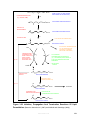
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The Handbook of Redox Biochemistry- ESA, Inc.
Survey
Document related concepts
Two-hybrid screening wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Citric acid cycle wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Butyric acid wikipedia , lookup
Proteolysis wikipedia , lookup
Point mutation wikipedia , lookup
Nitric oxide wikipedia , lookup
15-Hydroxyeicosatetraenoic acid wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
Biochemistry wikipedia , lookup
Biosynthesis wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Transcript
THE HANDBOOK OF REDOX BIOCHEMISTRY Ian N. Acworth, D.Phil., Oxon. This book is dedicated to Emma Louise and Kimberly Ann. I would like to thank the following people. For their help with editing – Debbie Aldrich, Kim Acworth, John Waraska, Bruce Kristal and Paul Gamache. For their support – Scott Freeto, Bruce Bailey, Wayne Matson, Walter DiGiusto, M. Bogdanov, and many other members of ESA, Inc. For his advice, willingness to help, and for the Sunrise Free Radical Schools – Prof. Garry Buettner. For continued help – Dr. Ken Hensley, Dr. K. Williamson, and Prof. R. Floyd (Oklahoma Medical Research Foundation). I would like to acknowledge all researchers in this field without whose work this handbook could not have been completed. ESA, Inc. 22 Alpha Road Chelmsford, MA 01824-4171 USA (978) 250-7000 Sales (800) 959-5095 Fax (978) 250-7090 www.esainc.com An ISO 9001 Company ESA Analytical, Ltd. Brook Farm, Dorton Aylesbury, Buckinghamshire HP18 9NH England, UK 01844 239381 Fax 01844 239382 ii Preface It has been several years since I co-wrote “The Handbook of Oxidative Metabolism” with my colleague Dr. Bruce Bailey, showing the use of electrochemical approaches in the study of free radical production, macromolecular damage and antioxidant protection. Since 1995, thousands of copies of the Handbook have been requested. It has been translated into other languages. It has even been used as a basic course work at university. But the field has moved rapidly and the original Handbook is now dated. I have now updated the old Handbook and renamed it “The Handbook of Redox Biochemistry” for reasons explained in the text. Although now greatly expanded it is, by necessity, selective in content. For readers wanting a more in depth view of the whole field I refer them to the excellent books by Halliwell and Gutteridge, Gilbert and Colton and several others mentioned in the reference section accompanying each chapter. I would very much appreciate having any errors, omissions or new findings brought to my attention. I can be contacted on: [email protected] Ian Acworth August 2003 iii CONTENTS Frontis Chapter 1. Introduction. Oxygen Toxicity – From Microbes To Man. Why Is Oxygen Toxic? Free Radical Pro-Oxidants. Reactive Oxygen Species, Reactive Nitrogen Species And Other Pro-Oxidants How Do Aerobic Organisms Survive Even When Pro-Oxidants Are Being Continuously Produced? Why Use Electrochemical Detection? Conclusions. References. Chapter 2. The Chemistry Of Reactive Species. Oxygen And The Reactive Oxygen Species (ROS). 1. Oxygen. Properties. Formation. Chemical Reactions And Biological Significance 2. Ozone. Properties. Formation. Chemical Reactions And Biological Significance. Measurement. 3. Singlet Oxygen. Properties. Formation. Chemical Reactions And Biological Significance Measurement. 4. Superoxide (Radical Anion). Properties. Formation. Electron Transport Chains. Immune Defense. Enzymes Reactions. Oxygen-Heme Interaction Metal-Catalyzed Auto-Oxidation Chemical Reactions Biological Significance. The Pro. The Con. Control. Measurement. Auto-Oxidation And Redox Cycling 5. Hydrogen Peroxide. Properties. Formation. Chemical Reactions And Biological Significance. Measurement. 6. The Hydroxyl Free Radical. i-ix 2 8 9 17 18 23 24 25 37 37 37 37 38 42 42 42 42 45 46 46 46 48 49 49 49 50 50 54 54 54 54 55 56 56 58 59 59 59 63 63 63 64 66 67 iv Properties. Formation. Chemical Reactions And Biological Significance. Measurement. EPR. HPLC. Nitrogen And The Reactive Nitrogen Species (RNS). 1. Nitrogen. Properties. Formation. Chemical Reactions. 2. The Oxides Of Nitrogen. 2.1 Nitric Oxide. Physical Properties. Formation. Chemical Reactions And Biological Significance. Measurement. 2.2 The Nitroxyl Anion And Nitrosonium Ion. 2.3 Peroxynitrite. Properties. Formation. Chemical Reactions And Biological Significance. Measurement. 2.4 Nitrosoperoxycarbonate And Nitrocarbonate. 2.5 Nitrogen Dioxide, The Nitronium Cation, And Nitrite. Properties. Formation. Chemical Reactions And Biological Significance. Measurement. 2.6 The Higher Oxides Of Nitrogen – Dinitrogen Trioxide, Dinitrogen Tetroxide And Dinitrogen Pentoxide. Properties. Formation. Chemical Reactions And Biological Significance. Measurement. 2.7 S-Nitrosothiols. Properties. Formation. Chemical Reactions And Biological Significance. Measurement. Halogenated Reactive Species (RHS). 1. Chlorine And Hypochlorous Acid. Properties. Formation. Chemical Reactions And Biological Significance. Measurement. 2. Nitrosyl Chloride, Nitryl Chloride And Related Compounds. Properties. Formation. Chemical Reactions And Biological Significance. Measurement. Sulfur, Thiols And Thiyl Radicals (Some Reactive Sulfur Species [RSS]). Properties. Chemical Reactions And Biological Significance. Measurement. Carbonyl Compounds. 67 67 67 70 70 72 82 82 82 82 82 84 85 85 86 89 94 97 98 98 98 100 106 106 107 107 107 107 109 110 110 110 110 111 112 112 112 113 114 114 114 114 114 116 118 118 118 118 119 120 121 121 121 125 126 v Properties. Formation. Chemical Reactions And Biological Significance. Measurement. The Pro-Oxidant Activity Of Low Molecular Weight Compounds And Other Xenobiotics References. 126 126 128 129 131 134 Appendix 2.1 Background To Electrode Potentials. Thermodynamics Of Reversible Cells. Standard Electrode Potentials. Some Comments On SEPs. Coupled Redox Reactions. References. 150 150 153 155 158 158 Appendix 2.2 Background To Kinetics. First-Order Processes. Second-Order and Pseudo-First-Order Processes. Some Published Second-Order Rate Constants. Measurement Of Reaction Order And Reaction Rates. References. 159 160 160 161 165 165 Appendix 2.3 Background To The White Blood Cell. Granulocytes. Lymphocytes. Monocytes. 167 168 168 169 Chapter 3. Damage And Repair. DNA Introduction. The DNA Molecule. DNA Damage. The Consequences Of Oxidative DNA Damage. Repair Of ROS/RNS-Induced Damage. Base Excision Repair. Nucleotide Excision Repair. Mitochondrial DNA Repair. Single Strand DNA Damage And PARP Activation. What Do The Levels Of DNA Adducts Mean? Steady State Levels. Total Adduct Levels. Measurement Of DNA Damage. Gas- And Liquid-Chromatography-Mass Spectrometry. HPLC. Postlabeling assays. Immunochemical detection. The Measurement Of 8-Hydroxy-2’deoxyguanosine In Urine. DNA Damage In Health And Disease. Amino Acids And Proteins. Introduction Protein Molecular Structure Pro-oxidants And Protein Damage. The Indirect Pathway. The Direct Pathway. Oxidative Damage To Tyrosine. Protein Repair And Degradation. Amino Acid And Protein Damage In Aging And Disease. 171 171 171 175 185 187 188 190 190 190 191 191 198 203 205 206 208 209 210 213 214 214 215 218 218 222 226 230 234 vi Measurement Of Amino Acid And Protein Damage. Whole Protein. Protein Hydrolysates. Measurement Of Free Modified Amino Acids And Modified Residues In Whole Proteins And Protein Hydrolysates 1. Protein Carbonyls. 2. Methionine Sulfoxide. 3. 2-Oxohistidine. 4. Tyrosine Markers. 3-Nitrotyrosine. 3-Chlorotyrosine. Dityrosine. Other Tyrosine Oxidation Products. Lipids. Introduction. Structure Of Biological Membranes. Lipid Damage. The Role Of Metals In Lipid Peroxidation. Lipid Oxidation Products. Malondialdehyde. 4-Hydroxyalkenals. Other Reactive Carbonyls. Cholesterol Oxidation. The Isoprostanes. Lipid Repair. Lipid Damage And Disease. Measurement Of Lipid Damage. Diene Conjugates. TBAR. Carbohydrates. Introduction. Ribose And Deoxyribose Damage. Glycation, Glyoxidation, Advanced Glycation End Products (AGES) And Age-Related Pigments. References. Appendix 3.1 Typical DNA Extraction And Hydrolysis. DNA Extraction Procedure. DNA Hydrolysis Procedure. Chapter 4. Protection. Introduction. Enzymes. Catalases. Peroxidases. The Biological Significance Of Catalase And Glutathione Peroxidase. Glutathione-S-Transferase. Heme Oxygenases. Superoxide Dismutases. The Catabolism Of Nitric Oxide. Sequestration Of Metal Ions. The Metabolism Of Iron And Copper. Iron And Copper Species As Pro-Oxidants. Measurement Of Iron And Copper. Low Molecular Weight Molecules. 237 237 240 241 241 241 241 242 242 246 248 248 248 248 249 251 256 257 260 262 264 264 266 268 269 270 272 272 276 276 277 278 280 305 305 307 309 310 315 317 319 320 320 321 323 324 326 329 330 330 vii Water-soluble antioxidants. Albumin. Ascorbic Acid. Antioxidant Properties. Pro-oxidant Properties. Measurement. Thiols. 1. Glutathione. Biological Roles Of Glutathione. Protection. Detoxification And Bioactivation. Cofactor. Storage Of Cysteine In A Non-Toxic Form. Amino Acid Transport. Regulation. Compartmentalization. Conditions And Diseases Affecting Glutathione. Measurement Of Glutathione And Its Disulfide. 2. Homocysteine. 3. Miscellaneous Endogenous Sulfur-Containing Compounds. Uric Acid. Formation. Xanthine Oxidase And Tissue Injury. Antioxidant And Pro-Oxidant Activities. Measurement. Fat-Soluble Antioxidants. Carotenoids Carotenoids And Disease. Antioxidant And Pro-Oxidant Activities Of Carotenoids. Retinoids. The Biological Activity Of The Retinoids. Antioxidant And Pro-Oxidant Activities Of The Retinoids. Measurement Of Carotenoids And Retinoids. Quinones And Hydroquinones. Coenzyme Q (Ubiquinone, Ubiquinol). Biology Of Coenzyme Q. Antioxidant And Pro-Oxidant Activities Of Coenzyme Q Measurement Of Coenzyme Q. Plastoquinone. Vitamin K. Pyrroloquinoline Quinone. Tocopherols Biology Of Tocopherols Antioxidant, Pro-Oxidant And Other Reactions Of The Tocopherols Tocopherol And Disease. Measurement Of Tocopherols And Their Metabolites. Other Endogenous And Exogenous Metabolites Proposed As Antioxidants. Bile Pigments. Biogenic Amines. Estrogen. 330 330 340 340 344 345 346 346 347 347 349 350 351 351 351 352 352 352. 355 358 359 359 360 361 363 363 363 364 365 367 368 369 369 372 372 373 375 377 378 378 380 380 382 383 387 387 391 391 391 395 viii Histidine Derivatives. Indoles And Related Compounds. α-Ketoacids. α-Lipoic, Dihydrolipoic Acids And Analogs. Melanins Melatonin. Phytochemicals. Simple Phenolic Acids. Flavonoids. Phytoestrogens. Resveratrol. Phytic Acid. Sulfur-Containing Compounds. Pteridines. Antioxidant Therapy. Enzymes. Chelators. Low Molecular Weight Molecules. Estimating The Total Antioxidant Capacity. Antioxidants As Food Preservatives. References. 396 397 398 399 401 402 406 406 410 415 418 419 419 420 421 421 422 424 432 438 441 Index. 480 ix Chapter 1 Introduction Mention a pure science such as chemistry or biology and most people will have a fair idea about the subject matter. Unfortunately, for those interested in studying the effects of reactive species on living organisms, no succinct and accurate descriptor of this field exists. Several general titles have been used over the years including free radical biology, redox chemistry and redox biology, yet none of them do justice to this complex, multi-disciplined field. While free radical biology ignores the fact that many chemical species being studied are not free radicals, redox chemistry implies a disregard for any biological aspects. Oxidative metabolism has been used but this is usually associated with energy metabolism. Although still not perfect I prefer the term Redox Biochemistry. I will discuss free radicals and redox reactions in greater detail below. WWW.ESAINC.COM 1 OXYGEN TOXICITY – FROM MICROBES TO MAN Oxygen is toxic to aerobic (and anaerobic) organisms, yet paradoxically oxygen is essential for their survival. Today terrestrial aerobes (both animals and plants) have successfully adapted to live in an atmosphere composed of approximately 21% oxygen and can survive minor fluctuations in the level of respired oxygen without disastrous consequences. True anaerobes, on the other hand, tolerate oxygen poorly, and some cannot survive even a brief exposure to atmospheric oxygen (Table 1.1). Anaerobes were the first living organisms on the planet. These evolutionary simple organisms show a wide range of oxygen tolerance. Strict or obligate anaerobes will only grow if oxygen is absent. While some obligate anaerobes are killed almost immediately following exposure to oxygen (aerophobic) (e.g., Clostridia species) others can survive for many days but cannot reproduce (e.g., Bacteroides fragilis). Another group of organisms, microaerophiles actually require some oxygen for growth but cannot survive when exposed to atmospheric oxygen concentrations. Most bacteria that reduce nitrate (producing nitrite, nitrous oxide or nitrogen) are called facultative anaerobes as they are not affected by exposure to oxygen and in fact will preferentially use oxygen, rather than nitrate, during respiration. Anaerobes can be found in any environment where oxygen levels are decreased to less toxic levels including muds and other sediments; bogs and marshes; polluted waters; certain sewage-treatment systems; rotting material; deep underground areas such as oil pockets; the sources of springs; decaying teeth and gangrenous wounds; the colon; and inappropriately canned foods. Rather than using oxygen during respiration (they usually lack terminal cytochromes that transfer electrons to oxygen) they use other electron acceptors such as ferric ions, sulfate or carbon dioxide which become reduced to ferrous ions, hydrogen sulfide and methane, respectively, during the oxidation of NADH (reduced nicotinamide adenine dinucleotide is a major electron carrier in the oxidation of fuel molecules) (Figure 1.1). Oxygen is toxic to anaerobes as it can affect the organism’s internal homeostasis by altering its reductive capacity, consuming compounds such as NAD(P)H, thiols and other chemicals essential for biosynthetic reactions and inactivating key enzymes. Although anaerobes had free range during the early stages of the evolution of living organisms, this was eventually curtailed by the success of oxygenproducing photosynthetic plants. With the levels of oxygen rising in the atmosphere, anaerobes had three choices, adapt, find niches where oxygen would not penetrate, or die. Organisms eventually evolved that not only survived in an oxygen-enriched atmosphere but prospered. Evidence suggests that the atmospheric oxygen levels have fluctuated markedly over time, increasing from 15-18% in the late Devonian to as high as 35% in the late Carboniferous and early Permian periods. This hyperoxia has been suggested to be one of the WWW.ESAINC.COM 2 possible causes of the mass extinction of terrestrial vertebrates (Graham et al. 1995). Atmospheric oxygen finally stabilized at today’s level (at least to date). 1) Sulfate Reduction (e.g., Desulfovibrio (water- logged soils), Desulfomaculum (spoilage of canned foods), Desulfomonas (intestines), Archaeglobus (a thermophile)): Step 1: Sulfate is activated. ATP + SO42- Adenosine phosphosulfate (APS) + PPi Step 2: A hydrogenase splits molecular hydrogen. Reduction of APS produces sulfite. APS + H2 SO32- + AMP + H2O Cyt c3 Step 3: Electrons derived from hydrogen reduce sulfite to hydrogen sulfide SO32- + 6H + + 6e - H2S + H2O + 2OH- 2) Methanogenesis pathway (e.g., Methanebacterium thermoautotrophicum Formylmethanofuran Carbon Dioxide H2O H4MPT Methanofuran + 2H + + 2e - N5-Formyl-5,6,7,8-tetrahydro methanopterin Methanofuran H2O N5, N10-Methylenetetrahydro methanopterin F420 F420H2 ) N5, N10-Methenyltetrahydro methanopterin F420H2 F420 Methyl-Coenzyme M 5-Methyl-5,6,7,8-tetra hydromethanopterin HTP CoM H4MPT CoM-S-S-HTP Methane CO2 + 8H + + 8e - = CH4 + 2H 2O Figure 1.1 Anaerobic Metabolism. WWW.ESAINC.COM 3 Glucose ATP -ATP ADP Glycogen Glucose 6-phosphate Fructose 6-phosphate ATP -ATP ADP Fructose 1,6-bisphosphate Dihydroxyacetone phosphate 2 x Glyceraldehyde 3-phosphate 2NAD+ + 2Pi NADH + H+ 2NADH NAD+ 2 x 3-Phosphoglyceroyl phosphate ADP 2ADP Substrate-level phosphorylation ATP Glycerol +2ATP 2ATP 2 x 3-Phosphoglycerate Fatty Acids Triglycerides 2 x 2-Phosphoglycerate 2 x Phosphoenolpyruvate 2ADP Anaerobic Glycolysis 2 xLactate 2ATP 2 x Pyruvate 2NAD+ 2NADH 2CO2 +2ATP Some amino acids Σ = +2ATP 2 x Ethanal 2NADH Anaerobic Fementation To Tricarboxylic Acid Cycle 2NAD+ 2 x Ethanol Figure 1.2 The Glycolytic Pathway And The Production Of ATP. WWW.ESAINC.COM 4 Group Oxidizing Conditions Growth Reducing Conditions No growth AerobeFacultative Growth Growth Not required but better if oxygen is present Anaerobeaerophobic (obligate; strict) Death Growth Harmful Anaerobeaerotolerant (moderate) Growth Growth Not required but better if oxygen is present Microaerophile Growth if oxygen level is not too high Growth if oxygen level not too low Required but at only low levels AerobeObligate Effect of Oxygen Example Essential Many bacteria, most fungi, algae, protozoa, all higher plants and animals Bacteria such as enteric and pathogenic species; some protozoa, yeasts (e.g., Saccharomyces) and fungi Many bacteria some protozoa. Bacteroides, Clostridia, Fusobacterium, Methanobacteriu m, and Ruminococcus Bacteroides fragilis, Treponema pallidum Campylobacter jejuni Table 1.1 The Effects Of Environment And Oxygen On Growth Of Aerobes And Anaerobes Facultative aerobes (Table 1.1) can survive in the presence or absence of oxygen. They obtain their energy either by oxidative phosphorylation or fermentation and do not require oxygen for synthesis. When oxygen is lacking this group of organisms can oxidize some organic compounds (which act as both electron donors and acceptors) with a small release of energy, in a process called fermentation. A variety of compounds can be fermented including most sugars, many amino acids, some organic acids, purines, pyrimidines and a variety of miscellaneous products. The energy is captured as two molecules of adenosine triphosphate (ATP) in a process termed substrate level phosphorylation. ATP is the cell’s immediate energy providing molecule and is used for growth, movement, and in biochemical processes e.g., biosynthesis and maintenance of ionic gradients. The stepwise breakdown of glucose into pyruvate is called glycolysis and occurs in both facultative and obligate aerobes (Figure 1.2). In fermentation, pyruvate produced by glycolysis is converted to ethanol or lactate (Figure 1.3). In the presence of oxygen however, glycolysis is WWW.ESAINC.COM 5 followed by aerobic respiration and pyruvate is completely oxidized to carbon dioxide and water (Figure 1.4). The oxidation of pyruvate takes place in a series of steps called the tricarboxylic acid cycle (TCA) (also called the citric acid or Krebs cycle) that occurs in the mitochondrion (Figure 1.4). During aerobic respiration the oxidation of glucose generates 36 molecules of ATP. Two ATP molecules are generated by substrate level phosphorylation (part of the cytosolic glycolytic pathway) and two are produced by substrate level phosphorylation occurring in the mitochondrion. However, the vast majority, thirty-two ATP molecules, are produced by mitochondrial oxidative phosphorylation when electrons are transferred from NADH or flavin adenine dinucleotide (reduced) (FADH2) to oxygen by a series of electron carriers. Thus it can be seen that aerobic respiration generates much more energy than anaerobic processes. For example, if pyruvate is completely oxidized by the TCA cycle then yeast will be able to form 19 times more energy from a given amount of glucose when growing aerobically than when growing anaerobically. ACETALDEHYDE GLUCOSE Glycolysis ETHANOL LACTATE PYRUVATE ANAEROBIC Oxidative Phosphorylation (TCA/electron transport) AEROBIC CO2 + H2O Figure 1.3 The Metabolic Fate Of Pyruvate. Obligate aerobes (e.g., higher plants and animals) use oxygen in respiration and for the biosynthesis of a variety of biomolecules. All higher organisms are obligate aerobes but they can make use of both anaerobic and aerobic processes. For example, many tissues such as the red blood cell, the cornea of the eye, the skin, the kidney medulla and type IIb (fast twitch-glycolytic) skeletal muscle fibers make use of anaerobic glycolysis. Here the two molecules of ATP produced by the anaerobic conversion of glucose to lactate is sufficient to supply most of these tissues’ normal energy needs. However, as the average human requires more than 40kg/day of ATP, and as much as 0.5kg/minute when undergoing strenuous exercise, anaerobic respiration simply cannot keep pace with this demand. Rather, higher organisms must obtain the vast majority of their energy from aerobic respiration, and that is why oxygen is essential for their survival. WWW.ESAINC.COM 6 Glycolysis Pyruvate CoA + NAD+ Amino Acids xNADH, xFADH2 NADH + CO2 Acetoacetyl CoA Acetyl CoA Fatty Acids 3ATP O2 Oxaloacetate Citrate NADH H2O NAD+ Malate Isocitrate H2O NAD+ 2ATP Fumarate O2 FADH2 FAD Amino Acids a-ketoglutarate (2-oxoglutarate) H2O Succinate H2O Succinyl CoA GTP + CoASH ADP NADH + CO2 NAD+ 3ATP O2 NADH + CO2 GDP + Pi Substrate-level Phosphorylation ATP GDP H2O 3ATP O2 Electron transport chain Figure 1.4 The Tricarboxylic Acid Cycle. Obligate aerobes are very oxygen sensitive. A total lack of oxygen is referred to as anoxia and rapidly results in cell death. For example, brain damage can result from perhaps as little as three minutes of anoxia. An acute decrease in respired oxygen leads to hypoxia, a situation where oxygen is still delivered to the tissue, but at a rate insufficient to maintain normal cellular processes. The effects of hypoxia depend upon the tissue and the degree and duration of the hypoxic event. For example, the brain is a very aerobic tissue and is exquisitely sensitive to oxygen tension. In higher animals an acute reduction in arterial oxygen tension WWW.ESAINC.COM 7 leads to altered mental function, analgesia and loss of muscle coordination (Blass and Gibson (1979); Gibson and Blass (1976); Gibson et al. (1978; 1981)). A more marked drop can result in unconsciousness, progressive depression of the central nervous system, circulatory failure and death. Ischemia is a consequence of mechanical disruption of blood flow to a tissue resulting in decreased oxygen, glucose and ATP levels. For example, the occlusion of essential blood vessels to the heart (a consequence of atherosclerosis and/or blood clots) results in ischemia. This leads to myocardial damage and heart attack. It has been estimated that irreversible myocardial damage can occur after about 20 minutes of ischemia (Sobel (1974)). The affected tissue eventually dies. Exposure to elevated levels of oxygen results in hyperoxia and is deleterious to aerobic microorganisms, plants and animals. The growth of aerobic bacteria is inhibited following exposure to pure oxygen. Plants show decreased chloroplast development and leaf damage when exposed to oxygen levels above normal. Animals exposed to 100% oxygen show a variety of symptoms depending upon the duration of exposure (Crapo et al. (1980); Francica et al. (1991)). Humans suffer chest soreness, coughing and sore throats following several hours of exposure to pure oxygen. Longer periods cause alveolar damage, edema and permanent irreversible lung damage. Hyperoxia also leads to damage to most of the major organs. Unfortunately, earlier this century unintentional retinal damage and blindness (retrolental fibroplasia) was caused to premature babies when they were maintained on high oxygen levels in their incubators. Fortunately, the level of oxygen to which premature babies are exposed is now more carefully monitored. It should be noted, however, that hyperoxia can also be beneficial. For example, hyperbaric oxygen is used to treat gangrene because of its toxicity to the obligate anaerobes that cause it. Correct oxygen tension is important to deep sea divers, astronauts, mountain climbers, athletes going from low to high elevations and those undergoing general anesthesia. Oxygen tension is also important in preventing the growth of harmful anaerobic pathogens in canned and bottled foods and beverages. WHY IS OXYGEN TOXIC? Over the years, several theories have been put forward to explain oxygen’s toxicity. This subject was reviewed recently by Gilbert (1999) so only an overview will be presented here. • One early hypothesis as to oxygen’s toxicity was that oxygen exerted its action through enzyme inhibition. For example, oxygen can inhibit nitrogenase and the first enzyme in the dark reactions of photosynthesis, WWW.ESAINC.COM 8 • • • • ribulose 1,5-bisphosphate decarboxylase, and at high concentrations some thiol-containing enzymes (Haugaard (1946); Stadie et al. (1944)). However, enzyme inhibition is far too slow and limited to explain oxygen’s toxic effect, and not all enzymes are affected by oxygen. Abundant evidence showed that irradiation caused DNA damage and cancer through a free radical mechanism and that oxygen had a sensitizing effect (von Sonntag (1991) and references therein). In the mid 1950s Gerschman and Gilbert proposed that oxygen, itself a diradical, may exert its toxic action through the formation of free oxygen radicals. These could then damage biologically important macromolecules such as DNA, proteins and lipids (see Gerschman (1981); Gerschman et al. (1954); and reviews by Gilbert (1999); Halliwell and Gutteridge (1993)). This breakthrough proposal, however, was initially strongly criticized by researchers who proposed that free radicals were far too reactive to exist in any great quantity in biological materials. These objections were finally laid to rest by the detection of free radicals both in dry biological tissues and in living organisms by electron spin resonance (Commoner et al. (1954, 1957)). In 1954 Harman developed his free radical theory of aging that postulated that “a single common process, modifiable by genetic and environmental factors, was responsible for the aging and death of all living things” (Harman (1956; 1992a,b)). His theory proposed that the accumulating irreversible damage to biologically important macromolecules over time led to disease and aging. Free radicals were further implicated by the discovery of the enzyme superoxide dismutase (SOD). Fridovich theorized that the superoxide radical anion was the major toxic form of oxygen and that SOD protected against it (Fridovich (1983, 1986a,b); McCord and Fridovich (1969)). The superoxide theory of oxygen toxicity, though not completely correct, was responsible for a great deal of experimental work and a better understanding of the field as a whole (reviewed in Halliwell and Gutteridge (1993)). We now know that oxygen mediates its toxic effects through a variety of compounds, not just free radicals, many of which contain other atoms in addition to oxygen. The properties of these species will be dealt with in Chapter 2. FREE RADICAL PRO-OXIDANTS. The term radical originally used by chemists referred to an ionic group that had either positive or negative charges associated with it (e.g., carbonate, sulfate etc.). A free radical is now defined as an atom or molecule that has one or more unpaired electrons (i.e., electrons that occupy atomic or molecular orbitals by themselves) and is capable of independent existence. In the strictest sense the free of free radical, is redundant. It may come as some surprise that oxygen is a WWW.ESAINC.COM 9 free radical (in fact a diradical) as are metals that have incomplete 3d shells (e.g., transition metals and their various oxidation (valency) states) (Table 1.2). Scandium 1s22s22p63s2 3p63d14s2 Sc3+ (NT) …3p6 Titanium 1s22s22p63s23p63d24s2 Ti2+ …3p6d2 Ti3+ …3p6d1 Vanadium 1s22s22p63s23p63d34s2 V2+ …3p6d3 V3+ …3p6d2 Chromium x 1s22s22p63s23p63d44s2 1s22s22p63s23p63d54s1 Cr2+ Mn2+ …3p6d4 …3p6d5 Cr3+ Mn3+ …3p6d3 …3p6d4 Iron 1s22s22p63s23p63d64s2 Fe2+ …3p6d6 Fe3+ …3p6d5 Cobalt 1s22s22p63s23p63d74s2 Co2+ …3p6d7 Co3+ …3p6d6 Nickel 1s22s22p63s23p63d84s2 Ni2+ …3p6d8 Copper 1s22s22p63s23p63d104s1 Cu2+ …3p6d9 Cu+ (NT) …3p6d10 Zinc (NT) 1s22s22p63s23p63d104s2 Zn2+ (NT) …3p6d10 Table 1.2 The Electronic Configuration Of The Atoms Of First Transition Series And Some Of Their Ions. (NT – non-transition. Note that NT compounds are also non-radicals.) Free radicals can be formed when a non-radical either gains or loses a single electron (Table 1.3). Free radicals can be formed during homolytic fission of covalent bonds. The energy required to cause bond dissociation can be brought about by several different processes, including exposure to heat or electromagnetic radiation, or by chemical reaction. Remember that covalent bonds are formed when two atoms share electrons (usually one from each atom). During homolytic fission one electron of the bonding pair is retained by atom A, while the other is retained by atom B forming the free radicals A• and B•, respectively. During homolysis of water, for example, the hydroxyl free radical (HO•) and the hydrogen atom (H•) are produced. Radical reactions are much more common in the gas phase and at high temperatures, e.g., combustion. Readers should be aware that many radical reactions found in the literature (especially chemistry texts) may be for gas phase reactions and are not always applicable to biological systems. Having said this, gas phase free radical chemistry is extremely important to those investigating the effects of atmospheric pollution and cigarette smoke on biological systems. WWW.ESAINC.COM 10 1. Heat. Radicals produced during combustion or by heating in absence of oxygen, e.g., C—C, C—H bonds typically require 450-600oC 2. Electromagnetic radiation. Including ionizing irradiation (e.g., x-rays, γ-rays) and photolysis (e.g., UV absorption) 3. Redox reactions. Radicals are produced in reactions involving one-electron transfer: • inorganic ions (e.g., ArN2+ + Cu+ → Ar• + N2 + Cu2+; Sandmeyer reaction) • metals (e.g., H2O2 + Fe2+ → Fe3+ + HO• + OH-, Fenton reaction) • electrolysis (e.g., 2RCO2- - e- → 2RCO2• → → R—R; Kolbe synthesis) • hydroquinone-semiquinone-quinone systems (e.g., production of superoxide from oxygen by ubiquinol/ubiquinone redox couple) 4. Enzymatic. Radicals are produced by the action of peroxidases (e.g., horseradish peroxidase) or oxidases (e.g., xanthine oxidase) 5. Chemical. By the reaction of hydroxyl free radical with a variety of substrates By the reaction of peroxynitrite with a variety of substrates As part of enzyme catalyzed reactions By reactions involved in the generation of O2•- during mitochondrial respiration By the reaction of oxygen with other radicals: • Production of lipid peroxyl radical when oxygen reacts with an alkyl radical • Production of peroxynitrite radical when oxygen reacts with nitric oxide By thermal decomposition of azo initiators (R-N=N-R): • 2,2’-azo-bis(2-amidinopropane) dihydrochloride [AAPH] for aqueous systems • 2,2’-azo-bis(2,4-dimethylvaleronitrile) [AMVN] for lipophilic systems By thermal decomposition of organic peroxides: • Di-tert-butyl peroxide • Dibenzoyl peroxide 6. Ultrasound. Also called sonochemical production. Primary radicals (e.g., H• and HO•) are produced due to pyrolysis of molecules located within collapsing cavitation microbubbles, while secondary radicals are formed by hydrogen abstraction or addition of primary radicals to other molecular species 7. Lithotripsy. Radicals are produced when high-energy shock waves are used to destroy solid objects, e.g., kidney stones 8. Lyophilization. Radicals can be produced by freeze-drying/thawing processes WWW.ESAINC.COM 11 Table 1.3 Free Radicals Can Be Produced In A Variety Of Ways. This table summarizes both in vitro and in vivo approaches for free radical production. (Crum et al. (1987); Doss and Swartz (1984); Fuciarelli et al. (1995); Halliwell and Gutteridge (1999); Heckly and Dimmick (1967); Hendrickson et al. (1970); Kondo et al. (1993); Misik and Riesz (1999); Misik et al. (1996, 1999); Morgan et al. (1988); Ostrowski (1969); Seel et al. (1991); Suhr et al. (1994); Vreugdenhil et al. (1991); Worthington et al. (1997)). A wide variety of radicals can exist (Table 1.4). Like any other chemical, radicals show a broad spectrum of physical and chemical properties. Some are stable and unreactive, whereas others react extremely rapidly. Some are hydrophobic while others hydrophilic. Radicals may share certain common characteristics and can be grouped together as presented in the following table. Unfortunately, as will be readily apparent such classification is not perfect as some radicals can belong to more than one category. For example, some sigma radicals are also carbon-centered monoradicals. • • • • • • • • • • • Radical σ (sigma) π (pi) Monoradicals Polyradicals Carbon centered Oxygen centered Sulfur centered Nitrogen centered Reducing Oxidizing Metal Examples H• (hydrogen atom), R• (carbon-centered radical), R3C• Ascorbyl•, Tocopheryl•, NAD• R•, R3C•, NO• O2 (a diradical) R•, R3C• LO2• RS•, RSO2• NO•, R2•NO, •NO2 CO2•-, PQ•HO•, LO2• Cu2+, Fe2+, Fe3+ Table 1.4 Different Types Of Radicals. R is used as an abbreviation for an alkyl group, L represents a lipid (e.g., fatty acid). Based on an original by G.R. Buettner. Of all the radicals that can be formed sigma (σ) radicals (e.g., the methyl radical, CH3•) are generally much more reactive than pi (π) (e.g., the tocopherol-derived radical, tocopheryl•) as their lone electron cannot be spread throughout the molecule (delocalized). π-Radicals are generally less reactive than σ ones because the lone electron is not confined to just one atom, but is delocalized through the conjugated π-bond system (Sykes (1975)). A physiological consequence is that σ-radicals play an important role in initiating lipid peroxidation while chain-breaking antioxidants prevent lipid peroxidation by reacting with the σ-radicals forming a much less energetic and less dangerous πradical species. WWW.ESAINC.COM 12 Most free radicals have very short lifetimes. Without stabilizing features (e.g., delocalization or steric hindrance) they decompose rapidly, often in the absence of external agents. Decomposition is usually through: 1. Unimolecular reactions (e.g., fragmentation or rearrangement), 2. Bimolecular reactions between radicals including dimerization (e.g., the formation of peroxynitrite from nitric oxide and the superoxide radical anion or the formation of hydrogen peroxide from two hydroxyl free radicals) or disproportionation (e.g., the formation of hydrogen peroxide and oxygen from two hydroperoxyl radicals1) which can involve electron or hydrogen atom transfer or 3. Bimolecular reactions between radicals and other molecules (e.g., addition, displacement, or atom [often H] abstraction). Further information can be found in good chemistry texts. Phase Example Initiation Fe2+ + H2O2 → Fe3+ + HO• + OHL—H + HO• → L• + H2O Propagation L• + O2 → LO2• LO2• + L—H → LO2H + L• Termination L• + L• → L—L (dimerization) LO2• + L• → L—L + O2 2LO2• → non-radical products 2C2H5• → C2H6 + C2H4 (disproportionation) reduced oxidized Table 1.5 The Three Phases Of Chain Reactions. (L represents a lipid undergoing peroxidation.) In biological systems the most infamous free radical cascade is the lipid peroxidation chain reaction (Table 1.5). Here a single initiation process can lead to the destruction of many poly-unsaturated fatty acid molecules. Unfortunately, not only does this affect membrane fluidity and thus many biochemical processes, but it can also lead to the production of cytotoxic carbonyl breakdown products (Chapter 3). Lipid peroxidation is also the major process responsible for food spoilage. Like any other chain reaction, lipid peroxidation consists of three phases termed a) initiation, b) propagation and c) termination. Biological systems are equipped with several mechanisms designed to prevent lipid peroxidation. Such processes include prevention of radical formation (inhibiting initiation) or 1 Note during disproportionation one species is reduced while the other is oxidized. involving electron or hydrogen atom transfer), WWW.ESAINC.COM 13 interception of fatty acid radicals once formed (inhibiting propagation). Biological systems are also capable of repairing damage that occurs. Several techniques can be used to measure free radicals. Electron paramagnetic resonance [EPR] (also called electron spin resonance, or ESR) is a very useful technique and is the only way to directly measure radicals. EPR makes use of the fact that the unpaired electron in a free radical has spin (either +1/2 or –1/2) and thus behaves as a small magnet (i.e., is paramagnetic). When placed in an external magnetic field the unpaired electron can align itself, either parallel or antiparallel, to that field (i.e., the free electron only has two possible energy levels). Exposure to electromagnetic radiation of the correct energy will move the electron from the lower energy level to a higher excited one. Thus an absorption spectrum is obtained which can be used for quantitation as well as gaining information about the environment surrounding the free radical (see Halliwell and Gutteridge (1993)). Direct EPR methods have a sensitivity limit of 0.1nmol/L and have been used extensively for in vitro work (e.g., to study the mechanism of enzyme action) but are often not selective enough for most in vivo work. Some researchers are, however, developing these techniques. Many free radicals are too reactive (e.g., HO•) and have too short a half-life for direct EPR methods. This can be overcome by using spin-trap agents that react with the free radical to produce a longer-lived species that is still paramagnetic (Figure 1.5). Interestingly, spin traps are also proving to be beneficial in the treatment of diseases thought to involve oxidative stress where they probably act to scavenge damaging free radicals. For example, α-phenyl-tert-butylnitrone (PBN) is being used at pharmacological levels to decrease ischemia-reperfusion injury in brain (Floyd (1990), Folbegrova et al. (1995)) and dog heart (Bolli et al. (1988)); reduce the size of liver edema in carbon tetrachloride intoxicated rats (Towner et al. (1993)); reduce the mortality associated with endotoxic shock in rodents (Miyajima and Kotake (1997) and references therein) and prolong the life span of the senescence-accelerated mouse model (Edamatsu et al. (1995)). The correct choice of a spin-trap agent is important. The ideal spin-trap should readily and specifically react with the radical of interest. It must also produce an adduct of sufficient longevity which possesses a characteristic EPR spectrum. It should never decompose during experimentation producing free radicals (see Halliwell and Gutteridge (1993)). Further limitations are placed upon a spin-trap by biological systems. The ideal reagent must not be toxic and should readily pass though any biological barrier (e.g., the blood-brain barrier) to reach the site of free radical production. A major problem with some spin-trap adducts is that they can be reduced in vivo by cellular reducing agents such as ascorbic acid and thiols, resulting in the production of diamagnetic (non EPR active) species. WWW.ESAINC.COM 14 CH3 CH3 N=O CH3 + R CH3 Radical (less stable) tert -Nitrosobutane + CH=N O CH3 O N R CH3 Spin-trap adduct (more stable) O + HO C(CH3)3 CH-N C(CH3)3 OH α−Phenyl- tert- butylnitrone Spin-trap adduct PBN PBN-OH Figure 1.5 Spin Traps React with Free Radicals to Produce Paramagnetic Products that can be Measured using EPR. A different approach to spin trapping is radical scavenging. Here the free radical reacts with an aromatic scavenging agent (e.g., salicylic acid). The aromaticradical adduct can then be quantified using HPLC-based techniques. This approach is much more versatile than spin trapping as neither the scavenging agent nor the product needs to be a radical. Scavengers are usually less toxic than spin traps. Furthermore, as scavenging agents and products are electrochemically active they can be measured at biologically relevant levels using HPLC with electrochemical detection (see ESA Application Notes: 70-1749 Hydroxyl Free Radical Measurement; 70-4820 Alternative Method for Hydroxyl Free Radical Measurement). The use of aromatic scavenging agents will be revisited in Chapter 2. WWW.ESAINC.COM 15 Reactive Oxygen Species (ROS) Reactive Nitrogen Species (RNS) Other A) Free Radicals Alkoxyl LO• Nitric Oxide (monoxide) NO• Hydroperoxyl HO2• Nitrogen Dioxide NO2• Hydroxyl Peroxyl Superoxide HO• LO2• O2- Peroxynitrite radical ONO2• Hydrogen Peroxide H2O2 Alkyl Peroxynitrite LO2NO- Lipid Peroxides Oxygen LO2H O2 Chloramine Dinitrogen Pentoxide NH2Cl N2O5 Ozone O3 Dinitrogen Tetroxide N2O4 Singlet Oxygen 1 Dinitrogen Trioxide N2O3 Nitrate NO3- Nitrite Nitrocarbonate Nitronium (Nitryl) Nitrosonium (Nitrosyl) Nitrosoperoxycarbonate Nitrosonium Chloride Nitroxyl Nitronium Chloride Peroxynitrite Taurine monochloramine Thionitrites (S-nitrosothiols) NO2O2NOCO2NO2+ NO+ ONO2CO2NOCl NONO2Cl ONO2SO3(CH3)2NHCl RSNO Carboncentered Radicals Disulfide Radical Hydrogen Atom Thiyl Radical e.g., CCl3• RSSR•H• RS• B) Non Radicals Singlet Oxygen ∆g O2 1 + Σg O2 Aldehydes (e.g., 4hydroxynonenal) Disulfide Hypohalous Acid Hypothiocyanic Acid Malondialdehyde Transition metal ions RCHO RSSR e.g., HOCl and HOBr HOSCN CHOCH2CHO e.g., Fe2+, Fe3+ Table 1.6 The Different Pro-Oxidants And Other Species Of Importance To Biological Systems. (L – alkyl; 1∆g and 1Σg+ represent the two forms of singlet oxygen; X• – a radical species). WWW.ESAINC.COM 16 REACTIVE OXYGEN SPECIES, REACTIVE NITROGEN SPECIES AND OTHER PRO-OXIDANTS. Although often referred to as free radicals, many of the compounds of interest to the field of redox biochemistry are not free radicals and include many non-radical species (Table 1.6). The term reactive (or reduced) oxygen species (ROS) is also commonly used despite the fact that not all of the oxidizing species are reactive (e.g., the hydroxyl free radical is typically ten million times more reactive and much less selective than hydrogen peroxide), or are produced by the reduction of oxygen (e.g., ozone and singlet oxygen are not reduced forms of oxygen). Furthermore, the use of the term ROS does not take into account that many species contain nitrogen, chlorine or sulfur. Reactive nitrogen species (RNS) is commonly used to distinguish those compounds that contain nitrogen in addition to oxygen, again with disregard for the variation in reactivity between members of the group. As no suitable descriptors can be found, I will use the word prooxidant. Pro-oxidant Species Ferryl species Hydrogen peroxide Hydrogen peroxide and tyrosine radicals Hydrogen peroxide Hydrogen peroxide Hydrogen peroxide, phenoxyl radicals Hydrogen peroxide Hydrogen peroxide Hydrogen peroxide Hydrogen peroxide, superoxide and nitric oxide Comments Essential to catalytic activity of cytochrome P450 and peroxidases. The explosive oxidation of hydroquinone by hydrogen peroxide in the presence of catalase and peroxidase is used to generate a hot defensive spray by the bombardier beetle. Required for the production of thyroxine by the thyroid peroxidase enzyme. Estrogen-induced uterine peroxidase activity plays a role in estrogen catabolism and may confer bactericidal activity too. Involved in the bioluminescence of several animal species. Involved in the formation of lignin. Oxidation and polymerization of tyrosine and phenylalanine residues catalyzed by peroxidases bound to the plant cell wall. With peroxidases are used by fungi to degrade lignin. Involved in fruit ripening. Fertilization of sea urchin eggs causes the rapid uptake of oxygen and production of hydrogen peroxide that is used by a peroxidase to produce tyrosyl radicals from tyrosine residues. These radicals readily dimerize to dityrosine cross-linking a fertilization membrane that prevents further spermatozoa from entering the egg. Redox regulation of gene expression, signal transduction and intracellular redox signaling. Activation of a transcription factor such as SoxS WWW.ESAINC.COM 17 leads to the stimulation of transcription thereby permitting bacteria to gain resistance to oxidants, antibiotics and immune cells that generate nitric oxide. Nitric oxide can activate the Ras oncoprotein by Snitrosylation of essential cysteine residue, stimulating GTPase activity and downstream signaling through activation of extracellular signal regulated kinase (ERK kinase). Hydrogen peroxide produced by a plasma membrane-bound NAD(P)H oxidase is activated by insulin and may act as an intracellular signal for this hormone promoting uptake of glucose and preventing triglyceride hydrolysis in adipocytes. Platelet derived growth factor uses hydrogen peroxide as intracellular messenger. Lipid peroxides and carbonyl metabolites Lipid centered radicals Nitric oxide Nitric oxide Nitric oxide, (nitrosothiols) – endothelialderived relaxing factor ROS, RNS, HOBr, HOCl, Cl2 Tyrosine, tryptophan, glycine and thiyl radicals Vitamin K hydroquinone and semiquinone Possibly act as antifungal and antibacterial agents protecting damaged plants from infection. Prostaglandin and leukotriene metabolism. Retrograde neurotransmitter. Bone synthesis, degradation and remodeling. Blood pressure regulation. Immune system – defense. Essential to catalytic activity of several enzymes such as ribonucleoside diphosphate reductase and pyruvate dehydrogenase. Required for carboxylation of glutamate to γcarboxylglutamic acid by microsomal glutamic acid carboxylase. Important in blood clotting. Table 1.7 Pro-oxidants Are Beneficial Too. (Halliwell and Gutteridge (1999) and references therein; and other references at the end of this chapter). HOW DO AEROBIC ORGANISMS SURVIVE EVEN WHEN PROOXIDANTS ARE BEING CONTINUOUSLY PRODUCED? The cells of aerobes are constantly being exposed to pro-oxidants. Consequently, their DNA, proteins, and lipids are continuously being damaged. During evolution one option would have been to prevent the formation of prooxidant species. This, however, would be virtually impossible to achieve in an oxygen-enriched environment as pro-oxidants are unavoidable side reactions of other important biochemical processes. Instead nature accepted that prooxidants would be produced so protective mechanisms evolved to repair and replace damaged molecules. In addition we are equipped with a suite of antioxidant defenses designed to prevent the formation of pro-oxidants, or to WWW.ESAINC.COM 18 intercept and destroy them if formed. Interestingly, aerobes also make good use of pro-oxidants as messengers, signals and defense molecules (Table 1.7). Under normal conditions the production of pro-oxidants is presumed to be in balance with antioxidant defenses. However, the overproduction of pro-oxidants and/or decreased antioxidant protection can lead to tissue damage and disease. Thus, in individuals with a genetic predisposition or for those exposed to environmental stressors such as cigarette smoke, sunlight and pollution, the prooxidant/antioxidant balance can be upset (Figure 1.6). The overproduction of prooxidant species or the failure of antioxidant defenses results in a condition called oxidative stress, a causal, or at least ancillary, factor in the pathology of many diseases (Sies (1985, 1997)). Oxidative Balance Antioxidants Antioxidants Oxidants Foods Foods Vitamins-H Vitamins-H22O O Sol. Sol. Fat Fat Soluble Soluble Vit. Vit. Dietary Dietary Sup. Sup. Small Small Molecules Molecules Enzymes Enzymes Smoking Cell Activity Pollutants Radiation UV Light Cell Damage Activation State of Oxidative Stress Cell Repair Deactivation Cellular Injury Figure 1.6. Oxidative Balance Between Pro-Oxidant And Antioxidant Species. Normally The Production Of Oxidants Is Matched By Antioxidant Defenses. Under Some Circumstances Oxidant Production Can Overwhelm These Defenses Resulting In Oxidative Stress, Cellular Damage And Disease. A continuously growing list of diseases and conditions, especially those involving inflammation, are reported to be associated with oxidative stress (Table 1.8). It is interesting to note that a number of these diseases are being treated by manipulation of antioxidant levels or by the use of drugs with antioxidant activity (Sies (1991)). WWW.ESAINC.COM 19 Disease/condition Abetalipoproteinaemia Active pulmonary sarcoidosis Adult respiratory distress syndrome AIDS/HIV Aging Alcohol related diseases Alzheimer’s disease Amyotrophic lateral sclerosis Apoptosis Arthritis Asbestosis Asthma Atherosclerosis Autoimmune diseases (general) Autoimmune vasculitis Batten’s disease Behcet's disease Bloom’s syndrome Bone disease (general) Bronchopulmonary dysplasia Cancer Cardiovascular disease Cataracts Chediak-Higashi syndrome Chronic granulomatous disease Crohn’s disease Reference Mimo (1992) Calhoun et al. (1988) Ballmer et al. (1994); Choi and Alam (1996) Baruchel and Wainberg (1992); Dobmeyer et al. (1997); McLemore et al. (1998); Revillard (1991) Balin and Allen (1986); Beckman and Ames (1998); Benzi and Moretti (1995); Bohr and Anson (1995); Cutler (1991); Harman (1988, 1992a,b); Hocman (1981); Knight (1995); Lebel and Bondy (1992); Leibovitz and Siegel (1980); Nohl (1993); Papa and Skulachev (1997); Scarfiotti et al. (1997); Simic (1992); Sohal (1993); Suzuki (1993) Goebel and Schneider (1981); Guemouri et al. (1993); Lieber (1997); Thome et al. (1997); Zhao et al. (1996) Beal (1997); Behl et al. (1994); Choi (1995); Markesbery (1997); McIntosh et al. (1997); Swerdlow et al. (1997); Volicer and Crino (1990) Beal (1997); Chou (1997); Migheli et al. (1994) Monti et al. (1992); Samali et al. (1996); Slater et al. (1995); Stoian et al. (1996) Biemond et al. (1988); Greenwald (1991); Kaur et al. (1996); Moulton (1996); Schiller et al. (1996); Stichtenoth and Frolich (1998) Kamp and Weitzman (1997); Kamp et al. (1992); Lenz et al. (1996); Rom et al. (1987) Hilterman et al. (1997); Smith et al. (1997) Bankson et al. (1993); Devaraj and Jialal (1996); Gambhir and Gambhir (1997); Napoli (1997) Bashir et al. (1993); Yoshida and Gershwin (1993) Bashir et al. (1993); Belch et al. (1989); Bruce et al. (1997) Clausen et al. (1988); Garg et al. (1982) Ohno et al. (1997); Pronai et al. (1990) Emerit and Cerutti (1981) Ralston (1997) Banks et al. (1998) Bankson et al. (1993); Borek (1993); De Flora et al. (1991); Emerit (1994); Hochstein and Atallah Klotz (1998); Hocman (1981); Oberley and Buettner (1979); Oberley and Oberley (1997); Ockner et al. (1993); Palmer and Paulson (1997); Pryor (1997); Slaga (1995); Troll (1991); Trush and Kensler (1991); Weinberg (1996) De Meyer and Herman (1997); Marin and RodriguezMartinez (1997); Welch and Loscalzo (1994) Bhuyan et al. (1986); Niwa and Iizawa (1994); Varma et al. (1984, 1995); Walsh and Patterson (1991); Zigler and Hess (1985) Falloon and Gallin (1986); Quie (1997); Volkman et al. (1984) Umeki (1994); Volkman et al. (1984) Allgayer (1991); Baldassano et al. (1993); Curran et al. (1991); Kimura et al. (1997); McKenzie et al. (1996); Rachmilewitz et al. (1997); Solis-Herruzo et al. (1993) WWW.ESAINC.COM 20 Cystic fibrosis Diabetes Down’s syndrome Duchenne’s muscular dystrophy Exercise Favism Friedreich’s ataxia Gastritis Gerstmann-Straussler Syndrome Glomerular injury Gout Guillain Barre syndrome Hashimoto’s thyroiditis Hemolytic diseases Hepatitis Huntington’s disease Hutchinson-Gilford syndrome Hypercholesterolaemia Hypersensitivity pneumonitis Idiopathic hemochromatosis Inborn errors of metabolism Infectious mononucleosis Inflammation (general) Inflammatory bowel disease Brown et al. (1994, 1995, 1996); Graseman et al. (1998); Percival et al. (1995); Portal et al. (1995); Winklhofer-Roob (1994); Worlitzsch et al. (1998) Dandona et al. (1997); Giugliano et al. (1995); Semenkovich and Heinecke (1997); Wolff et al. (1991) Brugge et al. (1992); Kedziora and Bartosz (1988); Lott (1982); Reiter et al. (1996) Burr et al. (1987); Dioszeghy et al. (1989); Haycock et al. (1996); Ragusa et al. (1997) Fielding and Meydani (1997); Higuchi et al. (1985); Ji (1996); Lawson et al. (1997); Leeuwenburgh et al. (1994); Ortenblad et al. (1997); Packer (1997) Gaetani et al. (1996); Mavelli et al. (1984); Musci et al. (1987); Winterbourn et al. (1986) Rotig et al. (1997) Beno et al. (1993, 1994); Durak et al. (1994); Mannick et al. (1996) Migheli et al. (1994) Rohrmoser and Mayer (1996) Marcolongo et al. (1988); Rosen et al. (1986) Gutowski et al. (1998) Bagchi et al. (1990); Sugawara et al. (1988); Szabo et al. (1996) Fritsma (1983); Lachant and Tanaka (1986); Stack et al. (1989); Stocks et al. (1971); Vertongen et al. (1981); Winterbourn (1990); Yenchitsomanus and Wasi (1983) Arthur et al. (1985); Biasi et al. (1994); Biemond et al. (1988); Bonkovsky et al. (1997); De Maria et al. (1996); Yu et al. (1997) Beal (1995, 1996, 1997); Bondy (1995); Borlongan et al. (1996); Browne et al. (1997); Shapira (1996) Goldstein (1971) Cohen (1995); Devaraj and Jialal (1994); Harrison and Ohara (1995); Verhaar et al. (1998); Wennmalm (1994) Calhoun (1991) Britton and Brown (1985); Gutteridge et al. (1985); Houglum et al. (1997); Selden et al. (1980); Young et al. (1994) Bird et al. (1995); Blau et al. (1996); Brown and Squier (1996); Delgado and Calderon (1979); Jansen and Wanders (1997); Kavanagh et al. (1994); Loscalzo (1996); Moyano et al. (1997); Patel and Leonard (1995); Pitkanen and Robinson (1996); Prohaska (1986); Quie (1977); Welch et al. (1997); Whitin and Cohen (1988); Yoshida et al. (1995) Hokama et al. (1986); Niwa et al. (1984); Ritter et al. (1994) Billiar (1995); Chapple (1997); Cirino (1998); Connor and Grisham (1996); Dallegri and Ottonello (1997); Halliwell et al. (1988); Morris et al. (1995); Parke and Parke (1996); Pyne (1994); Southorn and Powis (1988); Stichtenoth and Frolich (1998); Trenam et al. (1992); Weitzman and Gordon (1990); Winrow et al. (1993); Winyard and Blake (1997) Buffinton and Doe (1995); Macdonald (1998) WWW.ESAINC.COM 21 Ischemia/reoxygenation injury; reperfusion injury Kashin-Beck disease Keshan disease Leprosy Liver disease (general) Lupus Macular degeneration Malaria Motor neuron disease Multiple sclerosis Neuronal ceroid lipofuscinosis Pancreatitis Parkinson’s disease Periodontal disease Porphyria Prion Diseases Renal dialysis Retrolental fibroplasia Rheumatic diseases Salmonella typhimurium infection Septic shock Skin inflammation Smoking Stroke Transplantation Ar' Rajab et al. (1996); Bulkley (1994); Flaherty and Weisfeldt (1988); Gutteridge and Halliwell (1990); Hudson (1994); Johnson and Weinberg (1993); Maxwell (1997); McCord (1987); Szabo (1996); Waxman (1996); Weight et al. (1996) Peng et al. (1992); Wu and Xu (1987) Hensrud et al. (1994); Levander et al. (1997) Agnihotri et al. (1996); Sethi et al. (1996); Sharp and Banerjee (1985) Abrams et al. (1995) Belmont et al. (1997); Benke et al. (1990); Cooke et al. (1997); Mohan and Das (1997); Suryaprabha et al. (1991) Anderson et al. (1994); Nicolas et al. (1996); Van der Hagen et al. (1996) Delmas-Beauvieux et al. (1995); Ginsburg and Atamna (1994); Mishra et al. (1994); Postma et al. (1996); Vennerstrom and Eaton (1988) Anderson et al. (1997); Donohoe and Brady (1996); Lyras et al. (1996); Morrison (1995); Sendtner and Thoenen (1994); Shaw et al. (1995); Wong and Borchelt (1995); Zeman et al. (1994) Calabrese et al. (1994); Clausen et al. (1997); Cooper et al. (1997); Hooper et al. (1998); Langemann et al. (1992); Nagra et al. (1997); Parkinson et al. (1997) Garg et al. (1982); Gutteridge et al. (1983); Marklund et al. (1981); Santavuori et al. (1989) Sanfey (1986) Beal (1997); Cadet and Brannock (1998); Ciccone (1998); Di Momte et al. (1992); Fahn and Cohen (1992); Gerlach et al. (1994); Hirsch et al. (1997); Jenner (1996); Jenner and Olanow (1996); Koller (1997); Owen et al. (1997); Simonian and Coyle (1996); Youdin et al. (1988, 1990) Ellis et al. (1998); Kimura et al. (1993); Moore et al. (1994); Scmidt et al. (1996) Monteiro et al. (1986, 1989); Thunell et al. (1997) Brown et al. (1997); Wiseman and Goldfarb (1996) Biasioli et al. (1997); Cristol et al. (1994); Westhuyzen et al. (1995) Anderson et al. (1994); Cunningham (1987); Johnson et al. (1974); Southorn and Powis (1988) Miesel et al. (1996) Mehta et al. (1998) Brigham (1991); Goode and Webster (1993); Keusch (1993); Kilbourn et al. (1997); Kuhl and Rosen (1998); Novelli (1997); Taylor and Piantadosi (1995) Trenam et al. (1992) Cantin and Crystal (1985); Chow (1993); Crystal (1991); Kohlmeier and Hastings (1995); McCusker (1992); Pryor, W.A. (1997); Rahman and MacNee (1996) Chang et al. (1998); Fisher and Bogousslavsky (1998); Keli et al. (1996); Mattson (1997); Meldrum (1995) Hernandez and Granger (1988); Keith (1993); Lehr and WWW.ESAINC.COM 22 Ulcerative colitis Viral infection Werner’s syndrome Wilson’s disease Xeroderma pigmentosum Messmer (1996); McCord (1985); Meyer et al. (1998); Paller (1992); Toledo-Pereyra (1991) Keshavarzian et al. (1997); Holmes et al. (1998); Lundberg et al. (1994); McKenzie et al. (1996); Ramakrishna et al. (1997); Reimund et al. (1998); Sedghi et al. (1994) Peterhans (1997) Marklund et al. (1981) Britton and Brown (1995); Carmichael et al. (1995); Ogihara et al. (1995); Sokol et al. (1994) Crawford et al. (1988); Runger et al. (1995); Schallreuter et al. (1991) Table 1.8 Diseases And Conditions Associated With Oxidative Stress. WHY USE ELECTROCHEMICAL DETECTION? Oxidation can be defined as a gain in oxygen, a loss of hydrogen, a loss of protons or the loss of electrons. Conversely, reduction is the loss of oxygen, a gain of hydrogen, or the gain of electrons. The two processes are complementary and no oxidation process can take place without a corresponding reduction; these complimentary reactions are typically referred to as REDuction-OXidation or REDOX reactions. Of all the different detectors that are used in the study of redox biochemistry, perhaps the most useful is the electrochemical detector (ECD). This detector actually measures the flow of electrons (current) when an electron-rich compound loses electrons to the working electrode’s surface while this compound undergoes oxidation (conversely, electron-poor compounds can also be measured as they accept electrons from the working electrode’s surface while undergoing reduction). When coupled to the high resolution achievable with high-performance liquid chromatography (HPLC) an analytical instrument is produced that can be used to measure many different pro-oxidant, antioxidant, and damaged species (Chapter 2 and 3). Electrochemical detection is one of the most sensitive and selective detection techniques available for use with HPLC. The theory behind it has been extensively reviewed elsewhere (Acworth and Bowers (1997) and references therein; Acworth et al. (1997a,b,c; 1998)). Of all the ECDs on the market place, ESA’s coulometric detectors are the most sensitive and selective, and are virtually maintenance free. ESA, Inc., offers two electrochemical detectors (Figure 1.7). The Coulochem® detector offers a high-sensitivity DC mode along with pulsed and cyclic capabilities. The CoulArray® is the only electrochemical detector that can work with even the most aggressive gradients. Practical examples using these detectors will be presented throughout this handbook. WWW.ESAINC.COM 23 Figure 1.7 The Coulochem® III (Upper Figure) And CoulArray® Detectors. WWW.ESAINC.COM 24 CONCLUSIONS. Oxygen is toxic and exerts its toxicity through the production of a variety of prooxidant species. During evolution living organisms either remained anaerobic surviving in oxygen poor conditions or became aerobic, adapting to the increased atmospheric levels of oxygen. Aerobic organisms tolerate continued production of pro-oxidants and have evolved mechanisms to repair or remove damaged molecules or to prevent the formation and to intercept and deactivate the prooxidant species. Normally there is a balance between production of pro-oxidant species and destruction by the antioxidant defenses. However, under certain conditions this balance is upset in favor of overproduction of the pro-oxidants leading to oxidative stress and disease. HPLC-ECD is one of the most sensitive analytical techniques for the measurement of pro-oxidants, antioxidants, and damage markers. REFERENCES. Abrams, G.A., Trauner, M., and Nathanson, M.H. (1995). Nitric oxide and liver disease. Gastroenterologist, 3, 220-233. Acworth, I.N., Bailey, B.B., and Maher, T.J. (1998). The use of HPLC with electrochemical detection to monitor reactive oxygen and nitrogen species, markers of oxidative damage and antioxidants: application to the neurosciences. In: Neurochemical Markers of Degenerative Nervous Diseases and Drug Addiction. Qureshi, G., Parvez, H., Caudy, P., and Parvez, S. (Eds.). Progress in HPLC-HPCE, 7. VSP Publications, The Netherlands. Pp. 3-56. Acworth, I.N., and Bowers, M. (1997a). An introduction to HPLC-based electrochemical detection: From single electrode to multi-electrode arrays. In: Coulometric Electrode Array Detectors for HPLC. Progress in HPLC-HPCE 6. Acworth, I. N., Naoi, M., Parvez, S., and Parvez, H. (Eds.). VSP Publications, The Netherlands. Pp. 1-48. Acworth, I.N., McCabe, D.R., and Maher, T.J. (1997b). The analysis of free radicals, their reaction products and antioxidants. In: Oxidants, Antioxidants and Free Radicals. Baskin, S.I., and Salem, H., (Eds.). Taylor and Francis, Washington DC. Pp. 23-77. Acworth, I.N., Naoi, M., Parvez, S., and Parvez, H. (Eds.). (1997c). Coulometric Electrode Array Detectors for HPLC. Progress in HPLC-HPCE, 6. VSP Publications, The Netherlands. Agnihotri, N. Ganguly, N.K., Kaur, S., Khullar, M., Sharma, S.C., and Chugh, K.S. (1995). Role of reactive oxygen species in renal damage in experimental leprosy. Lepr. Rev., 66, 201-209. Allgayer, H. (1991). Clinical relevance of oxygen radicals in inflammatory bowel disease – facts and fashion. Klin. Wochenschr., 69, 1001-1003. Andersen, P.M., Nilsson, P., Keranen, M.L., Forsgren, L., Hagglund, J., Karlsborg, M., Ronnevi, L.O., Gredal, O., and Marklund, S.L. (1997). Phenotypic heterogeneity in motor neuron disease patients with CuZn- superoxide dismutase mutations in Scandinavia. Brain, 120, 1723-1737. Anderson, R.E., Kretzer, F.L., and Rapp, L.M. (1994). Free radicals and ocular disease. Adv. Exp. Med. Biol., 366, 73-86. Ar' Rajab, A., Dawidson, I., and Fabia, R. (1996). Reperfusion injury. New Horiz., 4, 224-234. Arthur, M.J., Bentley, I.S., Tanner, A.R., Saunders, P.K., Millward-Sadler, G.H., and Wright, R. (1985). Oxygen-derived free radicals promote hepatic injury in the rat. Gastroenterology, 89, 1114-1122. Bagchi, N., Brown, T.R., Herdegen, D.M., Dhar, A., and Sundick, R.S. (1990). Antioxidants delay the onset of thyroiditis in obese strain chickens. Endocrinology, 127, 1590-1595. Baldassano, R.N., Schreiber, S., Johnston, R.B. Jr., Fu, R.D., Muraki, T., and MacDermott, R.P. (1993). Crohn's disease monocytes are primed for accentuated release of toxic oxygen metabolites. Gastroenterology, 105, 60-66. Balin, A.K., and Allen, R.G. (1986). Mechanisms of biologic aging. Dermatol. Clin., 4, 347-358. Ballmer, P.E., Reinhart, W.H., and Gey, K.F. (1994). Antioxidant vitamins and disease risk of a suboptimal supply. Ther. Umsch., 51, 467-474. Banks, B.A., Ischiropoulos, H., McClelland, M., Ballard, P.L., and Ballard, R.A. (1998). Plasma 3-nitrotyrosine is elevated in premature infants who develop bronchopulmonary dysplasia. Pediatrics, 101,870-874. Bankson, D.D., Kestin, M., and Rifai, N. (1993). Role of free radicals in cancer and atherosclerosis. Clin. Lab. Med., 13, 463-480. Baruchel, S., and Wainberg, M.A. (1992). The role of oxidative stress in disease progression in individuals infected by the human immunodeficiency virus. J. Leukoc. Biol., 52, 111-114. Bashir, S., Harris, G., Denman, M.A., Blake, D.R., and Winyard, P.G. (1993). Oxidative DNA damage and cellular sensitivity to oxidative stress in human autoimmune diseases. Ann. Rheum. Dis., 52, 659-666. Beal, M.F. (1995). Aging, energy, and oxidative stress in neurodegenerative diseases. Ann. Neurol., 38, 357-366. Beal, M.F. (1996). Mitochondria, free radicals, and neurodegeneration. Curr. Opin. Neurobiol., 6, 661-666. WWW.ESAINC.COM 25 Beal, M.F. (1997). Oxidative damage in neurodegenerative diseases. Neuroscientist, 3, 21-27. Beckman, K.B., and Ames, B.N. (1998). The free radical theory of aging matures. Physiol. Rev., 78, 547-581. Behl, C., Davis, J.B., Lesley, R., and Schubert, D. (1994). Hydrogen peroxide mediates amyloid β protein toxicity. Cell, 77, 817-827. Belch, J.J., Chopra, M., Hutchison, S., Lorimer, R., Sturrock, R.D., Forbes, C.D., and Smith, W.E. (1989). Free radical pathology in chronic arterial disease. Free Radic. Biol. Med., 6, 375-378. Belmont, H.M., Levartovsky, D., Goel, A., Amin, A., Giorno, R., Rediske, J., Skovron, M.L., and Abramson, S.B. (1997). Increased nitric oxide production accompanied by the up-regulation of inducible nitric oxide synthase in vascular endothelium from patients with systemic lupus erythematosus. Arthritis Rheum., 40, 1810-1816. Benke, P.J., Levcovitz, H., Paupe, J., and Tozman, E. (1990). Scavengers of free radical oxygen affect the generation of low molecular weight DNA in stimulated lymphocytes from patients with systemic lupus erythematosus. Metabolism, 39, 1278-1284. Beno, I., Volkovova, K., Batovsky, M., and Staruchova, M. (1993). Increased mucosal antioxidant enzyme activities in chronic gastritis and benign gastric polyps. Eur. J. Cancer Prev., 2, 461-465. Beno, I., Volkovova, K., Staruchova, M., and Koszeghyova, L. (1994). The activity of Cu/Zn-superoxide dismutase and catalase of gastric mucosa in chronic gastritis, and the effect of alpha-tocopherol. Bratisl. Lek. Listy., 95, 9-14. Benzi, G., and Moretti, A. (1995). Are reactive oxygen species involved in Alzheimer’s disease? Neurobiol. Aging, 16, 661-674. Bhuyan, K.C., Bhuyan, D.K., and Podos, S.M. (1986). Lipid peroxidation in cataract of the human. Life Sci., 38, 14631471. Biasi, F., Chiarpotto, E., Lanfranco, G., Capra, A., Zummo, U., Chiappino, I., Scavazza, A., Albano, E., and Poli, G. (1994). Oxidative stress in the development of human ischemic hepatitis during circulatory shock. Free Radic. Biol. Med., 17, 225-233. Biasioli, S., Schiavon, R., Petrosino, L., Cavallini, L., Zambello, A., De Fanti, E., and Giavarina, D. (1997). Free radicals and oxidative stress challenge dialysis patients: effects of two different membranes. ASAIO J., 43, M766-772. Biemond, P., Swaak, A.J., van Eijk, H.G., and Koster, J.F. Superoxide dependent iron release from ferritin in inflammatory diseases. Free Radic. Biol. Med., 4, 185-198. Billiar, T.R. (1995). Nitric oxide. Novel biology with clinical relevance. Ann. Surg., 221, 339-349. Bird, S., Miller, N.J., Collins, J.E., and Rice-Evans, C.A. (1995). Plasma antioxidant capacity in two cases of tyrosinaemia type 1: one case treated with NTBC. J. Inherit. Metab. Dis., 18, 123-126. Blass, J.P., and Gibson, G.E. (1979). Consequences of mild, graded hypoxia. Adv. Neurol., 26, 229-250. Blau, N. de Klerk, J.B., Thony, B., Heizmann, C.W., Kierat, L., Smeitink, J.A., and Duran, M. (1996). Tetrahydrobiopterin loading test in xanthine dehydrogenase and molybdenum cofactor deficiencies. Biochem. Mol. Med., 58, 199-203. Bohr, V.A., and Anson, R.M. (1995). DNA damage, mutation and fine structure DNA repair in aging. Mutat. Res., 338, 2534. Bolli, R., Patel, B.S., Jeroudi, M.O., Lai, E.K., and McCay, P.B. (1988). Demonstration of free radical generation in “stunned” myocardium of intact dogs with the use of the spin trap N-tert-phenyl -α-butylnitrone. J. Clin. Invest., 82, 476485. Bondy, S.C. (1995). The relation of oxidative stress and hyperexcitation to neurological disease. Proc. Soc. Exp. Biol. Med., 208, 337-345. Bonkovsky, H.L., Banner, B.F., and Rothman, A.L. (1997). Iron and chronic viral hepatitis [see comments]. Hepatology, 25, 759-768. Borek, C. (1993). Molecular mechanisms in cancer induction and prevention. Environ. Health Perspect., 101, Suppl 3, 237-245. Borlongan, C.V., Kanning, K., Poulos, S.G., Freeman, T.B., Cahill, D.W., and Sanberg, P.R. (1996). Free radical damage and oxidative stress in Huntington's disease. J. Fla. Med. Assoc., 83, 335-341. Brigham, K.L. (1991). Oxygen radicals--an important mediator of sepsis and septic shock. Klin. Wochenschr., 69, 10041008. Britton, R.S., and Brown, K.E. (1995). Genetic hemochromatosis and Wilson's disease: role for oxidant stress? Hepatology, 21, 1195-1197. Brown, D. R., Schulz-Schaeffer, W.J., Schmidt, B., and Kretzschmar, H.A. (1997). Prion protein-deficient cells show altered response to oxidative stress due to decreased SOD-1 activity. Exp. Neurol., 146, 104-112. Brown, G.K., and Squier, M.V. (1996). Neuropathology and pathogenesis of mitochondrial diseases. J. Inherit. Metab. Dis., 19, 553-572. Brown, R.K., and Kelly, F.J. (1994). Evidence for increased oxidative damage in patients with cystic fibrosis. Pediatr. Res., 36, 487-493. Brown, R.K., McBurney, A., Lunec, J., and Kelly, F.J. (1995). Oxidative damage to DNA in patients with cystic fibrosis. Free Radic. Biol. Med., 18, 801-806. Brown, R.K., Wyatt, H., Price, J.F., and Kelly, F.J. (1996). Pulmonary dysfunction in cystic fibrosis is associated with oxidative stress. Eur. Respir. J., 9, 334-339. Browne, S.E., Bowling, A.C., MacGarvey, U., Baik, M.J., Berger, S.C., Muqit, M.M., Bird, E.D., and Beal, M.F. (1997). Oxidative damage and metabolic dysfunction in Huntington's disease: Selective vulnerability of the basal ganglia. Ann. Neurol., 41, 646-653. Bruce, I., McNally, J., and Bell, A. (1997). Enhanced monocyte generation of reactive oxygen species in primary systemic vasculitis. J. Rheumatol., 24, 2364-2370. Brugge, K.L., Nichols, S., Delis, D., Saitoh, T., and Truaner, D. (1992). The role of alterations in free radical metabolism in mediating cognitive impairments in Down's syndrome. EXS, 62, 190-198. Buffinton, G.D., and Doe, W.F. (1995). Altered ascorbic acid status in the mucosa from inflammatory bowel disease patients. Free Radic. Res., 22, 131-143. WWW.ESAINC.COM 26 Bulkley, G.B. (1994). Reactive oxygen metabolites and reperfusion injury: aberrant triggering of reticuloendothelial function [see comments]. Lancet, 344, 934-936. Burr, I.M., Asayama, K., and Fenichel, G.M. (1987). Superoxide dismutases, glutathione peroxidase, and catalase in neuromuscular disease. Muscle Nerve, 10, 150-154. Cadet, J.L., and Brannock, C. (1998). Free radicals and the pathobiology of brain dopamine systems. Neurochem. Int., 32, 117-131. Calabrese, V., Raffaele, R., Cosentino, E., and Rizza, V. (1994). Changes in cerebrospinal fluid levels of malondialdehyde and glutathione reductase activity in multiple sclerosis. Int. J. Clin. Pharmacol. Res., 14, 119-123. Calhoun, W.J. (1991). Enhanced reactive oxygen species metabolism of air space cells in hypersensitivity pneumonitis. J. Lab. Clin. Med., 117, 443-452. Calhoun, W.J., Salisbury, S.M., Chosy, L.W., and Busse, W.W. (1988). Increased alveolar macrophage chemiluminescence and airspace cell superoxide production in active pulmonary sarcoidosis. J. Lab. Clin. Med., 112, 147-156. Cantin, A., and Crystal, R.G. (1985). Oxidants, antioxidants and the pathogenesis of emphysema. Eur. J. Respir. Dis. Suppl., 139, 7-17. Carmichael, P.L., Hewer, A., Osborne, M.R., Strain, A.J., and Phillips, D.H. (1995). Detection of bulky DNA lesions in the liver of patients with Wilson's disease and primary haemochromatosis. Mutat. Res., 326, 235-243. Chang, C.Y., Lai, Y.C., Cheng, T.J., Lau, M.T., and Hu, M.L. (1998). Plasma levels of antioxidant vitamins, selenium, total sulfhydryl groups and oxidative products in ischemic-stroke patients as compared to matched controls in Taiwan. Free Radic. Res., 28, 15-24. Chapple, I.L., (1997). Reactive oxygen species and antioxidants in inflammatory diseases. J. Clin. Periodontol., 24, 287296. Choi, B.H. (1995). Oxidative stress and Alzheimer’s disease. Neurobiol. Aging, 16, 675-678. Choi, A.M., and Alam, J. (1996). Heme oxygenase-1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am. J. Respir. Cell Mol. Biol., 15, 9-19. Chou, S.M. (1997). Neuropathology of amyotrophic lateral sclerosis: new perspectives on an old disease. J. Formos. Med. Assoc., 96, 488-498. Chow, C.K. (1993). Cigarette smoking and oxidative damage in the lung. Ann. N. Y. Acad. Sci., 686, 289-298. Ciccone, C.D. (1998). Free-radical toxicity and antioxidant medications in Parkinson's disease. Phys. Ther., 78, 313-319. Cirino, G. (1998). Multiple controls in inflammation. Extracellular and intracellular phospholipase A2, inducible and constitutive cyclooxygenase, and inducible nitric oxide synthase. Biochem. Pharmacol., 55, 105-111. Clausen, J., Jensen, G.E., and Nielsen, S.A. (1988). Selenium in chronic neurologic diseases. Multiple sclerosis and Batten's disease. Biol. Trace Elem. Res., 15, 179-203. Cohen, R.A. (1995). The role of nitric oxide and other endothelium-derived vasoactive substances in vascular disease. Prog. Cardiovasc. Dis., 38, 105-128. Commoner, B., Heise, J.J., Lippincott, B.B., Norberg, R.E., Passonneau, J.V., and Townsend, J. (1957). Biological activity of free radicals. Science, 126, 57-63. Commoner, B., Townsend, J., and Pake, G.E. (1954). Free radicals in biological materials. Nature, 174, 689-691. Conner, E.M., and Grisham, M.B. (1996). Inflammation, free radicals, and antioxidants. Nutrition, 12, 274-277. Cooke, M.S., Mistry, N., Wood, C., Herbert, K.E., and Lunec, J. (1997). Immunogenicity of DNA damaged by reactive oxygen species – implications for anti-DNA antibodies in lupus. Free Radic. Biol. Med., 22, 151-159. Cooper, R.L. (1997). Multiple sclerosis: an immune legacy? Med. Hypotheses, 49, 307-311. Crapo, J.D., Barry, H.A., Foscue, H.A., and Shelburne, J. (1980). Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am. Rev. Respir. Dis., 122, 123-143. Crawford, D., Zbinden, I., Moret, R., and Cerutti, P. (1988). Antioxidant enzymes in xeroderma pigmentosum fibroblasts. Cancer Res., 48, 2132-2134. Cristol, J.P., Canaud, B., Rabesandratana, H., Gaillard, I., Serre, A., and Mion, C. (1994). Enhancement of reactive oxygen species production and cell surface markers expression due to haemodialysis. Nephrol. Dial. Transplant., 9, 389-394. Crystal, R.G. (1991). Oxidants and respiratory tract epithelial injury: pathogenesis and strategies for therapeutic intervention. Am. J. Med., 91, 39S-44S. Cunningham, A.S. (1987). Breast-feeding, antioxidants, and retinopathy of prematurity [letter]. Am. J. Obstet. Gynecol., 156, 1040-1041. Curran, F.T., Allan, R.N., and Keighley, M.R. (1991). Superoxide production by Crohn's disease neutrophils. Gut, 32, 399402. Cutler, R.G. (1991). Antioxidants and aging. Am. J. Clin. Nutr., 53, 373S-379S. Dallegri, F., and Ottonello, L. (1997). Tissue injury in neutrophilic inflammation. Inflamm. Res., 46, 382-391. Dandona, P., Thusu, K., Cook, S., Snyder, B., Makowski, J., Armstrong, D., and Nicotera, T. (1996). Oxidative damage to DNA in diabetes mellitus. Lancet, 347, 444-445. De Flora, S., Izzotti, A., D'Agostini, F., and Cesarone, C.F. (1991). Antioxidant activity and other mechanisms of thiols involved in chemoprevention of mutation and cancer. Am. J. Med., 91, 122S-130S. Delgado, W., and Calderon, R. (1979). Acatalasia in two Peruvian siblings. J. Oral Pathol., 8, 358-368. Delmas-Beauvieux, M.C., Peuchant, E., Dumon, M.F., Receveur, M.C., Le Bras, M., and Clerc, M. (1995). Relationship between red blood cell antioxidant enzymatic system status and lipoperoxidation during the acute phase of malaria. Clin. Biochem., 28, 163-169. De Maria, N., Colantoni, A., Fagiuoli, S., Liu, G.J., Rogers, B.K., Farinati, F., Van Thiel, D.H., and Floyd, R.A. (1996). Association between reactive oxygen species and disease activity in chronic hepatitis C. Free Radic. Biol. Med., 21, 291-295. De Meyer, G.R., and Herman, A.G. (1997). Vascular endothelial dysfunction. Prog. Cardiovasc. Dis., 39, 325-342. Devaraj, S., and Jialal, I. (1996). Oxidized low-density lipoprotein and atherosclerosis. Int. J. Clin. Lab. Res., 26, 178-184. WWW.ESAINC.COM 27 Di Monte, D.A., Chan, P., and Sandy, M.S. (1992. Glutathione in Parkinson’s disease: A link between oxidative stress and mitochondrial damage. Ann. Neurol., 32, S111-S115. Dioszeghy, P., Imre, S., and Mechler, F. (1989). Lipid peroxidation and superoxide dismutase activity in muscle and erythrocytes in adult muscular dystrophies and neurogenic atrophies. Eur. Arch. Psychiatry Sci., 238, 175-177. Dobmeyer, T.S., Findhammer, S., Dobmeyer, J.M., Klein, S.A., Raffel, B., Hoelzer, D., Helm, E.B., Kabelitz, D., and Rossol, R. (1997). Ex vivo induction of apoptosis in lymphocytes is mediated by oxidative stress: role for lymphocyte loss in HIV infection. Free Radic. Biol. Med., 22, 775-785. Donohoe, D.J., and Brady, B. (1996). Motor neuron disease: etiology, pathogenesis and treatment--a review. Ir. J. Med. Sci., 165, 200-209. Durak, I., Ormeci, N., Akyol, O., Canbolat, O., Kavutcu, M., and Bulbul, M. (1994). Adenosine deaminase, 5'-nucleotidase, xanthine oxidase, superoxide dismutase, and catalase activities in gastric juices from patients with gastric cancer, ulcer, and atrophic gastritis. Dig. Dis. Sci., 39, 721-728. Ellis, S.D., Tucci, M.A., Serio, F.G., and Johnson, R.B. (1998). Factors for progression of periodontal diseases. J. Oral Pathol. Med., 27, 101-105. Edamatsu, R., Mori, A., and Packer, L. (1995). The spin trap N-tert-phenyl-α-butylnitrone prolongs the life span of the senescence-accelerated mouse. Biochem. Biophys. Res. Comms., 211, 847-849. Emerit, I. (1994). Reactive oxygen species, chromosome mutation, and cancer: Possible role of clastogenic factors in carcinogenesis. Free Radic. Biol. Med., 16, 99-109. Emerit, I., and Cerutti, P. (1981). Clastogenic activity from Bloom syndrome fibroblast cultures. Proc. Natl., Acad. Sci. USA, 78, 1868-1872. Fahn, S., and Cohen, G. (1992). The oxidant stress hypothesis in Parkinson's disease: evidence supporting it. Ann. Neurol., 32, 804-812. Falloon, J., and Gallin, J.I. (1986). Neutrophil granules in health and disease. J. Allergy Clin. Immunol., 77, 653-662. Fielding, R.A., and Meydani, M. (1997). Exercise, free radical generation, and aging. Aging (Milano), 9, 12-18. Fisher, M., and Bogousslavsky, J. (1998). Further evolution toward effective therapy for acute ischemic stroke [see comments]. JAMA, 279, 1298-1303. Flaherty, J.T., and Weisfeldt, M.L. (1988). Reperfusion injury. Free Radic. Biol. Med., 5, 409-419. Floyd, R.A. (1990). Role of oxygen free radicals in carcinogenesis and brain ischemia. FASEB, J., 4, 2587-2597. Folbegrova, J., Zhao, Q., Katsura, K., and Siesjo, B. (1995). N-tert-butyl-α-phenylnitrone improves recovery of brain energy state in rats following transient focal ischemia. Proc. Natl. Acad. Sci. USA, 92, 5057-5061. Fracica, P.J., Knapp, M.J., Piantadosi, C.A., Takeda, K., Fulkerson, W.J., Coleman, R.E., Wolfe, W.G., and Crapo, J.D. (1991). Responses of baboons to prolonged hyperoxia: Physiology and qualitative pathology. J. Appl. Physiol., 71, 2352-2362. Fritsma, G.A. (1983). Vitamin E and autoxidation. Am. J. Med. Technol., 49, 453. Gaetani, G.F., Rolfo, M., Arena, S., Mangerini, R., Meloni, G.F., and Ferraris, A.M. (1996). Active involvement of catalase during hemolytic crises of favism. Blood, 88, 1084-1088. Gambhir, D.S., and Gambhir, J.K. (1997). Oxidized low density lipoprotein, antioxidants and coronary atherosclerosis [editorial]. Indian Heart J., 49, 19-22. Garg, H.S., Awasthi, Y.C., Neff, W.A., Ansari, N.H., Srivastava, S.K. (1982). Studies in neuronal ceroid lipofuscinosis: enzymes of liver and brain tissues involved in the defense against oxidative damage. J. Neurosci. Res., 7, 305-311. Gerlach, M., Ben-Scachar, D., Riederer, P., and Youdim, M.B.H. (1994). Altered brain metabolism of iron a cause of neurodegenerative diseases? J. Neurochem., 63, 793-807. Gerschman, R. (1981). Historical introduction to the “free radical theory” of oxygen toxicity. In: Oxygen and Living Processes: An Interdisciplinary Approach. Gilbert, D.L. (Ed). Springer-Verlag, Berlin. Pp. 44-46. Gerschman, R., Gilbert, D.L., Nye, S.W., Dwyer, P., Fenn, W.O. (1954). Oxygen poisoning and x-irradiation: A mechanism in common. Science, 119, 623-626. Gibson, G.E., and Blass, J.P. (1976). Impaired synthesis of acetylcholine in brain accompanying mild hypoxia and hypoglycemia. J. Neurochem., 27, 37-42. Gibson, G.E., Pulsinelli, W., Blass, J.P., and Duffy, T.E. (1981). Brain dysfunction in mild to moderate hypoxia. Am. J. Med., 70, 1247-1254. Gibson, G.E., Shimada, M., and Blass J.P. (1978). Alterations in acetylcholine synthesis and cyclic nucleotides in mild cerebral hypoxia. J. Neurochem., 31, 757-760. Gilbert, D.L. (1999). From the breath of life to reactive oxygen species. In: Reactive Oxygen Species in Biological Systems. Gilbert, D.L., and Colton, C.A. (Eds.). Kluwer Academic/Plenum Publishers, New York. Pp. 3-31. Ginsburg, H., and Atamna, H. (1994). The redox status of malaria-infected erythrocytes: an overview with an emphasis on unresolved problems. Parasite, 1,5-13. Giugliano, D., Ceriello, A., and Paolisso, G. (1995). Diabetes mellitus, hypertension, and cardiovascular disease: which role for oxidative stress? Metabolism, 44, 363-368. Goebel, K.M., and Schneider, J. (1981). Erythrocyte membrane fluidity, lipid peroxidation and lysis in alcoholic liver disease. Acta Biol. Med. Ger., 40, 571-576. Goldstein, S. (1971). The biology of aging. N. Engl. J. Med., 285, 1120-1129. Goode, H.F., and Webster, N.R. (1993). Free radicals and antioxidants in sepsis. Crit. Care Med., 21, 1770-1776. Graham, J. B., Dudley, R., Agullar, N. M., and Gans, C. (1995). Implications of the late Palaeozoic oxygen pulse for physiology and evolution. Nature, 375, 117-120. Grasemann, H., Ioannidis, I., Tomkiewicz, R.P., de Groot, H., Rubin, B.K., and Ratjen, F. (1998). Nitric oxide metabolites in cystic fibrosis lung disease. Arch. Dis. Child., 78, 49-53. Greenwald, R.A. (1991). Oxygen radicals, inflammation, and arthritis: Pathophysiological considerations and implications for treatment. Semin. Arthritis. Rheum., 20, 219-240. Guemouri, L., Lecomte, E., Herbeth, B., Pirollet, P., Paille, F., Siest, G., and Artur, Y. (1993). Blood activities of antioxidant enzymes in alcoholics before and after withdrawal. J. Stud. Alcohol, 54, 626-629. WWW.ESAINC.COM 28 Gutowski, N.J., Pinkham, J.M., Akanmu, D., Chirico, S., and Murphy, R.P. (1998). Free radicals in inflammatory neurological disease: increased lipid peroxidation and haptoglobin levels in Guillain Barre syndrome. Ir. J. Med. Sci., 167, 43-46. Gutteridge, J.M.C., and Halliwell, B. (1990). Reoxygenation injury and antioxidant protection: A tale of two paradoxes. Arch. Biochem. Biophys., 283, 223-226. Gutteridge, J.M.C., Rowley, D.A., Griffiths, E., and Halliwell, B. (1985). Low-molecular-weight iron complexes and oxygen radical reactions in idiopathic haemochromatosis. Clin. Sci., 68, 463-467. Gutteridge, J.M.C., Westermarck, T., and Santavuori, P. (1983). Iron and oxygen radicals in tissue damage: implications for the neuronal ceroid lipofuscinoses. Acta Neurol. Scand., 68, 365-370. Halliwell, B., and Gutteridge, J.M.C. (1999). Free Radicals in Biology and Medicine. Oxford University Press. Halliwell, B., Hoult, J.R., and Blake, D.R. (1988). Oxidants, inflammation, and anti-inflammatory drugs. FASEB J., 2, 28672873. Harman, D. (1956). Aging: A theory based on free radical and radiation chemistry. J. Gerontol., 11, 298-304. Harman, D. (1988). Free radicals in aging. Mol. Cell Biochem., 84, 155-161. Harman, D. (1992a). Free radical theory of aging: history. EXS, 62, 1-10. Harman, D. (1992b). Free radical theory of aging. Mutat. Res., 275, 257-266. Harrison, D.G., and Ohara, Y. (1995). Physiologic consequences of increased vascular oxidant stresses in hypercholesterolemia and atherosclerosis: Implications for impaired vasomotion [see comments]. Am. J. Cardiol., 75, 75B-81B. Haugaard, N. (1946). Oxygen poisoning XI. The relation between inactivation of enzymes and essential sulfhydryl groups. J. Biol. Chem., 164, 265-270. Haycock, J.W., MacNeil, S., Jones, P., Harris, J.B., and Mantle, D. (1996). Oxidative damage to muscle protein in Duchenne muscular dystrophy. Neuroreport, 8, 357-361. Hensrud, D.D., Heimburger, D.C., Chen, J., and Parpia, B. (1994). Antioxidant status, erythrocyte fatty acids, and mortality from cardiovascular disease and Keshan disease in China. Eur. J. Clin. Nutr., 48, 455-464. Hernandez, L.A., and Granger, N. (1988). Role of antioxidants in organ preservation and transplantation. Crit. Care Med., 16, 543-549. Higuchi, M., Cartier, L.J., Chen, M., and Holloszy, J.O. (1985). Superoxide dismutase and catalase in skeletal muscle: adaptive response to exercise. J. Gerontol., 40, 281-286. Hiltermann, T.J., de Bruijne, C.R., Stolk, J., Zwinderman, A.H., Spieksma, F.T., Roemer, W., Steerenberg, P.A., Fischer, P.H., van Bree, L., and Hiemstra, P.S. (1997). Effects of photochemical air pollution and allergen exposure on upper respiratory tract inflammation in asthmatics. Am. J. Respir. Crit. Care Med., 156, 1765-1772. Hirsch, E.C., Faucheux, B., Damier, P., Mouatt-Prigent, A., and Agid, Y. (1997). Neuronal vulnerability in Parkinson's disease. J. Neural. Transm. Suppl., 50, 79-88. Hochstein, P., and Atallah, A.S. (1988). The nature of oxidants and antioxidant systems in the inhibition of mutation and cancer. Mutat. Res., 202, 363-375. Hocman, G. (1981). Biochemistry of aging and cancer. Int. J. Biochem., 13, 659-672. Hokama, Y., Dayaon, E., Iwamoto, L., Yanagisawa, R., Reichert, E., Sato, D., and Ching, C.Y. (1986). Significant enhanced superoxide anion (O2-) production in vitro by peripheral blood monocytes of lepromatous leprosy patients stimulated with liposome and suppression by C-reactive protein (CRP). J. Med., 17, 299-311. Holmes, E.W., Yong, S.L., Eiznhamer, D., and Keshavarzian, A. (1998). Glutathione content of colonic mucosa: evidence for oxidative damage in active ulcerative colitis. Dig. Dis. Sci., 43, 1088-1095. Hooper, D.C., Spitsin, S., Kean, R.B., Champion, J.M., Dickson, G.M., Chaudhry, I., and Koprowski, H. (1998). Uric acid, a natural scavenger of peroxynitrite, in experimental allergic encephalomyelitis and multiple sclerosis. Proc. Natl. Acad. Sci. USA, 95, 675-680. Houglum, K., Ramm, G.A., Crawford, D.H., Witztum, J.L., Powell, L.W., and Chojkier, M. (1997). Excess iron induces hepatic oxidative stress and transforming growth factor beta1 in genetic hemochromatosis. Hepatology, 26, 605-610. Hudson, K.F. (1994). A phenomenon of paradox: myocardial reperfusion injury. Heart Lung, 23, 384-393. Jansen, G.A., and Wanders, R.J. (1997). Plasmalogens and oxidative stress: evidence against a major role of plasmalogens in protection against the superoxide anion radical. J. Inherit. Metab. Dis., 20, 85-94. Jenner, P. (1996). Oxidative stress in Parkinson's disease and other neurodegenerative disorders. Pathol. Biol., 44, 5764. Jenner, P., and Olanow, C.W. (1996). Oxidative stress and the pathogenesis of Parkinson's disease. Neurology, 47, S161-170. Ji, L.L. (1996). Exercise, oxidative stress, and antioxidants. Am. J. Sports Med., 24, S20-24. Johnson, K.J., and Weinberg, J.M. (1993). Postischemic renal injury due to oxygen radicals. Curr. Opin. Nephrol. Hypertens., 2, 625-635. Johnson, L., Schaffer, D., and Boggs, T.R. Jr. (1974). The premature infant, vitamin E deficiency and retrolental fibroplasia. Am. J. Clin. Nutr., 27, 1158-1173. Kamp, D.W., and Weitzman, S.A. (1997). Asbestosis: clinical spectrum and pathogenic mechanisms. Proc. Soc. Exp. Biol. Med., 214, 12-26. Kamp, D.W., Graceffa, P., Pryor, W.A., and Weitzman, S.A. (1992). The role of free radicals in asbestos-induced diseases. Free Radic. Biol. Med., 12, 293-315. Kaur, H., Edmonds, S.E., Blake, D.R., and Halliwell, B. (1996). Hydroxyl radical generation by rheumatoid blood and knee joint synovial fluid. Ann. Rheum. Dis., 55, 915-920. Kavanagh, T.J., Raghu, G., White, C.C., Martin, G.M., Rabinovitch, P.S., and Eaton, D.L. (1994). Enhancement of glutathione content in glutathione synthetase – deficient fibroblasts from a patient with 5-oxoprolinuria via metabolic cooperation with normal fibroblasts. Exp. Cell Res., 212,69-76. Kedziora, J., and Bartosz, G. (1988). Down's syndrome: a pathology involving the lack of balance of reactive oxygen species. Free Radic. Biol. Med., 4, 317-330. WWW.ESAINC.COM 29 Keith, F. (1993). Oxygen free radicals in cardiac transplantation. J. Card. Surg., 8, 245-248. Keli, S.O., Hertog, M.G., Feskens, E.J., and Kromhout, D. (1996). Dietary flavonoids, antioxidant vitamins, and incidence of stroke: the Zutphen study. Arch. Intern. Med., 156, 637-642. Keshavarzian, A., Haydek, J.M., Jacyno, M., Holmes, E.W., and Harford, F. (1997). Modulatory effects of the colonic milieu on neutrophil oxidative burst: a possible pathogenic mechanism of ulcerative colitis. J. Lab. Clin. Med., 130, 216225. Keusch, G.T. (1993). Antioxidants in infection. J. Nutr. Sci. Vitaminol., 39, S23-33. Kilbourn, R.G., Traber, D.L., and Szabo, C. (1997). Nitric oxide and shock. Dis. Mon., 43, 277-348. Kimura, H., Miura, S., Shigematsu, T., Ohkubo, N., Tsuzuki, Y., Kurose, I., Higuchi, H., Akiba, Y., Hokari, R., Hirokawa, M., Serizawa, H., and Ishii, H. (1997). Increased nitric oxide production and inducible nitric oxide synthase activity in colonic mucosa of patients with active ulcerative colitis and Crohn's disease. Dig. Dis. Sci., 42, 1047-1054. Kimura, S., Yonemura, T., and Kaya, H. (1993). Increased oxidative product formation by peripheral blood polymorphonuclear leukocytes in human periodontal diseases. J. Periodontal Res., 28, 197-203. Klotz, T., Bloch, W., Volberg, C., Engelmann, U., and Addicks, K. (1998). Selective expression of inducible nitric oxide synthase in human prostate carcinoma. Cancer, 82, 1897-1903. Knight, J.A. (1995). The process and theories of aging. Ann. Clin. Lab. Sci., 25, 1-12. Kohlmeier, L., and Hastings, S.B. (1995). Epidemiologic evidence of a role of carotenoids in cardiovascular disease prevention. Am. J. Clin. Nutr., 62, 1370S-1376S. Koller, W.C. (1997). Neuroprotective therapy for Parkinson's disease. Exp. Neurol., 144, 24-28. Kuhl, S.J., and Rosen, H. (1998). Nitric oxide and septic shock. From bench to bedside [clinical conference]. West. J. Med., 168, 176-181. Lachant, N.A., and Tanaka, K.R. (1986). Antioxidants in sickle cell disease: the in vitro effects of ascorbic acid. Am. J. Med. Sci., 292, 3-10. Langemann, H., Kabiersch, A., and Newcombe, J. (1992). Measurement of low-molecular-weight antioxidants, uric acid, tyrosine and tryptophan in plaques and white matter from patients with multiple sclerosis. Eur. Neurol., 32, 248-252. Lawson, D.L., Chen, L., and Mehta, J.L. (1997). Effects of exercise-induced oxidative stress on nitric oxide release and antioxidant activity. Am. J. Cardiol., 80, 1640-1642. LeBel, C.P., and Bondy, S.C. (1992). Oxidative damage and cerebral aging. Progress Neurobiol., 38, 601-609. Leeuwenburgh, C., Fiebig, R., Chandwaney, R., and Ji, L.L. (1994). Aging and exercise training in skeletal muscle: responses of glutathione and antioxidant enzyme systems. Am. J. Physiol., 267, R439-445. Lehr, H.A., and Messmer, K. (1996). Rationale for the use of antioxidant vitamins in clinical organ transplantation. Transplantation, 62, 1197-1199. Leibovitz, B.E., and Siegel, B.V. (1980). Aspects of free radical reactions in biological systems: Aging. J. Gerontol., 35, 45-56. Lenz, A.G., Costabel, U., and Maier, K.L. (1996). Oxidized BAL fluid proteins in patients with interstitial lung diseases. Eur. Respir. J., 9, 307-312. Levander, O.A., and Beck, M.A. (1997). Interacting nutritional and infectious etiologies of Keshan disease. Insights from coxsackie virus B-induced myocarditis in mice deficient in selenium or vitamin E. Biol. Trace Elem. Res., 56, 5-21. Lieber, C.S. (1997). Role of oxidative stress and antioxidant therapy in alcoholic and nonalcoholic liver diseases. Adv. Pharmacol., 38, 601-628. Loscalzo, J. (1996).The oxidant stress of hyperhomocyst(e)inemia. J. Clin. Invest., 98, 5-7. Lott, I.T. (1982). Down's syndrome, aging, and Alzheimer's disease: a clinical review. Ann. N. Y. Acad. Sci., 396, 15-27. Lundberg, J.O., Hellstrom, P.M., Lundberg, J.M., and Alving, K. (1994). Greatly increased luminal nitric oxide in ulcerative colitis [see comments]. Lancet, 344, 1673-1674. Lyras, L., Evans, P.J., Shaw, P.J., Ince, P.G., and Halliwell, B. (1996). Oxidative damage and motor neuron disease difficulties in the measurement of protein carbonyls in human brain tissue. Free Radic. Res., 24, 397-406. MacDonald, T.T. (1998). Viral vectors expressing immunoregulatory cytokines to treat inflammatory bowel disease. Gut, 42, 460-461. Mannick, E.E., Bravo, L.E., Zarama, G., Realpe, J.L., Zhang, X.J., Ruiz, B., Fontham, E.T., Mera, R., Miller, M.J., and Correa, P. (1996). Inducible nitric oxide synthase, nitrotyrosine, and apoptosis in Helicobacter pylori gastritis: effect of antibiotics and antioxidants. Cancer Res., 56, 3238-3243. Marcolongo, R., Calabria, A.A., Lalumera, M., Gerli, R., Alessandrini, C., and Cavallo, G. (1988). The "switch-off" mechanism of spontaneous resolution of acute gout attack. J. Rheumatol., 15, 101-109. Marin, J., and Rodriguez-Martinez, M.A. (1997). Role of vascular nitric oxide in physiological and pathological conditions. Pharmacol. Ther., 75, 111-134. Markesbery, W.R. (1997). Oxidative stress hypothesis in Alzheimer's disease. Free Radic. Biol. Med., 23, 134-147. Marklund, S., Nordensson, I., and Back, O. (1981). Normal CuZn superoxide dismutase, Mn superoxide dismutase, catalase and glutathione peroxidase in Werner's syndrome. J. Gerontol., 36, 405-409. Marklund, S.L., Santavuori, P., and Westermarck, T. (1981). Superoxide dismutase, catalase and glutathione peroxidase in infantile, late infantile and juvenile neuronal ceroid- lipofuscinosis. Clin. Chim. Acta, 116, 191-198. Mattson, M.P. (1997). Neuroprotective signal transduction: relevance to stroke. Neurosci. Biobehav. Rev., 21, 193-206. Mavelli, I., Ciriolo, M.R., Rossi, L., Meloni, T., Forteleoni, G., De Flora, A., Benatti, U., Morelli, A., and Rotilio G. (1984). Favism: A hemolytic disease associated with increased superoxide dismutase and decreased glutathione peroxidase activities in red blood cells. Eur. J. Biochem., 139, 13-18. Maxwell, S.R., and Lip, G.Y. (1997). Reperfusion injury: A review of the pathophysiology, clinical manifestations and therapeutic options. Int. J. Cardiol., 58, 95-117. McCord, J.M. (1987). Oxygen-derived radicals: a link between reperfusion injury and inflammation. Fed. Proc., 46, 24022406. McCord, J.M., and Fridovich, I. (1969). Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem., 244, 6049-6053. WWW.ESAINC.COM 30 McIntosh, L.J., Trush, M.A., and Troncoso, J.C. (1997). Increased susceptibility of Alzheimer's disease temporal cortex to oxygen free radical-mediated processes. Free Radic. Biol. Med., 23, 183-190. McKenzie, S.J., Baker, M.S., Buffinton, G.D., and Doe, W.F. (1996). Evidence of oxidant-induced injury to epithelial cells during inflammatory bowel disease. J. Clin. Invest., 98, 136-141. McLemore, J.L., Beeley, P., Thorton, K., Morrisroe, K., Blackwell, W., Dasgupta, A. (1998). Rapid automated determination of lipid hydroperoxide concentrations and total antioxidant status of serum samples from patients infected with HIV: elevated lipid hydroperoxide concentrations and depleted total antioxidant capacity of serum samples. Am. J. Clin. Pathol., 109, 268-273. McCord, J.M. (1985). Oxygen-derived free radicals in postischemic tissue injury. N. Engl. J. Med., 312, 159-163. McCusker, K.(1992). Mechanisms of respiratory tissue injury from cigarette smoking. Am. J. Med., 93, 18S-21S. McKenzie, S.J., Baker, M.S., Buffinton, G.D., and Doe, W.F. (1996). Evidence of oxidant-induced injury to epithelial cells during inflammatory bowel disease. J. Clin. Invest., 98, 136-141. Mehta, A., Singh, S., and Ganguly, N.K. (1998). Impairment of intestinal mucosal antioxidant defense system during Salmonella typhimurium infection. Dig. Dis. Sci., 43, 646-651. Meldrum, B.S. (1995). Cytoprotective therapies in stroke. Curr. Opin. Neurol., 8, 15-23. Meyer, K.C., Love, R.B., and Zimmerman, J.J. (1998). The therapeutic potential of nitric oxide in lung transplantation. Chest, 113, 1360-1371. Miesel, R., Hartung, R., and Kroeger, H. (1996). Priming of NADPH oxidase by tumor necrosis factor alpha in patients with inflammatory and autoimmune rheumatic diseases. Inflammation, 20, 427-438. Migheli, A., Cavalla, P., Piva, R., Giordana, M.T., and Schiffer, D. (1994). Bcl-2 protein expression in aged brain and neurodegenerative diseases. Neuroreport, 5, 1906-1908. Mino, M. (1992). Clinical uses and abuses of vitamin E in children. Proc. Soc. Exp. Biol. Med., 200, 266-270. Mishra, N.C., Kabilan, L., and Sharma, A. (1994). Oxidative stress and malaria-infected erythrocytes. Indian J. Malariol., 31, 77-87. Miyajima, T., and Kotake, Y. (1997). Optimal time and dosage of N-tert-phenyl-α-butylnitrone (PBN) for the inhibition of nitric oxide synthase induction in mice. Free Radic. Biol. Med., 22, 463-470. Mohan, I.K., and Das, U.N. (1997). Oxidant stress, anti-oxidants and essential fatty acids in systemic lupus erythematosus. Prostaglandins Leukot. Essent. Fatty Acids, 56, 193-198. Monteiro, H.P., Abdalla, D.S., Augusto, O., and Bechara, E.J. (1989). Free radical generation during delta-aminolevulinic acid autoxidation: Induction by hemoglobin and connections with porphyrinpathies. Arch. Biochem. Biophys., 271, 206216. Monteiro, H.P., Abdalla, D.S., Faljoni-Alario, A., and Bechara, E.J. (1986). Generation of active oxygen species during coupled autoxidation of oxyhemoglobin and delta-aminolevulinic acid. Biochim. Biophys. Acta., 881, 100-106. Monti, D., Troiano, L., Tropea, F., Grassilli, E., Cossarizza, A., Barozzi, D., Pelloni, M.C., Tamassia, M.G., Bellomo, G., and Franceschi, C. (1992). Apoptosis--programmed cell death: A role in the aging process? Am. J. Clin. Nutr., 55, 1208S-1214S. Moore, S., Calder, K.A., Miller, N.J., and Rice-Evans, C.A. (1994). Antioxidant activity of saliva and periodontal disease. Free Radic. Res., 21, 417-425. Morris, C.J., Earl, J.R., Trenam, C.W., and Blake, D.R. (1995). Reactive oxygen species and iron--a dangerous partnership in inflammation. Int. J. Biochem. Cell Biol., 27, 109-122. Morrison, K.E. (1995). Mechanisms in motor neuron disease: Clues from genetic studies. Mol. Med. Today, 1, 195-201. Moulton, P.J. (1996). Inflammatory joint disease: the role of cytokines, cyclooxygenases and reactive oxygen species. Br. J. Biomed. Sci., 53, 317-324. Moyano, D., Vilaseca, M.A., Pineda, M., Campistol. J., Vernet, A., Poo, P., Artuch, R., and Sierra, C. (1997). Tocopherol in inborn errors of intermediary metabolism. Clin. Chim. Acta, 263, 147-155. Musci, G., Mavelli, I., and Rotilio, G. (1987). Evidence for superoxide generation from the autoxidation of the favisminducing aglycone divicine. Biochim. Biophys. Acta, 926, 369-372. Nagra, R.M., Becher, B., Tourtellotte, W.W., Antel, J.P., Gold, D., Paladino, T., Smith, R.A., Nelson, J.R., and Reynolds, W.F. (1997). Immunohistochemical and genetic evidence of myeloperoxidase involvement in multiple sclerosis. J. Neuroimmunol., 78, 97-107. Napoli, C. (1997). Low density lipoprotein oxidation and atherogenesis: From experimental models to clinical studies. G. Ital. Cardiol., 27, 1302-1314. Nicolas, M.G., Fujiki, K., Murayama, K., Suzuki, M.T., Shindo, N., Hotta, Y., Iwata, F., Fujimura, T., Yoshikawa, Y., Cho, F., and Kanai, A. (1996). Studies on the mechanism of early onset macular degeneration in cynomolgus monkeys. II. Suppression of metallothionein synthesis in the retina in oxidative stress. Exp. Eye Res., 62, 399-408. Niwa, Y., and Iizawa, O. (1994). Abnormalities in serum lipids and leukocyte superoxide dismutase and associated cataract formation in patients with atopic dermatitis. Arch. Dermatol., 130, 1387-1392. Niwa, Y., Sakane, T., Miyachi, Y., Kanoh, T., and Somiya, K. (1984). Decrease in generation of reactive oxygen species by neutrophils from patients with infectious mononucleosis: role of suppressor T lymphocytes. Blood, 64, 994-999. Nohl, H. (1993). Involvement of free radicals in AGEing: A consequence or cause of senescence. Br. Med. Bull., 49, 653667. Novelli, G.P. (1997). Role of free radicals in septic shock. J. Physiol. Pharmacol., 48, 517-527. Oberley, L.W., and Buettner, G.R. (1979). Role of superoxide dismutase in cancer: A review. Cancer Res., 39, 11411149. Oberley, T.D., and Oberley, L.W. (1997). Antioxidant enzyme levels in cancer. Histol. Histopathol., 12, 525-535. Ockner, R.K., Kaikaus, R.M., and Bass, N.M. (1993). Fatty-acid metabolism and the pathogenesis of hepatocellular carcinoma: review and hypothesis. Hepatology, 18, 669-676. Ogihara, H., Ogihara, T., Miki, M., Yasuda, H., and Mino, M. (1995). Plasma copper and antioxidant status in Wilson's disease. Pediatr. Res., 37, 219-226. WWW.ESAINC.COM 31 Ohno, E., Ohtsuka, E., Watanabe, K., Kohno, T., Takeoka, K., Saburi, Y., Kikuchi, H., and Nasu, M. (1997). Behcet's disease associated with myelodysplastic syndromes. A case report and a review of the literature. Cancer, 79, 262-268. Ortenblad, N., Madsen, K., and Djurhuus, M.S. (1997). Antioxidant status and lipid peroxidation after short-term maximal exercise in trained and untrained humans. Am. J. Physiol., 272, R1258-1263. Owen, A.D., Schapira, A.H., Jenner, P., and Marsden, C.D. (1997). Indices of oxidative stress in Parkinson's disease, Alzheimer's disease and dementia with Lewy bodies. J. Neural. Transm. Suppl., 51, 167-173. Packer, L. (1997). Oxidants, antioxidant nutrients and the athlete. J. Sports Sci., 15, 353-363. Paller, M.S. (1992). Free radical-mediated postischemic injury in renal transplantation. Ren. Fail., 14, 257-260. Palmer, H.J., and Paulson, K.E. (1997). Reactive oxygen species and antioxidants in signal transduction and gene expression. Nutr. Rev., 55, 353-361. Papa, S., and Skulachev, V.P. (1997). Reactive oxygen species, mitochondria, apoptosis and aging. Mol. Cell Biochem., 174, 305-319. Parke, D.V., and Parke, A.L. (1996). Chemical-induced inflammation and inflammatory diseases. Int. J. Occup. Med. Environ. Health, 9, 211-217. Parkinson, J.F., Mitrovic, B., and Merrill, J.E. (1997). The role of nitric oxide in multiple sclerosis [see comments]. J. Mol. Med., 75, 174-186. Patel, J.S., and Leonard, J.V. (1995). Ketonuria and medium-chain acyl-CoA dehydrogenase deficiency. J. Inherit. Metab. Dis., 18, 98-99. Peng, A., Yang, C., Rui, H., and Li, H. (1992). Study on the pathogenic factors of Kashin-Beck disease. J. Toxicol. Environ. Health, 35, 79-90. Percival, S.S., Bowser, E., and Wagner, M. (1995). Reduced copper enzyme activities in blood cells of children with cystic fibrosis. Am. J. Clin. Nutr., 62, 633-638. Peterhans, E. (1997). Oxidants and antioxidants in viral diseases: disease mechanisms and metabolic regulation. J. Nutr., 127, 962S-965S. Pitkanen, S., and Robinson, B.H. (1996). Mitochondrial complex I deficiency leads to increased production of superoxide radicals and induction of superoxide dismutase. J. Clin. Invest., 98, 345-351. Portal, B.C., Richard, M.J., Faure, H.S., Hadjian, A.J., and Favier, A.E. (1995). Altered antioxidant status and increased lipid peroxidation in children with cystic fibrosis [see comments]. Am. J. Clin. Nutr., 61, 843-847. Postma, N.S., Mommers, E.C., Eling, W.M., and Zuidema, J. (1996). Oxidative stress in malaria; implications for prevention and therapy. Pharm. World Sci., 18, 121-129. Prohaska, J.R. (1986). Genetic diseases of copper metabolism. Clin. Physiol. Biochem., 4, 87-93. Pronai, L., Ichikawa, Y., Nakazawa, H., and Arimori, S. (1990). Superoxide scavenging activity of leukocytes in rheumatoid arthritis and Behcet's diseases. Tokai J. Exp. Clin. Med., 15, 93-97. Pryor, W.A. (1997). Cigarette smoke radicals and the role of free radicals in chemical carcinogenicity. Environ. Health Perspect., 105 Suppl 4, 875-882. Pyne, D.B. (1994). Exercise-induced muscle damage and inflammation: A review. Aust. J. Sci. Med. Sport, 26, 49-58. Quie, P.G. (1977). Disorders of phagocyte function: Biochemical aspects. Prog. Clin. Biol. Res., 13, 157-169. Rachmilewitz, D., Okon, E., and Karmeli, F. (1997). Sulfhydryl blocker induced small intestinal inflammation in rats: a new model mimicking Crohn's disease. Gut, 41, 358-365. Ragusa, R.J., Chow, C.K., and Porter, J.D. (1997). Oxidative stress as a potential pathogenic mechanism in an animal model of Duchenne muscular dystrophy. Neuromuscul. Disord., 7, 379-386. Rahman, I., and MacNee, W. (1996). Role of oxidants/antioxidants in smoking-induced lung diseases. Free Radic. Biol. Med., 21, 669-681. Ralston, S.H. (1997). The Michael Mason Prize Essay 1997. Nitric oxide and bone: what a gas! Br. J. Rheumatol., 36, 831-838. Ramakrishna, B.S., Varghese, R., Jayakumar, S., Mathan, M., and Balasubramanian, K.A. (1997). Circulating antioxidants in ulcerative colitis and their relationship to disease severity and activity. J. Gastroenterol. Hepatol., 12, 490-494. Reimund, J.M., Allison, A.C., Muller, C.D., Dumont, S., Kenney, J.S., Baumann, R., Duclos, B., and Poindron, P. (1998). Antioxidants inhibit the in vitro production of inflammatory cytokines in Crohn's disease and ulcerative colitis. Eur. J. Clin. Invest., 28, 145-150. Reiter, R.J., Barlow-Walden, L., Poeggeler, B., Heiden, S.M., and Clayton, R.J. (1996). Twenty-four hour urinary excretion of 6-hydroxymelatonin sulfate in Down syndrome subjects. J. Pineal Res., 20, 45-50. Revillard, J.P. (1991). Oxidative stress: A marker of progression and a therapeutic target in HIV infection. Int. Conf. AIDS, 7, 73 (abstract no. TU.A.66). Ritter, K., Kuhl, R.J., Semrau, F., Eiffert, H., Kratzin, H.D., and Thomssen, R. (1994). Manganese superoxide dismutase as a target of autoantibodies in acute Epstein-Barr virus infection. J. Exp. Med., 180, 1995-1998. Rohrmoser, M.M., and Mayer, G. (1996). Reactive oxygen species and glomerular injury. Kidney Blood Press. Res., 19, 263-269. Rom, W.N., Bitterman, P.B., Rennard, S.I., Cantin, A., and Crystal, R.G. (1987). Characterization of the lower respiratory tract inflammation of nonsmoking individuals with interstitial lung disease associated with chronic inhalation of inorganic dusts. Am. Rev. Respir. Dis., 136, 1429-1434. Rosen, M.S., Baker, D.G., Schumacher, H.R. Jr., and Cherian, P.V. (1986). Products of polymorphonuclear cell injury inhibit IgG enhancement of monosodium urate-induced superoxide production. Arthritis Rheum., 29, 1473-1479. Rotig, A., de Lonlay, P., Chretien, D., Foury, F., Koenig, M., Sidi, D., Munnich, A., and Rustin, P. (1997). Aconitase and mitochondrial iron-sulfur protein deficiency in Friedreich ataxia. Nat. Genet., 17, 215-217. Rotilio, G. (1984). Favism: a hemolytic disease associated with increased superoxide dismutase and decreased glutathione peroxidase activities in red blood cells. Eur. J. Biochem., 139, 13-18. Runger, T.M., Epe, B., and Moller, K. (1995). Repair of ultraviolet B and singlet oxygen-induced DNA damage in xeroderma pigmentosum cells. J. Invest. Dermatol., 104, 68-73. WWW.ESAINC.COM 32 Samali, A., Gorman, A.M., and Cotter, T.G. (1996). Apoptosis – the story so far. Experientia, 52, 933-941. Sanfey, H., Sarr, M.G., Bulkley, G.B., and Cameron, J.L. (1986). Oxygen-derived free radicals and acute pancreatitis: A review. Acta Physiol. Scand. Suppl., 548, 109-118. Santavuori, P., Heiskala, H., Autti, T., Johansson, E., and Westermarck, T. (1989). Comparison of the clinical courses in patients with juvenile neuronal ceroid lipofuscinosis receiving antioxidant treatment and those without antioxidant treatment. Adv. Exp. Med. Biol., 266, 273-282. Scarfiotti, C., Fabris, F., Cestaro, B., and Giuliani, A. (1997). Free radicals, atherosclerosis, aging, and related dysmetabolic pathologies: Pathological and clinical aspects. Eur. J. Cancer Prev., 6, Suppl 1, S31-36. Schallreuter, K.U., Pittelkow, M.R., and Wood, J.M. (1991). Defects in antioxidant defense and calcium transport in the epidermis of xeroderma pigmentosum patients. Arch. Dermatol. Res., 283, 449-455. Schapira, A.H. (1996). Oxidative stress and mitochondrial dysfunction in neurodegeneration. Curr. Opin. Neurol., 9, 260264. Schiller, J., Arnhold, J., Sonntag, K., and Arnold, K. (1996). NMR studies on human, pathologically changed synovial fluids: role of hypochlorous acid. Magn. Reson. Med., 35, 848-853. Schmidt, A.M., Weidman, E., Lalla, E., Yan, S.D., Hori, O., Cao, R., Brett, J.G., and Lamster, I.B. (1996). Advanced glycation endproducts (AGEs) induce oxidant stress in the gingiva: A potential mechanism underlying accelerated periodontal disease associated with diabetes. J. Periodontal Res., 31, 508-515. Schoenberg, M.H., and Beger, H.G., (1993). Reperfusion injury after intestinal ischemia. Crit. Care Med., 21, 1376-1386. Sedghi, S., Keshavarzian, A., Klamut, M., Eiznhamer, D., and Zarling, E.J. (1994). Elevated breath ethane levels in active ulcerative colitis: evidence for excessive lipid peroxidation. Am. J. Gastroenterol., 89, 2217-2221. Selden, C., Seymour, C.A., and Peters, T.J. (1980). Activities of some free-radical scavenging enzymes and glutathione concentrations in human and rat liver and their relationship to the pathogenesis of tissue damage in iron overload. Clin. Sci., 58, 211-219. Semenkovich, C.F., and Heinecke, J.W. (1997). The mystery of diabetes and atherosclerosis: time for a new plot. Diabetes, 46, 327-334. Sendtner, M., and Thoenen, H. (1994). Neurodegenerative disease. Oxidative stress and motorneuron disease. Curr. Biol., 4, 1036-1039. Sethi, N.C., Madadi, A.J., and Bhandari, S. (1996). Serum zinc, copper, magnesium, proteins and superoxide dismutase in leprosy patients on multidrug therapy – a follow-up study. Indian J. Lepr., 68, 325-333. Sharp, A.K., and Banerjee, D.K. (1985). Hydrogen peroxide and superoxide production by peripheral blood monocytes in leprosy. Clin. Exp. Immunol., 60, 203-206. Shaw, P.J., Ince, P.G., Falkous, G., and Mantle, D. (1995). Oxidative damage to protein in sporadic motor neuron disease spinal cord. Ann. Neurol., 38, 691-695. Sies, H. (1985). Oxidative stress: Introductory remarks. In: Oxidative Stress. Sies, H. (Ed.). Academic Press, London. Sies, H. (1991). Oxidative stress: From basic research to clinical applications. Am. J. Med., 91, 31S-38S. Sies, H. (1997). Oxidative stress: Oxidants and antioxidants. Exp. Physiol., 82, 291-295. Simic, M.G. (1992). The rate of DNA damage and aging. EXS, 62, 20-30. Simonian, N.A., and Coyle, J.T. (1996). Oxidative stress in neurodegenerative diseases. Annu. Rev. Pharmacol. Toxicol., 36, 83-106. Slaga, T.J. (1995). Inhibition of the induction of cancer by antioxidants. Adv. Exp. Med. Biol., 369, 167-174. Slater, A.F., Nobel, C.S., and Orrenius, S. (1995). The role of intracellular oxidants in apoptosis. Biochim. Biophys. Acta, 1271, 59-62. Smith, L.J., Shamsuddin, M., Sporn, P.H., Denenberg, M., and Anderson, J. (1997). Reduced superoxide dismutase in lung cells of patients with asthma. Free Radic. Biol. Med., 22, 1301-1307. Sobel, B.E. (1974). Biochemical and morphological changes in infarcting myocardium. In: The Myocardium: Failure and Infarction. Braunwald, E. (Ed.).. N.P. Publishing Co. Inc., New York. Pp. 247-260. Sohal, R.S. (1993). The free radical hypothesis of aging: an appraisal of the current status [see comments]. Aging, 5, 317. Sokol, R.J., Twedt, D., McKim, J.M., Jr. Devereaux, M.W., Karrer, F.M., Kam, I., von Steigman, G., Narkewicz, M.R., Bacon,.B.R., and Britton, R.S. Oxidant injury to hepatic mitochondria in patients with Wilson's disease and Bedlington terriers with copper toxicosis. Gastroenterolog, 107, 1788-1798. Solis-Herruzo, J.A., Fernandez, B., Vilalta-Castell, E., Munoz-Yague, M.T., Hernandez-Munoz, I., de la Torre-Merino, M.P., and Balsinde, J. (1993). Diminished cytochrome b content and toxic oxygen metabolite production in circulating neutrophils from patients with Crohn's disease. Dig. Dis. Sci., 38, 1631-1637. Southorn, P.A., and Powis, G. (1988). Free radicals in medicine. II. Involvement in human disease. Mayo Clin. Proc., 63, 390-408. Stadie, W.C., Riggs, B.C., and Haugaard, N. (1944). Oxygen poisoning. Am. J. Med. Sci., 207, 84-114. Stichtenoth, D.O., and Frolich, J.C. (1998). Nitric oxide and inflammatory joint diseases. Br. J. Rheumatol., 37, 246-257. Stack, T.A. (1989). Haemolytic anemia due to oxidative stress in the preterm infant [letter]. Early Hum. Dev., 20, 79-80. Stocks, J., Kemp, M., and Dormandy, T.L. (1971). Increased susceptibility of red-blood-cell lipids to autooxidation in hemolytic states. Lancet, 1, 266-269. Stoian, I., Oros, A., and Moldoveanu, E. (1996). Apoptosis and free radicals. Biochem. Mol. Med., 59, 93-97. Sugawara, M., Kita, T., Lee, E.D., Takamatsu, J., Hagen, G.A., Kuma, K., Medeiros-Neto, G.A. (1988). Deficiency of superoxide dismutase in endemic goiter tissue. J. Clin. Endocrinol. Metab., 67, 1156-1161. Suryaprabha, P., Das, U.N., Ramesh, G., Kumar, K.V., and Kumar, G.S. (1991). Reactive oxygen species, lipid peroxides and essential fatty acids in patients with rheumatoid arthritis and systemic lupus erythematosus. Prostaglandins Leukot. Essent. Fatty Acids, 43, 251-255. Suzuki, K. (1993). Origin of senescence: A review. Horm. Res., 39, Suppl 1, 5-8. WWW.ESAINC.COM 33 Swerdlow, R.H., Parks, J.K., Cassarino, D.S., Maguire, D.J., Maguire, R.S., Bennett, J.P. Jr., Davis, R.E., and Parker, W.D. Jr. (1997). Cybrids in Alzheimer's disease: A cellular model of the disease? [see comments]. Neurology, 49, 918925. Szabo, C. (1996). The pathophysiological role of peroxynitrite in shock, inflammation, and ischemia-reperfusion injury. Shock, 6, 79-88. Szabo, J., Foris, G., Mezosi, E., Nagy, E.V., Paragh, G., Sztojka, I., and Leovey, A. (1996). Parameters of respiratory burst and arachidonic acid metabolism in polymorphonuclear granulocytes from patients with various thyroid diseases. Exp. Clin. Endocrinol. Diabetes, 104, 172-176. Taylor, D.E., and Piantadosi, C.A. (1995). Oxidative metabolism in sepsis and sepsis syndrome. J. Crit. Care, 10, 122135. Thome, J., Zhang, J., Davids, E., Foley, P., Weijers, H.G., Wiesbeck, G.A., Boning, J., Riederer, P., and Gerlach, M. (1997). Evidence for increased oxidative stress in alcohol-dependent patients provided by quantification of in vivo salicylate hydroxylation products. Alcohol Clin. Exp. Res., 21, 82-85. Thunell, S., Andersson, D., Harper, P., Henrichson, A., Floderus, Y., and Lindh, U. (1997). Effects of administration of antioxidants in acute intermittent porphyria. Eur. J. Clin. Chem. Clin. Biochem., 35, 427-433. Toledo-Pereyra, L.H. (1991). Liver transplantation reperfusion injury. Factors in its development and avenues for treatment. Klin. Wochenschr., 69, 1099-1104. Towner, R.A., Janzen, E.G., Zhang, Y.-K., and Yamashiro, S. (1993). MRI study of the inhibitory effects of new spin traps on in vivo CCl4-induced hepatotoxicity in rats. Free Radic. Biol. Med., 14, 677-681. Traub, O., and Van Bibber, R. (1995). Role of nitric oxide in insulin-dependent diabetes mellitus-related vascular complications. West. J. Med., 162, 439-445. Trenam, C.W., Blake, D.R., and Morris, C.J. (1992). Skin inflammation: reactive oxygen species and the role of iron. J. Invest. Dermatol., 99, 675-682. Troll, W. (1991). Prevention of cancer by agents that suppress oxygen radical formation. Free Radic. Res. Commun., 1213 Pt 2, 751-757. Trush, M.A., and Kensler, T.W. (1991). An overview of the relationship between oxidative stress and chemical carcinogenesis. Free Radic. Biol. Med., 10, 201-209. Umeki, S. (1994). Mechanisms for the activation/electron transfer of neutrophil NADPH-oxidase complex and molecular pathology of chronic granulomatous disease. Ann. Hematol., 68, 267-277. van der Hagen, A.M., Yolton, D.P., Kaminski, M.S., and Yolton, R.L. (1993). Free radicals and antioxidant supplementation: a review of their roles in age-related macular degeneration. J. Am. Optom. Assoc., 64, 871-878. Varma, S.D., Chand, D., Sharma, Y.R., Kuck, J.F. Jr., and Richards, R.D. (1984). Oxidative stress on lens and cataract formation: role of light and oxygen. Curr. Eye Res., 3, 35-57. Varma, S.D., Devamanoharan, P.S., and Morris, S.M. (1995). Prevention of cataracts by nutritional and metabolic antioxidants. Crit. Rev. Food Sci. Nutr., 35, 111-129. Vennerstrom, J.L., and Eaton, J.W. (1988). Oxidants, oxidant drugs, and malaria. J. Med. Chem., 31, 1269-1277. Verhaar, M.C., Wever, R.M., Kastelein, J.J., van Dam, T., Koomans, H.A., and Rabelink, T.J. (1998). 5methyltetrahydrofolate, the active form of folic acid, restores endothelial function in familial hypercholesterolemia. Circulation, 97, 237-241. Vertongen, F., Heyder-Bruckner, C., Fondu, P., and Mandelbaum, I. (1981). Oxidative hemolysis in protein malnutrition. Clin. Chim. Acta, 116, 217-222. Volicer, L., and Crino, P.B. (1990). Involvement of free radicals in dementia of the Alzheimer type: A hypothesis. Neurobiol. Aging, 11, 567-571. Volkman, D.J., Buescher, E.S., Gallin, J.I., and Fauci, A.S. (1984). B cell lines as models for inherited phagocytic diseases: abnormal superoxide generation in chronic granulomatous disease and giant granules in Chediak-Higashi syndrome. J. Immunol., 133, 3006-3009. Walsh, S., and Patterson, J.W. (1991). Effects of oxidants on lens transport. Invest. Ophthalmol. Vis. Sci., 32, 1648-1658. Waxman, K. (1996). Shock: Ischemia, reperfusion, and inflammation. New Horiz., 4, 153-160. Weight, S.C., Bell, P.R., and Nicholson, M.L. (1996). Renal ischemia – reperfusion injury. Br. J. Surg., 83, 162-170. Weinberg, E.D. (1996). The role of iron in cancer. Eur. J. Cancer Prev., 5, 19-36. Weitzman, S.A., and Gordon, L.I. (1990). Inflammation and cancer: role of phagocyte-generated oxidants in carcinogenesis. Blood, 76, 655-663. Wennmalm, A. (1994). Nitric oxide (NO) in the cardiovascular system: role in atherosclerosis and hypercholesterolemia. Blood Press., 3, 279-382. Welch, G., and Loscalzo, J. (1994). Nitric oxide and the cardiovascular system. J. Card. Surg., 9, 361-71. Welch, G.N., Upchurch, G.R., and Loscalzo, J. (1997).Homocysteine, oxidative stress, and vascular disease [see comments]. Hosp. Pract., 32, 81-2 and 85, 88-92. Westhuyzen, J., Adams, C.E., and Fleming, S.J. (1996). Evidence for oxidative stress during in vitro dialysis. Nephron, 70, 49-54. Whitin, J.C., and Cohen, H.J. (1988). Disorders of respiratory burst termination. Hematol. Oncol. Clin. North Am., 2, 289299. Winklhofer-Roob, B.M. (1994). Oxygen free radicals and antioxidants in cystic fibrosis: the concept of an oxidantantioxidant imbalance. Acta Paediatr. Suppl., 83, 49-57. Winrow, V.R., Winyard, P.G., Morris, C.J., and Blake, D.R. (1993). Free radicals in inflammation: second messengers and mediators of tissue destruction. Br. Med. Bull., 49, 506-522. Winterbourn, C.C. (1990). Oxidative denaturation in congenital hemolytic anemias: The unstable hemoglobins. Semin. Hematol., 27, 41-50. Winterbourn, C.C., Benatti, U., and De Flora, A. (1986). Contributions of superoxide, hydrogen peroxide, and transition metal ions to auto-oxidation of the favism-inducing pyrimidine aglycone, divicine, and its reactions with hemoglobin. Biochem. Pharmacol., 35, 2009-2015. WWW.ESAINC.COM 34 Winyard, P.G., and Blake, D.R. (1997). Antioxidants, redox-regulated transcription factors, and inflammation. Adv. Pharmacol., 38, 403-421. Wiseman, A., and Goldfarb, P. (1996). Is there an optimal membrane protein content determined by membrane stability to lipid peroxidation? Disease and aging consideration. Biochem. Soc. Trans., 24, 376S. Wolff, S.P., Jiang, Z.Y., and Hunt, J.V. (1991). Protein glycation and oxidative stress in diabetes mellitus and aging. Free Radic. Biol. Med., 10, 339-352. Wong, P.C., and Borchelt, D.R. (1995). Motor neuron disease caused by mutations in superoxide dismutase 1. Curr. Opin. Neurol., 8, 294-301. Worlitzsch, D., Herberth, G., Ulrich, M., and Doring, G. (1998). Catalase, myeloperoxidase and hydrogen peroxide in cystic fibrosis. Eur. Respir. J., 11, 377-383. Wu, J., and Xu, G.L. (1987). Plasma selenium content, platelet glutathione peroxidase and superoxide dismutase activity of residents in Kashin-Beck disease affected area in China. J. Trace Elem. Electrolytes Health Dis., 1, 39-43. Yenchitsomanus, P., and Wasi, P. (1983). Increased erythrocyte superoxide dismutase activities in beta 0thalassaemia/hemoglobin E and in hemoglobin H diseases. J. Clin. Pathol., 36, 329-333. Young, I.S., Trouton, T.G., Torney, J.J., McMaster, D., Callender, M.E., and Trimble, E.R. (1994). Antioxidant status and lipid peroxidation in hereditary haemochromatosis. Free Radic. Biol. Med., 16, 393-397. Yoshida, S., and Gershwin, M.E. (1993). Autoimmunity and selected environmental factors of disease induction. Semin. Arthritis Rheum., 22, 399-419. Yoshida, Y. Machigashira, K., Suehara, M., Arimura, H., Moritoyo, T., Nagamatsu, K., and Osame, M. (1995). Immunological abnormality in patients with lysinuric protein intolerance. J. Neurol. Sci., 134, 178-182. Youdim, M.B.H. (1988). Iron in the brain: Implications for Parkinson’s and Alzheimer’s diseases. Mount Sinai J. Med., 55, 97-101. Youdim, M.B., Ben-Shachar, D., and Riederer, P. (1990). The role of monoamine oxidase, iron-melanin interaction, and intracellular calcium in Parkinson's disease. J. Neural. Transm. Suppl., 32, 239-248. Yu, M.W., Chiang, Y.C., Lien, J.P., and Chen, C.J. (1997). Plasma antioxidant vitamins, chronic hepatitis B virus infection and urinary aflatoxin B1-DNA adducts in healthy males. Carcinogenesis, 18, 1189-1194. Zeman, S., Lloyd, C., Meldrum, B., and Leigh, P.N. (1994). Excitatory amino acids, free radicals and the pathogenesis of motor neuron disease. Neuropathol. Appl. Neurobiol., 20, 219-231. Zhao, M., Matter, K., Laissue, J.A., and Zimmermann, A. (1996). Copper/zinc and manganese superoxide dismutases in alcoholic liver disease: Immunohistochemical quantitation. Histol. Histopathol., 11, 899-907. Zigler, J.S. Jr., and Hess, H.H. (1985). Cataracts in the Royal College of Surgeons rat: evidence for initiation by lipid peroxidation products. Exp. Eye Res., 41, 67-76. WWW.ESAINC.COM 35 Chapter 2 The Chemistry of The Reactive Species As discussed in Chapter 1, the discovery that oxygen is toxic led to the immediate consideration of the potential role of free radicals as the damaging species. Data rapidly revealed that not all oxygen-based noxious compounds were free radicals. Furthermore, pro-oxidants other than the ROS were discovered that contained atoms in addition to oxygen. This chapter primarily reviews the formation, reaction chemistry, and biological significance of the various important pro-oxidants, including those based on oxygen, nitrogen, halogens, sulfur and carbonyls. Some of the analytical approaches used to measure them will also be discussed. The chapter concludes with an overview of the pro-oxidant activities of a variety of xenobiotics (foreign or man-made substances) and environmental pollutants. Remember though, that just because a reaction can be made to occur in a test tube does not mean that such a reaction is important biologically. WWW.ESAINC.COM 36 OXYGEN AND THE REACTIVE OXYGEN SPECIES (ROS). 1. Oxygen. Properties. Oxygen (dioxygen) is a colorless and odorless diatomic gas. It was discovered independently by Karl W. Scheele (1742-1786) in 1772 and Joseph Priestley (1733-1804) in 1774 (see Gilbert (1999) for an excellent review). As Scheele’s work was not published until 1777, Priestley is often credited with the discovery of oxygen. Priestley named the new gas “dephlogisticated air” which was eventually called “oxygene” (acid former) by Antoine Lavoisier (1743-1794). Oxygen is particularly abundant in the earth’s crust (~54% by weight), occurs in the atmosphere (~21% by volume, 23% by weight for dry air), and is the major component of water’s structure (89% by weight). Oxygen has a melting point of –219oC and a boiling point of –183oC. Oxygen is only slightly soluble in water (~280mmol/dm3 at 25oC) — enough to support aquatic life. Oxygen is about five times more soluble in organic solvents. Oxygen is the first member of Group 6B of the periodic table and possesses eight electrons with an electronic configuration of 1s2, 2s2, 2p4. Oxygen does not possess available d orbitals so it is limited to a valency of 2. As shown in Figure 2.1, oxygen (3Σg-O2) is a diradical, possessing two unpaired electrons. Oxygen is therefore paramagnetic and can be measured using EPR. Formation. Oxygen can be formed in the laboratory by: a) thermal decomposition of metal oxides low in the electrode potential series (e.g., Eqn 2.1); b) thermal decomposition of higher oxides (e.g., Eqn 2.2); c) catalytic decomposition of peroxides (Eqn 2.3); d) the reaction between solid peroxides and water (Eqn 2.4); e) thermal decomposition of salts containing oxygen-enriched anions (e.g., Eqns 2.5 and 2.6); or f) the electrolysis of aqueous solutions of acids or alkalis. Industrially, oxygen is obtained from the atmosphere by the liquefaction of air. Biologically, oxygen is produced as a waste product of photosynthesis (Eqn 2.7). WWW.ESAINC.COM 37 Oxygen and ROS Electronic Configuration σ*2p π *2p π2p σ 2p GroundState Oxygen (3Σg-O2) Singlet Oxygen (1∆gO2) Singlet Oxygen (3Σg+O2) Superoxide Radical Anion (O2.-) Peroxide Anion (O22-) Figure 2.1 Molecular Orbital Diagram Of Molecular Oxygen And Some ROS (Based On An Original Figure By Halliwell And Gutteridge (1999)). 2HgO → 2Hg + O2 2Pb3O4 → 6PbO + O2 2H2O2 → 2H2O + O2 2(Na)2O2 + H2O → 4NaOH + O2 2KNO3 → 2KNO2 + O2 2KMnO4 → K2MnO4 + MnO2 + O2 6CO2 + 6H2O → C6H12O6 + 6O2 Eqn 2.1 Eqn 2.2 Eqn 2.3 Eqn 2.4 Eqn 2.5 Eqn 2.6 Eqn 2.7 Chemical Reactions and Biological Significance. The reactions of oxygen are often slower than would be predicted from its electronegativity (3.5)1 which is second only to fluorine (4.0), a very reactive, strong oxidizing agent. The reason for oxygen’s inertness is that its double bond dissociation energy is relatively high so that reactions that require this double 1 Electronegativity is a measure of the ability of an atom to attract electrons and involves both ionization energy and electron affinity. Pauling gave fluorine, the most electronegative element, an arbitrary value of 4.0 and related the electronegativities of the atoms of other elements to it. The bond formed between two atoms of similar electronegativity will be essentially covalent. An increase in the electronegativity of one atom will attract the electrons involved in the covalent bond, causing it to be polarized. Further increases in electronegativity will result in increased polarity until the electron pair will reside almost entirely on one atom, i.e., an electrovalent bond will be established. WWW.ESAINC.COM 38 bond to be broken occur only at high temperatures. If this were not the case, spontaneous combustion of animals and plants would be a very common event! Once initiated by an external energy source most reactions involving the breaking of oxygen’s double bond are self-sustaining, due to their exothermic nature. The majority of oxygen reactions are found to occur at temperatures considerably higher than room temperature, but oxygen can be made to react at physiological temperatures by a variety of enzymes (Table 3.1). The most facile reactions of oxygen are those in which its double bond is not completely broken, such as in the formation of superoxide radical anion and peroxides. An explanation for oxygen’s lack of reactivity can best be understood from its electronic structure (Figure 3.1). Ground state oxygen has two unpaired (parallel spin) electrons occurring in two degenerate antibonding π*2p orbitals (i.e., dioxygen is a triplet molecule in the ground state). For oxygen to oxidize a chemical species in a two-electron reaction, the compound undergoing reaction must have two unpaired (antiparallel spin) electrons to enter the π*2p orbitals of the ground state oxygen molecule. Due to Pauli’s exclusion principle, an electron pair would not fulfill this criterion. As a result, oxygen tends to accept electrons singularly. This explains why molecular oxygen is kinetically unreactive with most compounds but readily reacts with σ-radicals (such as R•) and transition metal complexes. This is important when proposing that a compound undergoes autooxidation (see below). σ-Radicals possess an unpaired electron which can readily enter one of oxygen’s π*2p orbitals. Readers interested in a more in-depth discussion of the reactivity of oxygen are referred to Malmstrom (1982), and Naqui et al. (1986). Oxygen reacts with most metals except the less reactive ones (e.g., silver and gold). Lithium forms oxides (Eqn 2.8). Sodium forms oxides and, in excess oxygen, peroxides (Eqn 2.9). The remaining group 1A elements form superoxides (e.g., KO2). For transition metals, the oxidation state of the metal in the product depends upon the reaction conditions and the complexation of the transition metal, as discussed in Chapter 2 and in greater detail below. The oneelectron reduction of oxygen by transition metal complexes is extremely important to redox biochemistry. All non-metals, with the exception of the noble gases and halogens, react directly with oxygen (Eqn 2.10). Oxides of the halogens and the heavier noble gases can be prepared only by indirect means. 4Li + O2 → 2Li2O 2Na + O2 → Na2O2 S + O2 → SO2 WWW.ESAINC.COM Eqn 2.8 Eqn 2.9 Eqn 2.10 39 Enzyme Oxidases that reduce dioxygen to hydrogen peroxide Comments Flavin-containing Oxidases a) Simple - D- and L-amino acid oxidase; Glucose oxidase; Some monoamine oxidases; “Old Yellow Enzyme”; Lactate oxidase b) Metalloflavoproteins Xanthine oxidase; Aldehyde oxidase Reference Malmstrom (1982) Metal-containing Oxidases Galactose oxidase; Some monoamine oxidases; benzylamine oxidases Oxidases that reduce dioxygen to water Blue Oxidases Ascorbate oxidase; Ceruloplasmin; Laccase Malmstrom (1982) Cytochrome Oxidase Oxygenases Dioxygenases a) Flavin-containing: Few exist b) Metal-containing: Heme – Tryptophan-2,3-dioxygenase; Indoleamine-2,3-dioxygenase Non-heme – Lipoxygenase Malmstrom (1982); White and Coon (1980) Monoxygenases a) Flavin-containing: p-Hydroxybutylate hydroxylase b) Metal-containing: Heme – Cytochrome P450 Non-heme – Tyrosinase; Dopamine-β- hydroxylase Table 2.1 Some Enzymes That Utilize Oxygen. Oxygen is converted to its allotrope2 ozone (O3) by silent (non-thermal) electrical discharge (Eqn 2.11). Formation of ozone is an endothermic process so that thermal energy produced during sparking would decompose it. 2 Allotropy is the ability of a substance to exist in two or more physical forms. WWW.ESAINC.COM 40 3O2 → 2O3 O2 + 4H+ + 4e- Eqn 2.11 Eqn 2.12 → 2H2O Oxygen is transformed into water through successive additions of electrons, which produce a series of reduced intermediates collectively known as the reactive oxygen species (ROS) (Figure 2.2). The four-electron, four-proton reduction of oxygen to water is biologically very important as it is the terminal reaction of aerobic respiration (Eqn 2.12). Unfortunately, respiration is not perfect and electrons can “leak” from the electron transport chains, resulting in the formation of potentially damaging ROS.3 Although mitochondrial respiration is one of the major producers of ROS, it is by no means the only source (see below). The steady-state levels of the ROS under biological conditions are typically kept low with hydrogen peroxide, superoxide, and hydroxyl free radical levels being 10-7-10-9, 10-10-10-11 and 10-15- 10-20M, respectively (Chance et al. (1979); Floyd (1997)). Even so it is estimated that a 70kg human would be expected to produce more than 1kg of superoxide annually! '∆g O 2 HO2 SINGLET OXYGEN HYDROPEROXYL H+ pKa = 4.5 e- 2/3O3 OZONE O2 DIOXYGEN -330mV 2H+ + e- Ο2− +940mV SUPEROXIDE H+ + e- Η2Ο2 H+ + e- H2 O ΗΟ +380mV +2330mV HYDROGEN HYDROXYL PEROXIDE FREE RADICAL Η 2Ο WATER SUPEROXIDE DISMUTASE CATALASE Figure 2.2 The Relationship Between Oxygen And ROS. The Potentials (in mV) are Electrode Potentials for the Reaction (see Appendix 2.1) 3 Estimates for ROS production vary widely but it appears that about 2-3% of oxygen uptake by isolated mitochondria in state 4 is reduced to H2O2 (Bovis and Chance (1973)); the production of superoxide is about 2-3nmol/min/mg protein 4 (Beal, (1997)); Escherichia coli produce 3 superoxide molecules for every 10 electrons transferred along their respiratory chains (Imlay and Linn (1988)). WWW.ESAINC.COM 41 The question is sometimes asked as to why oxygen and not some other compound was chosen by nature as the terminal oxidant of the respiratory chain. This question was elegantly answered by George (1965). He hypothesized that “the kinetic activity of the halogens make them unsuitable as biological oxidants, and nitrogen is too poor an oxidizing agent. Thus oxygen is the only element in the most appropriate physical state, with a satisfactory solubility in water and with desirable combinations of kinetic and thermodynamic properties.” Oxygen was therefore the only option even though it came with a high price – its reduction to pro-oxidant and biologically damaging species. As shown in Figure 2.2 the ROS include both reduced and non-reduced forms of oxygen. Before examining the reduced forms of oxygen in more detail, we will first explore the chemistries of ozone and the electronically excited form of oxygen — singlet oxygen. 2. Ozone. Properties. Ozone (O3) is an unstable, toxic, pale blue diamagnetic gas with a distinctive odor. It has a melting point of –250oC and a boiling point of –112oC. It is only slightly more soluble in water than oxygen, but unlike oxygen can also react with it. Formation. Ozone is produced in the stratosphere by the action of sunlight on atmospheric oxygen during the Chapman cycle (reviewed by Mustafa (1990) and Madronich (1999)). The ozone layer extends from 17km above the equator (8km above the poles) to about 50km above the Earth’s surface. This beneficial layer absorbs UV energy and protects living species from the mutagenic effects of electromagnetic radiation. In the lower troposphere, however, ozone is a major pollutant and one of the main components of photochemical smog. Ozone can be formed in the vicinity of electrical machinery and may cause health problems in poorly ventilated areas. Ozone can be formed in the laboratory by exposing dry oxygen gas to silent electrical discharge (Eqn 2.11). Chemical Reactions and Biological Significance. Ozone is thermodynamically unstable with respect to oxygen and, at high concentrations, is dangerously explosive. It is a very reactive compound (similar in reactivity to gaseous chlorine and fluorine) and even reacts slowly with water to produce a variety of ROS (Table 2.2). Ozone is a strong oxidizing agent. It is WWW.ESAINC.COM 42 only a little less reactive than the hydroxyl free radical and a much more powerful oxidant than oxygen. Ozone can oxidize sulfide to sulfate, iodide to iodine and Fe (II) to Fe (III). Ozone’s reaction with thiols (e.g., albumin and glutathione (GSH)) is complex. Ozone oxidizes GSH to its disulfide in a two-electron process (note that the valency of sulfur does not change) (Eqn 2.13). It can also react with thiols to produce compounds containing sulfur in its higher valencies. For example, thiols or thiol anions (mercaptide ions) can act as nucleophiles and undergo additional reactions with ozone, resulting in the formation of alkyl sulfinates (RSO2H) and alkyl sulfonates (RSO3H) (note that sulfur’s valency has increased from 2 to 4 and 2 to 6, respectively) (sulfur’s valency changes are explored in greater detail below). Ozone can react with mercaptide ions in a oneelectron process, leading to the formation of the thiyl radical and ozone radical anion (Eqn 2.14). The latter decomposes under acidic conditions and produces hydroxyl free radicals (Eqn 2.15). The thiyl (RS•) radical can also undergo several other reactions, including dimerization with itself or other thiols, forming the disulfide (RSSR) and mixed disulfide (R’SSR”), respectively. It can react with the mercaptide anion, forming the disulfide radical anion (RSSR•-) that can reduce oxygen to superoxide (Eqn 2.16). We will explore the formation and reactions of the thiyl radical in greater detail below. 