* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Ancient Gunpowder and Novel Insights Team Up Against
Survey
Document related concepts
Electrocardiography wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Remote ischemic conditioning wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Jatene procedure wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Cardiac surgery wikipedia , lookup
Coronary artery disease wikipedia , lookup
Heart failure wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Myocardial infarction wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
Transcript
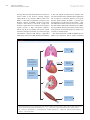
JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY VOL. 66, NO. 15, 2015 ª 2015 BY THE AMERICAN COLLEGE OF CARDIOLOGY FOUNDATION ISSN 0735-1097/$36.00 PUBLISHED BY ELSEVIER INC. http://dx.doi.org/10.1016/j.jacc.2015.08.028 EDITORIAL COMMENT Ancient Gunpowder and Novel Insights Team Up Against Heart Failure With Preserved Ejection Fraction* Walter J. Paulus, MD, PHD,y Loek van Heerebeek, MD, PHDyz E xercise intolerance due to dyspnea or muscle These beneficial effects of sodium nitrite on exercise fatigue is the most common clinical symptom hemodynamics are consistent with novel insights on in heart failure with preserved ejection frac- inadequate myocardial availability of NO and cyclic tion (HFpEF). Improvement of exercise tolerance is therefore correctly used as the primary endpoint in recent HFpEF trials. In this issue of the Journal, Borlaug et al. (1) report on invasively established improvement of exercise hemodynamics when HFpEF patients received an intravenous infusion of sodium nitrite before the exercise stress test. SEE PAGE 1672 guanosine monophosphate (cGMP) in HFpEF (3,4). EXERCISE INTOLERANCE AND NITRITE High left ventricular (LV) diastolic stiffness, LV systolic shortening deficit, chronotropic incompetence, and deficient pulmonary or systemic vasodilation have all been proposed as mechanisms critically limiting exercise tolerance in HFpEF (5,6). Their involvement in the beneficial effect of nitrite deserves closer scrutiny. Inorganic nitrite originates from the reduction of First among the proposed mechanisms for exercise inorganic nitrate, a compound known since ancient intolerance in HFpEF is high LV diastolic stiffness (5), times as gunpowder. In the human body, both inor- which elicits a prompt and large increase in PCWP (7), ganic nitrate and nitrite are converted to nitric oxide even at low-level exercise, as illustrated in the study (NO), with nitrate requiring 2 reductions and nitrite by Borlaug et al. (1). High LV diastolic stiffness results only 1 reduction (2). In contrast to organic nitrates, from a modest deposition of interstitial collagen and tolerance does not develop because there is no need from cardiomyocytes with a less distensible cyto- for enzymatic separation from small carbon-based skeletal protein titin (4,8,9). Low titin distensibility molecules (2). The improvement of exercise toler- is presumed to derive from hypophosphorylation ance observed by Borlaug et al. (1) was evident at a by protein kinase G (PKG), a presumption supported comparable workload from a lower pulmonary capil- by in vitro experiments in which PKG administration lary wedge pressure (PCWP), pulmonary artery pres- corrects distensibility of isolated HFpEF cardiomyo- sure, and systemic vascular resistance (SVR), as well cytes and by the presence in HFpEF myocardium of as from increased cardiac output (CO), left ventricular low PKG activity and an impressive 10-fold reduction stroke work (LVSW), oxygen consumption (V O2), and of the concentration of cGMP, the cofactor of PKG (3). slope of the D CO/D VO 2 relation (exercise factor). The low cGMP concentration derives from deficient upstream activation of soluble guanylate cyclase by *Editorials published in the Journal of the American College of Cardiology NO and of particulate guanylate cyclase by natriuretic reflect the views of the authors and do not necessarily represent the peptides. In HFpEF myocardium, both NO and natri- views of JACC or the American College of Cardiology. uretic peptide contents are low, respectively, because From the yInstitute for Cardiovascular Research, VU University Medical of microvascular endothelial inflammation induced Center, Amsterdam, the Netherlands; and the zOnze Lieve Vrouwe by comorbidities and because of low wall stress in a Gasthuis, Amsterdam, the Netherlands. Supported by grants from the European Commission (FP7-Health-2010; MEDIA-261409) and CVON concentrically remodeled left ventricle (3,4). In the (ARENA, RECONNECT). Both authors have reported that they have no absence of LV volume measurements, the current relationships relevant to the contents of this paper to disclose. study (1) did not directly assess diastolic LV stiffness. 