* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Answers
Elias James Corey wikipedia , lookup
Marcus theory wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
Aldol reaction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
George S. Hammond wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Ene reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Asymmetric induction wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Stille reaction wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
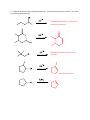
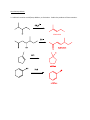
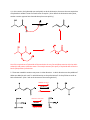
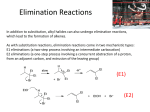
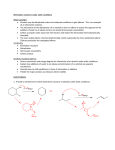
Elimination reactions under basic conditions Major concepts -hydroxy carbonyls can be eliminated under basic conditions in a two-step mechanism Alkyl halides can be eliminated under basic conditions in a one-step mechanism Vocabulary -hydroxyl carbonyl unsaturated carbonyl Students should be able to: Draw a mechanism for the basic elimination of a beta-hydroxy carbonyl Draw a mechanism for the basic elimination of an alkyl halide Explain the basis of the thermodynamic favorability of basic eliminations Predict the product of elimination reactions Daily Problems 1. Draw a mechanism for each of these elimination reactions. What is the factor that drives this reaction to completion (makes it thermodynamically favorable)? The reaction is thermodynamically favored since the product pi bond is conjugated. The reaction is thermodynamically favored because Br- is more stable the OH. 2. Predict the products of these elimination reactions. Two of them do not occur as written. For these, say, NO RXN, and explain why. NO RXN because alcohols only eliminate under acidic conditions NO RXN because there is no beta H to eliminate major (Zaitsev) product Cumulative problems 3. Label each reaction as acid/base, addition, or elimination. Predict the products of these reactions: elimination 4. In this reaction, the hydroxide acts catalytically to do the elimination, but more than one equivalent of hydroxide is needed. Draw a full mechanism to explain. (Hint: Before the elimination takes place, another reaction type we have learned takes place more quickly.) One of the equivalents of hydroxide will be used to do the very fast acid/base reaction with the acidic carboxylic acid proton to become water. The catalytic amount (0.1 equiv.) of hydroxide left will do the beta hydroxycarbonyl elimination. 5. These two metabolic reactions may occur in either direction. In which direction are they additions? What was added in each case? In which direction are they eliminations? Do they follow an acidic or basic elimination? (Hint: look at the structure of the starting alcohol.) addition of H2O elimination under basic elimination under basic addition of H2O Extension problem 6. So far, we have considered what happens when hydroxide acts as a base and removes a betahydrogen to do an elimination reaction. It is possible, though, that a different reaction occurs. What if the hydroxide acts as a nucleophile and attacks the electrophilic atom (the Lewis Acid.) What will the product be in this case?