* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download * Abundant! * Able to share 4 outer valence electrons! * Versatile
Survey
Document related concepts
Two-hybrid screening wikipedia , lookup
Microbial metabolism wikipedia , lookup
Isotopic labeling wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Western blot wikipedia , lookup
Peptide synthesis wikipedia , lookup
Genetic code wikipedia , lookup
Drug discovery wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Biosynthesis wikipedia , lookup
Protein structure prediction wikipedia , lookup
Proteolysis wikipedia , lookup
Transcript
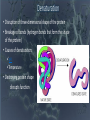
SUPER CARBON Abundant! Able to share 4 outer valence electrons! Versatile! Stable! O-Chem (organic chemistry) • Organic compounds are critical to the structure and function of all living things. • There are 4 main categories (classes/groups) of organic compounds they are all polymers (very large structures); 1) Carbohydrates 2) Proteins 3) Nucleic Acids 4) Lipids • All of these (1-4) will be made out of different monomers (single, smaller structures that bond together) How can we get 4 different functioning groups (classes) of organic compounds from the same basic component? (Carbon) • By using things called functional groups! • Functional groups are lone atoms or clusters of atoms, bonded to a carbon chain (backbone)* the bonding of those functional groups alters the properties of the carbon skeleton. * C-C-C-C-C-C-C-C Functional groups Carbohydrates OH Proteins NH2 Lipids C=C-OH Nucleic Acids P Functional groups change THE FUNCTION of an organic compound! Carbohydrates • Large compounds (polymer) • Used in the body for energy and structure • Functional Group = OH • Made up of monomers called saccharides (simple sugars, like glucose) glucose fructose • Monosaccharides (like glucose) bond together to form disaccharides (like sucrose) bond together to form polysaccharides or complex carbohydrates (like starch) • Examples: Pasta, potatoes Proteins • Large compounds (polymer) • Used in the body to form tissues, cell membrane gates • Functional Group – NH2 • Made up of monomers called amino acids • Single amino acids are bonded together using peptide bonds. • EX: Meat, eggs, fish Make a Prediction • Would your body like to use a single enzyme for more than one reaction? Why or why not? • Will your body want to regulate (start and stop) chemical reactions at different times throughout your life? Denaturation • Disruption of three-dimensional shape of the protein • Breakage of bonds (hydrogen bonds that form the shape of the protein) • Causes of denaturation: pH Temperature • Destroying protein shape disrupts function Nucleic Acids • Large compounds (polymer) • DNA and RNA • Made of monomers called nucleotides which are a phosphate, sugar and a nitrogenous base. (A,T,G,C or U) • Functional Group = P • Large compounds (polymer) • Used in the body for insulation, cell membrane structure and energy • Made up of the monomers; 3 fatty acids and 1 glycerol • Functional group C=O-OH • All are hydrophobic • EX: Butter, oil LIPIDS Modified lipids • If the fatty acids are in a ring format, they are called sterols. Ex: cholesterol and steroids • If we remove one fatty acid and add a phosphate we get phospholipids.