* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Programmed Cell Death in Plants: A Role for Mitochondrial
Survey
Document related concepts
Cell encapsulation wikipedia , lookup
Cell growth wikipedia , lookup
Signal transduction wikipedia , lookup
Cell culture wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cell membrane wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Endomembrane system wikipedia , lookup
Cytokinesis wikipedia , lookup
Transcript
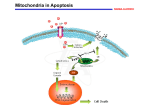
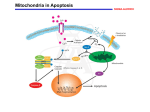
The Plant Cell, Vol. 18, 2097–2099, September 2006, www.plantcell.org ª 2006 American Society of Plant Biologists IN THIS ISSUE Programmed Cell Death in Plants: A Role for Mitochondrial-Associated Hexokinases ‘‘By the time I was born, more of me had died than survived. It was no wonder I cannot remember; during that time I went through brain after brain for nine months, finally contriving the one model that could be human, equipped for language.’’ This quote from Lewis Thomas (1992) speaks to the importance of programmed cell death (PCD) in human development. PCD is a fundamental process in both plants and animals (reviewed in Greenberg, 1996; Gilbert, 2001). A major pathway of PCD in animals is known as apoptosis, a highly regulated process of cell death that differs from autophagy (lysosomal degradation of organelles and certain proteins) and from necrosis (which results from acute tissue injury and provokes an inflammatory response in animals). Kerr et al. (1972) coined the term apoptosis from Greek words (apo ¼ from, and ptosis ¼ falling) used to describe plant leaf abscission, and they correctly hypothesized that it plays a broad role in normal cell metabolism and that abnormalities in this process contribute to a variety of diseases, including cancer. PCD plays a number of important roles in plant developmental pathways, including xylogenesis, formation of woody tissues in trees and perennials, leaf abscission, self-incompatibility, and defense responses to a wide variety of pathogens and environmental stresses. Researchers have noted a number of similarities between apoptosis in animals and PCD in plants (Greenberg, 1996), and comparing the functions of key components involved in these processes between kingdoms may serve fruitful for understanding the regulation of PCD in both. For example, cytochrome c release from mitochondria is a key event in apoptosis in animal cells and has also been shown to be an early event in PCD in plants (Balk et al., 1999; Balk and Leaver, 2001). Interestingly, it has been shown that preventing mito- chondrial cytochrome c release inhibits apoptosis in animal cells, in a manner that is intimately involved with the activity of mitochondrial hexokinase activity. In human tumor cells, elevated levels of mitochondria-bound hexokinases HK-I and HK-II prevent apoptosis and allow the cells to continue proliferating. Majewski et al. (2004) provided evidence that the protein kinase Akt functions in the suppression of apoptosis by eliciting translocation of hexokinase to the mitochondria. AzoulayZohar et al. (2004) showed that HK-I binds directly to the voltage-dependent anion channel (VDAC), an integral protein of the mitochondrial outer membrane that forms a large channel that transports ions, adenine nucleotides, and other metabolites into and out of the mitochondria. Binding of HK-1 to the VDAC induces closure of this channel, thereby preventing cytochrome c release and inhibiting apoptosis (AzoulayZohar et al., 2004). It also has been shown that excessive VDAC closure leads to mitochondrial swelling and apoptosis (Majewski et al., 2004; Rostovtseva et al., 2005). Because it is such a major factor in the life of a cell, perhaps it should come as no surprise that glucose metabolism (and hexokinase activity) also plays a key role in PCD. Some of the major routes of glucose metabolism are glycolysis (the principal pathway for cellular energy production in the form of ATP), the pentose phosphate pathway (which generates NADPH and precursors for a variety of anabolic pathways), and biosynthesis of structural and storage polysaccharides (e.g., starch, cellulose, and glycogen). The first step in glucose metabolism, ATP-dependent phosphorylation to yield glucose-6-phosphate, is catalyzed by hexokinase. Plants and animals contain a number of isozymes of hexokinase, which differ in subcellular localization and in their catalytic and regulatory properties, and selective expression of the various hexokinases is thought to be a major factor in