* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Small Is Beautiful: Issues in Nanomedicine
Survey
Document related concepts
Psychopharmacology wikipedia , lookup
Pharmaceutical marketing wikipedia , lookup
Compounding wikipedia , lookup
Neuropharmacology wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Drug design wikipedia , lookup
Drug interaction wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Nicholas A. Peppas wikipedia , lookup
Theralizumab wikipedia , lookup
Pharmacognosy wikipedia , lookup
Patent medicine wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Drug discovery wikipedia , lookup
Transcript
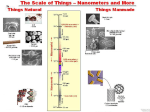
Small Is Beautiful: Issues in Nanomedicine1 Raj Bawa, MS, PhD Patent Law Department, Bawa Biotech LLC, Ashburn, Virginia, USA Department of Biological Sciences, Rensselaer Polytechnic Institute, Troy, New York, USA What a long strange trip it’s been for me regarding nano.2 Back in 2002, I presented on nanomedicine at the Canadian Space Agency, a few hours’ drive from Montreal. The audience was primarily composed of materials scientists and engineers. After a few excellent questions from the audience, I realized that physical scientists and pharmaceutical scientists view the nanoworld quite differently and that there is tension between the two camps. This was back then, but the state of affairs is the same today, maybe even more profound. Since then, nano has become more sectorized, with nanomedicine3 at the forefront along with nanoelectronics.4 However, there is still a clear need for “true” interdisciplinarily because of the different approaches of physical scientists versus pharmaceutical scientists with respect to generation, examination, analysis and discussion of nanomedicine data. 1 Copyright © 2016 Raj Bawa. All rights reserved. This excerpt by the author is derived from the preface of the following text: R. Bawa (Editor); G. F. Audette and B. E. Reese. (Assistant Editors): Handbook of Clinical Nanomedicine: Law, Business, Regulation, Safety and Risk, Pan Stanford Publishing, Singapore (2016) [ISBN 978‐ 981‐4669‐22‐1 (Hardcover), 978‐981‐4669‐23‐8 (eBook)] 2 What a Long Strange Trip It’s Been, subtitled The Best of the Grateful Dead, is a 1977 Warner Brothers Records compilation album by the Grateful Dead, the iconic American rock band formed in 1965 in Palo Alto, California. 3 Nanomedicine, an offshoot of nanotechnology, may be defined as the science and technology of diagnosing, treating and preventing disease and improving human health via nano. This exciting and interdisciplinary arena is driven by collaborative research, patenting, commercialization, business development and technology transfer within diverse areas such as biomedical sciences, chemical engineering, biotechnology, physical sciences, and information technology. Optimists see nano as an enabling technology, a sort of next industrial revolution that could enhance the wealth and health of nations. They promise that at least within the medical sector of nano, nanodrugs will be a healthcare game‐changer by offering patients access to personalized medicine. Pessimists, on the other hand, take a cautionary position, preaching instead a “go‐slow” approach, pointing to a lack of sufficient scientific information on health risks and general failure on the part of regulatory agencies and patent offices. As usual, the reality is somewhere between such extremes. Whatever your stance, nano has already permeated virtually every sector of the global economy, with potential applications consistently inching their way into the marketplace. Nano continues to evolve and play a pivotal role in various industry segments, spurring new directions in research and development and translational efforts. 4 The prefix “nano” in the SI measurement system denotes 10−9 or one‐billionth. There is no firm consensus over whether the prefix “nano” is Greek or Latin. While the term “nano” is often linked to the Greek word for “dwarf,” the ancient Greek word for “dwarf” is actually spelled “nanno” (with a double “n”) while the Latin word for dwarf is “nanus” (with a single “n”). While a nanometer refers to one‐billionth of a meter in size (10−9 m = 1 nm), a nanosecond refers to one billionth of a second (10−9 s = 1 ns), a nanoliter refers to one billionth of a liter (10−9 l = 1 nl) and a nanogram refers to one billionth of a gram (10−9 g = 1 ng). The diameter of an atom ranges from about 0.1 to 0.5 nm. Some other interesting comparisons: fingernails grow around a nanometer/second; in the time it takes to pick a razor up and bring it to your face, the beard will have grown a nanometer; a single nanometer is how much the Himalayas rise in every 6.3 seconds; a sheet of newspaper is about 100,000 nanometers thick; it takes 20,000,000 nanoseconds (50 times per second) for a hummingbird to flap its wings