1684 Paulus and van Heerebeek JACC VOL. 66, NO. 15, 2015 OCTOBER 13, 2015:1683–6 Nitrite for Exercise Intolerance in HFpEF The large increase after nitrite infusion in exercise LV of titin can explain the improved LV diastolic stiff- stroke volume (7 ml), however, strongly suggests ness after nitrite infusion. Nitrite infusion apparently improvement of LV diastolic stiffness with lower did not improve LV diastolic stiffness at rest given PCWP (11 mm Hg) at comparable or larger LV end- that the small reduction in PCWP (3 mm Hg) was diastolic volumes. In HFpEF, increased LV stroke accompanied by a reduced LV stroke volume (5 ml). volume is more likely to arise from a larger end- The difference between exercise and rest is compat- diastolic volume than from a smaller LV end-systolic ible with nitrite administration delivering more NO to volume because of high end-systolic elastance, the myocardium during exercise than at rest because which only allows for limited end-systolic volume of the presence of myocardial hypoxia, which boosts reductions during LV unloading or positive inotropic conversion of nitrite to NO. interventions. Increased NO delivery, replenished The drastic reduction of cGMP in HFpEF myocar- cGMP stores, and restored PKG-mediated destiffening dium is also relevant to the LV systolic shortening F I G U R E 1 Exercise Intolerance and Microvascular Dysfunction PVREX = or Comorbidities •Obesity, MS, DM •Renal Insufficiency • Salt-Sensitive HT Titin-PO4 Collagen Diastolic LV StiffnessRest LV Contractile ReserveEX Microvascular Inflammation/ Rarefaction Na+ RetentionRest Δ(CaO2 - CvO2)EX Microvascular dysfunction in lungs, skeletal muscle, heart, and kidneys induces exercise intolerance in patients with heart failure with preserved ejection fraction through organ-specific mechanisms. CaO2 CvO2 ¼ arteriovenous oxygen content difference; DM ¼ diabetes mellitus; EX ¼ exercise; HT ¼ hypertension; LV ¼ left ventricular; MS ¼ metabolic syndrome; Naþ ¼ sodium; PVR ¼ pulmonary vascular resistance; Titin-PO4 ¼ titin phosphorylation. Paulus and van Heerebeek JACC VOL. 66, NO. 15, 2015 OCTOBER 13, 2015:1683–6 Nitrite for Exercise Intolerance in HFpEF deficit frequently reported in HFpEF, both at rest and circulation. This produces impaired oxygen delivery during exercise (6). The dose-response curve for to exercising muscles, as evident by a smaller arte- cGMP and cardiac muscle contractile performance has riovenous oxygen content difference (Ca O 2 Cv O2) an ascending and descending limb explained by (12). The present study partially confirms the im- competing effects of cGMP on calcium handling and portance of blunted muscle perfusion for exercise myofilamentary desensitization (10). At the very low intolerance in HFpEF as it observed lower SVR cGMP levels observed in HFpEF myocardium, the and higher V O 2 but unaltered Ca O2 Cv O2 after nitrite cardiomyocytes could well be operating on the infusion. ascending limb of the dose-response curve with a concomitant LV systolic shortening deficit and the large increase in LVSW during exercise after nitrite infusion could be consistent with increased myocardial cGMP eliciting a move up the ascending limb. Another mechanism recently suggested to contribute to exercise intolerance in HFpEF is chronotropic incompetence (6). Evidence suggests that chronotropic incompetence not only reduces CO during exercise because of a blunted heart rate response, but also forces the left ventricle at the onset of exercise to use its preload reserve because the surge in venous return is not matched by an appropriate increase in heart rate. The latter phenomenon profoundly impairs exercise tolerance in transplant recipients. In the current study, there was no difference in the heart rate response to exercise, suggesting that chronotropic incompetence does not seem to be involved in the effects of nitrite on exercise tolerance. Deficient pulmonary and systemic vasodilation have also been implicated