the regulation of glucose metabolism (Wilson, 2003). Thus, hexokinase is well-positioned to be a major player in controlling life and death processes within the cell. In this issue of The Plant Cell, Kim et al. (pages 2341–2355) show that mitochondrial-associated hexokinase functions in the control of PCD in plants. The authors present evidence that virus-induced gene silencing (VIGS) of Hxk1, which encodes a mitochondrial hexokinase, caused PCD in Nicotiana benthamiana. In addition, overexpression of mitochondrial hexokinases in Arabidopsis led to enhanced survival of cells exposed to PCD-inducing conditions, such as treatment with H2O2. First, the authors examined expression of an Hxk1:GFP fusion construct and found that Hxk1:GFP was localized primarily in Phenotype of Hxk1-Silenced N. benthamiana. www.plantcell.org/cgi/doi/10.1105/tpc.106.046623 TRV:Hxk1-silenced plant (right) shows necrotic lesions, abnormal leaf development, and reduced plant height relative to control plant (left) infected with a TRV empty vector construct. 2098 The Plant Cell IN THIS ISSUE mitochondria, dependent on the presence of an N-terminal putative membrane anchor sequence. Expression analysis in wild-type plants showed that Hxk1 mRNA was most abundant in flowers, flower buds, and young leaves and was detected at lower levels in mature leaves, stems, and roots. Furthermore, Hxk1 expression was found to be induced in leaves following H2O2, heat treatment, or treatment with the chemical thapsigargin, which is known to induce apoptosis in animal cells. The authors next employed VIGS, using the tobacco rattle virus (TRV) vector described by Ratcliff et al. (2001), to examine the effect of suppression of Hxk1 expression in N. benthaniana. VIGS is based on the principle that virus vectors carrying host-plant-derived sequence inserts induce silencing of the corresponding genes in infected plants. The TRV vector designed by Ratcliff et al. (2001) mediates VIGS of endogenous plant genes in Nicotiana species without causing virus-induced symptoms and is able to target mRNAs in plant meristems. VIGS provides a means of rapidly assessing gene function through silencing without the need of creating transgenic plants; construction of the virus vector, including the target gene sequence, and monitoring symptoms on infected plants can be completed in a matter of weeks instead of months (Ratcliff et al., 2001). The TRV:Hxk1-silenced plants created by Kim et al. were found to have reduced hexokinase activity and exhibited necrotic lesions on leaves, abnormal leaf development, and reduced plant height, relative to control plants infected with a TRV empty vector (see figure on previous page). The areas surrounding necrotic lesions showed some of the hallmarks of PCD, such as DNA laddering and increased reactive oxygen species production, indicating that suppression of Hxk1 activity activates a PCD pathway in plants. Enhanced cell death in the Hxk1-silenced plants was also found to be accompanied by disruption of mitochondrial membrane potential and release of cytochrome c—two other hallmarks of PCD. Mitochondrial membrane potential, measured using a fluorescent lipophilic cationic dye (TMRM) that accumulates in mitochondria in proportion to the mitochon- drial membrane potential, declined in Hxk1silenced plants to 12.5% of that measured in controls (plants infected with the empty VIGS vector). Cytochrome c, measured by immunoblot analysis, was found to be associated mainly with the mitochondrial protein fraction in leaves of control plants but mainly with the cyotsolic fraction in Hxk1silenced plants, indicating that mitochondrial release of cytochrome c accompanied PCD in the Hxk1-silenced plants. Kim et al. also analyzed transgenic Arabidopsis plants that overexpressed the Arabidopsis hexokinase genes HXK1 or HXK2, which have N-terminal membrane anchor sequences and are localized primarily to the mitochondria. Isolated protoplasts from these plants were treated with H2O2 and a-picolinic acid (in separate experiments) to induce cell death, stained with propidium iodide, and assessed using flow cytometry (cells with disrupted membrane potential allow propidium iodide to enter the cell and fluoresce red). In each case, the percentage of live cells remaining following treatment was significantly higher in the HXK-overexpressing cells than in wild-type controls, suggesting that elevated hexokinase activity conferred partial protection from either H2O2- or a-picolinic acid– induced PCD. Finally, experiments were performed to examine the possibility