et al., 1984, 1998; Medina and Reiner, 1994, 1995; Striedter, 1997; Aste et al., 1998; Puelles et al., 2000). The immunoreactivity of cad6B, cad7, and Rcad is generally weak to moderate in the subpallial mantle. It is relatively diffuse and apparently related to both neuropil and scattered cells, with the exception of some regions (for example, the dorsal pallidum) in which stronger cadherin immunoreactivity is mostly related to the presence of immunoreactive neurons (described below; Table 1). Basal ganglia. The striatal mantle is characterized, in general, by low to moderate levels of cad6B immunoreactivity (Figs. 2– 4; but see below). Part of the nucleus accumbens (Acc in Figs. 2, 7) also expresses moderate levels of cad6B. The medial and lateral striatum (Figs. 2– 4, 11–15) express generally low levels of cad7 and Rcad, with the exception of the periventricular area of the medial striatum (STm) that is characterized by moderate to strong immunoreactivity for cad6B, cad7, and Rcad (Figs. 2– 4, 9). Such immunoreactivity is less intense in the periventricular region that is ventral to it (Fig. 3). This change in cadherin expression is gradual and does not have a prominent cytoarchitectonic correlate. It is located at or close to the striatopallidal limit revealed by the expression of the pallidum-related gene Nkx2.1 in the caudoventral part of the parolfactory lobe at E10.5 (Puelles et al., 2000). For this reason, we have called this region, which is classically considered as a caudoventral part of the parolfactory lobe, the medial part of the pallidum (or PAm). The avian dorsal pallidum (PAd, classically called paleostriatum primitivum) shows generally weak cad7 immunoreactivity (Figs. 3, 4, 11, 13) and contains groups of dispersed, large neurons that are strongly immunoreactive for either cad6B or Rcad (Figs. 4, 11, 13). These immunoreactive neurons are nonuniformly distributed throughout the dorsal pallidum, showing opposed gradients extending from medial to lateral. Neurons immunoreactive for cad6B are located mainly in the medial (deeper) part of PAd, whereas neurons immunoreactive for Rcad are located mostly at intermediate and lateral (superficial) levels. However, the rostral pole of PAd appears free of neurons immunoreactive for these cadherins (Fig. 3), thus, revealing a substantial heterogeneity of cadherin expression in the PA. The ventral pallidum (PAv) and the medial, periventricular part of the pallidum (PAm) are weakly immunoreactive for cad6B, cad7, and Rcad (Figs. 3, 4). Adjacent to the pial surface of the striatum and the pallidum, the olfactory tubercle (TO in Fig. 7D) does not show significant expression levels for the cadherin subtypes studied here. Other basal forebrain cell groups. The intrapeduncular nucleus (INP) can be clearly distinguished from the rostrally and dorsally adjacent dorsal pallidum by its neuropil and cells that are strongly and uniformly immunoreactive for cad6B and Rcad (Figs. 4, 11, 13). The innominate substance (SI) is understood as a diffuse area that is populated, amongst other cells, by large dispersed cholinergic neurons around and within the lateral forebrain bundle, where it enters the subpallium (Medina and Reiner, 1994). It is located caudal and ventromedial to the intrapeduncular nucleus and lateral to the ventral pallidum. It strongly expresses cad6B but only in patches (Figs. 11, 13). The SI also contains some scattered cells immunoreactive for Rcad (Figs. 11, 13). Adjacent to the SI, the lateral forebrain bundle (lfb) contains numerous fibers immunoreactive for cad6B, cad7, or Rcad (Figs. 4, 11, 13). The anterior part of the bed nucleus of the stria terminalis (BSTa) contains moderate to strong immunoreactivity for cad6B, cad7, Rcad, and Ncad (Figs. 4, 5, 9, 11). The BSTa can be divided into subregions on the basis of cadherin immunoreactivity, in agreement with the descrip- CADHERIN EXPRESSION IN CHICKEN TELENCEPHALON tion of Aste et al. (1998). Close to the BSTa, the stria terminalis contains numerous fibers positive for Rcad, and also some fibers positive for cad6B and cad7 (st; Figs. 6, 9, 11). More caudally, the posterior part of the bed nucleus of the stria terminalis (BSTp) shows weak immunoreactivity for Rcad but appears free of cad6B and cad7 immunoreactivity (Fig. 5). Septum. The septal region shows a heterogeneous cadherin expression pattern. In general, immunoreactivity for cad6B is low laterally and immunoreactivity for cad7, Rcad, and Ncad is generally moderate to high (Figs. 4, 5, 7, 9). Both medial and lateral septal areas (SM and SL, respectively) contain various subdivisions that differentially express cad6B, cad7, and Rcad. This heterogeneity reflects uncharted complexities of this region. The fiber tracts or commissures associated with the septal region contain fibers immunoreactive for different cadherins (Table 1). The septo-mesencephalic tract (tsm) contains numerous fibers positive for cad7 and a few fibers positive for cad6B (Figs. 4, 9). In addition, the anterior commissure (ca) contains numerous fibers strongly positive for cad7 and also fibers positive for Rcad, whereas the pallial commissure (cpa) contains only fibers positive for Rcad (Figs. 5, 7, 9). Pallium The pallium lies dorsal to the dorsal medullary lamina. In general, it shows weak immunoreactivity for cad6B, but it contains numerous regions that are strongly immunoreactive for cad7 and Rcad (Figs. 1–15). The main pallial divisions described in the classic literature, i.e., the hippocampus (Hp), the parahippocampal area (APH), the Wulst, the dorsal ventricular ridge (DVR, with its two parts, the neostriatum and ventral hyperstriatum) and the archistriatum can be readily distinguished by means of their cadherin immunoreactivity patterns. Each pallial division contains distinct cadherin-related secondary subdivisions (cell groups, nuclei, areas, strata, bands, or laminae), most of which represent known functional centers, whereas others are newly detected parts. As in the case of the subpallium, the immunoreactivity observed for the different cadherins in the pallial cell groups is partially complementary. It is relatively diffuse and apparently related to neuropil or cell aggregates and sometimes to scattered cells. Hippocampus and parahippocampal area. The Hp and the APH appear rostrally in the medial wall of the hemisphere, and gradually, they expand dorsally and laterally into the caudolateral telencephalic pole. The APH establishes contact laterally with the caudal piriform cortex (CPi; Figs. 6, 16). Guided by cytoarchitecture and the cadherin immunostaining profile, we distinguish four divisions of the parahippocampal area (APH) that each extend radially from the ventricular to the pial surface. These divisions are the medial APH (APHm), the intermediate APH (APHi), the lateral APH (APHl), and the caudolateral APH (APHcl). We introduce the novel term APHcl to denote a superficial part of the classic CDL region (Karten and Hodos, 1967), based on the evidence presented below. The APHl is the part of the complex that extends most rostrally, invading the medial wall of the hemisphere. It typically shows (1) a broad cell-poor periventricular layer; (2) a cortical cell plate that is divided into an inner, dense, and strongly basophilic sublayer and an outer sublayer 257 with less abundant and paler neurons; and (3) a thin marginal layer with few cells (panels D and E in Figs. 1–16). The outer cortical plate sublayer is strongly immunoreactive for cad7, whereas the periventricular layer weakly expresses Rcad and cad6B (APHl; Figs. 1– 6, 16A,B). The APHi consists of (1) a periventricular layer, which is populated by small neurons and is narrower than its counterpart in APHl; (2) a broader cortical cell plate subdivided also into inner and outer sublayers; and (3) a marginal cell-poor layer (AHPi; panels D,E in Figs. 1– 6). In APHi, both the inner and outer cortical plate sublayers show prominent to very strong cad7 immunoreactivity (Figs. 2–16). The signal is distinctly stronger in the outer sublayer. The periventricular layer of APHi is weakly cad6B immunoreactive and strongly expresses Rcad (Figs. 2A,C, 3A,C). The APHm is more compact but shows also a threelayered structure. It has (1) a thin periventricular layer with small neurons; (2) a broad cortical plate that is again divided into inner and outer sublayers, of which the inner one is more cell-dense; and (3) a characteristic superficial layer of medium neurons that bulges out at the brain surface, particularly at middle and caudal section levels (Figs. 4 –15, 16C,D). At E11, the APHm shows weak cad7 immunoreactivity in the superficial cell layer. The ependyma stains for cad6B and Rcad (Figs. 4 – 6, 16A). At E15, the inner sublayer of the cortical plate of APHm shows moderate Rcad immunoreactivity, whereas the protruding superficial layer displays strong cad7 expression (Figs. 7–15, 16C). The APHcl appears only in caudal sections through the telencephalon, at levels where the ventricle starts to expand lateralward. At its rostralmost appearance, it is difficult to separate it from the caudal end of the Wulst (described below; Fig. 4). However, the APHcl shows a three-layered structure similar to that of other APH subdivisions. It has a cell-poor periventricular layer similar to that of the APHl, but its cortical plate is thinner and denser. It is not divided into sublayers (Fig. 5D). Cad7 expression in it is similar but weaker than that in the APHl. At E15, the APHcl contains distinct patches of cad7 expression (Fig. 13B). More caudally, it contacts laterally the caudal piriform cortex (CPi in Figs. 5, 6) and can, thus, be conceived as a transition zone between the parahippocampal cortex and the CPi. The hippocampus proper (Hp) shows (1) a periventricular cell layer with small neurons, (2) a dense cortical plate with slightly larger neurons, and (3) a marginal layer traversed by fiber bundles converging caudally on the fimbria or rostrally on the septo-mesencephalic tract. The Hp expresses Rcad at the ependymal lining. Moderate cad7 immunostaining is observed at its ventralmost tip, close to the fimbria fornicis (f), and in the fibers of the marginal layer and fimbria (Hp in Figs. 3–16). The fimbria expands considerably at the back of the hemisphere and borders upon the telencephalic choroid plexus caudomedially (Fig. 6D). Wulst. The Wulst is surrounded on all sides by the hyperstriatum and the APH, which meet each other rostral and caudal to the Wulst (Kuhlenbeck, 1938). The Wulst appears rostrally just lateral to the APH at the top of the ventricle. A large medial part of it forms a superficial protrusion that is limited laterally by the vallecula (va in Figs. 2D, 3D, 15D), a shallow longitudinal furrow at the 258 C. REDIES ET AL. TABLE 1. Gray Matter Derivatives of Telencephalic Subdivisions, Fiber Tracts, and Their Cadherin Expression Profile in Chicken Embryos of 11 Days of Incubation1 Immunoreactivity Structure Cad6B Cad7 Rcad Ncad Abbr.2 Shown in Figure(s) Subpallium (SbP) Pallidum, dorsal portion Pallidum, ventral part Pallidum, medial part s (⫹) (⫹) Pallidum(PA) (⫹) s (⫹) (⫹) (⫹) (⫹) ⫺ ⫺ ⫹ PAd PAv PAm 3, 4, 11–14 3, 4, 10, 16 3, 4 Striatum, medial portion Striatum, lateral portion ⫹ (⫹) Striatum (ST) p p (⫹) (⫹) ⫹ ⫹ STm STl 2–6, 9, 10, 16 2–5, 11–16 ⫹ ⫺ ⫹ ⫺ ⫹ p p p ⫺ INP SI Acc TO BSTa S SL SM Tn 4, 11, 13, 14 11–14 2, 7 7, 8 4, 5, 9–12, 16 2, 3 4, 5, 7, 8 3, 4, 7, 10, 16 5 APH APHl APHi APHm APHcl Hp 1, 15, 17 1–14 2–16 3–16 4–8, 13, 15, 16 3–16 Ncad ⫺ ⫺ ⫹ (⫹) (⫹) (⫹) ⫹ HA HIS HISp HD HDs HDp CDL 1–3, 7–10, 13–16 1–3, 7–11, 16 2, 3 1–3, 7–12, 16 3, 15 7, 8 4, 15, 16 HVC HVCp 4–6, 13–15 4, 7–10 Other Intrapeduncular nucleus Innominate substance Accumbens nucleus Olfactory tubercle Bed nucleus of the terminal stria, anterior part Septum Lateral septum Medial septum Nucleus taeniae ⫹ p p ⫺ p p ⫺ p ⫺ (⫹) ⫺ ⫺ ⫺ p p p p ⫺ ⫹ s ⫺ ⫺ ⫹ p p p ⫹ Pallium (P) Medial pallium (MP) Parahippocampal area Lateral part Intermediate part Medial part Caudolateral part Hippocampus Cad6B Cad7 Rcad Ncad (p)3 (p)3 ⫺ (p) ⫺ p p p4 p p (p)3 p3 ⫺ (p) ⫺ (⫹) (⫹) (⫹) (⫹) (⫹) Cad6B ⫺ (⫹) (⫹) ⫺ ⫺ ⫺ ⫺ Cad7 ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ Cad6B ⫺ ⫺ Cad7 ⫹ ⫹ Rcad ⫺ ⫹ Ncad ⫹ ⫹ (⫹) (⫹) ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫺ ⫹ (s) ⫹ ⫹ ⫹ ⫹ s p s ⫹ ⫺ ⫹ ⫹ ⫺ ⫹ s p s ⫹ ⫹ (⫹) (⫹) (⫹) (⫹) (⫹) (⫹) (⫹) HVp HVn 1, 2, 3, 7, 8 2, 11, 13–16 HVd HVds HVv HVvs HVF HVFs 2, 2, 2, 2, 1, 1 ⫺ ⫺ ⫹ ⫺ ⫺ (p) ⫹ ⫹8 (⫹) ⫺ ⫺ ⫺ ⫹ (⫹) (⫹) ⫺ TPO CPi NCL NCLp 4 4–6, 13–16 4–6, 11–17 4, 17 Cad6B ⫺ ⫺ Cad7 ⫹ ⫹ Rcad ⫺ ⫺ Ncad (⫹) ⫹ ⫺ ⫺ ⫹ ⫹ ⫹ ⫹ ⫹ ⫺ ⫺ ⫺ ⫺ ⫺ 4, 5, 11–14, 16 4, 5, 7–10, 16 5–8, 10, 16 9–11, 16 9, 10, 16 9, 10, 16 4–6, 11–16 ⫺ ⫺ p p ⫺ ⫺ (⫹) ⫹ ⫹ ⫹ ⫹ ⫺ ⫹ ⫺ (p) ⫺ (⫹) (⫹) ⫺ ⫹ (⫹) (⫹) (⫹) ⫹ NC NCp L L1 L2 L3 NIF — is NI NId NIv NIp E Ec pE mE rE EIF — is rlis NL NF Dorsal pallium (DP) Accessory hyperstriatum Supreme intercalate hyperstriatum Periventricular portion Dorsal hyperstriatum5 Superficial portion Periventricular portion Dorsolateral corticoid area Rcad ⫺ ⫹ ⫹ ⫺ ⫺ ⫺ ⫺ Lateral pallium (LP) Ventral hyperstriatum, caudal portion Ventral hyperstriatum, caudal portion, periventricular part Ventral hyperstriatum, periventricular portion Nucleus of ventral hyperstriatum Ventral hyperstriatum, intermediate portion Dorsal part Superficial part Ventral part Superficial part Ventral hyperstriatum, frontal portion Ventral hyperstriatum, frontal portion, superficial stratum Temporo-parieto-occipital area Piriform cortex6 Neostriatum, caudal portion, lateral subregion7 Periventricular part 3, 9–13, 16 3, 13, 14 3, 9–13, 16 3, 15, 16 7–12, 14, 16 Ventral pallium (VP) Caudal neostriatum Periventricular portion Field L8 Portion 1 (ventral) Portion 2 (intermediate) Portion 3 (dorsal) Neostriatal island field Matrix Islands Intermediate neostriatum Dorsal portion Ventral portion Periventricular portion Ectostriatum Core Periectostriatal belt Medial belt area Retroectostriatal portion Ectostriatal island field Matrix Islands Rostrolateral island Lateral neostriatum Frontal neostriatum (p) ⫺ ⫺ p (p) ⫺ (p) ⫺ (p) ⫹ (⫹) (⫹) ⫺ (⫹) ⫹ ⫺ p (⫹) ⫹ (⫹) ⫺ ⫹ ⫹ ⫺ ⫺ ⫺ p ⫺ ⫹ (⫹) (⫹) ⫹ 11–15 3, 11–14 3, 9 3, 9 3 2, 3, 11–16 2, 3 4, 13–16 2, 3, 11–16 3 (arrows), 11, 13, 15 3, 15, 16 1–3, 15, 16 1, 2, 11–14 CADHERIN EXPRESSION IN CHICKEN TELENCEPHALON 259 TABLE 1. (continued) Immunoreactivity Structure Dorsal portion Ventral portion Periventricular portion Basal nucleus Nucleus of the lateral olfactory tract Anterior olfactory nucleus Olfactory bulb Prepiriform cortex Neostriatal marginal zone Cad6B Cad7 Rcad Ncad p ⫺ (p) ⫺ (⫹) ⫺ s ⫺ ⫺ ⫹ ⫹ (⫹) ⫹ ⫹ p p ⫹ ⫹ ⫺ (⫹) p ⫹ ⫹ ⫹ p ⫹ ⫺ ⫹ ⫹ ⫹ ⫹ (⫹) ⫹ p (⫹) (⫹) Rcad Ncad Shown in Figure(s) Abbr.2 NFd NFv NFp Bas LOT OA OB CPP mz 2 2 2, 9, 10, 16 2, 11–16 2, 3, 15, 16 1, 7–10, 16 1, 7–10, 16 1, 10, 13, 14 1–3, 13, 15, 16 Archistriatal complex Cad6B Cad7 ⫺ Subpallial part ⫺ ⫺ ⫺ Aa 4, 13–16 Intermediate archistriatum, ventral part Medial archistriatum (p) ⫺ Ventral pallial part p ⫹ ⫺ ⫹ ⫺ ⫺ Aiv Am 4–6, 13–16 5, 6, 13–16 Intermediate archistriatum, dorsal part Intermediate archistriatum, intermediate part Medial portion Posterior archistriatum ⫺ ⫺ ⫹ ⫺ Lateral pallial part (⫹) ⫹ ⫹ ⫺ ⫹ ⫺ (⫹) ⫹ (⫹) ⫺ ⫺ ⫺ Aid Aii *(Aim) Ap 4–6, 15, 16 4–6, 15–17 5, 6, 15–17 5, 6, 13–16 Anterior archistriatum Diencephalic/telencephalic transition zone Nucleus taeniae Nucleus of the anterior commissure Nucleus of the pallial commissure Eminentia thalami Cad6B ⫺ ⫺ ⫺ ⫺ Cad7 ⫺ ⫺ ⫺ ⫺ Ansa lenticularis Anterior commissure Fimbria fornicis Lateral forebrain bundle Olfactory tract Occipito-mesencephalic tract Pallial commissure Septo-mesencephalic tract Stria medullaris Stria terminalis Cad6B (⫹) (⫹) ⫺ (p) ⫺ p ⫺ (p) ⫺ (⫹) Cad7 ⫺ ⫹ ⫹ p ⫺ ⫹ ⫺ p ⫺ ⫹ Rcad ⫹ ⫺ ⫺ ⫺ Ncad ⫹ ⫹ ⫹ ⫺ Tn CA CPA EmT 5, 16 5, 9 5 — Rcad ⫹ (p) ⫺ p ⫺ p ⫹ ⫺ p p Ncad (⫹) ⫺ ⫺ p (⫹) ⫺ ⫺ ⫺ (⫹) ⫺ al ca f lfb olf om cpa tsm sme st 4, 5, 11 5, 7–11 3–8, 12, 13 3–5, 11, 13 2, 3, 11, 15, 16 5, 13 5, 7, 9 4, 9 3–5 6, 9, 11 Fiber tracts 1 Symbols are as follows: ⫺, structure is not immunoreactive; ⫹, structure is immunoreactive; n, only neuropil is immunoreactive; p, only parts of the structure are immunoreactive; and s, only scattered cells are immunoreactive. Parentheses denote weak immunoreactivity. For abbreviations, see list. Abbreviation used in the present work. 3 Only periventricular layer positive. 4 Superficial layer positive at embryonic day 15. 5 Possibly derived from lateral pallium (see Discussion section). 6 Comprises also dorsopallial parts (see Discussion section and Figs. 5, 6). 7 Possibly ventral pallial and amygdaloid (see Discussion section). 8 Immunoreactivity at embryonic day 15 (see Results section). 2 brain surface. From lateral to medial, the Wulst consists of three adjacent radial domains (for review, see Medina and Reiner, 2000), also referred to as layers (Shimizu and Karten, 1990; Karten and Shimizu, 1991): the dorsal hyperstriatum (HD), the superior intercalated hyperstriatum (HIS), and the accessory hyperstriatum (HA). The latter includes the intercalated nucleus of the HA, a narrow (radial) thalamorecipient band of HA adjacent to HIS (Karten et al., 1973; Shimizu and Karten, 1990; Medina and Reiner, 2000). At E11, the HD is distinguished cytoarchitectonically by its lower cell density (e.g., see Figs. 2D, 3D). Along its radial dimension, a periventricular stratum of the HD (HDp) can be distinguished from a massive intermediate stratum (Figs. 2D, 3D). The HD shows moderate cad7 immunostaining. Its marginal layer displays slightly weaker cad7 immunoreactivity (HDs in Figs. 3, 13, 15). The HD is sharply limited from the strong cad7expression in HIS. It has a less distinct border with the adjacent ventral hyperstriatum (HV). The HIS can be distinguished from both HD and HA by its relatively high level of Rcad and cad7 expression, weak cad6B expression and a markedly higher cell density (Figs. 1–3, 9). At E11, the HA has a lower cell density than the HIS, shows only a moderate immunostaining for cad7, and lacks detectable levels of cad6B and Rcad expression (Figs. 1–3, 7, 9). Caudal to the section shown in Fig. 3, the entire Wulst rapidly diminishes in size, both mediolaterally and radially, and its periventricular stratum finally disappears at the level where the APH starts to expand lateralward. At the caudal transition from the Wulst to the APH, a different corticoid domain becomes apparent more laterally at the brain surface. This area is known in the literature as the caudolateral corticoid area (CDL). It borders the caudal part of the HV and the APHl, and it overlies the lateral angle of the lateral ventricle, just before the lateral ventricle expands caudolaterally (Fig. 4D). The thionine stain shows that the “classic” CDL consists of a rather 260 C. REDIES ET AL. Fig. 1. Cadherin expression in adjacent transverse sections through the telencephalon of the embryonic day 11 chicken at the level of the frontal part of the ventral hyperstriatum (HVF). Sections were immunostained with antibodies against cadherin-6B (cad6B, A), cadherin-7 (cad7, B), and R-cadherin (Rcad, C). D: Thionine (thio) staining of an adjacent section. E: Schematic diagrams of the telen- cephalic embryonic divisions (represented by different shadings; Puelles et al., 2000). F: Gray matter structures that are apparently derived from each division. In E and F, the solid lines represent divisional borders and the dashed lines represent borders of additional subregions within telencephalic gray matter. For abbreviations, see list. Scale bar ⫽ 0.5 mm in E (applies to A–F). thick and moderately dense periventricular layer, a cellpoor intermediate stratum, a thin cortical plate, and a thin, fiber-rich marginal layer (Fig. 4D). This arrange- ment probably can be best understood as the result of sectioning through an oblique boundary between the APH and the caudolateral end of the Wulst complex. This obliq- CADHERIN EXPRESSION IN CHICKEN TELENCEPHALON Fig. 2. Cadherin expression in adjacent transverse sections through the telencephalon of the embryonic day 11 chicken at the level of the basal nucleus of the neostriatum (Bas). Sections were immunostained with antibodies against cadherin-6B (cad6B, A), cadherin-7 (cad7, B), and R-cadherin (Rcad, C). D: Thionine (thio) staining of an adjacent section. E: Schematic diagrams of the telencephalic embryonic divisions (represented by different shadings; Puelles et al., 2000). F: Gray matter structures that are apparently 261 derived from each division. In E and F, the solid lines represent divisional borders and the dashed lines represent borders of additional subregions within telencephalic gray matter. The asterisk in B indicates an artefact. The asterisk in F indicates a cell-dense lamina of the intermediate ventral hyperstriatum (HVv) that is supradjacent to the hyperstriatal lamina (lh). For abbreviations, see list. Scale bar ⫽ 0.5 mm in E (applies to A–F). Fig. 3. Cadherin expression in adjacent transverse sections through the telencephalon of the embryonic day 11 chicken at the level of the caudal part of the ectostriatum. Sections were immunostained with antibodies against cadherin-6B (cad6B, A), cadherin-7 (cad7, B), and R-cadherin (Rcad, C). D: Thionine (thio) staining of an adjacent section. E: Schematic diagrams of the telencephalic embryonic divisions (represented by different shadings; Puelles et al., 2000). F: Gray matter structures that are apparently derived from each division. In E and F, the solid lines represent divisional borders and the dashed lines represent borders of additional subregions within telencephalic gray matter. The arrows indicate ectostriatal islands. The asterisk in C indicates an artifact. For abbreviations, see list. Scale bar ⫽ 0.5 mm in E (applies to A–F). CADHERIN EXPRESSION IN CHICKEN TELENCEPHALON Fig. 4. Cadherin expression in adjacent transverse sections through the telencephalon of the embryonic day 11 chicken at the level of the anterior archistriatum (Aa). Sections were immunostained with antibodies against cadherin-6B (cad6B, A), cadherin-7 (cad7, B), and R-cadherin (Rcad, C). D: Thionine (thio) staining of an adjacent section. E: Schematic diagrams of the telencephalic embryonic divi- 263 sions (represented by different shadings; Puelles et al., 2000). F: Gray matter structures that are apparently derived from each division. In E and F, the solid lines represent major divisional borders and the dashed lines represent borders of additional subregions within telencephalic gray matter. For abbreviations, see list. Scale bar ⫽ 0.5 mm in E (applies to A–F). Fig. 5. Cadherin expression in adjacent transverse sections through the telencephalon of the embryonic day 11 chicken at the level of the anterior commissure (ca). Sections were immunostained with antibodies against cadherin-6B (cad6B, A), cadherin-7 (cad7, B), and R-cadherin (Rcad, C). D: Thionine (thio) staining of an adjacent section. E: Schematic diagrams of the telencephalic embryonic divisions (represented by different shadings; Puelles et al., 2000). F: Gray matter structures that are apparently derived from each division. A color-coded superposition of the immunostaining results is shown in Figure 16A. In E and F, the solid lines represent major divisional borders and the dashed lines represent borders of additional subregions within telencephalic gray matter. The asterisks in A–C and F indicate the position of a cad6B-positive medial part of the intermediate archistriatum. For abbreviations, see list. Scale bar ⫽ 0.5 mm in E (applies to A–F). Fig. 6. Cadherin expression in adjacent transverse sections through the telencephalon of the embryonic day 11 chicken at the level of the posterior part of field L (L). Sections were immunostained with antibodies against cadherin-6B (cad6B, A), cadherin-7 (cad7, B), and R-cadherin (Rcad, C). D: Thionine (thio) staining of an adjacent section. E: Schematic diagrams of the telencephalic embryonic divisions (represented by different shadings; Puelles et al., 2000). F: Gray matter structures that are apparently derived from each division. A color-coded superposition of the immunostaining results is shown in Figure 16B. In E and F, the solid lines represent major divisional borders and the dashed lines represent borders of additional subregions within telencephalic gray matter. The asterisks in A–C and F indicate the position of the cad6B-positive medial part of the intermediate archistriatum. For abbreviations, see list. Scale bar ⫽ 0.5 mm in E (applies to A–F). 266 C. REDIES ET AL. Fig. 7. Cadherin expression in adjacent parasagittal sections through the telencephalon of the embryonic day 15 chicken at the level of the olfactory tubercle (TO). Sections were immunostained with antibodies against cadherin-6B (cad6B, A), cadherin-7 (cad7, B), and R-cadherin (Rcad, C). D: Thionine (thio) staining of an adjacent section. Corresponding schematic diagrams of the telencephalic embryonic divisions and their derivative gray matter are shown in Figure 8. The asterisk in B indicates an artifact (missing part of the section). For abbreviations, see list. Scale bar ⫽ 1 mm in D (applies to A–D). uity may be a consequence of the change in position of the ventricle and associated formations as they approach the caudal telencephalic pole. In this interpretation, the periventricular stratum represents the deep intermediate or periventricular strata of the caudalmost Wulst. Consistent with the rostral Wulst pattern, it shows weak to moderate immunostaining for cad7. On the other hand, the dense cortical plate seems to represent the beginning of the APHcl, as judged by more caudal sections. Its cell plate is cad7 immunoreactive (Fig. 4B), as is in general typical of APH. Accordingly, the “classic” CDL would be heterogeneous and would consist of apparently super- CADHERIN EXPRESSION IN CHICKEN TELENCEPHALON 267 Fig. 8. Embryonic divisions and derived structures of gray matter for the sections displayed in Figure 7. A: The telencephalic divisions (Puelles et al., 2000) are represented schematically by different shadings. B: Gray matter structures that are apparently derived from each division. The solid lines represent divisional borders, and the dashed lines represent borders of additional subregions within telencephalic gray matter. For abbreviations, see list. Scale bar ⫽ 1 mm in A (applies to A,B). posed (but radially independent) Wulst and APH portions. The Wulst component disappears at more caudal levels of the telencephalon, where the HV and the piriform cortex are in direct contact with the APH (Fig. 5). Ventral hyperstriatum. The ventral hyperstriatum (HV) extends practically all along the rostrocaudal extent of the telencephalon and represents the dorsal part of the avian DVR. Classic work on the avian telencephalon divides the HV into frontal, intermediate, and caudal portions (HVF, HVI, and HVC, respectively) as well as into ventral and dorsal parts (HVv and HVd; Huber and Crosby, 1929). The current data on cadherin immunoreactivity support radial and dorsoventral divisions of HV, but do not clearly distinguish the rostrocaudal divisions. Use of the classic terms, nevertheless, is useful for topographical reference, and it allows us to relate our findings to previously published data. Radial HV divisions appear as superposed strata that can be followed throughout the rostrocaudal axis, as one proceeds from the ependymal to the pial surface: (1) a dense periventricular stratum, (2) a massive intermediate stratum that can be divided into inner and outer parts, and (3) a superficial, less densely populated marginal stratum. The continuity of these strata is partly obscured by drastic changes in the relative position of the ventricular zone and the corresponding pial surface as the sectioning plane advances from rostral to caudal levels. The periventricular layer of the entire HV (HVp and HVCp) displays rather strong cad7 staining and some weaker immunoreactivity for cad6B and Rcad (Figs. 2– 4, 7–10, 16A–C). Many strongly immunoreactive neurons stand out individually among less immunoreactive neurons. At the section level shown in Figure 3B, expression of cad7 expands from the HVp layer into the adjacent deep part of the intermediate stratum, particularly in the dorsal part of HV (HVd, see below). This cad7-positive deep intermediate domain diminishes in thickness more caudally (HVC; Fig. 4B), but it can still be followed caudolaterally