once; a single drop of water is ~50,000 nanoliters; a grain of table salt weighs ~50,000 nanograms. Ever since that talk at the Canadian Space Agency in 2002, I have been involved in almost all aspects of nanoscience, nanomedicine and nanotechnology—patent prosecution, teaching, research, FDA regulatory filings, conference organizer and speaker. I have intimately seen the evolution of nanomedicine. I have cheered on the cutting‐edge discoveries and inventions but also stood up to criticize inept governmental regulatory policies, spotty patent examination at patent offices, hyped‐up press releases from eminent university labs and inaccurate depiction of nano by the press and politicians.5 While the nano die was cast long ago, it is well entrenched now and there is no turning back. Although the air may be thick with news of nano‐breakthroughs and a hot topic for discussion in industry, pharma, patent offices and regulatory agencies, the average citizen on the street knows very little about what constitutes a nanoproduct, a nanomaterial or a nanodrug. This was apparent to me early on when the CBS station in Albany, NY, while interviewing me for a story on nanotechnology, approached people on the street and asked them to define nano. To my amazement, not a single person could come up with even a functional description of the term. At the time, I was well aware that there were close to two thousand marketed products that incorporated nano in some fashion. This issue persists. Mind you, these interviews were taking place a few blocks from the massive, world‐class education and research complex comprising Albany Nanotech (now SUNY Polytechnic Institute’s Colleges of Nanoscale Science and Engineering), where billions have been invested each year since the early 1990s. Recognizing that the so‐called “nanorevolution” will have limited impact unless all stakeholders, especially the public, are fully on board, I immediately initiated an annual nano‐conference that continues to this day. This annual conference is free to all attendees and is sponsored by the American Bar Association. For the first dozen years was held at Rensselaer Polytechnic Institute in Troy, NY, while the latest one was held this fall at Albany College of Pharmacy and Health Sciences. Louis Pasteur famously said: “Science knows no country, because knowledge belongs to humanity, and is the torch which illuminates the world. Science is the highest personification of the nation because that nation will remain the first which carries the furthest the works of thought and intelligence.” Perhaps, nothing is truer in this regard than nanoscience, nanotechnology and nanomedicine. However, we are in a rapidly changing, globalized world— politically, economically, societally, environmentally and technologically. At the present time in our history, national boundaries are rendered artificial or eroding, there are few true leaders on the global stage, the world power dynamics are shifting and the outdated bureaucratic structures firmly in place are unable to deal effectively with the emerging knowledge economy. These changes are also causing a certain degree of chaos in the world of nano. From my 5 Too often though, start‐ups, academia and companies exaggerate basic research developments as potentially revolutionary advances and claim these early‐stage discoveries as confirmation of downstream novel products and applications to come. Nanotechnology’s potential benefits are frequently overstated or inferred to be very close to application when clear bottlenecks to commercial translation exist. Many have desperately tagged or thrown around the “nano” prefix to suit their purpose, whether it is for federal research funding, patent approval of the supposedly novel technologies, raising venture capital funds, running for office or seeking publication of a journal article. perspective, there is a combination of excitement, potential, confusion, hype, and misinformation in this regard—all of this seen more than in other fields when they were evolving. It is true that in the heady days of any new, emerging technology, definitions tend to abound and are only gradually documented via reports, journals, books and dictionaries. Ultimately, standard‐setting organizations like the International Organization for Standardization (ISO) and American Society for Testing and Materials (ASTM) International produce technical specifications. This evolution is typical and essential, as the development of terminology is a prerequisite for creating a common language needed for effective communication in any field. Clearly, a similar need for an internationally agreed definition for key terms like nanotechnology, nanoscience, nanomedicine, nanobiotechnology, nanodrug, nanotherapeutic, nanopharmaceutical and nanomaterial, has gained urgency. Nomenclature, technical