that PCD in the Hxk1-silenced plants was not due to a direct effect of Hxk1 but occurred as an indirect result of perturbed carbon metabolism. Using a mitochondria-enriched fraction isolated from leaves, the authors showed that exogenous addition of Hxk1 blocked cytochrome c release induced by clotrimazole and H2O2. Clotrimazole is an antifungal azole derivative that acts to dissociate hexokinases from mitochondria in a dosedependent manner. The authors first showed that clotrimazole had no effect on cytochrome c release alone, but it enhanced or potentiated H2O2-induced cytochrome c release when the two agents were combined. The addition of Hxk1 blocked this induction of cytochrome c release in a dosedependent manner and was also dependent on the presence of the Hxk1 N-terminal membrane anchor, as recombinant protein lacking the N-terminal frag- ment had no effect. Other experiments showed that Hxk1 did not bind directly to cytochrome c, but rather, Hxk1 prevented disruption of mitochondrial membrane potential. These results strongly suggest that Hxk1 association with mitochondria inhibits cytochrome c release associated with PCD by acting to maintain mitochondrial membrane potential. The authors speculate that, as in animal cells, mitochondrial-associated hexokinase influences mitochondrial membrane potential and PCD by binding to the VDAC protein, which has been shown to play a role in both plant and animal PCD (Godbole et al., 2003). This intriguing possibility will need to be addressed in future experiments. Nancy A. Eckardt News and Reviews Editor [email protected] REFERENCES Azoulay-Zohar, H., Israelson, A., Abu-Hamad, S., and Shoshan-Barmatz, V. (2004). In selfdefence: Hexokinase promotes voltagedependent anion channel closure and prevents mitochondria-mediated apoptotic cell death. Biochem. J. 377, 347–355. Balk, J., and Leaver, C.J. (2001). The PET1CMS mitochondrial mutation in sunflower is associated with premature programmed cell death and cytochrome c release. Plant Cell 13, 1803–1818. Balk, J., Leaver, C.J., and McCabe, P.F. (1999). Translocation of cytochrome c from the mitochondria to the cytosol occurs during heat-induced programmed cell death in cucumber plants. FEBS Lett. 463, 151–154. Gilbert, S.F. (2001). Developmental Biology, 6th ed. (Sunderland, MA: Sinauer Associates). Godbole, A., Varghese, J., Sarin, A., and Mathew, M.K. (2003). VDAC is a conserved element of death pathways in plant and animal systems. Biochim. Biophys. Acta 1642, 87–96. Greenberg, J.T. (1996). Programmed cell death: A way of life for plants. Proc. Natl. Acad. Sci. USA 93, 12094–12097. Kerr, J.F., Wyllie, A.H., and Currie, A.R. (1972). Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26, 239–257. September 2006 2099 IN THIS ISSUE Kim, M., Lim, J.-H., Ahn, C.S., Park, K., Kim, G.T., Kim, W.T., and Pai, H.-S. (2006). Mitochondria-associated hexokinases play a role in the control of programmed cell death in Nicotiana benthamiana. Plant Cell 18, 2341–2355. Majewski, N., Noqueira, V., Bhaskar, P., Coy, P.E., Skeen, J.E., Gottlob, K., Chandel, N.S., Thompson, C.B., Robey, R.B., and Hay, N. (2004). Hexokinasemitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol. Cell 16, 819–830. Ratcliff, F., Martin-Hernansez, A.M., and Baulcombe, D.C. (2001). Tobacco rattle virus as a vector for analysis of gene function by silencing. Plant J. 25, 237–245. Rostovtseva, T.K., Tan, W., and Colombini, M. (2005). On the role of VDAC in apoptosis: Fact and fiction. J. Bioenerg. Biomembr. 37, 129–142. Thomas, L. (1992). The Fragile Species. (New York: Macmillan), p. 18. Wilson, J.E. (2003). Isozymes of mammalian hexokinase: Structure, subcellular localization and metabolic function. J. Exp. Biol. 206, 2049–2057. Programmed Cell Death in Plants: A Role for Mitochondrial-Associated Hexokinases Nancy A. Eckardt Plant Cell 2006;18;2097-2099 DOI 10.1105/tpc.106.046623 This information is current as of June 15, 2017 References This article cites 10 articles, 3 of which can be accessed free at: /content/18/9/2097.full.html#ref-list-1 Permissions https://www.copyright.com/ccc/openurl.do?sid=pd_hw1532298X&issn=1532298X&WT.mc_id=pd_hw1532298X eTOCs Sign up for eTOCs at: http://www.plantcell.org/cgi/alerts/ctmain CiteTrack Alerts Sign up for CiteTrack Alerts at: http://www.plantcell.org/cgi/alerts/ctmain Subscription Information Subscription Information for The Plant Cell and Plant Physiology is available at: http://www.aspb.org/publications/subscriptions.cfm © American Society of Plant Biologists ADVANCING THE SCIENCE OF PLANT BIOLOGY