as the ventricle expands in that direction. Finally, it seems to connect to a region called caudolateral neostriatum in the classic literature (NCL; Figs. 4 – 6). The NCL comes to overlie the archistriatal complex at the caudolateral pole of the telencephalon and also expresses cad6B (Fig. 17A) but no Rcad. As seen on sagittal sections (Fig. 15), it extends rostrally into a narrow region with a similar profile of cadherin expression. This region extends below the HD and continues below the lh. The periventricular lining of NCL is negative for the cadherins at E11 (NCLp in Fig. 4), but it expresses cad7 at E15 (Figs. 13, 15, 17A). The intermediate stratum of the HV has at least two distinct dorsoventral subdivisions, which correspond to the dorsal and ventral HV portions of Karten and Hodos (1967; HVd and HVv in Figs. 2, 3, 9 –13, 16C). These sectors are intercalated between the hyperstriatal lamina (lh; limit between the neostriatum and the HV) and the superior frontal lamina (limit between the HV and the HD) that is barely visible on histologic grounds at E11 and E15 (lfs in Figs. 2D, 9). As discussed in detail below, the two sectors show some differential immunostaining for the three cadherins, as well as cytoarchitectonic differences. Due to the obliquity of the topologic radial dimension relative to our cross-sections, it is not easy to identify the two sectors in transverse sections cut at extreme frontal and caudal levels (HVF and the HVC, respectively). However, parasagittal sections (Figs. 7–14) show clearly 268 C. REDIES ET AL. Fig. 9. Cadherin expression in adjacent parasagittal sections through the telencephalon of the embryonic day 15 chicken at the level of the medial striatum (STm). Sections were immunostained with antibodies against cadherin-6B (cad6B, A), cadherin-7 (cad7, B), and R-cadherin (Rcad, C). D: Thionine (thio) staining of an adjacent section. Corresponding schematic diagrams of the telencephalic em- bryonic divisions and their derivative gray matter are shown in Figure 10. A color-coded superposition of the immunostaining results is shown in Figure 16C. The asterisks indicate an artifact (cut at brain surface). For abbreviations, see list. Scale bar ⫽ 1 mm in D (applies to A–D). that the two sectors extend rostrally into the HVF and caudally into the HVC. The HVv is nonhomogeneous. Its neuronal cell density increases toward the hyperstriatal lamina (lh in Figs. 2, 9, 10, 13–16). On top of this boundary, there is a very dense band of cells that expresses moderate levels of cad7 and Rcad (asterisk in Fig. 2F). Near the rostral brain surface, a well delimited, denser aggregate of HVv neurons is CADHERIN EXPRESSION IN CHICKEN TELENCEPHALON 269 Fig. 10. Embryonic divisions and derived structures of gray matter for the sections displayed in Figure 9. A: The telencephalic divisions (Puelles et al., 2000) are represented schematically by different shadings. B: Gray matter structures that are apparently derived from each division. The solid lines represent divisional borders, and the dashed lines represent borders of additional subregions within telencephalic gray matter. For abbreviations, see list. Scale bar ⫽ 1 mm in A (applies to A,B). negative for cad7 and Rcad. This nucleus (HVn in Figs. 2A–E, 9A–D, 11A–D) contains only a few weakly cad7positive scattered neurons and shows moderate Ncad and weak cad6B staining. The HVn overlies topographically the ectostriatum, a similar, though somewhat larger condensation of cells in the neostriatum (Fig. 2). Apart from this lighter part, the rest of HVv displays medium to strong cad7 expression and weak or moderate Rcad expression (same figures). The boundary of the HVd with the HVv is best seen on cad7-immunostained parasagittal sections (Figs. 9B, 10B). The superficial half of the intermediate stratum of the HV (as defined in the present work) shows only weak immunoreactivity for Rcad (Fig. 4) and Ncad. Caudally, it largely corresponds to the classic temporo-parietooccipital area (TPO) that is found at section levels where the APHcl appears at the neighboring brain surface (Fig. 4F). The marginal layer of the HVv and the HVF, found only rostrally, is rather cell poor and is populated by scattered cells that express moderate levels of cad7 and Rcad (HVvs and HVFs in Figs. 1–3, 15, 16). The marginal layer of the HVd is more cell dense (HVds in Figs. 2, 3, 13, 14) and displays moderate cad7 expression. Neostriatum and olfactory structures. The neostriatum is a large ventral DVR territory that is separated from the subpallium (the striatum, in particular) by the dorsal medullary lamina (lmd) and from the hyperstriatum by the hyperstriatal lamina (lh). The lh limit is partially characterized by a dorsal-to-ventral drop in cad7 expression (Figs. 2– 4, 9). Rostrolaterally, the lh is also underlined by an Rcad-negative band (Figs. 2C, 3C, 11C, 13C, 15C). The neostriatum extends rostrocaudally throughout the telencephalon, and it includes several spe- cialized separate sensory regions (basal nucleus, ectostriatum, field L) and some superficial olfactory structures. At the rostral pial surface, these latter structures compose the nucleus of the lateral olfactory tract and the prepiriform cortex (LOT, CPP, respectively; Figs. 1–3, 13–15). At its rostral end, the neostriatum contacts the anterior olfactory nucleus and the olfactory bulb (OA, OB; Figs. 9, 10; Puelles et al., 1999, 2000). Caudolaterally, the neostriatum borders anterior parts of the archistriatal complex (Figs. 4 – 6, 15). As a special added feature, the neostriatum contains multiple islands of cells that differ in their cadherin expression from the surrounding matrix. These islands vary in size and occur in regions that are either associated with the ectostriatum (termed “ectostriatal island field”) or with other parts of the neostriatum (termed “neostriatal island field;” treated below). Neostriatum. Standard terminology (Karten and Hodos, 1967) distinguishes frontal, intermediate, and caudal divisions of the neostriatum (NF, NI, and NC, respectively). These terms are useful for topographical reference, but the corresponding divisions show largely a common pattern of cytoarchitecture and cadherin immunoreactivity. For practical reasons, we will first describe a cross-section at the level of the ectostriatum (Fig. 3) and then follow the distinctly stained areas rostrally and caudally. A coronal section passing through the ectostriatum shows that the periventricular stratum of the intermediate N (NIp) is characterized by lack of cad6B and cad7 expression and weak to moderate Rcad expression at E11 (Fig. 3A,B). Lateral to this stratum, there appears a domain subdivided into dorsal and ventral sectors. The dorsal sector is cad7 negative, whereas the ventral one is cad7 positive (Figs. 3B, 4B). The ventral sector appears to cor- 270 C. REDIES ET AL. Fig. 11. Cadherin expression in adjacent parasagittal sections through the telencephalon of the embryonic day 15 chicken at the level of the innominate substance (SI). Sections were immunostained with antibodies against cadherin-6B (cad6B, A), cadherin-7 (cad7, B), and R-cadherin (Rcad, C). D: Thionine (thio) staining of an adjacent section. Corresponding schematic diagrams of the telencephalic embryonic divisions and their derivative gray matter are shown in Figure 12. The asterisk in A indicates an artifact. For abbreviations, see list. Scale bar ⫽ 1 mm in D (applies to A–D). respond to the target in NI of the thalamic dorsolateral posterior nucleus (Wild, 1987b, 1989; Korzeniewska and Güntürkün, 1990). Still more laterally, the neostriatum is enlarged and contains what may be called the “ectostriatal complex.” Here, the conventional ectostriatum, or ectostriatal core (Ec), is surrounded by several other areas that are related to it, at least topographically, but possibly also functionally. The Ec is a specialized visual thalamorecipient subregion of the NI (Karten and Hodos, 1970; Kröner and Güntürkün, 1999) that typically contacts the dorsal medullary lamina (lmd) and bulges inside the NI without reaching the hyperstriatal lamina (lh). It ex- CADHERIN EXPRESSION IN CHICKEN TELENCEPHALON 271 Fig. 12. Embryonic divisions and derived structures of gray matter for the sections displayed in Figure 11. A: The telencephalic divisions (Puelles et al., 2000) are represented schematically by different shadings. B: Gray matter structures that are apparently de- rived from each division. The solid lines represent major divisional borders, and the dashed lines represent borders of additional subregions within telencephalic gray matter. For abbreviations, see list. Scale bar ⫽ 1 mm in A (applies to A,B). presses cad6B, cad7, and Rcad weakly or not at all (Figs. 3, 11–15). This core area is surrounded medially by a distinct medial belt area with a similar cadherin expression profile (mE; Figs. 2, 3). Dorsally and laterally, the Ec is covered by a cad7-negative periectostriatal belt area (pE) that strongly expresses Rcad. At some points, interdigitations are observed between the Rcad-negative Ec and the Rcad-positive pE (Fig. 3C, arrows; Fig. 13C, is). At E11, but not at E15, the pE shows also basophilic cellular clumps, presumably islands of aggregated neurons, that are centered within the Rcad-negative digitations (arrows in 3D). We have called this domain of pE the “ectostriatal island field” (EIF; Figs. 2, 3, 11–15, 16C). The islands of the EIF express low to moderate levels of Ncad (only at E11), cad6B, and cad7. These markers are largely absent from the Ec and from the EIF matrix around the islands. In contrast, Rcad appears restricted to the matrix around the islands. Rostrolateral to the Ec, a particularly large and compact island is found, called here the “rostrolateral island” (rlis; Figs. 3, 15, 16D). Lateral to the EIF and close to the pial surface, there appears a “lateral neostriatal region” that can be distinguished by its very low levels of immunoreactivity for the four cadherins studied. This lateral neostriatal region (NL in Figs. 1–3, 15) expands ventrally into a superficial domain lying under the nucleus of the lateral olfactory tract (LOT; Figs. 2, 3; see below). At more rostral section levels, the periventricular stratum of the N remains visible (NFp in Fig. 2), whereas the deep intermediate domain gradually expands in front of the diminishing subpallium (NF in Fig. 2). Its ventral cad7-positive portion enlarges and acquires a more superficial position, encompassing the basal nucleus (Bas in Figs. 2, 11–14). The dorsal cad7-negative part also expands slightly in front of the diminishing ectostriatal complex. The NL, together with the cad7-positive marginal zone, caps rostrally the neostriatum, whereas the LOT is substituted most rostrally by the prepiriform cortex (CPP) that expresses strongly both cad7 and Rcad (Figs. 1, 13– 15, 16D). Now tracing neostriatal regions caudalward from the level of the ectostriatum, we observe that the periventricular stratum and associated cad7-positive intermediate domain progressively increase in size, encompassing finally the field L (L; Figs. 5–9, 16A–C). The different areas or zones of field L described in the literature (L1, L2, L3 of Bonke et al., 1979; Wild et al., 1993) can be discerned by their cadherin staining pattern at E15 but not at E11. At E15, field L1 shows strong Rcad immunoreactivity but only low to moderate levels of cad7 immunoreactivity (L1 in Figs. 9, 16C). In contrast, field L2 shows strong cad7 immunoreactivity and lacks the other cadherins (L2 in Figs. 9, 16C). Finally, field L3 expresses cad6B and cad7 at E15 (Figs. 9, 16C). The periventricular stratum of the caudal neostriatum (NCp) expresses cad7 more strongly at E15 than at E11 (Figs. 5B, 9B). Except for the ependymal layer, it is Rcad negative (Figs. 5C, 6C, 9C, 11C). Laterally, the cad7-positive intermediate domain ends as a tail-like lateral extension behind the ectostriatum (Figs. 4 – 6). At this level, the outer intermediate domain occupied before by the ectostriatal complex transforms into two Rcad-positive areas. One of them is a flattened cell aggregate that appears adjacent to the lmd. We provisionally named it the retro-ectostriatal nucleus (rE; Figs. 4, 13–15, 16D). Rcad staining in this region can be followed back into the Rcad-positive medial part of the archistriatum (Am in Figs. 5, 6, 15, 16A,B,D). The other Rcad-positive area is larger, and, like the EIF, it continues showing islands interspersed with the matrix. We have called it the “neostriatal island field” (NIF; Figs. 4 – 6, 11–16; see below). The rE and NIF contact one another laterally (Fig. 4C,F). 