specifications, standards, guidelines and best practices are critically needed to advance nanotechnologies in a safe and responsible manner. Contrary to some commentators, terminology does matter because it prevents misinterpretation and confusion. It is essential for research activities, harmonized regulatory governance, accurate patent searching and prosecution, standardization of procedures, manufacturing and quality control, assay protocols, decisions by granting agencies, effective review by policymakers, ethical analysis, public dialogue, safety assessment, and more. Also, nomenclature is critical to any translational and commercialization efforts. All definitions of nanotechnology based on size or dimensions must be dismissed, especially in the context of medicine and pharmaceutical science.6 Viable sui generis definition of nano having a bright‐line size range as applied to nanodrugs blurs with respect to what is truly nanoscale; it is unnecessary, misleading, and in fact, may never be feasible. Since the 100 nm boundary has no solid scientific or legal basis, this series will avoid using the sub‐100 nm inaccurate and random definition of nano.7 6 Nanoscale therapeutics may have unique properties (nanocharacter) that can be beneficial for drug delivery but there is no specific size or dimensional limit where superior properties reside. Hence, a size limitation below 100 nm cannot be touted as the basis of novel properties of nanotherapeutics. In fact, properties other than size can also have a dramatic effect on the nanocharacter of nanodrugs: shape/geometry, zeta potential, specific nanomaterial class employed, composition, delivery route, crystallinity, aspect ratio, surface charge, etc. See: Bawa, R. (2016). What’s in a name? Defining “nano” in the context of drug delivery. In: Bawa, R., Audette, G. and Rubinstein, I. eds. Handbook of Clinical Nanomedicine: Nanoparticles, Imaging, Therapy, and Clinical Applications, Chapter 6, Pan Stanford Publishing, Singapore. 7 Definitions along these lines should instead be employed: The design, characterization, production, and application of structures, devices, and systems by controlled manipulation of size and shape at the nanometer scale (atomic, molecular, and macromolecular scale) that produces structures, devices, and systems with at least one novel/superior characteristic or property.” This flexible definition has four key features: (i) It recognizes that the properties and performance of the synthetic, engineered “structures, devices, and systems” are inherently rooted in their nanoscale dimensions. The definition focuses on the unique physiological behavior of these “structures, devices, and systems” that is occurring at the nanoscale; it does not focus on anyshape, aspect ratio, specific size or dimensionality. (ii) It focuses on “technology” that has commercial potential from a consumer perspective, not “nanoscience” or “basic R&D” conducted in a lab setting that lacks commercial applications. (iii) The “structures, devices, and systems” that result from or incorporate nano must be “novel/superior” compared to their bulk/conventional counterparts. (iv) The concept of “controlled manipulation” (as compared to “self‐assembly”) is critical. Also see: See: Bawa, R. (2007). Patents and nanomedicine. Nanomedicine (London), 2(3), 351–374. Advances in drug delivery8 are leading all developments in nanomedicine. The following points serve to highlight the major impact of nanotechnology to drug delivery: • Novel nanodrugs and nanocarriers are being developed that address fundamental problems of traditional drugs because of the ability of these compounds to overcome poor water solubility issues, alter unacceptable toxicity profiles, enhance bioavailability, and improve physical/chemical stability. Additionally, via tagging with targeting ligands, these nanodrug formulations can serve as innovative drug delivery systems for enhanced cellular uptake or localization of therapeutics into tissues of interest (i.e., site‐specific targeted delivery). Various FDA‐approved liposomal, solid nanoparticle‐based, antibody‐drug conjugate (ADC) and polymer‐drug conjugate delivery platforms overcome associated issues such as (i) low solubility (Abraxane), (ii) extremely high drug toxicity (Brentuximab vedotin and Trastuzumab emtansine), and (iii) side effects related to high doses of free drug (Doxil, Marqibo, DaunoXome). • Reformulation of old, shelved therapeutics into nanosized dosage forms could offer the possibility of adding new life to old therapeutic compounds. Classic examples include drugs developed as nanocrystalline products (Rapamune, Emend, Triglide). • Coupled with advances in pharmacogenomics, smart nanomachines, information technology and personalized medicine, upcoming innovations in nanomedicine may even generate multifunctional entities enabling simultaneous