2GSH + O3 → GSSG + O2 + H2O RS- + O3 → RS• + O3•O3•- + H+ → HO• + O2 RSSR•- + O2 → RSSR + O2•- Eqn 2.13 Eqn 2.14 Eqn 2.15 Eqn 2.16 Ozone readily reacts with organic compounds containing unsaturated bonds and forms a variety of chemical species — hydrogen peroxide, carbonyl compounds, Criegee ozonides, aliphatic radicals and hydroxy-hydroperoxides (Figure 2.3) — depending on the environment in which the reactions are taking place (Pryor (1993); Pryor and Church (1991)). Ozone also attacks unsaturated compounds and produces free radicals capable of promoting lipid peroxidation. Unlike all the other ROS, ozone is the only pro-oxidant not produced endogenously. Ozone is extremely toxic to living organisms. For example, it can cause respiratory problems (airway inflammation and decreased pulmonary function), and can damage the skin of humans and animals as well as the surface tissues of plants (Menzel (1984); Menzel and Meacher (1999); Mustafa (1990); Runeckles (1994); Thiele et al. (1997)). Such biological damage is complex but is thought to be due to direct oxidation, through the formation of free radicals and by the production of other reactive intermediates. The reactions of ozone of biological importance are summarized in Table 2.2. WWW.ESAINC.COM 43 Reaction Comments In aqueous solution ozone decomposes to give a variety of ROS including H2O2, O2•-, HO• and HO2•. Ozone may be able to abstract H• (hydrogen atoms) from many organic compounds leading to chain reactions in the presence of O2. Fatty acids with one double bond and cholesterol react to give epoxides. Water Saturated compounds Unsaturated compounds PUFAs (polyunsaturated fatty acids) undergo lipid peroxidation giving RO2•, RO2H, RO•, TBAR-reactive (thiobarbituric acid reactive) substances such as MDA (malondialdehyde), aldehydes, conjugated dienes, alkanes (e.g., ethane, pentane), and epoxides. Many reactive aldehydes are cytotoxic (see below). Damage to the lipid bilayer leads to altered membrane fluidity and permeability. Many functional groups are oxidized including thiols, amines, alcohols, and aldehydes. Amino acids such as cysteine, cystine, histidine, methionine, tyrosine and tryptophan can be oxidized. Enzymes such as those involved in prostaglandin synthesis, cholinesterase and α1-antiproteinase can be damaged by ozone. Other macromolecules such as structural proteins, DNA, RNA and membranes can also be damaged. Ozone readily reacts with antioxidants such as albumin, ascorbic acid, GSH, tocopherol, and uric acid. Amino acids, proteins, enzymes, DNA and RNA Antioxidants Table 2.2 Some Important Reactions Of Ozone. OH OH R1 H H R2 DIOL O R1 O2 H H R2 DIRADICAL R1 Reduction H O R1 R2 O O O R1 O R1CHO O O H H R2 TRIOXOLANE NONAQUEOUS O O O H R2 CRIEGEE OZONIDE O R2 OH H CARBONYL OXIDE AQUEOUS R2 H O2H R2CHO H2O2 HYDROXYHYDROPEROXIDE Figure 2.3 The Reaction Of Ozone With Alkenes Such As PUFAs Under Aqueous And Non-Aqueous Conditions. Based On Mechanisms Originally Proposed By Pryor And Church (1991). WWW.ESAINC.COM 44 Ozone is an important atmospheric pollutant so its biological actions have been studied in several animal and plant models. Studies have focused on the action of respired ozone on pulmonary function. Exposure to ozone caused airway hyperreactivity, neutrophil infiltration, increased epithelial macromolecular permeability, and the promotion of mucus secretion that eventually culminates in lung inflammation. The first biological fluid that comes into contact with inhaled ozone is the respiratory tract lining fluids (RTLF) which not only serve to absorb and detoxify ozone but also limit its passage to more vulnerable areas (e.g., the peripheral gas exchange regions of the lung). It is extremely difficult to obtain an RTLF sample to study possible antioxidant defenses so it is common to use plasma (Cross et al. (1992); Van der Vleit et al. (1995a)) or skin as models (Thiele et al. (1997)). In the plasma model ozone quickly and directly reacted with uric acid and ascorbic acid, but only slowly reacted with protein-thiol groups. In skin, ozone depleted both ascorbic acid and α-tocopherol while increased production of the lipid peroxidation product, malondialdehyde. RTLF contains high levels of glutathione (GSH) that not only reacts directly with ozone (Eqn 2.13) but also can control the toxic effects of some of its secondary products (e.g., aldehydes and hydrogen peroxide). The biological consequences of inhalation cannot be due totally to ozone itself. Ozone will react primarily with RTLF antioxidants so that it will not be able to deeply penetrate lung tissue. Pryor (1993) proposed ozone’s toxicity may be due to a cascade mechanism whereby ozonolysis products act as messengers capable of inducing biological changes far removed from the initial site of ozone attack. Such products include aldehydes, hydroxyhydroperoxides, and Criegee ozonides that can activate lipases. Lipases can then release endogenous cellular signal transduction molecules and mediators of inflammation, such as eicosonides and platelet-activating factors. Ozonolysis products may also be responsible for some of the other problems caused by respired ozone such as carcinogenesis, damage to the hematopoietic system, and altered central nervous system functionality (Mustafa (1990)). Measurement. The presence of ozone can be determined by its ability to “tail” mercury (the surface of this metal is partially converted to its oxide so that it sticks to the walls of the vessel containing it). In the laboratory ozone can be quantified by reacting it with acidified potassium iodide. The iodine so liberated can then be titrated with a standard solution of sodium thiosulfate. Ozone also can be measured using chemiluminescence-based detectors (MacDougal et al. (1998); van Heusden and Mans (1978)). WWW.ESAINC.COM 45 3. Singlet Oxygen. Properties. The input of appropriate energy can excite the unpaired electrons of the oxygen molecule, thereby forming singlet oxygen. There are two forms of singlet oxygen (Figure 2.1). 1∆gO2 has two electrons with opposite spins in a common π*2p orbital; therefore, and therefore is not a free radical. It has an energy of 93.7kJ mol-1 above the ground state. 1Σ+O2 has two electrons with opposite spins in different π*2p orbitals. It is even more reactive than 1∆gO2 with an energy of 156.9kJ mol-1 above the ground state. In biological systems 1Σ+O2 usually decays rapidly (t1/2=10-11s) to the 1∆gO2 state and is usually ignored. Once formed the 1 ∆gO2 molecule is not long-lived (t1/2=2 x 10-6s at 37oC) due to its extreme reactivity. Formation. Singlet oxygen can be formed in the laboratory by: a) the action between hypochlorite and hydrogen peroxide (Eqn 2.17); b) the thermal dissociation of endoperoxides (e.g., 3,3'-(1,4-naphthylidene) dipropionate); c) the disproportionation of superoxide and hydroperoxide; d) decomposition of primary and secondary peroxyl radicals (Russel reaction), and e) the Haber-Weiss reaction (reviewed by Huie and Neta (1999)). As discussed in greater detail below, phagocyte activation during the immune response produces hypochlorous acid from hydrogen peroxide and chloride ions (Harrison and Schultz (1976)) (Eqn 2.18). The subsequent reaction between hydrogen peroxide and hypochlorite forms singlet oxygen (Eqn 2.17) is used to kill pathogens (Kiryu et al (1999)). OCl- + H2O2 → Cl- + H2O + 1∆gO2 Cl- + H+ + H2O2 → HOCl + H2O WWW.ESAINC.COM Eqn 2.17 Eqn 2.18 46 N CH3 N CH3 S Methylene Blue* O CH3 N N CH3 O2 Excited State Ground N R State (3Σg-O2) UV 260nm NH HO N NH2 8-Hydroxy2-Deoxy-Guanosine O N N CH3 N S N CH3 CH3 CH3 NH O2 Singlet State (1∆gO2) N R N NH2 2-Deoxy-Guanosine Methylene Blue Ground State Figure 2.4 The Production Of 1∆gO2 By Photosensitization Can Lead To Damage Of Biologically Important Compounds (e.g., DNA Bases). Based On Some Reactions Presented By Halliwell And Gutteridge (1989)). Singlet oxygen is also produced through photosensitizing reactions. Here the absorption of light of the correct energy can excite a molecule into a higher energy state. This energy can then be transferred to an oxygen molecule in close proximity, exciting it to its singlet state. The photosensitizer simultaneously returns to its ground state. For example, singlet oxygen generated by the interaction of UV light with the dye, methylene blue, can be used to explore the chemical reactions of singlet oxygen (see Figure 2.4 above). In this case singlet oxygen caused oxidative damage to DNA producing the very mutagenic lesion, 8-hydroxy-2’deoxyguanosine (Chapter 3). Many exogenous compounds can act as photosensitizing agents, including dyes (e.g., acridine orange, merocyanine540, blue) and other compounds (e.g., psoralen, meso-substituted porphyrins). Endogenous compounds can act as photosensitizing agents as well, including porphyrins and corrins (e.g., heme), linear pyrroles (e.g., bilirubin/biliverdin), conjugated polyenes (e.g., retinal) and flavins (e.g., FAD, FMN and riboflavin). Some drugs (e.g., tetracycline antibiotics (Hassan and Khan (1986)) and constituents of cosmetics may also act as photosensitizing agents (Halliwell and Gutteridge (1989)). Not all photosensitization damage, however, occurs through the generation of singlet oxygen (type II mechanism). The excited photosensitizing agent itself can inflict damage directly (type I mechanism). Furthermore, excited photosensitizing agents (e.g., merocyanine-540) are also capable of generating other ROS, such as superoxide and hydroxyl free radicals (e.g., Feix and Kalyanaraman (1991)). WWW.ESAINC.COM 47 Compound Amino acids (free and protein bound) • Cysteine (R-SH) • Histidine (free and part of carnosine) • Methionine • Tryptophan Ascorbic acid Cholesterol Phenols (e.g., tyrosine) Polyene • β-Carotene Purine • Guanine • 2’-Deoxy-guanosine Pyrroles • Bilirubin α-Tocopherol Consequence Cystine (RSSR) and sulfonic acid (RSO3H) Endoperoxide, oxohistidine, other products Methionine sulfoxide Hydroperoxide, dioxetene, N-formylkynurenine Quenching, some oxidation products 5α-Hydroperoxide, minor products Quenching, some oxidation products formed Quenching, oxidation products 8-Hydroxyguanine 8-Hydroxy-2’deoxyguanosine Quenching, some oxidation products Quenching, some oxidation products (e.g., αtocopherylquinone) Table 2.3 The Reaction Of Singlet Oxygen With Some Biologically Important Species. Chemical Reactions and Biological Significance. Singlet oxygen is a much more powerful oxidizing agent than oxygen because the spin restriction that encumbers oxygen is removed. Not surprisingly, the typical basal singlet oxygen levels found in vivo are kept low, e.g., ~1 x 10-16 to 1 x 10-18M, for isolated hepatocytes and whole liver, respectively. Singlet oxygen can react by two mechanisms: 1) it can transfer its excitation energy to another molecule (which in turn becomes excited) while subsequently returning to its ground state (i.e., quenched); or 2) it can chemically modify another molecule. Chemical modification depends upon the structure of the compound being attacked (Table 2.3). Compounds containing carbon-carbon double bonds are particularly abundant in nature (e.g., carotenoids and polyunsaturated fatty acids) and are readily damaged by singlet oxygen (fatty acids form hydroperoxides; phenols form endoperoxides that can undergo further decomposition; tryptophan forms a dioxetane that then undergoes ring opening). Singlet oxygen has both beneficial and detrimental effects. As a beneficial molecule it, along with a variety of other pro-oxidants, plays an important role in the active defense mechanisms of the immune system. Photosensitization reactions are also used in disease treatment (photodynamic therapy of Herpes WWW.ESAINC.COM 48 simplex and jaundice)(Halliwell and Gutteridge (1989)). Unfortunately, singlet oxygen also poses a major problem for biological systems. It directly reacts with unsaturated fatty acids (causing lipid peroxidation), and, with DNA (producing strand breaks and mutagenic lesions) (Devasagayam et al. (1991)) (Chapter 3). Singlet oxygen is also a problem for biological systems involved in light transduction (e.g., the chloroplast and the eye) or in humans who are sensitive to light (e.g., patients exhibiting porphyrias). The major defense against singlet oxygen-induced damage appears to be quenching by ascorbic acid (forming an unstable reactive, hydroperoxide, that can decompose to potentially toxic compounds – L-threonolactone and oxalic acid), carotenoids, and tocopherols (forming α-tocopherol hydroperoxide, that decomposes to α-tocopherylquinone) (Fukuzawa et al. (1998); Halliwell and Gutteridge (1989); Kaiser et al. (1990); Kwon and Foote (1988)). See Table 2.3 (above). Dietary flavonoids can also protect against singlet oxygen damage but their role in vivo needs to be evaluated further (Tournaire et al. (1993)). Measurement. Several approaches, varying in their degree of specificity, can be used to measure singlet oxygen levels or to assess its involvement in a reaction of interest (Basu-Modak and Tyrrell (1993); Egorov et al. (1997); Halliwell and Gutteridge (1989); Motohashi and Mori (1989)). These include: • • • • • Measurement of light emission (monomol emission at 1270nm; dimol emission at 634 and 703nm); The use of EPR with sterically hindered heterocyclic amines or bipyrazole derivatives; Measurement of novel markers produced from ß-carotene, cholesterol, phenol and tryptopan; The use of HPLC-ECD using the electrochemically active adduct, 2,2,6,6tetramethyl-4-piperidone-N-oxyl which is formed when 2,2,6,6-tetramethyl-4piperidone; and The use of scavengers (e.g., azide, carnosine and diphenyl-isobenzofuran) to inhibit singlet oxygen production. 4. Superoxide (Radical Anion). Properties. When oxygen is reduced in a single-electron process (Eqn 2.19), the additional electron must enter one of oxygen’s π*2p antibonding orbitals (Figure 2.1). The resulting molecule is both an anion and free radical, called superoxide, or, more correctly, the superoxide radical anion. The addition of an extra electron to the WWW.ESAINC.COM 49 oxygen molecule weakens the double bond, producing a more reactive molecule with only one and one-half bonds. It is relatively unstable and has a half-life of 10-5s at 37oC. O2 + e- → O2•- Eqn 2.19 Formation. Superoxide can be produced in the laboratory by using pulse radiolysis of aqueous solutions, electrochemical reduction of oxygen, or from ionic salts such as potassium- or tetramethylammonium-superoxide. There are many example of the superoxide production in vivo including: • The electron transport chains. Located in mitochondria, the endoplasmic reticulum, nuclear membrane, and chloroplasts, these along with immune defense, are probably the most important sources of superoxide in vivo. Mitochondria are both important sources – and important targets – of reactive species. Acute exposure to relatively high levels of oxidants, especially in the presence of calcium, can induce an event termed the mitochondrial permeability transition, uncouple oxidative phosphorylation, and may contribute to cytotoxicity via necrosis and/or apoptosis (through release of cytochrome c, apoptosis-inducing factor, and other proteins). Longer exposure of mitochondria to milder oxidants appears to lead to progressive mitochondrial impairment and eventual dysfunction, possibly, in some systems, by reducing mitochondrial DNA (mtDNA) expression. Even if mitochondria do not undergo catastrophic failure through one of these mechanisms, oxidant-mediated mitochondrial dysfunction may proceed due to oxidant damage to lipids, proteins, and nucleic acids. The potential for such oxidant-mediated damage is increased because mitochondria are also the major source of reactive species in eukaryotes. Mitochondrial respiration generates ROS, and their generation may be increased in damaged mitochondria and in cells with compromised mitochondrial function. This potential feed-forward interaction between oxidative stress and mitochondrial dysfunction may lead to a deleterious spiral and eventual mitochondrial collapse and cell death. At a crude level, mitochondrial structure may be described as consisting of an inner compartment (termed the matrix), surrounded by two lipid bilayers (the inner and outer mitochondrial membranes). The matrix primarily houses the elements involved in mitochondrial gene expression and energetics. The mitochondrial gene expression system includes the mitochondrial genome, mitochondrial ribosomes, and the transcription and translation machinery needed to regulate and conduct gene expression as well as mtDNA WWW.ESAINC.COM 50 replication and repair. Machinery involved in energetics includes the enzymes of the Kreb's citric acid or TCA (tricarboxylic acid) cycle, some of the enzymes involved in fatty acid catabolism (β-oxidation), and the proteins needed to help regulate these systems. The inner membrane is central to mitochondrial physiology and, as such, contains multiple protein systems of interest. These include the protein complexes involved in the electron transport component of oxidative phosphorylation and proteins involved in substrate and ion transport. Mitochondrial roles in, and effects on, cellular homeostasis extend far beyond the production of ATP, but the transformation of energy is central to most mitochondrial functions. For example, mitochondria play a central role in the regeneration of antioxidants both directly, and indirectly, through the production of reducing equivalents. Reducing equivalents are also used for anabolic reactions. The energy produced by mitochondria is most commonly thought of to come from the pyruvate that results from glycolysis, but it is important to keep in mind that the chemical energy contained in both fats and amino acids can also be converted into NADH and FADH2 through mitochondrial pathways. The major mechanism for harvesting energy from fats is β-oxidation; the major mechanism for harvesting energy from amino acids and pyruvate is the TCA cycle. Once the chemical energy has been transformed into NADH and FADH2, these compounds are fed into the mitochondrial respiratory chain. The mitochondrial respiratory chain consists of five proteins complexes: NADH dehydrogenase (complex I), succinate dehydrogenase (complex II, also part of the TCA cycle), cytochrome bc1 complex (complex III), cytochrome c oxidase (complex IV) and the FoF1ATPase (complex V). The components of each of these protein complexes are listed in Table 2.4. The first four components are also referred to collectively as the mitochondrial electron transport chain. WWW.ESAINC.COM 51 Complex Name (alternative name) Comments Prosthetic Group Substrate Binding Site Products I NADH: ubiquinone Oxido-reductase (NADH-Q Reductase) (NADH dehydrogenase) Composed of ~39 subunits (7 coded by mitochondrial DNA; ~32 by nuclear DNA) (Wallace [1992]). Others report 16 subunits (MW ~500kd) (Newsholme and Leech [1992]) or 25 subunits (MW ~850kd) (Stryer [1988]). FMN Fe-S NADH NADH - matrix NAD Ubiquinone Ubiquinone membrane Ubiquinol Succinate: ubiquinone Oxido-reductase (Succinate-Q Reductase) (Succinate Dehydrogenase) Composed of 4 subunits (MW ~140kd) all encoded by nuclear DNA (Wallace [1992]; Stryer [1988]), FAD Fe-S Ubiquinol:ferricytochrome C Oxido-reductase (Cytochrome bc1 complex) (Cytochrome reductase) (Ubiquinone Dehydrogenase) Composed of ~10 subunits (MW ~250kd) with 1 subunit encoded by mitochondrial DNA and ~9 by nuclear DNA (Wallace [1992]). Ferrocyto-chrome C:oxygen Oxidoreductase (Cytochrome oxidase) FoF1 ATPase (ATP synthase) II III IV V + Succinate Succinate matrix Fumarate Ubiquinone Ubiquinone membrane Ubiquinol Heme b-562 Heme b-566 Heme c1 Fe-S Ubiquinol Ubiquinone membrane Ubiquinone Cyt C-Fe 3+ Cyt C – intermembrane space Cyt C-Fe 2+ Composed of 6 (MW ~160kd (Newsholme and Leech [1992])) to ~13 subunits (Wallace [1992]). Of the ~13 subunits 3 are encoded by mitochondrial DNA and 10 by nuclear DNA. Heme a Heme a3 Cu Cyt C-Fe 2+ Cyt C Intermembrane space Cyt C-Fe 3+ F1: MW ~380kd. Composed of five types of subunits (α, β, γ,δ, ε). Contains the catalytic site for ATP synthesis. Located as a spherical headpiece on matrix side. None? H , ADP Oxygen + + H intermembrane space Water ATP F0: MW ~66kd. Composed of four subunits. Functions as a transmembrane proton channel. Four additional subunits, including the F1 inhibitor, are located in the stalk between F0 and F1 Table 2.4 Mitochondrial Respiratory Chain And ATP-Synthesizing Complex. Under physiological conditions, electrons generally enter either through complex I (NADH-mediated, examined in vitro using substrates such as glutamate/malate) or complex II (FADH2-mediated, examined in vitro using succinate) (Figures 2.5). Electrons are then sequentially passed through a series of electron carriers. The energy released during the transfer of WWW.ESAINC.COM 52 electrons from carrier to carrier is used to pump protons from the inner mitochondrial matrix to the intermembrane space at three points in the chain (complexes I, III, and IV). The progressive transfer of electrons (and resultant proton pumping) converts the chemical energy stored in carbohydrates, lipids, and amino acids into potential energy in the form of the proton gradient. The potential energy stored in this gradient is used to phosphorylate ADP forming ATP. Two Electrons Enter Here MATRIX Fumarate + 2H + Succinate E-FAD E-FADH2 [(Fe-S)Red]3 Succinate-CoQ Reductase Complex II [(Fe-S)Ox]3 H+ H+ 2e- NADH + H+ FMN (Fe-S)Red Q Cyt b [Fe 2+] (Fe-S)Ox Cyt c 1 [Fe2+] Cyt c [Fe 3+] QH2 Cyt b [Fe 3+] (Fe-S)Red Cyt c 1 [Fe3+] Cyt c [Fe 2+] Two Electrons Enter Here NAD+ (Fe-S)Ox FMNH2 Q Cycle H+ H+ QH2-Cytochrome c Reductase Complex III NADH-CoQ Reductase Complex I INTERMEMBRANE SPACE MATRIX H+ Cyt c [Fe 3+] Cyt a [Fe 2+] Cyt a 3 [Fe3+] Cu+ O2 + 4H+ Four Electrons Transferred Here Cyt c [Fe 2+] Cyt a [Fe 3+] Cyt a 3 [Fe2+] Cu2+ 2H2O H+ Cytochrome c Oxidase Complex IV INTERMEMBRANE SPACE Figure 2.5 Components Of The Electron Transport Chain. Mitochondrial pathways of energy production culminate in the electron transport chain and the coupled transfer of four electrons (and four protons) to molecular oxygen to form water. This final reaction, catalyzed by cytochrome oxidase, is “safe,” in that the coordinate, sequential transfer of four single electrons is rarely, if ever, associated with free radical damage. It has been WWW.ESAINC.COM 53 generally estimated that electron leak from the respiratory chain is ~1-4% of total oxygen consumption (Boveris and Chance (1973); Nohl and Hegner (1978); Turrens et al. (1985)), although these estimates may be 10-fold too high (Beckman and Ames (1998); Hansford et al. (1997)). Electron leak predominantly occurs in complexes I and III. Mitochondrial sources of oxidative stress other than the respiratory chain include: monoamine oxidase (production of superoxide and hydrogen peroxide); mitochondrial nitric oxide synthase (RNS formation); the NADH reductase on the mitochondrial outer membrane (ROS formation); and the flavoprotein dihydroorotate dehydrogenase (ROS formation). • • • Immune defense. The phagocytosis of pathogens by neutrophils, eosinophils and mononuclear phagocytes involves production of superoxide through the activation of membrane-bound NADPH oxidase complex (see below). Enzymatic reactions. A variety of enzymes can produce superoxide including peroxidases, oxidases and dioxygenases (Table 2.1). The ability of xanthine oxidase to produce superoxide and the role of this enzyme in the damage associated with ischemia-reperfusion injury is reviewed in Chapter 4. Nitric oxide synthase, the enzyme responsible for the endogenous production of nitric oxide from arginine can, under certain circumstances, lead to the formation of superoxide (Griffith and Steuhr (1995)). Thus the same enzyme can produce both precursors of the aggressive pro-oxidant, peroxynitrous acid (see below). Cytochrome P450 (isoforms) found in the endoplasmic reticulum of many animal and some plant tissues is a mono-oxygenase (mixed–function oxidase) that uses oxygen and a reducing agent NADPH (mediated by the flavoprotein NADPH-cytochrome P450 reductase (EC 1.6.2.3)) in the oxidation of many substrates, especially xenobiotics (Chapter 5). Under certain circumstances it can produce both superoxide and hydrogen peroxide. Oxygen-heme interaction. The binding of oxygen to the heme ring of deoxyhemoglobin (or deoxymyoglobin) forms oxyhemoglobin (or oxymyoglobin), a Fe (II)-oxygen complex (Eqn 2.20). Sometimes this complex decomposes with the production of superoxide and methemoglobin (containing Fe (III) state) at about 3%/day (Eqn 2.21) (Halliwell and Gutteridge (1989)). Methemoglobin is scavenged by reduction to hemoglobin by methemoglobin reductase in a NADPH-based mechanism. Fe2+(heme) + O2 → Fe2+(heme)—O2 ↔ Fe3+(heme)—O2Fe2+(heme)—O2 → Fe3+(heme) + O2•- • Eqn 2.20 Eqn 2.21 Metal-catalyzed auto-oxidation of carbohydrates, thiols, monoamines and other endogenous metabolites and redox cycling of quinones (see below). WWW.ESAINC.COM 54 Chemical Reactions. In comparison to many other pro-oxidants, superoxide is not that reactive (typical second order rate constants4 of 103 to 106 M-1s-1]). However, it does show selective reactivity towards some molecules including other σ-radicals (e.g., nitric oxide). I will limit the chemistry of superoxide to aqueous conditions. Superoxide can act both as a weak reducing and oxidizing agent. Superoxide can reduce Fe (III) to Fe (II) and Cu (II) to Cu (I) (e.g., as part of the superoxide dismutation reaction). The Reduction of Fe (III) to Fe (II) is biologically important as Fe (II) has the potential to take part in the Fenton reaction, ultimately leading to the production of hydroxyl free radicals. In this example, the Fenton reaction is being promoted by superoxide so it is known as the superoxide-Fenton, or Haber-Weiss, reaction (we will return to the Fenton and Haber-Weiss reactions below). Some researchers have challenged the role of superoxide in the promotion of the Fenton reaction in vivo. For example, based on reaction kinetics, superoxide is much more likely to take part in dismutation by SOD than to play a role in the Haber-Weiss reaction (Wardman and Candeias (1996)). Superoxide can oxidize Fe (II) (or Cu (I)), ascorbic acid and compounds containing a thiol group (for an example see Eqn 2.22)). Whether superoxide oxidizes or reduces iron is dependent upon the experimental conditions, whether the iron is free or bound, and what iron-chelator is present (Miller et al., (1990)). Metal2+ + O2•- + 2H+ → Metal3+ + H2O2 Eqn 2.22 Superoxide is a weak base (pKa 4.5-4.8) and accepts protons to form the hydroperoxyl radical (HO2•) (Eqn 2.23). Under physiological conditions only about 1% of superoxide is protonated. Acidic-conditions promote hydroperoxyl radical formation. Therefore, when the pH is decreased (e.g., in the lysozome, in the microenvironment of biological membranes, and following acidosis, ischemia, and prolonged exercise), the chemistry of the hydroperoxyl radical becomes more important. The hydroperoxyl radical is a relatively long-lived species and is more reactive (a stronger oxidizing and reducing agent) than superoxide. Furthermore, the hydroperoxyl radical is lipophilic (i.e., it can readily pass through membranes) and, unlike superoxide, is a promoter of lipid peroxidation (Chapter 3). Superoxide, due to its charge, cannot pass through membranes unless a carrier is present. Superoxide can enter the erythrocyte by using the anion transporter through which chloride and bicarbonate anions normally pass. 4 See Appendix 2.2 for typical rate constants. WWW.ESAINC.COM 55 O2•- + H+ ↔ HO2• Base Acid Eqn 2.23 Superoxide spontaneously dismutates (disproportionates) into hydrogen peroxide (Eqn 2.24). The rate of dismutation is pH sensitive and is most rapid under acidic conditions. Under physiological conditions, the rate of dismutation is found to be approximately 105 M-1s-1 so that any reaction involving superoxide must be in competition with dismutation. Therefore, any reaction producing superoxide will also be producing hydrogen peroxide. 2 O2•- + 2H+ → 2H2O2 + O2 Eqn 2.24 Superoxide readily reacts with other radicals. The reaction between superoxide and nitric oxide, which forms peroxynitrite (ONO2-), is rapid, with a typical second-order rate constant of 107-109 M-1s-1 (Pryor and Squidrito (1995); Radi et al. (1991a,b)) (Eqn 2.25). The importance of peroxynitrite formation is discussed further below. Superoxide also reacts with hypochlorous acid, forming hydroxyl free radicals (Eqn 2.26). O2•- + NO• → ONO2O2•- + HOCl → HO• + Cl- + O2 Eqn 2.25 Eqn 2.26 Biological Significance. Superoxide can be a benefit or a detriment to the living organism. Superoxide helps the body in its defense against invading pathogens. However, the unwanted production of superoxide is a problem causing enzyme inhibition, release of redox active iron, and increasing oxidative stress. We now explore these extremes by giving two examples, the use of superoxide in destroying pathogens, and the problems of superoxide production in the brain. The Pro: Superoxide is a major pro-oxidant and precursor for many of the other aggressive cytotoxic species used by the defense system to control pathogens. Once stimulated neutrophils, eosinophils, monocytes (with the exception of macrophages), and B lymphocytes show increased oxygen consumption, often, but incorrectly referred to as the respiratory burst (Babior (1978); Badwey et al. (1979); Robinson and Badwey (1995)) (See Appendix 2.3). The increase in oxygen consumption is associated with increased glucose flux through the pentose phosphate pathway leading to increased NADPH production. It is caused by the activation of a membrane-bound NADPH oxidase complex WWW.ESAINC.COM 56 responsible for the reduction of oxygen to superoxide (Eqn 2.27). Leukocyte NADPH oxidase is a highly complex protein whose components are distributed between the cytosol and membranes of a variety of organelles, including the plasma membrane, secretory vesicles, and granules (Babior (1999)). The active enzyme is composed of heterodimeric flavohemoprotein cytochrome b558, and two guanine nucleotide-binding proteins (one is a cytosolic Rac2 protein of the Rho family; the other a membrane bound Rap1 protein of the Ras family) (Babior (1999)). Upon stimulation the various components of the NADPH oxidase complex come together and specifically organize within the membrane so that NADPH oxidation occurs on the cytosolic side while oxygen reduction occurs on the extracellular side. NADPH oxidation involves a flavin that either reduces oxygen to superoxide directly or passes its electron to oxygen via cytochrome b558. The passage of electrons across the membrane appears to be accompanied by an outward movement of protons through membrane channels, in order to maintain electroneutrality. Readers interested in an in-depth discussion of enzyme activation, deactivation, and electron transport by the oxidase are referred to Babior (1999). NADPH + 2O2 → NADP+ + H+ + 2O2•- Eqn 2.27 Superoxide can then produce other pro-oxidants. Once dismutated to hydrogen peroxide hydroxyl free radicals can be produced by the Fenton reaction (see below). Hydrogen peroxide is also used by myeloperoxidase (MPO) in the production of hypochlorous acid and by eosinophil myeloperoxidase (EPO) for synthesis of hypobromous acid (see below). Phagocytes also contain an inducible-form of nitric oxide synthase that can produce nitric oxide in large amounts. Nitric oxide reacts with superoxide to form peroxynitrite, another potent pro-oxidant in the cells’ armamentarium (see below). WWW.ESAINC.COM 57 AlcDH CH 2 CH=NH CH 2 CHO AldDH HO HO H2O NH 3 OH OH CATECHOLIMINE CATECHOLALDEHYDE H2O 2 MAO O2 CO 2 H CH 2 OH OH CATECHOLACID HO OH CATECHOLALCOHOL SUBSTITUTED CATECHOLACIDS SUBSTITUTED CATECHOLALCOHOLS HO CH 2 CH 2 NH 2 AlcDH AldDH O2 HO O 2- + H + OH CATECHOLAMINE H2O 2 H2O 2 MAO O2 CH 2 CH 2 NH 2 O2 CH 2 CH 2 NH 2 + HO Nu HO AUTO-OXIDATION OH OH 5-substituted6-substitutedCATECHOLAMINE CATECHOLAMINE SEMIQUINONE O2 ROS O 2- + H + O O QUINONE H2O 2 CATECHOL AUTO-OXIDATION (METAL INDUCED) HO HO OTHER INTERMEDIATES + OH CH 2 CH 2 NH 2 CH 2 CH 2 NH 2 Nu O H2O 2 Catecholamine Metabolism N H O2 LEUCOCHROME (e.g., nucleophiles: Nu: cysteine, homocysteine, glutathione, protein thiols; free radicals: HO .) O O HO O 2- + H + H2O 2 N H O2 O O 2- + H + O N H -O + N H DOPACHROME H2O 2 Figure 2.6 The Catabolism And Auto-Oxidation Of Catecholamines Are Intimately Involved With ROS Production. This Example Shows The Metabolism Of Dopamine. (Adapted From Acworth et al. (1998a)). The Con: The oxidation of the monoamine neurotransmitters is very interesting because not only can they produce superoxide and other ROS but also a number of biologically active and potentially toxic molecules. Together, these compounds are being proposed to cause neuronal oxidative stress that may be one of the mechanisms that eventually lead to neuronal degeneration. The catecholamine neurotransmitters are notoriously unstable – either in the presence of transition metals or when exposed to a basic pH – and undergo metal-induced “autooxidation,” producing reactive semiquinone intermediates, quinones, and ROS (Bindoli et al. (1992); Miller et al. (1990, 1996)) (Figure 2.6). The reactive intermediates can undergo intramolecular cyclization to form cytotoxic aminochromes (e.g., dopachrome), polymerize to form neuromelanin, or react with a variety of nucleophiles (e.g., cysteine) to produce a spectrum of potentially neurotoxic compounds (Acworth et al. (1998a)). Neuromelanin is a complex polymer (of oxidized catecholamine residues) bound to lipofuscin granules. It is capable of binding Fe (III) and reducing it to biologically available Fe (II) capable of producing hydroxyl free radicals (Bindoli et al. (1989); Graham (1978); Graham et al. (1978) (Chapter 4). This finding, in WWW.ESAINC.COM 58 conjunction with the discovery that neuromelanin can also generate other ROS, has led some to hypothesize that it may play a role in progression of Parkinson’s disease (Gerlach et al. (1994)). The brown/black skin melanins (eumelanins) are pigments formed by the oxidation and polymerization of tyrosine, are devoid of bound iron, and are actually ROS scavengers. Pheomelanins are either yellow or red-brown pigments found in the skin and hair of redheaded people. These are less effective radical scavengers and may even degrade with the formation of superoxide upon exposure to strong light. Control. The cellular level of superoxide is maintained by the enzyme superoxide dismutase (SOD). Several forms of SOD exist in higher animals and these will be discussed further in Chapter 5. Together, these enzymes keep the cellular levels of superoxide <10-11M (rat liver cytosol) to 10-10M (liver and heart mitochondria). Measurement. Superoxide can be monitored using a number of approaches (Halliwell and Gutteridge (1999); Livovich and Scheeline (1997); McNeil et al. (1992); Riley et al. (1991); Shoaf et al. (1991); Suzaki et al. (1994)). These include: measurement of its spectrum using EPR at low temperatures; measurement of its absorbance at 245nm; the use of differential pulse polarography; chemiluminescence detection; the use of reporter molecules (e.g., cytochrome c, dianisidine, epinephrine, luminol, nitroblue tetrazolium, and tetranitromethane); and voltammetric detection. Auto-oxidation and Redox Cycling Reactions. Many biologically relevant compounds are reported to react spontaneously with oxygen in a one- or two-electron process, producing superoxide and hydrogen peroxide, respectively. These include carbohydrates (ascorbic acid, glucose, glyceraldehydes, and glycoxidation processes), catechols, cysteine, hemoglobin and myoglobin, lipids (cholesterol, polyunsaturated fatty acids, and lipid peroxidation processes), and monoamines (Burkitt and Gilbert (1991); Ford et al. (1993); Kachur et al (1998); Kon (1978); Mansouri and Perry (1987); Miyata et al. (1998); Pryor et al. (1976); Saez et al. (1982); Sevanian and McLeod (1987); Smith (1987); Thornalley et al. (1984); Tomoda et al. (1981); Wolff and Dean (1987); Wolman (1975)). This process is called auto-oxidation and in the strictest sense can be defined as the “spontaneous oxidation in air of a compound in a process that does not require a catalyst” (Miller et al. (1990)). WWW.ESAINC.COM 59 But do these auto-oxidation reactions really occur in vivo? It is unlikely (Miller et al. (1990); Reilly and Aust, (1999)). Firstly, the one electron reduction of oxygen is a thermodynamically unfavorable reaction (Eo’=-330mV) due to the energy needed to add an extra electron to the partially filled π* orbitals of the triplet dioxygen molecule (Reilly and Aust, (1999)). Therefore, the reduction of oxygen will occur only if it is coupled with energetically favorable processes that can drive the reduction reaction. Since the only biological molecules capable of reducing dioxygen are the reduced flavins, the “auto-oxidation” of the compounds mentioned above could not possibly produce superoxide and hydrogen peroxide. Secondly, although the reduction of dioxygen to hydrogen peroxide by ascorbate is favorable thermodynamically it is hindered kinetically due to spin restrictions (Reilly and Aust, (1999)). Ascorbic acid, lipids, thiols, etc. can promote the reduction of dioxygen, but only in the presence of a transition metal catalyst. The transition metals are characterized by incompletely filled 3d orbitals and depending upon their complexation, can exist in a variety of spin states. Therefore, such redox-active metal complexes can react with oxygen to form a superoxo-metal complex, thereby reducing the triplet nature of the oxygen molecule, and relieving the spin restriction for the reaction between oxygen and biomolecules (Reilly and Aust (1999)). Compounds such as ascorbate can reduce Fe (III) in a one-electron process, producing a radical species (ascorbyl radical) and Fe (II). The Fe (II) can then react with oxygen (part of the Haber-Weiss reaction) producing superoxide and Fe (III), and eventually leading to the formation of hydrogen peroxide and hydroxyl free radicals; the radical species is no longer spinrestricted and can either reduce oxygen directly or form an addition reaction with it (Reilly and Aust (1999)). The cycle continues until all the reductant is used up and iron can no longer be reduced. In redox cycling the reductant is continuously regenerated, thereby providing substrate for the “auto-oxidation” reaction. For example, partially oxidized compounds can be enzymatically reduced, enabling the auto-oxidative generation of superoxide and other ROS to start again. Several enzymes (e.g., NADPH-cytochrome P450 reductase, NADPH-cytochrome b5 reductase [EC 1.6.2.2] NADPH-ubiquinone oxidoreductase [EC 1.6.5.3], and xanthine oxidase [EC 1.2.3.2]), can reduce quinones into semiquinones in a single electron process. The semiquinone can then reduce dioxygen to superoxide during its oxidation to a quinone (Figure 2.7). WWW.ESAINC.COM 60 OH O2 - + H+ O2 O Metal OH O2 - + H+ O2 O Metal OH e.g. NADPHCytochrome P450 Reductase e.g. NADPH- O Cytochrome P450 Reductase DT-Diaphorase Figure 2.7 The Involvement Of NADPH-Cytochrome P450 Reductase And DT-Diaphorase In Redox Cycling. A number of xenobiotics can undergo redox cycling, in part accounting for their beneficial or detrimental activity in biological systems. Such compounds include the bipyridyl herbicides (diquat and paraquat), which produce ROS and release redox active iron from ferritin; the diabetogenic agent, alloxan; antibiotics (e.g., actinomycin D, mitomycin C and streptonigrin); antitumor drugs (e.g., anthracyclines, etoposides, tirapazamine, diaziridinylbenzoquinones); and the hydroxylated metabolites of the antimalarial drug primaquine (Butler (1998); Halliwell and Gutteridge (1999); Newsholme and Leech (1992); Vasquez-viva and Augusto (1992)) (Figure 2.8). Redox cycling is thought to play a role in carcinogenesis. For example, the naturally occurring estrogen metabolites (the catecholestrogens) have been implicated in hormone-induced cancer, possibly as a result of their redox cycling and production of ROS (Yager and Liehr (1996)). Furthermore, the banned synthetic estrogen, diethylstilbestrol, is believed to exert its carcinogenicity through the production of ROS by redox cycling (Liehr et al. (1986); Wyllie and Liehr (1997)). Roy et al. (1991) reported that diethylstilbestrol causes the production of the mutagenic lesion 8-hydroxy-2’deoxyguanosine (Chapter 3). Redox cycling can also cause DNA strand breakage. For example, redox cycling of 2,5-dihydroxypyridine, a metabolite of 3-hydroxypyridine found in cigarette smoke, can cause DNA strand scission (Kim and Novak (1990)). WWW.ESAINC.COM 61 Cytochrome c (Fe 2+) O2 H2 O N H3 C Cyt c Oxidase N CH3 O2 - Oxidized Paraquat Cytochrome c (Fe 3+) H3C N NADPH P450 Reductase N CH3 O2 Reduced Paraquat NADPH O2 - NADPH P450 Reductase H3 C N N CH3 Oxidized Paraquat 2H+, 2e- O O Thioredoxin? HN HN NH O NH O O O H O Alloxan OH Dialuric Acid ROS O2 Figure 2.8 The Redox Cycling Of Paraquat And Alloxan. Paraquat undergoes one electron oxidation producing a paraquat radical and superoxide. The paraquat radical can be reduced by either the electron transport chain (cytochrome c) or by NADPH cytochrome P450 reductase in a process requiring NADPH. In the islets of Langerhans of the pancreas alloxan is thought to undergo a two-electron reduction by thioredoxin. In the presence of metals dialuric acid undergoes oxidation with the production of superoxide, hydrogen peroxide and hydroxyl free radicals. In many cases redox cycling is deleterious to the organism and must be prevented. DT diaphorase [(EC 1.6.99.2) also called NAD(P)H dehydrogenase (quinone), NAD(P)H oxidoreductase, quinone reductase or azo-dye reductase] is a flavoprotein that uses NADH or NADPH to reduce quinones, quinoneimines, and nitrogen oxides in a two-electron process (Cadenas (1995)) (Figure 2.7). The action of DT-diaphorase is to prevent redox cycling by removing quinones, thereby preventing their partial reduction by other enzymes and generation of superoxide. DT-diaphorase is a Phase II detoxifying enzyme that can be induced WWW.ESAINC.COM 62 in a number of tissues by a wide variety of compounds, including dithiolethiones and isothiocyanates (Chapter 4). Not only does DT-diaphorase inactivate xenobiotics, it also plays a role in the activation of a number of quinonecontaining chemotherapeutic prodrugs (Rauth et al. (1997)). Redox cycling is also essential to aerobic respiration and components of the electron transfer chain (cytochromes and coenzyme Q10) redox cycle because electrons are passed from NADH to the terminal electron acceptor, oxygen. Unlike the examples given above, however, the redox cycling associated with aerobic respiration is more tightly controlled and only a minor proportion of electrons “leak”, producing ROS. 5. Hydrogen Peroxide. Properties. Hydrogen peroxide (H2O2) is a pale blue, viscous liquid with a melting point of -0.9oC and a boiling point of +150oC. It is stable in the absence of reducing agents. In the presence of such contaminants its half-life is of the order of minutes to hours at 37oC under aqueous conditions, depending upon its concentration and conditions. Hydrogen peroxide is formed in the single-electron reduction of superoxide or the two-electron reduction of oxygen (Figure 2.2). During single-electron reduction of superoxide, the extra electron enters the remaining partially filled π*2p orbital (Figure 2.1). Consequently, the resulting peroxide anion (O22-) has its π*2p orbitals completely filled. The peroxide anion is not a radical and is therefore diamagnetic. It has a relatively weak, single oxygen-oxygen bond. The peroxide anion exists only under extremely basic conditions, so under physiological conditions it is protonated and exists as hydrogen peroxide. Formation. Hydrogen peroxide is made in the laboratory by acidification of ionic peroxides (e.g., barium peroxide). Industrially, it is made either by the catalytic reduction of 2-butylanthraquinone to 2-butylanthraquinol – which is then oxidized with oxygen enriched air to hydrogen peroxide — or the oxidation of 2-propanol with oxygen under slight pressure. Hydrogen peroxide is produced in vivo by the two-electron reduction of oxygen or by superoxide dismutation (see above). As a result, superoxide produced by the electron transport chains – cytochrome P450, phagocytosis, etc. – will always produce hydrogen peroxide. It is also formed by several oxidases (e.g., by monoamine oxidase as shown in Figure 2.5). WWW.ESAINC.COM 63 Chemical Reactions and Biological Significance. Hydrogen peroxide is thermodynamically unstable with respect to oxygen and water and is readily decomposed by heat or by contact with finely divided solids (e.g., manganese (IV) oxide and metals) and traces of alkali (Eqn 2.28). Homolytic fission (e.g., by irradiation) yields the hydroxyl free radical (Eqn 2.29). High levels of H2O2 (10-5-10-8M) have been reported in water obtained from UVprotected water stills. This can pose a problem for the measurement of the hydroxyl free radical, especially if redox active metals are present (see below). 2H2O2 (g) → O2 (g) + H2O (g) ∆Go(298)= -126kJ mol-1 H2O2 H2O2 → 2HO• Eqn 2.28 Eqn 2.29 Hydrogen peroxide can act as both a weak oxidizing and reducing agent. For example, it acts as a weak oxidizing agent, converting sulfide, Fe (II) and iodide ions into sulfate ions, Fe (III) and iodine, respectively (Eqn 2.30). Strong oxidizing agents (e.g., silver oxide, and acidified potassium permanganate) force H2O2 to assume the role of reducing agent (Eqn 2.31). H2O2 + 2I- + 2H+ → 2H2O + I2 Ag2O + H2O2 → 2Ag + H2O + O2 Eqn 2.30 Eqn 2.31 Like superoxide, hydrogen peroxide is not particularly reactive (the second order rate constants are typically 101 to 105 M-1s-1). Under physiological conditions, the reactions of H2O2 are mainly confined to its oxidizing ability. It can oxidize thiols and by so doing, inactivate enzymes that contain an essential thiol group (Chapter 3). As hydrogen peroxide is fairly stable and can readily pass through membranes it can react with biological molecules far removed from its site of production (Makino et al. (1994)). A significant problem for living organisms is the consequence of the reaction between hydrogen peroxide and oxidizable metals, the Fenton reaction. The Fenton reaction originally described the oxidation of an α-hydroxy acid (tartaric acid) to an α-keto acid in the presence of hydrogen peroxide (or hypochlorite) and low levels of iron salts (Fenton (1876, 1894)). Although the Fenton reaction is often presented as a straightforward equation (Eqn 2.32) this is a gross simplification because many reactions are possible (e.g., Eqns 2.33 to 2.35). For example, when the Fenton reaction is carried out in the presence of HCl, alkenes are chlorohydroxylated (Sawyer et al. (1995)). Readers wanting a more comprehensive explanation of the Fenton reaction are WWW.ESAINC.COM 64 referred to the following references Goldstein et al. (1993); Halliwell and Gutteridge (1990, 1992); Koppenol (1993); Liochev (1999); and Wardman and Candeias (1996). Although hydroxyl free radicals are thought to be the major prooxidant species formed there remains considerable controversy about whether they exist in a free form (Wardman and Candeias (1996) and references therein). Pro-oxidant metal species have also been proposed as the pro-oxidant species (Buxton and Mulazzani (1999)). For example, the ferryl radical (e.g., Fe2+-O), where iron is in its IV valency, may also be formed in conjunction with the hydroxyl free radical (Eqns 2.36 and 2.37). However, it is doubtful that ferryl radicals are the primary pro-oxidant species formed in vivo (Halliwell and Gutteridge (1999); Koppenol (1993)). Similarly, a perferryl species (e.g., Fe2+-O2 ⇔ Fe3+-O2•-) may be formed when Fe (III) reacts with superoxide (e.g., as part of the Haber-Weiss reaction); however, it is unlikely to be the major reactive species (Eqn 2.38) (Halliwell and Gutteridge (1999)). Qian and Buettner (1999) have challenged these ideas, suggesting that an unknown “Fe2+ + O2” species was indeed capable of initiating free radical oxidations. Their finding was based, in part, on the fact that the Fenton reaction has only a small rate constant (103105 M-1s-1), while the reaction of Fe (II) with superoxide is much greater (106-107 M-1s-1). Therefore, under physiological conditions, the latter reaction will effectively limit the availability of Fe (II) to take part in the Fenton reaction. Qian and Buettner reported that when the [oxygen]/[hydrogen peroxide] ratio <10 the Fenton reaction dominated, but when this ratio >100 (under physiological conditions this ratio ~1000), then the Fenton reaction played only a subservient role to the “Fe2+ + O2” species. Oxidant + reduced metal → oxidized metal + superior oxidant e.g., H2O2 + Fe2+ → Fe3+ + OH- + HO• HO• + Fe2+ → OH- + Fe3+ HO• + H2O2 → H2O + H+ + O2•O2•- + Fe3+ → O2 + Fe2+ (part of the Haber-Weiss reaction) H2O2 + Fe2+ → FeOH3+ + OHor H2O2 + Fe2+ → FeO2+ + H2O FeO2+ + H2O2 → Fe2+ + H2O + O2 O2•- + Fe3+ ↔ [Fe3+-O2- ↔ Fe2+-O2] ↔ O2 + Fe2+ Eqn 2.32 Eqn 2.33 Eqn 2.34 Eqn 2.35 Eqn 2.36 Eqn 2.37 Eqn 2.38 Currently, many researchers are not convinced of iron’s role in pro-oxidant production and suggest that with the exception of iron-related disorders, there is little or no direct proof that iron plays an important role in the Fenton reaction in vivo (Chapter 4). Several metals besides iron are capable of undergoing changes in oxidation status (e.g., copper, chromium, vanadium, etc.) and can reduce hydrogen peroxide to hydroxyl free radicals. Whether they are involved in Fenton-like reactions in vivo is still a matter of debate (Masarwa et al. (1988)). Interestingly, WWW.ESAINC.COM 65 Cu (I) salts can react with hydrogen peroxide to form both hydroxyl free radicals and also the powerful oxidizing agent Cu (III) (Eqn 2.39). This strongly oxidizing form of copper can be also formed by the action of Cu (II) ions with superoxide. H2O2 + Cu+ → Cu2+ + HO• + OH- Eqn 2.39 Hydrogen peroxide is beneficial too. Like superoxide, it plays an important role in the immune response. It does so both directly by inhibiting key enzymes within the pathogen and indirectly as the “safe” precursor to the hydroxyl free radical (Chapter 4). Hydrogen peroxide is also essential for the synthesis of thyroxine in the thyroid gland (Dupuy et al. (1991)). The typical steady-state cellular hydrogen peroxide concentration is estimated to be 10-7-10-9M in the liver and 10-5M in the human eye lens. These concentrations represent a balance between hydrogen peroxide production and destruction. Its level is primarily controlled by two groups of enzymes, the catalases (Eqn 2.40) and glutathione peroxidases (Eqn 2.41) (see Chapter 4). 2H2O2 → 2H2O + O2 H2O2 + 2GSH → 2H2O + GSSG Eqn 2.40 Eqn 2.41 Measurement. In the laboratory, hydrogen peroxide can be measured using chemical titration with acidified potassium permanganate, but this approach is not selective and is too insensitive for its measurement in vivo. Hydrogen peroxide can be measured in biological systems by peroxidase-based methods with fluorometric detection (Corbett (1989) and by HPLC-chemiluminescence methods (Yamamoto and Ames (1987)). It can also be determined by the measurement of evolved oxygen using an oxygen electrode following the addition of catalase (Halliwell and Gutteridge (1999)), or evolved 14CO2 using scintillation counting when it reacts with labeled 2-oxoglutarate (Varma (1989)). Hydrogen peroxide is electrochemically active and can be measured voltammetrically in “real time,” using either a platinum-disk (Yokoyama et al. (1998)) or enzyme-modified electrodes (Livovich and Scheeline (1997); Tatsuma et al. (1992, 1994)). WWW.ESAINC.COM 66 6. The Hydroxyl Free Radical. Properties. The hydroxyl free radical (HO•) is the most reactive ROS formed in vivo. It has a half-life of 10-9 to 10-10 s and shows typical second-order rate constants of 109 to 1010 M-1s-1. The hydroxyl free radical is formed by the single electron reduction of the peroxide ion. During this process the extra electron enters the empty σ*2p molecular orbital. The single oxygen-oxygen bond of the peroxide ion is weakened and cleaves, forming the hydroxyl free radical and hydroxide ion (Figure 2.2). The addition of two electrons to the peroxide ions also cleaves the oxygen-oxygen bond but, in this case, two oxide (O2-) ions are formed. Formation. The hydroxyl free radical can be formed by a number of processes including the Fenton reaction, the Haber-Weiss reaction, and the homolytic fission of water molecules (e.g., by ionizing radiation). It can also be produced by the decomposition of ozone under aqueous conditions (Table 2.2) (Hoigne and Bader (1975)), the microsomal ethanol oxidizing system (part of the endoplasmic reticulum), and the reaction between the superoxide radical anion and hypochlorous acid (Eqn 2.26) (Candeias et al. (1993)). Typical steady-state levels in vivo are ~10-20M. Chemical Reactions and Biological Significance. The hydroxyl free radical is extremely reactive. It will react with most, if not all, compounds found in the living cell (including DNA, proteins, lipids and a host of small molecules). The hydroxyl free radical is so aggressive that it will react within 5 (or so) molecular diameters from its site of production. The damage caused by it, therefore, is very site specific (Pryor (1986)). The reactions of the hydroxyl free radical can be classified as hydrogen abstraction, electron transfer, and addition (Figure 2.9). • • • Hydrogen abstraction, which typically occurs with aliphatic compounds, causes lipid peroxidation and DNA damage (Chapter 3). Electron transfer produces secondary radicals of varying reactivity, such as the carbonate radical anion. Aromatic compounds typically react with the hydroxyl free radical by addition. The products that are formed depend upon the species being attacked and the reaction conditions. For example, the fast addition of the hydroxyl free radical to benzene produces the unstable hydroxycyclohexadienyl radical. This can regain aromatic stability by either dimerization or oxidation (Kaur and Halliwell (1994b)). The action of WWW.ESAINC.COM 67 the hydroxyl free radical with a substituted benzene can produce a spectrum of products. For example, the reaction between salicylic acid (2hydroxybenzoic acid) and the hydroxyl free radical produces 2,3- and 2,5dihydroxybenzoic acid and the decarboxylation product, phenol (Figure 2.9). Tyrosine undergoes dimerization with the production of dityrosine or oxidation forming 3,4-dihydroxyphenylalanine (Chapter 3). Aromatic hydroxylation is favored by the presence of oxygen, Fe (III), and Cu (II) ions, whereas decarboxylation is favored by a lack of these compounds (Halliwell and Gutteridge (1999)). Consequently, under physiological conditions aromatic hydroxylation tends to be the predominant reaction. The reaction between the hydroxyl free radical and an aromatic compound is referred to as scavenging, and is sometimes used to trap this prooxidant prior to detection (Chapter 1 and see below). Readers should be aware that some of the addition reactions of the hydroxyl free radical are mimicked by peroxynitrite (see below). The formation of the hydroxyl free radical can be disastrous for living organisms. Unlike superoxide and hydrogen peroxide, which are mainly controlled enzymatically, the hydroxyl free radical is far too reactive to be restricted in such a way – it will even attack antioxidant enzymes. Instead, biological defenses have evolved that reduce the chance that the hydroxyl free radical will be produced and, as nothing is perfect, to repair damage. Redox active metals are chelated (Chapter 4); hydrogen peroxide is catabolized enzymatically. The repair of damaged molecules (e.g., enzymatic repair processes) will be discussed further in Chapter 3. Even though low molecular weight antioxidants readily react with hydroxyl free radicals it is doubtful that they play an important role in controlling its level (Chapter 4). Remember, for an antioxidant to be effective it would have to occur at the site of hydroxyl free radical production and be at sufficient (probably unphysiological) concentration to compete with all the other chemical species for reaction with this pro-oxidant. WWW.ESAINC.COM 68 O2 a) ABSTRACTION O2 C OH H H C2H5 C C2H5 HO OH OH C C2H5 H Peroxyl Radical X2 H Propanol H Hydroxypropyl Radical C2H5 C OH C2H5 C OH H Hexan-3,4-Diol b) ADDITION OH O OH OH HO O H R R R Tyrosyl Tyrosine H X2 -H2O H R O H R Di-Tyrosine Radical (Keto form) OH OH R R Di-Tyrosine c) ELECTRON TRANSFER (Enol form) HO + Cl - Cl HO + CO 3- CO3- + OH - + OH - Figure 2.9 Some Reactions Of The Hydroxyl Free Radical. The Hydroxyl Free Radical Can React With Molecules By Hydrogen Abstraction (A), Electron Transfer (B) Or Addition (C). In This Figure, Abstraction Forms A Carbon-Based Radical Capable Of Reacting With Another Radical (E.G., Oxygen Or Even Itself [Dimerization]) Through The Formation Of A σ-Bond. Addition Forms A π-Radical That Can Regain Aromatic Stability Through Dimerization To Dityrosine. Electron Transfer Can Produce Very Reactive Radicals Such As The Chlorine Radical (Cl•) And Carbonate Radical Anion (CO3•-). WWW.ESAINC.COM 69 Measurement. A variety of approaches, differing in their specificity, sensitivity, applicability and ease of use are used in to detect the hydroxyl free radical (See Halliwell and Gutteridge (1999) and references therein). So far EPR and HPLC-based approaches have proven to be the most useful. EPR: Under special circumstances, EPR can be used directly to measure hydroxyl free radicals (Halliwell and Gutteridge (1999)). However, it is more common to use a spin trap, as discussed in Chapter 1. The use of EPR and spin traps to measure the hydroxyl free radical is well documented in vitro but care must be taken when interpreting data as the spin trap adduct themselves may produce ROS (Finkelstein et al. (1980); Floyd (1983); Kaur et al. (1981); Yamazaki and Piette (1987)). The measurement of the hydroxyl free radical in vivo is particularly challenging. Interestingly, Dugan et al. (1995) coupled in vivo microdialysis sampling procedures with on-line EPR to study free radical production following focal cerebral ischemia-reperfusion in spin trap-treated animals. Although this approach showed promise, the authors did caution about spin trap stability, toxicity, and the spontaneous formation of the spin trap-HO• adduct. Some spin trap adducts are also electrochemically active. Consequently, HPLCECD is being used currently to overcome the sensitivity and quenching problems associated with EPR (Floyd et al. (1984a,b), Iwahashi (1996); Motohashi and Mori (1989); Stronks et al. (1984); Towel and Kalyanaraman (1991)). Fast-scan voltammetry is also being used to explore the reaction mechanisms of some spin traps (Baur et al. (1996)). WWW.ESAINC.COM 70 COOH COOH OH OH OH + CO2 O2N NO2 2-Hydroxy-5-NitroBenzoic Acid NO2 2-Hydroxy-3-Nitro Benzoic Acid 2-Nitrophenol CO.Glucuronate OH ONO2H Salicyl acyl glucuronide Glucuronidation COOH O.Glucuronate COOH Salicyl phenolic glucuronide OH CONHCH 2COOH OH Salicylic acid Salicyluric acid HO Cytochrome P450 COOH COOH OH OH OH + CO2 OH OH 2,3-Dihydroxybenzoic acid (~49%) Catechol (~11%) HO 2,5-Dihydroxybenzoic acid (~40%) Figure 2.10 Metabolism Of Salicylate And Its Reaction With The Hydroxyl Free Radical And Peroxynitrite. (The % represent the abundance of the products during the reaction of salicylic acid with the hydroxyl free radical in vitro (Kaur and Halliwell (1994b)). WWW.ESAINC.COM 71 HPLC: The other major approach to studying the formation of the hydroxyl free radical in vitro and in vivo is based on HPLC separation. As with EPR, the hydroxyl free radical is too reactive to be measured directly. It must first be trapped in a stable form that is also amenable to HPLC analysis so such methods are indirect. The production of the hydroxyl free radical is inferred from the abundance of a product formed when this radical is scavenged, by either an endogenous substrate or an administered reagent (see reviews by Acworth et al. (1997, 1998a,b); Halliwell and Gutteridge (1999)). See Table 2.5. An issue with many of the HPLC-based approaches used is that often only the reaction products are quantified. A change in the level of a product is assumed to reflect a change in radical production. Obviously, this is not always the case as a change in the level of a marker could entirely be due to altered availability of the scavenging agent (e.g., hepatic metabolism or excretion). A better approach is to simultaneously measure both the scavenging agent and products, thereby permitting normalization of the data.5 Target Endogenous Markers Product Comments Creatinine Creatol and methyl-guanidine Products are used as markers of oxidative stress. 2’-Deoxy-cytidine 5-Hydroxy2’deoxycytidine 2’-Deoxyguanosine 8-Hydroxy 2’-deoxyguanosine Histidine 2-Oxohistidine (free and protein Can be measured by GC-MS or HPLC with UV or ECD. HPLC-ECD is ideally suited to measure low tissue levels (Chapter 4). This marker is not exclusive to the hydroxyl free radical. Urinary levels may be of use as a global oxidative stress marker. Can be measured using ELISA, HPLC-ECD, GC-MS, GC-MS-MS and TLC-32P approaches (Chapter 4). HPLC-ECD is ideally suited to measure low tissue levels. Not specific as it can be formed by the action of singlet oxygen also. Tissue DNA requires careful hydrolysis to prevent artificial formation. It is extremely difficult to measure in urine. Can be measured using HPLC-based methods. Exists as both free and References Aoyagi et al. (1998a,b); Nakamura et al. (1996); Yokozawa et al. (1997) Wagner et al. (1992) Schneider et al. (1989); Floyd et al. (1986) Lewisch and Levine (1995); 5 One exception is microdialysis. This approach is used to monitor analyte levels in the living organism in real time. As the scavenger is perfused through a microdialysis probe directly into the tissue it will be unaffected by peripheral metabolism. Consequently, data need not be normalized. WWW.ESAINC.COM 72 bound) Methionine Methionine sulfoxide (free and protein bound) L-Phenyl-alanine (L-Phe) o-, m-Tyrosine (free and protein bound) L-Tyrosine 3,4- (and 2,4)-LDOPA) (free and protein bound) L-Tyrosine Dityrosine (free and protein bound) protein bound forms (Chapter 4). The protein bound form needs to be hydrolyzed before analysis by HPLC. Can be measured using HPLC-based methods (Chapter 4). It exists as both free and protein bound forms. The protein bound form needs to be hydrolyzed before analysis by HPLC. It can be formed by hydroxyl free radicals and other ROS. Both target and product can be measured using HPLC-UV, but this approach may not be adequate for most tissue analyses. Improved sensitivity can be obtained using either HPLC-fluorescence or HPLCECD following derivatization (e.g., OPA/βME). All tyrosine isomers are electrochemically active and can be measured directly using HPLC-ECD. Protein bound targets require hydrolysis before analysis by HPLC. Both target and product can be measured using HPLC-UV, but may not be adequate for most tissue analyses. Improved sensitivity can be obtained using either HPLCfluorescence or HPLC-ECD following derivatization (e.g., OPA/βME). All tyrosine isomers are electrochemically active and can be measured directly using HPLC-ECD. Protein bound targets require hydrolysis before analysis by HPLC. Free 3,4-isomer is also formed enzymatically by tyrosine hydroxylase and this will limit the use of this assay in catecholaminergic tissue. The 2,4isomer is only a minor product and is not commercially available. Protein bound 3,4-L-DOPA requires hydrolysis before analysis by HPLC. Can be measured using GC-MS, TLC and HPLC with UV, fluorescence or ECD (Chapter 4). HPLC-ECD is most often used due to the very low tissue level of this marker. May be formed from other pathways including reaction of tyrosine with either hypochlorous acid or peroxynitrous acid. Used as a marker for hydrogen peroxide stress. Protein bound form requires hydrolysis before analysis by HPLC. WWW.ESAINC.COM Uchida and Kawakishi (1990, 1993) Levine et al. (1996); Li et al. (1995a,b); Vogt (1995) Ishimitsu et al. (1986); Nair et al. (1995); Sontag et al. (1997) Hensley et al. (1997) Giulivi and Davies (1994); Heinecke et al. (1993); Huggins et al. (1993); Ischiropoulos et al. (1992); Leeuwenburgh et al. (1997); van der Vleit (1995); Vissers and Winterbourne (1991) 73 Exogenously Administered Agent and In Vitro Examples 4-Amino-salicylic acid (4HAS) 5-Amino-salicylic acid (5HSA) N-Acetyl-4-aminosalicylic acid; dihydroxy-4aminobenzoic acids, and many other analytes N-Acetyl-5-aminosalicylic acid; dihydroxy-5aminobenzoic acids, and many other analytes Aniline o- and pAminophenol Dimethylsulfoxide DMPO (5,5dimethylpyrroline-Noxide) Methanesulfinic acid 4-Hydroxybenzoic acid (4HBA) 3,4-DHBA (minor amounts of 2,4isomer formed) 2-Methyl-2nitroso-propane (t-nitrosobutane) Depends on system being studied PBN (α-phenyl tert-butylnitrone Aminoxyl and other adducts depending on the system being investigated Catechol, resorcinol Phenol DMPO-OH, DMPOOH2 Can be measured using EPR and HPLC-based approaches. Can produce complex chromatograms. Allgayer et al. (1992) Can be measured using HPLC with UV or ECD. Chromatograms may be complex due to the number of analytes produced. This agent has several metabolic effects including: reduction of leukotriene production, inhibition of interleukin-1 release, inhibition of prostaglandin synthetase or lipoxygenase, and interference of antibody production. Can be measured using HPLC with UV or ECD. Aniline is toxic and is not practical for biological experiments. So far only in vitro studies using HPLC-UV have been reported Can be measured using EPR but the product can be quenched in vivo. HPLC-ECD can overcome the problems associated with EPR and offers better sensitivity. Similar in reactivity to salicylate but with less physiological activity. Only one major product formed permitting lower LODs than Sal (where signal is split between two products). Intestinal microbes readily form 4HBA, which may be problematic, if gut is damaged. For microdialysis perfusion experiments the presence of metals in the fluid path must be minimized in order to prevent the spontaneous production of 3,4-DHBA. So far only in vitro studies using HPLC-UV have been reported. Fischer and Klotz (1994); Palumbo et al. (1997) This is usually measured using EPR. It can be measured using HPLC-ECD but the chromatography can be complicated. Can be measured using HPLC-UV or ECD but is not practical for biological experiments. WWW.ESAINC.COM Radzik et al. (1983) Fukui et al. (1993) Floyd et al. (1984a) Acworth et al. (1998b); Bogdanov (1998a,b,c); Montgomery et al. (1995); Ste-Marie et al. (1996) Hiraoka et al. (1989, 1990); Inami et al. (1986, 1987) Chen et al. (1990; 1994); Cheng et al. (1993); Stronks et al. (1984) Floyd et al. (1984b); Radzik et al. (1983) 74 D-Phe o-, m- D-Tyrosine See L-Phe above. D- and L-forms cannot be resolved unless a chiral column is used. D-Phe to L-Phe isomerization will deplete target molecule thereby affecting its availability for reaction with the hydroxyl free radical (Acworth et al. (1997)). This approach may not be practical for study of the central nervous system as L-Phe can affect dopamine synthesis and release in brain (During et al. (1988)) See L-Phe above. May not be practical for study of the central nervous system as L-Phe can affect dopamine synthesis and release in brain (During et al. (1988)). The rate of reaction is slower than for Sal. May be a useful marker of food irradiation. Tyrosine isomers may also be formed by the action of peroxynitrite on LPhe. L-Phe o-, m-Tyrosine isomers 4-POBN (α-(4pyrisyl-1-oxide)N-tertbutylnitrone) 4-POBN radical adduct Usually measured using EPR. HPLCECD permits the study of reaction mechanisms. This is an in vitro method only. Salicylic acid (Sal) 2,3- and 2,5-DHBA Can be measured using GC-MS and HPLC with a variety of detection systems including UV, ECD, ECD with UV, ECD with fluorescence, and MS. The 2,3-isomer better reflects hydroxyl free radical production as the 2,5- isomer is formed by cytochrome P450 (Figure 2.10). The 2,5-DHBA isomer is also formed due to singlet oxygen activity (Kalyanaraman et al. (1993)). Sal has physiological affects albeit at higher concentrations. Perfusion through a microdialysis probe can lead to spontaneous production of DHBA isomers. Both Sal and the DHBAs can bind ferric iron thereby perturbing the iron-dependent generation of the hydroxyl free radical. DHBAs may be formed by the action of peroxynitrite on Sal. Peroxynitrite also reacts with Sal to form the marker, 2-hydroxy-5nitrobenzoate (Skinner et al. (1996)). See Figure 2.11. WWW.ESAINC.COM Kaur and Halliwell (1994a,b) Gelvan et al. (1992); Kaur and Halliwell (1994a,c); Kaur et al. (1988, 1996); Halliwell and Kaur (1997)); Ishimitsu et al. (1984); Karam and Simic (1988); Liu (1993), Ramezanian et al. (1996); Sontag et al. (1997); Sun et al. (1993); van der Vliet et al. (1994) Cheng et al. (1993); Iwahashi (1996); Stoyanovsky et al. (1999) Acworth et al. (1997); Bickford et al. (1999); Blandini et al. (1999); Floyd et al. (1984b); Halliwell and Kaur (1997); Liu et al. (1999); Luo and Lehotay (1997); McCabe et al. (1997); Tabatabaei and Abbott (1999) 75 L-Tyrosine 3,4-Dihydroxyphenylalanine L-Tryptophan Complex mixture of metabolites Can be measured using HPLC with UV or ECD. This has been used for in vitro studies only. Can be measured using HPLC with UV or ECD. This has been used for in vitro studies only. Ramezanian et al. (1996) Maskos et al. (1992) Table 2.5 Endogenous Markers And Exogenous Agents Used To Study Hydroxyl Free Radical Formation. Based Upon Acworth et al. (1998a). The major problem with measuring endogenous markers is interpreting what their levels really represent. Many of these markers are not produced exclusively by the hydroxyl free radical and can be formed by other pro-oxidants (e.g., singlet oxygen and peroxynitrite). Furthermore, as the hydroxyl free radical will react with any compound it encounters, the measurement of just one endogenous marker is likely to underestimate the total production of this pro-oxidant. Currently, some endogenous markers are being proposed as a useful measures of total “oxidative stress” e.g., 8-hydroxy-2’deoxyguanosine in urine (Chapter 3). The use of exogenous scavengers also has limitations. For an exogenous scavenger to be effective, enough of it must get to the site of hydroxyl free radical production in order to compete with the other compounds capable of reacting with this radical. Remember the radical scavenger is unlikely to react with all hydroxyl free radicals produced so, like endogenous markers, it will underestimate the total production of this pro-oxidant. Consequently, scavengers are usually given at high doses (typically hundreds of milligrams per kilogram of body weight). This may be a problem if the scavenger is toxic, suffers from distribution problems or possesses adverse biological activity. The ideal scavenger must be non-toxic, have limited or no biological activity, readily reach the site of hydroxyl free radical production (i.e., pass through barriers such as the blood-brain barrier), react rapidly with the free radical, be specific for this radical, and neither the scavenger nor its product(s) should undergo further metabolism. As it may be appreciated, no scavenger has successfully fulfilled all of these criteria. Of all of the approaches outlined in Table 2.5, the use of salicylate and 4hydroxybenzoic acid as hydroxyl free radical-scavenging agents are by far the most common (reviewed in Acworth et al. (1997; 1998a,b)). See ESA Application Notes for further experimental details (70-1749 Measurement of The Hydroxyl Free Radical; 70-4820 Alternate Method for the Measurement of The Hydroxyl Free Radical). Salicylic acid reacts rapidly (five times faster than phenylalanine and 22 times faster than guanosine) with the hydroxyl free radical, producing readily quantifiable products (2,3- and 2,5-dihydroxybenzoic acids (Figure 2.10)). It readily distributes throughout the body, even passing through the blood-brain barrier, making it a useful tool to study central metabolism. Unfortunately it also suffers from some problems: WWW.ESAINC.COM 76 • • • It possess physiological activity if used at too high a concentration, so the amount administered in vivo must be chosen carefully; A growing body of evidence suggests that hydroxylated products are formed as a consequence of peroxynitrite attack (see below). One approach to distinguish between true hydroxyl free radical-dependent aromatic hydroxylation and that involving peroxynitrite is to measure aromatic nitration products along with the DHBA isomers (Halliwell and Kaur (1997)); and A number of publications now report a significant level of spontaneous formation of DHBAs when salicylic acid is dissolved in microdialysis perfusion medium. This situation can be further exacerbated by metals in the flow path (e.g., the syringe needle and metal in the probe itself) (Acworth et al. (1998b); McCabe et al. (1997a,b); Montgomery et al. (1995)). Whether conditions do exist in which salicylic acid can be effectively used as a hydroxyl free radicalscavenger, while coupled to microdialysis perfusion, is yet to be determined. WWW.ESAINC.COM 77 I. post amphetamine/saline rat striatal tissue sample II. post amphetamine/SAL rat striatal tissue sample III. 650 nM standard 2,3-DHBA 2,5-DHBA A II I III I. post amphetamine/saline rat striatal tissue sample II. post amphetamine/SA rat striatal tissue sample III. 360 µM standard SA B II III I Figure 2.11 Chromatograms Showing The Simultaneous Measurement Of DHBAs And Salicylic Acid For The Detection Of The Hydroxyl Free Radical In Rat Striatal Tissue. The DHBAs were selectively detected on the first coulometric electrode (A; +250mV, 100nA) while salicylic acid (SA) was measured on the second electrode (B; +750mV, 20µA gain). The isocratic system consisted of a pump, an autosampler, a thermal chamber and a Coulochem III detector. LC Conditions: Column: Mobile Phase: DHBA-250. 