272 C. REDIES ET AL. Fig. 13. Cadherin expression in adjacent parasagittal sections through the telencephalon of the embryonic day 15 chicken at the level of the medial archistriatum (Am). Sections were immunostained with antibodies against cadherin-6B (cad6B, A), cadherin-7 (cad7, B), and R-cadherin (Rcad, C). D: Thionine (thio) staining of an adjacent section. Corresponding schematic diagrams of the telencephalic em- bryonic divisions and their derivative gray matter are shown in Figure 14. The arrow in C points to periventricular Rcad immunoreactivity that is continuous with the Rcad-immunoreactive matrix of the neostriatal island field (NIF). For abbreviations, see list. Scale bar ⫽ 1 mm in D (applies to A–D). Neostriatal island field. This field represents a caudal continuation of the EIF, but it does not overlie any obvious equivalent of the ectostriatal core. The neostriatal island field (NIF) is not conspicuous in Nissl-stained sections but can be clearly identified by its Rcad immu- noreactivity, which labels its matrix cell population even more strongly than in the case of the EIF (Figs. 4 – 6, 11–15, 16A,B,D). The NIF extends to caudal neostriatal regions, where it reaches the caudal pole of the ventricle (Figs. 11C, 13C). In general, the size of the CADHERIN EXPRESSION IN CHICKEN TELENCEPHALON 273 Fig. 14. Embryonic divisions and derived structures of gray matter for the sections displayed in Figure 13. A: The telencephalic divisions (Puelles et al., 2000) are represented schematically by different shadings. B: Gray matter structures that are apparently de- rived from each division. The solid lines represent major divisional borders, and the dashed lines represent borders of additional subregions within telencephalic gray matter. For abbreviations, see list. Scale bar ⫽ 1 mm in A (applies to A,B). islands is smaller in the caudalmost NIF than in the EIF. Like the EIF, the NIF contains interdigitations of Rcadpositive neostriatal matrix and Rcad-negative islands. The islands consist of small to large cell aggregates that display a cadherin expression profile similar to the overlying NCL region (Figs. 11, 13, 15, 16A). Islands are smaller caudally than rostrolaterally. Caudally, some islands have connecting bridges with the NCL at some section levels (is, NIF; Figs. 11–15). Rostrolaterally, the islands seem to connect to the thin Rcad-negative stripe below the lh (Figs. 13C, 15). Some of the islands have a central clump of cells moderately positive for cad7 (Figs. 11B, 13B, 15B, 16D). Some islands can be distinguished also by their higher cell density, which is similar to that of the supradjacent NCL region (Fig. 15D), or by their Ncad expression (data not shown). At the NCLp/NIF interface at E11 (Fig. 5), the matrix contains a different type of much smaller islands that display lower levels of Rcad expression. These smaller islands show weak to moderate cad7 expression and no cad6B. They contribute to an overall patchy appearance of the Rcad immunostaining expression in NIF at this stage (Figs. 5C, 6C, 16A,B). The caudal NIF area also fuses laterally with the dorsal intermediate archistriatum (Aid; Fig. 5) and, most caudally, with the posterior archistriatum (Ap; Fig. 6). These two areas express Rcad very strongly. The Aid is intercalated between the cad7-positive NCL and the cad7-positive intermediate portion of the intermediate archistriatal domain (Aii; Figs. 5B,C, 6B,C, 16B). Dorsal to the Ap, the Rcad-positive matrix of NIF reaches the ventricular surface (arrow in Fig. 13C). Olfactory bulb and other olfactory structures. The external layers of the olfactory bulb (OB), including the olfactory nerve layer and glomerular layer express moderately Ncad. The external plexiform layer and the neu- ropil surrounding the mitral cells show strong expression of Rcad (Figs. 7C, 9C). The anterior olfactory nucleus (OA) also shows strong Rcad immunoreactivity, which appears to be stronger in its medial part. It displays also weak to moderate cad7 immunoreactivity, particularly at the boundary between OB and OA (Figs. 1, 7, 9, 16D). More caudally, the prepiriform cortex (CPP), the nucleus of the lateral olfactory tract (LOT), and the marginal zone found dorsal to the olfactory tract are all located at the surface. The CPP and the LOT show cad7 and Rcad immunoreactivity (Figs. 1–3, 13, 15). The LOT also stains weakly for cad6B (Figs. 2, 3, 15). The brain surface over the NL shows a cad7-positive marginal zone (mz; Figs. 1B, 2B, 13B, 15B), which expands ventrally across the lateral olfactory tract into the LOT (Fig. 15). The cad7 expression can be followed caudalward into the ventral intermediate archistriatum (Aiv; Figs. 3B, 4B). The olfactory tract itself appears negative for all these cadherins (olf in Figs. 2, 3, 11, 15, 16). Close to the dorsal intermediate archistriatum, there appears superficially a thick marginal cortical domain with cad7 and Rcad expression. This domain apparently corresponds to the caudal piriform cortex (CPi; Figs. 5, 6). In the literature (e.g., Reiner and Karten, 1985), the term CPi is applied indistinctly also to the subpial neostriatum (our mz; see Figs. 2, 3) and to free, ependyma-lined cortex at the caudolateral tip of the lateral ventricle. These structures differ in their cytoarchitecture and cadherin expression. The latter (our CPi in Figs. 5, 6) resembles the APHcl (see above and Discussion section). In the present study, piriform cortex is identified exclusively where it appears to be cytoarchitectonically distinct. Olfactory projections have also been suggested to end in the subpial N by Reiner and Karten (1985) who used autoradiographic tract tracing methods. Fig. 15. Cadherin expression in adjacent parasagittal sections through the telencephalon of the embryonic day 15 chicken at the level of the nucleus of the lateral olfactory tract (LOT). Sections were immunostained with antibodies against cadherin-6B (cad6B, A), cadherin-7 (cad7, B), and R-cadherin (Rcad, C). D: Thionine (thio) staining of an adjacent section. E: Schematic diagrams of the telencephalic embryonic divisions (represented by different shadings; Puelles et al., 2000). F: Gray matter structures that are apparently derived from each division. A color-coded superposition of the immunostaining results is shown in Figure 16D. In E and F, the solid lines represent major divisional borders and the dashed lines represent borders of additional subregions within telencephalic gray matter. The asterisks in A,C,F indicate the position of a cad6B-positive medial part of the intermediate archistriatum. For abbreviations, see list. Scale bar ⫽ 1 mm in E (applies to A–F). CADHERIN EXPRESSION IN CHICKEN TELENCEPHALON 275 Fig. 16. Color-coded overlays of immunostained sections for cadherin-6B (cad6B), cadherin-7 (cad7), and R-cadherin (Rcad). The images displayed in each panel are the result of the superposition of adjacent sections. A: Results from adjacent transverse sections through the telencephalon of the embryonic day 11 chicken at the level of the anterior commissure. The data are the same as those shown in Figure 5A–C. B: Results from adjacent transverse sections through the telencephalon of the embryonic day 11 chicken at the level of the posterior part of field L (L). The data are the same as those shown in Figure 6A–C. C: Results from adjacent parasagittal sections through the telencephalon of the embryonic day 15 chicken at the level of the medial striatum (STm). The data are the same as those shown in Figure 9A–C. D: Results from adjacent parasagittal sections through the telencephalon of the embryonic day 15 chicken at the level of the nucleus of the lateral olfactory tract (LOT). The data are the same as those shown in Figure 15A–C. The different colors represent the cadherin-immunostaining results, as indicated by the boxes in C. Note the partial overlap of cadherin expression indicated by the mixed colors (pink for cad6B/cad7, turquoise for cad6B/Rcad, and yellow for cad7/Rcad). The lines represent the borders of embryonic divisions and subregions of telencephalic gray matter (compare with Figs. 5E,F, 6E,F, 10, and 15E,F). The asterisks in A,B,D indicate the position of a cad6B-positive medial part of the intermediate archistriatum. For abbreviations, see list. Scale bars ⫽ 0.5 mm in A,B, 1 mm in C,D. Archistriatal complex. The archistriatal complex includes anterior, intermediate, posterior, and medial divisions (Aa, Ai [divided into Aid, Aii, and Aiv], Ap, and Am, see Table 1). The identification of these divisions in the present study tentatively followed the adult schema proposed by Zeier and Karten (1971) but was adapted to the slightly different topography of the archistriatum and its parts observed at E11 and E15 (see also Puelles et al., 2000), as well as to the apparent limits of the different domains of cadherin expression. Some partial inconsistencies between our embryonic and the adult divisional scheme may require further studies in the future. 276 Fig. 17. Cadherin-6B expression in adjacent transverse sections through the telencephalon of the embryonic day 15 chicken at the level of the caudolateral neostriatum (NCL). A,C: A section that was immunostained with antibodies against cadherin-6B (cad6B). B: Thionine (thio) staining of an adjacent section. C represents an enlarge- C. REDIES ET AL. ment of the boxed area in A. The arrows in A and C point to cad6Bimmunoreactive fibers that connect the NCL and the medial part of the intermediate archistriatum (Aim). For abbreviations, see list. Scale bars ⫽ 0.5 mm in B (applies to A,B), 0.1 mm in C. CADHERIN EXPRESSION IN CHICKEN TELENCEPHALON Both the Aid and Ap express Rcad very strongly and lack noticeable expression of cad7 and cad6B, whereas the Aii lacks Rcad expression, but show moderate to strong cad7 immunoreactivity (Figs. 4 – 6, 15, 16A,B,D). The Rcad-positive Aid reaches the ventricular surface at the caudolateral edge of the lateral ventricle (Fig. 6C). With the medially adjacent NCL, the Aid forms a border that runs orthogonal to the ventricular surface. The Aii contacts a medially protruding, rounded portion (Aim) that strongly expresses cad6B (asterisks in Figs. 5, 6, 15, 16A,B,D, 17). This part of the Ai is connected to the overlying cad6B-positive NCL by cad6B-positive nerve fibers (Fig. 17C). The medial nucleus (Am) and the ventral intermediate nucleus of the archistriatum (Aiv) are found in topographic contiguity with the caudalmost NIF and NC domains. Similarly to the adjacent parts of the NIF, these nuclei of the archistriatum express Rcad strongly (Aiv) or even very strongly (Am). In these nuclei, cad7 expression ranges from no expression to moderate expression (Figs. 4 – 6, 13, 15). The small Aa is found medially under the dorsal medullary lamina (Figs. 4, 13, 15). Like the adjacent subpallial regions, such as the striatum, the Aa is weakly immunoreactive or negative for Rcad or cad7. The nucleus taeniae (Tn) is a superficial cell group positive for Rcad; it is found medial to the pallio-subpallial boundary at the caudal end of the archistriatum (Fig. 5). Puelles et al. (2000) recently suggested a possible extratelencephalic origin for this formation. Solitary cad7-positive cells of the pallium. Apart from the regionalized expression of cad7 by cell aggregates, there are also solitary cad7-positive cells that are widely dispersed at relatively regular intervals throughout most pallial regions. These cells have small to medium cell nuclei, relatively little cytoplasm, and a few short processes (data not shown). This type of cell is more widely distributed in the pallium at E15 than at E11, and it is rare in the subpallium. A similar type of cad7-positive cell was not found in other parts of the brain (data not shown). DISCUSSION Regionalized and complementary expression of cadherins in multiple telencephalic (sub)divisions Three of the four cadherins studied in the current work (cad6B, cad7, and Rcad) are expressed in a specific and combinatorial manner in multiple areas widely distributed over most of the major telencephalic (sub)divisions. Examples are the distinctive and sometimes complementary expression patterns of cad7 and Rcad in the sensory and/or thalamorecipient structures of the neostriatum (such as field L, ectostriatum, and basal nucleus, as well as the specific target for the thalamic dorsolateral posterior nucleus in the intermediate neostriatum), in the multimodal association area called caudolateral neostriatum (NCL, Figs. 5, 6), or in the different areas of the avian (para)hippocampal complex (APH and Hp in Figs. 1–16). These data suggest an association of cadherin expression to specific functional areas, aggregates, or nuclei of the telencephalon. The current cadherin results also indicate the existence of other gray matter structures not previously described. Frequently, these structures are also characterized by particular cytoarchitectonic features. A 277 typical example is the cell group in the HV, which we have called the nucleus of the ventral hyperstriatum (HVn; see below); it was first delimited by the expression of the three cadherins and corroborated cytoarchitectonically by the compact aggregation of its cells (Fig. 2). Data reported for songbirds and the parrot suggest the existence of an HN nucleus also in these adult brains (oval nucleus of HV: Striedter, 1994; Durand et al., 1997; Brauth et al., 1994; Hvo complex: Jarvis and Mello, 2000). More research is needed to determine whether the HVn of the chicken embryo has an adult correlate and whether it is identical to any of the HV elements in other birds. Other novel entities presented here are the NL, EIF, NIF, mE, NFv/d, HVs, rE, Aim, and some APH subdivisions (see below). Thus, cadherin expression is a useful tool for identifying novel areas or nuclei within the major divisions and subdivisions of the telencephalon. It should be stressed that we did not assume cadherin expression alone to be sufficient to define any given gray matter region. Instead, a careful analysis of several features was carried out in each case by weighing results in light of additional data available in the literature, especially when the cadherin expression data suggested a novel divisional scheme or the existence of a novel nucleus or area. A similarly complex distribution of the same cadherin types has been observed previously in the chicken diencephalon (Redies et al., 1993, 2000; Yoon et al., 2000), as well as in the chicken mesencephalon and rhombencephalon (Arndt and Redies, 1996, 1998; Arndt et al., 1998; Wöhrn et al., 1999; Redies et al., unpublished results). Other types of cadherins are also known to be expressed in specific regions widely distributed throughout the brain in chicken (e.g., cadherin-10: Fushimi et al., 1997) and mouse (e.g., cadherin-8: Korematsu and Redies, 1997; cadherin-6: Inoue et al., 1998; OL-cadherin: Hirano et al., 1999). Many of the gray matter structures expressing a specific cadherin subtype are functionally connected (Redies et al., 1993; for review, see Redies, 2000). The widespread expression of Ncad by radial glia results in a more diffuse pattern of expression of this molecule, except for a few areas displaying relatively high levels of Ncad expression (Table 1). The discrete expression of each cadherin subtype in multiple brain divisions differs from the expression of some homeobox or regulatory genes (or the transcription factors these genes codify), which frequently are restricted to one or only a few of the major brain divisions or subdivisions (Puelles and Rubenstein, 1993; Shimamura et al., 1995). For example, the neuronal expression of Emx-1 and Tbr-1 is restricted to the whole pallium or to several of its divisions at early developmental stages in the mouse and in the chicken (Simeone et al., 1992; Smith-Fernandez et al., 1998; Puelles et al., 1999, 2000). Another example is the expression of Dlx-2 and Nkx-2.1 which is restricted, early in development, to the entire subpallium (Dlx-2) or to one of its subdivisions (Nkx-2.1; Bulfone et al., 1993; Puelles and Rubenstein, 1993; Smith-Fernandez et al., 1998; Puelles et al., 1999, 2000). Such genes and transcription factors are good candidates for specifying general properties of major brain regions (e.g., general identity and special differentiation features of cerebral cortex, basal ganglia, or thalamus). 