diagnosis, delivery and monitoring of APIs. Emerging technologies are particularly problematic for governmental regulatory agencies, given their independent nature, slow response rate, significant inertia and a general mistrust of industry. Major global regulatory systems, bodies and regimes regarding nanomedicines are not fully mature, hampered in part by a lack of specific protocols for preclinical development and characterization. Additionally, in spite of numerous harmonization talks and meetings, there is a lack of consensus on the different procedures, assays and protocols to be employed during pre‐clinical development and characterization of nanomedicines. On the other hand, there is a rise of diverse nanospecific regulatory arrangements and systems, contributing to a dense global regulatory landscape, full of gaps and devoid of central coordination. It is often observed that governmental regulatory bodies lack technical and scientific knowledge to support risk‐ 8 Although numerous nanodrugs have been routinely used in medicinal products for decades without any focus or even awareness of their nanocharacter, it is only within the past two decades that they have been highlighted due to their potential of revolutionizing drug delivery. Obviously, the Holy Grail of any drug delivery system is to deliver the correct dose of a particular active agent to a specific disease or tissue site within the body while simultaneously minimizing toxic side effects and optimizing therapeutic benefit. This is often not achievable via conventional drugs. However, the potential to do so may be greater now via engineered nanodrugs. Often, nanodrugs transport active agents to their target binding sites (ligands, receptors, active sites, etc.) to impart maximum therapeutic activity with maximum safety (i.e., protect the body from adverse reactions) while preventing the degradation/denaturation/inactivation of the active agent during delivery/transit. Targeting can be achieved or enhanced by (i) linking specific ligands or molecules (e.g., antibodies, glycoproteins, etc.) to the carrier, or (ii) by altering the surface characteristics of the carrier. Furthermore, therapeutics that have side effects due to triggering an immune response (e.g., complement activation) or cleared by the reticuloendothelial (RES) system can be entrapped, encapsulated or embedded within a nanoparticle coat or matrix. based regulation, thereby leaving a significant regulatory void. In fact, the “baby steps” undertaken by the FDA over the past decade have led to regulatory uncertainty. There are potentially serious and inhibitory consequences if nanomedicine is overregulated. A balanced approach is required here, at least on a case‐by‐case basis, which addresses the needs of commercialization against mitigation of inadvertent harm to patients or the environment. Obviously, not everything “nanomedical” needs to be regulated. However, more is clearly needed from regulatory agencies like the FDA and EMA than a stream of guidance documents that are generally in draft format, position papers that lack any legal implication, presentations that fail to identify key regulatory issues and policy papers that are often short on specifics. There is a very real need for regulatory guidelines that follow a science‐based approach (not policy‐based) that are responsive to the associated shifts in knowledge and risks. However, is nano arriving faster than other previous technologies like steam engines, telephones, digital computers, genetic engineering and synthetic biology? Is it proceeding too rapidly or in a vacuum to generate any meaningful discussion, analysis or formulation of FDA and EPA regulations, safety guidelines, governmental policy, patent prosecution parameters? Ultimately, the true value of a particular nanoproduct lies in its clinical utility balanced against any potential adverse effects. Therefore, effective translation of nanomedicine candidates requires a “technological push” coupled to a “clinical pull” guided or catalyzed by regulatory agencies, patent offices and venture community. All of this has to be bridged by logical intermediary data that mechanistically demonstrate the efficacy and biosafety. Whether the FDA eventually creates new regulations, tweaks existing ones, or establishes a new regulatory center to handle nanoproducts, for the time being it should at least look at nanoproducts on a case‐by‐case basis. The FDA should not attempt regulation of nanomedicine by applying existing statutes alone, especially where scientific evidence suggests otherwise. Incorporating nanomedicine regulation into the current regulatory scheme is unwise. Regulation of nanotech must balance innovation and R&D with the principle of ensuring maximum public health protection and safety. Moreover, it is important to note that regulatory oversight must