50mM Sodium acetate, 50mM Citric Acid, 25% Methanol, 5% Isopropanol, pH 2.5 with Phosphoric Acid. Flow Rate: 0.5mL/min. Temperature: Ambient. Injection Volume: 10 µL. Guard Cell, Model 5020 EGC = +775mV Analytical Cell, Model 5010 E1 = +250mV; E2 = +750mV See 70-1749 Measurement of The Hydroxyl Free Radical for more details. WWW.ESAINC.COM 78 A variety of HPLC-based procedures have been used to measure salicylic acid and DHBAs (Acworth et al. (1997, 1998b); McCabe et al. (1997a)). UV detection tends to be too insensitive for routine biological work, requiring use of large amounts of salicylic acid in order to render the DHBAs detectable by HPLC-UV. Unfortunately, high doses can exacerbate physiological problems. HPLC-ECD is much more sensitive and selective and has been used to supplement the UV approach. In this example the upstream ECD is used for the sensitive measurement of the DHBAs while the downstream UV detector measures the greater abundance of salicylic acid (Jen et al. (1998); Sloot and Gramsbergen (1995)). Analytical approaches requiring two detectors are cumbersome, expensive, and unnecessary. HPLC-ECD can also be used to measure both precursor and product. Both salicylic acid and DHBAs can be measured simultaneously on a single amperometric thin-layer electrode but, due to the high applied potential necessary to measure salicylate, detection can suffer from noise and co-elutions (Floyd et al. (1984b, 1986); Kaur and Halliwell (1994b)). Perhaps a better approach to overcome these problems is to use a dual coulometric detector that makes use of the inherent differences in the electrochemical behavior of the DHBAs and salicylate (McCabe et al. (1997a,b) (Figure 2.11). A major advantage of the high selectivity and sensitivity of this approach is that less salicylic acid has to be administered to the animal, thereby minimizing possible physiological side effects. A similar coulometric approach this time using 4-hydroxybenzoic acid as the scavenging agent can also be used to measure the presence of hydroxyl free radicals (Figure 2.12). This has several advantages over the salicylic method as discussed in Table 2.5. WWW.ESAINC.COM 79 I. basal microdialysis sample II. post 4-HBA microdialysis sample III. 10 nM standard 5-HIAA DOPAC 3,4-DHBA A I II B 4-HBA III I. basal microdialysis sample II. post 4-HBA microdialysis sample III. 10 µM standard II III I Figure 2.12 Chromatograms Showing The Simultaneous Measurement Of 3,4-DHBA And 4-Hydroxybenzoic Acid (4-HBA) For The Detection Of The Hydroxyl Free Radical In Rat Brain Microdialysis Samples. 3,4-DHBA and neurotransmitter metabolites were selectively detected on the first coulometric electrode (A; +150mV, 10nA) while 4-HBA was measured on the second electrode (B; +700mV, 10µA gain). The isocratic system consisted of a pump, an autosampler, a thermal chamber and a Coulochem III detector. LC Conditions: Column: Mobile Phase: Flow Rate: Temperature: Injection Volume: Analytical cell, Model 5011: Super ODS (4.6 x 50mm; 2µm) TosoHaas. 100mM Sodium Phosphate Buffer (pH2.8) Containing Methanol (6.5% v/v). 1.0mL/min. 29oC . 20µL. E1 = +150mV; E2 = +700mV See 70-4820 Alternative Method for the Measurement of The Hydroxyl Free Radical for more details. WWW.ESAINC.COM 80 3-hydroxybenzoic acid 2,4-dihydroxybenzoic acid 2,3-dihydroxybenzoic acid 4-hydroxybenzoic acid 2,5-dihydroxybenzoic acid 10.0 3,5-dihydroxybenzoic acid Response (µA) 15.0 resorcinol 3-nitrotyrosine catechol 3,4-dihydroxybenzoic acid tyrosine meta-tyrosine 20.0 hydroquinone ortho-tyrosine homogentisic acid 3-chlorotyrosine In recent papers the chromatography used to measure the DHBAs on a single thin-layer electrode was extended to simultaneously measure a variety of monoamines and metabolites in brain and CSF tissues (e.g., Sloot and Gramsbergen (1995)). When dealing with such complex chromatography it is vitally important to fully characterize each eluting peak (both chromatographically and voltammetrically) to ensure its authenticity, and to avoid a possible co-elution or misidentification. Unfortunately, such approaches are usually incredibly tedious and time-consuming, unless coulometric electrode array detection is used. Beal used a gradient coulometric array method to measure salicylic acid, the DHBAs, 3-nitrotyrosine, 3-aminotyrosine and twenty-four neurochemicals simultaneously (Beal et al. 1990, 1995). Recently, Acworth et al. (1998b) developed a coulometric array method capable of resolving a number of possible markers of oxidative stress (Figure 2.13). 5.0 0.0 mV 830 810 670 630 570 500 450 400 R1 R2 10.0 20.0 30.0 40.0 50.0 60.0 Retention time (minutes) Figure 2.13 Isocratic Coulometric Array Chromatogram Showing The Simultaneous Measurement Of Several ROS And RNS Markers And Precursors (standards at 10µg/mL on column each). Dityrosine elutes just after tyrosine but is not shown for clarity. WWW.ESAINC.COM 81 The isocratic system consisted of a pump, an autosampler, a thermal chamber and a CoulArray detector. LC Conditions: Column: Mobile Phase: TSKgel ODS-80TM (4.6 x 250mm; 5µm) TosoHaas. 20mM Sodium Phosphate Buffer (pH3.2) Containing Methanol (8% v/v). 1.0mL/min. 31oC . 20µL. +400, +450, +500, +570, +630, +670, +810 and +830mV Flow Rate: Temperature: Injection Volume: Array Potentials: NITROGEN AND THE REACTIVE NITROGEN SPECIES (RNS). 1. Nitrogen. Properties. Nitrogen is a colorless and odorless diatomic gas that occurs in the atmosphere to the extent of about 78% by volume. Nitrogen has a melting point of -210oC and a boiling point of -196oC. Nitrogen is the first member of Group 5B of the periodic table and possesses seven electrons with an electronic configuration of 1s2, 2s2, 2p3. Unlike oxygen, nitrogen does not possess unpaired electrons (Figure 2.14) and is therefore considered diamagnetic. Nitrogen does not possess available d orbitals so it is limited to a valency of 3. It can show a range of oxidation states from -3 (ammonia) to +6 (nitrate radical). Formation. Nitrogen can be formed in the laboratory by the oxidation of ammonia (Eqn 2.42). Industrially, nitrogen is obtained from the atmosphere by liquefaction of air. Biologically, nitrogen is produced as part of the nitrogen cycle. 2NH3 + 3CuO → N2 + 3H2O + 3Cu Eqn 2.42 Chemical Reactions. Chemically, nitrogen is fairly inert, due to the very large N≡N bond energy (946kJ mol-1) but it can be forced to react if conditions are correct. For example, it can WWW.ESAINC.COM 82 form ionic nitrides with electropositive elements (e.g., lithium) (Eqn 2.43), covalent nitrides with non-metals (e.g., carbon and boron), and — under extreme temperature and pressure and in the presence of a catalyst — it can be reduced to ammonia (Eqn 2.44). In the presence of oxygen, nitrogen can produce nitric oxide when sparked (Eqn 2.45): this takes place in the atmosphere during lightning flashes. 6Li + N2 → 2(Li+)3N3N2 + 3H2 → 2NH3 N2 + O2 → 2NO• Eqn 2.43 Eqn 2.44 Eqn 2.45 Nitrogen and Nitric Oxide Electronic Configuration σ *2p π *2p π2p σ 2p GroundState Nitrogen Nitric Oxide (NO) Nitrosonium Cation (NO+ ) Triplet Nitroxyl Anion (NO-) Singlet Nitroxyl Anion (NO-) Figure 2.14 Molecular Orbital Diagram Of Molecular Nitrogen And Nitric Oxide. Unlike oxygen, in which a variety of oxidases and oxygenases (Table 2.1) make use of this gas in biochemically important reactions, the high energy of the N≡N bond renders nitrogen biochemically inert. Only one enzyme, the microbial nitrogenase complex, “fixes” nitrogen. This enzyme catalyzes the reduction of nitrogen to ammonia at a great energetic cost (Eqn 2.46). One subunit of the nitrogenase complex is a strong reducing agent with an Eo’= -0.4V. Ammonia can then be assimilated, by the action of glutamate dehydrogenase and glutamine synthetase, into the nitrogen cycle through the production of amino acids. WWW.ESAINC.COM 83 N2 + 6e- + 12ATP +12H2O → 2NH4+ +12ADP + 12PI + 4H+ Eqn 2.46 2. The Oxides of Nitrogen. In the field of oxidative metabolism, it is the oxides of nitrogen that are the most important. Readers are referred to Beckman (1996a) for an excellent review. At first sight the chemistry of and the interrelationships between the nitrogen oxides may appear pretty daunting. However, it should be remembered that many of the reactions described in a typical chemistry textbook are for those obtained in the gas phase and for high concentrations of reactants. These reactions are usually less relevant to biological systems. Readers should be aware that many articles in the literature fail to make such a distinction. However, gas phase reactions are important in exposure to air pollution (e.g., ozone and a variety of nitrogen oxides are formed in the atmosphere by lightning discharge and irradiation; nitrogen oxides are produced by the internal combustion engine and in tobacco smoke). The relationship between the different RNS is presented in Figure 2.15. The dimeric nitrogen oxides (N2O2 and N2O4) and acid anhydrides (N2O3 and N2O5) are usually formed only at higher concentrations of nitric oxide and/or nitrogen dioxide and are unlikely to be formed from the low concentrations of nitric oxide (typically 10-400nM) and nitrogen dioxide usually found in biological systems. The one exception is with the immune system which, when activated, can produce large quantities of nitric oxide (Hibbs et al. (1988)). WWW.ESAINC.COM 84 Reactive Nitrogen Species (RNS) R-SH NO+ Nitrosonium Cation NONitroxyl Anion Citrulline -e- NO2++ HONitronium Cation -RS +RSH +eO 2NO Nitric Oxide Arginine RSNO Nitrosothiol +H+ O=NOO Peroxynitrite -H+ CO 2/ HCO 3- HOCl NOCl Nitrosyl Chloride O2NOCO 2Nitrocarbonate NO2Nitrate (III) O=NOOH Peroxynitrous Acid ONO 2CO 2Nitrosoperoxycarbonate O=NO---OH Caged Pair NO3- + H+ Nitrate (V) NO3Nitrate (V) HOCl ClONO + ClNO2 Nitryl Chlorine Nitrite Chloride Figure 2.15 The Relationship Between The Different RNS. 2.1 Nitric Oxide. Physical Properties. Nitric oxide (NO•) is a colorless monomeric gas that can also exist as a blue liquid and blue solid consisting mainly of centrosymmetric dimers. Nitric oxide is quite stable in pure water and can be dissolved to 1.93mM at 25oC and at a partial pressure of 1 atm. The solubility of nitric oxide at physiological ionic strength and temperature is 1.55mM. Its solubility in membranes is approximately 6-7 fold higher than in the aqueous phase. Under physiological conditions the half-life of nitric oxide is only a few seconds (see below). Nitric oxide has a single unpaired electron in its π*2p antibonding orbital (Figure 2.14) and is therefore paramagnetic. This unpaired electron also weakens the overall bonding seen in diatomic nitrogen molecules so that the nitrogen and oxygen atoms are joined by only 2.5 bonds. The structure of nitric oxide is a resonance hybrid of two forms (Figure 2.16). • The loss of an electron (from the π*2p antibonding orbital) produces the nitrosonium ion (NO+), a molecule isoelectronic to nitrogen and carbon WWW.ESAINC.COM 85 • monoxide. The triple nitrogen-oxygen bond in the nitrosonium ion is much more stable (bond distance 0.114nm; bond energy 1048kJ mol-1) than the 2.5 bonds in nitric oxide (bond distance 0.120nm; bond energy 627kJ mol1 ) (Beckman (1996a)). The nitrosonium ion forms several ionic salts (e.g., nitrosonium perchlorate [NO+ClO4-] and nitrosonium hydrogen sulfate [NO+HSO4-]). When nitric oxide gains one electron, the nitroxyl anion (NO-) is formed which is isoelectronic to oxygen. Like oxygen, the nitroxyl anion has two unpaired electrons of parallel spin in two π*2p molecular orbitals in its lowest energy configuration (triplet state) (Figure 2.14). The nitroxyl anion also exists in a singlet state (where the two electrons form an antiparallel spin pair residing in a single π*2p molecular orbital). As expected this state is much higher in energy (~87.9kJ mol-1) than the triplet molecule (Standbury (1989)). N N O O Figure 2.16 The Resonance Forms Of Nitric Oxide. Formation. Nitric oxide can be produced in the laboratory by the action of 50% nitric acid on copper metal (Eqn 2.47). Nitric oxide so produced is contaminated with nitrogen dioxide, but can be purified by passing it through a concentrated iron (II) sulfate solution. Nitric oxide can then be liberated from FeSO4.NO by heating it in the absence of air. Nitric oxide is also formed during the electrical discharge of nitrogen (Eqn 2.45). 3Cu + 8HNO3 → 3Cu2+ + 6NO3- + 4H2O + 2NO• Eqn 2.47 A wide selection of NO-donor reagents now exist and can be used to generate nitric oxide in test systems (Table 3.5). These NO-donors vary in stability, pHand oxygen-sensitivity, water solubility, and contamination. For example, some reagents can also produce nitrosonium ions, nitroxyl anions, and other nitrogen oxides. Care must be exercised when interpreting data obtained using these donors (reviewed by Feelisch and Stamler (1996)). WWW.ESAINC.COM 86 Class Metal nitrosyls Inorganic NO donors Nitroxyl generating compounds Hydroxylamine N-Hydroxyguanidines O-Nitro and O-Nitroso compounds S-Nitro and S-Nitroso compounds N-Nitroso compounds Diazeniumdiolates (NONOates) C-Nitro and C-nitroso compounds Heterocyclic NO donors Examples Nitroprusside, dinitrosyl-iron (II) complexes, nitrosyl complexes of ironsulfur clusters, and nitrosyl complexes of other transition metals Acidified nitrite, nitrosonium salts and nitrosyl halides, peroxynitrite, and sodium azide Angeli’s salt, Piloty’s acid, cyanamide, and sodium nitroxyl Organic nitrates, and organic nitrites Thionitrates, and thionitrites N-Nitrosamines, N-hydroxy-Nnitrosamines, N-nitrosamides, Nnitrosoguanidines, N-nitrosohydrazines, and N-nitrosimines Oxadiazoles, oxatriazoles, and sydnonimines Table 2.6 Examples Of Nitric Oxide-Donor Molecules. In living organisms nitric oxide is produced enzymatically. Microbes can generate nitric oxide by the reduction of nitrite or oxidation of ammonia. In mammals nitric oxide is produced by stepwise oxidation of L-arginine catalyzed by nitric oxide synthase (NOS). Nitric oxide is formed from the guanidino nitrogen of the Larginine in a reaction that consumes five electrons and requires flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN) tetrahydrobiopterin (BH4), and iron protoporphyrin IX as cofactors (Figure 2.17). The primary product of NOS activity may be the nitroxyl anion that is then converted to nitric oxide by electron acceptors (see below). WWW.ESAINC.COM 87 . NH2 N H2N O OH H2N H2N NH O2 H2O O2 NH NH H2O + NADPH NADP+ 0.5 NADPH NH3 O L-Arginine O 0.5 NADP+ NH3 NH3 O O O N O O N G-HydroxyL-Arginine L-Citrulline Figure 2.17 The Formation Of Nitric Oxide From Arginine. To date, all sequenced NOS cDNAs show homology with the cytochrome P450 reductase family. Based on molecular genetics there appears to be at least three distinct forms of NOS: • • • A Ca2+/calmodulin-requiring constitutive enzyme (c-NOS; ncNOS or type I) (which produces relatively low levels of nitric oxide and is important in neurotransmission, maintenance of vascular tone, and inhibition of platelet aggregation); A calcium-independent inducible enzyme (i-NOS; type II), which is primarily involved in the mediation of the cellular immune response; and A second Ca2+/calmodulin-requiring constitutive enzyme found in aortic and umbilical endothelia (ec-NOS or type III) (Michel et al. (1996)). The important roles of these different NOS isoforms have been reviewed elsewhere (Feldman, et al. (1993); Kostka (1992); Snyder and Bredt (1992)). Recently, a mitochondrial form of the enzyme, which appears to be similar to the endothelial form, has been found in brain and liver tissue (Bates et al. (1995); Giulivi et al. (1998); Tatoyan and Giulivi (1998)). Although the exact role of nitric oxide in the mitochondrion remains elusive, it may play a role in the regulation of cytochrome oxidase (Giulivi (1998)). Nitric oxide appears to regulate its own production through a negative feedback loop (Griscavage et al. (1995)). The binding of nitric oxide to the heme prosthetic group of NOS inhibits this enzyme, thereby preventing the production of more nitric oxide. Interestingly, c-NOS and ec-NOS are much more sensitive to this regulation than i-NOS. This suggests that, in the brain, nitric oxide can regulate its own synthesis and therefore the neurotransmission process. Furthermore, inhibition of ec-NOS will prevent the cytotoxicity associated with excessive nitric WWW.ESAINC.COM 88 oxide production. Conversely, the insensitivity of i-NOS to nitric oxide will enable high levels of nitric oxide to be produced for cytotoxic effects. Several endogenous inhibitors of NOS (mainly the guanidino-substituted derivatives of arginine) occur in vivo as a result of post-translational modification of proteincontained arginine residues by S-adenosylmethionine (Kostka (1992)). For example, the dimethylarginines (NG,NG-dimethyl-L-arginine and NG,N’G-dimethylL-arginine) occurs in tissue proteins, plasma, and urine of humans (Tsikas et al. (1998)).6 These NOS inhibitors are thought to act as both regulators of NOS activity and reservoirs of arginine for the synthesis of nitric oxide. Numerous studies have reported that NOS can form peroxynitrite, suggesting that both its precursors, nitric oxide and superoxide, can be produced by this enzyme (see Miller et al. (1997) and references therein). This ability can be explained by examining of the structure of the enzyme. c-NOS, for example, consists of a flavin-containing reductase domain and an heme-containing oxygenase domain linked together by a sequence of amino acids that contains a calmodulin binding site. Binding of calmodulin brings these two domains together and allows the transfer of NADPH-derived electrons from the reductase domain to the oxygenase domain, resulting in the conversion of arginine to citrulline and the concomitant formation of nitric oxide. Under certain circumstances, such as a deficiency in arginine, or in the presence of NOS inhibitors, the activated hemeoxygenase complex dissociates and forms superoxide. Ec-NOS can also produce superoxide in a process modulated by the tetrahydrobiopterin cofactor (Vasquez-Vivar et al. (1998)). Recently, i-NOS was also shown to produce superoxide, but mainly by the action of the flavin-binding site of the reductase domain of this enzyme (Xia et al. (1998)). Chemical Reactions and Biological Significance. Much has been written about the chemistry of nitric oxide, particularly its reactions in the gas phase. For example, it is a reducing agent and is readily oxidized by oxygen to nitrogen dioxide (Eqn 2.48) and by chlorine in the presence of a catalyst to nitrosyl chloride (NOCl) (Eqn 2.49). Interestingly, both nitrogen dioxide and nitrosyl chloride can also be formed under aqueous conditions in vivo, albeit by very different mechanisms. 2NO•(g) + O2(g) → 2NO2•(g) 2NO•(g) + Cl2(g) → 2NOCl(g) Eqn 2.48 Eqn 2.49 Before we turn to the reactions of nitric oxide under physiological conditions, it must remembered that, although many of nitric oxides reactions can occur in the 6 These inhibitors can be measured using HPLC fluorescence following OPA derivatization (Meyer et al. (1997)). WWW.ESAINC.COM 89 aqueous phase, its reactivity is greatly accelerated within the hydrophobic interior of biological membranes. It has been calculated that even though membrane makes up about 3% of the total tissue volume, 90% of the reaction of nitric oxide with oxygen occurs within this compartment (Liu et al. (1998)). Thus the membrane is an important site for nitric oxide chemistry. There are two major aspects to nitric oxide chemistry. First, it can undergo single electron oxidation and reduction reactions producing nitrosonium and nitroxyl, respectively. These will be dealt with in greater detail in their own section below in recognition of their importance to redox biochemistry. Second as it has a single unpaired electron in its π*2p molecular orbital it will react readily with other molecules that also have unpaired electrons, such as free radicals and transition metals (Fukuto and Wink (1999)). Examples of the reaction of nitric oxide with radical species include: • • • • • Nitric oxide will react with oxygen to form the peroxynitrite (nitrosyldioxyl) radical (ONO2•) and with superoxide to form the powerful oxidizing and nitrating agent, peroxynitrite anion (ONO2-) (Eqn 3.24). Peroxynitrite causes damage to many important biomolecules and has been implicated in a variety of diseases (see below); Nitric oxide reacts with thiyl radicals to form nitrosothiols that are important in the regulation of blood pressure (see below); Nitric oxide reacts with alkylperoxyl radicals and thereby terminates lipid peroxidation (Chapter 3). The alkyl peroxynitrites (RO2NO) formed may be cytotoxic decomposing to nitrogen dioxide and other pro-oxidants; Nitric oxide can react rapidly (second order rate constant 10-9 M-1s-1) with tyrosyl radicals forming 3-nitrosotyrosine and/or 4-O-nitrosotyrosine. This reaction can affect the activity of enzymes that utilize tyrosyl radicals in their mechanisms. It is a common misconception that nitric oxide will react with tyrosine to form 3-nitrotyrosine, a compound currently being used as a marker of RNS activity. Nitric oxide will not react with tyrosine directly. Furthermore, it has yet to be proven that nitrosotyrosine formed as above can be oxidized to 3-nitrotyrosine in biological systems. Nitric oxide rapidly reacts with oxyhemoglobin producing nitrate — the primary route of its destruction in vivo (see below). Of great importance to redox biochemistry is the reaction between nitric oxide and transition metal complexes (Cooper (1999)). During this reaction a “ligand” bond is formed (the unpaired electron of nitric oxide is partially transferred to the metal cation), resulting in a nitrosated (nitrosylated) complex. For example, such complexes can be formed with free iron ions, iron bound to heme or iron located in iron-sulfur clusters (Salerno (1996)). Ligand formation allows nitric oxide to act as a signal, activating some enzymes while inhibiting others (Table 2.7). Thus, the binding of nitric oxide to the Fe (II)-heme of guanylate (guanalyl) cyclase [GTP-pyrophosphate lyase: cyclizing] is the signal transduction mechanism by WWW.ESAINC.COM 90 which nitric oxide acts as a messenger molecule (Eqn 2.50). Guanylate cyclase exists as cytosolic and membrane-bound isozymes. The soluble form (sGC) occurs as a heterodimer (MW 150kD), which also contains 1mol heme. The binding of nitric oxide to the iron of the heme molecule activates sGC, which then converts GTP to cyclic-GMP. Cyclic GMP can then activate protein kinase C, phosphodiesterase, and ion channels, thereby amplifying the original nitric oxide signal. These actions, as well as the subsequent decrease in intracellular calcium levels, mediates some of the biological effects of nitric oxide. E(inactive)-Fe2+(heme) + NO• → E(active)-Fe2+(heme)-NO Eqn 2.50 When produced in appropriate amount and periodicity, nitric oxide fulfills several significant biological roles. It is now regarded as a general short-lived secondary messenger (Bredt and Snyder (1994)) and, on the whole, as a beneficial molecule. First, it plays a role in blood pressure regulation as the endotheliumderived relaxing factor (EDRF) (Moncada et al. (1991)). Second, it acts as a retrograde neurotransmitter implicated in the formation of long-term memory (Schuman and Madison (1991)). Third, it contributes to the regulation of bone metabolism (Ralston (1997) Fourth, nitric oxide has a major function in the immune system and is produced by both macrophages and neutrophils. It inhibits the activity of key enzymes in the pathogen by forming transition metal complexes and its product, peroxynitrite, acts as a pro-oxidant with cytotoxic actions (Hibbs, et al., (1988); Marletta et al. (1988)). Finally, it may play a role as an antioxidant; however, this hypothesis is controversial: • • • • • Nitric oxide dissolves into membranes and can intercept lipid-based radicals, preventing lipid peroxidation processes (Hogg and Kalyanaraman (1999)). However, the products may be toxic and unstable resulting in the release of nitrogen dioxide and other RNS; Nitric oxide can detoxify the hydroxyl free radical by forming nitrous acid. However, this can occur only at unphysiological levels of nitric oxide (mM range) in order for it to compete with other compounds for reaction with hydroxyl free radical. Furthermore, nitric oxide can stimulate hydroxyl free radical production by reducing Fe (III) to Fe (II) that can then take part in the Haber-Weiss reaction; The reaction between nitric oxide and oxygen produces nitrogen dioxide, while its reaction with superoxide produces peroxynitrite, two very reactive pro-oxidant species; Nitric oxide is converted to a nitrosonium ion-like species capable of attacking important biomolecules (see below); and Nitric oxide may inhibit glutathione peroxidase, preventing detoxification of lipid hydroperoxides (Chapter 4). WWW.ESAINC.COM 91 Thiol Metal Extracellular Albumin GSH Membrane Adenylyl cyclase (type I) Calcium ATPase G Proteins KCa+ Channel NADPH Oxidase NMDA Receptor Protein Kinase C Cytosolic/ mitochondrial Actin Alcohol Dehydrogenase Aldolase Aldehyde Dehydrogenase GAP-43 Glyceraldehyde 3phosphate Dehydrogenase γ-Glutamylcysteinyl Synthetase Glutathione Glutathione peroxidase SNAP-25 Tissue Plasminogen Activator 2,3-Indolamine Aconitase Complex I Complex II Complex IV Cyclo-oxygenases Cytochrome P450 Guanylate Cyclase Dioxygenase Hemoglobin NO-Synthase Tryptophan Hydroxylase Nuclear AP-1 OMDM Transferase NF-κB SoxRS Tyrosyl/ Tryptophyl Radicals Cytochrome-c Peroxidase Prostaglandin H Synthase Ribonucleotide Reductase Table 2.7 Some Biologically Important Targets Of Nitrogen Oxides. (From Eiserich et al. (1995); Kuhn and Arthur (1996); Moncada and Higgs (1993); Raddi (1996); Stamler (1994); and See Table 3.11). OMDM – O-methylguanine DNA methyltransferase. See references and Glossary for definitions of abbreviations used. Unfortunately, nitric oxide also represents a major problem for biological systems (Gross and Wolin (1995)). Uncontrolled production of nitric oxide (e.g., overactivity of the immune system or uncontrolled central release following ischemia) can directly damage enzymes and other proteins. Nitric oxide also promotes ADP-ribosylation of proteins (Brune et al. (1994)), can directly damage DNA (Nguyen et al. (1992); Wink et al. (1991)) and may deplete cellular antioxidants (d’Ischia and Novellino (1996); Gorbunov et al. (1996)). The detrimental action of nitric oxide can be further exacerbated by the formation of peroxynitrite (see below). Cells have developed different mechanisms to protect themselves against chronic nitric oxide exposure. For example, E. coli activate the soxRS WWW.ESAINC.COM 92 response to prevent damage by nitric oxide-producing macrophages (Nunoshiba et al. (1993)). Exposure of mammalian cells, including neurons, causes adaptive resistance possibly mediated through the induction of heme oxygenase 1 (Chapter 4) (Bishop et al. (1999); Hartsfield et al. (1997); Takahashi et al. (1997)). Although the exact mechanism of heme oxygenase is hard to pinpoint, it could either be direct, through the reaction of nitric oxide with the heme moiety of the enzyme (similar to that described for nitric oxide destruction in blood below), or indirect, through the production of the antioxidant bilirubin. Consequently, heme oxygenase 1 may play a role in regulating nitric oxide-dependent age- and disease-related neurodegeneration. NO Hb-Fe2+-O2 O2 Oxyhemoglobin Hb-Fe2+O2NO Hb-Fe2+ Hemoglobin NADPHdependent reduction Hb-Fe3+ NO3- Methemoglobin Figure 2.18 The Metabolism Of Nitric Oxide To Nitrate. The • • destruction of nitric oxide proceeds via two mechanisms: First, in an oxygenated solution, nitric oxide undergoes a complex series of reactions involving many different ROS, culminating in the formation of nitrite (Eqn 2.51) (Beckman (1996a)). Nitrate is also formed (Eqn 2.52 to 2.54). Overall excess nitrite is produced. Under aqueous conditions the oxidation of nitric oxide can produce a wide variety of RNS. Second, in the blood system in which high levels are typically produced, nitric oxide can be oxidized to nitrate following its binding to oxyhemoglobin (or oxymyoglobin) (Figure 2.18). During this process WWW.ESAINC.COM 93 the Fe (II) contained within the heme ring is oxidized to the Fe (III) form, producing methemoglobin (cf. Eqns 3.20 and 3.21). Hemoglobin is regenerated in the erythrocyte by the enzymatic reduction of methemoglobin with NADPH. Interestingly, hemoglobin can be Snitrosylated in the lung and can release nitric oxide during arterialvenous transit (Gow and Stamler (1998); Jia et al. (1996)). Hemoglobin not only regulates nitric oxides level but it may also act as a carrier of nitric oxide and therefore a regulator of blood pressure. 4NO• + O2 + 2H2O ↔ 4HNO2 2NO• + O2 ↔ 2NO2• 2NO2• ↔ N2O4 N2O4 + H2O ↔ NO2- + NO3- + 2H+ Eqn 2.51 Eqn 2.52 Eqn 2.53 Eqn 2.54 Measurement. Many approaches are used to measure nitric oxide both directly and indirectly and these, along with their advantages and disadvantages, are presented in Table 2.8. Approach Bioassays e.g., aortic strips Comments Indirect. Not specific for nitric oxide. Not quantitative. Inconvenient. Reference Cocks, et al. (1985); Furchgott (1984); Griffith, et al. (1984); Martin, et al. (1985); Rapaport and Murad (1983); Rubanyi, et al. (1985) cGMP radioimmunoassay Indirect. Measures formation of [32P]cGMP from [α-32P]-GTP when nitric oxide activates guanylyl cyclase. Determined by liquid scintillation counting. cGMP levels increased by NO-donors and decreased by NOS inhibitors. In some cases it lacks selectivity and qualitative information. Can be made more nitric oxide-specific by the use of reporter cells such as RFL-6 cells. Does not always work reliably. Forstermann and Ishi (1996); Ignarro et al. (1984); Palacios et al. (1989) Chemiluminescence with hydrogen peroxide-luminol, or ozone Direct. Requires photomultiplier tube (an ozone generator is required for ozone approach). Failure to extract all nitric oxide from the biological sample into the gas phase will underestimate its level. Biological samples may foam during the Aoki, T. (1990); Hampl et al. (1996); Kojima et al. (1997) WWW.ESAINC.COM 94 Citrulline levels. [14C]-Citrulline formation from [14C]-arginine procedure that can cause problems with the detector (may be overcome using microdialysis). Humidity can affect sensitivity. Indirect. • Measured as OPA/βME derivative using HPLC-ECD in brain microdialysis samples. Citrulline levels were increased following kainic acid and decreased following NOS inhibition. Other biochemical pathways may also form citrulline. • Measured as OPA/βME derivative using HPLC-fluorescence in endothelial cells. Other biochemical pathways may also form citrulline. Indirect. • Measured in microdialysis samples. Does not take into account loss of label when other pathways metabolize citrulline. Only uses simple column extraction so signal from [14C]-Citrulline may be contaminated. Does not take into account loss of radiolabel during sample processing. • Thin layer chromatography of endothelial cell extracts. Does not take into account loss of label when other pathways metabolize citrulline. • Moncada, and Palmer (1990); Wang and Maher (1992) • Hecker and Billiar (1996) • Bhardwaj et al. (1995) • Hecker and Billiar (1996) EPR Direct or indirect. Lacks sensitivity to measure free nitric oxide in vivo. The angular momentum of the radical electron can couple with the angular momentum of the nitric oxide molecule to diminish the paramagnetism of nitric oxide. Useful in examining endogenous nitric oxidecomplexes. EPR can be extended by the use of nitric oxide-spin traps including Nmethyl-D-glucamine dithiocarbamate/ferrous ions, nitroxides, deoxyhemoglobin, and hemoglobin. May require freeze quenching of samples. Cheletrophic biradical traps are showing some promise. Arroya et al. (1990); Henry et al. (1993); Henry and Singel (1996); Korth and Weber (1996); Kosaka and Shiga (1996); Lai and Komarov (1994); Singel and Lancaster (1996) Electrochemical Direct. Various voltammetric probes exist. Probes differ in sensitivity, selectivity and kinetically. Burlet and Cespuglio (1997); Canini et al. (1997); Desvignes et al. (1997); Friedemann et al.(1996); Malinski, and Taha (1992); Rivot et al. (1997); Shibuki (1990); Indirect. Measurement of nitrite. WWW.ESAINC.COM 95 Strehlitz et al. (1996); Yao et al. (1995) Fluorescent Probes Mass Spectrometry Nitrite/nitrate Direct. Fluorescent probes, such as 4,5diaminofluorescein or 2,3-diamino naphthalene, permit real time imaging of nitric oxide production within cells. Direct or indirect. Useful for measuring nitric oxide in the gas phase. Probably not the best approach for in vivo studies. Indirect. • Nitric oxide is readily converted in vivo to nitrite and then nitrate (nitrate is normally two orders of magnitude higher). Total nitridergic involvement can be estimated following a) nitrate reduction to nitrite by cadmium or nitrate reductase, b) reaction with Greiss reagent and c) monitoring spectrophotometrically at 540nm. Kojima et al. (1998); Nakatsubo et al. (1998) Payne et al. (1996) Muscara and Nucci (1996); Pratt et al. (1995); Preik-Steinhoff and Kelm (1996); Salter et al. (1996); Schmidt and Kelm (1996); Stratford (1999); Yamada and Nabeshima (1997) Problems include incomplete reduction of nitrate, influence of diet, bacterial metabolism production unrelated to the arginine-nitric oxide pathway. • • • • HPLC-UV following reduction and Greiss reaction (nitrite and nitrate) HPLC-coulometric ECD (nitrite only) and UV (nitrate only) Chemiluminescence (nitrite and nitrate) Voltammetric nitrite-sensor (nitrite only) Approaches that measure both nitrite and nitrate do so for lack of sensitivity. Techniques measuring nitrite alone may be more representative of nitric oxide production, but care must be exercised when interpreting data. Spectrophotometric Indirect. Nitric oxide trapped by oxyhemoglobin and measured using a spectrophotometer. Maybe problematic for routine use in vivo. Coupling this approach to microdialysis has been used successfully to measure central NO• levels in vivo. Balcioglu and Maher (1993); Feelisch et al. (1996) and references therein; Zou and Cowley (1997) Table 2.8 The Different Approaches Used To Measure Nitric Oxide (see also Kishnani and Fung (1996)). WWW.ESAINC.COM 96 2.2 Nitroxyl Anion And The Nitrosonium Ion. As discussed above nitric oxide can take part in redox reactions. Chemically, it can act as both an oxidizing agent (becoming reduced to the nitroxyl anion) (Eqn 2.55) and a reducing agent (becoming oxidized to the nitrosonium ion) (Eqn 2.56) (Hughes (1999)). The nitroxyl anion and nitrosonium ion show distinct chemistries and their biological significance is still being evaluated (Crow and Beckman (1995), Stamler et al. (1992b)). NO• + e- → NONO• - e- → NO+ Eqn 2.55 Eqn 2.56 Under physiological conditions nitric oxide is a moderate oxidizing agent (Eo=+390mV). As discussed above (see also Figure 2.14), the nitroxyl anion can exist in a triplet and singlet state. The triplet nitroxyl anion can act as a oneelectron reductant, thereby reforming nitric oxide. It can react with other radicals. For example, it reacts with oxygen (second order rate constant of 3.4 x 107 M-1s-1) forming peroxynitrite (Eqn 2.57) (Huie and Padmaja (1993)). It also reacts reversibly with transition metal ions. For example, it is similar in size and shape to superoxide and readily reacts with copper ions found in Cu,Zn-superoxide dismutase forming nitric oxide (Eqn 2.58) (Beckman (1996a)). Indeed, some have proposed that NOS does not generate nitric oxide but rather the nitroxyl anion; the latter is then converted to the former by SOD and other electron acceptors (Hobbs et al. (1994); Schmidt et al. (1996b)). The reaction is freely reversible so nitric oxide can readily be reduced to the nitroxyl anion as well (Beckman (1996a)). In solution the nitroxyl radical readily undergoes a series of reactions producing nitrite and nitrous oxide (N2O) (a simplified reaction is shown Eqn 2.59). The singlet nitroxyl anion is more energetic than the triplet form. As it does not have an unpaired electron it does not react with oxygen (Hughes (1999); Stanbury (1989)). It also shows different chemical reactivity. NO- + O2 → ONO2NO- + Cu2+(SOD) ↔ NO• + Cu+(SOD) NO- + 2NO• → N2O + NO2- Eqn 2.57 Eqn 2.58 Eqn 2.59 The nitrosonium ion is a strong oxidizing agent (E0=1210mV) so its direct formation from nitric oxide is unlikely to occur under physiological conditions. However, a nitrosonium-like species can be formed in vivo when nitric oxide reacts with transition metal complexes (Eqns 2.60). For example, the formation of nitrosothiols by the reaction presented in Eqn 2.61 will not occur unless an electron acceptor (e.g., nitrogen dioxide or transition metals) is present (Eqn 2.62). Although this may be important in the synthesis of nitrosothiols (below), it WWW.ESAINC.COM 97 can be a major problem if this were to affect a thiol group critical to an enzyme’s function (Laval and Wink (1994); Stamler (1994)). In general nitrosation reactions can be a major problem for any living organism, as they can generate a variety of reactive and potentially toxic products. We will return to nitrosation reactions when we discuss nitrous acid below. NO• + Fe3+(heme) → Fe2+(heme)–NO NO• + RSH → RSNO + H+ + eFe2+heme)–NO + RSH → Fe2+(heme) + RSNO + H+ Eqn 2.60 Eqn 2.61 Eqn 2.62 The extent of nitrosation in vivo can be estimated by the measurement of a variety of nitrosated amino acid products (e.g., nitrosoproline, Nnitrosothiazolidine-4-carboxylic acid and N-nitroso-2-methylthiazolidine-4carboxylic acid) in urine using GC with a thermal energy analyzer ((Ohshima and Bartsch (1999)). 2.3 Peroxynitrite. Properties. Peroxynitrous acid (ONO2H) has a pKa of 6.8. Dilute basic solutions of peroxynitrite (~200mM) are relatively stable and are yellow in color. Under these conditions peroxynitrite can be kept safely at -20oC for many weeks. At physiological pH, the unstable and highly reactive peroxynitrous acid is formed. Peroxynitrite also forms highly colored, relatively stable salts (e.g., the tetramethylammonium salt is a yellow-orange solid). These salts are free from other ROS and RNS and should be used when accurate determination of reaction stoichiometry is critical. Peroxynitrite occurs in both cis- and transisomers (Beckman (1996a)). Formation. For readers interested in a review of the discovery of peroxynitrite see Beckman (1996a). The peroxynitrite anion can be produced in the laboratory by several methods (Table 2.9) (Saha et al. (1998); Uppu et al. (1996a)). The most common synthesis of peroxynitrite comes from acidified nitrite and hydrogen peroxide (Eqns 2.63 and 2.64) (Koppenol et al. (1996)). Peroxynitrite and its salts are now commercially available. HNO2 + H+ <=> H2O + NO+ NO+ + H2O2 → ONO2H + H+ WWW.ESAINC.COM Eqn 2.63 Eqn 2.64 98 • Reaction of ozone with azide ions. • Auto-oxidation of hydroxylamine. • 1. 2. 3. Reaction of hydrogen peroxide with: Acidified nitrite or nitrous acid. Alkyl nitrites (water-soluble or water-insoluble). Nitric oxide. • Reaction of nitric oxide with: 1. Solid potassium superoxide. 2. Tetramethylammonium superoxide. • Photolysis of solid potassium nitrate. Table 2.9 Different In Vitro Methods For The Synthesis Of Peroxynitrite. Peroxynitrite can be formed in vivo by at least three possible reactions (Beckman (1996a): • • • The reaction between nitric oxide and the superoxide radical anion (Eqn 2.25) is, without a doubt, the major source of peroxynitrite production in vivo. This reaction proceeds at a near diffusion-limited rate (6.7 x 109 M-1s-1) which is approximately 3-6 times the rate at which superoxide is dismuted by SOD. Thus, both nitric oxide and superoxide can modulate the effects of the other. For example, superoxide can block the hypotensive effects of nitric oxide by diverting it to form peroxynitrite. SOD can increase the hypotensive effects of nitric oxide by decreasing the availability of superoxide. Due to the synthesis of peroxynitrite, nitric oxide can be considered to “detoxify” superoxide (Feigl (1988); Kanner et al. (1991); Rubanyi et al. (1991)).7 As discussed above, these beneficial antioxidant properties are far outweighed by the formation of peroxynitrite, a toxic and highly reactive pro-oxidant; Peroxynitrite can also be formed by the reaction between the nitroxyl anion (e.g., formed by reduced SOD) and oxygen (Eqn 2.57); and The reduction of the peroxynitrite radical (Eqn 2.65). ONO2• + O2•- → ONO2- + O2 Eqn 2.65 7 The probability that one chemical species will attack another depends not only on the rate of the reaction but also on the concentration of the target (Crow and Beckman (1995)). The multiple of the reaction rate and target concentration is called the target area, and under normal conditions, the target area of SOD exceeds the target area of nitric oxide by about 30 fold. However, during pathological conditions such as reperfusion following ischemia, the target area of nitric oxide can exceed that of SOD such that under these conditions peroxynitrite is preferentially produced. WWW.ESAINC.COM 99 Although peroxynitrite can be formed by the reaction between the nitrosonium ion and hydrogen peroxide it is highly unlikely to occur under physiologically conditions. Chemical Reactions and Biological Significance. The chemistry of peroxynitrite has recently been the topic of several reviews and numerous papers (e.g., Beckman (1996); Beckman et al. (1994); Daiber et al. (1998); Groves (1999); Pryor and Squadrito (1995); Squadrito and Pryor (1998)). The reactivity of peroxynitrite not only depends upon the pH of the reaction and which chemical species are present, but also on the fact that peroxynitrite exists in vivo in the cis-isomer. The cis-isomer is much more reactive than the transisomer; the latter readily isomerizes to nitrate without further reaction. Since the barrier for isomerization is about 110kJ mol-1, cis-trans isomerization is unlikely to occur in vivo. Peroxynitrite is therefore locked in the more reactive form (Beckman (1996a)). A summary of the many reactions of peroxynitrite/peroxynitrous acid with various biomolecules is presented in Table 2.10. Molecule Damaged DNA Comments • • • • Lipids Proteins/ enzymes/ amino acids • • • • • • Forms oxazolone, 8-oxo-2’d-adenosine and 8OH2’d-guanosine when adenine and guanine nucleosides incubated with peroxynitrite. Oxazolone and 8-oxo-2’d-adenosine levels are elevated when double stranded DNA is incubated with peroxynitrite. Forms 8-nitroguanine when guanine is treated with peroxynitrite in vitro. Causes DNA strand breaks. Activates the DNA repair enzyme, poly(ADP)ribosyltransferase. Damages 2-deoxyribose. Promotes lipid peroxidation. Forms F2-isoprostanes during oxidation of human low-density lipoproteins. Nitrated tyrosine can alter protein conformation and activity. Nitrated tyrosine can disable tyrosine phosphorylation regulatory mechanism and target proteins for degradation. Oxidizes thiols. The two-electron oxidation pathway, mediated by peroxynitrite anion, predominates over the one-electron pathway mediated by peroxynitrous acid and its derivatives. In vivo, nitrocarbonate favors the one-electron oxidation of thiols to thiyl radicals. These are involved in chain reactions that ultimately oxidize thiols to disulfides. This can WWW.ESAINC.COM Reference Beckman et al. (1990); Chabot et al. (1997); Douki and Cadet (1996); Szabo et al. (1997); Tamir et al. (1996); Yermilov et al. (1995a,b); Zingarelli et al. (1996). Moore et al. (1995); Radi et al. (1991); Rubbo et al. (1994). Alvarez et al. (1996); Beckman (1996); Beckman et al. (1992); Bouton et al. (1997); Briviba et al. (1998); Cooper et al. (1998); Crow et al. (1995); Frears et al. (1996); Galli et al. (1998); Gow et al. (1996); Halliwell (1997); Kaur et al. (1997); 100 Ascorbic acid, bilirubin and uric acid effect the activity of enzymes requiring participation of thiol groups in their reaction mechanisms (e.g., alcohol dehydrogenase, and glyceraldehyde-3-phosphate dehydrogenase). Interestingly, cysteine and GSH are peroxynitrite scavengers. • Procollagenase activation. May affect the extracellular matrix and lead to disease (e.g., arthritis). • Inactivation of the inhibitor of metalloproteinase1. Metalloproteinases are a group of enzymes including collagenases, gelatinases and stromelysins that are critical in controlling connective tissue remodeling. • Inactivates α1-antiproteinase, the major inhibitor of serine proteases such as elastase. • Inactivates E. coli Mn/Fe-SOD but not bovine CuZn-SOD. • Inhibits glutathione peroxidase by oxidation of selenol to selenocysteine in enzyme’s active site. • Inhibits GTP binding to Rac2 by tyrosine oxidation. • Inactivates sarcoplasmic reticulum calciumATPase. • Converts xanthine dehydrogenase to xanthine oxidase, an enzyme capable of generating ROS. • Modulates iron regulatory protein. • Inhibits mitochondrial electron transport. • Forms protein carbonyls. • Reacts with methemoglobin to generate globinbound free radical species that may play a role in vascular disease. • Tryptophan produces protein tryptophan radicals, nitrotryptophan, N-formylkynurenine or hydropyrroloindole depending upon reaction conditions. • Tyrosine produces protein tyrosyl radicals, 3nitrotyrosine, dityrosine and 3,4-DOPA depending upon reaction conditions (Figure 3.9). These products can be formed by reactions not involving peroxynitrite (see Chapter 5). • Phenylalanine produces tyrosine isomers and 4nitrophenylalanine. • Oxidation of methionine to methionine sulfoxide (Figure 2.19) and/or ethylene. Ascorbic and uric acids scavenge peroxynitrite but produce ascorbyl and uric acid radicals that then have to be removed. Uric acid also forms a nitrosated/nitrated adduct that can act as a nitric oxide donor. Bilirubin does not react directly with peroxynitrite but is effective at scavenging secondary oxidants formed during the oxidation WWW.ESAINC.COM Ischiropoulos and AlMehdi (1995); Ischiropoulos et al. (1992); Kato et al. (1997); Kong et al. (1996): Mohr et al. (1994); Moreno and Pryor (1992); Muijsers et al. (1997); Okamoto et al. (1997); Padmaja et al. (1998); Pietraforte and Minetti (1997a,b); Pryor et al. (1994); Quijano et al. (1997); Radi et al. (1991a); Ramezanian et al. (1996); Rohn et al. (1999); Sakuma et al. (1997); Scorza and Minetti (1998); Souza and Radi (1998); van der Vleit et al. (1994, 1995); Viner et al. (1996); Whiteman and Halliwell (1996). Bartlett et al. (1995); Minetti et al. (1998); Skinner et al. (1998); Vasquez-Vivar et al. (1996). 101 Flavonoids Inorganic anions Monoamines Salicylic acid Tocopherol process. Quercetin, rutin and epigallocatechin gallate are peroxynitrite scavengers. Reacts with carbonate, cyanide, iodide, and thiocyanate. The reaction with sulfite produces an intermediate that prolongs peroxynitrite reactivity and may play a role in sulfite’s neurotoxicity. This intermediate eventually decays to sulfate and nitrate. Nitrates catecholamines in vitro forming 6nitrocatecholamines (nitration may be due to peroxynitrite radical or other oxides of nitrogen). Now found in vivo and may act as a potential signal molecule linking the actions of norepinephrine and nitric oxide. Peroxynitrite can hydroxylate salicylate forming 2,3and 2,5-dihydroxybenzoic acid and nitrate salicylate forming 5-nitrosalicylic acid. Thus peroxynitrite can interfere with assays for hydroxyl free radical measurement (Figure 3.11). Nitrates γ-tocopherol to form 5-nitro-γ-tocopherol. Fiala et al. (1996); Haenen et al. (1997). Groves (1999) and references therein; Huie and Nita (1999) and references therein; Reist et al. (1998) d’Ischia and Costantini (1995); Shintani et al. (1996) Halliwell and Kaur (1997); Kaur et al. (1997); Narayan et al. (1997); Ramezanian et al. (1996). Christen et al. (1997) Table 2.10 Many Molecules React With Peroxynitrite. Peroxynitrite shows five distinct reactions pathways: 1. Following protonation, the decomposition of peroxynitrous acid produces a species showing both hydroxyl free radical- and nitrogen dioxide-like reactivity (see Figure 2.19). Although originally it was hypothesized that both free hydroxyl free radicals and nitrogen dioxide were produced by a homolytic cleavage of peroxynitrite (Eqn 2.66), evidence now suggests that neither of these free radicals is actually formed to any extent in vivo. Rather, both hydroxyl free radical and nitrogen dioxide-like activity may exist within the same molecule, the “radical ends” proposed by Crow and Beckman (1995). Such reactivity can be explained by either a caged-pair (geminate pair) or activated conformer (ONO2H*) of peroxynitrous acid (Pryor and Squadrito (1995)).8 The attack of a molecule by one end of peroxynitrite will form nitrated products, while the attack by the other end will lead to hydroxylation. ONO2- + H+ → HO•…….•NO2 Eqn 2.66 For some of its reactions, peroxynitrite can be regarded as a more stable form of the hydroxyl free radical and is capable of transporting this reactive species 8 * -1 The vibrationally active state ONO2H is 71kJ mol above the energy level of the ground state. WWW.ESAINC.COM 102 to places far removed from its site of production. Remember that the hydroxyl free radical reacts as soon as it is produced. The half-life of peroxynitrite, however, is on the order of ms to s time scale under physiological conditions (this may be somewhat reduced by its reaction with endogenous thiols and bicarbonate). Furthermore cell membranes offer no significant barrier to peroxynitrite diffusion (Groves (1999) and references therein). Thus when compared to the hydroxyl free radical, peroxynitrite can diffuse a considerable distance in vivo before reacting and causing damage (Table 2.11). Species Hydrogen Peroxide Steady State Level 10-9 to 10-8M “Half-Life”# Years – when pure; minutes to hours in presence of reducing agents; unknown in vivo Diffusion Distance Freely diffusible over great distances unless intercepted Reference Cadenas (1999)** Hydroxyl free radical 10-20M 10-9s 3nm Hutchinson (1957) Nitric oxide ~10-8 to 10-7M (e.g., in cell signaling) 30s buffer 700µm 1s heart* 130µm Crow and Beckman (1995); Kelm and Schrader (1988) Unknown 1s buffer 100µm 9 x 10-3s “biological conditions” 9µm 10-5s 1-4µm intracellular Peroxynitrous acid Superoxide 10-11 to 10-10 M Radi et al. (1991a,b); Zhu et al. (1992). Beckman (1996) 15µm vascular Table 2.11 Comparison Of Steady-State Level, Half-Life And Diffusion Distance Between Different ROS And RNS (See Beckman (1996) and references therein for further details. See also Boveris, A., and Cadenas, E. (1997) and Pryor, W.A. (1986). *Blood free, isolated perfused heart. **Personal communications. #Half-life is used here although, in the strictest sense, it should only be applied to species whose decay is first order. 2. Peroxynitrite reacts with metals to produce a potent nitrating agent similar to the nitronium ion (heterolytic cleavage of peroxynitrite) (Eqn 2.67). ONO2- + M2+ → NO2+ + OH- + M+ WWW.ESAINC.COM Eqn 2.67 103 The importance of this reaction in vivo is not clear, as the amount of free metals required are well above biological levels (Crow and Beckman (1995)). The copper atom in CuZn-SOD can, however, react with peroxynitrite to form a species capable of nitrating phenol whereas free copper and chelated copper cannot. This suggests that SOD is acting as more than a species capable of chelating copper. SOD thus appears to play two critical roles in peroxynitrite chemistry in vivo. First it can regulate peroxynitrite formation by affecting the availability of superoxide. Second, it can enhance the peroxynitrite’s nitration pathway. In the absence of a compound capable of undergoing nitration (e.g., phenol) CuZn-SOD undergoes self-nitration, without affecting its activity (Beckman (1996)). It has been proposed that mutations of CuZn-SOD associated with familial amyotrophic lateral sclerosis permit easier access of peroxynitrite into the enzyme’s active site (Beckman et al. (1993)). The production of a pronitration species may then explain the increased protein nitration and free nitrotyrosine found in affected neurons (Abe et al. (1995); Bruijn et al. (1997)). 3. Peroxynitrite causes the oxidation of sulfur-containing groups (e.g., the oxidation of methionine produces methionine sulfoxide) (Figure 2.19). 4. Peroxynitrite reacts with carbon dioxide/bicarbonate and forms nitrocarbonate or other potent nitrating species (see below). Due to the abundance of carbon dioxide/bicarbonate in biological systems, this redirection of peroxynitrite’s reactivity is very important in vivo. 5. Peroxynitrite indirectly leads to the nitrosation of nucleophiles possibly through an intermediate X-N=O (where X is –OONO2, -NO2, or –CO3-) (Uppa et al. (1998)). The level of peroxynitrite is mainly controlled by the availability of its precursors, superoxide and nitric oxide (Briviba et al. (1999) and references therein) and by the action of scavengers. Depending upon the tissue, SOD, nitric oxide synthase, and oxyhemoglobin all appear to play a major role in controlling peroxynitrite formation. A number of antioxidant scavengers such as ascorbic acid, uric acid, and thiols (GSH, cysteine and methionine) may also affect peroxynitrite levels (Table 2.10 and references therein). In order for a scavenger to be effective it must react with peroxynitrite in a bimolecular fashion and rapidly enough to compete with carbon dioxide. Although ascorbic acid is much too slow to be an effective antioxidant, thiols rapidly react with peroxynitrite (Briviba et al. (1999)). Some synthetic compounds (e.g., ebselen and iron (III) porphyrin) are extremely reactive towards peroxynitrite and are of potential use in antioxidant treatment (Chapter 4) (Squidrito and Pryor (1998)). Interestingly, glutathione peroxidase (Chapter 4) is even more effective than ebselen at protecting against peroxynitrite-mediated oxidation and nitration reactions (Sies et al. (1997)). WWW.ESAINC.COM 104 Peroxynitrite is implicated in a variety of diseases and conditions including Alzheimer’s, apoptosis, atherosclerosis, cystic fibrosis, endotoxic shock, gastritis, idiopathic pulmonary fibrosis, inflammation, ischemia/reperfusion injury, pneumonia, respiratory distress syndrome, rheumatoid arthritis, sepsis, and viral infection (Halliwell (1997) and references therein; Kaur and Halliwell (1994c); Kooy et al. (1994); Moriel and Abdalla (1997); Saleh et al. (1997); Smith et al. (1997); Szabo (1996); van der Veen et al. (1997)). Care should be exercised though as many reports use the presence of 3-nitrotyrosine as an indicator of the involvement of peroxynitrite. Unfortunately, 3-nitrotyrosine is difficult to measure accurately and can also be formed by mechanisms not involving peroxynitrite (Chapter 4). A) ONO2H NO2 OH OH O -H2O OH O NO2 H H R R OH NO2 H NO2 R R R B) OH ONO2H HO /NO2 OH OH +H2O OH2 OH -H+, -H OH H H R R R R NO2 OH OH NO2 H -H + NO2 H R R C) O NH2 OH S CH3 O H O N O -NO2- O NH2 OH OH S CH3 -H+ O NH2 OH O S CH3 Figure 2.19 Mechanisms For The Reaction Of Peroxynitrite With Substituted Phenols (A And B) And The Oxidation Of Methionine To Methionine Sulfoxide (C). Reaction A shows the generally accepted reaction sequence for the nitration of substituted aromatic compounds. Reaction B takes into account that aromatic compounds can be hydroxylated or nitrated by peroxynitrite and is based on that proposed by Pryor and Squadrito (1995). Reaction C shows the oxidation and change in valency of sulfur in methionine. WWW.ESAINC.COM 105 Measurement. Although peroxynitrite levels can be determined using spectrophotometric techniques by measuring its absorbance at 302nm (Beckman et al. (1994)), the lack of specificity and sensitivity may render this approach inadequate for most in vivo investigations. Other methods indirectly measure ONO2-/ONO2H levels by determining 3-nitrotyrosine concentrations and use either a qualitative immunological approach or quantitative HPLC techniques. These are discussed in greater detail in Chapter 3 so will not be dealt with here. 2.4 Nitrosoperoxycarbonate and Nitrocarbonate. It has been known for many years that peroxynitrite is less stable in carbonate than in phosphate buffers (Keith and Powell (1969)). Peroxynitrite reacts with carbon dioxide in a second-order process with a rate constant of 3 x 104 M-1s-1 at 25oC (Lymar and Hurst (1995a,b); Uppa et al. (1996b)). In biological systems carbon dioxide is in equilibrium with bicarbonate. Because bicarbonate is present at a concentration typically >25mM (normal carbon dioxide levels are ~1.3mM), its involvement in peroxynitrite-mediated reactions will be significant. Under aqueous conditions the most likely reaction is hydrolysis producing carbonate and nitrate (Vesela and Wilhelm (2002)) thereby preventing peroxynitritemediated damage including the inhibition of the oxidation of thiols, oxyhemoglobin and cytochrome c2+, and prevention of aromatic oxidation (hydroxylation) (Denicola et al. (1996); Gow et al. (1996a); Lemercier et al. (1997); Radi et al. (1999); Uppa and Pryor (1996); Uppa et al. (1996b); Zhang et al. (1997)). However, in the non-polar environment of membranes reactions will include aromatic nitration (e.g., production of 3-nitrotyrosine from tyrosine and 8nitroguanine from guanine) and oxidative damage. Several compounds have been suggested to mediate peroxynitrite/carbon dioxide reactions. The unstable nitrosoperoxycarbonate anion (ONOOCO2-) and its product, the more stable but still reactive nitrocarbonate (O2NOCO2-) anion, are the most likely candidates. Alternatively, the weak peroxo O-O bond in nitrosoperoxycarbonate could undergo either homolytic cleavage, to produce the very reactive carbonate radical anion (CO3•-) and nitrogen dioxide (Eqn 2.68), or heterolytic cleavage, to produce carbonate and nitronium ions (Eqn 2.69) (Bonini et al. (1999); Denicola et al. (1996); Lymar and Hurst (1995a); Uppa et al. (1996b)). ONOOCO2- → CO3•- + NO2• ONOOCO2- → CO32- + NO2+ WWW.ESAINC.COM Eqn 2.68 Eqn 2.69 106 Lymar and Hurst (1996) have suggested that nitrosoperoxycarbonate may serve two important biological functions. First, it acts as a scavenger of peroxynitrite that, due to its instability, will limit the area of damage caused by this pro-oxidant (Vasela and Wilhelm (2002)). Second, it may be a superior microbicide to hydrogen peroxide, as it will not be deactivated by microbial catalase. Again, unlike hydrogen peroxide that can diffuse a long way from its generation site and cause damage in areas remote from the site of production, the activity of nitrosoperoxycarbonate will be limited to the area in which it is produced. 2.5 Nitrogen Dioxide, The Nitronium Cation and Nitrite. Properties. Nitrogen dioxide (NO2•) exists as a dense, poisonous, dark brown gas, a pale yellow liquid with a boiling point of 22oC and, at low temperatures, a pale yellow solid composed almost entirely of its dimer dinitrogen tetroxide (N2O4). The nitronium cation (NO2+) is a linear molecule and is isoelectronic to carbon dioxide. It can be isolated as its stable but very reactive perchlorate salt. Nitrite (NO2-) is the salt of the weak and unstable acid, nitrous acid (HNO2). With a pKa of ~3.5, nitrous acid formation is favored by acidic pH. Formation. Nitrogen dioxide is made in the laboratory by the reaction of copper with concentrated nitric acid, by oxidation of nitric oxide, and the thermal decomposition of metallic nitrates (Eqn 2.70). Biologically nitrogen dioxide is primarily formed by the oxidation of nitric oxide and possibly by the oxidation of nitrite by peroxidases (Eqns 2.71-2.73) (Klebanoff (1993)). Nitrogen dioxide is very soluble in water producing both nitric and nitrous acids (Eqn 2.74). 2Pb(NO3)2 → 2PbO + 4NO2• + O2 Peroxidase + H2O2 → Peroxidase Compound I + H2O Peroxidase Compound I + NO2- → Peroxidase Compound II + NO2• Perxoidase Compound II + NO2- → Peroxidase + NO2• 2NO2• + H2O → HNO3 + HNO2 Eqn 2.70 Eqn 2.71 Eqn 2.72 Eqn 2.73 Eqn 2.74 Chemical Reactions and Biological Significance. Like nitric oxide, nitrogen dioxide is a radical that shows redox behavior. It can undergo single electron reduction9 to nitrite (NO2-) (Eqn 2.75) or single-electron 9 It can also undergo a two-electron reduction to nitric oxide (e.g., with the oxidation of hydrogen sulfide to sulfur, iodide to iodine, and sulfur dioxide to sulfate). WWW.ESAINC.COM 107 oxidation to the nitronium cation (NO2+) (Eqn 2.76). Like nitric oxide it also can take part in radical-radical interactions (e.g., Eqn 2.77). NO2• + e- → NO2NO2• - e- → NO2+ NO2• + LO2• → LO2NO2 Eqn 2.75 Eqn 2.76 Eqn 2.77 Nitrogen dioxide is a strong one-electron oxidant (Eo’=+0.99V) that in turn is reduced to nitrite. Nitrite is further metabolized to nitrate by oxyhemoglobin (Eqn 2.78). The methemoglobin so formed is reduced to hemoglobin enzymatically using NADPH (see above). A similar reaction occurs when humans consume foods high in nitrite – used as an antioxidant “cured meats.” This can lead to methemoglobinemia in humans (Eqn 2.79) (Beckman (1996a)) (Anon. (1992)). Excessive nitrite consumption can even be fatal (Chilcote et al. (1977); Ellis et al. (1992)). 