278 C. REDIES ET AL. Cadherin expression interpreted within a model of topologically radial divisions in chicken telencephalon In the Results section, the immunoreactivity patterns for the cadherins were described almost exclusively in conventional, atlas-derived terminology and subdivisions. Here, we interpret them also in the context of a recently proposed divisional scheme of the avian and mammalian telencephalon (Puelles et al., 1999, 2000), as demonstrated in the schematic diagrams for each set of cadherin immunostained sections (Figs. 1E,F– 6E,F, 8, 10, 12, 14, 15E,F) and supplementary diagrams (Fig. 18). This hypothetical divisional scheme was based on a comparative analysis of the expression of several gene transcription factors in chicken and mouse embryos, as well as on differential hodology, in these and other vertebrate species (see also Smith-Fernandez et al., 1998). Our present analysis neither proves nor disproves this scheme but rather serves to point out possible alternative interpretations to more conventional ones. The scheme by Puelles et al. (1999, 2000) groups the major pallial regions of classic and modern studies (Karten and Hodos, 1967; Reiner and Karten, 1983, 1985; Striedter, 1997; Dubbeldam, 1998) into the following four molecularly distinct histogenetic territories that are continuous along the entire telencephalon (we indicate the corresponding conventional avian telencephalic regions in parentheses): (1) the medial pallium (hippocampal and parahippocampal complexes), (2) the dorsal pallium (the Wulst and other related corticoid regions), (3) the lateral pallium (the ventral hyperstriatum and associated corticoid areas, including at least a posterior part of the piriform olfactory cortex), and (4) the ventral pallium (the neostriatum, the olfactory bulb, the anterior olfactory nucleus, and the prepiriform olfactory cortical area). The caudal portions of the lateral and ventral pallium also include the dorsolateral and ventromedial parts of the archistriatum, respectively, which are conceived as parts of the amygdaloid complex (Puelles et al., 1999, 2000). Note that, in classic terminology, the archistriatum forms the posterior part of the dorsal ventricular ridge (DVR). In the proposed model, each of the pallial divisions represents a histogenetic unit of the telencephalic wall that radially extends from the ependymal to the pial surface. In the present work, we refer to such units as “radial units.” By using this term, we do not wish to exclude the possibility that minor cell populations migrate tangentially across the divisional boundaries, partially colonizing other radial units (e.g., see Anderson et al., 1997), and we do not suggest that the radial glia, which help to define the units, persist into adulthood (although they are visible from the ventricle to the pia in proper sectioning planes at E11; Puelles unpublished observations). The same applies to the striatal and pallidal subpallial portions, likewise defined molecularly (Puelles et al., 1999, 2000). As described in more detail below, some of the boundaries between the postulated pallial and subpallial divisions coincide with abrupt changes in the cadherin expression profile, even though this applies sometimes only to parts of their full extent, due to the regional expression of the cadherins (e.g., see cad7 expression along the lh). Other postulated boundaries do not coincide with changes in cadherin expression (see, e.g., the boundary between the lateral and ventral pallium in the archistriatal com- Fig. 18. Schematic representation of the embryonic divisions of the chicken telencephalon, as viewed from above (A, modified after Puelles et al., 1999, 2000) and in a “flat-mount” projection of the telencephalon (B). The telencephalon is composed of the subpallium (SbP) and the pallium. The two divisions are separated by the lamina medullaris dorsalis (lmd). The subpallium consists mainly of the striatum (ST) and the pallidum (PA). The pallium can be further divided into ventral pallium (VP), lateral pallium (LP), dorsal pallium (DP), and medial pallium (MP; see Discussion section). The divisions are indicated by different shadings. For other abbreviations, see Table 1 and list. CADHERIN EXPRESSION IN CHICKEN TELENCEPHALON plex and in the area dorsally adjacent to it). The cadherin expression profile varies characteristically within each telencephalic pallial or subpallial division, primarily according to strata superposed one upon another from the ventricular to the pial surface (see Results section). The cadherin expression profile secondarily relates to specific neuronal cell groups (layers or nuclei) within each stratum (see, e.g., sagittal sections in Fig. 9). Examples for these types of staining patterns are discussed below for each telencephalic division. Pallium Medial pallium. A comparison of the cadherin expression profile to the cytoarchitecture of the medial pallium allowed us to identify four to five radial divisions, namely, the previously described Hp, plus at least three distinct radial divisions of APH, which we call APHm, APHi, and APHl, and a possible novel one, APHcl. Within each division, both the cytoarchitecture and the cadherin expression are layered in parallel to the ventricular and outer surfaces of the brain (for details, see Results section) and the layers are oriented orthogonally to radial glial fibers in the region (Striedter and Beydler, 1997). These findings are consistent with the notion that such divisions represent true radial subunits and, thus, cytoarchitectonically distinct areal subdivisions of the medial pallial cortex. The APHm, APHi, and APHl divisions can be followed from rostromedial to caudolateral areas of the medial pallium, as reported in the classic literature (see below). Moreover, at caudal levels, a portion of the piriform cortex may coincide partly with the APHcl (see Figs. 5, 6, 15, 18), which is distinguished here from the neighboring caudal dorsolateral corticoid area (Reiner and Karten, 1985). Architectonic subdivisions of the avian medial pallium were noted by several previous authors (Craigie, 1935, 1939, 1940; Bures et al., 1960; Kuhlenbeck, 1938, 1973). With regard to Craigie’s classification, which seems at the root of the other versions, it should be noted that he included our APHm as subfield H4 of his four hippocampal subfields H1–H4, whereas our APHi possibly includes his parts a, b, and c, and our APHl then corresponds to part d of his parahippocampal cortex. The additional chemoarchitectonic and hodologic subdivisions proposed by Krebs and collaborators (Krebs et al., 1991; Montagnese et al., 1993, 1996; Erichsen et al., 1994; Szekely and Krebs, 1996) did not aim to represent or construe histogenetic radial units, but can be reduced easily to the present divisional model, thus suggesting a fundamental consensus. The dorsomedial subdivision of these authors corresponds to the superficial layers of APHm. If the underlying strata that compose the area 4 and the dorsal part of the area 3 of Erichsen et al. (1991) are added to this dorsomedial subdivision, the full radial unit of our APHm is constituted. Note that this correlates also with a radial domain defined by a minimal density of neurotensin binding sites (Brauth et al., 1986; their Figs. 5, 6). The rest of the dorsolateral subdivision of Krebs and collaborators correlates with our APHi, whereas their ventral subdivision (area 2 and ventral part of area 3 of Erichsen et al., 1991) coincides with our Hp. The area 7 of Erichsen et al. (1991) apparently coincides with our APHl. Interestingly, both the Hp and APHi areas stand out by the density of zinc-containing neuropil, whereas APHm and APHl are largely negative for this marker (Faber et al., 1989). 279 Dorsal pallium (Wulst). Based on its distinct cadherin expression profile and cytoarchitecture, the dorsal pallium can be clearly distinguished from the medially and laterally adjacent divisions of the pallium (Figs. 2– 4, 9 –15), which encroach upon each other not only caudolaterally, but also at the rostral end of the brain (Fig. 18; see also Puelles et al., 2000). The HD, HIS, and HA subdivisions of the dorsal pallium (or Wulst) have characteristic cadherin expression profiles and cytoarchitecture (see Results section). Their boundaries can be followed from the periventricular stratum to the brain surface, consistent with the suggestion that these subdivisions represent separate radial units (Medina and Reiner, 2000) rather than cortical laminae (Shimizu and Karten, 1990; Karten and Shimizu, 1991). At the caudal transition from the Wulst to the APH, the CDL corticoid domain becomes apparent at the surface of the dorsal pallium. It is intercalated between the caudal superficial part of the HV and the APHl and APHcl areas, and it overlies the lateral angle of the lateral ventricle just as it starts to expand caudolaterally (Fig. 4D; compare with the area labeled “HA?” in Fig. 10n of Puelles et al., 2000). In the Results section, we have argued that the classic CDL seems heterogeneous and apparently consists of obliquely superposed medial and dorsal pallial parts. We propose that the name CDL be reserved for the dorsopallial derivative, which disappears at more caudal levels of the telencephalon, where the lateral pallium (our CPi) comes to be in direct contact with the medial pallium (APHcl; Fig. 5). Ventral and lateral pallium. The cadherin expression profile allows a clear distinction between the lateral and ventral pallium from rostral to caudal levels of the telencephalon, even at the level of the island fields (see below); thus, it can be used for delineating the boundary between the two pallial territories. The expression profile shows only minor differences at the frontal, intermediate, and caudal portions of the ventral and lateral pallial subdivisions (N and HV, respectively). At all levels, cad7 typically labels moderately to strongly the deep and intermediate portions of the ventral hyperstriatum (HV, in the lateral pallium) whereas the entire HV shows weak to moderate Rcad immunostaining. In contrast, most of the ventral pallium (neostriatum) represents a patchwork of areas that express Rcad or cad7, in a complementary manner. This patchiness is especially prominent in the island fields (EIF and NIF) that extend continuously from the caudal periventricular area to the rostrolateral telencephalic surface (Figs. 11–15). The quantitative architectonic studies of Rehkämper et al. (1984, 1985) are, in our view, largely coincident with the present interpretation of the boundaries and the extension of the lateral and ventral pallial territories (see below). Both pallial subdivisions have their maximal periventricular and ventricular zone representations caudomedially in the hemisphere, from where their radial dimension extends rostrolaterally to the brain surface, as best seen on parasagittal sections (Figs. 7–15), and on radial glial preparations (Striedter and Beydler, 1997; L. Puelles, unpublished data). Caudolateral neostriatum. The boundary between the lateral and ventral pallial divisions at the caudal telencephalon has been less well-defined in the literature. This is partly because the hyperstriatal lamina (lh), a relatively cell-free landmark that separates the ventral hyperstria- 280 tum from the neostriatum, is clearly visible only at rostral and intermediate levels of the telencephalon and cannot be observed laterally at caudal levels (Karten and Hodos, 1967). The absence of this histologic landmark, together with the restricted distribution of some neuropeptide receptors in the HV and medialmost caudal regions (Wächtler, 1985; Brauth et al., 1986; Reiner et al., 1989, 1994; Durstewitz et al., 1998, 1999) have led to the widespread notion that the caudal part of the HV is restricted only to the medial periventricular region (for example, see Reiner et al., 1989; Wild et al., 1993; Veenman et al., 1995; Metzger et al., 1996; Kröner and Güntürkün, 1999; Metzdorf et al., 1999). All the rest of the caudal pole of the dorsal ventricular ridge, bridging the space from the HV to the lateropallial part of the archistriatum and the neighboring CPi, has been interpreted, therefore, as “caudolateral neostriatum” (NCL). However, there is developmental, architectonic, or hodologic evidence that the NCL also shares features with lateropallial structures. First, during embryogenesis, the NCL expresses the gene Emx1 that is typically absent in the ventral pallium, or neostriatum (N) (Puelles et al., 1999, 2000), although this marker is partially downregulated at more advanced stages (Fig. 10p of Puelles et al., 2000). Likewise, cad7 expression at E11 is continuous along the entire periventricular HV and extends into the NCL area (Figs. 4B– 6B). Second, data on cell density measurements by Rehkämper et al. (1984) indicate that the caudal HV, if restricted to the medial part as defined in the literature, only correlates with characteristics of the periventricular HV at more rostral levels, where intermediate to superficial areas of HV are significantly less dense (their Figs. 1, 5, 7, 8). Moreover, the study by Rehkämper et al. (1985) of the pigeon neostriatum revealed a similarity in cell density and myeloarchitecture between the caudal HV and the caudal periventricular region of the neostriatum (our NCLp in our Fig. 5; their area Ne15 in their Figs. 7–9). Zeier and Karten (1973), in their experimental analysis of connections of the pigeon anterior commissure, illustrated dense terminals over the rostral superficial stratum of the HV, which they