* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Dr. Ali Ebneshahidi
Supramolecular catalysis wikipedia , lookup
History of chemistry wikipedia , lookup
Organic chemistry wikipedia , lookup
Acid–base reaction wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Molecular orbital diagram wikipedia , lookup
Water splitting wikipedia , lookup
Click chemistry wikipedia , lookup
Computational chemistry wikipedia , lookup
Electrochemistry wikipedia , lookup
Chemical reaction wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Metallic bonding wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
Implicit solvation wikipedia , lookup
Molecular dynamics wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Marcus theory wikipedia , lookup
George S. Hammond wikipedia , lookup
Abiogenesis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Electrolysis of water wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Atomic theory wikipedia , lookup
History of molecular theory wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Chemical bond wikipedia , lookup
Energy applications of nanotechnology wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Transition state theory wikipedia , lookup
Physical organic chemistry wikipedia , lookup
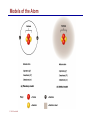
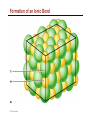
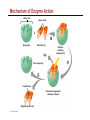
Dr. Ali Ebneshahidi © 2009 Ebneshahidi A . Introduction Chemistry – science that deals with the composition of substances and the changes that take place in their composition. Organic chemistry – chemistry that deals with organic substances (those that contain carbon and hydrogen). Biochemistry - chemistry of living organisms; essential for understanding physiology because body functions involve chemical changes that occur within cells. Matter – anything that has weight (or mass) and takes up space. It can be solids, liquids, or gases. © 2009 Ebneshahidi Energy Energy – the ability to do work . Potential energy (PE) is stored energy in matters ; Kinetic energy (KE) is working energy produced by the motion of matters. Energy occurs in 4 forms in the human body: chemical, electrical, radiant, and mechanical energy. Chemical energy is the most important form in terms of actually driving chemical reactions. © 2009 Ebneshahidi Models of the Atom © 2009 Ebneshahidi Atomic number (AN) = number of protons = number of electrons. Atomic weight (AW) = number of protons + number of neutrons. © 2009 Ebneshahidi IONS In addition to neutrons, the electrons of atoms tend to change also – atoms that have either lost or gained electrons are called ions. Atoms that have lost electrons (as a result, now contain more p+ than e-) are called cations which carry positive charges, while atoms that have gained excessive electrons (as a result, now contain more ethan p+) are called anions which carry negative charges. © 2009 Ebneshahidi Chemically Reactive Elements Reactive elements do not have their outermost energy level fully occupied by electrons. © 2009 Ebneshahidi Bonding of atoms Ionic bonding = formed by attraction of opposite charges of a cation and an anion. (e.g. Na+ + Cl- →NaCl) © 2009 Ebneshahidi Formation of an Ionic Bond © 2009 Ebneshahidi Covalent bond formed by sharing of electrons between two atoms (e.g. Cl + Cl →Cl2). The strongest type of bonding. © 2009 Ebneshahidi Hydrogen Bond formed by weak attraction between H+ and nitrogen (N) or oxygen (O) [e.g. H of a water molecule attracting to O of another water molecule]. The weakest type of bonding. © 2009 Ebneshahidi Four Types of Chemical Reactions Chemical reactions involve the formation, breaking, or rearrangement of chemical bonds. There are 4 general types: Dehydration synthesis: A + B → AB + water Decomposition (or hydrolysis): AB + water → A + B Exchange: AB + CD → AD + CB Reversible: A + B < - - - > AB © 2009 Ebneshahidi The rate of Chemical Reactions size of reacting molecules: smaller molecules have greater kinetic energy which produces faster reaction rate. Temperature: higher temperature creates greater kinetic energy and faster reaction rate. Concentration of reactants: higher concentration produces faster rate . Presence of catalysts: inorganic catalysts or organic catalysts (enzymes) increase reaction rate. © 2009 Ebneshahidi Electrolytes = compounds that release ions when dissolved in water (e.g. NaCl + water → Na+ + Cl- ). Acids = electrolytes that release H+ (e.g. H2 CO3 → H+ + HCO3- ). Bases = electrolytes that release anions that can combine with H+ (e.g. NaOH → Na+ + OH- ). Salts = substances formed by the reaction between an acid and a base (e.g. HCl + NaOH → H2O + NaCl ). © 2009 Ebneshahidi PH measurement of H+ concentration in a solution - More H+ = lower PH = more acidic - Less H+ = higher pH = less acidic -Ph scale is form 0 to 14, where the midpoint (pH 7.0) is neutral. From pH 0 to 6.9, it is acid; while from pH 7.1 to 14 is base. © 2009 Ebneshahidi PH Scale © 2009 Ebneshahidi Organic substances = chemicals that contain C and H (e.g. Carbohydrates, Protein, Fat, and nucleic acid). Inorganic substances = chemicals that do not contain C and H (e.g. table salt or NaCl, carbon dioxides or CO2 , ammonia or NH3). (Most inorganic substances are small, electrolytes and usually use ionic bonding, while most organic substances are large, non electrolytes, and usually use covalent bonding ). © 2009 Ebneshahidi An Organic Compound (cholestrol) © 2009 Ebneshahidi Protein Figure 2.3 © 2009 Ebneshahidi Carbohydrate © 2009 Ebneshahidi Nucleic Acid © 2009 Ebneshahidi Solution and concentration When a substance is dissolved in a liquid (ex. water), a solution is formed. The substance that is dissolved is the solute and the liquid in which the dissolution occurred is the solvent. Concentration: The measure of dissolution of a particular solute in a given volume of solvent. it is measured in molarity. Molarity: The number of solute molecule per unit volume of solution. Buffer: A substance that can react with an acid or a base and thus resist a change in PH. © 2009 Ebneshahidi Tonicity the ability of a solution to change the tone or shape of cells by changing their internal H2O volume. - Hypertonic: solutions with higher osmotic pressure cells in a Hypertonic solution lose H2O and shrink. - Hypotonic : solution with a lower osmotic pressure cells in hyportonic solution gain H2O and swell. - Isotonic : same tonicity - cell in isotonic solutions neither gain, nor lose H2O. © 2009 Ebneshahidi The effect of solutions of varying tonicities on red blood cell © 2009 Ebneshahidi Enzymes 1. Are always made of globular proteins. 2. Can promote the rate of chemical reactions by billions of times. 3. Can lower the activation energy – energy necessary to start a reaction – resulting in a conservation of energy. © 2009 Ebneshahidi 4. Are usually reusable or recycled. 5. Are always very specific – using its active site, each enzyme is designed to bind to only one specific substance, the substrate and rapidly transforms the substrate into a product. 6. Many enzymes would not achieve their optimum efficiency unless they are bound to a cofactor (i.e. ions, metals) or to a coenzyme (organic cofactors such as vitamins). 7. Most enzymes' names end with "ase“ (ex. DNAse, Sucrase). © 2009 Ebneshahidi Mechanism of Enzyme Action Active site Amino acids 1 Enzyme (E) Substrates (s) H20 Enzymesubstrate complex (E–S) 2 Free enzyme (E) 3 Peptide bond Internal rearrangements leading to catalysis Dipeptide product (P) © 2009 Ebneshahidi Many factors affect enzyme activity: Since all enzymes are made of globular proteins, and proteins are made of amino acids linked by peptide bonds, enzymes can be affected or denatured very easily. Factors that could affect or denature enzymes include heat, radiation , electricity, certain chemical substances, and extreme PH. © 2009 Ebneshahidi Metabolism Anabolic metabolism uses dehydration synthesis reaction to build large molecules from small molecules. Each reaction releases a water molecule and requires energy input Example – monosaccharide + energy → polysaccharide + water amino acids + energy → protein + water. Synthesis and Hydrolysis of Sucrose : © 2009 Ebneshahidi Catabolic metabolism Uses hydrolysis (or decomposition) reaction to break up large molecules into smaller molecules. Each reaction requires a water molecule and releases energy. Example -energy. © 2009 Ebneshahidi triglyceride + water → fatty acids + Adenosine Triphosphate (ATP) High- energy molecule that is derived from the nucleotide, adenine. Contains 3 phosphate groups (PO4) and high-energy chemical bonds that each time the bonds are broken, a large amount of energy is generated. Energy is released by ATP is broken down by hydrolysis reaction: ATP + water → ADP + PO4+ energy [ADP = adenosine diphosphate] ADP + water → AMP + PO4 + energy [AMP = adenosine monophosphate] © 2009 Ebneshahidi ATP © 2009 Ebneshahidi