4HbO2 + 4NO2- + 4H+ → 4metHb + 4NO3- + O2 + 2H2O NO2- + 2H+ + Fe2+ → Fe3+ + H2O + NO2• Eqn 2.78 Eqn 2.79 At the low concentrations typically found in vivo nitrogen dioxide readily initiates free radical oxidation of proteins and unsaturated lipids (inducing lipid peroxidation through hydrogen atom abstraction) (Eqn 2.80) (Beckman (1996a)). At higher concentrations nitrogen dioxide will rapidly react with the radical produced in (Eqn 2.80) forming organic nitro-derivatives (e.g., nitro-lipid adducts are produced from lipid radicals and 5-nitro-γ-tocopherol from γ-tocopherol radicals) (Eqn 2.81) (Cooney et al. (1993, 1995); Huie (1994)). Nitrogen dioxide can also react with itself or with nitric oxide forming higher oxides (see below). Nitrogen dioxide can react with the superoxide radical anion to produce peroxynitrate (O2NO2-). Peroxynitrate shows similar 2-electron oxidation behavior to peroxynitrite, but different reactivities to carbon dioxide, pH stability and decomposition pathways (Goldstein, et al., (1998); Olson et al., (2003)). Whether peroxynitrate is biologically important is debatable as one of its precurorsors, nitrogen dioxide, is biochemically scarce. Peroxynitrate could possibly play a role in the phagosome. γ-Tocopherol-H + NO2• → γ-Tocopheryl• + NO2- + H+ NO2• + γ-Tocopheryl• → γ-Tocopherol-NO2 Eqn 2.80 Eqn 2.81 Under acidic conditions nitrite forms nitrous acid – the latter is in equilibrium with the nitrosonium ion (Eqn 2.82); consequently, many of the reactions reported WWW.ESAINC.COM 108 specifically for nitrous acid are a result of this ion, e.g., the nitrosation of amines. Primary amines produce unstable intermediates that undergo a series of reactions consisting of N-nitrosation, diazotization, and decomposition. The final product is dependent upon whether the structure of the original amine is aliphatic or aromatic in nature. Aliphatic primary amines will lead to the formation of reactive carbonium ions (Eqn 2.83) while aromatic primary amines form deaminated products. For example, guanine is deaminated to form xanthine that can disrupt base-pairing in the DNA molecule (Eritja et al. (1986); Nguyen et al. (1992); Wink et al. (1991)). Secondary amines (such as dimethylamine and morpholine) form relatively stable but cytotoxic nitrosamines (Eqn 2.84). The nitrosonium ion reacts with water and superoxide, producing nitrite and peroxynitrite, respectively. Thiols produce S-nitrosothiols (Eqn 2.85). HNO2 + H+ ↔ H2O—NO+ R-NH2 + NO+ → R-NH-N=O + H+ → R-N=N-OH → R-N2+ + OH- → R+ + N2 R2NH + NO+ → R2NNO + H RSH + NO+ → RSNO + H+ Eqn 2.82 Eqn 2.83 Eqn 2.84 Eqn 2.85 The nitronium cation is a strong oxidizing agent (Eo’=1600mV). It is also an aggressive electrophile, readily taking part in electrophilic substitution reactions of aromatic systems, in which the formation of the carbon-nitrogen bond is the rate-determining step (Eqn 2.86). In the laboratory, it is formed by the reaction between concentrated nitric and sulfuric acids (Eqn 2.87). In biological systems, it appears that a nitronium-like species is produced when peroxynitrite reacts with metal ions at physiological pH (Beckman et al. (1992); Ischiropoulos et al. (1992b); Koppenol et al. (1992)). Ar-H + NO2+ → Ar-NO2 + H+ HNO3 + 2H2SO4 → NO2+ + H3O+ + 2HSO4- Eqn 2.86 Eqn 2.87 Measurement. Atmospheric nitrogen dioxide can be determined using chemiluminescent or voltammetric methods (Goldman and Macrae (1994)). Measurement of aqueous levels of nitrogen dioxide is difficult due to its reactivity. Nitrogen dioxide can be determined by measuring of nitrate using voltammetric, reduction, and spectrophotometric methods (Eaton et al. (1995)) (Table 2.8). Nitrite can be determined using a variety of methods (Table 2.8). WWW.ESAINC.COM 109 2.6 The Higher Oxides of Nitrogen – Dinitrogen Trioxide, Dinitrogen Tetroxide and Dinitrogen Pentoxide Properties Dinitrogen trioxide (N2O3), the acid anhydride of nitrous acid, exists as an unstable blue liquid and solid. Dinitrogen tetroxide (N2O4) is in equilibrium with nitrogen dioxide and at its freezing point (-11.2oC) is pale yellow, due to the presence of 0.1% nitrogen dioxide. Dinitrogen pentoxide (N2O5), the acid anhydride of nitric acid, exists as a colorless non-ionic gas and an ionic solid composed of nitronium nitrate (NO2+NO3-). Formation. The formation of these higher oxides requires the interaction of two nitrogen oxides. Dinitrogen trioxide is formed by the reaction between nitric oxide and nitrogen dioxide (Eqn 2.88). Dinitrogen tetroxide is formed when two molecules of nitrogen dioxide react together (Eqn 2.89). Dinitrogen pentoxide is ultimately formed from nitric oxide and nitrogen dioxide (Beckman (1996a)). NO• + NO2• <=> N2O3 2NO2• <=> N2O4 Eqn 2.88 Eqn 2.89 Chemical Reactions and Biological Significance. As nitrogen dioxide is not particularly abundant in vivo it is more likely that it will react preferentially with nitric oxide to form dinitrogen trioxide than dimerize to form dinitrogen tetroxide. In the presence of superoxide, the production of any of these dimers will be in direct competition with the production of peroxynitrite and peroxynitrate (see above). Dinitrogen trioxide is unstable and readily decomposes to nitric oxide and nitrogen dioxide. As the acid anhydride of the unstable nitrous acid, it reacts with water to produce nitrite (Eqn 2.90). It is a strong (two-electron) oxidizing and nitrosating agent. N2O3 + H2O → 2HNO2 <=> 2H+ + 2NO2- WWW.ESAINC.COM Eqn 2.90 110 Dinitrogen tetroxide ionizes under aqueous conditions producing the nitrosonium ion (Eqn 2.91). It is a strong (two-electron) oxidizing, nitrosylating and nitrating agent (Eqn 2.92). Dinitrogen pentoxide is a strong oxidizing and nitrating agent. N2O4 <=> NO+ + NO3N2O4 + 2H+ + 2e- → 2HNO2 (Eo=+1.07V) Eqn 2.91 Eqn 2.92 As all but nitric oxide occurs at low levels, the biological significance of these compounds is questionable. The one exception is during phagocytosis where high levels of nitric oxide and ROS can result in the formation of dinitrogen trioxide and dinitrogen tetroxide which, in turn are then used to kill pathogens. Measurement. Spectrophotometric methods exist for the measurement of dinitrogen oxides (Feelisch and Stamler (1996)). The measurement of the low levels of these compounds found in biological systems is difficult. NH2 NH2 O O S-N=O S-N=O OH OH S -Nitrosohomocysteine O=N S HOOC S -Nitrosocysteine O=N-S O H N COOH O N H S-N=O NH2 S -Nitrosoglutathione S -Nitrosoprotein Figure 2.20 Some S-Nitrosothiols Reported In Vivo. WWW.ESAINC.COM 111 2.7 S-Nitrosothiols. Properties. S-Nitrosothiols (also called thionitrite, sulfenyl nitrites, and thionitrous acid esters) are highly colored solids and liquids. In general, tertiary S-nitrosothiols are more stable than primary ones. Several S-nitrosated low molecular weight thiols and proteins-thiols have been found in vivo (Figure 2.20). Due to their instability only a few nitrosothiols have been isolated in solid form. Of these, nitroso-albumin can be kept lyophilized or in solution for several months. Glutathione nitrosothiol can be kept desiccated in the dark at –20oC for several months and is commercially available. Cysteinyl nitrosothiol is relatively unstable in solution (seconds to hours) depending upon temperature, pH, oxygen pressure, and presence of redox-active species, nucleophiles and trace metals in the solution. In general Snitrosothiol stability is favored by acidic pH. Formation. S-Nitrosothiols were first synthesized by Tasker and Jones (1909). Although often cited in literature, S-nitrosothiols cannot be formed by the reaction between nitric oxide and a thiol unless a strong electron acceptor is present (Beckman (1996a)). There are several possible routes in vivo for S-nitrosothiol production (Crow and Beckman (1995)): • • • • • Thiols can auto-oxidize (in the presence of metal catalysts), forming thiyl radicals that are capable of reacting with nitric oxide thereby forming nitrosothiols (Eqn 2.93 and 2.94). Nitrosothiols can be produced by the action of a nitrosating species, such as nitrogen dioxide or the nitrosonium ion (from N2O3) – with thiols or thiyl radicals (Eqns 2.95 and 2.96). They can be formed by the metal-induced nitrosation of thiols (Eqns 2.97 and 2.98). Nitrosothiols can be produced by the action of peroxynitrite on thiols (Eqn 2.99). S-nitrosothiols can be formed by the direct nucleophilic nitrosation of thiols by peroxynitrite with elimination of hydrogen peroxide (van der Vleit et al. (1998)). Of these mechanisms the one catalyzed by a transition metal is probably the most important biologically, as it occurs at a higher rate and efficiency than all the other pathways (Crow and Beckman (1995)). RSH + O2 → RS• + O2•- + H+ RS• + NO• → RSNO WWW.ESAINC.COM Eqn 2.93 Eqn 2.94 112 RSH + NO2• → RS• + H+ + NO2(then RS• + N2O3 → RSNO + NO2•) RSH + N2O3 → RSNO + HNO2 NO• + Fe3+ → Fe2+….NO+ Fe2+….NO+ + RSH → Fe2+ + H+ + RSNO RSH + ONO2- → RSNO + O2•- + H+ Eqn 2.95 Eqn 2.96 Eqn 2.97 Eqn 2.98 Eqn 2.99 Chemical Reactions and Biological Significance. S-Nitrosothiols are of great interest to biochemists because they release nitric oxide under physiological conditions and thus mimic many of the biological effects reported for nitric oxide. S-nitrosothiols can be regarded as slow releasing nitric oxide-reservoirs, capable of prolonging the activity of nitric oxide (Keaney et al. (1993); Stamler et al. (1992a)). The mechanism(s) by which nitric oxide is released from an S-nitrosothiol under biological conditions is not clear and still remains controversial. The homolytic cleavage of the S-N bond is unlikely (Eqn 2.100) even though the reaction is favored by the formation of a disulfide from two thiyl radicals (Eqn 2.101). The most likely biological mechanism appears to require additional thiol (or reduced transition metal) in order to promote the reductive release of nitric oxide from Snitrosothiols (Crow and Beckman (1995)) (Eqn 2.102). An alternate mechanism requires the formation of a disulfide radical anion (Eqn 2.103) (Beckman (1996a)). RSNO → RS• + NO• 2RSNO → RSSR + 2NO• 2RSNO + 2R1SH → 2NO• + 2RSH + R1SSR1 RSNO + RSH → RS•--SR + NO• + H+ Eqn 2.100 Eqn 2.101 Eqn 2.102 Eqn 2.103 S-Nitrosoproteins, formed by post-translational nitrosation of protein cysteinyl groups, have been proposed to possess a signaling function distinct from nitric oxide’s ability to directly stimulate cGMP production (see above) (Lipton et al. (1993); Sucher and Lipton (1991)). Examples include: • • • The S-nitrosation of a thiol in the active site of cathepsin B that inhibits the action of this thiol-proteinase; S-nitrosation of the NMDA receptor infers neuroprotection; Nitrosation of tissue type plasminogen activator endows this enzyme with vasodilatory and platelet-inhibitory properties; and WWW.ESAINC.COM 113 • Inhibition of glyceraldehyde dehydrogenase by ADP-ribosylation is possibly mediated by a nitrosothiol intermediate (see Upchurch et al. (1995) and references therein). In recognition of the involvement of nitrosothiols in biochemical reactions, Stamler and Feelisch (1996) have suggested that S-nitrosation may represent a novel cell regulatory mechanism perhaps as important as phosphorylation. Measurement. S-Nitrosothiols have been measured using direct spectroscopy, infrared spectroscopy, nuclear magnetic resonance, chemiluminescence, colorimetric assays, CZE-absorbance, HPLC-UV following derivatization, HPLC-ECD, voltammetry, electrospray ionization mass spectrometry, LC-MS, and EPR of released nitric oxide (Akaike et al. (1997); Ewing and Janero (1998); Fang et al. (1998); Kluge et al. (1997); Kostka and Park (1999); Pfeiffer et al. (1998); Samouilov and Zweier (1998); Tsikas et al. (1999); Vukomanovic et al. (1998); Wink et al. (1999); also reviewed by Stamler and Feelisch (1996)). REACTIVE HALOGENATED SPECIES (RHS). 1. Chlorine and Hypochlorous Acid. Properties. Chlorine (Cl2) is a greenish-yellow poisonous, diatomic gas that is moderately soluble in water (reacting to form hydrochloric and hypochlorous acids), but much more soluble in organic solvents. Chlorine has a melting point of –102oC and a boiling point of –34.6oC. It is the second member of Group 7B of the periodic table – the halogens – and has 17 electrons with an electronic configuration of 1s2, 2s2, 2p6, 3s2, 3p5. Hypochlorous acid is unstable and cannot be isolated in pure form. It commonly occurs as a dilute aqueous solution. Formation. Chlorine is prepared in the laboratory by the action of potassium permanganate on concentrated hydrochloric acid or by heating the latter with manganese dioxide. Chlorine is also produced when bleaching powder is treated with dilute acids. Hypochlorous acid is formed when chlorine is passed into water or cold dilute sodium hydroxide solution (Eqn 2.104). Cl2 + 2OH- ⇔ OCl- + Cl- + H2O WWW.ESAINC.COM Eqn 2.104 114 Both chlorine and hypochlorous acid can be produced by biological systems (Dunford (2000)). These reactive oxidants produced by phagocytes constitute part of the host defense mechanism but also play an active role in inflammation. As discussed above, phagocytosis leads to the production of superoxide by activation of the NADPH-oxidase complex. The hydrogen peroxide produced by superoxide dismutation is then used by myeloperoxidase (MPO) for the production of a variety of bactericidal and cytotoxic species (Kettle and Winterbourn (1991)). MPO is a green, heme-containing, non-specific glycoprotein (composed of two subunits (60,000 and 12,000 Daltons)) that is secreted by activated phagocytes. It is the most abundant protein found in neutrophils (comprising 5% of their dry weight) and plays a major role in immune defense mechanisms (Klebanoff (1988); Weiss and LoBuglio (1982); Weiss and Ward (1982)). MPO uses hydrogen peroxide to oxidize a variety of halides (and pseudohalides) to their corresponding hypohalous acids (Eqn 2.105) (Nauseef et al. (1988); van Dalen et al. (1997)). It is the only human enzyme so far discovered that reacts with physiological levels of chloride to produce the highly toxic hypochlorous acid (HOCl) (Eqn 3.18) (Foote et al. (1981); Harrison and Schultz (1976)) – the active ingredient found in household bleach! MPO is also capable of oxidizing nitrite, producing the pro-oxidant cytotoxic species, nitrogen dioxide and nitryl chloride (Eiserich et al. (1997); Klebanoff (1993); van der Vleit et al. (1997)). 3Chlorotyrosine and 3,5-dichlorotyrosine are being used as markers of hypochlorous acid activity (see Chapter 3 and Figure 2.13). R- (Cl-, Br-, I-, SCN-) + H2O2 → HOR (HOCl, HOBr, HOI, HOSCN) + H2O Eqn 2.105 Other reactions of MPO include the novel superoxide-dependent hydroxylation of a variety of substrates in a process that does not require hydrogen peroxide and is unaffected by hydroxyl radical scavengers (Kettle and Winterbourn (1994)), and the one electron oxidation of tyrosine producing tyrosyl radicals. These radicals are very reactive and can polymerize to form dityrosine and other addition products (e.g., trityrosine, pulcherosine and isodityrosine) (Jacob et al. (1996)) (Chapter 3). Differences in reaction products (halogenation, nitration and oxidation) formed intaphagosomally vs. extracellularly were reviewed by Jiang and Hurst (1997). Interestingly, eosinophils contain a similar enzyme eosinophil peroxidase (EPO) that shows different substrate specificity to MPO (Mayeno et al. (1989)). EPO preferentially uses bromide to generate a brominating agent (hypobromous acid (HOBr)), even though physiological levels of bromide (20-100µM) are 1000 fold less than chloride (140mM). Remarkably, at least 25-30% of the oxygen used by WWW.ESAINC.COM 115 stimulated eosinophils is directed towards generation of halogenating species (Mayeno et al. (1989); Weiss et al. (1986)). The major in vivo markers of hypobromous acid production appear to be 3-bromotyrosine, 3,5-dibromotyrosine (Wu et al. (1999a)) and a variety of novel brominated-oxysterols. These products are probably formed by the action of reactive intermediates such as bromamine (NH2Br) or N-bromamine derivatives (RNHBr) with tyrosine (Hazen and Wu (1998)). Like MPO, EPO can oxidize nitrite to other RNS, can form tyrosyl radicals, dityrosine, trityrosine, pulcherosine and 3NT from tyrosine (McCormick et al. (1998); Wu et al. (1999b)). Chemical Reactions and Biological Significance. Chlorine is hydrolyzed by water and bases forming chloride and hypochlorite (Eqn 2.106). Consequently, under aqueous conditions chlorine gas is in equilibrium with hypochlorous acid (Eqn 2.106). The production of chlorine will therefore be favored by acidic pH and chloride ions (Hazen et al. (1996a)). Both chlorine and hypochlorous acid are extremely oxidizing. Like hydrogen peroxide, hypochlorous is a poor one-electron (Eqn 2.107) but a strong two-electron oxidizing agent (Eqn 2.108) (Koppenol (1994)). Chlorine is a powerful twoelectron oxidizing agent (Eqn 2.109). Cl2 + H2O ⇔ HOCl + Cl- + H+ HOCl + H+ + e- = H2O + Cl• HOCl + H2O + 2e- = H+ + Cl- + 2OHCl2 (aq) + 2e- → 2Cl- Eqn 2.106 Eo=-460V Eqn 2.107 o E ’=+1.08V Eqn 2.108 Eo=+1.40V Eqn 2.109 Hypochlorous acid can give rise to hydroxyl free radicals by taking part in a “Fenton-like” reaction with Fe (II) (Eqn 2.110). The hydroxyl free radical can also be produced by the reaction between hypochlorous acid and superoxide (Eqn 2.26) (Wardman and Candeias (1996)). In fact, this reaction is seven orders of magnitude faster than the production of hydroxyl free radicals from hydrogen peroxide (Wardman and Candeias (1996)).10 Interestingly, hydroxyl free radicals react with chloride ions to produce the hypochlorite radical (Eqn 2.111). These decompose to produce highly reactive chlorine atoms (Eqns 2.112) (Saran et al. (1999)). Chlorine atoms can abstract hydrogen atoms from a variety of molecules (Eqn 2.113) or react with chloride, eventually forming chlorine molecules (Eqns 2.114 and 2.115). 10 Similarly, the pseudohalogen equivalent of hypochlorous acid hypothiocyanate (HOSCN) can take part in “Fenton-like” reactions. Hypothiocyanate is formed from thiocyanate (SCN ) by the action of hydrogen peroxide. Thiocyanate, in turn, is a constituent of saliva (Wardman and Candeias (1996) and references therein). WWW.ESAINC.COM 116 Fe2+ + HOCl → Fe3+ + HO• + ClHO• + Cl- → HOCl•HOCl•- + H+ → Cl• R-H + Cl• → R• + HCl Cl• + Cl- → Cl2•2Cl2•- → Cl2 + 2Cl- Eqn 2.110 Eqn 2.111 Eqn 2.112 Eqn 2.113 Eqn 2.114 Eqn 2.115 The pattern of reaction products formed by the hypochlorous acid/chlorine system is dependent, in part, on the pH of the reaction medium. For example, under neutral pH (favoring hypochlorous acid) aliphatic amines are chlorinated to chloramines (Thomas et al. (1982); Weil and Morris (1949); Weiss et al. (1982)). Several chloramines can be formed in vivo. Monochloramine, formed by the action of hypochlorous acid on ammonia (Eqn 2.116), is a lipophilic, short-lived oxidant that can oxidize thiols, ascorbate, and other compounds. Taurine-Nmonochloramine, formed by the action of hypochlorous acid on taurine (Eqn 2.117), is a hydrophilic, long-lived oxidant that shows limited reactivity. Although this compound is capable of producing monochloramine (Eqn 2.118) (Grisham et al. (1984); Weiss et al. (1983)) its intracellular production appears to protect neutrophils. Chloramine derivatives can decompose to produce reactive carbonyl compounds (e.g., the chloramine derivative of free serine decomposes to produce glycoaldehyde, free threonine derivative produces both 2hydroxypropanal and acrolein, while the free tyrosine derivative produces phydroxy-phenylacetaldehyde) (Anderson et al. (1997); Hazen et al. (1996b, 1997)). HOCl + NH3 → NH2Cl HOCl + -SO3(CH2)2NH2 → H2O + -SO3(CH2)2NHCl SO3(CH2)2NHCl + NH3 → NH2Cl + -SO3(CH2)2NH2 Eqn 2.116 Eqn 2.117 Eqn 2.118 Of all the amino acid residues found in a protein only lysine has a free amine group that can take part in reaction with hypochlorous acid. This protein-bound, lysine-derived chloramine then undergoes an intermolecular reaction resulting in the conversion of a nearby tyrosine residue into 3-chlorotyrosine (Domigan et al. (1995)). Under acidic conditions (favoring chlorine production), direct chlorination of the tyrosine’s aromatic ring takes place (Hazen et al. (1996b)). The formation, reactions and detection of 3-chlorotyrosine are discussed further in Chapter 3. Hypochlorous acid can react with hydrogen peroxide to produce singlet oxygen (Eqn 2.18). However, as the production of singlet oxygen is not favored by acidic or neutral pH, the biological importance of this reaction is yet to be established. The myeloperoxidase-hydrogen peroxide-chloride system also oxidizes thiol groups; converts methionine residues to methionine sulfoxide; converts cysteine to cysteine sulfinic acid; bleaches (decolorizes) heme proteins; reacts with ironsulfur centers; produces chlorohydrins from unsaturated fatty acids and WWW.ESAINC.COM 117 cholesterol; and destroys carotenoids (Albrich et al. (1981); Carr et al. (1997); Heinecke et al. (1994); Panasenko et al. (1997a); Winterbourn et al. (1992)). Circulating hypochlorous acid can inhibit α1-antiprotease by oxidizing an essential methionine residue (Clark et al. (1981)). The extent of inactivation of this enzyme is dependent upon the site of hypochlorous acid production and the levels of extracellular antioxidants. For example, the antioxidants albumin and ascorbic acid can readily react with hypochlorous acid, thereby minimizing its toxicity. Thus α1-antiprotease present in tissues rich in these antioxidants (e.g., blood) will be protected from hypochlorous acid inactivation. Conversely, in tissues with low levels of albumin and ascorbate (e.g., inflamed rheumatoid joints) α1-antiprotease will be inactivated. Although taurine (2-aminoethanesulfonic acid) has been proposed as an antioxidant, it reacts with hypochlorous acid to form taurine-N-chloramines species that are less oxidizing than hypochlorous acid, but yet are still capable of inactivating α1-antiprotease (see above). Hypochlorous acid can also directly affect antioxidant enzymes. Low levels of hypochlorous acid readily inactivate glutathione peroxidase while moderate levels are required for inhibition of catalase. On the other hand, superoxide dismutase reacts only slowly with hypochlorous acid, suggesting that this enzyme may play an important role in controlling hypochlorous acid toxicity (Aruoma and Halliwell (1987)). The release of hypochlorous acid has been implicated in the pathogenesis of diseases ranging from atherosclerosis to ischemia-reperfusion injury and cancer (Hazell et al. (1996); Hazen et al. (1996b) and references therein). Measurement. Atmospheric chlorine can be measured using ion chromatography with conductivity (NIOSH (1994)). Aqueous chlorine can be measured using voltammetric and colorimetric approaches (Eaton et al. (1995)). Chlorinated adducts can be measured using a variety of techniques including GC- and HPLC-based approaches (Chapter 3). 2. Nitrosyl Chloride, Nitryl Chloride, and Related Compounds. Properties. Nitrosyl chloride (NOCl) and nitryl chloride (ClNO2) can be considered formally as the halide salts of the nitrosonium and nitronium ions, respectively. Nitrosyl chloride is an orange-yellow colored gas with a melting point of –62oC and boiling point of –6oC. Nitryl chloride is a colorless gas with a melting point of –145oC and boiling point of –15oC. WWW.ESAINC.COM 118 Formation. Nitrosyl chloride can be made in the laboratory by mixing chlorine and nitric oxide gases. Nitrosyl chloride (NOCl) is also the active ingredient of aqua regia. This can be made in the laboratory by mixing concentrated hydrochloric and nitric acids (3:1 v/v). Recently, evidence suggests that nitrosyl chloride and nitryl chloride (nitronium chloride) (ClNO2) can be formed in biological systems and may act as possible cytotoxic agents. Koppenol (1994) hypothesized that neutrophils and macrophages might form the highly reactive compound, nitrosyl chloride. Based upon the energetics of possible reactions, he concluded that the reaction between hypochlorous acid and peroxynitrite is most likely to produce nitrosyl chloride (Eqn 2.119). HOCl + ONO2H → NOCl + O2 + H2O (∆Go’= -79.5kJ mol-1) Eqn 2.119 Based on the work of Kono (1995), Eiserich et al. (1996) showed that the reaction between nitrite and hypochlorous acid produces a reactive species capable of producing 3-nitrotyrosine, 3-chlorotyrosine, and dityrosine from tyrosine. Initially the reactive specie(s) responsible for these reactions was thought to be nitryl chloride or the cis- or trans- isomers of chlorine nitrite (ClONO) (Figure 2.15). Eiserich et al. (1996) hypothesized that such reactive species can be formed as shown in Eqn 2.120. This is in contrast to the generally accepted reaction (Eqn 2.121).11 Recent evidence suggests that the pro-oxidant species formed by the action of MPO on nitrite and hypochlorous acid are nitryl chloride and nitrogen dioxide (Eiserich et al. (1998)). 2HOCl + 2NO2- → ClONO + ClNO2 + 2OHHOCl + NO2- → HCl + NO3- Eqn 2.120 Eqn 2.121 Chemical Reactions and Biological Significance. Both nitrosyl chloride and nitryl chloride react with water to produce a variety of products including nitrous acid, nitric acid, nitric oxide, and chloride. Nitrosyl 11 This suggests that the approaches that use the measurement of nitrite as an estimate of nitric oxide production in tissues or fluids from patients with acute or chronic inflammation may be in error due to the removal of nitrite by its reaction with hypochlorous acid. WWW.ESAINC.COM 119 chloride is a very reactive substance and its presence in aqua regia enables this reagent to even dissolve gold and platinum. Nitrosyl chloride is an electrophilic nitrosating agent and can be regarded as a carrier of the nitrosonium ion. Thus many of its biological reactions are due to production of the nitrosonium ion (see above). It does not appear, however to cause aromatic nitration (Whiteman et al., (2003)). Nitryl chloride promotes lipid peroxidation and has been suggested to play a role in low-density lipoprotein modification (Panasenko et al. (1997b)) and DNA damage (Spencer et al., (2000)). Measurement. Unfortunately, the direct measurement of the low levels of nitrosyl chloride and nitryl chloride is difficult, especially in vivo (Feelisch and Stamler (1996)). 3d 3p 3s 2p 2s Sulfur - valency 4 Sulfur - valency 2 1s2, 2s 2, 2p 6, 3s 2, 3p 4 1s2, 2s 2, 2p 6, 3s 2, 3p 3, 3d 1 Sulfur - valency 6 1s2, 2s 2, 2p 6, 3s 2, 3p 3, 3d 2 Figure 2.21 The Electronic Configuration Of Sulfur’s Different Valencies. WWW.ESAINC.COM 120 SULFUR, THIOLS, AND THIYL RADICALS (SOME REACTIVE SULFUR SPECIES [RSS]). Properties. Sulfur is a yellow solid. The two major allotropes of sulfur consist of S8 molecules in which single bonds unite the sulfur atoms into puckered octagonal rings. Rhombic sulfur is the form of sulfur most commonly encountered while monoclinic sulfur exists at temperatures >95.6oC. This type of allotropy, in which a definite transition point exists where two forms become equally stable, is called enantiotropy. Sulfur has a melting point of +118.95oC (monoclinic) and boiling point of 444.6oC. Sulfur is the second member of Group 6B of the periodic table. Unlike oxygen, the first member of Group 6B that only has a valency of 2, sulfur due to its vacant 3d orbitals can show valencies of 2, 4 and 6 (Figure 2.21). Each of these show various oxidation states. Examples showing the different valencies of sulfur are presented in Figure 2.22. Chemical Reactions and Biological Significance. Sulfur and its compounds show a wide variety of reactions and these can be found in any good chemical text. Some of these reactions important to the field of redox biochemistry have been recently reviewed elsewhere (Stamler and Slivka (1996)). Possibly the best known of all sulfur-based reactions in this field involves thiols (sulfhydryls). Thiols share many of the chemical characteristics of the corresponding alcohols but also show many unique reactions. This is due to the physical properties of the S-H bond, which determines thiol reactivity and chemistry. The S-H bond is longer and weaker than the O-H bond which affects its pKa (thiols tend to react as RS- and show nucleophilic activity) and renders it more easily oxidized. Thiols are therefore very good reducing agents. The oxidation behavior of a thiol is more complex than the corresponding alcohol. Alcohol oxidation to aldehydes and acids involves a change in the oxidation state of the carbon to which the alcohol group is attached (the valency of carbon and oxygen cannot change) (Eqn 2.122). Oxidation of sulfur is much more complicated. Mild oxidation of thiols results in the formation of a disulfide (the valency of sulfur does not change) (Eqn 2.123) and not the corresponding aldehyde or acid. Under more vigorous conditions the sulfur atom itself can be oxidized into its higher valencies (e.g., a thiol is first oxidized to a sulfinate and finally a sulfonate) (Figure 2.22). For example, hypochlorous acid oxidizes methionine (sulfur valency 2) into methionine sulfoxide (sulfur valency 4) (Eqn 2.124). Ozone can also oxidize sulfur into its highest valency (6) (see above). WWW.ESAINC.COM 121 R-CH2OH → R-CHO + 2H+ + 2e2R-SH → R-SS-R + 2H+ + 2eR-S-CH3 + HOCl → R-SO-CH3 + HCl Eqn 2.122 Eqn 2.123 Eqn 2.124 A) Valency 2 HO γ-Glu-Cys-Gly S CH3 METHIONINE γ-Glu-Cys-Gly S-NITROSOHOMOCYSTEINE HO S S SH O S NH2 γ-Glu-Cys-Gly CYSTEINE GLUTATHIONE DISULFIDE DIALLYLSULFIDE SH HS HO S NH2 NH2 GLUTATHIONE S-NO O O SH HO SH S O HOMOCYSTEINE DIALLYLDISULFIDE CO2H H NH2 LIPOIC ACID (Reduced) B) Valency 4 C) Valency 6 O HO S CH3 O NH2 METHIONINESULFOXIDE γ-Glu-Cys-Gly γ− Glu-Cys-Gly SO2H SO3H GLUTATHIONESULFINIC ACID GLUTATHIONESULFONIC ACID SO3H H2 N TAURINE Figure 2.22 Compounds Illustrating The Different Valencies Of Sulfur. The thiol-disulfide redox couple is very important to oxidative metabolism (Chapter 4). For example, GSH is a reducing cofactor for glutathione peroxidase, an antioxidant enzyme responsible for the destruction of hydrogen peroxide (Eqn 2.125). The importance of the antioxidant role of the thiol-disulfide redox couple is discussed further in Chapter 4. 2GSH + H2O2 → GSSG + H2O WWW.ESAINC.COM Eqn 2.125 122 Thiols and disulfides can readily undergo exchange reactions, forming mixed disulfides (Eqn 2.126). Thiol-disulfide exchange is biologically very important. For example, GSH can react with protein cystine groups and influence the correct folding of proteins (Hwang et al. (1992); Zingler (1985)). GSH may also play a direct role in cellular signaling through thiol-disulfide exchange reactions with membrane bound receptor proteins (e.g., the insulin receptor complex), transcription factors (e.g., nuclear factor κB), and regulatory proteins in cells (Powis et al. (1995)). Conditions that alter the redox status of the cell can have important consequences on cellular function. R1-SH + R2-SS-R3 → R1-SS-R2 + R3-SH R R R -2H+, -2e- R'SH OH R'S O OH OH OH O Catechol Catechol-quinone 5-Thiol Substituted Catechol NH2 NH2 NH2 H2N Eqn 2.126 CO2H H2N CO2H HO S S S HO OH NH2 S HO CO2H OH NH2 HO CO2H OH 2-S-CysteinylDopamine 5-S-CysteinylDopamine 2,5-bi-S-CysteinylNorepinephrine NH2 NH2 H3C H GS H S-G HO S-Protein HO OH OH 5-S-GlutathionylDopamine Protein-Dopamine 5-S-Adduct OH H HO OH 2-Glutathionyl4-Hydroxy-Estradiol Figure 2.23 Formation And Examples Of Biologically Relevant Thiol Adducts WWW.ESAINC.COM 123 Thiols are good nucleophiles and readily react with carbonyl compounds to form hemithioacetals and thioacetals. Consequently, thiols form conjugates with a variety of biochemicals including purines, estrogens, and monoamine neurotransmitters (Figure 2.23). Some of these thiol adducts are potentially toxic and their biological implications will be discussed further in Chapter 4. Unfortunately, thiols can also undergo one-electron oxidation to produce chemically reactive thiyl radicals (Eqn 2.127 and 2.128) (D’Aquino et al. (1994); Hartmann et al. (1999); (Saez et al. (1982)). This can be formed by: • • • • • • • • • Enzymes (e.g., glutathione reductase, lipoamide dehydrogenase; peroxidases, (horseradish and lactoperoxidase) thioredoxin reductase, xanthine oxidase); Metal-containing proteins (e.g., myoglobin); Components of the electron transport pathway; Redox-active metals (e.g., iron, copper); Hydrogen peroxide; Other free radicals; Thermolysis, radiolysis and photolysis of disulfides; Some sulfur-containing drugs (e.g., penicillamine). This may account for the autoimmune side effects of some of these compounds (Halliwell and Gutteridge (1999)). The hemolytic action of diphenyl disulfide is possibly mediated, at least in part, by thiyl radical production; and The zinc fingers of some DNA-binding regulatory proteins (Sarkar (1995)). HO• (RO• or RO2•) + R’SH → H2O (ROH, or RO2H) + R’S• RSH + Cu2+ → Cu+ + H+ + RS• Eqn 2.127 Eqn 2.128 Although thiyl radicals are less reactive than hydroxyl free radicals, they still show considerable reactivity and readily take part in electron transfer, hydrogen transfer, and addition reactions that can be problematic for the cell (Figure 2.24). The reaction between the glutathiyl radical and the glutathiolate anion (GS-) or GSH produces the strongly reducing glutathione disulfide radical (GSSG•-) (Eqns 2.129 and 2.130) that can reduce oxygen to superoxide (Eqn 2.132). Thiyl radicals can also lead to singlet oxygen production (Wefers and Sies (1983)). Conversely, thiyl radicals may also be beneficial to the cell. Both free- and protein contained-thiyl radicals are essential in detoxification of more potent prooxidants (Kalyanaraman (1995)). 2GSH + H2O2 → 2GS• + 2H2O (e.g., Horseradish peroxidase or lactoperoxidase) GS• + GSH → (GSSG)•- + H+ WWW.ESAINC.COM Eqn 2.129 Eqn 2.130 124 (GSSG)•- + O2 → GSSG + O2•- Eqn 2.131 Thiyl radicals can be detoxified by superoxide dismutase in mammalian cells and by a thiol-specific enzyme in bacterial cells (Kalyanaraman (1995) and references therein). GSH + R hydrogen abstraction GSSG + O2 - RSH + NAD + H+) (e.g., NADH + RS electron transfer GSOO GSNO RH addition Photolysis O2 O2 GSH 1/2 GSSG One-electron Oxidation* Thermolysis Photolysis Radiolysis GS- GS GSSG addition R-CH=CH-R R R GS GS- + R + electron abstraction R-CH-CH-R addition (e.g., leukotrienes, carotenoids, retinoids) GSSG + R electron transfer *One-electron oxidation can be promoted by: the hydroxyl free radical, superoxide, copper II (and other metal ions), hydrogen peroxide/iron II, alkyl radical, alkyl peroxyl radical, ozone and peroxynitrite. Figure 2.24 Production And Reaction Of Thiyl Radicals. GSH Is Used As An Example. (D’Aquino et al. (1994); Forni and Wilson (1986a,b); Hartmann et al. (1999); Karoui et al. (1996); Monig et al. (1987); Mortenson et al. (1997); Pryor (1984); Quijano et al. (1997); Scorza and Minetti (1998); Singh et al. (1996); Vasquez-Vivar et al. (1996)). Measurement. Thiols and disulfides have been determined using a variety of approaches including colorimetric, spectrophotometric, enzymatic, and HPLC-based techniques (see glutathione, Chapter 5). Thiyl radicals can be studied using optical spectroscopy and EPR in conjunction with spin traps such as PBN and DMPO (Davies et al. (1987); Kalyanaraman (1995); Kalyanaraman et al. (1996)). WWW.ESAINC.COM 125 CARBONYL COMPOUNDS. The carbonyl group (C=O) is one of the most important structures in organic chemistry. It is found in a wide variety of compounds including aldehydes (RCHO), ketones (R2CO), amides (RCONH2), carboxylic acids (RCO2H), esters (RCO2R), acid anhydrides ((RCO)2O) and acid chlorides (RCOCl). To cover the chemistry and biochemistry of all carbonyl compounds found in biological systems is way beyond the scope of this handbook. We will instead concentrate on some biologically significant reactive aldehydes. Properties. The simplest aliphatic aldehyde is formaldehyde (HCHO), a pungent-smelling gas that is extremely soluble in water. Short chain (carbon length 2 to 9) aliphatic aldehydes are clear liquids with distinct odors. Their solubility in water decreases with increasing chain length. Formation. Aldehydes can be formed in the laboratory by a variety of approaches, including careful oxidation of a primary alcohol, Rosenmund’s synthesis (chemical reduction of an acid chloride), ozonolysis of alkenes, or by heating formic acid and a carboxylic acid with manganous oxide at 300oC. A number of reactive aldehydes are found in vivo (Figure 2.25) and are: • • • • Derived from the diet (e.g., glucose can be considered as a reactive aldehyde and is capable of undergoing glycation and glyoxidation) Formed as part of normal metabolism (e.g., the production of catecholaldehyde by the action of monoamine oxidase on catecholamines). Produced by the action of reactive species on amino acids (e.g., the formation of acrolein, glycolaldehyde and 2-hydroxypropanal when hypochlorous acid reacts with amino acids). Result from lipid peroxidation (e.g., 4-hydroxynonenal and malondialdehyde) and this will discussed further in Chapter 3. Cytotoxic aldehydes are also found in automobile exhaust, cigarette smoke, cooking emissions, and drinking water. They are also produced during the metabolism of some anticancer drugs (e.g., oxazaphosphorines) (Ghilarducci and Tjeerdema (1995); Jakab (1977); Sladek (1987)). WWW.ESAINC.COM 126 NH 2 HO HOCl O O HO OH Glycolaldeyhde Serine NH 2 OH OH HOCl O CH + H2O OH Threonine H2 N O O 2-Hydroxypropanal Acrolein CO 2H CHO CH 2 CH 2 HOCl OH OH 4-Hydroxyphenyl acetaldehyde Tyrosine R CHO OH 4-Hydroxynonenal CO 2H Poly-unsaturated Fatty Acid H O=C H C=O Malondialdehyde H2 O2 O2 , H 2 O O NH NH O 4,9-Diazadodecanedial O Spermine/ Spermidine NH 2 Serum Amine Oxidase Acrolein O H2N 3-Aminopropanal HN O N-(4-Aminobutyl)3-aminopropanal CH 2CH 2NH 2 O2 MAO HO OH Catecholamine CH 2CH=NH H2 O2 HO OH H2O Catecholimine CH 2CHO NH 3 HO OH Catecholaldehyde Figure 2.25 Some Reactive Carbonyls Formed In Vivo. WWW.ESAINC.COM 127 Chemical Reactions and Biological Significance. The chemical reactions of aldehydes will not be dealt with in depth here. Aldehydes are fairly reactive and can undergo reduction (to alcohols), oxidation (to acids), or nucleophilic addition with alcohols (forming hemi-acetals and acetals), thiols (forming hemi-thioacetals and thioacetals), hydrogen cyanide (forming cyanohydrin) and hydrogen sulfite (forming hydrogen sulfonic acid salts). Unsaturated aldehydes where the carbon-carbon double bond is located in the 2-position (e.g., 2-alkenals – acrolein and 4-hydroxynonenal) are much more reactive than the corresponding saturated aldehydes. Acrolein will therefore show the chemical reactions typical for compounds containing a carbonyl and a double bond. 4-Hydroxynonenal has three functional sites, and will undergo reactions typical of the carbonyl, double bond and alcohol groups. 2-Alkenals undergo Michael addition with nucleophiles, such as thiols and amines, and lead to the formation of cyclic hemi-acetals and protein adducts, respectively (Figure 2.26) (Sayre et al. (1993)). SR SR R O R O OH Michael Adduct RSH Cyclic Hemiacetal Protein Protein-NH2 R O OH NH O R OH OH 4-Hydroxy-2-nonenal Michael Adduct Protein-NH2 R R NH N O OH Schiff Base Protein Protein OH -H2O R R N Protein N Protein Pyrrole Figure 2.26 The Reaction Of The Cytotoxic Lipid Peroxidation Product 4-Hydroxy-2-Nonenal With Thiols (RSH) Or Proteins (Protein-NH2). WWW.ESAINC.COM 128 Aldehydes can react with nucleophiles that contain an acidic proton. A subsequent elimination is then possible, leading to complete substitution. A typical example of this is the Schiff base (imine) formation between an aldehyde and a primary amine (e.g., amino acid, purine or pyrimidine) (Eqn 2.132). The formation of Schiff bases is important to the activity of many enzymes. Here the ε-amine group of a lysine residue located on the enzyme reacts with carbonyl substrate (e.g., aldose, keto acid or pyridoxal phosphate). Examples of enzymes that make use of Schiff bases include aldolase, aminotransferases, lysyl oxidase, phosphorylase, rhodopsin, and transaldolases – the production of unwanted Schiff bases however is a major problem for the cell. It is the first step in several non-enzymatic reactions. For example, the reaction between aldehydes and DNA bases can lead to the formation of base adducts and, in the case of 4hydroxynonenal and malondialdehyde, to DNA-DNA and DNA-protein crosslinks. Formation of these metabolites can interfere with normal DNA replication (Chapter 3). RICHO + RIINH2 ⇔ RICH=NRII + H2O Eqn 2.132 Proteins can react with reducing (aldehyde-containing) sugars in a nonenzymatic, non-oxidative process termed glycation. The irreversible autooxidation of glycated products, glycoxidation, leads to the formation of advanced glycation end products (AGEs) such as carboxymethyl lysine and pentosidine (Figure 2.27). Proteins can also react with reactive carbonyls (e.g., 2-alkenals form fluorescent pyrrole derivatives) called “advanced lipid peroxidation end products” (ALPs) or “advanced lipoxidation end products” (ALEs) (Figure 3.23) (Sayre et al. (1993, 1996)). AGEs and ALPs are associated with aging and the oxidative stress pathophysiology of neurodegenerative diseases, diabetes, and uremia (Calingasan et al. (1999); Miyata et al. (1999); Xu and Sayre (1998)). Patients presenting with carbonyl overload are said to be under “carbonyl stress” (Miyata et al. (1999)). The biological importance of the reactions between aldehydes, DNA and proteins is discussed in Chapter 3. Measurement. The presence of aldehydes can be determined by their ability to restore the magenta color to Schiff’s reagent (sulfur dioxide treated rosanaline hydrochloride). Alternatively, as they are reducing agents, their presence is indicated by the production of a silver mirror when treated with Tollen’s reagent (ammoniacal silver nitrate) or a precipitate of red Cu (I) oxide when treated with Fehling’s solution (an alkaline complex of copper tartrate). WWW.ESAINC.COM 129 Aldehydes present in biological samples can be quantified using the thiobarbituric acid test (TBAR), GC, GC-MS, and following derivatization (e.g., 2,4-dinitrophenylhydrazine), by HPLC with UV, fluorescence or electrochemical detection (Chapter 3). A) GLYCATION R R R (CH 2 )4 (CH 2 )4 (CH 2 )4 NH NH NH CH CH Lysine -H 2 O H H R (CH 2 )4 NH 2 O H C C OH (CHOH) 3 CH 2 OH C OH C (CHOH) 3 Amadori Rearrangement OH (CHOH) 3 CH 2 C O (CHOH) 3 CH 2 OH CH 2 OH CH 2 OH Schiff Base (Imine) Eneaminol Fructose-Lysine (Amadori Product) Glucose HOCH 2 CHO N (CH 2 )4 CH H 2N CO 2 H Pyrraline R B) GLYCOXIDATION C) OTHER AGEs (CH 2 )4 NH R CH 2 (CH 2 )4 O Arginine Imidazolone Methylglyoxal Lysine Imidazole Carboxymethyl Lysine (CML) Metal n+ CH 2 C CO 2 H [O 2 ] NH Glyoxal Arginine (CHOH) 3 CH 2 OH Lysine Fructose-Lysine (Amadori Product) NH Arginine CH NH 2 CH NH 2 N NH CO 2 H CO 2 H N N N Pentosidine (CH 2 )4 (CH 2 )4 H 3C + N N (CH 2 )4 (CH 2 )4 CH H 2N CO 2 H Methylglyxoyllysine Dimer CH H 2N CO 2 H Glyoxallysine Dimer Figure 2.27 A) Glycation Reactions, B) Glycoxidation Reactions and C) Some Additional AGEs. WWW.ESAINC.COM 130 THE PRO-OXIDANT ACTIVITY OF LOW MOLECULAR WEIGHT COMPOUNDS AND OTHER XENOBIOTICS. A number of xenobiotic compounds owe their biological activity to their ability to generate pro-oxidants. However, this ability is also responsible for their undesirable side effects and unwanted toxicity. Table 2.12 presents a brief, nonexhaustive list of several classes of pro-oxidant xenobiotic compounds. Readers wanting a more comprehensive review of the toxicology of these compounds are referred to Halliwell and Gutteridge (1999), Kehrer (1993) and Sies and de Groot (1992). Compound Class or Drug Class Acrylates Antibiotics Example References Bone cement Cephalosporin, chloramphenicol, gentamicin, rifamycin, tetracyclines Vale et al. (1997) Halliwell and Gutteridge (1999); Muller et al. (1998) Antihypertensives Hydralazine Sodium nitroprusside Artimisinin Methotrexate Anthracyclins and other quinone containing drugs (daunomycin, doxorubicin, mitomycins, steptonigrin) Cisplatin Metal chelators (bleomycins, tallysomycin) Protein antitumor antibiotics (macromycin, neocarzinostatin) Other - tirapazamine Benzene and aniline and their derivatives Rauhala et al. (1998) Antimalarial agents Antirheumatics Antitumor agents • • • • • Aromatic hydrocarbons Aromatic polyphenols (also quinones and hydroxylatedmethoxylated compounds) Aromatic quinones and derivatives Bipyridylium compounds Diabetogenics L-DOPA, dopamine, etoposides, 6-hydroxydopamine, Anthraquinones, benzoquinones, indolequinones and naphthaquinones and their derivatives Diquat, paraquat Alloxan, streptozotocin WWW.ESAINC.COM Meshnick (1998) Gressier et al. (1994) Baliga et al. (1998); Evans et al. (1998); Gewirtz (1999); Keizer et al. (1990); Muller et al. (1998); Sinha and Politi (1990) Brennan and Schiestl (1997); Shen et al. (1996) Halliwell and Gutteridge (1999); Woodgate et al. (1999) Everett et al. (1998); Gatto et al. (1996); Fukushima et al. (1993); Halliwell and Gutteridge (1999); Yamada and Fukushima (1993) Halliwell and 131 • • • Gutteridge (1999) El-Bachs, et al. (1999); Yu et al. (1999) Halliwell and Gutteridge (1999); Henderson et al. (1999) Crebelli et al. (1999); Halliwell and Gutteridge (1999) Halliwell and Gutteridge (1999); Sugiyama (1992) Halliwell and Gutteridge (1999); Munday (1989) • • Parman et al. (1999) Daniel et al. (1995); Lund and Aust (1991); Vallyathan (1994) Hiramoto et al. (1995) Avent et al. (1996); Behl et al. (1996); McNaught et al. (1998); Naoi et al. (1998) Halliwell and Gutteridge (1999) Dopaminergics Apomorphine, cocaine Ethanol Halogenated hydrocarbons Bromobenzene, carbon tetrachloride, chloroform, dibromoethane, halothane Heavy metals Chromium, lead, manganese, mercury, titanium, vanadium Hemolytic agents Immunomodulator Mineral dusts Aminothiols, thiophenols Favism agents (convicine, vicine) Hydrazines (acetylphenylhydrazine, phenylhydrazine, iproniazid, isoniazid) • Quinones (juglone, lawsone, plumbagin and menadione) Thalidomide Silicates e.g., asbestos Mushroom toxins Neurotoxins Nitro-aromatics Organic hydroperoxides and peroxides Photosensitizing agents Quinoneimines Benzenediazonium salts Amphetamine and derivatives, 6-hydroxydopamine, isoquinolines, haloperidol metabolites, MPTP • • • • Fungal agents (sporidesmin) Radiosensitizing agents/hypoxic cell sensitizers (chloramphenicol, furazolidone, metronidazole, misonidazole, nitrofurantoin, nitrofurazone) Benzoyl peroxide, cumene hydroperoxide, tertbutyl hydroperoxide Dyes (e.g., indocyanine green) Furocoumarins (psoralens) Porphyrins Quinines and antimalarials (e.g., chloroquine, primaquine and quinacrine) E.g., acetaminophen metabolite Halliwell and Gutteridge (1999); Sestili et al. (1998) Baumler et al. (1999); Bonnett and Berenbaum (1989); Moreno (1986); Potapenko (1991); Spikes (1998) Halliwell and Gutteridge (1999) Table 2.12 The Pro-Oxidant Activity Of Some Low Molecular Weight Compounds And Other Xenobiotics. The separation of compounds based upon their compound class or drug class is not perfect as some compounds may fall into more than one category. For example, some compounds are antiobiotics possess a quinones structure and are antitumor agents. WWW.ESAINC.COM 132 The mechanism by which a pro-oxidant xenobiotic produces oxidative stress is dependent, in part, upon its chemical structure. A number of xenobiotics can undergo redox cycling producing ROS. For example, Figure 3.24 shows redox cycling of bipyridyl herbicide paraquat and the diabetogenic agent alloxan. Other compounds that can redox cycle include antibiotics (e.g., actinomycin D, mitomycin C and streptonigrin); antitumor drugs (e.g., anthracyclines, etoposides, tirapazamine, diaziridinylbenzoquinones, and EO9); and the hydroxylated metabolites of the antimalarial drug primaquine (Butler (1998); Halliwell and Gutteridge (1999); Newsholme and Leech (1992); Vasquez-viva and Augusto (1992)). The generation of ROS by redox cycling is only one possible explanation for the action of many drugs. Rifamycin not only owes its activity to ROS generation but also to its ability to block bacterial RNA synthesis as well. Quinones (and/or semiquinones) can also form adducts with nucleophiles, especially thiols (Figure 2.6; Figure 2.23; Chapter 4). These adducts may act as toxins directly or indirectly through the inhibition of key enzymes (e.g., by reacting with essential cysteinyl residues) or the depletion of GSH. To go through all the mechanisms of action for the list of compounds presented in Table 2.11 is beyond the scope of this book. Rather we have chosen to give four brief examples: • The bipyridyl herbicides (e.g., paraquat and diquat) are toxic to both plants and animals (reviewed by Halliwell and Gutteridge (1999)). In animals paraquat is converted to its radical by microsomal NADPH cyctochrome P450 reductase (or cytochrome c oxidase). The paraquat radical can then reduce oxygen to superoxide (Figure 2.8). The ROS produced by this redox cycling readily explains why paraquat causes such damage to tissues especially the lungs (pulmonary edema and alveolar inflammation). • MPTP (1-methyl-4-phenyl-1,2,3,4-tetrahydropyridine), a neurotoxin first discovered in 1979 as a contaminant in a “designer drug” preparation of a meperidine analog, causes a Parkinson’s syndrome, a consequence of its damage to the dopaminergic nigrostriatal pathway. For its toxic action, MPTP must first be converted to its neurotoxic pyridinium analyte MPP+ through oxidation by glial monoamine oxidase (MAO B). MPP+, is then actively accumulated by dopaminergic neurons by the dopamine transporter. Although MPP+ shows some structural similarity to paraquat it does not appear to exert its pro-oxidant action through redox cycling. Instead MPP+ exerts its toxicity by inhibiting the mitochondrial electron transport chain at complex I. This not only prevents the production of the essential metabolite, ATP but also produces ROS and leads to oxidative stress. Other neurotoxic drugs include a pyridinium metabolite of the neuroleptic agent, haloperidol, that may account for some of haloperidol’s unwanted neurotoxic side effects, and the isoquinolinium metabolites of the WWW.ESAINC.COM 133 isoquinolines, that some suggest might be endogenous Parkinson-causing agents. Interestingly, these pyridinium and isoquinolinium compounds are structurally similar to MPP+. • Halogenated hydrocarbons exert their pro-oxidant activity through the formation of carbon-centered radicals and/or corresponding peroxy radicals (through their reaction with oxygen) (Halliwell and Gutteridge (1999)). We shall concentrate on tetrachloromethane (CCl4; TCM) (a constituent of dry cleaning fluid) as a representative example. TCM is an active pro-oxidant, affecting many organs but particularly the liver. The first step in TCM activation is the production of the trichloromethyl radical (CCl3•) by microsomal P450 (other enzymes can also promote this reaction). The trichloromethyl radical can then follow three pathways. First, it can directly attack macromolecules forming covalent bonds. Second, it can abstract hydrogen atoms directly from lipids and initiate lipid peroxidation. Third, it can react with oxygen to form the reactive trichloromethylperoxy radical (CCl3O2•) that effectively initiates lipid peroxidation (Chapter 3) and can covalently bind to important macromolecules. • Finally, some pro-oxidants act through the production of singlet oxygen (above). Such compounds include a diverse group of photosensitizing agents, some of which are used as sensitizing agents in photodynamic therapy. REFERENCES. Abe, K., Pan, L.H., Watanabe, M., Kato, T., and Itoyama, Y. (1995). Induction of nitrotyrosine-like immunoreactivity in the lower motor neuron of amyotrophic lateral sclerosis. Neurosci. Lett., 199, 152-154. Acworth, I.N., McCabe, D.R., and Maher, T.J. (1997). The analysis of free radicals, their reaction products, and antioxidants. In: Oxidants, Antioxidants, and Free Radicals. Baskin, S.I., and Salem, H. (Eds.). Taylor and Francis, Washington, DC. Pp. 23-77. Acworth, I.N., Bailey, B.B., and Maher, T.J. (1998a). The use of HPLC with electrochemical detection to monitor reactive oxygen and nitrogen species, markers of oxidative damage and antioxidants: application to the neurosciences. In: Neurochemical Markers of Degenerative Nervous Diseases and Drug Addiction. Qureshi, G., Parvez, H., Caudy, P., and Parvez, S. (Eds.). Progress in HPLC-HPCE, 7. VSP Publications, The Netherlands. Pp. 3-56. Acworth, I.N., Bogdanov, M.B., McCabe, D.R., and Beal, M.F. (1998b). Improved methods for estimation of hydroxyl free radical levels in vivo based on liquid chromatography with electrochemical detection. Meth. Enzymol., 300, 297-313. Akaike, T., Inoue, K., Okamoto, T., Nishino, H., Otagiri, M., Fujii, S., and Maeda, H. (1997). Nanomolar quantification and identification of various nitrosothiols by high performance liquid chromatography coupled with flow reactors of metals and Griess reagent. J. Biochem., 122, 459-466. Albrich, J.M., McCarthy, C.A., and Hurst, J.K. (1981). Biological activity of hypochlorous acid: Implications for microbicidal mechanisms of leukocyte myeloperoxidase. Proc. Natl. Acad. Sci. USA, 78, 210-214. Aleryani, S., Milo, E., Rose, Y., and Kostka, P. (1998). Superoxide-mediated decomposition of S-nitrosothiols. J. Biol. Chem., 273, 6041-6045. Allgayer, H., Hofer, P., Schmidt, M., Bohne, P., Kruis, W., and Gugler, R. (1992). Hydroxyl and fatty acid radical scavenging by aminosalicylates. Biochem. Pharmacol., 43, 259-262. Alvarez, B., Rubbo, H., Kirk, M., Barnes, S., Freeman, B., and Radi, R. (1996). Peroxynitrite-dependent tryptophan nitration. Chem. Res. Toxicol., 9, 390-396. Anderson, M.M., Hazen, S.L., Hsu, F.F., and Heinecke, J.W. (1997). Human neutrophils employ the myeloperoxidasehydrogen peroxide-chloride system to convert hydroxy-amino acids into glycoaldehyde, 2-hydroxypropanal, and acrolein. J. Clin. Invest., 99, 424-432. Anon. (1974). Nitrates and nitrites in food. Med. Lett. Drugs Ther., 16, 75-76. WWW.ESAINC.COM 134 Aoki, T., (1990). Continuous flow determination of nitrite with membrane separation/chemi-luminescence detection. Biomed. Chromatogr., 4, 128-130. Ayoyagi, K., Akiyama, K., Kuzure, Y., Takemura, K., Nagase, S., Ienaga, K., Nakamura, K., Koyama, A., and Narita M. (1998a). Synthesis of creatol, a hydroxyl radical adduct of creatinine and its increase by puromycin aminonucleoside in isolated rat hepatocytes. Free Radic. Res., 29, 221-226. Aoyagi, K., Nagase, S., Koyama, A., Narita, M., and Tojo, S. (1998b). Products of creatinine with hydroxyl radical as a useful marker of oxidative stress in vivo. Methods Mol. Biol., 108, 157-164. Arroya, C.M., Forray, C., El-Fakahany, E.E., and Rosen, G.M. (1990). Receptor-mediated generation of an EDRF-like intermediate in a neuronal cell line detected by spin-trapping techniques. Biochem. Biophys. Res. Commun., 170, 1177-1183. Aruoma, O.I., and Halliwell, B. (1987). Action of hypochlorous acid on the antioxidant protective enzymes superoxide dismutase, catalase and glutathione peroxidase. Biochem. J., 248, 973-976. Babbs, C.F., and Griffin, D.W. (1989). Scatchard analysis of methane sulfinic acid production from dimethyl sulfoxide: a method to quantify hydroxyl radical formation in physiologic systems. Free Radic. Biol. Med., 6, 493-503. Babior, B.M. (1978). Oxygen dependent microbial killing by phagocytes. New Engl. J. Med., 298, 659-668; 721-725. Badwey, J.A., Curnutte, J.T., and Karnovsky, M.L. (1979). The enzymes of granulocytes that produce superoxide and peroxide. New Engl. J. Med., 300, 1157-1160. Balcioglu, A., and Maher, T.J. (1993). Determination of kainic acid-induced release of nitric oxide using a novel hemoglobin trapping technique with microdialysis. J. Neurochem., 61: 2311-2313. Bartlett, D., Church, D.F., Bounds, P.L., and Koppenol, W. (1995). The kinetics of the oxidation of L-ascorbic acid by peroxynitrite. Free Radic. Biol. Med., 18, 85-92. Basu-Modak, S., and Tyrrell, R.M. (1993). Singlet oxygen: a primary effector in the ultraviolet A/near-visible light induction of the human heme oxygenase gene. Cancer Res., 53, 4505-4510. Bates, T.E., Loesch, A., Burnstock, G., and Clark, J.B. (1995). Immunocytochemical evidence for a mitochondrially located nitric oxide synthase in brain and liver. Biochem. Biophys. Res. Commun., 213, 896-900. Baur, J. E., Wang, S., and Brandt, M. C. (1996). Fast-scan voltammetry of cyclic nitroxide free radicals. Anal. Chem., 68, 3815-3821. Beal, M.F. (1997). Oxidative damage in neurodegenerative diseases. Neuroscientist, 3, 21-27. Beauchamp, C., and Fridovich, I. (1970). A mechanism for the production of ethylene from methional. The generation of the hydroxyl radical by xanthine oxidase. J. Biol. Chem., 245, 4641-4646. Beckman, K.B., and Ames, B.N. (1998). Free radical theory of aging matures. Physiol. Rev., 71, 547-581. Beckman, J.S. (1996a). The physiological and pathological chemistry of nitric oxide. In: Nitric Oxide: Principals and Actions. Lancaster, J. (Ed.). Academic Press, Inc., New York. Pp. 1-82. Beckman, J.S. (1996b). Oxidative damage and tyrosine nitration from peroxynitrite. Chem. Res. Toxicol., 9, 836-844. Beckman, J.S., Beckman, T.W., Chen, J., Marshall, P.A., and Freeman, B.A. (1990). Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA, 87, 1620-1624. Beckman, J.S., Carson, M., Smith, C.D., and Koppenol, W.H. (1993). ALS, SOD, and peroxynitrite. Nature, 364, 584. Beckman, J.S., Chen, J., Ischiropoulos, H., and Crow, J.P. (1994). Oxidative chemistry of peroxynitrite. Meth. Enzymol., 233, 229-240. Beckman, J.S., Ischiropoulos, H., Zhu, H., van der Woerd, M., Smith, C., Chen, J., Harrison, J., Martin, J.C., and Tsai, M. (1992). Kinetics of superoxide dismutase and iron catalyzed nitration of phenolics by peroxynitrite. Arch. Biochem. Biophys., 298, 438-445. Bhardwaj, A., Northington, F.J., Koehler, R.C., Stiefel, T., Hanley, D.F., and Traystman, R.J. (1995). Adenosine modulates n-methyl-d-aspartate-stimulated hippocampal nitric oxide production in vivo. Stroke, 26, 1627-1633. Bindoli, A., Rigobello, M.P., and Deeble, D.J. (1992). Biochemical and toxicological properties of the oxidation products of catecholamines. Free Radic. Biol. Med., 13, 391-405. Bindoli, A., Rigobello, M.P., and Galzinga, L. (1989). Toxicity of aminochromes. Toxicol. Lett., 48, 3-20. Bishop, A., Marquis, J.C., Cashman, N.R., and Demple, B. (1999). Adaptive resistance to nitric oxide in motor neurons. Free Radic. Biol. Med., 26, 978-986. Bogdanov, M.B., Ferrante, R.J., Kuemmerle, S., Klivenyi, P., and Beal, M.F. (1998a). Increased vulnerability of 3nitropropionic acid in an animal model of Huntington’s disease. J. Neurochem., 71, 2642-2644. Bogdanov, M.B., Ramos, L.E., Xu, X., and Beal, M.F. (1998b). Elevated hydroxyl radical generation in vivo in an animal model of amyotrophic lateral sclerosis. J. Neurochem., 71, 1321-1324. Bogdanov, M.B., Ramos, L.E., Martinou, J-C., and Beal, M.F. (1998c). Oxidative stress is attenuated in mice overexpressing BCL-2. J. Neurochem. Accepted for publication. Bonini, M.G., Radi, R., Ferrer-Sueta, G., Ferreira, A.M., and Augusto, O. (1999). Direct EPR detection of the carbonate radical anion produced from peroxynitrite and carbon dioxide. J. Biol. Chem., 274, 10802-10806. Bors, W., Michel, C., and Saran, M. (1979). On the nature of biochemically generated hydroxyl radicals. Studies using the bleaching of p-nitrosodimethylaniline as a direct assay method. Eur. J. Biochem., 95, 621-627. Bouton, C., Hirling, H., and Drapier, J.-C. (1997). Redox modulation of iron regulatory proteins by peroxynitrite. J. Biol. Chem., 272, 19969-19975. Boveris, A., and Cadenas, E. (1997). Cellular sources and steady-state levels of reactive oxygen species. In: Oxygen, Gene Expression, and Cellular Function. Lung Biology in Health and Disease. Clerch, L.B., and Massaro, D.J. (Eds.). Volume 105, Marcel Dekker, New York. Pp. 1-25); Bovis, A., and Chance, B. (1973). The mitochondrial generation of hydrogen peroxide. Biochem. J., 134, 707-716. Bredt, D.S., and Snyder, S. (1994). Nitric oxide: A physiologic messenger molecule. Ann. Rev. Biochem., 63, 175-195. Briviba, K., Devasagayam, T.P., Sies, H., and Steenken, S. (1993). Selective para hydroxylation of phenol and aniline by singlet molecular oxygen. Chem. Res. Toxicol., 6, 548-553. WWW.ESAINC.COM 135 Briviba, K., Kissner, R., Koppenol, W.H., and Sies, H. (1998). Kinetic study of the reaction of glutathione peroxidase with peroxynitrite. Chem. Res. Toxicol., 11, 1398-1401. Briviba, K., Klotz, L.-O., and Sies, H. (1999). Defenses against peroxynitrite. Meth. Enzymol., 301, 301-311. Bruijn, L.I., Beal, M.F., Becher, M.W., Schulz, J.B., Wong, P.C., Price, D.L., and Cleveland, D.W. (1997). Elevated free nitrotyrosine levels, but not protein-bound nitrotyrosine or hydroxyl radicals, throughout amyotrophic lateral sclerosis (ALS)-like disease implicate tyrosine nitration as an aberrant in vivo property of one familial ALS-linked superoxide dismutase 1 mutant. Proc. Natl. Acad. Sci. USA, 94, 7606-7611. Brune, D., Dimmeler, S., Molina, V., and Lapetina, E.G. (1994). Nitric oxide: A signal for ADP-ribosylation of proteins. Life Sci., 54, 61-70. Burkitt, M.J., and Gilbert, B.C. (1991). The autoxidation of iron(II) in aqueous systems: The effects of iron chelation by physiological, non-physiological and therapeutic chelators on the generation of reactive oxygen species and the inducement of bio-molecular damage. Free Radic. Res. Commun., 14, 107-123. Burlet, S., and Cespuglio, R. (1997). Voltammetric detection of nitric oxide (NO) in the rat brain: Its variations throughout the sleep-wake cycle. Neurosci. Lett., 226, 131-135. Butler, J. (1998). Redox cycling antitumor drugs. In: DNA and Free Radicals: Techniques, Mechanisms and Applications. Aruoma, O.I., and Halliwell, B. (Eds.). OICA, London, 131-159. Buxton, G.V., and Mulazzani, Q.G. (1999). Free radicals as a source of uncommon oxidation states of transition metals. In: Metal Ions in Biological System, 36. Sigel, A., and Sigel, H. (Eds.). Marcel Dekker, Inc., New York. Pp. 103-123. Cadenas, E. (1995). Antioxidant and pro-oxidant functions of DT-diaphorase. Biochem. Pharmacol., 49, 127-140. Calingasan, N.Y., Uchida, K., and Gibson, G.E. (1999). Protein-bound acrolein: A novel marker of oxidative stress in Alzheimer’s disease. J. Neurochem., 72, 751-756. Candeias, L.P., Patel, K.B., Stratford, M.R.L., and Wardman, P. (1993). Free hydroxyl radicals are formed from the reaction between the neutrophil-derived species, superoxide anion and hypochlorous acid. FEBS Letts. 333, 151-153. Canini, F., Bourdon, L., Cespuglio, R., and Buguet, A. (1997). Voltammetric assessment of brain nitric oxide during heatstroke. Neurosci. Lett., 231, 67-70. Carr, A.C, Vissers, M.C., Domigan, N.M., and Winterbourn, C.C. (1997). Modification of red cell membrane lipids by hypochlorous acid and hemolysis by preformed chlorohydrins. Redox Rep., 3, 263-271. Chabot, F., Mitchell, J., Quinlan, G., and Evans, T. (1997). Characterization of the vasodilator properties of peroxynitrite on rat pulmonary artery: role of poly (adenosine 5’-diphosphoribose) synthase. Br. J. Pharmacol., 121, 485-490. Chance, B., Sies, H., and Boveris, A. (1979). Hydroperoxide metabolism in mammalian organs. Physiol. Rev., 59, 527605. Chen, G., Griffin, M., Poyer, J.L., and McCay, P.B. (1990). HPLC procedure for the pharmacokinetic study of spin-trapping agent, alpha-phenyl-N-tert-butyl nitrone. Free Radic. Biol. Med., 9, 93-98. Chen, G., Janzen, E.G., and Bray, T.M. (1994). Identification of 3-MI-derived N-centered radicals obtained from incubation of 3-MU with microsomal-NADPH system by EPR-HPLC spin trapping. Free Radic. Biol. Med., 17, 19-25. Cheng, H.-Y., Liu, T., Feuerstein, G., and Barone, F.C. (1993). Distribution of spin-trapping compounds in rat blood and brain: In vivo microdialysis determination. Free Radic. Biol. Med., 14, 243-250. Chilcote, R.R., Williams, B., Wolff, L.J., and Baehner, R.L. (1977). Sudden death in an infant from methemoglobinemia after administration of "sweet spirits of nitre". Pediatrics, 59, 280-282. Christen, S., Woodall, A.A., Shigenaga, M.K., Southwell-Keely, P.T., Duncan, M.W., and Ames, B.N. (1997). γ-Tocopherol traps mutagenic electrophiles such as NOx and complements α-tocopherol: Physiological implications. Proc. Natl. Acad. Sci., 94, 3217-3222. Clark, R.A., Stone, P.J., El Hag, A., Calore, J.D., and Franzblau, C. (1981). Myeloperoxidase-catalyzed inactivation of α1protease inhibitor by human neutrophils. J. Biol. Chem., 256, 3348-3353. Cocks, T.M., Angus, J.A., Campbell, J.H., and Campbell, G.R. (1985). Release and properties of endothelium-derived relaxation factor (EDRF) from endothelial cells in culture. J. Cell Physiol., 123, 310-320. Cooney, R.V., Franke, A.A., Harwood, P.J., Hatch-Pigott, V., Custer, L.J., and Mordan, L.J. (1993). γ-Tocopherol detoxification of nitrogen dioxide: Superiority to α-tocopherol. Proc. Natl. Acad. Sci, USA, 90, 1771-1775. Cooney, R.V., Hardwood, P.J., Franke, A.A., Narala, K., Sundstrom, A-K., Berggren, P.-O., and Mordan, L.J. (1995). Product of γ-tocopherol reaction with NO2 and their formation in rat insulinoma (RINm5F) cells. Free Radic. Biol. Med., 19, 259-269. Cooper, C.E. (1999). Nitric oxide and iron proteins. Biochim. Biophys. Acta, 1411, 290-309. Cooper, C.E., Torres, J., Sharpe, M.A., Wilson, M.T., and Svistunenko, D.A. (1998). Peroxynitrite reacts with methemoglobin to generate globin-bound free radical species. Implications for vascular disease. Adv. Exp. Med. Biol., 454, 195-202. Corbett, J.T. (1989). The scopoletin assay for hydrogen peroxide: A review and a better method. J. Biochem. Biophys. Meth., 256, 297-308. Cross, C.E., Motchnik, P.A., Bruener, B.A., Jones, D.A., Kaur, H., Ames, B.N., and Halliwell, B. (1992). Oxidative damage to plasma constituents by ozone. FEBS Lett. 298, 269-272. Crow, J.P., and Beckman, J.S. (1995). Reactions between nitric oxide, superoxide, and peroxynitrite: footprints of peroxynitrite in vivo. Adv. Pharmacol., 34, 17-43. Crow, J.P., Beckman, J.S., and McCord, J.M. (1995). Sensitivity of the essential zinc-thiolate moiety of yeast alcohol dehydrogenase to hypochlorite and peroxynitrite. Biochem., 34, 3544-3552. Daiber, A., Mehl, M., and Ullrich, V. (1998). New aspects in the reaction mechanisms of phenol with peroxynitrite: The role of phenoxy radicals. Nitric Oxide, 2, 259-269. D’Aquino, M., Bullion, C., Chopra, M., Devi, D., Devi, S., Dunster, C., James, G., Komuro, E., Kundo, S., Nike, E., Raza, F., Robertson, F., Sharma, J., and Willson, R. (1994). Sulfhydryl free radical formation enzymatically by sonolysis, by radiolysis, and thermally: Vitamin A, curcumin, muconic acid, and related conjugated olefins as references. Meth. Enzymol., 233, 34-46. WWW.ESAINC.COM 136 Davies, M.J., Forni, L.G., and Shuter, S.L. (1987). Electron spin resonance and pulse radiolysis studies on the spin trapping of sulfur-centered radicals. Chem. Biol. Interact., 61, 177-188. Denicola, A., Freeman, B.A., Trujillo, M., and Radi, R. (1996). Peroxynitrite reaction with carbon dioxide/bicarbonate: Kinetics and influence on peroxynitrite-mediated oxidations. Arch. Biochem. Biophys., 333, 49-58. Desvignes, C., Robert, F., Vachette, C., Chouvet, G., Cespuglio, R., Renaud, B., and Lambas-Senas, L. (1997). Monitoring nitric oxide (NO) in rat locus coeruleus: Differential effects of NO synthase inhibitors. Neuroreport, 8, 13211325. Devasagayam, T.P., Steenken, S., Obendorf, M.S., Schulz, W.A., and Sies, H. (1991). Formation of 8-hydroxy (deoxy) guanosine and generation of strand breaks at guanine residues in DNA by singlet oxygen. Biochemistry, 30, 62836289. D’Ischia, M., and Costantini, C. (1995). Nitric oxide-induced nitration of catecholamine neurotransmitters: A key to neuronal degeneration. Bioorg. Med. Chem., 3, 923-927. D’Ischia, M., and Novellino, L. (1996). Nitric oxide-induced oxidation of α-tocopherol. Bioorg. Med. Chem., 4, 1747-1753. Domigan, N.M., Charlton, T.S., Duncan, M.W., Winterbourn, C.C., and Kettle, A.J. (1995). Chlorination of tyrosyl residues in peptides by myeloperoxidase and human neutrophils. J. Biol. Chem., 270, 16542-16548. Douki, T., and Cadet, J. (1996). Peroxynitrite mediated oxidation of purine bases of nucleosides and isolated DNA. Free Radic. Res., 24, 369-380. Dugan, L.A., Lin, T-S., He, Y.