misinterpreted as the HIS (HIS is supposed to lie medial to HD, not lateral to it; see their Figs. 2, 3, 4; Medina and Reiner, 2000). This projection domain is clearly continuous caudolaterally with an unidentified area that lies dorsal to the archistriatum and precisely correlates with our NCL (their Figs. 2– 4). Finally, a mapping of zinc in the chicken brain (Faber et al., 1989) shows continuity of dense zinc-containing neuropil from the rostral and intermediate HV (lateral pallium) into our NCL domain (their Fig. 1D–K). In several aspects, the avian NCL appears comparable to an amygdaloid nucleus in reptiles called the dorsolateral amygdala (DLA; Lanuza, 1997). First, both the avian NCL and reptilian DLA are ventrally adjacent to the caudolateral edge of the telencephalic ventricle, and both appear to be a caudal continuation of the dorsolateral DVR. In reptiles, this part of DVR has been suggested to be part of the lateropallial domain and comparable to part of the avian HV (Guirado et al., 2000). Moreover, both NCL and DLA show a dense dopaminergic and cholinergic innervation and/or high acetylcholinesterase activity (Smeets et al., 1986; Medina et al., 1993; Metzger et al., 1996; Lanuza, 1997; Lanuza et al., 1998; Kröner and Güntürkün, 1999; Riters et al., 1999; Puelles, unpublished observations in the chicken). Finally, both structures re- C. REDIES ET AL. ceive a great variety of information from various pallial regions (limbic, sensory, and motor input) and project to the striatum and to major amygdaloid output centers (archistriatum in birds; Lanuza et al., 1998; Metzger et al., 1998; Kröner and Güntürkün, 1999). Interestingly, the reptilian DLA has been suggested to be comparable to the mammalian basolateral amygdala, precisely according to the similarities pointed out above in sauropsids. Therefore, the same could be true of NCL (Lanuza, 1997; Lanuza et al., 1998). The basolateral amygdala is a derivative of the lateropallial domain of mammals, characterized by its expression of the gene Emx1 during development (Puelles et al., 2000). However, as noted above, many neurochemical features of the NCL indicate that this structure is in some ways different from the periventricular HVC (e.g., see Brauth et al., 1986; Reiner et al., 1989). This may be explained by the dispersion of cell clones along the caudorostral radial dimension within the DVR (Szele and Cepko, 1996). An alternative view that is consistent with the present results as well as with the previously published neurochemical data is that NCL may represent the caudal part of a radial domain that is intercalated between the ventropallial neostriatum and the lateropallial HV. This domain, not contemplated in Puelles et al. (2000), would be characterized by a lack of Rcad expression and weak to moderate cad6B and cad7 expression. Rostrolaterally, it would thin out and underline the lh, as best seen on parasagittal sections (see Figs. 9C, 11C). Whether the NCL belongs to the (ventropallial) neostriatum, or to a specialized caudal part of the (lateropallial) HV, or forms a third independent domain, which is intercalated between the neostriatum and the HV, remains unclear at present. To account for the provisional status of this issue, we traced the borders of the NCL by dotted lines in the schematic diagrams. Further studies are needed to evaluate the merits of each of the three possibilities considered here. One novel neostriatal domain observed in the Rcadimmunostained preparations lies at the caudal confluence of the NIF (see below) with the medial archistriatum and the pallio-subpallial boundary. This domain forms a spikeshaped area that diverts ventromedially from the NIF and adheres to the lmd at the back of the ectostriatum. This strikingly distinct area, provisionally named here the retro-ectostriatal nucleus (rE), presently lacks any correlation with published connectivity data but possibly coincides with a domain highlighted in mappings of muscarinic cholinergic receptors in the pigeon (Wächtler, 1985; his Fig. 1e; compare with our Fig. 4; Kohler et al., 1995, his Fig. 1f). The anterior olfactory nucleus and the olfactory bulb have a cadherin expression profile that supports inclusion among ventral pallial structures, as was likewise concluded by mapping the expression of gene transcription factors (Puelles et al., 1999, 2000). Both structures, therefore, are considered here the rostralmost representation of the ventral pallium. The tridimensional extent of the ventral and lateral pallia, as shown in the current study and by Puelles et al. (1999, 2000), corresponds well to the course of the radial glia in these subdivisions (Striedter and Beydler, 1997; L. Puelles, unpublished data). Moreover, assuming that radial glia serve as a scaffold for neuronal migration patterns (Rakic, 1988; Nieuwenhuys et al., 1998), these results are also in agreement with the predominantly CADHERIN EXPRESSION IN CHICKEN TELENCEPHALON oblique, caudorostral dispersion of cell clones within the neostriatum and within the ventral hyperstriatum, respectively (Szele and Cepko, 1996; Striedter et al., 1998). Together, these studies suggest that the ventral and lateral pallia (similarly to the medial and dorsal pallia) represent two adjacent but separate histogenetic fields, irrespective of the potential ingression of tangentially migrated cellular cohorts (Cobos, 2000). Island fields. At intermediate and caudal levels, a large lateral part of the ventral pallium is formed by what we here call the neostriatal island field (NIF), supplemented rostrally by the ectostriatal island field (EIF). The island fields are characterized by the presence of small islandlike, sometimes dense, cellular aggregates located in the dorsal neostriatum and ectostriatal belt area. Rostrolaterally, the islands become larger. The islands show a cadherin expression profile and cytoarchitecture similar to that found in the neighboring NCL domain (and also partly similar to the subjacent ectostriatal core), whereas the surrounding matrix is characterized by Rcad expression (for details, see Results section). A highly patchy distribution of cadherin expression in this area has been described before for Rcad and cadherin-10 but was not commented upon (see Fig. 4 in Arndt and Redies, 1996; and Fig. 11B in Fushimi et al., 1997; respectively). A similarly patchy expression pattern was found for cadherin-8 in the matrix of the striatum of the neonatal rat (Korematsu et al., 1998) and for cad7 in the caudolateral part of the E15 parahippocampal area (this study). Like the ventral and lateral pallial divisions, the Rcadpositive matrix and the Rcad-negative islands extend as complete radial units from periventricular regions at caudal neostriatal levels (Fig. 11) to the rostrolateral pial surface at intermediate neostriatal levels (Figs. 3, 15). One possible interpretation of the immunostaining results is that at least some islands represent interdigitated radial domains that exhibit differential cadherin expression, as is suggested by the occasional cellular bridges that connect some islands to the overlying neostriatal or NCL areas. As an alternative or supplementary explanation, similar clumps of cells might be produced by differential aggregation of cellular cohorts that originate in the same embryonic subdivision, but whose cadherin expression differentially correlates with specific birthdates. Birthdaterelated cell aggregates analogous in shape and position to our islands have been reported recently in the neostriatum (and the HV) of the chicken (Striedter and Keefer, 2000). These cell groups might express different adhesive properties related to their time origin and, thus, segregate into cellular aggregates in the matrix environment. Such homotypic cell sorting and aggregation of cell populations that differentially express cadherin subtypes has been demonstrated repeatedly in vitro and in vivo (for review, see Redies, 2000). The resulting patterns of cell mixing (e.g., whether layers, aggregates, or islands are formed) are likely to depend on the relative adhesive strengths and cell number ratios of the mixed cell populations (for review, see Steinberg, 1970; Redies, 2000), as well as on the extent of heterotypic interactions between cells expressing different cadherin subtypes (Shimoyama et al., 2000). Subpallium The cadherin expression profile also may be useful for delineating better the boundary between the major subdivisions of the subpallium, i.e., between the striatum and 281 the pallidum, especially in the periventricular area. Here, a large region termed the parolfactory lobe (LPO) was classically thought to represent the medial part of the avian striatum. This assumption was based on numerous neurochemical features and connectivity data. For example, a similarity of the LPO to the mammalian striatum has been suggested by its high acetylcholinesterase activity, dense dopaminergic innervation and large number of cells and fibers containing the neuropeptides substance P or enkephalin, as well as by reciprocal connections with the avian ventral tegmental area and substantia nigra (Karten and Dubbeldam, 1973; Reiner et al., 1984, 1998; Medina and Reiner, 1995). However, the LPO is chemically heterogeneous at caudal levels, where the avian dorsal pallidum (paleostriatum primitivum) appears laterally. At these caudal levels, the LPO typically shows a dorsal part that is laterally continuous with the avian lateral striatum (paleostriatum augmentatum) and a ventral part that is located medial to the dorsal pallidum (for example, see Fig. 1B in Karle et al., 1996). During embryonic development, the caudoventral part of LPO expresses the gene Nkx2.1, which is a pallidal marker in tetrapods (Puelles et al., 1999, 2000). This expression persists at least until E14.5 (Puelles, unpublished observations) and may well be present in the adult pallidum in Xenopus (O. Marı́n, personal communication; Marı́n et al., 1998). Consequently, the ventral part of LPO was termed here the “medial pallidum” (PAm in Figs. 3, 4), even though the literature usually interprets this area as homogeneously striatal. The change of periventricular cadherin expression in this region (Fig. 3A–C) possibly relates to the limit between the striatal and pallidal periventricular parts of the LPO. Note that, in general, many cells in the avian and mammalian pallidum have neurochemical characteristics typical of striatal cells (see, e.g., Sun and Reiner, 2000). The striatal-like features found in the caudoventral LPO (PAm) in the adult may be due to the apparent presence of a mixed population of both pallidal-type and striatal-type cells in this region. Whether the striatal-type cells originate in striatal (Nkx2.1 negative) or pallidal (Nkx2.1 positive) neuroepithelium remains unclear at present. Archistriatum The expression of transcription factors (Puelles et al., 1999, 2000) indicate that the ventral and lateral pallial divisions as well as the subpallium can be followed to the caudal and ventrolateral pole of the telencephalon, invading the archistriatum, where each of the corresponding subdivisions consists of specific nuclei. Several of these archistriatal nuclei differentially express cadherins and show a characteristic cytoarchitecture (Figs. 16, 18; Table 1). Lateropallial subdivision. Similar to neighboring parts of the lateral pallium, this subdivision of the archistriatum (which includes Aid, Aii, and Ap) generally shows mantle expression of the pallial genes Tbr1, Emx1, and ependymal expression of Pax6 (Puelles et al., 1999, 2000). Some of the nuclei in this subdivision (with the exception of Aid) show also strong cad7 immunoreactivity and moderate or weak staining for Rcad (Figs. 4 – 6). Ventropallial subdivison. Similar to the adjacent parts of the ventral pallium, this subdivision of the archistriatum (which includes Aiv and Am) generally contains higher levels of Rcad expression, whereas cad7 expression 282 is relatively low (Figs. 4 – 6). Like the ventral pallium, this archistriatal region expresses the pallial marker gene Tbr1 but lacks expression of Emx1, except at the pial surface (Puelles et al., 1999, 2000). Subpallial subdivision. The anterior archistriatum (Aa) shows a cadherin expression profile that resembles more the adjacent striatum than the neighboring ventropallial archistriatum (Figs. 4 – 6). The subpallial nature of Aa, which was postulated on molecular marker evidence (notably the abundance of migrated Pax-6 –positive cells) by Puelles et al. (2000), awaits corroboration with other comparative methods. It is appropriate to point out that some of the postulated divisional boundaries in the archistriatum do not coincide with abrupt changes in cadherin expression. For example, there is a prominent continuity of Rcad expression from Aid (postulated lateropallial subdivision) into NIF and Ap (postulated ventropallial subdivision). Moreover, the Rcad immunoreactivity in Aid seems to extend to the ventricular surface just medial to the lateral edge of the lateral ventricle. This unique pattern coincides with a similar band of Emx-1 expression in E8 embryos, which seems to separate the archistriatum from the lateral pallium (Figs. 10i,j in Puelles et al., 2000). These inconsistencies of cadherin staining with the postulated pallial divisional borders will require further analysis. Given their provisional status, we indicated these borders by dotted lines in the schematic diagrams. Relation of cadherin expression to specific functional cell groups and neural circuits The functional systems and neural circuits of the brain typically consist of several interconnected neuronal populations or gray matter areas, which are distributed in a particular way through the brain, following usually highly specific rules of connectivity. The cell populations participating in a given circuit are often derived from several of the major (sub)divisions of the vertebrate brain. The development of such complex neural circuitry requires connectional chemospecificity for axonal navigation, contact guidance and target recognition. Pioneering axons probably use different cues than axons following their trail. The distribution