in HFpEF exercise intol- EXERCISE INTOLERANCE AND MICROVASCULAR DYSFUNCTION Microvascular dysfunction induced by comorbidities appears to be a common denominator for most of the mechanisms responsible for exercise intolerance in HFpEF as it is involved in high LV diastolic stiffness, LV systolic shortening deficit, and blunted pulmonary or systemic vasodilation (Figure 1). It is also involved in renal sodium retention because low renal NO content shifts the renal perfusion pressure–natriuresis curve to the right. The extent of microvascular dysfunction in pulmonary, systemic, coronary, and renal beds probably varies in relation to the duration of HFpEF or comorbidity profile. This supports the hemodynamic evaluation of exercise intolerance in individual HFpEF patients to guide personalized treatment, as illustrated in this new study (1), which showed nitrite improved PCWP, CO, and LVSW but not PVR and Ca O2 CvO 2 during exercise. CONCLUSIONS erance (11–13). Patients with exertional pulmonary venous hypertension have a limited ability to lower Borlaug et al. (1) deserve to be commended for the pulmonary vascular resistance (PVR) during exercise current study. It demonstrates the clinical relevance and one-third of patients even experience a para- of invasive exercise hemodynamics before assigning doxical increase in PVR (11). The current study (1) HFpEF patients to therapy, establishes inorganic ni- observed a similar inability to lower PVR, which trite as acutely improving exercise hemodynamics in unfortunately remained unaffected by a previous HFpEF, and, because inorganic nitrite lacks tolerance nitrite infusion. Patients in both studies share an development, paves the way for its use in long-term important clinical characteristic: obesity, with an HFpEF trials. average body mass index of 33 kg/m 2. Obesity and metabolic syndrome are known to cause microvas- REPRINT REQUESTS AND CORRESPONDENCE: Dr. cular inflammation, reduced density of the micro- Walter J. Paulus, Department of Physiology, Institute vascular network (i.e., microvascular rarefaction), for Cardiovascular Research VU (ICaR-VU), VU Uni- and increased PVR. In obese HFpEF patients, micro- versity Medical Center Amsterdam, Van der Boe- vascular inflammation and rarefaction not only af- chorststraat 7, 1081 BT Amsterdam, the Netherlands. fect the pulmonary circulation but also the systemic E-mail: [email protected]. REFERENCES 1. Borlaug BA, Koepp KE, Melenovsky V. Sodium nitrite improves exercise hemodynamics and ventricular performance in heart failure with preserved ejection fraction. J Am Coll Cardiol 2015;66:1672–82. 2. Vanderpool R, Gladwin MT. Harnessing the nitrate-nitrite-nitric oxide pathway for therapy of heart failure with preserved ejection fraction. Circulation 2015;131:334–6. 3. Van Heerebeek L, Hamdani N, Falcão-Pires I, et al. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation 2012;126:830–9. 1685 1686 Paulus and van Heerebeek JACC VOL. 66, NO. 15, 2015 OCTOBER 13, 2015:1683–6 Nitrite for Exercise Intolerance in HFpEF 4. Paulus WJ, Tschoepe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013; 62:263–71. 5. Paulus WJ. Culprit mechanism(s) for exercise intolerance in heart failure with normal ejection fraction. J Am Coll Cardiol 2010;56:864–6. 6. Borlaug BA, Olson TP, Lam CS, et al. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol 2010;56:845–54. 7. Maeder MT, Thompson BR, Brunner-La Rocca HP, et al. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol 2010;56: 855–63. 8. Mohammed SF, Hussain S, Mirzayev SA, et al. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 2015;131:550–9. 9. Zile MR, Baicu CF, Ikonomidis JS, et al. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation 2015;131: 1247–59. 10. Mohan P, Brutsaert DL, Paulus WJ, et al. Myocardial contractile response to nitric oxide and cGMP. Circulation 1996;93:1223–9. 11. Santos M, Opotowsky AR, Shah AM, et al. Central cardiac limit to aerobic capacity in patients with exertional pulmonary venous hypertension: implications for heart failure with preserved ejection fraction. Circ Heart Fail 2015;8:278–85. 12. Dhakal BP, Malhotra R, Murphy RM, et al. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circ Heart Fail 2015;8:286–94. 13. Kitzman DW, Nicklas B, Kraus WE, et al. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol 2014;306:H1364–70. KEY WORDS nitrate, nitric oxide, nitrite