-Y., Hsu, C.Y., and Choi, D.W. (1995). Detection of free radicals by microdialysis/spin trapping EPR following focal cerebral ischemia-reperfusion and a cautionary note on the stability of 5,5-dimethyl-1pyrroline N-oxide (DMPO). Free Rad. Res. 23, 27-32. Dunford, H.B. (2000). Peroxidase-catalyzed halide ion oxidation. Redox. Rep., 5, 169-171 Dupuy, C., Virion, A., Ohayon, R., Kamiewski, J.M., Deme, D., and Pommier, J. (1991). Mechanism of hydrogen peroxide formation catalyzed by NADPH oxidase in thyroid plasma membrane. J. Biol. Chem., 266, 3739-3743. During, M.J., Acworth, I.N., and Wurtman, R.J. (1988). Phenylalanine administration influences dopamine release in the rat’s corpus striatum. Neurosci. Lett., 93, 91-95. Dzwigaj, S., and Pezerat, H. (1995). Singlet oxygen-trapping reaction as a method of (1)O2 detection: Role of some reducing agents. Free Radic. Res., 23, 103-115. Eaton, A.D., Clesceri, L.S., and Greenberg, A.E. (Eds.). (1995). Standard Methods for The Examination of Water and Wastewater. American Public Health Association, Washington, DC. Egorov, S., Yu, K., Kurella, E.G., Boldyrev, A.A., and Krasnovsky, A.A. (1997). Quenching of singlet molecular oxygen by carnosine and related antioxidants. Monitoring 1270-nm phosphorescence in aqueous media. Biochem. Mol. Biol. Int., 41, 687-694. Eiserich, J.P., Butler, J., Van Der Vleit, A., Cross, C.E., and Halliwell, B. (1995). Nitric oxide rapidly scavenges tyrosine and tryptophan radicals. Biochem. J., 310: 745-749. Eiserich, J.P., Cross, C.E., Jones, A.D., Halliwell, B., and van der Vleit, A. (1997). Formation of nitrating and chlorinating species by reaction of nitrite with hypochlorous acid. J. Biol. Chem., 271, 19199-19208. Ellis, M., Hiss, Y., and Shenkman, L. (1992). Fatal methemoglobinemia caused by inadvertent contamination of a laxative solution with sodium nitrite. Isr. J. Med. Sci. 28, 289-291. Eritja, R., Horowitz, D.M., Walker, P.A., Ziehler-Martin, J.P., Boosalis, M.S., Goodman, M.F., Itakura, K., and Kaplan, B.E. (1986). Synthesis and properties of oligonucleotides containing 2'-deoxynebularine and 2'-deoxyxanthosine. Nucleic Acids Res., 14, 8135-8153. Ewing, J.F., and Janero, D.R. (1998). Specific S-nitrosothiol (thionitrite) quantification as solution nitrite after vanadium (III) reduction and ozone-chemiluminescent detection. Free Radic. Biol. Med., 25, 621-628. Fang, K., Ragsdale, N.V., Carey, R.M., MacDonald, T., and Gaston, B. (1998). Reductive assay for S-nitrosothiols: Implications for measurements in biological systems. Biochem. Biophys. Res. Commun., 252, 535-540. Feelisch, M., and Stamler, J.S. (1996). Donors of Nitrogen Oxides. In: Methods in Nitric Oxide Research. (Feelisch, M., and Stamler, J.S. (Eds.). John Wiley and Sons Ltd. London. Pp 71-115. Feelisch, M., Kubitzek, D., and Weringloer, J. (1996). The oxyhemoglobin assay. In: Methods in Nitric Oxide Research. (Feelisch, M., and Stamler, J.S. (Eds.). John Wiley and Sons Ltd. London. Pp. 455-478. Feigl, E.O. (1988). EDRF-a protective factor. Nature, 331, 490-491. Feix, J.B., and Kalyanaraman, B. (1991). Production of singlet oxygen-derived hydroxyl radical adducts during merocyanine-540-mediated photosensitization: Analysis of ESR-spin trapping and HPLC with electrochemical detection. Arch. Biochem. Biophys., 291, 43-51. Feldman, P.L., Griffith, O.W., and Stuehr, D.J. (1993). The surprising life of nitric oxide. C&EN, December, 26-38. Fenton, H.J.H. (1876). On a new reaction of tartaric acid. Chem. News, 9, 190. Fenton, H.J.H. (1894), Oxidation of tartaric acid in the presence of iron. J. Chem. Soc., 65, 899-910. Fiala, E., Sodum, R., Bhattacharya, M., and Li, H. (1996). (-)-Epigallocatchin gallate, a polyphenolic tea antioxidant, inhibits peroxynitrite-mediated formation of 8-oxodeoxyguanosine and 3-nitrotyrosine. Experientia, 52, 922-926. Finkelstein, E., Rosen, G.M., and Rauckman, E.J. (1980). Spin trapping of superoxide and hydroxyl radical: practical aspects. Arch. Biochem. Biophys., 200, 1-16. Fischer, C., and Klotz, U. (1994). Radical-derived oxidation products of 5-aminosalicylic acid and N-acetyl-5aminosalicylic acid. J. Chromatogr. B. 661, 57-68. Floyd, R.A. (1983). Hydroxyl free-radical spin-adducts in rat brain synaptosomes observations on the reduction of the nitroxide. Biochem. Biophys. Acta., 756, 204-216. Floyd, R.A. (1997). Workshop. Oxygen Society Meeting, San Francisco. Floyd, R.A., Henderson, R., Watson, J., and Wong, P.K. (1986). Use of salicylate with high-pressure liquid chromatography and electrochemical detection (LCED) as a sensitive measure of hydroxyl free radicals in adriamycin treated rats. J. Free. Rad. Biol. Med., 2, 13-18. Floyd, R.A., Lewis, C.A., and Wong, P.K. (1984a). High-pressure liquid chromatography-electrochemical detection of oxygen free radicals. Meth. Enzymol., 105, 231-327. WWW.ESAINC.COM 137 Floyd, R.A., Watson, J.J., and Wong, P.K. (1984b). Sensitive assay of hydroxyl free radical formation utilizing high pressure liquid chromatography with electrochemical detection of phenol and salicylate hydroxylation products. J. Biochem. Biophys. Meth., 10, 221-235. Floyd, R.A., Watson, J.J., Wong, P.K., Altmiller, D.H., and Rickard, R.C. (1986). Hydroxyl free radical adduct of deoxyguanosine: Sensitive detection and mechanisms of formation. Free Rad. Res. Comm., 1, 163-172. Foote, C.S., Goyne, T.E., and Lehrer, R.I. (1981). Assessment of chlorination by human neutrophils. Nature, 301, 715716. Ford, P.C., Wink, D.A., and Stanbury, D.M. (1993). Autoxidation kinetics of aqueous nitric oxide. FEBS Lett., 326, 103. Forni, L.G., and Willson, R.L. (1986a). Thiyl free radicals and the oxidation of ferrocytochrome c. Direct observation of coupled hydrogen atom and electron transfer. Biochem. J., 240, 905-907. Forni, L.G., and Willson, R.L. (1986b). Thiyl and peroxyl free radicals and NADH. Direct observation of one-electron oxidation. Biochem. J., 240, 897-903. Forstermann, U., and Ishi, K. (1996). Measurement of cyclic GMP as an indicator of nitric oxide production. Feelisch, M., and Stamler, J.S. (Eds.). John Wiley and Sons Ltd., London. Pp. 555-566. Frears, E., Zhang, Z., Blake, D., O’Connell, J., and Winyard, P. (1996). Inactivation of tissue inhibitor of metalloproteinase1 by peroxynitrite. FEBS Lett., 381, 21-24. Fridovich, I. (1989). Superoxide dismutase: An adaptation to a paramagnetic gas. J. Biol. Chem., 264, 7761-7767. Friedemann, M.N., Robinson, S.W., and Gerhardt, G.A. (1996). o-Phenylenediamine-modified carbon fiber electrodes for the detection of nitric oxide. Anal. Chem., 68, 2621-2628. Fukuzawa, K., Inokami, Y., Tokumura, A., Terao, J., and Suzuki, A. (1998). Singlet oxygen scavenging by alphatocopherol and beta-carotene: Kinetic studies in phospholipid membranes and ethanol solution. Biofactors, 7, 31-40. Fukui, S., Hanasaki, Y., and Ogawa, S. (1993). High-performance liquid chromatographic determination of hydroxyl radicals. J. Chromatogr., 630, 187-193. Fukuto, J.M., and Wink, D.A. (1999). Nitric oxide (NO): Formation and biological roles in mammalian systems. In: Metal Ions in Biological System, 36. Sigel, A., and Sigel, H. (Eds.). Marcel Dekker, Inc., New York. Pp. 547-595. Furchgott, R.F. (1984). The role of endothelium in responses of vascular smooth muscle to drugs. Ann. Rev. Pharmacol. Toxicol., 24, 175-197. Galli, F., Rovidati, S., Ghibelli, L., and Canestrari, F. (1998). S-nitrosylation of glyceraldehyde-3-phosphate dehydrogenase decreases the enzyme affinity to the erythrocyte membrane. Nitric Oxide, 2, 17-27. Gee, P., and Davison, A.J. (1989). Intermediates in the aerobic autoxidation of 6-hydsroxydopamine: Relative importance under different reaction conditions. Free Radic. Biol. Med., 6, 271-284. Gelvan, D., Moreno, V., Gassmann, W., Hegenauer, J., and Saltman, P. (1992). Metal-ion-directed site specificity of hydroxy radical detection. Biochem. Biophys. Acta, 1116, 183-191. George, P. (1965). In: Oxidase and Redox Related Systems. King, T.E., Mason, H.S., and Morrison, M. (Eds.). Wiley, New York. Pp. 3-36. Gerlach, M., Ben-Shachar, D., Riederer, P., and Youdim, M.B.H. (1994). Altered brain metabolism of iron as a cause of neurodegenerative diseases. J. Neurochem., 63, 793-807. Ghilarducci, D.P., and Tjeerdema, R.S. (1995). Fate and effects of acrolein. Rev. Environ. Contam. Toxicol., 144, 95-146. Gilbert, D.L. (1999). From the breath of life to reactive oxygen species. In: Reactive Oxygen Species in Biological Systems. Gilbert, D.L., and Colton, C.A. (Eds.). Kluwer Academic/Plenum Publishers, New York. Pp. 3-31. Giulivi, C. (1998). Functional implications of nitric oxide produced by mitochondria in mitochondrial metabolism. Biochem. J., 332, 673-679. Giulivi, C., and Davies, K.J.A. (1994). Dityrosine: A marker for oxidatively modified proteins and selective proteolysis. Meth. Enzymol., 233, 363-371. Giulivi, C., Poderoso, J.J., and Boveris, A. (1998). Production of nitric oxide by mitochondria. J. Biol. Chem., 273, 1103811043. Gleu, K., and Hubold, R. (1935). Die einwirkung von wasserstoffsuperoxyd auf saltpetrige saure. Persalpetridge saure. Z. Anorg. Allg. Chem. 179, 233-266. Goldman, A.P., and Macrae, D.J. (1994). Nitrogen dioxide measurement in breathing systems. Lancet, 343, 1850. Goldstein, S., Czapski, G., Lind, J., and Merenyi, G. (1988). Mechanism of decomposition of peroxynitric ion. Evidence for the formation of superoxide and nitrogen dioxide radical. Inorg. Chem., 37, 3943-3947. Goldstein, S., Meyerstein, D., and Crapski, G. (1993). The Fenton reagents. Free Radic. Biol. Med., 15, 435-554. + Gorbunov, N.V., Osipov, A.N., Sweetland, M.A., Day, B.W., Elsayed, N.M., and Kagan, V.E. (1996). NO redox paradox: Direct oxidation of α-tocopherol-mediated oxidation of ascorbate. Biochem. Biophys. Res. Commun., 219, 835-841. Gow, A., and Stamler, J. (1998). Reactions between nitric oxide and hemoglobin under physiological conditions. Nature, 391, 169-173. Gow, A., Duran, D., Malcolm, S., and Ischiropoulos, H. (1996). Effects of peroxynitrite-induced modifications on tyrosine phosphorylation and degradation. FEBS Lett., 385, 63-66. Gow, A., Duran, D., Thom, S.R., and Ischiropoulos, H. (1996). Carbon dioxide enhancement of peroxynitrite-mediated protein tyrosine nitration. Arch. Biochem. Biophys., 333, 42-48. Graham, D. (1978). Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol. Pharmacol., 14, 633-643. Graham, D., Tiffany, S.M., Bell, W.R. and Gutknecht, W.F. (1978). Autoxidation versus covalent binding of quinones as mechanisms of toxicity of dopamine, 6-hydroxydopamine, and related compounds toward C1300 neuroblastoma cells in vitro. Mol. Pharmacol., 14, 644-653. Griffith, O.W., and Stuehr, D.J. (1995). Nitric oxide synthases: Properties and catalytic mechanisms. Ann. Rev. Physiol., 57, 707-736. Griffith, T.M., Edwards, D.H., Lewis, M.J., Newby, A.C., and Henderson, A.H. (1984). The nature of endothelium-derived vascular relaxant factor. Nature, 308, 645-647. WWW.ESAINC.COM 138 Griscavage, J.M., Hobbs, A.J., and Ignarro, L.J. (1995). Negative modulation of nitric oxide synthase by nitric oxide and nitroso compounds. Adv. Pharmacol., 34, 215-234. Grisham, M.B., Jefferson, M.M., Melton, D.F., and Thomas, E.L. (1984). Chlorination of endogenous amines by isolated neutrophils. Ammonia-dependent bacteriocidal, cytotoxic, and cytolytic activities of the chloramines. J. Biol. Chem., 259, 10404-10413. Gross, S.S., and Wolin, M.S. (1995). Nitric oxide: Pathophysiological mechanisms. Ann. Rev. Physiol., 57, 737-769. Groves, J.T. (1999). Peroxynitrite: Reactive, invasive and enigmatic. Curr. Opin. Chem. Biol., 3, 226-235. Haenen, G., Paquay, J., Korthouwer, R., and Bast, A. (1997). Peroxynitrite scavenging by flavonoids. Biochem. Biophys. Res. Commun., 236, 591-593. Halliwell, B. (1997). What nitrates tyrosine? Is nitrotyrosine specific as a biomarker of peroxynitrite formation in vivo? FEBS Lett., 411, 157-160. Halliwell, B., and Gutteridge, J.M.C. (1981). Formation of thiobarbituric-acid-reactive substance from deoxyribose in the presence of iron salts: the role of superoxide and hydroxyl radicals. FEBS Lett., 128, 347-352. Halliwell, B., and Gutteridge, J.M.C. (1990). Role of free radicals and catalytic metal ions in human disease: An overview. Meth. Enzymol., 186, 1-85. Halliwell, B., and Gutteridge, J.M.C. (1992). Biologically relevant metal ion-dependent hydroxyl radical generation. FEBS Lets., 307, 108-112. Halliwell, B., and Gutteridge, J.M.C. (1999). Free Radicals in Biology and Medicine. Clarendon Press, Oxford. Halliwell, B., Gutteridge, J.M.C., and Aruoma, O.I. (1987). The deoxyribose method: a simple "test-tube" assay for determination of rate constants for reactions of hydroxyl radicals. Anal. Biochem., 165, 215-219. Halliwell, B., and Kaur, H. (1997). Hydroxylation of salicylate and phenylalanine as assays for hydroxyl radicals: A cautionary note visited for the third time. Free Rad. Res., 27, 239-244. Halliwell, B., Hu, M.-L., Louie, S., Duvall, T., Tarkington, B., Motchnik, P., and Cross, C. (1992). Interaction of nitrogen dioxide with human plasma. FEBS Lett., 313, 62-66. Hampl, V., Walters, C.L., and Archer, S.L. (1996). Determination of nitric oxide by the chemiluminescence reaction with ozone. In: Methods in Nitric Oxide Research. Feelisch, M., and Stamler, J.S. (Eds.). John Wiley and Sons Ltd., London. Pp. 309-318. Hansford, R.G., Hogue, B.A., and Mildaziene, V. (1997). Dependence of H2O2 formation by rat heart mitochondria on substrate availability and donor age. J. Bioenerg. Biomem., 29, 89-95. Harrison, J.E., and Schultz, J. (1976). Studies on the chlorinating activity of myeloperoxidase. J. Biol. Chem., 251, 13711374. Hartmann, H.-J., Sievers, C., and Weser, U. (1999). Thiyl radicals in biochemically important thiols in the presence of metal ions. In: Metal Ions in Biological System, 36. Sigel, A., and Sigel, H. (Eds.). Marcel Dekker, Inc., New York. Pp. 389-413. Hartsfield, C.L., Alam, J., Cook, J.L., and Choi, A.M. (1997). Regulation of heme oxygenase-1 gene expression in vascular smooth muscle cells by nitric oxide. Am. J. Physiol., 273, L980-988. Hassan, T., and Khan, A.U. (1986). Phototoxicity of the tetracyclines: Photosensitized emission of singlet delta oxygen. Proc. Natl. Acad. Sci. USA, 83, 4604-4606. Hazell, L.J., Arnold, L., Flowers, D., Waeg, G., Malle, E., and Stocker, R. (1996). Presence of hypochlorite-modified proteins in human atherosclerotic lesions. J. Clin. Invest., 97, 1535-1544. Hazen, S.L., and Heinicke, J.W. (1997). 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J. Clin. Invest., 99, 2075-2081. Hazen, S.L., and Wu, W. (1998). 3-Bromo and 3,5-dibromo-tyrosine: Potential markers for eosinophil-dependent injury in vivo. Free Radic. Biol. Med., 25, Supplement 1, 74. Hazen, S.L., Gaut, J.P., Hsu, F.F., Crowley, J.R., d’Avignon, A., and Heinecke, J.W. (1997). pHydroxyphenylacetaldehyde, the major product of L-tyrosine oxidation by the myeloperoxidase-H2O2-chloride system of phagocytes, covalently modifies ε-amino groups of protein lysine residues. J. Biol. Chem., 272, 16990-16998. Hazen, S.L., Hsu, F.F., and Heinecke, J.W. (1996b). p-Hydroxyphenylacetaldehyde is the major product of L-tyrosine oxidation by activated human phagocytes. J. Biol. Chem., 271, 1861-1867. Hazen, S.L., Hsu, F.F., Mueller, D.M., Crowley, J.R., and Heinicke, J.W. (1996a). Human neutrophils employ chlorine gas as an oxidant during phagocytosis. J. Clin. Invest., 98, 1283-1289. Hecker, M., and Billiar, T.R. (1996). Detection of L-arginine, related metabolites and guanidino compounds. In: Methods in Nitric Oxide Research. Feelisch, M., and Stamler, J.S. (Eds.). John Wiley and Sons Ltd., London. Pp. 260-270. Heinecke, J.W., Li, W., Daehnke, H.L., and Goldstein, J.A. (1993). Dityrosine, a specific marker of oxidation, is synthesized by the myeloperoxidase-hydrogen peroxide system of human neutrophils and macrophages. J. Biol. Chem., 268, 4069-4077. Heinecke, J.W., Li, W., Mueller, D.M., Bohrer, A., and Turk, J. (1994). Cholesterol chlorohydrin synthesis by the myeloperoxidase-hydrogen peroxide-chloride system: Potential markers for lipoproteins oxidatively damaged by phagocytes. Biochem., 33, 10127-10136 Henry, Y.A., and Singel, D. (1996). Metal-nitrosyl interactions in nitric oxide biology probed by electron paramagnetic resonance spectroscopy. In: Methods in Nitric Oxide Research. Feelisch, M., and Stamler, J.S. (Eds.). John Wiley and Sons Ltd., London. Pp. 357-372. Henry, Y., Lepoivre, M., Drapier, J-C., Ducrocq, C., Boucher, J-C., and Guissani, A. (1993). EPR characterization of molecular targets for NO in mammalian cells and organelles. FASEB J., 7, 1124-1134. Hensley, K., Maidt, M.L., Pye, Q.N., Stewart, C.A., Wack, M., Tabatabaie, T., and Floyd, R.A. (1997). Quantitation of protein-bound 3-nitrotyrosine and 3,4-dihydroxyphenylalanine by high-performance liquid chromatography with electrochemical array detection. Anal. Biochem., 251, 187-195. Hibbs, J.B., Taintor, R.R., Vavrin, Z., and Rachlin, E.M. (1988). Nitric oxide: A cytotoxic activated macrophage effector molecule. Biochem. Biophys. Res. Commun., 157, 87-94. WWW.ESAINC.COM 139 Hiraoka, W., Kuwabara, M., and Sato, F. (1989). OH-induced free radicals in purine nucleoside monophosphates: ESR and spin-trapping. Int. J. Radiat. Biol., 55, 51-58. Hiraoka, W., Kuwabara, M., Sato, F., Matsuda, A., and Ueda, T. (1990). Free-radical reactions induced by OH-radical attack on cytosine-related compounds: A study by a method combining ESR, spin trapping and HPLC. Nucleic Acids Res., 18, 1217-1223. Hobbs, A.J., Fukuto, J.M., and Ignarro, L.J. (1994). Formation of free nitric oxide from L-arginine by nitric oxide synthase: Direct enhancement of generation by superoxide dismutase. Proc. Natl. Acad. Sci. USA, 91, 10992-10996. Hogg, N., and Kalyanaraman, B. (1999). Nitric oxide and lipid peroxidation. Biochim. Biophys. Acta, 1411, 378-384. Hoigne, J., and Bader, H. (1975). Ozonation of water: Role of hydroxyl radicals as oxidizing intermediates. Science, 190, 792. Huggins, T.G., Wells-Knecht, M.C, Detorie, N.A., Baynes, J.W., and Thorpe, S.R. (1993). Formation of o-tyrosine and dityrosine in proteins during radiolytic and metal-catalyzed oxidation. J. Biol. Chem., 268, 12341-12347. Hughes, M.N. (1999). Relationships between nitric oxide, nitroxyl ion, nitrosonium cation and peroxynitrite. Biochim. Biophys. Acta, 1411, 263-272. Huie, R.E. (1994). The reaction kinetics of NO2•. Toxicol., 89, 193-196. Huie, R.E., and Neta, P. (1999). Chemistry of reactive oxygen species. In: Reactive Oxygen Species in Biological Systems. Gilbert, D.L., and Colton, C.A. (Eds.). Kluwer Academic/Plenum Publishers, New York. Pp. 33-73. Huie, R.E., and Padmaja, S. (1993). The reaction rate of nitric oxide with superoxide. Free Radic. Biol. Med., 18, 195-199. Hutchinson, F. (1957). The distance that a radical formed by ionizing radiation can diffuse in a yeast cell. Radiat. Res., 7, 473-483. Hwang, C., Sinskey, A.J., and Lodish, H.F. (1992). Oxidized redox state of glutathione in the endoplasmic reticulum. Science, 257, 1496-1502. Igarashi, T., Sakurai, K., Oi, T., Obara, H., Oyha, H., and Kamada, H. (1999). New sensitive agents for detecting singlet oxygen by electron spin resonance spectroscopy. Free Radic. Biol. Med., 26, 1339-1345. Ignarro, L.J., Burke, T.M., Wood, K.S., Wolin, M.S., and Kadowitz, P.J. (1984). Association between cGMP accumulation and acetylcholine-elicited relaxation of bovine intrapulmonary artery. J. Pharmacol. Exp. Ther., 228, 682-690. Imlay, J.A., and Linn, S. (1988). DNA damage and oxygen radical toxicity. Science, 240, 1302-1309. Inami, O., Kuwabara, M., Endoh, D., and Sato, F. (1986). OH-induced free radicals in uridine studied by a method combining ESR, spin-trapping, and liquid chromatography. Radiat. Res., 108, 1-11. Inami, O., Kuwabara, M., and Sato, F. (1987). OH-induced free radicals in 3’-UMP and poly(U): Spin-trapping and radical chromatography. Radiat. Res., 112, 36-44. Ischiropoulos, H., and Al-Mehdi, A.B. (1995). Peroxynitrite-mediated oxidative protein modifications. FEBS Letts., 364, 279-282. Ischiropoulos, H., Zhu, L., Chen, J., Tsai, H.M., Martin, J.C., Smith, C.D., and Beckman, J.S. (1992b). Peroxynitritemediated tyrosine nitration catalyzed by superoxide dismutase. Arch. Biochem. Biophys., 298, 431-437. Ishimitsu, S., Fujimoto, S., and Ohara, A. (1986). Hydroxylation of phenylalanine by the hypoxanthine-xanthine oxidase system. Chem. Pharmaceut. Bull., 32, 4645-4649. Ishimitsu, S., Fujimoto, S., and Ohara, A. (1986). Determination of m-tyrosine and o-tyrosine in human serum by highperformance liquid chromatography with fluorometric detection. J. Chromatogr., 378, 222-225. Iwahashi, H. (1996). Quantitative detection of reduced, radical and oxidized forms of α-(4-pyridyl-1-oxide)-N-tertbutylnitrone radical adduct using high-performance liquid chromatography with electrochemical detection. J. Chromatogr. A, 753, 235-242. Jacob, J.S., Cistola, D.P., Hsu, F.F., Muzaffar, S., Mueller, D.M., Hazen, S.L., and Heinecke, J.W. (1996). Human phagocytes employ the myeloperoxidase-hydrogen peroxide system to synthesize dityrosine, trityrosine, pulcherosine, and isodityrosine by a tyrosyl radical-dependent pathway. J. Biol. Chem., 271, 19950-19956. Jahnke, L.S. (1999). Measurement of hydroxyl radical-generated methane sulfinic acid by high-performance liquid chromatography and electrochemical detection. Anal. Biochem., 269, 273-277. Jakab, G.J. (1977). Adverse effect of a cigarette smoke component, acrolein, on pulmonary antibacterial defenses and on viral-bacterial interactions in the lung. Am. Rev. Respir. Dis., 115, 33-38. Jen, J., Leu, M., and Yang, T.C. (1998). Determination of hydroxyl radicals in an advanced oxidation process with salicylic acid trapping and liquid chromatography. J. Chromatogr. A, 796, 283-288. Jia, L., Bonaventura, C., Bonaventura, J., and Stamler, J.S. (1996). S-nitrosohemoglobin: A dynamic activity of blood involved in vascular control. Nature, 380, 221-226. Jiang, Q., and Hurst, J.K. (1997). Relative chlorinating, nitrating, and oxidizing capabilities of neutrophils determined with phagocytosable probes. J. Biol. Chem., 272, 32767-32772. Jourd’heuil, D., Mai, C.T., Laroux, F.S., Wink, D.A., and Grisham, M.B. (1998). The reaction of S-nitrosoglutathione with superoxide. Biochem. Biophys. Res. Commun., 246, 525-530. Kachur, A.V., Tuttle, S.W., and Biaglow, J.E. (1998). Autoxidation of ferrous ion complexes: A method for the generation of hydroxyl radicals. Radiat. Res., 150, 475-482. Kaiser, S., Mascio, P.D., Murphy, M.E., and Sies, H. (1990). Quenching of singlet molecular oxygen by tocopherols. In: Antioxidants in Therapy and Preventive Medicine. Emerit et al. (Eds.). Plenum Press, New York. Pp. 117-124. Kalyanaraman, B. (1995). Thiyl radicals in biological systems: Significance or trivial? Biochem. Soc. Symp., 61, 55-63. Kalyanaraman, B., Karoui, H., Singh, R.J., and Felix, C.C. (1996). Detection of thiyl radical adducts formed during hydroxyl radical- and peroxynitrite-mediated oxidation of thiols – a high resolution ESR spin-trapping study at Q-band (35 GHz). Anal. Biochem., 241, 75-81. Kalyamaraman, B., Ramanujam, S., Singh, R.J., Joseph, J., and Feix, J.B. (1993). Formation of 2,5-dihydroxybenzoic 1 acid during the reaction between O2 and salicylic acid: Analysis of ESR oximetry and HPLC with electrochemical detection. J. Am. Chem. Soc., 115, 4007-4012. Kanner, J., Harel, S., and Granit, R. (1991). Nitric oxide as an antioxidant. Arch. Biochem. Biophys., 289, 130-136. WWW.ESAINC.COM 140 Karam, L.R., and Simic, M.G. (1988). Detecting irradiated foods: Use of hydroxyl radical biomarkers. Anal. Chem., 16, 1117A-1119A. Karoui, H., Hogg, N., Frejaville, C., Tordo, P., and Kalyanaraman, B. (1996). Characterization of sulfur-centered radical intermediates formed during the oxidation of thiols and sulfite by peroxynitrite. ESR-spin trapping and oxygen uptake studies. J. Biol. Chem., 271, 6000-6009. Kato, Y., Kawakishi, S., Aoki, T., Itakura, K., and Osawa, T. (1997). Oxidative modification of tryptophan residues exposed to peroxynitrite. Biochem. Biophys. Res. Commun., 234, 82-84. Kaur, H., and Halliwell, B. (1994a). Aromatic hydroxylation of phenylalanine as an assay for hydroxyl radicals: Measurement of hydroxyl radical formation from ozone and in blood from premature babies using improved HPLC methodology. Anal. Biochem. 220, 11-15. Kaur, H., and Halliwell, B. (1994b). Detection of hydroxyl radicals by aromatic hydroxylation. Meth. Enzymol. 233, 67-82. Kaur, H., and Halliwell, B. (1994c). Evidence for nitric oxide-mediated oxidative damage in chronic inflammation. Nitrotyrosine in serum and synovial fluid from rheumatoid patients. FEBS Letts. 350, 8-12. Kaur, H., Edmonds, S.E., Blake, D.R., and Halliwell, B. (1996). Hydroxyl radical generation by rheumatoid blood and knee-joint synovial fluid. Ann. Rheumatic Dis., 55, 915-920. Kaur, H., Fagerheim, I., Grootveld, M., Puppo, A., and Halliwell, B. (1988). Aromatic hydroxylation of phenylalanine as an assay for hydroxyl radicals: Application to activated human neutrophils and to heme protein leghemoglobin. Anal. Biochem., 172, 360-367. Kaur, H., Leung, K.H.W., and Perkins, M.J. (1981). J. Chem. Soc. Chem. Commun., 142-144. Kaur, H., Whiteman, M., and Halliwell, B. (1997). Peroxynitrite-dependent aromatic hydroxylation and nitration of salicylate and phenylalanine. Is hydroxyl radical involved. Free Radic. Res., 26, 71-82. Keaney, J.F., Simon, D.I., Stamler, J.S., Jaraki, O., Scharfstein, J., Vita, J.A., and Loscalzo, J. (1993). NO forms an adduct with serum albumin that has endothelium-derived relaxing factor-like properties. J. Clin. Invest., 91, 1582-1589. Keith, W.G., and Powell, R.E. (1969). J. Chem. Soc., A. 90. Kelm, M., and Schrader, J. (1988). Nitric oxide release from the isolated guinea pig heart. Eur. J. Pharmacol., 155, 317321. Kettle, A.J., and Winterbourn, C.C. (1991). The influence of superoxide on the production of hypochlorous acid by human neutrophils. Free Rad. Res. Comm., 12-13, 47-52. Kettle, A.J., and Winterbourn, C.C. (1994). Superoxide-dependent hydroxylation by myeloperoxidase. J. Biol. Chem., 269, 17146-17151. Kim, S.G., and Novak, R.F. (1990). Role of P450IIE1 in the metabolism of 3-hydroxypyridine, a constituent of tobacco smoke: Redox cycling and DNA strand scission by the metabolite 2,5-dihydroxypyridine. Cancer Res., 50, 5333-5339. Kiryu, C., Makiuchi, M., Miyazaki, J., Fujinaga, T., and Kakinuma, K. (1999). Physiological production of singlet molecular oxygen in the myeloperoxidase-hydrogen peroxide-chloride system. FEBS Lett., 443, 154-158. Kishnani, N.S., and Fung, H.-L. (1996). Nitric oxide generation from pharmacological nitric oxide donors. Meth. Enzymol., 268, 259-265. Klebanoff, S.J. (1988). In: Inflammation: Basic Principles and Clinical Correlates. Gallin, J.I., Goldstein, I.M., and Snyderman, R. (Eds.). Raven Press, New York. Pp. 391-443. Klebanoff, S.J. (1993). Reactive nitrogen intermediates and antimicrobial activity: Role of nitrite. Free Radic. Biol. Med., 14, 351-360. Klein, S.M., Cohen, G., and Cederbaum, A.I. (1981). Production of formaldehyde during metabolism of dimethyl sulfoxide by hydroxyl radical generating systems. Biochem., 20, 6006-6012. Kluge, I., Gutteck-Amsler, U., Zollinger, M., and Do, K.Q. (1997). S-Nitrosoglutathione in rat cerebellum: Identification and quantification by liquid chromatography-mass spectrometry. J. Neurochem., 69, 2599-2607. Kojima, H., Kikuchi, K., Hirobe, M., and Nagano, T. (1997). Real-time measurement of nitric oxide production in rat brain by the combination of luminol-H2O2 chemiluminescence and microdialysis. Neurosci. Letts., 233, 157-159. Kojima, H., Nakatsubo, N., Kikuchi, K., Kawahara, S., Kirino, Y., Nagoshi, H., Hirata, Y., and Nagano, T. (1998). Detection and imaging of nitric oxide with novel fluorescent indicators: Diaminofluoresceins. Anal. Chem., 70, 2446-2453. Kon, S.H. (1978). Biological autoxidation. I. Decontrolled iron: An ultimate carcinogen and toxicant: An Hypothesis. Med. Hypotheses, 4, 445-471. Kong, S.-K., Yim, M., Stadtman, E., and Chock, P. (1996). Peroxynitrite disables the tyrosine phosphorylation regulatory mechanism: Lymphocyte-specific tyrosine kinase fails to phosphorylate nitrated cdc2(6-20)NH2 peptide. Proc. Natl. Acad. Sci., USA, 93, 3377-3382. Kono, Y. (1995). The production of nitrating species by the reaction between nitrite and hypochlorous acid. Biochem. Mol. Biol. Int., 36, 275-283. Kooy, N.W., Royall, J.A., Ye, Y.Z., Kelley, D.R., and Beckman, J.S. (1994). Evidence for in vivo peroxynitrite production in human acute lung injury. Am. Rev. Respir. Dis., 151, 1250-1254. Koppenol, W.H. (1993a). A thermodynamic appraisal of the radical sink hypothesis. Free Radic. Biol. Med., 14, 91-94. Koppenol, W.H. (1993b). The centennial of the Fenton reaction. Free Radic. Biol. Med., 15, 645-651. Koppenol, W.H. (1994). Thermodynamic considerations on the formation of reactive species from hypochlorite, superoxide and nitrogen monoxide. Could nitrosyl chloride be produced by neutrophils and macrophages? FEBS Lett., 347, 5-8. Koppenol, W.H., Kissner, R., and Beckman, J.S. (1996), Synthesis of peroxynitrite: To go with the flow or on solid grounds? Meth. Enzymol., 269, 296-302. Koppenol, W.H., Moreno, J.J., Pryor, W.A., Ischiropoulus, H., and Beckman, J.S. (1992). Peroxynitrite a cloaked oxidant from superoxide and nitric oxide. Chem. Res. Toxicol., 5, 834-842. Korth, H.-G., and Weber, H. (1996). Detection of nitric oxide with nitric oxide-trapping agents. In: Methods in Nitric Oxide Research. Feelisch, M., and Stamler, J.S. (Eds.). John Wiley and Sons Ltd., London. Pp. 383-391. WWW.ESAINC.COM 141 Korth, H.G., Ingold, K.U., Sustmann, R., de Groot, H., and Sies, H. (1992). Tetramethyl-o-chinodiethan (NOCT-!) das erste mitgleid einer familie massgescheiderter chletroper singanger fur Stickstoffmonoxide. Angew. Chem., 104, 915917. Kosaka, H., and Shiga, T. (1996). Detection of nitric oxide by electron spin resonance using hemoglobin. In: Methods in Nitric Oxide Research. Feelisch, M., and Stamler, J.S. (Eds.). John Wiley and Sons Ltd., London. Pp. 373-391. Kostka, P. (1992). Free Radicals (Nitric Oxide). Anal. Chem., 67, 411R-416R. Kostka, P., and Park, J.K.J. (1999). Fluorometric detection of S-nitrosothiols. Meth. Enzymol., 301, 227-235. Kuhn, D.M., and Arthure, R.E. (1996). Inactivation of brain tryptophan hydroxylase by nitric oxide. J. Neurochem., 67, 1072-1077. Kwon, B-M., and Foote, C.S. (1988). Chemistry of singlet oxygen. 50. Hydroperoxide intermediates in the photooxygenation of ascorbic acid. J. Am. Chem. Soc. 110, 6582-6583. Lai, C-S., and Komarov, A.M. (1994). Spin-trapping of nitric oxide produced in vivo in septic shock mice. FEBS Lett., 345, 120-124. Laval, F., and Wink, D.A. (1994). Inhibition by nitric oxide of the repair protein, O6-methylguanine-DNA-methyltransferase. Carcinogen., 15, 443-447. Leeuwenburgh, C., Rasmussen, J.E., Hsu, F.F., Mueller, D.M., Pennathur, S., and Heinecke, J.W. (1997). Reactive nitrogen intermediates promote low density lipoprotein oxidation in human atherosclerotic intima. J. Biol. Chem., 272, 3520-3526. Lemercier, J.-N., Padmaja, S., Cueto, R., Squadrito, G.L., Uppa, R.M., and Pryor, W.A. (1997). Carbon dioxide modulation of hydroxylation and nitration of phenol by peroxynitrite. Arch. Biochem. Biophys., 345, 160-170. Levine, R.L., Mosoni, L., Berlett, B.S., and Stadtman, E.R. (1996). Methionine residues as endogenous antioxidants in proteins. Proc. Natl. Acad. Sci. USA, 93, 15036-15040. Lewisch, S.A., and Levine, R.L. (1995). Determination of 2-oxohistidine by amino acid analysis. Anal. Biochem., 231, 440446. Li, S., Schoneich, C., and Borchardt, R.T. (1995a). Chemical pathways of peptide degradation. VIII. Oxidation of methionine in small model peptides by pro-oxidant/transition metal ion systems: Influence of selective scavengers for reactive oxygen intermediates. Pharmacol. Res., 12, 348-355. Liehr, J.G., Ulubelen, A.A., and Strobel, H.W. (1986). Cytochrome P-450-mediated redox cycling of estrogens. J. Biol. Chem., 261, 16865-16870. Liochev, S.I. (1999). The mechanism of “Fenton-like” reactions and their importance for biological systems. A biologist’s view. In: Metal Ions in Biological System, 36. Sigel, A., and Sigel, H. (Eds.). Marcel Dekker, Inc., New York. Pp. 1-39. Lion, Y., Demelle, M., and Van de Vorst, A. (1976). New method of detecting singlet oxygen production. Nature, 263, 442443. Lipton, S.A., Choi, Y.B., Pan, Z.H., Lei, S.Z., Chen, H.S., Sucher, N.J., Loscalzo, J., Singel, D.J., and Stamler, J.S. (1993). A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitrosocompounds [see comments]. Nature, 364, 626-632. Liu, D. (1993). Generation and detection of hydroxyl radical in vivo in rat spinal cord by microdialysis administration of Fenton’s reagents and microdialysis sampling. J. Biochem. Biophys. Meth., 27, 281-291. Liu, X., Miller, M.J.S., Joshi, M.S., Thomas, D.D., and Lancaster, J.R. (1998). Accelerated reactions of nitric oxide with O2 within the hydrophobic interior of biological membranes. Proc. Natl. Acad. Sci. USA, 95, 2175-2179. Livovich, V., and Scheeline, A. (1997). Amperometric sensors for simultaneous superoxide and hydrogen peroxide detection. Anal. Chem., 69, 454462. Luo, X., and Lehotay, D.C. (1997). Determination of hydroxyl radicals using salicylate as a trapping agent by gas chromatography-mass spectrometry. Clin. Biochem., 30, 41-46. Lymar, S.V., and Hurst, J.K. (1995a). Rapid reaction between peroxynitrite ion and carbon dioxide: Implications for biological activity. J. Am. Chem. Soc., 117, 8867-8868. Lymar, S.V., and Hurst, J.K. (1995b). Role of compartmentalization in promoting toxicity of leukocyte-generated strong oxidants. Chem. Res. Toxicol., 8, 833-840. Lymar, S.V., and Hurst, J.K. (1996). Carbon dioxide: Physiological catalyst for peroxynitrite-mediated cellular damage or cellular protectant? Chem. Res. Toxicol., 9, 845-850. McCabe, D.R., Maher, T.J., and Acworth, I.N. (1997a). Improved method for the estimation of hydroxyl free radical levels in vivo based on liquid chromatography with electrochemical detection. J. Chromatogr. B. 691, 23-32. McCabe, D.R., Acworth, I.N., Maidt, M.L., and Floyd, R.A. (1997b). A sensitive and selective method for the determination th of tissue 8-hydroxy-2’deoxyguanosine using HPLC with electrochemical array detection. Presented at The 88 Annual Meeting of The American Association for Cancer Research. San Diego CA. McCord, J.M., and Day, E.D. (1978). Superoxide-dependent production of hydroxyl radical catalyzed by iron-EDTA complex. FEBS Lett., 86, 139-142. McCormick, M.L., Buettner, G.R., Lewis, T.S., Heinecke, J.W., and Britigan, B.E. (1998). Eosinophil peroxidase forms dityrosine via generation of tyrosyl radicals. Free Radic. Biol. Med., Supplement 1, S46. McNeil, C.J., Greenough, K.R., Weeks, P.A., and Self, C.H. (1992). Electrochemical sensors for direct reagentless measurement of superoxide production by human neutrophils. Free Radic. Biol. Med., 17, 399-406. MacDougal, C.S., Rigas, M.L., Ben-Jebria, A., and Ultman, J.S. (1998). A respiratory ozone analyzer optimized for high resolution and swift dynamic response during exercise conditions. Arch. Environ. Health, 53, 161-174. Madronich, S. (1999). Stratospheric ozone and its effects on the biosphere. In: Reactive Oxygen Species in Biological Systems. Gilbert, D.L., and Colton, C.A. (Eds.). Kluwer Academic/Plenum Publishers, New York. Pp. 317-334.. Makino, N., Mochizuki, Y., Bannai, S., and Sugita, Y. (1994). Kinetic studies on the removal of extracellular hydrogen peroxide by cultured fibroblasts. J. Biol. Chem., 269, 1020-1025. Malinski, T., and Taha, Z. (1992). Nitric oxide release from a single cell measured in situ by a porphyrinic-based microsensor. Nature, 358, 676-678. Malmstrom, B.G. (1982). Enzymology of oxygen. Ann. Rev. Biochem., 51, 21-59. WWW.ESAINC.COM 142 Mansouri, A., and Perry, C.A. (1987). Hemoglobin autoxidation at physiological concentrations. Hemoglobin, 11, 353-371. Marletta, M.A., Yoon, P.S., Iyengar, R., Leaf, C.D., and Wishnok, J.S. (1988). Macrophage oxidation of L-arginine to nitrite and nitrate: Nitric oxide is an intermediate. Biochem., 27, 8706-8711. Martin, W., Villani, G.M., Jothianandan, D., and Furchgott, R.F. (1985). Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J. Pharmacol. Exp. Ther., 232: 708-716. Masarwa, M., Cohen, H., Meyerstein, D., Hickman, D.L., Bacak, A., and Espenson, J.H. (1988). Reactions of low-valent + 2+ transition metal complexes with hydrogen peroxide. Are they “Fenton-like” or not? 1. The case of Cu (aq) and Cr (aq). J. Am. Chem. Soc., 110, 4293-4297. Maskos, Z., Rush, J.D., and Koppenol, W.H., (1992). The hydroxylation of tryptophan. Arch. Biochem. Biophys. 296, 514520. Menzel, D.B. (1984). Ozone: An overview of its toxicity in man and animals. J. Toxicol. Environ. Health., 13, 183-204. Menzel, D.B., and Meacher, D.M. (1999). Ozone and nitrogen dioxide. In: Reactive Oxygen Species in Biological Systems. Gilbert, D.L., and Colton, C.A. (Eds.). Kluwer Academic/Plenum Publishers, New York. Pp. 335-366. Meyer, J., Richter, N., and Hecker, M. (1997). High-performance liquid chromatographic determination of nitric oxide synthase-related arginine derivatives in vitro and in vivo. Anal. Biochem., 247, 11-16. Michel, T., Xie, Q-W., and Nathan, C. (1996). Molecular biological analysis of nitric oxide synthases. In: Methods in Nitric Oxide Research. Feelisch, M., and Stamler, J.S. (Eds.). John Wiley and Sons Ltd., London. Pp. 161-175. Miller, D.M., Buettner, G.R., and Aust, S.D. (1990). Transition metals as catalysts of “autoxidation” reactions. Free Radic. Biol. Med., 8, 95-108. Miller, J.W., Selhub, J., and Joseph, J.A. (1996). Oxidative damage caused by free radicals produced during catecholamine autoxidation: Protective effects of O-methylation and melatonin. Free Radic. Biol. Med., 21, 241-249. Miller, R.T., Martasek, P., Roman, L.J., Nishimura, J.S., and Masters, B.S. (1997). Involvement of the reductase domain of neural nitric oxide synthase in superoxide anion production. Biochem., 36, 15277-15284. Minetti, M., Mallozzi, C., Di Stasi, A.M., and Pietraforte, D. (1998). Bilirubin is an effective antioxidant of peroxynitritemediated protein oxidation in human blood plasma. Arch. Biochem. Biophys., 352, 165-174. Miyata, T., Inagi, R., Asahi, K., Yamada, Y., Horie, K., Sakai, H., Uchida, K., Kurokawa, K. (1998). Generation of protein carbonyls by glycoxidation and lipoxidation reaction with autoxidation products of ascorbic acid and polyunsaturated fatty acids. FEBS Lett., 437, 24-28. Miyata, T., van Ypersele de Strihou, C., Kurokawa, K., and Baynes, J.W. (1999). Alterations in nonenzymatic biochemistry in uremia: Origin and significance of “carbonyl stress” in long term uremic complications. Kidney Int., 55, 389-399. Moan, J., and Wold, E. (1979). Detection of singlet oxygen production by ESR. Nature, 279, 450-451. Mohr, S., Stamler, J.S., and Brune, B. (1994). Mechanism of covalent modification of glyceraldehyde-3-phosphate dehydrogenase at its active site thiol by nitric oxide, peroxynitrite and related nitrosating agents. FEBS Lett., 348, 223227. Moncada, S., and Higgs, A. (1993). The L-Arginine-nitric oxide pathway. N. Engl. J. Med., 329, 2002-2011. Moncada, S., and Palmer, R.M.J. (1990). The L-Arginine-nitric oxide pathway in the vessel wall. In: Nitric Oxide from LArginine: A Bioregulatory System. Moncada, S., and Higgs, E.A. (Eds.). Elsevier, Amsterdam. Pp. 19-33. Moncada, S., Palmer, R.M.J., and Higgs, E.A. (1991). Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev., 43, 109-141. Monig, J., Asmus, K.D., Forni, L.G., and Willson, R.L. (1997). On the reaction of molecular oxygen with thiyl radicals: A reexamination. Int. J. Radiat. Stud. Phys. Chem. Med., 52, 589-602. Montgomery, J., Ste-Marie, L., Boismenu, D., and Vachon, L. (1995). Hydroxylation of aromatic compounds as indices of hydroxyl radical production: A cautionary note revisited. Free Radic. Biol. Med., 19, 927-933. More, K.P., Darley-Usmar, V., Morrow, J., and Roberts, L.J. (1995). Formation of F2-isoprostanes during oxidation of human low-density lipoprotein and plasma by peroxynitrite. Circ. Res., 77, 335-341. Moreno, J.J., and Pryor, W.A. (1992). Inactivation of α-1-proteinase inhibitor by peroxynitrite. Chem. Res. Toxicol., 5, 425432. Moriel, P., and Abdalla, S. (1997). Nitrotyrosine bound to β-VLDL-apoproteins: A biomarker of peroxynitrite formation in experimental atherosclerosis. Biochem. Biophys. Res. Commun., 232, 332-335. Mortensen, A., Skibsted, L.H., Sampson, J., Rice-Evans, C., and Everett, S.A. (1997). Comparative mechanisms and rates of free radical scavenging by carotenoid antioxidants. FEBS Lett., 24, 91-97. Motohashi, N., and Mori, I. (1989). High-performance liquid chromatography-electrochemical detection of singlet oxygen by reaction with 2,2,6,6-teramethyl-4-piperidon. J. Chromatogr., 465, 417-421. Muijsers, R., Folkerts, G., Henricks, P., Sadeghi-Hashjin, G., and Nijkamp, F. (1997). Peroxynitrite: A two-faced metabolite of nitric oxide. Life Sci., 60, 1833-1845. Muscara, M., and de Nucci, G. (1996). Simultaneous determination of nitrite and nitrate anions in plasma, urine and cell culture supernatants by high-performance liquid chromatography with post-column reactions. J. Chromatogr. B., 686, 157-164. Mustafa, M.G. (1990). Biochemical basis of ozone toxicity. Free Radic. Biol. Med., 9, 245-265. Nair, U.J., Nair, J., Friesen, M.D., Bartsch, H., and Ohshima, H. (1995). Ortho- and meta-tyrosine formation from phenylalanine in human saliva as a marker of hydroxyl radical generation during betel quid chewing. Carcinogen. 16, 1195-1198. Nakamura, K., Ienaga, K., Nakano, K., Nakai, M., Nakamura, Y., Hasegawa, G., Sawada, M., Kondo, M., Mori, H., and Kanatsuna, T. (1996). Diabetic renal failure and serum accumulation of creatinine oxidative metabolites creatol and methylguanidine. Nephron, 73, 520-525. Nakatsubo, N., Kojima, H., Sakurai, K., Kikuchi, K., Nagoshi, H., Hirata, Y., Akaike, T., Maeda, H., Urano, Y., Higuchi, T., and Nagano, T. (1998). Improved nitric oxide detection using 2,3-diaminonaphthalene and its application to the evaluation of novel nitric oxide synthase inhibitors. Biol. Pharm. Bull., 21, 1247-1250. WWW.ESAINC.COM 143 Naqui, A., Chance, B., and Cadenas, E. (1986). Reactive oxygen intermediates in biochemistry. Ann. Rev. Biochem., 55, 137-166. Narayan, M., Berliner, L., Merola, A., Diaz, P., and Clanton, T. (1997). Biological reactions of peroxynitrite: Evidence for an alternative pathway of salicylate hydroxylation. Free Radic. Res., 27, 63-72. Nauseef, W.M., Olsson, I., and Arnljots, K. (1988). Biosynthesis and processing of myeloperoxidase – a marker for myeloid cell differentiation. Eur. J. Haematol., 40, 97-110. Newsholme, E.A., and Leech, A.R. (Eds.). (1992). Biochemistry for the Medical Sciences. John Wiley and Sons, New York. Nguyen, T., Brunson, D., Crespi, C.L., Penman, B.W., Wishnok, J.S. and Tannenbaum, S.R. (1992). DNA damage and mutation in human cells exposed to nitric oxide in vitro. Proc. Natl. Acad. Sci. USA, 89, 3030-3034. NIOSH (1994). Chlorine. Method 6011. Nunoshiba, T., deRojas-Walker, T., Wishnok, J.S., Tannenbaum, S.R., and Demple, B. (1993). Activation by nitric oxide of an oxidative-stress response that defends E. coli against activated macrophages. Proc. Natl. Acad. Sci. USA, 90, 99939997. Okamoto, T., Akaike, T., Negano, T., Miyajima, S., Suga, M., Ando, M., Ichimori, K., and Maeda, H. (1997). Activation of human neutrophil procollagenase by nitrogen dioxide and peroxynitrite: A novel mechanism for procollagenase activation involving nitric oxide. Arch. Biochem. Biophys., 342, 261-274. O’Donnell, V.B., Chumley, P.H., Hogg, N., Bloodsworth, A., Darley-Usmar, V.M., and Freeman, B.A. (1997). Nitric oxide inhibition of lipid peroxidation: Kinetics of reaction with lipid peroxyl radicals and comparison with alpha-tocopherol. Biochem., 36, 15216-15223. Ohshima, H., and Bartsch, H. (1999). Quantitative estimation of endogenous N-nitrosation in humans by monitoring Nnitrosoproline in urine. Meth. Enzymol., 301, 40-49. Olson, L.P., Bartlberger, M.D., and Houk, K.N. (2003). Peroxynitrate and peroxynitrite: A complete basic set onvestigation of similarities and differences between these Nox species. J. Am. Chem. Soc., 125, 3999-4006. Padmaja, S., Squadrito, G.L., and Pryor, W.A. (1998). Inactivation of glutathione peroxidase by peroxynitrite. Arch. Biochem. Biophys., 349, 1-6. Palacois, M., Knowles, R.G., Palmer, R.M.J., and Moncada, S. (1989). Nitric oxide from L-arginine stimulates the soluble guanylate cyclase in adrenal glands. Biochem. Biophys. Res. Comm., 165, 802-809. Palumbo, G., Carlucci, G., Mazzeo, P., Frieri, G., Pimpo, M.T. and Fanini, D. (1995). Simultaneous determination of 5aminosalicylic acid, acetyl-5-aminosalicylic acid and 2,5-dihydroxybenzoic acid in endoscopic intestinal biopsy samples in humans by high-performance liquid chromatography with electrochemical detection. J. Pharmaceut. Biomed. Anal. 14, 175-180. Panasenko, O.M., Briviba, K., Klotz, L.O., and Sies, H. (1997a). Oxidative modification and nitration of human low-density lipoproteins by the reaction of hypochlorous acid with nitrite. Arch. Biochem. Biophys., 343, 254-259. Panasenko, O.M., Panasenko, O.O., Briviba, K., and Sies, H. (1997b). Hypochlorite destroys carotenoids in low density lipoproteins thus decreasing their resistance to peroxidative modification. Biochem. (Mosc.), 62, 1140-1145. Payne, W.J., Le Gall, J., and Berlier, Y. (1996). Use of mass spectrometry for the detection of nitrogen oxides. In: Methods in Nitric Oxide Research. Feelisch, M., and Stamler, J.S. (Eds.). John Wiley and Sons Ltd., London. Pp. 393402. Pfeiffer, S., Schrammel, A., Schmidt, K., and Mayer, B. (1998). Electrochemical determination of S-nitrosothiols with a Clark-type nitric oxide electrode. Anal. Biochem., 258, 68-73. Pietraforte, D., and Minetti, M. (1997a). One-electron oxidation pathway of peroxynitrite decomposition in human blood plasma: Evidence for the formation of protein tryptophan-centered radicals. Biochem. J., 321, 743-750. Pietraforte, D., and Minetti, M. (1997b). Direct ESR detection of peroxynitrite-induced tyrosine-centered protein radicals in human blood plasma. Biochem. J., 325, 675-684. Pou, S., Nguyen, S., Gladwell, T., and Rosen, G. (1995). Does peroxynitrite generate hydroxyl radical? Biochim. Biophys. Acta, 1244, 62-68. Powis, G., Briehl, M., and Oblong, J. (1995). Redox signaling and the control of cell growth and death. Pharmacol. Ther. 68, 149-173. Pratt, P.F., Nithipatikom, K., and Campbell, W.B. (1995). Simultaneous determination of nitrate and nitrite in biological samples by multichannel flow injection analysis. Anal. Biochem., 231, 383-386. Preik-Steinhoff, H., and Kelm, M. (1996). Determination of nitrite in human blood by combination of a specific sample preparation with high-performance anion exchange chromatography and electrochemical detection. J. Chromatogr. B., 685, 348-352. Pryor, J.J., Jin, X., and Squadrito, G.L. (1994). One- and two-electron oxidations of methionine by peroxynitrite. Proc. Natl. Acad. Sci. USA, 91, 11173-11177. Pryor, W.A. (1986). Oxy-radicals and related species: Their formation lifetimes, and reactions. Annu. Rev. Physiol., 48, 657-663. Pryor, W.A., (1993). Ozone in all its reactive splendor. J. Lab. Clin. Med., 122, 483-486. Pryor, W.A. (1994). Mechanisms of radical formation from reactions of ozone with target molecules in the lung. Free Radic. Biol. Med., 17, 451-465. Pryor, W.A., and Church, D.F. (1991). Aldehydes, hydrogen peroxide and organic radicals as mediators of ozone toxicity. Free Radic. Biol. Med., 11, 41-46. Pryor, W.A., and Squadrito, G.L. (1995). The chemistry of peroxynitrite: a product from the reaction of nitric oxide with superoxide. Am. J. Physiol., 268, L699-L722. Pryor, W.A., Squadrito, G.L., and Friedman, M. (1995). The cascade mechanism to explain ozone toxicity: The role of lipid ozonation products. Free Radic. Biol. Med., 19, 935-941. Pryor, W.A., Stanley, J.P., and Blair, E. (1976). Autoxidation of polyunsaturated fatty acid: II. A suggested mechanism for the formation of TBA-reactive materials from prostaglandin-like endoperoxides. Lipids, 11, 370-379. WWW.ESAINC.COM 144 Qian, S.Y., and Buettner, G.R. (1999). Iron and dioxygen chemistry is an important route to initiation of biological free radical oxidations: An electron paramagnetic resonance spin trapping study. Free Radic. Biol. Med., 26, 1447-1456. Quijano, C., Alvarez, B., Gatti, R., Augusto, O., and Radi, R. (1997). Pathways of peroxynitrite oxidation of thiol groups. Biochem. J., 322, 167-173. Radi, R. (1996). Reactions of nitric oxide with metalloproteins. Chem. Res. Toxicol., 9, 828-835. Radi, R., Beckman, J.S., Bush, K.M., and Freeman, B.A. (1991a). Peroxynitrite-mediated sulfhydryl oxidation: The cytotoxic potential of superoxide and nitric oxide. J. Biol. Chem., 266, 4244-4250. Radi, R., Beckman, J.S., Bush, K.M., and Freeman, B.A. (1991b). Peroxynitrite-induced membrane lipid peroxidation: The cytotoxic potential of superoxide and nitric oxide. Arch. Biochem. Biophys., 288, 481-487. Radi, R., Denicola, A., and Freeman, B.A. (1999). Peroxynitrite reactions with carbon dioxide-bicarbonate. Meth. Enzymol., 301, 353-367. Radzik, D.M., Roston, D.A., and Kissinger, P.T. (1983). Determination of hydroxylated aromatic compounds produced via superoxide-dependent formation of hydroxyl radicals by liquid chromatography/electrochemistry. Anal. Biochem. 131, 458-464. Ralston, S.H. (1997). The Michael Mason Prize Essay 1997. Nitric oxide and bone: what a gas! Br. J. Rheumatol., 36, 831-838. Ramezanian, M.S., Padmaja, S., and Koppenol, W.H. (1996). Nitration and hydroxylation of phenolic compounds by peroxynitrite. Chem. Res. Toxicol. 9, 232-240. Rapaport, R.M., and Murad, F. (1983). Agonist induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cyclic GMP. Circ. Res., 52, 352-357. Rauth, A.M., Goldberg, Z., and Misra, V. (1997). DT-diaphorase: Possible roles in cancer chemotherapy and carcinogenesis. Oncol. Res., 9, 339-349. Reist, M., Marshall, K.-A., Jenner, P., and Halliwell, B. (1998). Toxic effects of sulphite in combination with peroxynitrite on neuronal cells. J. Neurochem., 71, 2431-2438. Riley, D.P., Rivers, W.J., and Weiss, R.H. (1991). Stopped-flow kinetic analysis for monitoring superoxide decay in aqueous systems. Anal. Biochem., 196, 344-349. Rivot, J.P., Barraud, J., Montecot, C., Jost, B., and Besson, J.M. (1997). Nitric oxide (NO): In vivo electrochemical monitoring in the dorsal horn of the spinal cord of the rat. Brain Res., 773, 66-75. Robinson, J.M., and Badwey, J.A. (1995). The NADPH oxidase complex of phagocytic leukocytes: A biochemical and cytochemical view. Histochem., 103, 163-180. Rohn, T.T., Nelson, L.K., Davis, A.R., and Quinn, M.T. (1999). Inhibition of GTP binding to Rac2 by peroxynitrite: Potential role for tyrosine modification. Free Radic. Biol. Med., 26, 1321-1331. Roy, D., Floyd, R.A., and Liehr, J.G. (1991). Elevated 8-hydroxydeoxyguanosine levels in DNA of diethylstilbestrol-treated Syrian hamsters: Covalent DNA damage by free radicals generated by redox cycling of diethylstilbestrol. Cancer Res., 51, 3882-3885. Rubanyi, G., Ho, E.H., Cantor, E.H., Lumma, W.C., and Parker Botelho, L.H. (1991). Cytoprotective function of nitric oxide: Inactivation of superoxide radicals produced by human leukocytes. Biochem. Biophys. Res. Commun., 181, 1392-1397. Rubanyi, G.M., Lorenz, R.R., and Vanhoutte, P.M. (1985). Bioassay of endothelium-derived relaxing factor(s): inactivation by catecholamines. Am. J. Physiol., 249, H95-H101. Rubbo, H., Darley-Usmar, V., and Freeman, B. (1996). Nitric oxide regulation of tissue free radical injury. Chem. Res. Toxicol., 9, 809-820. Rubbo, H., Radi, R., Trujillo, M., Telleri, R., Kalyanaraman, B., Barnes, S., Kirk, M., and Freeman, B.A. (1994). Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. J. Biol. Chem., 269, 26066-26075. Runeckles, V.C. (1994). The impact of UV-B radiation and ozone on terrestrial vegetation. Environ. Pollut., 83, 191-213. Sachs, C.H. and Johnson, G. (1975). Mechanisms of action of 6-hydroxydopamine. Pharmacol., 24, 1-8. Saez, G., Thornalley, P.J., Hill, H.A., Hems, R., and Bannister, J.V. (1982). The production of free radicals during the autoxidation of cysteine and their effect in isolated rat hepatocytes. Biochim. Biophys. Acta, 719, 24-31. Sagone, A.L., Decker, M.A., Wells, R.M., and De Mocko, C. (1980). A new method for the detection of hydroxyl radical production by phagocytic cells. Biochim. Biophys. Acta, 628, 90-97. Saha, A., Goldstein, S., Cabelli, D., and Czapski, G. (1998). Determination of optimal conditions for the synthesis of peroxynitrite by mixing acidified hydrogen peroxide with nitrite. Free Radic. Biol. Med., 24, 653-659. Sakuma, S., Fujimoto, Y., Sakamoto, Y., Uchiyama, T., Yoshioka, K., Nishida, H., and Fujita, T. (1997). Peroxynitrite induces the conversion of xanthine dehydrogenase to oxidase in rabbit liver. Biochem. Biophys. Res., Commun., 230, 476-479. Saleh, D., Barnes, P., and Giaid, A. (1997). Increased production of the potent oxidant peroxynitrite in the lungs of patients with idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med., 155, 1763-1769. Salerno, J.C. (1996), Nitric oxide complexes of metalloproteins: An introductory overview. In: Nitric Oxide: Principles and Actions. Lancaster, J. (Ed.). Academic Press, New York. Pp. 83-110. Salter, M., Duffy, C., Garthwaite, J., and Strijbos, J.L.M., (1996). Ex vivo measurement of brain tissue nitrite and nitrate accurately reflects nitric oxide synthase activity in vivo. J. Neurochem., 66, 1683-1690. Samouilov, A., and Zweier, J.L. (1998). Development of chemiluminescence-based methods for specific quantitation of nitrosylated thiols. Anal. Biochem., 258, 322-330. Saran, M., Beck-Speier, I., Fellerhoff, B., and Bauer, G. (1999). Phagocytic killing of microorganisms by radical processes: Consequences of the reaction of hydroxyl radicals with chloride yielding chlorine atoms. Free Radic. Biol. Med., 26, 482-490. Sarkar, B. (1995). Metal replacement in DNA-binding zinc finger proteins and its relevance to mutagenicity and carcinogenicity through free radical generation. Nutrition, 11, 646-649 WWW.ESAINC.COM 145 Satoh, S., Kimura, T., Toda, M., Maekawa, M., Ono, S., Narita, H., Miyazaki, H., Murayama, T., and Nomura, Y. (1997). Involvement of L-type amino acid transporters in S-nitrosocysteine-stimulated noradrenaline release in the rat hippocampus. J. Neurochem., 69, 2197-2205. Sawyer, D.T., Hage, J.P., and Sobkowiak, A. (1995). Iron(II)-induced activation of 1:1 HOOH/HCl for the chlorohydroxylation of olefins and the chlorination of hydrocarbons: Chlorinated Fenton chemistry. J. Am. Chem. Soc., 117, 106-109. Sayre, L.M., Arora, P.K., Iyer, R.S., and Salomon, R.G. (1993). Pyrrole formation from 4-hydroxynonenal and primary amines. Chem. Res. Toxicol., 6, 19-22. Sayre, L.M., Sha, W., Xu, G., Kaur, K., Nadkarni, D., Subbanagounder, G., and Salomon, R.G. (1996). Immunochemical evidence supporting 2-pentylpyrrole formation on proteins exposed to 4-hydroxy-2-nonenal. Chem. Res. Toxicol., 9, 1194-1201. Schmidt, H., and Kelm, M. (1996). Determination of nitrite and nitrate by the Greiss reaction. In: Methods in Nitric Oxide Research. Feelisch, M., and Stamler, J.S. (Eds.). John Wiley and Sons Ltd., London. Pp. 491-497. Schmidt, H., Hofmann, H., Schindler, U., Shutenko, Z., S., Cunningham, D.D., and Feelisch, M. (1996). NO NO from NO synthase. Proc. Natl. Acad. Sci. USA, 93, 14492-14497. Schneider, J.E., Browning, M.M., Zhu, X., Eneff, K.L. and Floyd, R.A. (1989). Characterization of hydroxyl mediated damage to plasmid pBR322 DNA. Mutation Res,. 214, 23-31. Schuman, E.M., and Madison, D.V. (1991). A requirement for the intercellular messenger nitric oxide in long-term potentiation. Science, 254, 1503-1506. Scorza, G., and Minetti, M. (1998). One-electron oxidation pathway of thiols by peroxynitrite in biological fluids: Bicarbonate and ascorbate promote the formation of albumin disulfide dimers in human blood plasma. Biochem. J., 15, 329, 405-413. Sevanian, A., McLeod, L.L. (1987). Cholesterol autoxidation in phospholipid membrane bilayers. Lipids, 22, 627-636. Shibuki, K. (1990). An electrochemical microprobe for detecting nitric oxide release in brain tissue. Neurosci. Res., 9, 6976. Shintani, F., Kinoshita, T., Kanba, S., Ishikawa, T., Suzuki, E., Sasakawa, N., Kato, R., Asai, M., and Nakaki, T. (1996). Bioactive 6-nitronorepinephrine identified in mammalian brain. J. Biol. Chem., 271, 13561-13565. Shoaf, A.R., Shaikh, A.U., Harbison, R.B., and Hinojosa, O. (1991). Extraction and analysis of superoxide free radicals from whole mammalian liver. J. Biolumin. Chemilumin., 6, 87-96. Sies, H., Sharov, V.S., Klotz, L.O., and Briviba, K. (1997). Glutathione peroxidase protects against peroxynitrite-mediated oxidations: A new function for selenoproteins as peroxynitrite reductase. J. Biol. Chem., 272, 27812-27817. Singel, D.J., and Lancaster, J.R. (1996). Electron paramagnetic resonance spectroscopy and nitric oxide biology. In: Methods in Nitric Oxide Research. Feelisch, M., and Stamler, J.S. (Eds.). John Wiley and Sons Ltd., London. Pp. 342356. Singh, A., Singh, H., Kremers, W., and Koroll, G.W. (1981). Involvement of singlet oxygen in biochemical systems. Bull. Eur. Physiopath. Resp., 17, 31-41. Singh, R.J., Hogg, N., Joseph, J., and Kalyanaraman, B. (1996). Mechanisms of nitric oxide release from S-nitrosothiols. J. Biol. Chem., 271, 18596-18603. Skinner, K.A., Wang, Z., and Parks, D.A. (1996). Detection of salicylate nitration and hydroxylation products after liver preservation. The Third Annual Meeting of The Oxygen Society, Florida. Poster #2-39. Skinner, K.A., White, C.R., Patel, R., Tan, S., Barnes, S., Kirk, M., Darley-Usmar, V., and Parks, D.A. (1998). Nitrosation of uric acid by peroxynitrite. Formation of a vasoactive nitric oxide donor. J. Biol. Chem., 273, 244491-24497. Sladek, N.E. (1987). Metabolism and action of oxazaphosphorines. In: Metabolism and Action of Anticancer Drugs. Powis, G., and Prough, R.A. (Eds.). Taylor and Francis, Washington, DC. Pp. 48-90. Sloot, W.N., and Gramsbergen, J.B.P. (1995). Detection of salicylate and its hydroxylated adducts 2,3- and 2,5dihydroxybenzoic acid as possible indices for in vivo hydroxyl radical formation in combination with catechol- and indoleamines and their metabolites in CSF and brain tissue. J. Neuroscience Meth., 60, 141-149. Smith, L.L. (1987). Cholesterol autoxidation 1981-1986. Chem. Phys. Lipids, 44, 87-125. Smith, M.A., Richey Harris, P., Sayre, L., Beckman, J., and Perry, G. (1997). Widespread peroxynitrite-mediated damage in Alzheimer’s disease. J. Neuroscience, 17, 2653-2657. Snyder, S.H., and Bredt, D.S. (1992). Biological roles of nitric oxide. Scientific American, May, 68-77. Sontag, G., Bernweiser, I., and Krach, C. (1997). HPLC with electrode array detection and its application in analytical food chemistry. In: Coulometric Electrode Array Detectors for HPLC. Acworth, I.N., Naoi, M., Parvez, S., and Parvez H. (Eds.). Progress in HPLC-HPCE 6, VSP Publications, The Netherlands. Pp. 75-126. Souza, J.M., and Radi, R. (1998). Glyceraldehyde-3-phosphate dehydrogenase inactivation by peroxynitrite. Arch. Biochem. Biophys., 360, 187-194. Spencer, J.P., Whiteman, M., Jenner, A., and Halliwell, B. (2000). Nitrite-induced deamination and hypochlorite-induced oxidation of DNA in intact human respiratory tract epithelial cells. Free Radic. Biol. Med., 28, 1039-1050. Stamler, J.S. (1994). Redox signaling: Nitrosylation and related target interactions of nitric oxide. Cell, 78, 931-936. Stamler, J.S., and Feelisch., M. (1996). Preparation and detection of S-nitrosothiols. In: Methods in Nitric Oxide Research. Feelisch, M., and Stamler, J.S. (Eds.). J. Wiley and Sons Ltd., New York. Pp. 521-539. Stamler, J.S., and Slivka, A. (1996). Biological chemistry of thiols in the vasculature and in vascular-related disease. Nutr. Rev., 54, 1-30. Stamler, J.S., Jaraki, O., Osborne, J., Simon, D.I., Keaney, J., Vita, J., Singel, D., Valeri, C.R., and Loscalzo, J. (1992). Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc. Natl. Acad. Sci. USA, 89, 7674-7677. Stamler, J.S., Singel, D.J., and Loscalzo, J. (1992). Biochemistry of nitric oxide and its redox-activated forms. Science, 258, 1898-1902. Stanbury, D.M. (1989). Reduction potentials involving inorganic free radicals in aqueous solution. Adv. Inorg. Chem., 33, 69-138. WWW.ESAINC.COM 146 Stoyanovsky, D.A., Melnikov, Z., and Cederbaum, A.I. (1999). ESR and HPLC-EC analysis of the interaction of hydroxyl radical with DMSO: Rapid reduction and quantification of POBN and PBN nitroxides. Anal. Chem., 71, 715-721. Strehlitz, B., Grundig, B., Schumacher, W., Kroneck, P., Vorlop, K.-D., and Kotte, H. (1996). A nitrite sensor based on a highly sensitive nitrite reductase mediator-coupled amperometric detection. Anal. Chem., 68, 807-816. Ste-Marie, L., Boismenu, D., Vachon, C., and Montgomery, J. (1996). Evaluation of sodium 4-hydroxybenzoate as an hydroxyl radical trap using gas chromatography-mass spectrometry and high-performance liquid chromatography with electrochemical detection. Anal. Biochem. 241, 67-74. Steuhr, D.J., and Marletta, M.A. (1985). Mammalian nitrate biosynthesis: Mouse macrophages produce nitrite and nitrate in response to E. coli lipopolysaccharide. Proc. Natl. Acad. Sci. USA, 82, 7738-7742. Stratford, M.R.L. (1999). Measurement of nitrite and nitrate by high-performance ion chromatography. Meth. Enzymol., 301, 259-269. Stronks, H.J., Janzen, E.G., and Weber, R.J. (1984). Electrochemical detection of spin adduct aminoxyls (nitroxides) separated by high performance liquid chromatography. Anal. Letts. 17, 321-328. Sucher, N.J., and Lipton, S.A. (1991). Redox modulatory site of the NMDA receptor-channel complex: Regulation by oxidized glutathione. J. Neurosci. Res., 30, 582-591. Suzaki, E., Kawai, E., Kodama, Y., Suzaki, T., and Masujima, T. (1994). Quantitative analysis of superoxide anion generation in living cells using chemiluminescence video microscopy. Biochim. Biophys Acta, 1201, 328-332. Szabo, C. (1996). The pathophysiological role of peroxynitrite in shock, inflammation, and ischemia-reperfusion injury. Shock, 6, 79-88. Szabo, C., Cuzzocrea, S., Zingarelli, B., O’Connor, M., and Salzman, A. (1997). Endothelial dysfunction in a rat model of endotoxic shock. J. Clin. Invest., 100, 723-735. Tabatabaei, A.R., and Abbott, F.S. (1999). LC/MS analysis of hydroxylation products of salicylate as an indicator of in vivo oxidative stress. Free Radic. Biol. Med., 26, 1054-1058. Takahashi, K., Hara, E., Ogawa, K., Kimura, D., Fujita, H., and Shibahara, S. (1997). Possible implications of the induction of human heme oxygenase-1 by nitric oxide donors. J. Biochem., 121, 1162-1168. Tamir, S., Burney, S., and Tannebaum, S.R. (1996). DNA damage by nitric oxide. Chem. Res. Toxicol., 9, 821-827. Tasker, H.S., and Jones, H.Q. (1909). The action of mercaptans on acid chlorides. Part II. The acid chlorides of phosphorus, sulfur, and nitrogen. J. Chem. Soc., 95, 1910-1912. Tatoyan, A., and Giulivi, C. (1998). Purification and characterization of nitric-oxide synthase from rat liver mitochondria. J. Biol. Chem., 273, 11044-11048. Tatsuma, T., Gondaira, M., and Watanabe, T. (1992). Peroxidase-incorporated polypyrrole membrane electrodes. Anal. Chem., 64, 1183-1187. Tatsuma, T., Watanabe, T., Tatsuma, S., and Watanabe, T. (1994). Substrate-purging enzyme electrodes. Peroxidase/catalase electrodes for H2O2 with an improved upper sensing limit. Anal. Chem., 66, 290-294. Thiele, J.J., Traber, M.G., Tsang, K., Cross, C.E., and Packer, L. (1997). In vivo exposure to ozone depletes vitamins C and E and induces lipid peroxidation in epidermal layers of murine skin. Free Radic. Biol. Med., 23, 385-391. Thomas, E.L., Jefferson, M.M., and Grisham, M.B. (1982). Myeloperoxidase-catalyzed incorporation of amines into proteins: Role of hypochlorous acid and dichloramines. Biochem., 21, 6299-6308. Thornalley, P., wolff, S., Crabbe, J., and Stern, A. (1984). The autoxidation of glyceraldehyde and other simple monosaccharides under physiological conditions catalyzed by buffer ions. Biochim. Biophys. Acta, 797, 276-287. Tomoda, A., Takizawa, T., Tsuji, A., and Yoneyama, Y. (1981). Kinetic analysis of myoglobin autoxidation by isoelectricfocusing electrophoresis. Biochem. J., 193, 181-185. Tournaire, C., Croux, S., Maurette, M.T., Beck, I., Hocquaux, M., Braun, A.M., and Oliveros, E. (1993). Antioxidant activity of flavonoids: Efficiency of singlet oxygen (1 delta g) quenching. J. Photochem. Photobiol. B., 19, 205-215. Towel, J., and Kalyanaraman, B. (1991). Detection of radical adducts of 5,5-dimethyl-1-pyrroline N-oxide by the combined use of high-performance liquid chromatography with electrochemical detection and electron spin resonance. Anal. Biochem., 196, 111-119. Tsikas, D., Junker, W., and Frolich, J.C. (1998). Determination of dimethylated arginines in human plasma by highperformance liquid chromatography. J. Chromatogr. B, 705, 174-176. Uchida, K., and Kawakishi, S. (1990). Site-specific oxidation of angiotensin 1 by copper(II) and L-ascorbate: Conversion of histidine into 2-imidazolones. Arch. Biochem. Biophys., 283, 20-26. Uchida, K., and Kawakishi, S. (1993). 2-Oxohistidine as a novel biological marker for oxidatively modified proteins. FEBS Lett., 332, 208-210. Uchida, K., and Kawakishi, S. (1994). Identification of oxidized histidine generated at the active site of Cu, Zn-superoxide dismutase exposed to H2O2. J. Biol. Chem., 269, 2405-2410. Upchurch, G.R., Welch, G.N., and Loscalzo, L. (1995). S-Nitrosothiol: Chemistry, biochemistry and biological actions. Adv. Pharmacol., 34, 343-349. Uppa, R.M., and Pryor, W.A. (1996). Carbon dioxide: Catalysis of the reaction of peroxynitrite with ethyl acetoacetate: An example of aliphatic nitration by peroxynitrite. Biochem. Biophys. Res. Commun., 229, 764-769. Uppa, R.M., Lemercier, J.N., Squadrito, G.L., Zhang, H., Bolzan, R.M., and Pryor, W.A. (1998). Nitrosation by peroxynitrite: Use of phenol as a probe. Arch. Biochem. Biophys., 358, 1-16. Uppa, R.M., Squadrito, G.L., Cueto, R., and Pryor, W.A. (1996). Selecting the most appropriate synthesis of peroxynitrite. Meth. Enzymol., 269, 285-295. Uppa, R.M., Squadrito, G.L., and Pryor, W.A. (1996). Acceleration of peroxynitrite oxidations by carbon dioxide. Arch. Biochem. Biophys., 327, 335-343. van Dalen, C.J., Whitehouse, M.W., Winterbourn, C.C., and Kettle, A.J. (1997). Thiocyanate and chloride as competing substrates for myeloperoxidase. Biochem. J., 327, 487-492. Van der Veen, R., Hinton, D., Incardonna, F., and Hofman, F. (1997). Extensive peroxynitrite activity during progressive stages of central nervous system inflammation. J. Neuroimmunol., 77, 1-7. WWW.ESAINC.COM 147 van der Vliet, A., Eiserich, J.P., Halliwell, B., and Cross, C.E. (1997). Formation of reactive nitrogen species during peroxidase-catalyzed oxidation of nitrite. J. Biol. Chem., 272, 7617-7625. van der Vliet, A., Eiserich, J.P., O’Niell, C.A., Halliwell, B., and Cross, C.E. (1995). Tyrosine modification by reactive nitrogen species: A closer look. Arch. Biochem. Biophys., 319, 341-349. van der Vliet, A., O’Neill, C.A., Halliwell, B., Cross, C.E., and Kaur, H. (1994). Aromatic hydroxylation and nitration of phenylalanine and tyrosine by peroxynitrite. FEBS Letts., 339, 89-92. van der Vleit, A., O’Neill, C.A., Eiserich, J.P., and Cross, C.E. (1995). Oxidative damage to extracellular fluids by ozone and possible protective effects of thiols. Arch. Biochem. Biophys., 321, 43-50. van der Vleit, A., t’Hoen, P.A., Wong, P.S., Bast, A., and Cross, C.E. (1998). Formation of S-nitrosothiols via direct nucleophilic nitrosation of thiols by peroxynitrite with elimination of hydrogen peroxide. J. Biol. Chem., 273, 3025530262. van Heusden, S., and Mans, L.G. (1978). Alternating measurement of ambient and cabin ozone concentrations in commercial jet aircraft. Aviat. Space Environ. Med., 49, 1056-1061. Varma, S.D. (1989). Radio-isotopic determination of sub-nanomolar amounts of peroxides. Free Rad. Res. Commun., 5, 359-368. Vasela, A., and Wilhelm, J. (2002). The role of carbon dioxide in free radical reactions of the oganism. Physiol. Res., 51, 335-339. Vasquez-Vivar, J., and Augusto, O. (1992). Hydroxylated metabolites of the antimalarial drug primaquine. Oxidation and redox cycling. J. Biol. Chem., 267, 6848-6854. Vasquez-Vivar, J., Santos, A.M., Junqueira, V.B., and Augusto, O. (1996). Peroxynitrite-mediated formation of free radicals in human plasma: EPR detection of ascorbyl, albumin-thiyl and uric acid-derived free radicals. Biochem. J., 314, 869-876. Vasquez-Vivar, J., Kalyanaraman, B., Martasek, P., Hogg, N., Masters, B.S., Karoui, H., Tordo, P., and Pritchard, K.A. (1998). Superoxide generation by endothelial nitric oxide synthase: The influence of cofactors. Proc. Natl. Acad. Sci. USA, 95, 9220-9225. Viner, R.I., Huhmer, A.F., Bigelow, D.J., and Schoneich, C. (1996). The oxidative inactivation of sarcoplasmic reticulum calcium-ATPase by peroxynitrite. Free Radic. Res., 24, 243-259. Vissers, M.C., and Winterbourne, C.C. (1991). Oxidative damage to fibronectin. II. The effects of H2O2 and the hydroxyl radical. Arch. Biochem. Biophys., 285, 357-364. Vogt, W. (1995). Oxidation of methionyl residues in proteins: Tools, targets, and reversal. Free Radic. Biol. Med., 18, 6173. Vukomanovic, D.V., Hussain, A., Zoutman, D.E., Marks, G.S., Brien, J.F., and Nakatsu, K. (1998). Analysis of nanomolar S-nitrosothiol concentrations in physiological media. J. Pharmacol. Toxicol. Meth. 39, 235-240. Wade, R., and Castro, C. (1990). Redox reactivity of iron III porphyrins and heme proteins with nitric oxide. Nitrosyl transfer to carbon, oxygen, nitrogen and sulfur. Chem. Res. Toxicol., 3, 289-291. Wagner, J.R., Hu, C.