of cadherins over the brain is widespread, although selective. This distribution includes the expression by developing fiber tracts, apart from the expression by specific cell groups (Table 1). The capacity of cadherins to foster homotypic binding interactions between fasciculating axons, neural cells (aggregation and segregation), or both, have led to their being postulated as being one of several possible regulators that build or stabilize specific neural circuits (for review, see Redies, 1995, 2000). This is possibly achieved by way of specific matching of growing axons, axonal pathways and target cells that express the same combination of cadherins (Redies et al., 1993; Arndt and Redies, 1996; Fannon and Colman, 1996; Wöhrn et al., 1998, 1999; Huntley and Benson, 1999; for review, see Redies, 1995, 2000). Although many other types of molecules are thought to also participate in circuitry development at specific brain locations, many of them do not show correlative expression along given pathways (for review, see Tessier-Lavigne and Goodman, 1996; O’Leary and Wilkinson, 1999; Raper, 2000). Previous studies on the expression of cad6B, cad7, and Rcad in chicken brain (Redies et al., 1993, 2000; Arndt and Redies, 1996; Wöhrn et al., 1998; Arndt et al., 1998) dem- C. REDIES ET AL. onstrated a relation of each of these cadherins (or combinations of them, see Wöhrn et al., 1999) to specific neural circuits. These studies, together with the current data in the telencephalon, suggest the possibility that the same cadherins may be related also to specific thalamopallial, intratelencephalic and basal ganglia circuits of the chicken embryo, as outlined below. It should be stressed that this hypothesis remains an extrapolation of findings from our previous studies on the diencephalon and the rhombencephalon (same references as above), to the present telencephalic data. To prove that telencephalic neural circuits differentially express specific cadherins, more detailed studies will be required. At the present level of analysis, though, we scarcely found clear-cut examples of mismatches in cadherin expression between specific projections and their target areas. One such exception is possibly found in the olfactory system, where neither the olfactory tract nor the mitral cells of the olfactory bulb expressed any of the cadherins studied, although the prepiriform cortex strongly expresses cad7 and Rcad, as does the nucleus of the lateral olfactory tract and the marginal zone of the neostriatum. These are areas reported to receive olfactory projections (Reiner and Karten, 1985). Based on the cadherin expression profile in specific dorsal thalamic nuclei (Redies et al., 2000) and in their telencephalic targets, the thalamopallial circuits appear to differentially express the three cadherins. These circuits include (1) the multimodal pathway from the thalamic dorsolateral posterior nucleus (DLP) to the thalamorecipient area of the NI (Gamlin and Cohen, 1986; Wild, 1994; Kröner and Güntürkün, 1999), which are apparently associated to cad7 or Rcad; (2) the auditory projections from the nucleus ovoidalis and some other periovoidal nuclei to field L and its subdivisions (Wild et al., 1993; Kröner and Güntürkün, 1999), which are apparently associated to cad7 or Rcad; (3) the visual pathway from the nucleus rotundus (part of the intermediate dorsal thalamic tier; Redies et al., 2000) to the ectostriatum and ectostriatal belt, including to the EIF region demonstrated in the present study (Revzin and Karten, 1967; Karten and Hodos, 1970; Kröner and Güntürkün, 1999), which are apparently associated to cad6B, cad7, or Rcad; (4) the telencephalic projections from “dorsal tier” thalamic nuclei (in the sense of Redies et al., 2000) to the Wulst, such as those from the lateral part of the dorsolateral nucleus (Karten et al., 1973; Shimizu and Karten, 1990), the dorsal intermediate ventral anterior nucleus (Wild, 1987a, 1989; Korzeniewska and Güntürkün, 1990; Kröner and Güntürkün, 1999), and the ventrointermediate area (Medina and Reiner, 1997; Medina et al., 1997), which are apparently associated to cad7 or Rcad. A relation of cadherins to specific thalamopallial pathways has also been suggested in mammals (Huntley and Benson, 1999; Obst-Pernberg et al., 2001). The current results show that cadherins are expressed in a restricted and partially complementary manner also in many structures related to specific intratelencephalic circuits. One particularly clear example of the association of a cadherin subtype to an intratelencephalic pathway is the projection of a specific region in the NCL to a restricted medial area of the intermediate archistriatum (Aim). These two structures as well as the fiber fascicles connecting them express cad6B (Fig. 17C). The intermediate archistriatum, in turn, projects to extratelencephalic targets CADHERIN EXPRESSION IN CHICKEN TELENCEPHALON (Wild et al., 1993; Wild, 1994; Kröner and Güntürkün, 1999). It is, thus, conceivable that these structures correspond to the high vocal center, the nucleus robustus archistriatalis and their connection, respectively, in songbirds (Nottebohm et al., 1976; Vicario, 1993; Wild, 1994). This possibility remains to be confirmed by a future, comparative study mapping the expression of cad6B and other molecular markers. Moreover, in the basal ganglia circuits, two cadherins (Rcad and cad6B) are related to particular groups of pallidal neurons. For example, the medial part of the dorsal pallidum contains cad6B-positive neurons, whereas the intermediate and lateral parts of the dorsal pallidum contain neurons expressing Rcad. The dorsal pallidum of birds is known to project to several targets in the subthalamus, the ventral and dorsal thalamus, the pretectum, and the diencephalic and midbrain tegmentum (Karten and Dubbeldam, 1973; Reiner et al., 1982, 1984; Medina and Reiner, 1997; Medina et al., 1997). As shown previously (Arndt and Redies, 1996; Redies et al., 2000), some of these targets express Rcad, such as the avian subthalamic nucleus (anterior nucleus of the ansa lenticularis), the ventrointermediate area (in the dorsal thalamus), a ventral part of the dorsointermediate posterior nucleus (in the dorsal thalamus), and a large part of the lateral spiriform nucleus (in the pretectum). Other targets of the dorsal pallidum express cad6B, e.g., parts of the lateral spiriform nucleus (Redies et al., 2000). ACKNOWLEDGMENTS The authors thank Meike Ast for expert technical assistance, Shinichi Nakagawa and Masatoshi Takeichi for generous gifts of antibodies, Reimund Düchting for help with photography, Min-Suk Yoon and Min Jeong Ju for assistance in preparing the figures, and G. Striedter for sharing unpublished data. C.R. received support from the Deutsche Forschungsgemeinschaft; L.M. from the Seneca Foundation; L.M. and L.P. from the MCYT and CICYT Governmental Grant Agency, Madrid; C.R. and L.P. from the Spanish Ministerio de Relaciones Exteriores and German Academic Exchange Service; and L.P. from the European Community BIOTECH Program. LITERATURE CITED Anderson SA, Eisenstat DD, Shi L, Rubenstein JLR. 1997. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science 278:474 – 476. Arndt K, Redies C. 1998. Development of cadherin-defined parasagittal subdivisions in the embryonic chicken cerebellum. J Comp Neurol 401:367–381. Arndt K, Redies C. 1996. Restricted expression of R-cadherin by brain nuclei and neural circuits of the developing chicken brain. J Comp Neurol 373:373–399. Arndt K, Nakagawa S, Takeichi M, Redies C. 1998. Cadherin-defined segments and parasagittal cell ribbons in the developing chicken cerebellum. Mol Cell Neurosci 10:211–228. Aste N, Balthazart J, Absil P, Grossmann R, Mulhbauer E, VigliettiPanzica C, Panzica GC. 1998. Anatomical and neurochemical definition of the nucleus of the stria terminalis in Japanese quail (Coturnix japonica). J Comp Neurol 396:141–157. Bixby JL, Zhang R. 1990. Purified N-cadherin is a potent substrate for the rapid induction of neurite outgrowth. J Cell Biol 110:1253–1260. Bonke BA, Bonke D, Scheich H. 1979. Connectivity of the auditory forebrain nuclei in the guinea fowl (Numida meleagris). Cell Tissue Res 200:101–121. 283 Brauth SE, Kitt CA, Reiner A, Quirion R. 1986. Neurotensin binding sites in the forebrain and midbrain of the pigeon. J Comp Neurol 253:358 – 373. Brauth SE, Heaton JT, Durand SE, Liang W, Hall WS. 1994. Functional anatomy of forebrain auditory pathways in the budgerigar (Melopsittacus undulatus). Brain Behav Evol 44:210 –233. Bulfone A, Puelles L, Porteus MH, Frohmann MA, Martin GR, Rubenstein JLR. 1993. Spatially restricted expression of Dlx-1, Dlx-2 (Tes-1), Gbx-2, and Wnt-3 in the embryonic day 12.5 mouse forebrain defines potential transverse and longitudinal segmental boundaries. J Neurosci 13:3155–3172. Bures J, Fifkova E, Marsala J. 1960. Leao’s spreading depression in pigeons. J Comp Neurol 114:1–10. Cobos I. 2000. Morfogénesis e histogénesis del telencéfalo: Estudio experimental en embriones de pollo. University of Murcia. Craigie EH. 1935. The hippocampal and parahippocampal cortex of the emu (Dromiceus). J Comp Neurol 61:563–591. Craigie EH. 1939. The cerebral cortex of Rhea americana. J Comp Neurol 70:331–353. Craigie EH. 1940. The cerebral cortex of palaeognathine and neognathine birds. J Comp Neurol 73:179 –234. Dubbeldam JL. 1998. Birds. In: Nieuwenhuys R, ten Donkelaar HJ, Nicholson C, editors. The central nervous system of vertebrates. Berlin: Springer. p 1525–1636. Durand SE, Heaton JT, Amateau SK, Brauth SE. 1997. Vocal control pathways through the anterior forebrain of a parrot (Melopsittacus undulatus). J Comp Neurol 377:179 –206. Durstewitz D, Kroner S, Hemmings H Jr, Güntürkün O. 1998. The dopaminergic innervation of the pigeon telencephalon: distribution of DARPP-32 and co-occurrence with glutamate decarboxylase and tyrosine hydroxylase. Neuroscience 83:763–779. Durstewitz D, Kroner S, Güntürkün O. 1999. The dopaminergic innervation of the avian telencephalon. Prog Neurobiol 59:161–195. Erichsen JT, Bingman VP, Krebs JR. 1991. The distribution of neuropeptides in the dorsomedial telencephalon of the pigeon (Columba livia): a basis for regional subdivisions. J Comp Neurol 314:478 – 492. Erichsen JT, Ciocchetti A, Fontanesi G, Bagnoli P. 1994. Neuroactive substances in the developing dorsomedial telencephalon of the pigeon (Columba livia): differential distribution and time course of maturation. J Comp Neurol 345:537–561. Espeseth A, Marnellos G, Kintner C. 1998. The role of F-cadherin in localizing cells during neural tube formation in Xenopus embryos. Development 125:301–312. Faber H, Braun K, Zuschratter W, Scheich H. 1989. System-specific distribution of zinc in the chick brain. A light- and electron-microscopic study using the Timm method. Cell Tissue Res 258:247–257. Fannon AM, Colman DR. 1996. A model for central synaptic junctional complex formation based on the differential adhesive specificities of the cadherins. Neuron 17:423– 434. Fushimi D, Arndt K, Takeichi M, Redies C. 1997. Cloning and expression analysis of cadherin-10 in the CNS of the chicken embryo. Dev Dyn 209:269 –285. Gamlin PDR, Cohen DH. 1986. A second ascending visual pathway from the optic tectum to the telencephalon in the pigeon (Columba livia). J Comp Neurol 250:296 –310. Gänzler SII, Redies C. 1995. R-cadherin expression during nucleus formation in chicken forebrain neuromeres. J Neurosci 15:4157– 4172. Guirado S, Davila JC, Real MA, Medina L. 2000. Light and electron microscopic evidence for projections from the thalamic nucleus rotundus to targets in the basal ganglia, the dorsal ventricular ridge, and the amygdaloid complex in a lizard. J Comp Neurol 424:216 –232. Gumbiner BM. 1996. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 84:345–357. Hamburger V, Hamilton H. 1951. A series of normal stages in the development of the chick embryo. J Morphol 88:49 –92. Hatta K, Takeichi M. 1986. Expression of N-cadherin adhesion molecules associated with early morphogenetic events in chick development. Nature 320:447– 449. Hirano S, Yan Q, Suzuki ST. 1999. Expression of a novel protocadherin, OL-protocadherin, in a subset of functional systems of the developing mouse brain. J Neurosci 19:995–1005. Huber GC, Crosby EC. 1929. The nuclei and fiber paths of the avian diencephalon, with consideration of telencephalic and certain mesencephalic centers and connections. J Comp Neurol 48:1–225. 