* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter Two:
Debye–Hückel equation wikipedia , lookup
Chemical element wikipedia , lookup
Atomic orbital wikipedia , lookup
Isotopic labeling wikipedia , lookup
Electronegativity wikipedia , lookup
Metallic bonding wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Coordination complex wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
List of phenyltropanes wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Atomic nucleus wikipedia , lookup
Electron configuration wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Chemical bond wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Drug discovery wikipedia , lookup
Organic chemistry wikipedia , lookup
History of chemistry wikipedia , lookup
History of molecular theory wikipedia , lookup
Homoaromaticity wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Atomic theory wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
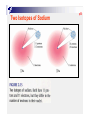
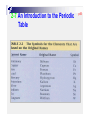
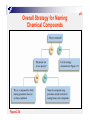
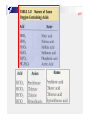
p38 Chapter Two: ATOMS, MOLECULES, AND IONS p39 2-1 The Early History of Chemistry p41 2-2 Fundamental Chemical Laws Three Important Laws Law of conservation of mass Mass Law A is neither created nor destroyed of definite proportion given compound always contains exactly the same proportion of elements by mass Three Important Laws (continued) Law of multiple proportions When two elements form a series of compounds, the ratios of the masses of the second element that combine with 1 gram of the first element can always be reduced to small whole numbers. P42 Ex 2.1 Illustrating the Law of Multiple Proportions The following data were collected for several compounds of nitrogen and oxygen: Sow how these data illustrate the law of multiple proportions. Solution: p43 2-3 Dalton’ s Atomic Theory Dalton’ s Atomic Theory (1808) Each element is made up of tiny particles called atoms. Dalton’ s Atomic Theory (1808) (continued) The atoms of a given element are identical; the atoms of different elements are different in some fundamental way or ways. Dalton’ s Atomic Theory (continued) Chemical compounds are formed when atoms combine with each other. A given compound always has the same relative numbers and types of atoms. Dalton’ s Atomic Theory (continued) Chemical reactions involve reorganization of the atoms - changes in the way they are bound together. The atoms themselves are not changed in a chemical reaction. Avogadro’ s Hypothesis (1811) At the same temperature and pressure, equal volumes of different gases contain the same number of particles. 5 liters of oxygen 5 liters of nitrogen Same number of particles! Representing Gay-Lussac’ s Results p43 Representing Gay-Lussac’ s Results p45 2-4 Early Experiments to Characterize the Atom p45 The Electron Figure 2.7 A Cathode-ray tube. The fast-moving electrons excite the gas in the tube, causing a glow between the electrodes. The green color in the photo is due to the response of the screen (coated with zinc sulfide) to the electron beam. p47 p47 Early Experiments to Characterize the Atom J. J. Thomson - postulated the existence of electrons using cathode ray tubes. Ernest Rutherford - explained the nuclear atom, containing a dense nucleus with electrons traveling around the nucleus at a large distance. Cathode Ray Tube p48 Milliken Oil Drop Experiment 2-5 The Modern View of Atomic Structure p49 The atom contains: electrons protons: found in the nucleus; positive charge equal in magnitude to the electron’ s negative charge. neutrons: found in the nucleus; no charge; virtually same mass as a proton. The Nuclear Atom Radioactivity p49 Rutherford’ s Gold Foil Experiment p50 Nuclear Atom Viewed in Cross Section p50 Two Isotopes of Sodium Ex 2.2 Writing the Symbols for Atoms P52 Write the symbol for the atom that has an atomic number of 9 and a mass number of 19. How many electrons and how many neutrons does this atom have? Solution: 2-6 Molecules and Ions p52 p53 Formation of Ionic Compounds p54 p55 Molecular vs. Ionic Compounds Covalent Bonding 2-7 An Introduction to the Periodic Table p55 p56 The Periodic Table 2-8 Naming Simple Compounds p57 Binary Ionic Compounds (Type I) p58 p58 Naming Binary Ionic Compounds P58 Ex 2.3 Naming Type I Binary Compounds Name each binary compound. a. CsF Solution b. AlCl3 c. LiH P59 Ex 2.4 Formulas from Names for Type I Binary Compounds Given the following systematic names, write the formula foe each compound: a. Potassium iodide, b. Calcium oxide, c. Gallium bromide Solution: Formulas from Names Binary Ionic Compounds (Type II) p59 Ex 2.5 Naming Type II Binary Compounds 1. Give the systematic name for each of the following compounds: a. CuCl b. HgO c. Fe2O3 2. Given the following systematic names, write the formula for each compound: a. Manganese(IV) oxide b. Lead(II) chloride P59 Solution: p60 Ex 2.6 Naming Binary Compounds 1. Give the systematic name for each of the following compounds: a. CoBr2 b. CaCl2 c. Al2O3 2. Given the following systematic names, write the formula for each compound: a. Chromium(III) chloride b. Gallium iodide P60 p61 S0lution: p61 Figure 2.22 The common cations and anions p62 P62 Ex 2.7 Naming Compounds Containing Polyatomic Ions 1. Give the systematic name for each of the following compounds: a. Na2SO4, b. KH2PO4, c. Fe(NO3)3, d. Mn(OH)2, e. Na2SO3, f. Na2CO3 2. Given the following systematic names, write the formula for each compound: a. Sodium hydrogen carbonate, b. Cesium perchlorate c. Sodium hypochlorite d. Sodium selenate e. Potassium bromate Solution: p63 p63 Ex 7.7 Solution (continued) p63 Binary Covalent Compounds (Type III) p64 N2O NO P64 Ex 2.8 Naming Type III Binary Compounds 1. Name each of the following compounds: a. PCl5, b. PCl3, c. SO2 2. From the following systematic names, write the formula for each compound: a. Sulfur hexafluoride, b. Sulfur trioxide, c. Carbon dioxide Solution: p64 Flowchart for Naming Binary Compounds Figure 2.23 p65 Overall Strategy for Naming Chemical Compounds Figure 2.34 p65 P65 Ex 2.9 Naming Various Types of Compounds 1. Give the systematic name for each of the following compounds: a. P4O10, b. Nb2O5, c. Li2O2, d. Ti(NO3)4 2. Given the following systematic names, write the formula for each compound: a. Vanadium(V) fluoride, b. Dioxygen difluoride, c. Rubidium peroxide, d. Gallium oxide p66 Solution: p66 Ex 2.9 Solution (continued): Flowchart for Naming Acids Figure 2.25 p67 p67 p67