-C., and Ames, B.N. (1992). Endogenous oxidative damage of deoxycytidine in DNA. Proc. Natl. Acad. Sci. USA, 89, 3380-3384. Wang, S.S., and Maher, T.J. (1992). Changes in hippocampal ECF amino acid levels in anesthetized rats during kainic acid-induced seizures: A microdialysis study. Pharmacologist, 34, 190. Wardman, P., and Candeias, L.P. (1996). Fenton Chemistry: An introduction. Rad. Res., 145, 523-531. Wefers, H., and Sies, H. (1983). Oxidation of glutathione by superoxide radical to the disulfide and the sulfonate yielding singlet oxygen. Eur. J. Biochem., 137, 29-36. Weil, L., and Morris, J.C. (1949). Kinetic studies on the chloramines. I. The rates of formation of monochloramine, Nchlormethylamine, and N-chlordimethylamine. J. Am. Chem. Soc., 71, 1664-1671. Weiss, S.J., and Slivka, A. (1982a). Monocyte and granulocyte-mediated tumor cell destruction. A role for the hydrogen peroxide-myeloperoxidase-chloride system. J. Clin. Invest., 69, 255-262. Weiss, S.J., and Ward, P.A. (1982). Immune complex induced generation of oxygen metabolites by human neutrophils. J. Immunol., 129, 309-313. Weiss, S.J., Klein, R., Slivka, A., and Wei, M. (1982). Chlorination of taurine by human neutrophils: Evidence for hypochlorous acid generation. J. Clin. Invest., 70, 598-607. Weiss, S.J., Lampert, M.B., and Test, S.T. (1983). Long-lived oxidants generated by human neutrophils: Characterization and bioactivity. Science, 222, 625-628. Weiss, S.J., Test, S.T., Eckmann, C.M., Roos, D., and Regiani, S. (1986). Brominating oxidants generated by human eosinophils. Science, 234, 200-203. White, R.E., and Coon, M.J. (1980). Oxygen activation by cytochrome P-450. Ann. Rev. Biochem 49, 315-356. Whiteman, M., and Halliwell, B. (1996). Protection against peroxynitrite-dependent tyrosine nitration and α1-antiproteinase inactivation by ascorbic acid. A comparison with other biological antioxidants. Free Radic. Res., 25, 275-283. Whiteman, M., Siau, J.L., and Halliwell, B. (2003). Lack of tyrosine nitration by hypochlorous acid in the presence of physiological concentrations of nitrite. Implication for the role of nitryl chloride in tyrosine nitration in vivo. J. Biol. Chem., 278, 8380-8384. Wink, D.A., Kasprzak, K.S., Maragos, C.M., Elespuru, R.K., Misra, M., Dunams, T.M., Cebula, T.A., Koch, W.H., Andrews, A.W., and Allen, J.S. (1991). DNA deaminating ability and genotoxicity of nitric oxide and its progenitors. Science, 254, 1001-1003. Wink, D.A., Kim, S., Coffin, D., Cook, J.C., Vodovotz, Y., Chistodoulou, D., Jourd’heuil, D., and Grisham, M.B. (1999). Detection of S-nitrosothiols by fluorometric and colorimetric methods. Meth. Enzymol., 301, 201-211. Winterbourn, C.C., Vandenberg, J.J.M., Roitman, E., and Kuypers, F.A. (1992). Chlorohydrin formation from unsaturated fatty acids reacted with hypochlorous acid. Arch. Biochem. Biophys., 296, 547-555. Wolff, S.P., and Dean, R.T. (1987). Glucose autoxidation and protein modification. The potential role of autoxidative glycosylation in diabetes. Biochem. J. 245, 243-250. Wolmann, M. (1975). Autoxidation in vivo. Isr. J. Med. Sci., 15, 30-57. WWW.ESAINC.COM 148 Wright, C.D., Hattori, R., Kosuga, K., Eizawa, H., Hiki, K., and Kawai, C. (1991). Purification of nitric oxide synthase from rat macrophages. Biochim. Biophys. Acta, 160, 813-819. Wu, W., Chen, Y., d’Avignon, A., and Hazen, S.L. (1999). 3-Bromotyrosine and 3,5-dibromotyrosine are major products of protein oxidation by eosinophil peroxidase: Potential markers for eosinophil-dependent tissue injury in vivo. Biochem., 38, 3538-3548. Wyllie, S., and Liehr, J.G. (1997). Release of iron from ferritin storage by redox cycling of stilbene and steroid estrogens metabolites: A mechanism of induction of free radical damage by estrogens. Arch. Biochem. Biophys., 346, 180-186. Xia, Y., Roman, L.J., Masters, B.S., and Zweier, J.L. (1998). Inducible nitric oxide synthase generates superoxide form the reductase domain. J. Biol. Chem., 273, 22635-22639. Xu, G., and Sayre, L.M. (1998). Structural characterization of a 4-hydroxy-2-nonenal-derived fluorophore that contributes to lipoperoxidation-dependent protein cross-linking in aging and degenerative diseases. Chem. Res. Toxicol., 11, 247251. Yager, J.D., and Liher, J.G. (1996). Molecular mechanisms of estrogen carcinogenesis. Ann. Rev. Pharmacol. Toxicol., 36, 203-232. Yamada, K., and Nabeshima, T. (1997). Simultaneous measurement of nitrite and nitrate levels as indices of nitric oxide release in the cerebellum of conscious rats. J. Neurochem., 68, 1234-1243. 2+ Yamazaki, I., and Piette, L.H. (1990). ESR spin-trapping studies on the reaction of Fe ions with H2O2-reactive species in oxygen toxicity in biology. J. Biol. Chem., 265, 13589-13594. Yamamoto, Y., and Ames, B.N. (1987). Detection of lipid hydroperoxides and hydrogen peroxide at picamole levels by an HPLC and isoluminol chemiluminescence assay. Free Radic. Biol. Med., 3, 359-361. Yao, S.J., Xu, W., and Wolfson, S.K. (1995). A micro carbon electrode for nitric oxide monitoring. ASAIO J., 41, M404409. Yermilov, V., Rubio, J., Becchi, M., Friesen, M.D., Pignatelli, B., and Ohshima, H. (1995). Formation of 8-nitroguanine with peroxynitrite in vitro. Carcinogen., 16, 2045-2050. Yermilov, V., Rubio, J., and Ohshima, H. (1995). Formation of 8-nitroguanine in DNA treated with peroxynitrite in vitro and its rapid removal from DNA by depurination. FEBS Lett., 376, 207-210. Yermilov, V., Yoshie, Y., Rubio, J., and Ahshima, H. (1996). Effects of carbon dioxide/bicarbonate on induction of DNA single-strand breaks and formation of 8-nitroguanine, 8-oxoguanine and base-propenal mediated by peroxynitrite. FEBS Lett., 399, 67-70. Yokoyama, H., Kasai, N., Ueda, Y., Niwa, R., Konaka, R., Mori, N., Tsuchihashi, N., Matsue, T., Ohya-Nishiguchi, H., and Kamada, H. (1998). In vivo analysis of hydrogen peroxide and lipid radicals in the striatum of rats under long-term administration of a neuroleptic. Free Radic. Biol. Med., 24, 1056-1060. Yokozawa, T., Fujitsuka, N., Oura, H., Ienaga, K., and Nakamura, K. (1997). In vivo effect of hydroxyl radical scavenger on methylguanidine production from creatinine. Nephron, 75, 103-105. Zhai, X., and Ashraf, M. (1995). Direct detection and quantification of singlet oxygen during ischemia and reperfusion in rat hearts. Am. J. Physiol., 269, H1229-H1236. Zhang, H., Squadrito, G.L., Uppa, R.M., Lemercier, J.-N., Cueto, R., and Pryor, W.A. (1997). Inhibition of peroxynitritemediated oxidation of glutathione by carbon dioxide. Arch. Biochem. Biophys., 339, 183-189. Zou, L., Gunn, C., and Beckman, J.S. (1992). Bactericidal activity of peroxynitrite. Arch. Biochem. Biophys., 298, 452-457. Zingaerlli, B., Day, B.J., Crapo, J.D., Salzman, A.L., and Szabo, C. (1997). The potential role of peroxynitrite in the vascular contractile and cellular energetic failure in endotoxic shock. Br. J. Pharmacol., 129, 259-267. Zingaerlli, B., O’Connor, M., Wong, H., Salzman, A., and Szabo, C. (1996). Peroxynitrite-mediated DNA strand breakage activates poly-adenosine diphosphate ribosyl synthetase and causes cellular energy depletion in macrophages stimulated with bacterial lipopolysaccharide. J. Immunol., 156, 350-358. Zingler, D.M. (1985). Role of reversible oxidation-reduction of enzyme thiols-disulfides in metabolic regulation. Ann. Rev. Biochem., 54, 305-329. Zou, A.-P., and Cowley, A.W. (1997). Nitric oxide in renal cortex and medulla. Hypertension, 29, 194-198. WWW.ESAINC.COM 149 Appendix 2.1 Background to Electrode Potentials As mentioned in Chapter 2, oxidation can be defined as a gain in oxygen, a loss of hydrogen, or the loss of electrons. Conversely, reduction is the loss of oxygen, a gain of hydrogen, or the gain of electrons. The two processes are complimentary and no oxidation process can take place without a corresponding reduction; these complementary reactions are typically referred to as REDuctionOXidation or REDOX reactions. Consider the reaction between hydrogen and oxygen (Eqn 2.1.1) and Fe (II) salts with hydrogen peroxide (Eqn 2.1.2). The substance that provides the oxygen or removes hydrogen (oxygen and hydrogen peroxide, respectively), and so becomes reduced, is the oxidizing agent; similarly, the substance that provides the hydrogen or removes oxygen (hydrogen and Fe (II), respectively), and so becomes oxidized, is the reducing agent. Sometimes redox reactions can be extremely complex (Eqn 2.1.3) so it is often easier to determine oxidation and reduction reactions from partial equations (Eqns 2.1.4 and 2.1.5). 2H2 + O2 → 2H2O Fe2+ + H2O2 → Fe3+ + HO• + OHMnO4- + 8H+ + 5Fe2+ → 5Fe3+ + Mn2+ + 4H2O MnO4- + 8H+ + 5e- → Mn2+ + 4H2O (reduction) 5Fe2+ → 5Fe3+ +5e (oxidation) Eqn 2.1.1 Eqn 2.1.2 Eqn 2.1.3 Eqn 2.1.4 Eqn 2.1.5 One important example of oxidation-reduction processes includes the reactions associated with aerobic respiration. Electrons from reducing agents such as NADH are passed along the mitochondrial electron transport chains to a terminal-oxidizing agent, oxygen. During this process the components of these chains (various cytochromes, flavoproteins, CoQ10, etc.) undergo redox reactions as electrons are passed from one to another. In this way, the free energy from the oxidation NADH by oxygen (∆Go’= -220 kJ mol-1) is utilized in a series of steps to synthesize 2.5 or 3 moles of ATP (from ADP and PI). THERMODYNAMICS OF REVERSIBLE CELLS. Perhaps the best way to illustrate the thermodynamics of redox processes is to give a simple example. School children learn that dropping zinc granules or iron filings into a solution of Cu (II) sulfate results in the surface of these metals being replaced by a coating of copper metal (Eqn 2.1.6). If, however, copper granules are added to a zinc sulfate solution nothing happens (Eqn 2.1.7). The reason that copper can be liberated from its solution while zinc cannot is a result of free WWW.ESAINC.COM 150 energy release. For the reaction of zinc granules with copper sulfate solution, the ∆Go is –213.4 kJ mol-1 at 25oC (corresponding to an equilibrium constant of 1037) and this is a spontaneous, irreversible reaction. The reaction of copper granules and zinc sulfate has a ∆Go of +213.4 kJ mol-1 so will not occur spontaneously. This reaction can be forced to occur if the appropriate energy is put into the system. Zn + Cu2+ → Zn2+ + Cu Cu + Zn2+ → Cu2+ + Zn √ X Eqn 2.1.6 Eqn 2.1.7 The free energy from a spontaneous reaction can be utilized in the form of electrical work. The ability for a reaction to do work can be studied by setting up an electrochemical cell (Figure 2.1.1). The overall reaction can be divided into a separate oxidation (Eqn 2.1.8) and reduction process (Eqn 2.1.9) commonly called a half-cell reaction. One of the two half-cell reactions takes place in each beaker shown in Figure 2.1.1. Electrical Electrical work work Current Current Zn Zn Cu Cu Salt Salt bridge bridge ZnSO ZnSO44 CuSO CuSO44 Figure 2.1.1 Electrochemical Cell For Zn/Cu Couple. Zn – 2e- → Zn2+ (oxidation) Cu2+ + 2e- → Cu (reduction) WWW.ESAINC.COM Eqn 2.1.8 Eqn 2.1.9 151 A salt bridge connects the two beakers (typically KCl gel). This allows electrical connection between the beakers, while also preventing the direct reaction that would result in the precipitation of copper. The circuit is completed when the zinc and copper electrodes are connected. Electrons will flow from the zinc to the copper electrode as zinc ions are formed and copper ions are reduced. Overtime, as the reaction proceeds to equilibrium, ∆G falls and the amount of electrical work obtained from the cell decreases. A familiar example of this is the dry-cell battery. When the reaction within the battery reaches equilibrium, no voltage is produced and the battery is “dead”. Battery + - A B G Zn/Cu Cell Galvanometer Figure 2.1.2 Measurement Of The EMF Of A Cell. The determination of free energy changes of an electrochemical cell can be obtained by measuring the electromotive force (EMF). If an external voltage is applied to the two electrodes shown in Figure 2.1.2 so as to oppose the direction of current of the cell, then at a certain point (A) the current flowing in the cell will be zero (the “null point”). Under these conditions the potential difference of the null point is the EMF. If the external voltage is further increased (B), the current will reverse its direction as the cell reaction is reversed (i.e., Cu + Zn2+ → Zn + Cu2+). At the null point the cell is behaving reversibly. The thermodynamics of the cell can now be explored. The ∆G (the amount of useful work) is related to the potential difference (E) of a reaction by Eqn 2.1.10. Measurement of the EMF (E) of a redox reaction gives ∆G directly (as long as the number of electrons (n) taking part in the reaction is known). For the cell shown in Figure 2.1.1, n=2 and the maximal amount of electrical work that can be obtained using Eqn 2.1.10 is 212.3 kJ mol-1 Zn. As a decrease in free energy (-∆G) is defined to be equal to WWW.ESAINC.COM 152 the maximal amount of electrical work that can be performed, then ∆G must be 212.3 kJ mol-1 Zn. Since the electrochemical cell contains substances in their standard states and the temperature is at 25oC, the free energy change of the system now becomes the standard free energy change. Therefore ∆Go =-212.3 kJ mol-1 Zn. ∆G = -nFE Eqn 2.1.10 (where F is the Faraday constant and n is the number of electrons transferred in the reaction). STANDARD ELECTRODE POTENTIALS. The half-cell potential cannot be measured directly (the very act of carrying out a measurement would introduce another metal into the solution that would set up its own electrode potential). However, as discussed above, the difference between the potentials of two half cells as part of an electrochemical cell can be measured. If one of the half-cells is a reference electrode then a series of relative values of electrode potentials can be obtained. The typical reference electrode is the hydrogen electrode (Eqn 2.1.11). The hydrogen electrode under standard conditions (H2, 1 atm; H+, 1M) is arbitrarily assigned a standard electrode potential (SEP) of 0mV. To obtain SEP values the reference and test electrodes (e.g., Zn/Zn2+) are connected together via a salt bridge as shown in Figure 2.1.3. It is then relatively simple to measure the electrode potential using a voltameter. As it is inconvenient to measure them in the laboratory, SEP values can readily be obtained from any good chemical textbook. SEPs are sometimes also referred to as redox potentials.1 H+ + e- → 1/2H2 (Pt) Eqn 2.1.11 Standard electrode potentials can be used to predict whether a reaction will occur or not. Let us return again to the zinc/copper reaction. If the EMFs of the half-cells are known, then the EMF for the total reaction can readily be calculated, as it is the algebraic difference between the two electrode potentials. By convention the total standard electrode potential (EoTOTAL) is the difference between the two half reactions (EoTOTAL = EoRHS - EoLHS). So, using the SEPs for the reaction of zinc with Cu (II) sulfate, EoTOTAL= +1.1V (Eqns 2.1.12 to 2.1.14). Thus it is thermodynamically possible for zinc to reduce copper. This is well 1 The electrode potential (E) of a reaction when carried out under standard state conditions (i.e., mole of substance under o 1 atmosphere of pressure) is denoted by E . In many biochemical processes there is a net uptake or release of protons as the reaction proceeds. A 1M solution of protons has a pH of 0 which is of little use to biochemists who normally study reactions at neutrality (~pH 7.0). To circumvent this problem the biochemical standard state can be used where all + -7 substances are in their standard state except H , which is present at 10 M. The biochemical standard electrode potential o’ is denoted by E . WWW.ESAINC.COM 153 supported by the ∆Go value for this reaction (∆Go= -213.4 kJ mol-1). For the reaction of copper with zinc sulfate, the Eo would be –1.10V indicating that it is thermodynamically impossible for copper to reduce zinc ions. A negative Eo (positive ∆Go) shows that the oxidized form is favored whereas a positive Eo (negative ∆Go) shows that the reduced state is favored. Thus using the standard electrode potentials shown in Table 2.5 it can be seen that H2 will not reduce Zn2+ to Zn but it will reduce Cu2+ to copper. Eo = -(-0.76V) Eo = +0.34V EoTOTAL = +1.10V Zn - 2e- → Zn2+ Oxidation Cu2+ + 2e- → Cu Reduction Zn + Cu2+ → Zn2+ + Cu Eqn 2.1.12 Eqn 2.1.13 Eqn 2.1.14 Valve Valve Voltmeter Voltmeter V Salt Salt bridge bridge Electron Electron flow flow ifif metal metal M M has has aa negative negative electrode electrode potential potential Electron Electron flow flow ifif metal metal M M has has aa positive positive electrode electrode potential potential Hydrogen Hydrogen Metal Metal M M n+ Metal Metal M Mn+ Molar Molar H+ H+ Figure 2.1.3 Apparatus For Measuring SEPs. Simple addition and subtraction cannot always combine standard electrode potentials. The number of electrons must also be considered. Consider the two half-cell reactions Eqn 2.1.15 and 2.1.16. Simply subtracting Eqn 2.1.16 from Eqn 2.1.15 cannot solve the conversion of Fe (III) to Fe (II) (Eqn 2.1.17). The reaction would not be balanced. The answer can only be found if the number of electrons are considered. The answer is thus obtained arithmetically [(3 x Eqn 2.1.15) – (2 x Eqn 2.1.16)] and is found to be -0.76V (Eqn 2.1.17). WWW.ESAINC.COM 154 Fe3+ + 3e- = Fe Fe2+ + 2e- = Fe Fe3+ + e- = Fe2+ -0.04V -0.44V -0.76V Eqn 2.1.15 Eqn 2.1.16 Eqn 2.1.17 SOME COMMENTS ON SEPS. Now that we have a clearer understanding of SEPs, we can apply them to the field of redox biochemistry. A wide variety of SEPs can be found in the literature and some of them are presented in Table 2.1.1. The SEPs are placed in descending order from the most positive (the reaction of the strongest oxidizing agent, the chlorine atom) to the most negative (the reaction of the strongest reducing agent, the electron). Compounds know to be antioxidants (see Chapter 4) have typical biochemical SEP values of <500mV, significantly below the Eo’ values of the reactions of the pro-oxidant species (+600 to +2310mV). Thus the reaction of antioxidants with reactive species such as alkyl radicals (L•) and lipid peroxyl radicals (LO2•) (Eqns 2.1.18 and 2.1.19) forms products which are much less oxidizing (Eqns 2.1.20 and 2.1.21). Closer examination of these reactions reveals that the Eo’ of LO2• is +400mV higher than that of L•, indicating that LO2• is a stronger oxidizing agent. This is significant biologically as σ-radicals like L• readily react with oxygen to form LO2•. Thus during lipid peroxidation processes very strong oxidizing agents are produced which can serve to promote this chain reaction. Based solely upon the biochemical SEPs presented in Table 2.1.1 there is an antioxidant hierarchy with the antioxidant activity of α-tocopherol being the least and lipoic acid being the most effective. This explains why during lipid peroxidation membrane bound α-tocopherol can be regenerated from its radical by membrane bound coenzyme Q10 or cytosolic ascorbic acid, GSH and lipoic acid. The biological importance of these antioxidants in lipid peroxidation is described in Chapters 3 and 4. LO2• + H+ + e- = LO2H L• + H+ + e- = LH TO• + H+ + e- = TOH (tocopherol) ½(Dehydroascorbate + 2H+ + 2e- = Ascorbate) Eo’ ≅ +1000mV (strong oxidant) Eo’ ≅ +600mV Eo’ ≅ +500mV Eqn 2.1.18 Eqn 2.1.19 Eqn 2.1.20 Eo’ = +40mV (weak oxidant) Eqn 2.1.21 Another interesting observation found in Table 2.1.1 is that the Eo’ value for the reaction H2O2 + H+ + e- = H2O + HO• is only +320mV (not much different in value from many antioxidant reactions). Although at first glance this reaction might appear unimportant, it is, in fact, a major problem for all aerobic organisms. WWW.ESAINC.COM 155 Hydrogen peroxide is readily decomposed to hydroxyl free radicals (e.g., by ferrous ions in the Fenton reaction). Once formed, the hydroxyl free radical is an incredibly strong oxidizing agent and the HO•, H+/H2O reaction has an Eo’=+2310mV. Just by reacting with iron, the standard electrode potential of one reactive oxygen species (hydrogen peroxide) can be increased by ≅+2000mV, forming one of the most oxidizing agents known! REDOX COUPLE One Electron Reactions Cl• + e- = ClSO4•- + e- = SO42HO• + H+ + e- = H2O (strongest oxidant under biochemical conditions) NO3• + e- = NO3Cl2•- + e- = 2ClBr• + e- = BrHO• + e- = OHC2H5• + H+ + e- = C2H6 O3•- + 2H+ + e- = O2 + H2O Br2•- + e- = 2BrRO• + H+ + e- = ROH (aliphatic) NO2+ + e- = NO2• ONO2- + 2H+ + e- = NO2• + H2O Mn3+ + e- = Mn2+ CO3- + e- = CO321 Σg O2 + e- = O2•NO+ + e- = NO• N2O4 + e- = NO• + NO3• CH2OH + H+ + e- = CH3OH O3 + e- = O3•• O2SO3- + e- = SO52CO2•- + H+ + e- = HCO2HO2• + H+ + e- = H2O2 Tryptophan Radical + H+ + e- = Tryptophan NO2• + e- = NO2HRP-II/HRP (horseradish peroxidase) Allyl• + H+ + e- = allyl-H HRP-I/HRP-II O2•- + 2H+ + e- = H2O2 RS• + e- = RS- (cysteine) Phenol• + H+ + e- = Phenol N2O3 + e- = NO• + NO2LO2• + H+ + e- = LO2H (alkylperoxyl) 1 ∆g O2 + e- = O2•SO3•- + e- = SO32PUFA• (L•) + H+ + e- = PUFA-H (L-H) HU•- + H+ + e- = UH2- (urate) CA-O• + H+ + e- = CA-OH (catechol) α-TO• + H+ + e- = α-TOH (tocopherol) ONO2• + e- = ONO2Cu2+ + H+ + e- = Cu+H (SOD) WWW.ESAINC.COM Eo(’)/mV STRONG OXIDANT +2550 +2430 +2310 +2300, +2600 +2300 +2000 +1900 +1900 +1800 +1800 +1600 +1600 +1600 +1510 +1500 +1270 +1210 +1200 +1200 +1140 +1100 +1100 +1060 +1000 +990 +970 +960 +950 +940 +920 +900 +800 +770, +1000, +1400 +650 +630 +600 +590 +530 +500 +430 +420 156 Cl2 + e- = Cl2•Br2 + e- = Br2•NO• + e- = NO- (triplet) FeO22+ + 2H+ + 2e- (myoglobin) H2O2 + H+ + e- = H2O + HO• Mn3+ + H+ + e- = Mn2+H (SOD) Fe3+ + O2 + e- = FeO22+ (hemoglobin) NAD• + H+ + e- = NADH FeO22+ + 2H+ + 2e- (myoglobin) Asc•- + H+ + e- = AscH- (ascorbate) Fe3+ + H+ + e- = Fe2+H (SOD) Fe3+ + e- = Fe2+ (cytochrome) Fe3+ + O2 + e- = FeO22+ (myoglobin) ONO2• + e- = ONO2CoQ•- + H+ + e- = CoQH2 (coenzyme Q10) Cu2+ + e- = Cu+ Fe3+ + e- = Fe2+ (hemoglobin) Fe3+(EDTA) + e- = Fe2+(EDTA) Fe3+ + e- = Fe2+ (aqueous, pH 7.0) Fe3+(citrate) + e- = Fe2+(citrate) Fe3+(ADP) + e- = Fe2+(ADP) Fe3+ + e- = Fe2+ (myoglobin) HOCl + e- = Cl- + HO• CoQ + H+ + e- = CoQ•Dehydroascorbate + H+ + e- = Ascorbate• HOBr + H+ + e- = H2O + Br• Fe3+ + e- = Fe2+ (ferritin) FADH• + H+ + e- = FADH2 Riboflavin + e- = riboflavin•O2 + e- = O2•Adriamycin + e- = Adriamycin semiquinone NO• + e- = NO- (singlet) HOBr + e- = Br- + HO• Fe3+ + e- = Fe2+ (transferrin) Paraquat2+ + e- = Paraquat+ O2 + H+ + e- = HO2• HOCl + H+ + e- = H2O + Cl• NAD+ + e- = NAD CH3CHO + H+ + e- = CH3C•HOH RSSR + e- = RSSR•(e.g., cystine or glutathione disulfide) NAD+ + H+ + e- = NADH+ CO2 + e- = CO2•H2O + e- = e-aq (hydrated electrons) Multiple Electron Reactions a) Two electron O3 + 2H+ + 2e- = O2 + H2O Cl2 (aq) + 2e- + 2ClHOBr + H+ + 2e- = H2O + BrHOCl + H+ + 2e- = H2O + ClUric diimine + 2H+ + 2e- = Uric acid O2 + 2H+ + 2e- = H2O2 CoQ + 2H+ + 2e- = CoQH2 WWW.ESAINC.COM +420 +410 +390 +390 +320 +310 +310 +300 +300 +282 +280 +260 +220 +200 +200, +350 +160 +140 +120 +110 +100 +100 +50 -40 -36, -230 -174 -180 -190 -240 -317 -330 -330 -350 -360 -400 (pH 7.3) -450 -460 -460 -930 -1380 -1500 -1580 -1800 -2870 STRONG REDUCTANT +2075 +1400 +1090 +1080 +400 +330 +100 157 Dehydroascorbate + 2H+ + 2e- = Ascorbate FAD+ + 2H+ + 2e- = FADH2 GSSG + 2H+ + 2e- = 2GSH S + 2H+ + 2e- = H2S Lipoic acid + 2H+ + 2e- = Dihydrolipoic acid NAD+ + H+ + 2e- = NADH b) Four electron O2 + 4H+ + 4e- = 2H2O +80 -180 -240 -270 -320 -320 +820 Table 2.1.1 SEPs For Different Reactions. (Based on Buettner (1993) but extended by Acworth et al. (1997a); Koppenol (1998) and references therein; Koppenol and Butler (1985)). COUPLED REDOX REACTIONS. The EMF values (E) can be considered as entirely analogous to free energy changes (∆G). Therefore as long as the number of electrons in the reaction are known, Eo values can be used to predict the position of equilibrium in a reaction. In the electron transport chain of the inner mitochondrial membrane, the redox couples (cytochromes, etc.) are arranged in order of increasing Eo’. As electrons are passed along the chain the total energy released in the oxidation of NADH is utilized to synthesize ATP from ADP and inorganic phosphate at several steps along the chain. If redox couples were not used and the energy of NADH oxidation was to be released in a single step much of it would be wasted as heat, with much less energy being available for the synthesis of ATP. REFERENCES Acworth, I.N., McCabe, D.R., and Maher, T.J. (1997a). The analysis of free radicals, their reaction products and antioxidants. In: Oxidants, Antioxidants and Free Radicals. Baskin, S.I., and Salem, H., (Eds.). Taylor and Francis, Washington DC. Pp. 23-77. Buettner, G.R. (1993). The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch. Biochem. Biophys. 300, 535-543. Koppenol, W.H. (1998). The basic chemistry of nitrogen monoxide and peroxynitrite. Free Radic. Biol. Med., 25, 385-391. Koppenol, W.H., and Butler, J. (1985). Energetics of interconversion reactions of oxy radicals. Adv. Free Radic. Biol. Med., 1, 91-131. WWW.ESAINC.COM 158 Appendix 2.2 Background to Kinetics Thermodynamics enables the prediction of whether a process or reaction will occur spontaneously. It cannot predict the rate at which such processes occur, for this we have to turn to the field of kinetics. The importance of the difference between thermodynamic and kinetic control of a reaction is illustrated by the following example. The hydrolysis of ATP is thermodynamically favorable (∆Go’= -31 kJ mol-1 at pH 7.0 and 25oC) yet a solution of ATP at pH 7.0 is fairly stable. This is because an activation energy has to be achieved before ATP hydrolysis will take place. A catalyst (such as ATPase) will lower the activation energy and hence speed up (increase the rate of) the reaction, but it will not effect the position of equilibrium. Kinetic measurements are of great interest to redox biochemists. For example, the measurement of reaction kinetics allows us to determine and compare the reactivity of pro-oxidants. Order Units R=k1[A] First s-1 R=k2[A]2 Second M-1s-1 R=k2[A] [B] Second M-1s-1 Table 2.2.1 Units of Reaction Order. The rate of a chemical reaction is dependent upon the concentration of reactants present, temperature, pressure, pH and the presence of inhibitors. For example, the reaction rate nearly doubles for every 10oC increase in temperature. The exact mathematical relationship between the rate of a reaction and the concentration of reactants is determined experimentally and is called the rate law. The order is defined as the power to which the concentration of reactant is raised in the rate law. Thus for the reaction where a moles of A combine with b moles of B (Eqn 2.2.1) the expression for the rate of formation (d[P]/dt) of product (P), might be Eqn 2.2.2 where k is a constant known as the rate constant. Once the reaction is started the concentrations of both A and B will fall and the reaction rate will fall too. That is why reaction rates are usually measured as soon as the reaction has started (initial rate measurement). The rate constant is an experimental quantity and can be either integral or non-integral (Table 2.2.1). The above reaction is ath order in A, bth order in B, with an overall order of (a+b). Order is not the same as molecularity. Molecularity is the minimum number of species involved in the rate-determining step (the slowest step of the reaction). WWW.ESAINC.COM 159 aA + bB → P d[P]/dt = k [A]a [B]b Eqn 2.2.1 Eqn 2.2.2 FIRST-ORDER PROCESSES. In this process the rate of the reaction depends only upon the reactant (R) Eqn 2.2.3. The rate law for this reaction (Eqn 2.2.4) upon integration yields Eqn 2.2.5 where c is a constant. If [R]0 is the initial concentration of R (t=0) then c=ln[R]0. Substitution of this expression in Eqn 2.2.6 yields Eqn 2.2.7. A plot of [R] versus time gives a straight line of slope –k. An example of a first order process is radioactive decay. The units for first-order rate constants is time (s-1) (Table 2.1.1). The half-life of a reaction is a very useful quantity. This is the time taken for [R] to fall to half of its initial value, i.e., the time taken for R to fall from [R]0 to 0.5[R]0 is the same as the time to go from 0.5[R]0 to 0.25[R]0. Therefore for a first order reactions, the half-life is independent of initial concentration of R. The expression of calculation of the half-life (t1/2) can be derived using Eqn 2.2.6 and is shown in Eqn 2.2.7. R → products -d[R]/dt = k [R] ln [R] = -kt + c ln [R]/[R]0 = -kt ln 2 = kt1/2 t1/2 = 0.693/k Eqn 2.2.3 Eqn 2.2.4 Eqn 2.2.5 Eqn 2.2.6 Eqn 2.2.7 SECOND-ORDER AND PSEUDO-FIRST-ORDER PROCESSES. Most often reactions are second-order or pseudo-first-order. In second-order reactions the rate depends upon two molecules reacting (Eqn 2.2.8). The rate law for this process for the condition [A]0≠[B]0 is presented in Eqn 2.2.9. Following integration, a plot of ln[B]0([A]0-x)/[A]0([B]0-x) versus t gives a straight line of slope k2([A]0-[B]0). If the initial concentrations of A and B are equal then the rate law becomes Eqn 2.2.10. The half-life is related to the initial concentration of A by Eqn 2.2.11. Therefore in second-order reactions the halflife is inversely proportional to the initial concentration of A, i.e., it takes twice as long for the initial concentration of A to go from 0.5[A]0 to 0.25[A]0 as it does for [A]0 to 0.5[A]0. The units for second-order rate constants are (concentration-1)(time-1) (e.g., M-1s-1, l mol-1 s-1, M-1h-1 etc.) (Table 2.1.1). WWW.ESAINC.COM 160 A + B → Products dx/dt = k2[A] [B] dx/dt = k2([A]0 – x)2 t1/2 = 1/k2[A]0 Eqn 2.2.8 Eqn 2.2.9 Eqn 2.2.10 Eqn 2.2.11 With pseudo-first order reactions the concentration of one of the reactants (say B) is in excess and will remain essentially constant throughout the reaction. Thus the rate law becomes Eqn 2.2.12. The process is apparently first order in A and zeroth order in B. Pseudo-first order reactions are in units of time (e.g., s-1). However, true second-order rate constants can be obtained by dividing by the concentration. -d[A]/dt=k[A] Eqn 2.2.12 SOME PUBLISHED SECOND-ORDER RATE CONSTANTS. Table 2.2.2 shows some rate constants published in literature. Be aware that rate constants will be affected by the experimental conditions under which they are obtained so care should be taken when comparing them to each other. REACTIONS HO• + General Metabolite HO• + Albumin HO• + Ascorbyl• HO• + N-Acetylcysteine HO• + Carnosine HO• + β-Carotene HO• + Cysteamine HO• + Cysteic acid HO• + Cysteinesulfinic acid HO• + Deoxyguanosine HO• + Deoxyribose HO• + Desferrioxamine HO• + DMSO HO• + “double bond” HO• + Ethanol HO• + Glucose HO• + Glutathione (reduced) HO• + Hypotaurine RATE CONSTANT (M-1s-1) 109 - 1010 >1010 1.0 x 1010 1.4 x 1010 2.5 x 109 <1.0 x 1011 5.9 x 109 5.3 x 107- 1.6 x 108 3.2 x 109 1.0 x 109 3.1 x 109 1.3 x 1010 6.6-7.1 x 109 REFERENCE Buxton et al. (1988); Ross et al. (1997) Halliwell et al. (1995) Bartlett et al. (1994) Aruoma et al. (1989a) Aruoma et al. (1989b) Lymar et al. (1995) Aruoma et al. (1988) Aruoma et al. (1988) Aruoma et al. (1988) ≥1010 1.9 x 109 1.0 x 109 8.8 x 109 Vieira et al. (1993) Halliwell et al. (1995) Denicola et al. (1995) Pryor and Squadrito (1995); Denicola et al. (1995) Breen and Murphy (1995) Pryor and Squadrito (1995) Halliwell et al. (1995) Aruoma et al. (1989a) 5 x 109 - 1.2 x 1010 Aruoma et al. (1988) WWW.ESAINC.COM 161 HO• + Mannitol HO• + NO• → HNO2 HO• + NO2• → ONO2H HO• + Phenylalanine 1.7 x 109 1.0 x 1011 4.5 x 109 1.9 x 109 HO• + Phosphate (e.g. DNA) HO• + R-H → R• + H2O HO• + Salicylic Acid <107 HO• + Taurine HO• + α-Tocopherol HO• + Trolox HO• + Unsaturated fatty acids O2•- + N-Acetylcysteine 1.0 x 109 5.0 x 109 - 1.0 x 1010 2.4 x 106 - 1.4 x 107 1.0 x 1010 8.0 x 1010 1.0 x 109 O2•- + Carnosine O2•- + Catechols 1.0 x 103 - 2.7 x 106 1.0 x 104 - 2.7 x 105 1.0 x 103 1.0 x 109 O2•- + Dihydrolipoic acid O2•- + Quinones 3.3-7.5 x 105 1.0 x 109 O2•- + Taurine O2•- + Fe3+ → Fe2+ + O2 2O2•- + H+ → H2O2 + O2 <1 x 103 1.0 x 106 5.0 x 105 - 2.4 x 109 2.4 x 105 - 8.0 x 107 O2•- + Ascorbate O2•- + HO2• + H+ → H2O2 + O2 O2•- + HOCl → HO• + Cl- + O2 O2•- + NO• → ONO2O2•- + NO2• → NO2- + O2 O2•- + O2•- + 2H+ → H2O2 + O2 HO2• + α-Tocopherol HO2• + HO2• → H2O2 + O2 H2O2 + 2Cysteine → Cystine + 2H2O H2O2 + Cu+ → HO• + HO- + Cu2+ H2O2 + Fe2+ → HO• + HO- + Fe3+ H2O2 + Fe2+-ADP → HO• + HO- + Fe3+ADP 7.5 x 106 3.4 x 107- 7 x 109 1.0 x 108 <0.3 - 5 x 105 2.5 x 106 8.0 x 105 Pryor and Squadrito (1995) Rubbo et al. (1995) Bartlett et al. (1994) Kaur and Halliwell (1994a); Kaur and Halliwell (1994b) Breen and Murphy (1995) Breen and Murphy (1995) Hiller et al. (1983) Aruoma et al. (1988) Lymar et al. (1995) Pryor and Squadrito (1995) Radi et al. (1991) Aruoma et al. (1989a) Halliwell and Gutteridge (1989); Radi et al. (1991) Aruoma et al. (1989b) Halliwell and Gutteridge (1989) Suzuki et al. (1991, 1993) Halliwell and Gutteridge (1989) Aruoma et al. (1988) Radi et al. (1991) Denicola et al. (1995); Beckman (1994) Bielski et al. (1985); Halliwell and Gutteridge (1989) Aruoma et al. (1989a) Pryor and Squadrito (1995); Radi et al. (1991) Alvarez et al. (1995) Halliwell and Gutteridge (1989) Rubbo et al. (1995) Halliwell and Gutteridge (1989) 1.3 x 101 Radi et al. (1991) 4.7 x 103 Beckman (1994) 7.6 x 101 Beckman (1994) 8.0 x 102 Beckman (1994) WWW.ESAINC.COM 162 H2O2 + Fe2+-EDTA → HO• + HO- + Fe3+EDTA H2O2 + ONO2H → O2•+ NO2• + H+ + H2O NO• + Fe2+(heme) NO• + Fe3+(heme) NO• + Heme NO• + HO• → HNO2 NO• + 1/2O2 → NO2• NO• + O2•- → ONO2NO• + Tryptophan• NO• + Tyrosine• NO2• + Fatty acid → NO2- + Fatty acid• + H+ NO2• + HO• → ONO2H NO2• + O2•- → NO2- + O2 NO2• + Tyrosine• → 3Nitrotyrosine NO2+ + Tyrosine → 3Nitrotyrosine + H+ ONO2- + SOD → SODCu+-O-NO2+ ONO2- + Albumin (single thiol) ONO2- + Cysteine (or glutathione) ONO2- + CO2 → ONO2CO2ONO2- + myeloperoxidase ONO2- + horseradish peroxidase ONO2- + cytochrome c2+ ONO2- + alcohol dehydrogenase ONO2H + Ascorbate ONO2H + H2O2 → O2•+ NO2• + H+ + H2O ONO2CO2- + Tyrosine → Tyr• + NO2• + HCO3AscH- + TO• → Asc•- + TOH 2Asc•- + H+ → AscH- + dehydro-Asc CCl3CO2• + ascorbate → CCl3CO2- + ascorbyl• CCl3CO2• + phenol → CCl3CO2- + phenol• CCl3CO2• + polyphenol 5.0 x 103 Beckman (1994) 1.0 x 105 Alvarez et al. (1995) 1 x 107 1 x 102 - 1 x 107 1 x 103 - 1 x 104 1.0 x 1011 3.5 x 106 (M-2.s-1) 3.4 x 107 - 7 x 109 1-2 x 109 1-2 x 109 1.0 x 105 Pryor and Squadrito (1995) Pryor and Squadrito (1995 Radi et al. (1991b) Rubbo et al. (1995) Crow and Beckman (1995) Pryor and Squadrito (1995); Radi et al. (1991) Radi (1996) Radi (1996) Radi et al. (1991b) 4.5 x 109 1.0 x 108 Bartlett et al. (1994) Alvarez et al. (1995) 3.0 x 109 van der Vleit et al. (1995) 1.0 x 10 Ischiropoulos et al. (1992) 1.0 x 105 Beckman (1994) 2.6 x 103 Radi et al. (1991a) 2.0-6.0 x 103 3.0 x 104 Bartlett et al. (1994); Radi et al. (1991a) Lymar and Hurst (1995) 2.0 x 107 Floris et al. (1993) 3.0 x 106 Floris et al. (1993) 2.0 x 105 4.0 x 105 Thompson et al. (1995) Crow et al. (1995) 2.4 x 102 1.0 x 105 Bartlett et al. (1994) Alvarez et al. (1995) >2.0 x 105 Lymar et al. (1996) 2.0 x 105 Scarpa et al. (1984) 2.0 x 105 Vieira et al. (1993) 1.3 x 108 Aruoma et al. (1997) 4 x 105 - 9.5 x 107 Aruoma et al. (1997) 8.4 x 105 - 4.0 x Aruoma et al. (1997) 8 WWW.ESAINC.COM 163 → CCl3CO2- + polyphenol• CCl3CO2• + αTocopherol → CCl3CO2- + αTocopheroxyl• CoQH2 + TO• → CoQ•+ TOH DNA• + GSH → DNA + GS• eaq- → H• + HFe3+ + e- → Fe2+ GS• + GS- → GSSG•GS• + O2 → GSO2• GSO2• → GS• + O2 GSSG•- → GS• + GSGSSG•- + O2 → O2- + GSSG L• + O2 → LO2• LO• (LO2•) + NO• LO2• + LH → L• + LO2H 2LO2• → Non-radical products LO2H + Fe2+ → LO2• + Fe3+ RCH2• + O2 → RCH2O2• TO + PUFA-O2• → PUFA-O2H + TO• Tyrosine• + Tyrosine• → Dityrosine 108 4.9 x 108 Aruoma et al. (1997) 2.0 x 105 Mukai et al. (1990) 1.0 x 107-108 Halliwell et al. (1995) 1.6 x 101 1.0 x 106 8.0 x 108 2.0 x 109 2.0 x 109 2.4 x 105 1.6 x 108 Breen and Murphy (1995) Beckman (1994) Buettner (1993) Buettner (1993) Buettner (1993) Buettner (1993) Buettner (1993) 3.0 x 108 1.3 x 109 1-5 x 101 1.0 x 106-7 Buettner (1993) Rubbo et al. (1996) Buettner (1993) Buettner (1993) 1.0 x 103 Beckman (1994) 3.0 x 109 8.0 x 104 Neta et al. (1990) Buettner (1993) 4.0 x 108 van der Vleit et al. (1995) Table 2.2.2 Rate Constants for Some Redox Biochemical Reactions. Modified from Acworth et al. (1997a). Rate constants are very useful to redox biochemists and can be used to compare the rates of different chemical reactions. For example both copper and iron can take part in the generation of hydroxyl free radicals, but which of these metals is more effective? As presented by Halliwell and Gutteridge, if equal concentrations of hydrogen peroxide are mixed with equal concentrations of ferrous (Eqn 2.2.13) or cuprous ions (Eqn 2.2.14), then the initial rate of hydroxyl free radical production by the copper-based reaction would be greater than the ferrous-based reaction by a factor of 62 (Halliwell and Gutteridge (1999)). Thus it appears that copper is a much more effective pro-oxidant than iron under these conditions. Furthermore they reported that if the hepatic concentrations of hydrogen peroxide and ferrous iron were mixed then the number of hydroxyl free radicals produced in one liter in one second would be in excess of 1013 molecules! WWW.ESAINC.COM 164 H2O2 + Fe2+ → Fe3+ + OH- + HO• H2O2 + Cu+ → Cu2+ + OH- + HO• k2 = 7.61 x 102 M-1s-1 Eqn 2.2.13 k2 = 4.70 x 103 M-1s-1 Eqn 2.2.14 MEASUREMENT OF REACTION ORDER AND REACTION RATES. The reaction order can be determined by comparing the concentrations of reactants (or products) as a function of time using the integrated rate laws discussed above. The determination of reaction order can often be simplified by using the half-times method (this can be found in many physical biochemistry texts). As many radical reactions proceed incredibly rapidly (e.g., the second order rate constant for HO•-based reactions are on the order of 109-10M-1s-1) and are beyond the standard approaches used by biochemists, two special approaches have been developed to measure their reaction rates: stopped flow and pulse radiolysis. These have been dealt with in detail elsewhere (e.g., Halliwell and Gutteridge (1999); Wardman (1978)). Stopped flow procedures are usually used when reaction rates are too rapid for the normal biochemical procedures. In this approach solutions of reactants are housed in separate syringes connected to a quartz cell. The outlet of the cell is connected to a third syringe that can only be filled to a predetermined volume until its plunger is abruptly stopped from moving. The cell is connected to a measuring device such as an absorbance detector. At the start of the experiment the flow from the two reaction syringes is initiated and flow rate is controlled to prevent significant reaction from taking place within the cell. When the plunger of the third syringe reaches the end of its travel it stops suddenly. The reaction in the cell then proceeds to completion and the rate is determined from the change in absorbance. With pulse radiolysis the compound under investigation is placed in a cell and subjected to a short pulse of radiation forming radical species (see below). With the correct conditions a reaction can be followed for microseconds or longer. Changes in absorbance can then be used to determine rate constants. REFERENCES Alvarez, B., Denicola, A., and Radi, R. (1995). Reaction between peroxynitrite and hydrogen peroxide: Formation of oxygen and slowing of peroxynitrite decomposition. Chem. Res. Toxicol., 8, 859-864. Aruoma, O.I., Halliwell, B., Hoey, B.M., and Butler, J. (1988). The antioxidant action of taurine, hypotaurine and their metabolic precursors. Biochem. J., 256, 251-255. Aruoma, O.I., Halliwell, B., Hoey, B.M., and Butler, J. (1989a). The antioxidant action of n-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide and hypochlorous acid. Free Radic. Biol. Med., 6, 593-597. Aruoma, O.I., Halliwell, B., and Williamson, G. (1997). In vitro methods for characterizing potential pro-oxidant and antioxidant actions of non-nutritive substances in plant foods. In: Antioxidant Methodology: In Vivo and In Vitro Concepts. Aruoma, O.I., and Cuppett, S. (Eds.). AOCS Press, Champaign. Pp. 173-204. WWW.ESAINC.COM 165 Aruoma, O.I., Laughton, M.J., and Halliwell, B. (1989b). Carnosine, homocarnosine and anserine: could they act as antioxidants in vivo? Biochem. J., 264, 863-869. Bartlett, D.B., Church, D.F., Bounds, P.L., and Koppenol, W.H. (1994). The kinetics of the oxidation of l-ascorbic acid by peroxynitrite. Free Radic. Biol. Med., 18, 85-92. Beckman, J. S. (1994). Peroxynitrite versus hydroxyl radical: the role of nitric oxide in superoxide-dependent cerebral injury. N.Y. Acad. Sci., 738, 69-75. Bielski, B.H.J., Cabelli, D.E., Arudi, R.L., and Ross, A.B. (1985). Reactivity of perhydroxyl/superoxide radicals in aqueous solution. J. Phys. Chem. Ref. Data, 14, 1041-1100. Breen, A.P., and Murphy, J.A. (1995). Reactions of the oxyl radicals with DNA. Free Radic. Biol. Med., 18, 1033-1077. Buettner, G.R. (1993). The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch. Biochem. Biophys. 300, 535-543. Buxton, G.V., Greenstock, C.L., Helman, W.P., and Ross, A.B. (1988). Critical review of rate constants for reactions of . . hydrated electrons, hydrogen atoms and hydroxyl radical ( OH/ O ) in aqueous solution. J. Phys. Chem. Ref. Data, 17, 513-886. Crow, J.P., and Beckman, J.S. (1995). Reactions between nitric oxide, superoxide, and peroxynitrite: Footprints of peroxynitrite in vivo. Adv. Pharmacol., 34, 17-43. Crow, J.P., Beckman, J.S., and McCord, J.M. (1995). Sensitivity of the essential zinc thiolate moiety of yeast alcohol dehydrogenase to hypochlorite and peroxynitrite. Biochem., 34, 3544-3552. Denicola, A., Souza, J.M., Gatti, R.M., Augusto, O., and Radi, R. (1995). Desferrioxamine inhibition of the hydroxyl radical-like reactivity of peroxynitrite: Role of the hydroxamine groups. Free Radic. Biol. Med., 19, 11-19. Floris, R., Piresma, S.R., Yang, C., Jones, P., and Wever, R. (1993). Interaction of myeloperoxidase with peroxynitrite – a comparison to lactoperoxidase, horseradish peroxidase and catalase. Eur. J. Biochem., 215, 765-775. Halliwell, B. and Gutteridge, J.M.C. (Eds.). (1999). Free Radicals in Biology and Medicine. Oxford: Clarendon Press. Halliwell, B., Aeschbach, R., Loliger, J., and Aruoma, O.I. (1995). The characterization of antioxidants. Fd. Chem. Toxic., 7, 601-617. Hiller, K.D., Hodd, P.L., and Wilson, R.L. (1983). Anti-inflammatory drugs: Protection of a bacterial virus as an in vitro biological measure of free radical activity. Chem. Biol. Inter., 47, 293-305. Ischiropoulos, H., Zhu, L., Chen, J., Tsai, M., Martin, J.C., Smith, C.D., and Beckman, J.S. (1992). Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch. Biochem. Biophys., 298, 431-437. Kaur, H., and Halliwell, B. (1994a). Detection of hydroxyl radicals by aromatic hydroxylation. Meth. Enzymol., 233, 67-82. Kaur, H., and Halliwell, B. (1994b). Aromatic hydroxylation of phenylalanine as an assay for hydroxyl radicals: measurement of hydroxyl radical formation from ozone and in blood from premature babies using improved HPLC methodology. Anal. Biochem., 220, 11-15. Lymar, S.V., and Hurst, J.K. (1995). Rapid reaction between peroxynitrite ion and carbon dioxide: Implications for biological activity. J. Am. Chem. Soc., 117, 8867-8868. Lymar, S.V., Jiang, Q., and Hurst, J.K. (1997). Mechanism of carbon dioxide-catalyzed oxidation of tyrosine by peroxynitrite. Biochem. In Press. Mukai, K., Kikuchi, S., and Urano, S. (1990). Stopped-flow kinetic study of the regeneration reaction of tocopheroxyl radical by reduced ubiquinone-10 in solution. Biochim. Biophys. Acta, 1035, 77-82. Neta, P., Huie, R.E., and Ross, A.B. (1990). Rate constants for reactions of peroxyl radicals in fluid solutions. J. Phys. Chem. Ref. Data, 19, 413-513. Pryor, W.A., and Squadrito, G.L. (1995). The chemistry of peroxynitrite: A product from the reaction of nitric oxide with superoxide. Am. J. Physiol., 268, L699-L722. Radi, R. (1996). Reactions of nitric oxide with metalloproteins. Chem. Res. Toxicol., 9, 828-835. Radi, R., Beckman, J.S., Bush, K.M., and Freeman, B.A. (1991a). Peroxynitrite oxidation of sulfhydryls. J. Biol. Chem., 266, 4244-4250. Radi, R., Beckman, J.S., Bush, K.M., and Freeman, B.A. (1991b). Peroxynitrite-induced membrane lipid peroxidation: The cytotoxic potential of superoxide and nitric oxide. Arch. Biochem. Biophys., 288, 481-487. Rubbo, H., Darley-Usmar, V., and Freeman, B. (1996). Nitric oxide regulation of tissue free radical injury. Chem. Res. Toxicol., 9, 809-820. Rubbo, H., Radi, R., Trujillo, M., Telleri, R., Kalynararaman, B., Barnes, S., Kirk, M., and Freeman, B.A. (1995). Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation: Formation of novel nitrogen-containing oxidized lipid derivatives. J. Biol. Chem., 269, 26066-26075. Scarpa, M., Rigo, A., Maiorino, M., Ursini, F., and Gregolin, C. (1984). Formation of α-tocopherol radical and recycling of α-tocopherol by ascorbate during peroxidation of phosphatidylcholine. Biochim. Biophys. Acta, 801, 215-219. Suzuki, Y.J., Tsuchiya, M., and Packer, L. (1991). Thioctic acid and dihydrolipoic acid are novel antioxidants which interact with reactive oxygen species. Free Radic. Biol. Med., 15, 255-263. Suzuki, Y.J., Tsuchiya, M., and Packer, L. (1993). Antioxidant activities of dihydrolipoic acid and its structural analogs. Free Radic. Res. Comms., 18, 115-122. Thomson, L., Trojillo, M., Telleri, R., and Radi, R. (1995). Kinetics of cytochrome c2 – oxidation by peroxynitrite: Implications for superoxide measurements in nitric oxide producing biological systems. Arch. Biochem. Biophys., 319, 491-497. van der Vleit, A., Eiserich, J.P., O’Neill, C.A., Halliwell, B., and Cross, C.E. (1995). Tyrosine modification by reactive nitrogen species. Arch. Biochem. Biophys., 319, 341-349. Vieira, A.J.S.C., Candeias, L.P., and Steenken, S. (1993). Hydroxyl radical induced damage to the purine bases of DNA: In vivo studies. J. Chim. Phys., 90, 881-897. Wardman, P. (1978). Application of pulse radiolysis methods to study the reactions and structure of biomolecules. Rep. Prog. Phys., 41, 259-265. WWW.ESAINC.COM 166 Appendix 2.3 Background to The White Blood Cell The purpose of the white blood cells (leukocytes) is to defend the body. As discussed in Chapter 3 the ability for some leukocytes to produce a variety of ROS, RNS and RHS is essential for their phagocytic activity. However, not all leukocytes are phagocytes and not all require pro-oxidants for their biological action. Here we briefly explore the different types of leukocytes found in the body. Reticulum Cell Myeloblast Lymphocytic Reticulum Cell Monoblast Promyelocyte Lymphoblast Monocyte Eosinophil Basophil Neutrophil Lymphocyte Figure 2.3.1 The Relationship Between Granulocytes, Lymphocytes And Monocytes And Their Development From A Common Stem Cell Located In The Bone Marrow, The Reticulum Cell. WWW.ESAINC.COM 167 There are three different types of leukocyte – the granulocytes, lymphocytes and monocytes – differing in their morphology, abundance and biological function. Granulocytes can be further subdivided into neutrophils, eosinophils and basophils. As shown in Figure 2.1.1 all lymphocytes form from a common stem cell, the reticulum cell. Granulocytes These cells are the first to be mobilized to deal with injury and infection. They are amoeboid in nature, phagocytic and are involved in controlling allergic reactions. They are referred to as granulocytes because their cytoplasm contains numerous small granules. • • • Basophils typically make up ~0.5% of the total leukocyte population and have an abundance of 0-160/µL blood. They resemble polymorphonuclear neutrophils but contain an irregular nucleus. They are called basophils as they readily absorb basic stains. Basophils contain heparin (or an heparinlike substance), histamine and 5HT. These cells are involved in hypersensitivity reactions. Eosinophils typically make up 1-4% of the total leukocyte population and have an abundance of 50-250/µL blood. Morphologically they resemble the neutrophil but contain larger cytoplasmic granules. These cells readily stain with eosin. They are phagocytic but less active than neutrophils. Eosinophils carry about 1/3rd of the total amount of blood histamine. These cells are involved in allergic and hypersensitivity responses (e.g., asthma). Interestingly these cells posses a special myeloperoxidase capable of promoting bromination of a variety of substrates. Neutrophils make up ~50-70% of the total leukocyte population and have an abundance of 1500-6000/µL blood. They are typically 12µm in diameter and contain an irregular (polynuclear) nucleus. Neutrophils are stained by neutral dyes. Neutrophils readily phagocytize bacteria and other pathogens. Lymphocytes These cells play a major role in the immune response. They are mononuclear, possessing a typical spherical nucleus. These cells are typically either 6-10µm (small) or 12-15µm (large) in diameter. They are present in the blood at 15004000/µL. The B-lymphocytes are derived from the bone marrow and are antibody producers. The T-lymphocytes are derived from the thymus and act on pathogens either through direct contact or through the production of lymphokines. WWW.ESAINC.COM 168 Monocytes This cell-type is the precursor to the macrophage. The monocyte contains a lobulated nucleus, is typically 14-20µm in diameter and is present in the blood at 100-1000/µL. At the later stages of inflammation monocytes leave the circulation and enter the damaged tissue. Here they differentiate forming macrophages. These are typically 20-40µm in diameter and do not contain a lobed nucleus. Macrophages are metabolically more mobile and active than monocytes and readily phagocytize bacteria, dead cells and other insoluble material. Macrophages can also be found in the lymphatic system and are also permanently located in a variety of tissues (e.g., the Kupffer cells of the liver). WWW.ESAINC.COM 169 Chapter 3 Damage And Repair The pro-oxidants can react with many molecules present in the cell. Rather than exploring all such reactions this chapter will focus on the damage that prooxidants cause to DNA, proteins, lipids and carbohydrates. Because these molecules are important to cellular homeostasis, a variety of mechanisms have evolved to protect them and detect any that are damaged. Affected molecules can then be repaired, or removed and eliminated. The failure to mend or remove damaged species is associated with a variety of diseases. WWW.ESAINC.COM 170 DNA. Introduction. DNA (deoxyribonucleic acid) is the genetic code of life. The faithful translation of DNA into messenger RNA (mRNA) and transcription of the latter into proteins is essential for normal growth, development and reproduction. It is thus imperative that the integrity and fidelity of a DNA molecule be maintained. Progressive accumulation of oxidative damage to DNA is thought to be central to the development of a number of diseases including cancer and neurodegenerative diseases, as well as aging (Ames (1989); Loft and Poulsen (1996); Wiseman and Halliwell (1996)). Weinberg (1989) noted that mutation of two types of critical genes, the proto-oncogenes1 and tumor suppressor genes, occurred during the development of human cancer. Often, a single base-pair substitution is all that is required to activate proto-oncogenes or to inhibit tumor suppressor genes, ultimately leading to cancer. To better understand how ROS/RNS might play a role in causing such base modifications and other types of DNA damage, we must first examine the structure of DNA. The DNA Molecule. The structure, synthesis and biological properties of the DNA molecule have been the topic of numerous articles and textbooks (e.g., Stryer (1988)) so will not be dealt with in depth here. DNA is a long, fragile, ribbon-like molecule. It is a polymer composed of deoxynucleotide monomers, each of which contains a base, a sugar and a phosphate group (Figure 3.1). The sugar-phosphate groups are linked together to form a structural backbone, while the bases carry genetic information. DNA utilizes four bases that contain either a purine (adenine and guanine) or pyrimidine (cytosine and thymine) (Table 3.1). The sugar in DNA is 2’-deoxyribose.2 A nucleoside consists of a base bonded to a sugar: the C-1 atom of 2’-deoxyribose forms a β (the base lies above the plane of the sugar ring) N-glycosidic bond with either the N-1 of pyrimidines or N-9 of purines. A nucleotide is a phosphate ester (typically at the 5’ position) of a nucleoside. mRNA is similar in structure to DNA but contains a ribose-phosphate backbone and uracil instead of thymine. In 1953 James Watson and Francis Crick showed that DNA is composed of a double helix, the two polymeric chains being held together by hydrogen bonding between specific base pairs located within the helix: adenine pairs with thymine while cytosine pairs with guanine (Figure 3.2). Such specific purine-pyrimidine 1 A proto-oncogene can be present in the human genome. It may have a role in the regulation of normal cell growth and proliferation. Involvement of these genes in a neoplastic process results from a somatic mutation that converts them into oncogenic alleles. 2 A primed number donates an atom of the sugar molecule whereas an unprimed number refers to an atom of the base. WWW.ESAINC.COM 171 pairing is also the consequence of steric factors imposed by the regular helical nature of the sugar phosphate backbone of each polynucleotide chain. NH2 NH2 BASE R N 7 1 N 3N NH 9 N 3 NH 1 Pyrimidine (Cytosine, R=H) O Purine (Adenine) NH2 NH2 N NUCLEOSIDE HO N N N N HO O N O O OH H OH H 2'-Deoxyadenosine 2'-Deoxycytidine NH2 N NUCLEOTIDE O O NH2 P O N N N O N O O O OH H 2'-Deoxyadenosine 5'-Monophosphate (dAMP) P O O O 4' N O 1' OH H 2'-Deoxycytidine 5'-Monophosphate (dCMP) Figure 3.1 Examples Of Bases, Nucleosides And Nucleotides. (The arrows show hot spots for oxidative damage). WWW.ESAINC.COM 172 Base Type Abbreviation Nucleoside Adenine Purine A Adenosine Cytosine Pyrimidine C Cytidine Guanine Purine G(ua) Guanosine Thymine Uracil Pyrimidine Pyrimidine T U Thymidine Uridine DNA or RNA DNA, RNA DNA, RNA DNA, RNA DNA RNA Complementary base T G(ua) C A A Table 3.1 Summary Of Bases, Nucleosides And Watson-Crick Complementary Base Pair. H2N O NH N N N N R O Thymine NH2 N Adenine O N N HN N N R R N O Cytosine R H2N Guanine Figure 3.2 Specific Base Pairing In The DNA Molecule. WWW.ESAINC.COM 173 The DNA molecule can come in a variety of sizes and shapes. For example, in prokaryotic organisms such as E. coli the DNA occurs as a continuous (circular) double stranded molecule. In this bacterium the DNA molecule consists of 4000kb (kilobases) and is 1360µm long and 2nm wide. The axis of the DNA helix can also be tightly twisted into a superhelix. This is not only important in packaging of the DNA molecule (supercoiling leads to a more compact shape) but also affects the ability of the double helix to unwind and interact with other molecules. The longest DNA molecule is found in the eukaryotic organism Drosophila melanogaster. In this organism the linear DNA molecule consists of 62,000kb and is 2.1cm long. Eukaryotic DNA is structurally much more complex than its prokaryotic counterpart. Unlike prokaryotic DNA, eukaryotic DNA is not naked but is tightly bound to a group of small basic proteins called histones. This nucleoprotein complex is called chromatin. Chromatin fibers consist of repeating units composed of DNA/histone complexes termed nucleosomes and these are chained together by “linker” DNA strands much like beads on a string. It has been proposed that nucleosomes are further packed into a solenoidal structure consisting of six nucleosomes per turn of the helix (Finch and Klug (1976)). The purpose of chromatin is to store DNA in a more condensed and manageable form. An inadvertent benefit of such storage is some degree of protection from pro-oxidant attack. NH2 N N O NH2 N cytosine O N O O P O NH2 O N N ROS N O N O NH2 O adenine OH N O O O O cytosine O OH P HO N N 8-hydroxy adenine N O O O NH O O P OH N O O O N O O P O O O O O thymine N O O O O N O O N OH P N O O O Strand Break O O thymine dimer NH HO NH2 guanine O HN OH NH OH O P OH P N HO OH NH N N NH2 O O O O O O 8-hydroxy guanine OH P O Figure 3.3 Examples Of ROS Induced Damage To DNA. For The Sake Of Clarity Only One Of The DNA Strands Is Shown. WWW.ESAINC.COM 174 DNA Damage. DNA is a fairly reactive molecule and is readily attacked by ionizing radiation, oxidizing agents and electrophiles (Figures 3.1 and 3.3). Oxidative damage to DNA includes covalent modification of bases (DNA adducts), production of alkali labile sites and strand breaks, either formed directly or as a consequence of repair processes (Aruoma and Halliwell (1998); Breen and Murphy (1995); Dizdaroglu (1991, 1994); von Sonntag (1991)) (Table 3.2). It must be remembered however that the vast majority of studies in this field have been conducted in vitro and may not always be applicable to circumstances in vivo. Damaging Species Alkylating Agents Hydrogen Peroxide Hydroxyl Free Radical Hypohalous Acids Consequences Exogenous (e.g., dimethylhydrazine and Nmethyl-N-nitrosourea) and endogenous (Sadenosylmethionine) alkylating agents can yield a variety of alkylated adducts. Depending upon the alkylating agent a number of adducts can be formed including 7-methylguanine, 7ethylguanine, 7-(2-hydroxyethyl) guanine, 3methylguanine, O-4 methylthymine, O-4ethylthymine and 0-6 methylguanine. No direct effect on bases but acts as a precursor for hydroxyl free radical production if encountering redox-active metals. Can cause strand scission, and protein-DNA cross-linking, and form a complex variety of DNA adducts and sugar-derived products (Figure 4.4, 4.5). DNA adducts include 8-hydroxy2’deoxyguanosine (8OH2’dG), 5-hydroxy2’deoxycytidine, 8-hydroxy-2’deoxyadenine, thymine glycol isomers, and 2,6-diamino-4hydroxy-5-formamidopyridine. 8-hydroxy2’deoxyadenine may undergo further oxidation to guanidinohydantoin. HOCl rapidly attacks pyrimidines forming thymine glycol isomers, 5-hydroxyuracil, 5-hydroxycytosine 5-hydroxyhydantoin, 5-chlorouracil and 5-chloro2’d-cytidine but see Whiteman et al., (2002). Purines (bases and nucleosides) form 8-chloroand 8-bromo derivatives. References Cushnir et al. (1993); Kang et al. (1992); Netto et al. (1992); van Delft et al. (1993); Tan et al. (1990) Dizdaroglu (1993); Halliwell and Aruoma (1991) Breen and Murphy (1995); Cadet et al. (1999); Dizdaroglu (1991); Duarte et al. (1999); Henle et al. (1996); Lloyd and Philips (1999); Luo et al. (1996); Raoul et al. (1995); von Sontag (1991), Wagner et al. (1992) Birnbaum et al., (1984); Kozumbo et al. (1992); Masuda et al., (2001); Shen et al., (2001); Whiteman et al. (1997, 1999a) Oxidation of some bases is promoted by nitrite due to formation of nitryl chloride. Can activate aryl xenobiotics that are capable of causing single strand breaks. WWW.ESAINC.COM 175 Irradiation Direct – absorbed energy forms DNA radical cations that can further react with water or undergo deprotonation reactions with bases and 2’-deoxyribose forming base-centered radicals and sugar-derived radicals, respectively. Breen and Murphy (1995); Dizdaroglu (1991); Menon (1995); von Sontag (1991) Indirect – solvated electrons do not cause strand breakage but can form a variety of base radicals. Hydroxyl free radicals cause considerable damage (see below). More than thirteen adducts are reported to be formed when DNA is irradiated. Lipid Peroxidation Products Can also lead to the formation of DNA hydroperoxides. Carbonyls – 4-hydroxnonenal, malondialdehyde and other alkyl aldehydes can form DNA adducts (e.g., 1-N2-propano-2’deoxyguanosine; deoxycytidine glyoxal). Hydroperoxides can cause single strand and double strand DNA breaks and produce a variety of DNA adducts. Miscellaneous Carbonyls – acrolein and crotonaldehyde form 1,N2-propanodeoxyguanosine adducts. Catecholestrogens can form a semiquinone (through redox cycling) capable of forming catecholestrogen-adducts with guanine. Superoxide produced when catecholestrogens recycle can react with nitric oxide to produce peroxynitrite that causes DNA strand breakage. Agarwal et al. (1994); Baker and He (1991); Cooke et al., (2003); Douki and Ames (1994); Esterbauer et al. (1990); Goda and Marnet (1991); Li et al. (1995a); Marnet (1985; 1996); Vaca et al. (1995); Yang and Schaich (1996); Wang and Liehr (1991; 1995) Eder and Hoffman (1993); Feinstein et al. (1993); Gebicki and Gebicki (1999); Liehr (1990; 1994); Mobley et al. (1999); (Nath et al. (1998); Spencer et al. (1994); Yoshie and Ohshima (1998) Protein hydroperoxides cause DNA-protein cross linking. Nitric Oxide, Nitrogen Dioxide, Peroxynitrite, other RNS The antitumor antibiotic adriamycin can also redox cycle. Once bound to DNA it can ultimately lead to the production of hydroxyl free radicals causing degradation of double and single stranded DNA. L-DOPA, dopamine and 3-O-methyl-DOPA in the presence of copper can promote oxidative damage to DNA. Can cause RNS-dependent nitration, nitrosation and deamination of bases. Nitrogen dioxide promotes single strand breaks. Peroxynitrite can directly oxidize and nitrate DNA bases producing 8-oxoguanine, 8-oxoguanosine, 8-oxoadenine, 8oxoadenosine, 8-nitroguanine, 2- and 8-nitro adenine (and adenosine), and 8-nitroxanthine. Peroxynitrite can also cause single strand breaks WWW.ESAINC.COM Bartsch and Frank (1996); Bittrich et al. (1993); Bermudez et al. (1999); Burney et al. (1999); Byun et al. (1999); Dawson and Dawson (1994); Gorsdorf et al. (1990); 176 and promote lipid peroxidation, thereby producing cytotoxic carbonyl compounds capable of forming DNA-carbonyl adducts. Peroxynitrite causes mutation in bacteria and human cells. Ozone Singlet Oxygen Superoxide Radical Anion Causes single-strand breaks. Forms DNA adducts (e.g., hydroxymethyluracil, thymine glycol, 8-hydroxyguanine, 8OH2’dG and other unidentified adducts). Can induce lipid peroxidation forming cytotoxic carbonyl compounds (e.g., malondialdehyde, 4hydroxynonenal) capable of damaging DNA. Selectively attacks guanine. Guanosine produces diastereomers of 4,8-dihydro-4-hydroxy-8-oxo2’deoxyguanosine while DNA primarily forms 8OH2’dG. Photochemical ROS generating systems induce the expression of several eukaryotic genes including adhesion molecules, early response genes, immunomodulatory cytokines, matrix metalloproteins, and stress proteins. No direct effect on bases but can act to promote hydroxyl free radical production through the Haber-Weiss reaction. Epe et al. (1996); Harris (1995); Juedes and Wogan (1996); Oshima and Bartsch (1994); Routledge et al. (1994); Sodum and Fiala (2001); Spencer et al. (1996); Szabo (1996); Yermilov et al. (1995, 1996) Bermudez et al. (1999); Cajigas et al. (1994); Calderon-Garciduenas et al. (1997); Ferng et al. (1997); Foksinski et al. (1999) Epe (1993); McCabe et al. (1997); Ravanat and Cadet (1995); Ryter and Tyrrell (1998) and references therein; Van den Akker et al. (1994) Dizdaroglu (1993); Halliwell and Aruoma (1991) Table 3.2 Oxidative Damage To DNA, Free Nucleosides and Bases. Ionizing radiation is arguably the most routinely used approach to promote DNA damage. It can be either absorbed directly by DNA leading to ionization of the bases (direct effect) or react with surrounding water molecules first (indirect effect) producing pro-oxidant species (e.g., hydroxyl free radical, hydrated electrons [e-aq], and hydrogen atoms). In the presence of oxygen it appears that most DNA damage is due to the production of the hydroxyl free radical. The hydroxyl free radical, whether produced through the homolytic effects of ionizing irradiation on water or from the Fenton reaction, adds at diffusion limited rates to double bonds (k>1010 M-1s-1), and efficiently abstracts hydrogen atoms from organic molecules (k=109 M-1s-1). It is much less reactive with phosphate groups of the DNA backbone (k<107 M-1s-1). Thus, hydroxyl free radical-induced damage to DNA is primarily a consequence of the addition to the π-bonds of bases or hydrogen abstraction from deoxyribose3 and the methyl group of thymine. Interestingly under normal physiological conditions neither superoxide 3 A number of modified sugar moieties are formed including both free (e.g., 2,5-dideoxypentos-4-ulose, 2,3-dideoxypentos-4-ulose, 2-deoxypentose-4-ulose and 2-deoxytetrodialose) and DNA-bound forms (e.g., 2-deoxypentanoic acid and erythrose) (Dizdaroglu (1991, 1998)). WWW.ESAINC.COM 177 nor hydrogen peroxide can react directly with deoxyribose or bases (Aruoma et al. (1989); Brawn and Fridovich (1981); Lesko et al. (1980); Rowley and Halliwell (1983)). However, the conversion of these ROS to hydroxyl free radicals or other pro-oxidant species will result in DNA damage (Halliwell and Gutteridge (1988); Nassi-Calo et al. (1989)). It is interesting to note that, of all the radicals found in aerobic cells, only the hydroxyl free radical readily attacks DNA. Pryor (1988) has hypothesized that the hydroxyl free radical is unique in that it has a rare combination of high electrophilicity, high thermokinetic reactivity, and a mechanism of production close to the DNA molecule. NH2 O O H N CH3 O NH OH O H N O N O O HN 8OHGua OCH3 NH 3MGua O O N N NH O NH2 NH2 OH OH O NH H CH3 N O NH CO2H 7MGua H2N TT-DIMER NH U C N O O NH 5OHCt HN H2N HN N NH O6MGua O HN OH N NH N H2N O NH2 NH2 N NH2 N FAPyAd 8OHAd HN NHCHO NH N CH3 N H2N NH2 N OH NH N HN OH NH 2-OHAd N N 5OHMU O N N O NH N NH2 CH2OH N CG O H2N H HO A O H OH OH NH H TG NH N HN NH N 8NitroG NH2 CH3 OH OH H NH N 5HH NH2 N HN NO2 H2N OH NH 5OH5MH HN HN H O N 5-OH-6-HC CO2H CT DIMER H OH H NH H H2N O NH O O H2N HN H HN O O OH CH3 HN OH H NH H N HN H H NH H O 5-OH-6-HT O O HN O H OH H NH H HN O H OH OH NH H O HN O UG 5-OH-6-HU H H NH H 5,6-DHU HN NH 5,6-DOHU O NH2 N OH O NH2 FAPyGua O H N H2N G 5,6-DHT NHCHO HN NH N H2N H2N TL DIMER O O O CH3 OH O OH OH NH CH3 HN O 5,6-DHC NH T Figure 3.4 A Selection Of Modified Bases (from Acworth et al. (1997); Dizdaroglu (1991); Dizdaroglu et al. (1993); Yermilov et al. (1995, 1996)). 2-OHAd – 2-hydroxyadenine; 3-MGua – 3-methylguanine; 7-MGua – 7-methylguanine; 6 O -MGua – O-6-methylguanine; WWW.ESAINC.COM 5-HH – 5-hydroxyhydantoin; 178 5-OHC – 5-hydroxycytosine; 5-OH-6-HC – 5-hydroxy-6-hydrocytosine; 5-OH-6-HT – 5-hydroxy-6-hydrothymine; 5-OH-6-HU – 5-hydroxy-6-hydrouracil; 5-OH5MH – 5-hydroxy-5-methylhydantoin; 5-OHMU – 5-(hydroxy-methyl) uracil; 5,6-DHC – 5,6dihydroxycytosine; 5,6-DHT – 5,6-dihydrothymine; 5,6-DHU – 5,6-dihydrouracil; 5,6-DOHU – 5,6-dihydroxyuracil; 8-NitroGua – 8-nitroguanine; 8-OHA – 8-hydroxyadenine; 8-OHGua – 8-hydroxyguanine; A – adenine; C – cytosine; CG(ua) – cytosine glycol; CT dimer – cytosine-tyrosine dimer; FAPyAd – 4,6-diamino-5-formamido-pyrimidine; FAPyGua – 2,6-diamino-4hydroxy-5-formamido-pyrimidine; G – guanine; T – thymine; TG(ua) – thymine glycol; TL dimer – thymine-lysine dimer; TT dimer – thymine-tyrosine dimer; U- uracil; UG(ua) – uracil glycol. Structures shown in red are electrochemically active and can be measured by HPLC with electrochemical detection. O O H CH2OH N O O N HN N N H2N N R OH O N R 5-OH2'dUd H2N N R 5-OH2'dCd N N N CH3 R N R 5,6-DiOH2'dUd OH N R NH2 NH2 OH OH H R Td-glycol N N H2N H FAPyG OH H O NH N H2N O H N HN 8,5'Cyclo-2'dAdo O N OH N O OH N OH N O N N O 8,5'Cyclo-2'dGuo N H N O H OH N N R 8-OH2'dAdo Figure 3.5 A Selection Of Modified DNA Nucleosides Dizdaroglu H 7-M2'dGuo N OH N O N NH2 HN H O HN O H2N R O6-M2'dGuo O OH N N N H2N 3-M2'dGuo NH2 N N HN R 8-OH2'dG O O N N H2N R 5-HOMdUd H N HN OH OCH3 CH3 N NH R FAPyA (From Acworth et al. (1997); (1994)). 