284 Huntley GW, Benson DL. 1999. Neural (N)-cadherin at developing thalamocortical synapses provides an adhesion mechanism for the formation of somatotopically organized connections. J Comp Neurol 407:453– 471. Inoue T, Tanaka T, Suzuki SC, Takeichi M. 1998. Cadherin-6 in the developing mouse brain: expression along restricted connection systems and synaptic localization suggest a potential role in neuronal circuitry. Dev Dyn 211:338 –351. Inuzuka H, Redies C, Takeichi M. 1991. Differential expression of R- and N-cadherin in neural and mesodermal tissues during early chicken development. Development 113:959 –967. Iwai Y, Usui T, Hirano S, Steward R, Takeichi M, Uemura T. 1997. Axon patterning requires DN-cadherin, a novel neuronal adhesion receptor, in the Drosophila embryonic CNS. Neuron 19:77– 89. Jarvis ED, Mello CV. 2000. Molecular mapping of brain areas involved in parrot vocal communication. J Comp Neurol 419:1–31. Källén B. 1962. Embryogenesis of brain nuclei in the chick telencephalon. Ergeb Anat Entwicklungsgesch 36:62– 82. Karle EJ, Anderson KD, Medina L, Reiner A. 1996. Light and electron microscopic immunohistochemical study of dopaminergic terminals in the striatal portion of the pigeon basal ganglia using antisera against tyrosine hydoxylase and dopamine. J Comp Neurol 369:109 –124. Karten HJ, Dubbeldam JL. 1973. The organization and projection of the paleostriatal complex in the pigeon (Columba livia). J Comp Neurol 148:61–90. Karten HJ, Hodos W. 1967. A stereotaxic atlas of the brain of the pigeon (Columba livia). Baltimore: Johns Hopkins University Press. Karten HJ, Hodos W. 1970. Telencephalic projections of the nucleus rotundus in the pigeon (Columba livia). J Comp Neurol 140:35–52. Karten HJ, Shimizu T. 1991. Are visual hierarchies in the brain of the beholders? Constancy and variability in the visual system of birds and mammals. In: Bagnoli P, Hodos W, editors. The changing visual system. New York: Plenum Press. p 51–59. Karten HJ, Hodos W, Nauta WJH, Revzin AM. 1973. Neural connections of the “visual Wulst” of the avian telencephalon. Experimental studies in the pigeon (Columba livia) and owl (Speotyto cunicularia). J Comp Neurol 150:253–278. Kohler EC, Messer WS Jr, Bingman VP. 1995. Evidence for muscarinic acetylcholine receptor subtypes in the pigeon telencephalon. J Comp Neurol 362:271–282. Korematsu K, Redies C. 1997. Expression of cadherin-8 mRNA in the developing mouse central nervous system. J Comp Neurol 387:291– 306. Korematsu K, Goto S, Okamura A, Ushio Y. 1998. Heterogeneity of cadherin-8 expression in the neonatal rat striatum: comparison with striatal compartments. Exp Neurol 154:531–536. Korzeniewska E, Güntürkün O. 1990. Sensory properties and afferents of the N. dorsolateralis posterior thalami of the pigeon. J Comp Neurol 292:457– 479. Krebs JR, Erichsen JT, Bingman VP. 1991. The distribution of neurotransmitters and neurotransmitter-related enzymes in the dorsomedial telencephalon of the pigeon (Columba livia). J Comp Neurol 314:467– 477. Kröner S, Güntürkün O. 1999. Afferent and efferent connections of the caudolateral neostriatum in the pigeon (Columba livia): a retro- and anterograde pathway tracing study. J Comp Neurol 407:228 –260. Kuenzel WJ, Masson M. 1988. A stereotaxic atlas of the brain of the chick (Gallus domesticus). Baltimore: The Johns Hopkins University Press. Kuhlenbeck H. 1938. The ontogenetic development and phylogenetic significance of the cortex telencephali in the chick. J Comp Neurol 69: 273–301. Kuhlenbeck H. 1973. The central nervous system of vertebrates. Berlin: Karger. Lanuza E. 1997. Fiber connections of the telencephalic amygdala of squamate reptiles. Doctoral Thesis, University of Valencia. Lanuza E, Belekhova M, Martinez-Marcos A, Font C, Martinez-Garcia F. 1998. Identification of the reptilian basolateral amygdala: an anatomical investigation of the afferents to the posterior dorsal ventricular ridge of the lizard Podarcis hispanica. Eur J Neurosci 10:3517–3534. Marı́n O, Smeets WJ, Gonzales A. 1998. Evolution of the basal ganglia in tetrapods: a new perspective based on recent studies in amphibians. Trends Neurosci 21:487– 494. Matsunaga M, Hatta K, Nagafuchi A, Takeichi M. 1988. Guidance of optic nerve fibers by N-cadherin adhesion molecules. Nature 334:62– 64. C. REDIES ET AL. Medina L, Reiner A. 1994. Distribution of choline acetyltransferase immunoreactivity in the pigeon brain. J Comp Neurol 342:497–537. Medina L, Reiner A. 1995. Neurotransmitter organization and connectivity of the basal ganglia in vertebrates: implications for the evolution of basal ganglia. Brain Behav Evol 46:235–258. Medina L, Reiner A. 1997. The efferent projections of the dorsal and ventral pallidal parts of the pigeon basal ganglia, studied with biotinylated dextran amine. Neuroscience 81:773– 802. Medina L, Reiner A. 2000. Do birds possess homologues of mammalian primary visual, somatosensory and motor cortices? Trends Neurosci 23:1–12. Medina L, Smeets WJAJ, Hoogland PV, Puelles L. 1993. Distribution of choline acetyltransferase immunoreactivity in the brain of the lizard Gallotia galloti. J Comp Neurol 331:261–285. Medina L, Veenman CL, Reiner A. 1997. Evidence for a possible avian dorsal thalamic region comparable to the mammalian ventral anterior, ventral lateral, and oral ventroposterolateral nuclei. J Comp Neurol 384:86 –108. Metzdorf R, Gahr M, Fusani L. 1999. Distribution of aromatase, estrogen receptor, and androgen receptor mRNA in the forebrain of songbirds and nonsongbirds. J Comp Neurol 407:115–129. Metzger M, Jiang S, Wang J, Braun K. 1996. Organization of the dopaminergic innervation of forebrain areas relevant to learning: a combined immunohistochemical/retrograde tracing study in the domestic chick. J Comp Neurol 376:1–27. Metzger M, Jiang S, Braun K. 1998. Organization of the dorsocaudal neostriatal complex: a retrograde and anterograde tracing study in the domestic chick with special emphasis on pathways relevant to imprinting. J Comp Neurol 395:380 – 404. Montagnese CM, Krebs JR, Szekely AD, Csillag A. 1993. A subpopulation of large calbindin-like immunopositive neurones is present in the hippocampal formation in food-storing but not in non-storing species of bird. Brain Res 614:291–300. Montagnese CM, Krebs JR, Meyer G. 1996. The dorsomedial and dorsolateral forebrain of the zebra finch, Taeniopygia guttata: a Golgi study. Cell Tissue Res 283:263–282. Nakagawa S, Takeichi M. 1998. Neural crest emigration from the neural tube depends on regulated cadherin expression. Development 125: 2963–2971. Nieuwenhuys R, ten Donkelaar HJ, Nicholson C. 1998. The central nervous system of vertebrates. Heidelberg: Springer. Nollet F, Kools P, van Roy F. 2000. Phylogenetic analysis of the cadherin superfamily allows identification of six major subfamilies besides several solitary members. J Mol Biol 299:551–572. Nottebohm F, Stokes TM, Leonard CM. 1976. Central control of song in the canary, Serinus canarius. J Comp Neurol 165:457– 486. O’Leary DD, Wilkinson DG. 1999. Eph receptors and ephrins in neural development. Curr Opin Neurobiol 9:65–73. Obst-Pernberg K, Medina L, Redies C. 2001. Expression of R-cadherin and N-cadherin by cell groups and fiber tracts in the developing mouse forebrain: relation to the formation of functional circuits. Neuroscience (accepted for publication). Puelles L, Rubenstein JLR. 1993. Expression patterns of homeobox and other putative regulatory genes in the embryonic mouse forebrain suggest a neuromeric organization. Trends Neurosci 16:472– 479. Puelles L, Kuwana E, Puelles E, Rubenstein JL. 1999. Comparison of the mammalian and avian telencephalon from the perspective of gene expression data. Eur J Morphol 37:139 –150. Puelles L, Kuwana E, Puelles E, Bulfone A, Shimamura K, Keleher J, Smiga S, Rubenstein J. 2000. Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx-2, Emx-1, Nkx-2.1, Pax-6, and Tbr-1. J Comp Neurol 424:409 – 438. Rakic P. 1988. Specification of cerebral cortical areas. Science 241:170 – 176. Raper JA. 2000. Semaphorins and their receptors in vertebrates and invertebrates. Curr Opin Neurobiol 10:88 –94. Redies C. 1995. Cadherin expression in the developing vertebrate brain: from neuromeres to brain nuclei and neural circuits. Exp Cell Res 220:243–256. Redies C. 1997. Cadherins and the formation of neural circuitry in the vertebrate CNS. Cell Tissue Res 290:405– 413. Redies C. 2000. Cadherins in the central nervous system. Prog Neurobiol 61:611– 648. CADHERIN EXPRESSION IN CHICKEN TELENCEPHALON Redies C, Inuzuka H, Takeichi M. 1992. Restricted expression of N- and R-cadherin on neurites of the developing chicken CNS. J Neurosci 12:3525–3534. Redies C, Engelhart K, Takeichi M. 1993. Differential expression of N- and R-cadherin in functional neuronal systems and other structures of the developing chicken brain. J Comp Neurol 333:398 – 416. Redies C, Ast M, Nakagawa S, Takeichi M, Martı́nez-de-la-Torre M, Puelles L. 2000. Morphological fate of diencephalic neuromeres and their subdivisions revealed by mapping cadherin expression. J Comp Neurol 421:481–514. Rehkämper G, Zilles K, Schleicher A. 1984. A quantitative approach to cytoarchitectonics: IX. The areal pattern of the hyperstriatum ventrale in the domestic pigeon, Columba livia f.d. Anat Embryol (Berl) 169: 319 –327. Rehkämper G, Zilles K, Schleicher A. 1985. A quantitative approach to cytoarchitectonics: X. The areal pattern of the neostriatum in the domestic pigeon, Columba livia f.d. A cyto- and myeloarchitectonical study. Anat Embryol (Berl) 171:345–355. Reiner A, Karten HJ. 1983. The laminar source of efferent projections from the avian Wulst. Brain Res 275:349 –354. Reiner A, Karten HJ. 1985. Comparison of olfactory bulb projections in pigeons and turtles. Brain Behav Evol 27:11–27. Reiner A, Brecha NC, Karten HJ. 1982. Basal ganglia pathways to the tectum: the afferent and efferent connections of the lateral spiriform nucleus of pigeon. J Comp Neurol 208:16 –36. Reiner A, Brauth SE, Karten HJ. 1984. Evolution of the amniote basal ganglia. Trends Neurosci 7:320 –325. Reiner A, Brauth SE, Kitt CA, Quirion R. 1989. Distribution of mu, delta, and kappa opiate receptor types in the forebrain and midbrain of pigeons. J Comp Neurol 280:359 –382. Reiner A, Karle EJ, Anderson KD, Medina L. 1994. Catecholaminergic perikarya and fibers in the avian nervous system. In: Smeets WJAJ, Reiner A, editors. Phylogeny and development of catecholamine systems in the cns of vertebrates. Cambridge: Cambridge University Press. p 135–181. Reiner A, Medina L, Veenman CL. 1998. Structural and functional evolution of the basal ganglia in vertebrates. Brain Res Rev 28:235–285. Revzin AM, Karten HJ. 1967. Rostral projections of the optic tectum and the nucleus rotundus in the pigeon. Brain Res 5:264 –276. Riters LV, Erichsen JT, Krebs JR, Bingman VP. 1999. Neurochemical evidence for at least two regional subdivisions within the homing pigeon (Columba livia) caudolateral neostriatum. J Comp Neurol 412: 469 – 487. Shimamura K, Hartigan DJ, Martı́nez S, Puelles L, Rubenstein JLR. 1995. Longitudinal organization of the anterior neural plate and neural tube. Development 121:3923–3933. Shimizu T, Karten HJ. 1990. Immunohistochemical analysis of the visual Wulst of the pigeon (Columba livia). J Comp Neurol 300:346 –369. Shimoyama Y, Tsujimoto G, Kitajima M, Natori M. 2000. Identification of three human type-II classic cadherins and frequent heterophilic interactions between different subclasses of type-II classic cadherins. Biochem J 349:159 –167. Simeone A, Acampora D, Gulisano M, Stornaiuolo A, Boncinelli E. 1992. Nested expression domains of four homeobox genes in developing rostral brain. Nature 358:687– 690. Smeets WJ, Hoogland PV, Voorn P. 1986. The distribution of dopamine immunoreactivity in the forebrain and midbrain of the lizard Gekko gecko: an immunohistochemical study with antibodies against dopamine. J Comp Neurol 253:46 – 60. Smith-Fernandez A, Pieau C, Reperant J, Boncinelli E, Wassef M. 1998. Expression of the Emx-1 and Dlx-1 homeobox genes define three molecularly distinct domains in the telencephalon of mouse, chick, turtle and frog embryos: implications for the evolution of telencephalic subdivisions in amniotes. Development 125:2099 –2111. 285 Steinberg MS. 1970. Does differential adhesion govern self-assembly processes in histogenesis? Equilibrium configurations and the emergence of a hierarchy among populations of embryonic cells. J Exp Zool 173: 395– 433. Striedter GF. 1994. The vocal control pathways in budgerigars differ from those in songbirds. J Comp Neurol 343:35–56. Striedter GF. 1997. The telencephalon of tetrapods in evolution. Brain Behav Evol 49:179 –213. Striedter GF, Beydler S. 1997. Distribution of radial glia in the developing telencephalon of chicks. J Comp Neurol 387:399 – 420. Striedter GF, Keefer BP. 2000. Cell migration and aggregation in the developing telencephalon: pulse-labeling chick embryos with bromodeoxyuridine. J Neurosci 20:8021– 8030. Striedter GF, Marchant TA, Beydler S. 1998. The “neostriatum” develops as part of the lateral pallium in birds. J Neurosci 18:5839 –5849. Sun Z, Reiner A. 2000. Localization of dopamine D1A and D1B receptor mRNAs in the forebrain and midbrain of the domestic chick. J Chem Neuroanat 19:211–224. Szekely AD, Krebs JR. 1996. Efferent connectivity of the hippocampal formation of the zebra finch (Taenopygia guttata): an anterograde pathway tracing study using Phaseolus vulgaris leucoagglutinin. J Comp Neurol 368:198 –214. Szele FG, Cepko CL. 1996. A subset of clones in the chick telencephalon arranged in rostrocaudal arrays. Curr Biol 6:1685–1690. Takeichi M. 1995. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol 7:619 – 627. Tessier-Lavigne M, Goodman CS. 1996. The molecular biology of axon guidance. Science 274:1123–1133. Uchida N, Honjo Y, Johnson KR, Wheelock MJ, Takeichi M. 1996. The catenin/cadherin adhesion system is localized in synaptic junctions bordering transmitter release zones. J Cell Biol 135:767–779. Veenman CL, Karle EJ, Anderson KD, Reiner A. 1995. Thalamostriatal projection neurons in birds utilize LANT6 and neurotensin: a light and electron microscopic double-labeling study. J Chem Neuroanat 9:1–16. Vicario DS. 1993. A new brain stem pathway for vocal control in the zebra finch song system. Neuroreport 4:983–986. Wächtler K. 1985. Regional distribution of muscarinic acetylcholine receptors in the telencephalon of the pigeon (Columbia livia f. domestica). J Hirnforsch 26:85– 89. Wild JM. 1987a. The avian somatosensory system: connections of regions of body representation in the forebrain of the pigeon. Brain Res 412: 205–223. Wild JM. 1987b. Thalamic projections to the paleostriatum and neostriatum in the pigeon (Columba livia). Neuroscience 20:305–327. Wild JM. 1989. Avian somatosensory system: II. Ascending projections of the dorsal column and external cuneate nuclei in the pigeon. J Comp Neurol 287:1–18. Wild JM. 1994. Visual and somatosensory inputs to the avian song system via nucleus uvaeformis (Uva) and a comparison with the projections of a similar thalamic nucleus in a nonsongbird, Columba livia. J Comp Neurol 349:512–535. Wild JM, Karten HJ, Frost BJ. 1993. Connections of the auditory forebrain in the pigeon (Columba livia). J Comp Neurol 337:32– 62. Wöhrn J-CP, Puelles L, Nakagawa S, Takeichi M, Redies C. 1998. Cadherin expression in the retina and retinofugal pathways of the chicken embryo. J Comp Neurol 396:20 –38. Wöhrn J-CP, Nakagawa S, Ast M, Takeichi M, Redies C. 1999. Combinatorial expression of cadherins and the sorting of neurites in the tectofugal pathways of the chicken embryo. Neuroscience 90:985–1000. Yoon M-S, Puelles L, Redies C. 2000. Formation of cadherin-expressing brain nuclei in diencephalic alar plate subdivisions. J Comp Neurol 421:461– 480. Zeier HJ, Karten HJ. 1973. Connections of the anterior commissure in the pigeon (Columba livia). J Comp Neurol 150:201–216.