evolve in concert with newer generations of nanomedical products. Two critically important and interrelated regulatory law issues topics include (i) nanosimilars (which combine generic drugs and nanocarriers as innovative excipient); and (ii) non‐biological complex drugs (NBCDs) that highlight critical issues in specific formulations (e.g., iron oxide nanoparticles, glatiramoids, liposomes, polymeric micelles). These are. Other topics are transnational regulatory harmonization, generic biowaivers, combination products (nanotheranostics), patent law, FDA law, ethics, personalized medicine, risk analysis, toxicology, nano‐characterization and commercialization activities. Let me highlight the translational issues and efforts pertaining to nanomedical products by using the classic example of drug R&D. Creating drugs today is time‐consuming, expensive and enormously challenging. De novo drug discovery and development is a10‐17 year process from idea to marketed drug. It may take up to a decade for a drug candidate to enter clinical trials with less than 10% of the tested candidates in trials reaching the market. In fact, more drugs come off patent each year than approved by the FDA. According to a 2014 study by the Tufts Center for the Study of Drug Development, developing a new prescription medicine that gains marketing approval is estimated to cost nearly $2.6 billion. It is clear to everyone that in the past decade, great strides have been made in basic science and research. This is obviously critical to any advanced society. However, the enormous medical advances that should have come from the large public investment in biomedical research are largely absent from a translation point‐of‐view. All stakeholders—pharma, patients, academia, NIH—have suffered and are to blame for the valley of death. Each needs to re‐examine their role and become an active, full partner in the drug development ecosystem so that translation is more robust. Similarly, although great strides have been made in nanomedicine generally at the “science” level, especially with respect to drug delivery and imaging, it continues to be dogged by challenges and bottlenecks at the “translational” level. In pharma, translational research involves the many elements that contribute to the successful conversion of an idea into a drug. Translation of drug products is a challenge faced by pharma at various levels. Bridging the chasm between drug discovery research activities and the successful translation of a drug to the market is a daunting task that requires varying degree of participation from key stakeholders— pharma, academia, nonprofit and for‐profit institutions, federal agencies and regulatory bodies, diseases foundations and patients. So far, the process of converting basic research in nanomedicine into commercially viable products has been difficult. Securing valid, defensible patent protection from the patent offices along with clearer regulatory/safety guidelines from regulatory agencies is critical to commercialization. This has been a mixed bag at best and much more is warranted. If translation of nanomedicine is to be a stellar success, it is important that some order, central coordination and uniformity be introduced at the transnational level. It is true that this decade has witnessed relatively more advances and product development in nanomedicine than the previous. In this context, many point to the influence of nanomedicine on the pharmaceutical, device and biotechnology industries. One can now truly say that R&D is in full swing and novel nanomedical products are starting to arrive in the marketplace. It is hoped that nanomedicine will eventually blossom into a robust industry. We will have to see whether there will be giant technological leaps (that can leave giant scientific, ethical and regulatory gaps) or paradigm‐shifting advances (that necessitate extraordinary proof and verification). In the meantime, tempered expectations are in order. Key players must come together on a global platform to address some of these crucial issues affecting effective translational efforts. It is important that the public’s desire for novel nanomedical products, venture community’s modest investment, federal infusion of funds and big pharma’s lingering interest in nanomedicine continues. In the end, the long‐term prognosis and development of nanomedicine will hinge on effective nanogovernance, issuance of valid patents, clearer safety guidelines, public transparency and full commitment of all the key players involved—big pharma, academia, governmental regulatory agencies, policy‐makers, the venture community and the consumer‐patient. Together, these stakeholders can help drive progress and make the future happen faster. Science fiction may become science fact. Not only is this possible, but it will happen. The Times They Are a‐Changin’.