2’Td-glycol – Thymidine glycol isomers; 3-M2’dGuo – 3-methyl-2’deoxyguanosine; 5-OH2’dCd – 5-hydroxy-2’deoxycytidine; 5-OH2’dUd – 5-hydroxy-2’dexoyuridine; 5-OHM2’dUd – 5-(hydroxymethyl)-2’deoxyuridine; 5,6-DiOH2’dUd – 5,6-dihydroxy2’deoxyuridine; 7-M2’dGuo – 7-methyl-2’deoxyguanosine; 8-OH2’dAdo – 8-hydroxy-2’deoxyadenosine; 8-OH2’dG – 8-hydroxy-2’deoxyguanosine; 8,5’Cyclo-2’dAdo – 8,5’-cyclo2’adenosine; 8,5’,Cyclo-2’dGuo – 8,5’-cyclo-2’deoxyguanosine (5’R- and 5’S-); FAPyA – 4-amino-5-formylamino-6-(2’6 deoxyribosyl)-aminopyrimidine; FAPyG – 2-amino-4-hydroxy-5-formylamino-6-(2’deoxyribosyl)-aminopyrimidine; O 6 M2’dGuo – O -methyl-2’deoxyguanosine; R – 2’deoxy-ribose. Structures shown in red are electrochemically active and can be measured by HPLC with electrochemical detection. Those in blue should be electrochemically active. WWW.ESAINC.COM 179 Early pulse-radiolytic studies suggest that about 20% of hydroxyl free radicals produced in close proximity to DNA damage deoxyribose molecules (Breen and Murphy (1995) and references therein). If such damage is not repaired (see below) it can lead to cleavage of the sugar-phosphate backbone resulting in single-strand breaks (SSB) in the DNA molecule.456 SSBs are not usually lethal as the complementary DNA strand serves as a template, holding the severed chain in place long enough for repair enzymes to take action. Indeed treatment of DNA with hydroxyl free radicals derived from hydrogen peroxide resulted in SSBs without significant cell death (Ward et al. (1985)). Double-strand breaks (DSB) are the common result of excessive irradiation. If the DSB are located too close to each other, this can result in severance of the DNA molecule, permanent damage and cell death. Readers interested in learning more about the possible mechanisms for hydroxyl radical-induced hydrogen atom abstraction and sugarphosphate cleavage are referred to Breen and Murphy (1995) and von Sonntag (1991). By far the most intense research in this field has been directed towards the chemistry and biology of DNA adduct formation. Attack of the free bases and nucleosides by pro-oxidants can yield a wide variety of adducts and DNA-protein cross-links (Figures 3.4 and 3.5). Such attack usually occurs at the C-4 and C-8 position of purines and C-5 and C-6 of pyrimidines (Breen and Murphy (1995)). A summary of the reaction products of the interaction between the hydroxyl free radical and the four nitrogen bases of DNA is presented in Table 3.3. Hydroxyl free radical-induced damage to purine bases and nucleosides can proceed through a C-8-hydroxy N-7 radical intermediate (Table 3.3). This radical can either undergo oxidation with the production of an 8-hydroxy purine, or reduction, probably by cellular thiols, followed by ring opening and the formation of FAPy (formamido-pyrimidine) metabolites (see Figure 3.6 for hydroxyl free radical-induced damage to guanosine). Although most research has focused on 8-hydroxy-purine adducts a growing number of publications are attempting to measure the FAPy derivative (see below). While many potential base lesions can be formed when DNA is irradiated, the relative abundance of such lesions varies considerably. Table 3.4 presents the relative abundance of base lesions when either naked DS-DNA or chromatin is exposed to irradiation. Under aqueous oxygenated conditions irradiation is synonymous to hydroxyl free radical damage. Note that the relative abundance of 4 A clastogen is an agent capable of causing breakage of chromosomes. Superoxide may indirectly induce genotoxicity by the formation of long-lived clastogenic factors (CFs). Emerit (1993) hypothesized that CFs induce the production of superoxide radical anions by competent cells which in turn release more CFs thereby promoting clastogenesis. This selfsustaining process may exceed the DNA repair system and ultimately lead to cancer. Increased cancer risk and CF formation are found in irradiated people, those with chronic inflammation, workers exposed to asbestos, HIV-infected people and those with Fanconi’s anemia. 5 DNA strand breaks can be measured using single-cell gel electrophoresis (SCGE, comet assay) (Collins (1998); Fairbairn et al. (1995); McKelvey-Martin et al. (1993); Piperakis et al. (1998)). 6 It has been estimated that 36,000 SSB and >40 DSB occur in each cell/day (Bernstein (1998)). WWW.ESAINC.COM 180 base lesions is much less than for naked DS-DNA, suggesting that the association of histones in chromatin confers some degree of protection.7 A second observation is that 8-hydroxyguanine is the most dominant lesion in both dsDNA and chromatin. Precursor Cytosine Thymine Adenine Intermediate C-6-hydroxy C-5 radical Product 5-Hydroxyhydantoin C-5-hydroxy C-6 radical 5,6-Dihydroxyuracil 5-Hydroxyuracil 5-Hydroxy-5,6-dihydrouracil Deoxyribose radical C-6-hydroxy C-5 radical Pyrimidine-derived cyclonucleoside 6-Hydroxy-5,6-dihydrothymine C-5-hydroxy C-6 radical Thymine glycol isomers N-Tartonoylurea Methylene radical 5-Hydroxymethyldeoxyuracil 5-Formyldeoxyuracil Deoxyribose radical C-4-hydroxy C-5 radical Pyrimidine-derived cyclonucleoside Hydrolyzed and reduced to adenine C-8-hydroxy C-4 radical Oxidized to 8-hydroxyadenine Reduced to FAPy-adenine C-5’ deoxyribose radical Guanine C-4-hydroxy C-5 radical Purine-derived cyclonucleoside (8.5’-cyclo2’deoxyadenosine) Dehydrated and reduced to guanine C-5-hydroxy C-4 radical Dehydrated and reduced to guanine C-8-hydroxy N-7 radical Oxidized to 8-hydroxyguanine Reduced to FAPy-guanine C-5’ deoxyribose radical Purine-derived cyclonucleoside (8.5’-cyclo2’deoxyguanosine) Table 3.3 Oxidative Damage To DNA Bases By The Hydroxyl Free Radical Under Oxygenated Conditions. DNA adducts can also be formed between bases and carbonyl compounds produced as a result of lipid peroxidation (see below). 4-Hydroxynonenal and 7 An alternative explanation is that DNA-protein cross-links can be formed that lead to decreased yields of “protein-free” modified bases (Gajewski et al. (1990)). WWW.ESAINC.COM 181 malondialdehyde are two extremely reactive carbonyl compounds that readily form adducts with DNA (and RNA) bases (Figure 3.7). These compounds are also capable of forming DNA-DNA and DNA-protein (most commonly with lysine) cross-links.8 The chemistries of carbonyl compounds are discussed in more detail below. Adducts can also be formed between bases and catecholestrogens (Chapter 4). O N HN N N H2N R Guanosine HO O N HN H2N OH H N N 8-OH2'dGuo O R O OH +H+ Reduction (Thiols?) +e- H2N N H N N N H2N N HN OH N HN R R +H+ +e- Reduction (Thiols?) O N HN O H N HN H O H2N N N OH H O R H2N N N -e-H+ Oxidation H N HN R H2N FAPyG OH N N R 8-OH2'dG R = deoxyribose Figure 3.6 Damage To 2’Deoxyguanosine By The Hydroxyl Free Radical Produces Both 8-Hydroxy-2’deoxyguanosine (8OH2’dG) And FAPyG. Based on Breen and Murphy (1995). 8 It has been estimated that ~37 (rat) and ~3 (human) DNA-cross links are formed in each cell/day (Bernstien (1998)). WWW.ESAINC.COM 182 Base C T A G Product Naked DNA (Radiation Yields nmolJ-1) Cytosine glycol 5,6-Dihydroxycytosine 5-Hydroxyhydantoin Thymine glycol 5-Hydroxymethyluracil 5-Hydroxy-5methylhydantoin FAPyAd 8-Hydroxyadenine FAPyGua 8-Hydroxyguanine 25.6 43.4 - Chromatin (Radiation Yields nmolJ-1) 2.3 0.3 0.2 0.4 0.05 0.4 5.9 15.8 3.6 46.7 1.0 3.5 1.8 8.1 Table 3.4 Abundance (Radiation Yields) Of DNA Base Adducts Formed Under Oxygenated Conditions As Determined Using GC-MS. (From Fuciarelli et al. (1990); Gajewski et al. (1990) and see Breen and Murphy (1995)). Nitric oxide damages DNA by two major pathways (Bartsch and Frank (1996); Bittrich et al. (1993); Dawson and Dawson (1994); Gorsdorf et al. (1990); Harris (1995); Oshima and Bartsch (1994); Routledge et al. (1994); Spencer et al. (1996); Szabo (1996); Yermilov et al. (1995, 1996)). The first route involves the nitrosation of the amines of the nucleic acid bases (Chapter 2). Primary aromatic amines produce deaminated products, while secondary amines form N-nitroso compounds. The second pathway involves the formation of peroxynitrite from nitric oxide. Peroxynitrite shows complex reactivity with DNA initiating DNA strand breakage, oxidation (e.g., formation of 8-hydroxyguanine, 8-OH2’dG, (5hydroxymethyl)-uracil, and FAPyGua), nitration (e.g., 8-nitroguanine), and deamination of bases (Table 3.2). Peroxynitrite can also promote the production of lipid peroxidation related active carbonyls and cause the activation of NAD+ ADP-ribosyltransferase (Szabo (1996) and references therein). WWW.ESAINC.COM 183 O O H N N O O H N N HN N N R O O N H εdGuo N N O R N M2Guo O N N R N N N N N N N O N O R M3Cd N N EdGuo M1dGuo OH O N N HO NH O N N N O N NH O H H H N N AdGuo 1,2 N N N AdGuo 3,4 N N H 3C NH H 3C NH N R M1dAdo N M1dCd N N R R CdGuo 1 O R O N N N N N N OH O OH H N R R N CdGuo 2 O H O N N H H N N O NH N N N N H O N O R HN N Cd-Guo-X N N N R N εdCd N N εdAdo R N N R M3dAdo Figure 3.7 Some DNA Base-Carbonyl Adducts. (Douki and Ames (1994); Eder and Hoffman (1993); Esterbauer at al. (1991) and references therein; Goda and Marnett (1991). AdGuo 1,2 and AdGuo 3,4 – 1,N2-propano-2’deoxyguanosine isomers derived from acrolein; CdGuo 1 and CdGuo 2 - 1,N2-propano-deoxyguanosine isomers derived from crotonaldehyde; Cd-Guo-X – malondialdehyde cross-link of cytidine and guanosine; εdAdo – 4-hydroxynonenaldeoxyadenosine adduct; εdCd – 4-hydroxynonenal-deoxy cytidine adduct; εdGuo – 4-hydroxynonenal-deoxyguanosine adduct; EdGuo – 1,N2-ethenodeoxy-guanosine; M1dAdo – (mono)-malondialdehyde-deoxyadenosine adduct; M1dGuo – (mono)-malondialdehydepyrimidopurinone adduct; M2Guo – (di)-malondialdehyde-deoxyguanosine adduct; M3Cd – (tri)malondialdehyde-deoxycytidine adduct; M3Ado – (tri)-malondialdehyde-deoxyadenosine adducts. WWW.ESAINC.COM 184 NH H2N O H2N N N N N NH N N O Syn 8-Oxoguanine Adduct Adenine Base Figure 3.8 Mispairing Of 8-Oxoguanine (8-Hydroxyguanine) With Adenine To Give G→T Transversion Mutations. Note That The Guanine Adduct Is Rotated On Its N-Glycosidic Bond Into The Syn Position. The Consequences of Oxidative DNA Damage. There is now considerable experimental evidence reporting the mutagenicity of ROS in prokaryotic (e.g., bacteriophage and plasmid DNA) and eukaryotic cells (De Flora et al. (1989); Hsei et al. (1986); Meneghini (1988); Moody and Hussan (1982); Retel et al. (1993)). Although all DNA bases can be oxidatively damaged, it is the modification of guanine that is the most frequent (Table 3.4). 8OH2’dG is the most abundant DNA adduct. This adduct was first discovered by Kasai and has recently been reviewed by him (Kasai (1997)). 8OH2’dG can exist in several tautomeric forms (e.g., 8-oxo-2’deoxyguanosine or 7,8-dihydro-8-oxo2’deoxyguanosine) that can affect its hydrogen bonding between base-pairs (Culp et al. (1989)). These base-pair substitutions are usually found clustered into areas called “hot spots”. As shown in Figure 3.2, guanine normally binds to cytosine. 8OH2’dG, however, can form hydrogen bonds with adenine (Figure 3.8). The formation of 8OH2’dG in DNA can therefore result in a G→T transversion.9 Upon replication this mutation has been found to occur with a frequency of 0.5-1.0% in bacteria and 2.5-4.8% in simian kidney cells (Moriya (1993); Wood et al. (1990)). 8-Hydroxyguanine was also shown to induce codon 12 activation of c-Ha-ras and K-ras in mammalian systems (Loft and Poulsen (1996) and references therein). Altered codon 12 activity has been postulated to 9 A transversion is the replacement of a purine with a pyrimidine or a pyrimidine with a purine. A transition is the replacement of one purine with another purine, or one pyrimidine with another pyrimidine. WWW.ESAINC.COM 185 contribute to the initiation and progression of human cancer (Capella et al. (1991); Seeburg et al. (1984); Yanez et al. (1987)). G→T transversions are also the most frequent hot spot mutations found in the p53 supressor gene which is associated with human tumors (Harris and Holstein (1993); Hollstein et al. (1991)). Singlet oxygen and 1,2-dioxetanes preferentially induce 8OH2’dG formation causing GC to AT base-pair substitutions (Loft and Poulsen (1996) and references therein). Apurinic sites10 are mispaired mainly with adenine. As is the case with 8OH2’dG , the frequency of such mispairing is dependent upon the adjacent base sequence and which polymerase is present (Loft and Poulsen (1996) and references therein). Adenine adducts (e.g., 8-hydroxy-2’deoxyadenosine) do not seem to mispair or lead to mutations (Shibutani (1993)). Oxidation products of cytosine (e.g., 5-hydroxycytosine, 5-hydroxyuracil) show sequence-context-dependent mispairing in vitro resulting in C→T transitions and C→G transversions (Breen and Murphy (1995) and references therein; Purmal et al. (1994)). Thymine glycol was shown to cause a low frequency T→C transition in a SS-DNA bacteriophage M13 (Basu et al. (1989)). However, this adduct was without effect on DS-DNA from another phage, probably due to the greater ability of its endonuclease III to repair thymine glycol lesions. Although not studied in great detail, another thymine adduct, 5-(hydroxymethyl)-2’deoxyuridine, may be worth investigating further. It appears to miscode during DNA replication and induces a high incidence of mutations in Salmonella typhimurium (Patel et al. (1992); ShirnameMore et al. (1987)). Thymine glycol, FAPyAdo, FAPyGuo and 8-hydroxyadenine appear not to induce base-pair modifications but rather act by blocking DNA and RNA polymerases and are thought to be lethal (Evans et al. (1993); Ide et al. (1994); Maccabee et al. (1994); O’Connor et al. (1998)). Several other mechanism by which ROS/RNS can lead to mutations have been proposed. Direct mechanisms include: conformational changes in the DNA template that reduces the accuracy of replication by DNA polymerases (Feig and Loeb (1993); Wiseman and Halliwell (1996) and references therein), and altered methylation of cytosine that affects gene control (Weitzman et al. (1994)). Indirect mechanisms include oxidative damage to proteins, including DNA polymerases and repair enzymes. Damage to lipids causes the production of mutagenic carbonyl compounds (Wiseman and Halliwell (1996) and references therein). Finally, Halliwell and Wiseman (1996) suggested that ROS/RNS might be involved in misalignment mutagenesis (“slippery DNA”) which is associated with 10 Apurinic and apyrimidinic (AP) sites result from hydrolysis of a weakened N-glycosyl linkage, a consequence of adduct formation. Such hydrolysis is also catalyzed by N-glycosylases as part of the excision-repair process (see below) and by treatment with heat, acid, alkylating and nitrating agents (Loeb and Preston (1986); Yermilov et al. (1995)). AP sites not only direct the incorporation of adenine opposite the lesion but can also inhibit DNA synthesis. Fortunately, such lesions can be bypassed by DNA polymerase. It has been estimated that ~7000 depurination events occur in each cell per day (Bernstein (1998)). WWW.ESAINC.COM 186 cancer and several genetic diseases such as myotonic dystrophy and Huntington’s disease (Kunkel (1993)). Repair of ROS/RNS-induced DNA Damage. The repair of damaged DNA is an ongoing and continuous process involving a number of repair enzymes (Table 3.5). Damaged DNA appears to be mended by two major mechanisms, base excision repair (BER) and nucleotide excision repair (NER) (Croteau and Bohr (1997); Singer and Hang (1998) and references therein). However, as isolated DNA is found to contain low levels of damaged bases, it would appear that these repair processes are not completely effective. Although sugar damage and double strand breaks are also critical lesions they have been extensively reviewed so will not be dealt with here (Lieber et al. (1996); Povirk (1996); Weaver (1996)). ENZYME DNA Alkyltransferase DNA Glycosylase ACTION Removes alkyl group from affected base. For example, O6-alkylguanine alkyltransferase repairs O6-methyl2’deoxyguanosine. Simple glycosylase - removes damaged base by cleaving the Nglycosidic bond forming an apurinic (or apyrimidinic) [AP] base. Phosphodiester bonds either side of the AP site are cleaved by endonucleases allowing insertion of intact nucleotide. Some glycosylases hydrolyze the N-glycosylic bond and possess lyase activity that cleaves resulting AP site producing a 3’ terminal and an unsaturated aldehyde that must be removed by endonuclease IV. DNA AP Endonuclease Some examples of glycosylases include: Uracil-DNA glycosylase (recognizing uracil) Hydroxymethyluracil-DNA glycosylase (recognizing hydroxymethyluracil) Hypoxanthine-DNA glycosylase (recognizing hypoxanthine) Pyrimidine hydrate-DNA glycosylase (recognizing thymine glycols, pyrimidine hydrates, ring-fragmented pyrimidines, urea) Formamidopyrimidine-DNA glycosylase (recognizing purines with a fragmented imidazole ring; 8-hydroxyguanine) 3-Methyladenine-DNA glycosylase (recognizing purines methylated at N3 or N7; pyrimidines at O2 position) 8-Oxoguanine glycosylase/lyase (recognizing 8-oxoguanine) – OGG1 and MutM Pyrimidine dimer-DNA glycosylase (recognizing cyclobutane pyrimidine dimers). Recognizes an AP site and nicks the phosphodiester bonds of DNA strand. Damaged DNA is then removed. Some enzymes such as endonuclease III possess both glycosylase and endonuclease activity for repair of oxidized pyrimidines. Endonuclease IV removes the 3’ blocking damage resulting from the action of Class 1 endonucleases. WWW.ESAINC.COM 187 DNA Exonuclease DNA Helicases DNA Ligase DNA Polymerases NAD+ ADPRibosyltransferase Degrades DNA by cleaving successive nucleotide residues (or short oligonucleotides) from either the 5’ or 3’ ends. For example, exonuclease III cleaves 5’ to the AP site. Unwinds DNA to facilitate separation of the two strands of the duplex. Joins newly synthesized DNA to the rest of the strand. Synthesizes DNA strand (fills the gaps left by exonucleases and endonucleases). Also called poly(ADP)ribose synthetase (PARS) and poly(ADP)ribose polymerase (PARP). This enzyme responds to DNA strand breaks by promoting the poly-ADP ribosylation of nuclear proteins. The alteration of the DNA-histone structure enhances the activity of DNA ligase for repairing strand breaks. Over-activity of the ribosyltransferase can be deleterious, resulting in rapid consumption of NAD+ and the depletion of ATP, eventually leading to cell death. Table 3.5 Some DNA Repair Enzymes. (See Demple and Harrison (1994)). Base Excision Repair. BER is first started by DNA glycosylases which recognize specific base modifications (e.g., 8OH2’dG) (Figure 3.9). For example, formamido-pyrimidineDNA glycosylase (Fpg protein) recognizes damaged purines such as 8oxoguanine and FAPyGua (and to a lesser extent adenine derivatives) (Boiteux et al. (1992)). Damaged pyrimidines are recognized by endonuclease III, which acts as both a glycosylase and AP endonuclease (Bohr et al. (1995); Croteau and Bohr (1997)). Glycosylases cleave the N-glycosylic bond between the damaged base and the sugar. There are two classes of glycosylases: simple glycosylases (that only hydrolyze N-glycosylic bonds between inappropriate base and deoxyribose sugar to release the base and produce an unmodified AP site) and glycosylase/AP lyase enzymes (that also cleave the resulting AP sites at the DNA-phosphate backbone). Following the glycosylase step, AP endonucleases then remove the 3'-deoxyribose moiety by cleavage of the phosphodiester bonds thereby generating a 3’-hydroxyl group that can then be extended by DNA polymerase. The final step in mending damaged DNA is the rejoining of the free ends of DNA by a DNA ligase (Seeberg et al. (1995)). It appears that the presence of 8-oxoguanine modified bases in DNA is not only a result of ROS attack on this macromolecule (Figure 3.9). Oxidized nucleosides and nucleotides from free cellular pools can also be incorporated into DNA by polymerases and cause AT to CG base substitution mutations (Kamiya et al. (1992); Shibutani et al. (1991)). To prevent this bacteria contain both BER and error avoidance mechanisms (Grollman and Moriya (1993); Mo et al. (1992); Taddei et al. (1997)). Three enzymes are involved in error avoidance including an 8-oxoguanine glycosylase/AP lyase (MutM or Fpg protein), adenine DNA glycosylase (MutY) and 8-hydroxy-2’deoxyGTPase (Fowler et al., (2003)). The MutY enzyme removes adenine that has been misincorporated opposite 8- WWW.ESAINC.COM 188 oxoguanine in DNA while the MuT enzyme prevents incorporation of damaged GTP into DNA (Michaels et al. (1992); Tajiri et al. (1995)). Functional homologs of these proteins are also found in higher eukaryotes (e.g., OGG1 corresponds to MutM; MTH1 to MutT; MYH to MutY) (Boiteux and Radicalla (1999); Croteau and Bohr (1997); Furuichi et al. (1994); Hayakawa et al. (1995); Hazra et al., 2001); Ide (2001); Nashimua (2002); Sekumi et al. (1993); Wani and D’Ambrosia (1995)). O N HN N H 2N NH Guanine ROS O (Acid Hydrolysis) N HN H 2N Glycosylase Diet OH N NH 8-Hydroxy-guanine OH ROS Transcription ROS OH OH mRNA Modified DNA DNA Modified mRNA (Enzymatic Hydrolysis) O Endonuclease O N HN H 2N Polymerase N N HO N HN OH OH H 2N N N HO O OH 8-Hydroxy-2'deoxyguanosine O OH OH 8-Hydroxy-guanosine Mut-T(MTH1) dGMP-OH dGTP-OH ROS dGTP Figure 3.9 Oxidative Damage Can Occur To DNA, mRNA, And The Free Nucleotide Pool. This figure uses guanine as an example and illustrates base damage and repair. Also shown in parenthesis are the in vitro methods used to liberate bases or nucleosides for analysis. WWW.ESAINC.COM 189 Nucleotide Excision Repair. Bacteria and eukaryotic cells supplement BER with NER mechanisms (Lindahl (1993); Satoh et al. (1993); Van Houten (1990)). NER involves two components, a global repair element and a transcription coupled repair mechanism (Croteau and Bohr (1997)). This explains why transcription coupled repair occurs more rapidly in transcriptionally active DNA rather than in the genome as a whole, a consequence of faster repair of lesions in the transcribed strand (Gowen et al. (1998) and references therein; Friedberg (1996)). The integration of DNA repair and transcription is the responsibility of the basal transcription factor TFIIH, which contains at least two DNA repair genes (Friedberg (1996)). To date very little is known about the role of NER in DNA repair. Mitochondrial DNA Repair. The mitochondrial genome consists of a circular DNA molecule (16,569 bp) encoding 13 polypeptides, 2 ribosomal RNA and 22 transfer RNA molecules, and each mitochondrion contains 10 copies (Wallace (1992)). The mitochondrion genome encodes the various complexes of the electron transport chain, but contains no genetic information for DNA repair enzymes. These enzymes must be obtained from the nucleus. As mitochondria are continuously producing DNAdamaging pro-oxidant species, effective DNA repair mechanisms must exist within the mitochondrial matrix in order for these organelles to function. However, these mitochondrial repair mechanisms do appear to operate slower than in the nucleus (Yakes and Van Houten (1997)). Fortunately mitochondria are not longlived and its been estimated that one mitochondrion turns over per cell every 15 minutes. Thus excessively damaged mitochondria will be quickly removed. The early finding that mitochondria could not repair UV-induced pyrimidine dimers led to the erroneous conclusion that they lack DNA repair enzymes (Clayton et al. (1974)). Indeed accumulating evidence now suggests that mitochondria contain many BER enzymes and are proficient at repair (Croteau and Bohr (1997); Bohr et al., (2002)). However, mitochondria do not appear to repair damaged DNA by NER mechanisms (Croteau and Bohr (1997); Van Houten (1998)). Single Strand DNA Damage and PARP Activation. Single strand DNA breakage activates NAD+ ADP-ribosyltransferase (PARP; Table 4.5). PARP is a protein-modifying, nucleotide-polymerizing enzyme and is found at high levels in the nucleus (Ueta and Hayashi (1985)). Activated PARP first cleaves NAD+ into ADP-ribose and nicotinamide, and then attaches the ADP-ribose units to a variety of nuclear proteins including histones and its own automodification domain. PARP then polymerizes the initial ADP-ribose WWW.ESAINC.COM 190 modification with other ADP-ribose units to form the nucleic acid-like polymer, poly (ADP) ribose. PARP only appears to be involved with BER and not NER. In BER PARP does not appear to play a direct role but rather it probably helps by keeping the chromatin in a conformation that enables other repair enzymes to be effective (Wiseman and Halliwell (1996) and references therein). It may also provide temporary protection to DNA molecules while it is being repaired. Interestingly, recent conflicting evidence suggests that PARP may not be an important DNA repair enzyme as cells from a PARP knockout mouse model have normal repair characteristics (Wang et al. (1995)). Other possible physiological roles for PARP include slowing of cellular metabolism as an adaptive response to environmental conditions, regulation of gene expression and cell differentiation, regulation of histone shuttling, and nucleosome unfolding. PARP is also involved in the expression of the major histocompatibility complex class II gene, ras, DNA methyltransferase gene, protein kinase C and i-NOS (Szabo (1996) and references therein). Activation of PARP can be dangerous to the cell. For each mole of ADP-ribose transferred, one mole of NAD+ is consumed, and through the regeneration of NAD+ four ATP molecules are wasted (Dawson and Dawson (1994)). Thus the activation of PARP can rapidly deplete a cell’s energy store and even lead to cell death. Some researchers suggest that this may be one mechanism whereby cells with excessive DNA damage are effectively removed. However, a variety of diseases may involve PARP overactivation including circulatory shock, CNS injury, diabetes, drug-induced cytotoxicity, and inflammation (Szabo (1996) and references therein). What Do The Levels Of DNA Adducts Mean? The measurement of oxidative base damage as an indicator of oxidative stress can fall into two broad categories – steady state (tissue concentration) and total levels (rate of excretion) (Poulsen and Loft (1998)). What category is measured is dependent upon the question that is being asked. Remember that a tissue steady-state level will not represent the total adduct formation of the whole organism. Conversely, the rate of excretion will not be due to just one tissue, but may be greatly or primarily influenced by one. Only direct experimental evidence will allow tissue contribution to be determined. Steady State Levels. The steady state DNA adduct level reflects the balance between damage, repair, dilution of unrepaired adducts during DNA replication as cells divide and incorporation of adduct nucleotides during DNA replication. Many different DNA adducts are currently being measured (Table 3.6) but 8OH2’dG is by far the most common (Collins et al. (1996)). Both nuclear and/or mitochondrial DNA levels of WWW.ESAINC.COM 191 8OH2’dG have been shown to be increased in a variety of diseases including colorectal cancer, coronary heart disease, diabetes, inflammation, neurodegeneration, and following irradiation (Beal (1997) and references therein; Collins et al. (1998a,b); Dandona et al. (1996); Shimoda et al. (1994); Wilson et al. (1993)). It should come as no surprise that the oxidative damage to mitochondrial DNA appears to be greater than nuclear DNA, a consequence of this organelle’s ability to generate ROS. This finding is complicated however by methodological issues (below and see Beckman and Ames (1999), and Suter and Richter (1999)). Lesion 2-Hydroxyadenine Species Human Tissue/ Cellular Source Of DNA Brain region: Temporal lobe Frontal lobe 5-Hydroxy cytosine Human Brain region: Temporal lobe Frontal lobe 5-Hydroxy2’deoxycytidine 5-Hydroxy2’deoxycytidine 5-Hydroxy2’deoxycytidine Calf Thymus Human Leukocyte Rat 5-Hydroxy2’deoxyuridine 5-Hydroxy2’deoxyuridine 5-Hydroxy2’deoxyuridine Calf Liver Kidney Brain Thymus Human Leukocyte Rat 5-Hydroxymethyluracil 5-Hydroxymethyluracil Human Liver Kidney Brain Leukocyte Human Lung 5-Hydroxyuracil Calf 5,6 Dihydroxy- Calf Level/Range Method Reference GC-MS Lyras et al. (1997) GC-MS Lyras et al. (1997) HPLC-UV or HPLC-ECD HPLC-UV or HPLC-ECD HPLC-UV or HPLC-ECD Wagner et al. (1992) Wagner et al. (1992) Wagner et al. (1992) HPLC-UV or HPLC-ECD HPLC-UV or HPLC-ECD HPLC-UV or HPLC-ECD Wagner et al. (1992) Wagner et al. (1992) Wagner et al. (1992) GC-MS Djuric et al. (1991) Jaruga et al. (1994) 0.13 nmol/mg DNA control 0.14 – Alzheimer’s 0.02 nmol/mg DNA control 0.04 – Alzheimer’s 0.67 nmol/mg DNA control 0.5 – Alzheimer’s 0.12 nmol/mg DNA control 0.12 – Alzheimer’s 10+2.5 fmol/µg DNA Thymus 3.2+1.6 fmol/µg DNA 10+3.5 fmol/µg DNA 9.9+4.4 22.6+3.4 10+4.0 to 75+0.25 fmol/µg DNA 2.1+1.8 fmol/µg DNA <0.5 fmol/µg DNA <0.5 <0.5 93+19 adducts/105 pb 4-15 adducts/105 pb normal 5-19 – cancer 0.5 nmol/mg DNA HPLC-ECD Thymus 10+4.0 fmol/µg DNA HPLC-UV or WWW.ESAINC.COM GC-MS-SIM Kaur and Halliwell (1996) Wagner et al. 192 dihydro2’deoxyuridine 5,6 Dihydroxydihydro2’deoxyuridine 5,6 Dihydroxydihydro2’deoxyuridine HPLC-ECD (1992) Human Leukocyte 6.2+4.6 fmol/µg DNA HPLC-UV or HPLC-ECD Wagner et al. (1992) Rat Liver HPLC-UV or HPLC-ECD Wagner et al. (1992) 8-Hydroxyadenine Calf Kidney Brain Thymus 8.5+3.5 fmol/µg DNA 10.3+4.0 14.6+4.5 0.8 nmol/mg DNA HPLC-ECD 8-Hydroxyadenine 8OH2’dG Human Lymphocyte C. elegans Cell culture 8OH2’dG Hamster Kidney Kaur and Halliwell (1996) Podmore et al. (1998) Bogdanov et al. (1999) Han and Liehr (1994) 8OH2’dG Human Food/ Beverages 8OH2’dG Human Liver Mixed diet 3000kcal/day Tea Coffee Blood – red blood cells Kidney dialysate Plasma Saliva Sweat Blood mononuclear cells 8OH2’dG Human 8OH2’dG Human CSF 8OH2’dG Human CSF 8OH2’dG Human CSF 8OH2’dG Human Placenta 8OH2’dG Human Brain 8OH2’dG Human Brain region 0.05+0.025 nmol/mg DNA 11.24+5.36 pg/mL GC-MS 3.6+1.2 adducts/105 pb 10.4+1.8 1.09+0.56 nmol/g HPLC-ECD 49 pmol/L 39 pmol/L 2.1+0.31 pg/mL – control 67.34+20.31 13.4+2.11 15.3+3.36 11.2+9.5 15.3 to 73.5 fmol/µg DNA control 96 to 223 – IDDM1 64 to 134 – MIDDM2 64.3+20 ng/mL – control 25.1+12 Alzheimer’s 0.98+0.03 pg/mL 1.5+0.2 pg/mL – control 2.1+0.2 – ALS 1.2+0.2 – other neurological disorders 0.2 to 1.0 adducts/105 pb 1.3 to 7.8 4.0+0.8 pmol/µg DNA – control 21.0+7.0 – Alzheimer’s 1.25+0.13 to 2.7+0.6 adducts/105 pb – nuclear WWW.ESAINC.COM HPLC-ECD HPLC-ECD Bogdanov et al. (1997) HPLC-ECD Bogdanov et al. (1999) HPLC-ECD Dandona et al. (1996) GC-MS Lovell et al. (1999). HPLC-ECD Bogdanov et al. (1999) Bogdanov et al. (2000) HPLC-ECD HPLC-ECD ELISA Yin et al. (1995) GC-MS Lovell et al. (1999) HPLC-ECD Mecocci et al. (1993) 193 15.78+8.3 to 27.34+14.7 – mitochondrial 5.1+3.3 adducts/105 pb – normal 3.6+1.9 – cancer tissue 3.6+1.1 adducts/105 pb – normal 5.6+2.3 – cancer tissue 3.9+0.26 adducts/105 pb 0.33+0.08 adducts/105 pb – control 0.51+0.25 – smokers 5.9+3.8 adducts/105 pb – control 7.1+4.3 – smokers 1.0+0.2 adducts/105 pb 8 adducts/105 pb control 16 to 112 – irradiated subjects 1.5+0.2 adducts/105 pb control 3.3+1 – smokers 4.5 to 13.4 adducts/105 pb – control 3.2 to 6.0 – cirrhotic tissue 1.6+0.7 adducts/105 pb – normal 3.2+2.1 – inflamed tissue 25-75 adducts/105 pb – normal 50-200 – cancer tissue 2.2 to 3.5 adducts/105 pb 0.58 to 0.90 adducts/105 pb male 0.33 to 0.51 female 8OH2’dG Human Breast tissue 8OH2’dG Human Kidney 8OH2’dG Human Leukocyte 8OH2’dG Human Leukocyte 8OH2’dG Human Leukocyte 8OH2’dG Human Leukocyte 8OH2’dG Human Leukocyte 8OH2’dG Human Leukocyte 8OH2’dG Human Liver 8OH2’dG Human Liver 8OH2’dG Human Lung 8OH2’dG Human Lymphocyte 8OH2’dG Human Lymphocyte 8OH2’dG Human Mononuclear leukocyte 1.16+0.4 adducts/105 pb Polymorphonuclear Leukocyte 1.13+0.4 adducts/105 pb WWW.ESAINC.COM HPLC-ECD Nagashima et al. (1995) HPLC-ECD Okamoto et al. (1994) HPLC-ECD Degan et al. (1995) Kiyosawa et al. (1990) HPLC-ECD HPLC-ECD Lagorio et al. (1994) HPLC-ECD Hanaoka et al. (1993) Wilson et al. (1993) TLC-32P, HPLC-ECD HPLC-ECD Lodovici et al., (2000) HPLC-ECD Carmichael et al. (1995) HPLC-ECD Shimoda et al. (1994) GC-MS-SIM Jaruga et al. (1994) HPLC-ECD Bashir et al. (1993) Collins et al. (1998) HPLC-ECD HPLC-ECD Takeuchi et al. (1994) 194 8OH2’dG Human Plasma 8OH2’dG Human Plasma cryoprecipitates 12.9+0.7 – control 17.7+1.2 – ALS 17.8+1.2 – other neurological disorders 0.37+0.04 to 2.27+0.06 pmol/µg DNA 0.61+0.05 to 1.89+0.07 10 to 100 adducts/105 pb – control 30-180 – hyperplastic tissue 200ng/mL HPLC-ECD Bogdanov et al. (2000) HPLC-ECD Lunec et al. (1994) GC-MS 8OH2’dG Human Prostate 8OH2’dG Human Serum 8OH2’dG Human Sperm 8OH2’dG Human Uterine Tumor 8OH2’dG Mouse Keratinocyte 8OH2’dG Mouse 8OH2’dG Mouse Liver – maternal Embryo Liver 8OH2’dG Mousehairless Skin cells 8OH2’dG Rat 0.32+0.009 pg/mL 0.7+0.1 pg/g HPLC-ECD HPLC-UV GC-MS HPLC-ECD GC-MS-SIM Olinski et al. (1995) ELISA Cooke et al. (1998) Fraga et al. (1991) Foksinski et al. (2000) 2.1+3.2 adducts/105 pb 0.81 adducts/105 bp – control 1.24 – tumor 0.56 – lymphocytes 1.4 adducts/106 pb HPLC-ECD 27+8 fmol/µg DNA HPLC-ECD ~8+6 0.8 to 1.8 adducts/104 pb 4.5+0.38 adducts/105 pb 8OH2’dG Rat Brain microdialysate Muscle microdialysate Feces 8OH2’dG Rat Heart 8OH2’dG Rat Calf Liver Thymus 8OH2’dG Rat Liver 8OH2’dG Rat Liver 8OH2’dG Rat Liver 8OH2’dG Rat Liver 1.00+0.1 nmol/mg DNA – control 1.50+0.1 – ischemia/reperfusion 1.74+0.6 adducts/105 pb 5.97+1.4 2.87+0.48 adducts/105 pb 0.96+0.37 adducts/105 pb 2.3 to 2.7 adducts/105 pb 20 adducts/106 pb 8OH2’dG Rat Various 8 to 73 adducts/106 HPLC-ECD HPLC-ECD HPLC-ECD HPLC-ECD HPLC-ECD Beehler et al. (1992) Liu and Wells (1995) Faux et al. (1992) HattoriNakakuki et al. (1994) Bogdanov et al. (1999) 0.19+0.008 WWW.ESAINC.COM Bogdanov et al. (1999) Cordis et al. (1998) HPLC-ECD Adachi et al. (1995) HPLC-ECD Denda et al. (1994) Adachi et al. (1993) Cattley and Glover (1993) Teixeira et al. (1995) Fraga et al. HPLC-ECD HPLC-ECD 195 Rat organs Kidney 8OH2’dG/ 8-hydroxyguanosine Human Liver Brain CSF 8OH2’dG/ 8-hydroxyguanosine Human Serum 8-hydroxy-2’ deoxyguanosine-5’monophosphate 8-Hydroxyguanine Human A549 cells Human Brain – substantia nigra 8-Hydroxyguanine 8-Hydroxyguanine Human Lymphocyte Rat Liver 8-Hydroxyguanosine (RNA) Human Liver – RNA Brain region: Temporal lobe 8OH2’dG Frontal lobe 8-Hydroxyguanosine (RNA) Human Plasma 8-Hydroxyguanosine (RNA) 8-Hydroxyguanosine (RNA) 8-Hydroxyguanosine (RNA) 8-Hydroxyguanosine (RNA) 8-Hydroxyguanosine (RNA) Human Plasma Human Serum Human CSF Human CSF Human Brain region: Temporal lobe pb 15 to 35 fmol/µg DNA 8 to 23 6 to 15 1.46+0.83 ng/mL – control 2.85+2.43 – Parkinoson’s 35.7+15 ng/mL – control 57.5+20.4 – Parkinoson’s 0.43+0.06 adducts/106 2.3 nmol/mg DNA – control 5.3 – Parkinson’s disease 0.25+0.07 nmol/mg DNA 1.06+0.55 adducts/105 pb 0.82+0.46 3.1nmol/mg DNA control 3.5 – Alzheimer’s 1.2 nmol/mg DNA control 0.7 – Alzheimer’s 1.42+0.59 nM Control 1.49+0.54 Parkinsons 127+14 fmol/mL 960+150 fmol/mL control 5000+690 diabetic 97+32pM – Control 500+213 – Alzheimer’s 97+32 pM Control 288+129 Parkinsons 3.1 nmol/mg DNA control 3.5 – Alzheimer’s WWW.ESAINC.COM HPLC-ECD (1990) Shigenaga and Ames (1991) ELISA Kikuchi et al., (2002) ELISA Kikuchi et al., (2002) HPLC-ECD Mei et al., (2003) GC-MS Alam et al. (1997) GC-MS Podmore et al. (1998) Fiala et al. (1989) HPLC-ECD GC-MS Lyras et al. (1997) HPLC-ECD Abe et al., (2003) HPLC-ECD Park et al. (1992) HPLC-ECD Shin et al., (2001) HPLC-ECD Abe et al. (2002) HPLC-ECD Abe et al., (2003) GC-MS Lyras et al. (1997) 196 Frontal lobe “DNA” Adducts Human Cervix FAPy-Ad Human Brain region: Temporal lobe Frontal lobe FAPy-Gua Calf Thymus FAPy-Gua Human Lung FAPy-Gua Human Brain region: Temporal lobe FAPy-Gua Human Brain – substantia nigra C8-Methylguanine Rat Liver Colon N7-Methylguanine Mouse Kidney Rat Liver Brain Liver Colon O4-Ethylthymine 6 O -Methylguanine Human Liver Human Leukocyte Liver 32 P postlabeling Simons et al. (1993) GC-MS Lyras et al. (1997) HPLC-ECD Kaur and Halliwell (1996) Jaruga et al. (1994) 0.22 nmol/mg DNA control 0.29 – Alzheimer’s 0.05 nmol/mg DNA control 0.04 – Alzheimer’s 1.0 nmol/mg DNA 25-33 adducts/105 pb normal 50-120 – cancer tissue Frontal lobe N7-Methylguanine 1.2 nmol/mg DNA control 0.7 – Alzheimer’s 3.81+2.13 adducts/108 pb – control 5.89+3.7 – smokers GC-MS-SIM GC-MS Lyras et al. (1997) GC-MS Alam et al. (1997) HPLC-Fl Netto et al. (1992) HPLC-ECD Tan et al. (1990) HPLC-Fl Netto et al. (1992) HPLC-UV and 32P postlabeling Kang et al. (1992) HPLC-UV and 32P postlabeling Kang et al. (1992) 10.5 nmol/mg DNA – control 9.5 – Alzheimer’s 0.8 nmol/mg DNA control 0.7 – Alzheimer’s 3.2 nmol/mg DNA – control 2.4 – Parkinson’s disease n.d. – basal 103 µmol/mol guan stim. n.d. – basal 139 – stimulated 13.5+0.5 to 31.5+6.5 µmol/mol DNA 10.4+1.0 to 23.6+3.0 14.8+0.6 to 18.6+1.4 387 µmol/mol guan – basal 7445 – stimulated 671 – basal 2318 – stimulated 1.9 to 8.7 adducts/108 n.d. 1.1 to 4.2 adducts/108 WWW.ESAINC.COM 197 O6-Methylguanine Rat Leukocyte Liver Colon 0.8 to 1.6 n.d. – basal 837 µmol/mol guan stim. n.d. – basal 99 – stimulated 3.9 to 7.5 adducts/108 O4-Methylthymine Human Liver Human Leukocyte Brain region: Temporal lobe n.d. Thymine glycol Frontal lobe 0.2 nmol/mg DNA control 0.35 – Alzheimer’s HPLC-Fl Netto et al. (1992) HPLC-UV and 32P postlabeling Kang et al. (1992) GC-MS Lyras et al. (1997) 1.4 nmol/mg DNA control 1.35 – Alzheimer’s Table 3.6 Some Tissue Levels Of DNA (and RNA) Adducts Reported In The Literature. 1IDDM - insulin-dependent diabetes mellitus. 2NIDDM - non- insulin-dependent diabetes mellitus. pb – parent base (or nucleoside); n.d. – not determined. MS-SIM – mass spectrometry with single ion monitoring. Based on the levels presented in Table 3.6, it can be seen that the frequency of adduct formation can be as high as 7 adduct/104 parent bases, but the range is typically on the order of 1-5 adducts/105 bases. An average of 3 adducts/105 bases translates into ~6 x 106 adducts per cell, a phenomenal amount (Helbock et al. (1998)). Critical evaluation of published values of DNA adduct formation has led some researchers to question artifactual production of adducts during sample preparation and analytical procedures (see below). Even with improved analytical procedures, 4 adducts/107 parent bases, the equivalent of 24,000 adducts per cell were reported (Helbock et al. (1998)). At present it is not clear whether such damage is occurring in introns or exons, or in active or quiescent genes. It is however most likely that such damage is taking place in exposed DNA rather than the condensed DNA occurring in chromatin (e.g., Table 3.4). Another question that still awaits an answer is what contribution dead or dying cells make to adduct levels. As Helbock points out, apoptotic cells may increase the overall tissue adduct level while having little deleterious biological effect. Total Adduct Levels. One estimation of total DNA damage is obtained from the measurement of urinary DNA adduct “markers”. Several possible markers are now being explored but most research has focused on 8OH2’dG (Table 3.8). This marker is unaffected by diet and is not produced from mRNA catabolism. In addition, the output of 8OH2’dG is significantly elevated in a variety of conditions that are thought to be associated with increased oxidative stress (e.g., Alzheimer’s disease, amyotrophic lateral sclerosis, cancer, cystic fibrosis, and smoking) (Bogdanov et al. (1997)). However, not all “stressful” conditions are associated WWW.ESAINC.COM 198 with higher levels (e.g., immediately following intense exercise) (Kasai (1997); Loft and Poulsen (1996)). Marker 5-Hydroxymethyl uracil 5-Hydroxy uracil 8-Hydroxyadenine 8-Hydroxy-2’deoxyadenosine 8-Hydroxyguanine 8-Hydroxyguanine 8-Hydroxyguanosine 8-Hydroxyguanosine Species Human Level 121+56 pmol/mL Human 58+23 pmol/mL Human 7+4 pmol/mL Human 0.3nM Human 583+376 pmol/mL Human 8-Hydroxyguanosine dGuo-Malondialdehyde adduct 8OH2’dG Human 138+83 nmol/24hr control 202+102 – cancer 333+125 pmol/kg/24h 2810+830 405+85 pmol/kg/24h – pre-exercise control 310+85 – post exercise 390+85 – post vitamin 335+125 pmol/kg/24h Rat Human 28.54+2.2 nmol/kg/24h 0.40+0.05 HPLC-Fl Human CE-ECD 8OH2’dG Human 8OH2’dG Human 8OH2’dG Human 13.51+5.1 nM – Control 35.3+28.0 – Cancer 4-19 µmol/mmol creatinine – oncological patients on radiotherapy 7.2 nmol/mmol creatinine – control 5.7 – H. pylori infected 9 nmol/mmol creatinine 8OH2’dG Human Human Rat Human 8OH2’dG Human 8OH2’dG Human 12+4 nmol/mmol creatinine – control 9+2 small cell carcinoma responders 7+0.4 control 11+3 small cell carcinoma non-responders 8 to 14 nmol/24h – cancer patients 31 to 40 – post radiotherapy 300+100 pmol/kg/24h WWW.ESAINC.COM Method HPLC then GC-MS HPLC then GC-MS HPLC then GC-MS LC-MS/MS Reference Ravanat et al. (1999) Ravanat et al. (1999) Ravanat et al. (1999) Weimann et al. (2001) ( HPLC then GC-MS GC-MS Ravanat et al. (1999) Rozalski et al. (2002) Park et al. (1992) Witt et al. (1992) HPLC-ECD HPLC HPLC CE-UV Park et al. (1992) Agarwal et al. (1994) Mei et al., (2003) Kvasnicova et al., (2003) ELISA Witherell et al. (1998) ELISA Cooke et al. (1998) Erhola et al. (1997) ELISA GC-MS Bergtold et al. (1990) GC-MS Simic and Bergtold (1991) 199 8OH2’dG Human 8OH2’dG Human 8OH2’dG Human 8OH2’dG Human 8OH2’dG Human 8OH2’dG Human 8OH2’dG Human 8OH2’dG Human 8OH2’dG Human 8OH2’dG Human 8OH2’dG Human 8OH2’dG Human 8OH2’dG Human 8OH2’dG Human 8OH2’dG Human 8OH2’dG Human 8OH2’dG Human 1.33+0.29 nmol/µmol creatinine – control 1.39+0.40 nmol/µmol creatinine – hemochromatosis patients 35+21 nmol/24hr control 36+15 – cancer 30+15 pmol/mL 1.44 nmol/mmol creatinine – male 1.68 – female 1.63 –smokers 1.56 – non-smokers 274 pmol/kg/24h – male 264 pmol/kg/24h – female 1.47+0.02 nmol/mmol creatinine – male 1.58+0.02 – female 5.34+0.03 – neonates 4.4+0.3 ng/mg creatinine – control 7.2+0.7 – ALS 4.6+0.3 – other neurological disorders 1.51+0.38 nmol/mmol creatinine – control 2.78+1.21 – cystic fibrosis 204+133 pmol/kg/24h 0.16 to 8.23 nmol/mmol creatinine 1.4+0.5 to 2.5+0.4 nmol/mmol creatinine 281.7+47 pmol/kg/day – human 333+47 – rat 213+84 pmol/kg/24h – control 320+99 – smokers 318+130 pmol/kg/24h – control 431+168 – smokers 629+218 pmol/kg 24h – control 15.2+5 ng/mg creatinine – control 20.4+8 – ileal neobladder 15.2+4 – colon neobladder 4.27+0.6 ng/mg creatinine – control 2.8+0.3 – +antiretroviral 2.03+1 µmol/mol creatinine – spot urine WWW.ESAINC.COM GC-MS Holmberg et al. (1999) GC-MS Rozalski et al. (2002) Ravanat et al. (1999) Bogdanov et al. (1997) HPLC then GC-MS HPLC-ECD HPLC-ECD Bogdanov et al. (1999) HPLC-ECD Bogdanov et al. (2000) HPLC-ECD Brown et al. (1995) HPLC-ECD Degan et al. (1991) Germadnik et al. (1997) Inoue et al. (1993) Lengger et al. (2000) HPLC-ECD HPLC-ECD HPLC-ECD HPLC-ECD Loft et al. (1992) HPLC-ECD Loft et al. (1994) HPLC-ECD Loft et al. (1995) Miyake et al. (2003) HPLC-ECD HPLC-ECD Paul et al. (2003) HPLC-ECD Pilger et al. (2002) 200 8OH2’dG Human 8OH2’dG Human 8OH2’dG Human 8OH2’dG Human 8OH2’dG Human 8OH2’dG Human 8OH2’dG Human 8OH2’dG Human 8OH2’dG Human 8OH2’dG Human 8OH2’dG Human 8OH2’dG Human 8OH2’dG 1.86+1.1 – 24h urine 130 to 300 pmol/kg/24h 4.46+2.0 µg/g creatinine 9.33+3.2 1.0+0.4 nmol/mmol creatinine – control 1.3+0.4 – smokers 1.6 to 3.7 nmol/mmol creatinine – control 1.4 to 14.7 – post exercise 14.9+7.8 nmol/24h – control 18+11 – cancer 950 pmol/kg/24h 3.3+1.9 µg/g creatinine – control 2.6+0.8 – asphalt exposure 300 to 630 pmol/kg/24h – vegetarian 210 to 490 – Brussel sprout diet 2.8+1.2 nmol/mmol creatinine – smokers 3.0+1.1 – smokers + βcarotene 480 to 520 pmol/kg/24h HPLC-ECD HPLC-ECD ELISA HPLC-ECD Shigenaga et al. (1990) Shimoi et al., (2002) Tagesson et al. (1992) HPLC-ECD Tagesson et al. (1992) HPLC-ECD Tagesson et al. (1995) HPLC-ECD Taylor et al. (1995) Toraason et al. (2001) HPLC-ECD HPLC-ECD van Poppel et al. (1995) HPLC-ECD Verhagen et al. (1995) HPLC-ECD Verhagen et al. (1995) Witt et al. (1992) HPLC-ECD Yamamoto et al. (1996) Human 405+85 pmol/kg/24h – control 310+85 – post exercise 747+425 pmol/kg/24h – control 1827+1500 pmol/kg/24h carcinoma 33+2 pmol/mL LC-MS/MS 8OH2’dG Human 20 pmol/mL LC-MS/MS 8OH2’dG Human 2 ng/mg creatinine LC-MS/MS 8OH2’dG Human 1 to 100nM LC-MS/MS 8OH2’dG ~120 to 300 pmol/kg/24h ~180 to 500 ~550 to 780 172+79 pmol/kg/24h 370+63 0.65 to 1.46 nmol/h HPLC-ECD 8OH2’dG Human Rat Mouse Human Rat Pig Pietta et al., (2003 Ravanat et al. (1998) Renner et al., (2000) Weimann et al. (2001) Shigenaga et al. (1989) HPLC-ECD 8OH2’dG Rat 490+70 pmol/kg/24h GC-MS 8OH2’dG WWW.ESAINC.COM HPLC-ECD HPLC-ECD Park et al. (1992) Loft et al. (1995) Teixeira et al. 201 8OH2’dG Rat Rat 165+66 to 481+163 pmol/kg/24h 100 to 450 pmol/kg/24h 8OH2’dG HPLC-ECD HPLC-ECD 8OH2’dG Rat 210+50 pmol/24h HPLC-ECD 8OH2’dG Rat 89.3+23.7 ng/mg creatinine HPLC-ECD N2-DimethylGuanine N2-EthylGuanine Human 0.022 to 0.185 mg/24h Human 0.0003 to 0.0007 mg/24h GC-MS or GC-MS-MS GC-MS or GC-MS-MS N7-(2-hydroxyethyl) guanine N2-MethylGuanine N7-Methylguanine Thymidine glycol Human 0.0006 to 0.003 mg/24h Human 0.352+0.09 mg/24h Human 3.03+1.46 mg/24h Human 390-435 pmol/kg/24h GC-MS or GC-MS-MS GC-MS or GC-MS-MS GC-MS or GC-MS-MS HPLC Thymidine glycol Human 110-250 pmol/kg/24h GC-MS Thymine glycol Human 100-174 pmol/kg/24h HPLC (1995) Fraga et al. (1990) Shigenaga and Ames (1991) Deng et al. (1998) De Martinis and Bianchi (2002) Cushnir et al. (1993) Cushnir et al. (1993) Cushnir et al. (1993) Cushnir et al. (1993) Cushnir et al. (1993) Loft and Poulsen (1998) Loft and Poulsen (1998) Loft and Poulsen (1998) Table 3.7 Some Urinary Levels Of DNA Markers Reported In The Literature. The use of urinary DNA adduct levels to estimate the total DNA damage is not without its problems. One potential issue with the use of 8OH2’dG as a marker is that it can be derived by action of ROS on the free deoxy-nucleotide pool (Mo et al. (1992); Sukumi et al. (1993)). Dephosphorylation of 8-hydroxy-dGTP by the MutT enzyme helps to limit incorporation of this adduct into DNA by phosphorylase, but by so doing produces free 8OH2’dG. Furthermore, some 8hydroxy-dGTP inevitably escapes MutT and will be incorporated into DNA. Following DNA repair this too will contribute to the 8OH2’dG pool (Figure 3.9). The contribution of oxidized dGTP to urinary 8-hydroxy-2’deoxyguanosine levels still needs to be critically evaluated. Another issue is that mitochondrial turnover and repair may contribute to the urinary excretion rates of DNA adducts. Finally apoptosis may also contribute to urinary excretion rates. All of these processes can lead to overestimation of the amount of DNA damage repaired each day. Conversely, several factors may lead to an underestimate of DNA repair. These include: WWW.ESAINC.COM 202 i) ii) iii) 8OH2’dG is susceptible to oxidation in vivo; as yet unknown salvage pathways may operate on this nucleotide; and in mammals the actual products of 8OH2’dG repair have not been definitively identified (Helbock et al. (1998)). Another confounding factor for the measurement of all urinary DNA adducts is the assumption that the analytical procedure being used is valid and that it is unequivocally and accurately identifying the analyte of interest. As will be discussed below, this is not always the case. Another proposed marker of DNA damage, 8-hydroxyguanine, is less useful as its level is affected by diet. Furthermore it is not specific to DNA as it can be formed by damage to both DNA and mRNA. Recently, 8-hydroxyguanosine is being determined as a marker of mRNA damage (Figure 3.9 and Table 3.6). Measurement of DNA Damage. A variety of techniques have been used to measure DNA damage (e.g., see Aruoma and Halliwell (1998)). Regardless of method used, extreme care must be taken to ensure that artifactual production of adducts does not occur during the sample extraction, preparation and analytical steps (Hofer and Moller (1998)). By diminishing artifactual base-modification production, analytical approaches must now possess sufficient sensitivity to measure one modification in 105 to 107 normal bases and in a few micrograms of DNA. Two main approaches have been developed to measure DNA damage based upon whether the DNA molecule is kept whole or hydrolyzed:11 • Intact DNA lesions can be measured using either immunological methods or by measuring the knicking activity of DNA repair enzymes (e.g., endonuclease III) in conjunction with sedimentation and gel-sequencing techniques in order to quantify the number of strand breaks (e.g., COMET assay – single cell gel electrophoresis) (Cadet et al. (1998); Collins et al. (1993); Gedik et al. (1998)). DNA damage can then be visualized by suitable staining followed by fluorescence microscopy or computer image analysis. • DNA is hydrolyzed using either acid (base release) or enzymatic digestion (producing nucleosides, nucleotides or short oligomers). Unfortunately, if due care is not taken, both isolation and hydrolysis can cause artifactual production of DNA adducts (reviewed by Kasai (1997)). When measuring 8-hydroxy-2’deoxyguanosine, evidence suggests that a number of 11 Although the measurement of adducts in urine does not require the use of either enzymatic or acid hydrolysis their analysis is especially challenging due to the low level of adducts and the number and abundance of other compounds in this sample matrix. Extensive sample cleaning procedures using solid-phase extraction or immunoaffinity columns are often used. A novel alternative method to simplify the analysis of urine samples makes use of the ability of certain carbonbased columns to selectively retain purines (Bogdanov et al. (1999)). WWW.ESAINC.COM 203 situations can contribute to formation of this adduct. These include organic solvents (e.g., chloroform), phenol, oxygen, light, reagent purity (e.g., metal content), pH, type of buffer, certain plastics, the quantity and type of tissue (e.g., hemolyzed blood is high in iron and can promote 8OH2’dG under acidic conditions), the times taken for DNA isolation and hydrolysis, and the analytical approach used (Adachi et al. (1995); Claycamp (1992); Kasai (1997); Nicotera et al. (1994); Floyd et al. (1990)). The contribution of these factors to artifactual adduct production continues to be evaluated and debated. For example, while some claim that phenol may be problematic Helbock et al. (1998) found that phenol extraction caused only a minor increase in 8OH2’dG levels. They also showed that the use of a “chaotropic12 sodium iodide method” to isolate DNA could lower the level of 8OH2’dG by an order of magnitude. Similarly, Hofer and Moller (1998) reported no effect of fresh and aged (pink) phenol and found that the inclusion of the spin-trap TEMPO during sample preparation could prevent artifactual production of 8OH2’dG. Taken together such observations are likely to explain some of the previously observed differences in 8OH2’dG levels. An up-to-date extraction and hydrolysis procedure for HPLC analysis is presented in Appendix 3.1. In order to address the inter-laboratory variability in the measurement of DNA adducts, The European Standards Commission on DNA Damage (ESCODD) was established. Over the past three years several laboratories (using different analytical procedures) have participated in the study and have been tested on their ability to detect 8OH2’dG (and sometimes 8OHGua) in artificial oligonucleotides, calf thymus DNA and HeLa DNA and to show dose response (Anon (2000, 2002); ESCODD (2002, 2003); Riis (2002)). The conclusions so far are: • • • • • Over the last few years the ability to overcome artifactual production of 8OH2’dG to some extent has been improved. The COMET assay possibly underestimates the 8OH2’dG level. GC-MS and HPLC with amperometric detection cannot be recommended due to artifacts. Immunological detection, 32P-postlabelling and LC-MS-MS lack precision and show no dose response. HPLC with coulometric detection shows good precision, shows dose response and is the preferred technique for 8OH2’dG detection. 12 The ability for certain substances to disrupt the structure of water promoting the solubility of certain non-polar substances (e.g., DNA) in polar solvents (e.g., water). WWW.ESAINC.COM 204 Gas- and Liquid- Chromatography-Mass Spectrometry. Gas chromatography-single ion monitoring mass spectrometry (GC-SIM-MS) has been routinely used to measure a wide variety of DNA adducts (e.g., Dizdaroglu (1991, 1994); Lyras et al. (1998)). It is one of the few techniques that can measure adducts formed from all four bases. After isolation DNA samples are first hydrolyzed using acid or enzymes. Formic acid is routinely used for DNA hydrolysis as most adducts are stable and few are formed as a result of this treatment. However, formic acid does cause deamination and dehydration of cytosine–derived adducts. Cytosine glycol either dehydrates to 5hydroxycytosine or dehydrates and deaminates to 5-hydroxyuracil. 5,6Dihydrocytosine, 5-hydroxy-6-hydrocytosine and 5,6-dihydroxycytosine deaminate to 5,6-dihydrouracil, 5-hydroxy-6-hydrouracil and 5,6-dihydroxyuracil, respectively (Dizdaroglu et al. (1993)). Alloxan, a product of cytosine, is decarboxylated to 5-hydroxyhydantoin. DNA bases and adducts must be converted into their volatile derivatives (e.g., trimethylsilyl derivatives) before GC/MS analysis. Many different isotopically enriched modified bases and nucleosides are now available, thus allowing isotope-dilution mass spectrometry to be used for the analysis of several DNA adducts (Dizdaroglu (1993b); Djuric et al. (1991b)). GC-SIM-MS routinely achieves high (picogram) sensitivity, high selectivity and structural information. The highest sensitivity can only be obtained when monitoring the most characteristic ion in its mass spectrum. A few characteristic ions of the analyte and its labeled analog must be monitored at the corresponding retention time in order to accurately and reliably identify the analyte of interest (Dizdaroglu (1997)). Recently Ravanat et al. (1995) reported that the conditions used for GCMS derivatization promote the formation of 8-OHGua from guanine. Furthermore, Douki et al. (1996) found that derivatization causes the artifactual formation of 5hydroxycytosine and 8-hydroxyadenine. Taken together these findings suggest that due care must be exercised when interpreting data on 8-OHGua obtained using GC-MS techniques (see the ESCODD conclusions, above). This may explain why those using GC-MS approaches report much higher levels of guanine adducts than those using HPLC-ECD (e.g., the CSF level of 8OH2’dG is ~60,000 fold higher when measured using GC/MS than HPLC-ECD (Table 3.6)). The use of anoxic conditions during preparation and derivatization, and the addition of a prepurification step prior to derivatization may overcome some of these issues (Dizdaroglu et al. (2003); Nakajima et al. (1996); Ravanat et al. (1998)) but renders GC-MS much less routine. The GC-MS approach has also been used to explore the specificity of glycosylase repair enzymes. Because many base lesions can be simultaneously measured in the same DNA sample, the base preference (which lesions are excised and which are ignored) for a given enzyme can readily be measured (Boiteux et al. (1992); Karakaya et al. (1997); Nackerdian et al. (1992)). This approach can also be used to study the kinetics of excision (Karakaya et al. WWW.ESAINC.COM 205 (1997)). Another GC-MS technique is also being used to study DNA damage: GC/electron capture negative ion mass spectrometry. Following initial isolation by reversed-phase HPLC, the modified bases are alkylated off-line to form the highly electrophoretic pentafluorobenzyl derivatives. This approach offers femtomole sensitivity. Over the past few years liquid chromatography with mass spectrometry (LC-MS) using electrospray interface has been applied to the measurement of various DNA adducts (Tables 3.6 and 3.7). Like with GC-MS, the DNA must be isolated and hydrolyzed before the adducts are measured (see below). With the “higherend” instruments used in a selected reaction monitoring mode high sensitivities (typically low to mid pg) can be achieved. LC-MS has been used to measure the ionization-induced decomposition of thymidine (Berger et al. (1992)), and to study the formation of malondialdehyde-DNA adducts (Chaudhary et al. (1996); Jajoo et al. (1992); Rouzer et al. (1997)). LC-MS (typically using triple-quads) is beginning to show promise for the analysis of 8OH2’dG in urine and even approaches HPLC-ECD in sensitivity (Poulsen et al. (1998); Ravanat et al. (1998)). However, LC-MS is not devoid of problems as nucleosides can artificially oxidize at the output of the HPLC column during the ionization process (Ravanat et al. (1998)). Furthermore, ESCODD do not recommend LC-MS approaches due to an inability to measure dose-dependent adduct formation (see above). HPLC. The measurement of DNA adducts using HPLC-based approaches first requires isolation of DNA from the tissue and then hydrolysis of the DNA molecule (see above). Reversed-phase HPLC permits the separation of a variety of nucleotides and bases. Several different detectors have been used including electrochemical, UV absorbance, fluorescence, and mass spectrometry (using different interfaces [thermospray, electrospray, fast atom bombardment and atmospheric pressure ionization approaches] and spectrometers [single quads, triple quads and ion traps] (Cadet and Weinfeld (1993); Douki et al. (2003); Poulsen et al., (2003)). They differ in selectivity, sensitivity, amount of DNA required, the ability to determine structure and ease of use. HPLC-UV or HPLC-photodiode array approaches are generally too insensitive to measure the low levels of adducts in typical DNA samples (1-100µg) (Cathcart et al. (1984)). HPLC-fluorescence was used to study the formation of DNA photoproducts (e.g., the reactions of furocoumarins, 3-carbethoxypsoralens and pyrimidones) (Cadet and Weinfeld (1993)) but not oxidized DNA adducts, probably due to their weak native fluorescence. HPLC-ECD is selective and sensitive (approximately 103-104 times greater than absorbance-based approaches), possesses good dynamic range (essential if adducts and precursor base/nucleoside are to be measured simultaneously), requires simple sample preparation and no derivatization, and is easy to operate. WWW.ESAINC.COM 206 Unfortunately, not all adducts are electrochemically active and unlike some MSbased analytical techniques HPLC-ECD does not offer structural information. The use of HPLC-ECD to measure DNA adducts, the advantages of coulometric detection over amperometric detection and the benefits of coulometric array detection have been reviewed elsewhere (Acworth et al. (1997; 1998); Poulsen et al. (2003)). A number of HPLC-ECD methods are currently being used (e.g., references in Tables 3.6 and 3.7). From the original work of Floyd et al. (1986), who first used an HPLC single electrode ECD approach to measure 8-hydroxy2’deoxyguanosine, the number of DNA adducts that can be well resolved and measured electrochemically has been considerably expanded. This is due in part to the use of gradient chromatography and improved voltammetric resolution achieved with coulometric arrays (Acworth et al. (1997)) (Figure 3.10) (see also ESA Application Note 70-5970 DNA, Nucleosides and Bases). Although there are previous reports of simultaneously measuring numerous adduct standards on a single electrode system, poor chromatography and a lack of voltammetric resolution severely limit the applicability of such an approach to the measurement of hydrolyzed DNA samples (Kaur and Halliwell (1996)). HPLCECD still remains one of the most accessible and preferred methods for DNA adduct analysis. [520 mV] [340 mV] uric acid [460 mV] 1.0 [400 mV] 2'deoxyadenosine adenosine thymidine 2'deoxyguanosine guanosine inosine 8-hydroxyadenine adenine thymine 2'deoxycytidine xanthine uridine hypoxanthine guanine [580 mV] 8-hydroxy-2'deoxyguanosine [640 mV] 8-hydroxyguanine [0 mV] 5-hydroxy-2'deoxycytidine 2.0 5-hydroxyuracil Response (µA) 3.0 cytidine 5-methylcytosine cytosine uracil 4.0 [280 mV] [220 mV] [160 mV] 0.0 [100 mV] 0.0 10.0 20.0 30.0 40.0 Retention time (minutes) Figure 3.10 Separation and Detection of DNA Adducts, Nucleosides and Bases Using Gradient HPLC With Coulometric Electrode Array And UVAbsorbance Detection (100ng each on column). (The UV channel is labeled 0mV). Electrochemically Active Compounds Can Be Found In Figures 3.5 and 3.6. WWW.ESAINC.COM 207 The gradient HPLC system consisted of two pumps, a PEEK pulse damper, a high-pressure mixer, a refrigerated autosampler, a thermostated organizer module, a twelve channel CoulArray and a dual channel UV detector. LC Conditions: Column: Mobile Phase A: Mobile phase B: TosoHaas TSK-GEL ODS-80TM, (4.6 x 250mm; 5µm). 50mM lithium acetate, pH 4.0 (with phosphoric acid). 50 mM lithium acetate-acetonitrile, pH 4.2 (with phosphoric acid); 85:15; (v/v). Gradient Conditions: 0-15 mins 0% B; 15 to 40 mins 50% B; 40 to 45 mins 100% B; 45 mins 0% B; 45 to 60 mins 0% B. Flow Rate: 1.0 mL/min. Temperature: 31oC. Injection Volume 20µL. Applied Potentials: 100 to 700 mV in 60mV increments (vs. Pd). Wavelength: 260nm (0.01 AUFS). See also ESA Application Note 70-5970 DNA, Nucleosides and Bases for more details. An interesting HPLC-ECD method for the measurement of DNA oxidation was recently developed (Beckman et al. (1998)). DNA is first isolated and is then treated with E. coli repair enzyme, formamidopyrimidine glycosylase, to release 8-hydroxyguanine. This adduct can then be easily measured using HPLC-ECD following separation from its parent DNA by ultrafiltration. This approach has the advantages of minimal sample treatment (thereby minimizing DNA oxidation) and the elimination of other bases and adducts from the sample resulting in simpler chromatography. Issues with this technique include the efficiency of adduct liberation and the lack of commercially available glycosylase. Postlabeling Assays. These assays include (Cadet et al. (1992)). 32 P postlabeling and chemical postlabeling methods Randerath and colleagues (1981) first developed the 32P postlabeling procedure to study carcinogen-DNA adducts. Isolated DNA is first digested enzymatically to produce nucleoside 3’ monophosphates (or short oligomers) that are then enzymatically radiolabeled by incubating with 32P-ATP and phage T4 polynucleotide kinase. Radiolabeled bases and adducts can then be separated using 2-D thin layer chromatography, polyacrylamide gel electrophoresis or HPLC (Gorelick (1993)). This approach enables high sensitivity measurement of a variety of DNA adducts (Keith and Dirheimer (1995); Poirier and Weston (1996)). For example, 5-hydroxmethylyuracil can be measured at the level of one modification per 107 normal bases in 1µg of DNA (Cadet et al. (1992)). Unfortunately this approach suffers from several disadvantages including the use of radioactive substances, artifact problems and is not suited for high sample throughput (Cadet et al. (1997)). The issues of this approach, including method development and its use in the study of DNA damage, have been reviewed WWW.ESAINC.COM 208 elsewhere (Cadet and Weinfeld (1993); Cadet et al. (1992); Marnett and Burcham (1993)). Using 32P postlabeling Randerath found a number of putative adducts in DNA extracts of tissues obtained from untreated animals (Randerath et al. (1986)). These spots were termed I-compounds as they occurred indigenously. Icompounds accumulate in an age-dependent, highly reproducible manner and their pattern is found to affected by gender, tissue and diet. I-compounds appear to arise via the interaction of DNA with endogenous reactants formed in the course of normal metabolism (see Marnett and Burcham (1993) and references therein). They exhibit a wide range of polarities suggesting that they are structurally diverse (Randerath et al. 1990)). Although their exact structure remains unknown, current evidence suggests that some I-compounds may contain DNA-lipid peroxidation adducts (Li et al. (1995b) and references therein). The biological role of the I-compounds remains controversial. Although many DNA adducts are found to increase the probability of mutation which may eventually lead to development of cancer, the levels of I-compounds do not positively correlate with cancer. Their levels do show diurnal variation suggesting that they are regulated. Consequently, some researchers have suggested that Icompounds may play a role in regulation of gene expression and proliferation (Marnett and Burcham (1993) and references therein). Others dispute this role and hypothesize that altered I-compound levels may just be the consequence of changes in cytochrome P450 activity. Chemical postlabeling follows a different procedure. Once separated using HPLC, nucleotides and nucleosides resulting from enzymatic hydrolysis can then be chemically postlabeled using acetic anhydride. The resulting acetylated nucleosides can then be resolved using another HPLC system (Frenkel et al. (1991)). However, with only picomole sensitivity this approach is not competitive with the other approaches described in this section. A second chemical method has nucleoside 5’-monophosphates reacting with the fluorogenic agents dansyl chloride/fluorescein isothiocyanate. This approach is capable of measuring 1 adduct per 106 normal nucleosides in a 100µg DNA sample (Sharma et al. (1990)). Immunochemical Detection. This approach makes use of the fact that antibodies raised to a specific DNA adduct can then be utilized to measure such lesions in DNA (see Cadet and Weinfeld (1993) and references therein; Herbert and Lunec (1998)). The major advantage of immunochemical detection is one of sensitivity (subfemtomol level), but this can only be achieved if the antibodies have been correctly generated. This approach can suffer from poor selectivity due to either inappropriate generation of the antibody (e.g., using DNA with a number of different lesions) or WWW.ESAINC.COM 209 cross reactivity with compounds containing similar chemical structure. The generation of monoclonal antibodies can improve the selectivity of this approach. The most widely used immunodetection method is probably the enzyme-linked immunoabsorbent assay (ELISA). Here, secondary antibodies that are covalently cross-linked to an enzyme, such as peroxidase or alkaline phosphatase, detect primary antibodies bound to the antigen. Such binding can then be visualized by applying a substrate that yields a product that can be measured using absorbance techniques. Alternatively, a secondary antibody covalently bound to a fluorogen (e.g., fluorescein isothiocyanate) can be used and measured using fluorescence approaches. Radioimmunoassay involves the coupling of a radioisotope to the primary antibody. Binding can then be measured using scintillation counting or phosphor imaging. The Measurement of 8OH2’dG in Urine. The accurate, reliable and routine measurement of urinary 8OH2’dG levels, as an estimate of total oxidative stress, has been the focus of many laboratories. Unfortunately, this is not as straight forward as it may first appear. The measurement of 8OH2’dG in urine represents a considerable challenge. This adduct is polar in nature and occurs at extremely low levels in an extremely complex and variable matrix. The complicated nature of the urine makes it necessary to process the sample prior to analysis to help minimize matrix interferences and to concentrate the adduct. A variety of approaches have been used including: • • • • • • Solid phase extraction (SPE) (Brown et al. (1995); Faux et al. (1994); Lunec et al. (1994); Shigenaga et al. (1989; 1990); Vigue et al. (1993)); SPE in conjunction with an immunoaffinity column (Fraga et al. (1990); Park et al. (1992); Shigenaga and Ames (1991); Shigenaga et al. (1994)); triple column switching (Loft et al. (1992, 1995); Verhagen et al. (1995)) prior to HPLC-ECD analysis; SPE and triple column switching (Lagorio et al. (1994); Tagesson et al. (1995)) prior to HPLC-ECD analysis; SPE and trimethylsilylation (Lunec et al. (1994)) prior to GC-MS analysis; Evaporation, acetylation and pentafluorobenzylation (Teixeira et al. (1993)) prior to GC-MS analysis; and SPE, evaporation, acetylation, pentafluorobenzylation, preparative HPLC and evaporation (Teixeira et al. (1995)) prior to GC-MS analysis. In general, the sample processing for HPLC-ECD is much simpler than those for GC-MS and are less likely to lead to artifactual adduct levels (see above). ode Although the original methods using SPE followed by HPLC-ECD were quite simple to perform in the laboratory, the results given by a single electr WWW.ESAINC.COM 210 detector may be erroneous (Brown et al. (1995); Faux et al. (1994); Lunec et al. (1994); Shigenaga et al. (1989; 1990); Vigue et al. (1993)). Using a coulometric array detector we have found that an 8OH2’dG peak that appears to be “pure” on a single electrode system is actually due to the co-elution of several analytes (Acworth et al. (1997); Bogdanov et al. (1999)) (Figure 3.11). This readily illustrates the danger of basing peak purity solely upon matching retention times of unknown samples to external standards. Methods using no or limited sample preparation, HPLC-single electrode detection and claiming to measure urinary 8OH2’dG levels should be viewed with extreme caution. One method that may eventually prove useful for isolating and analyzing 8hydroxy-2’deoxyguanosine from urine is SPE followed by an immunoaffinity column and HPLC-ECD (Fraga et al. (1990); Park et al. (1992); Shigenaga and Ames (1991); Shigenaga et al. (1994)). Here a monoclonal antibody specific for 8OH2’dG is used to clean the urine sample. The method appears to be quite specific and sensitive for the determination of 8OH2’dG. However, the lifetime of the columns may be a problem, with decreased binding efficiency occurring over time (Shigenaga et al. (1994)). We have observed that a coelution occurs when this column irreversibly ages. 8-OH2´DG In Urine Conventional 8-OH-2´DG 1.0 0.5 0.0 CoulArray 8-OH-2´DG What appears to be a single 8OH2’dG peak by conventional HPLCECD is actually found to be a co-elution of several metabolites by CoulArray detection! 8 7 6 5 4 3 9 10 11 12 13 14 15 16 Time (min) Figure 3.11 Analysis of 8OH2’dG In The Same Urine Sample Using A Conventional Single Electrode Detector (Top Chromatogram) And An Electrode Array Detector (Bottom Chromatogram). WWW.ESAINC.COM 211 Some approaches to sample preparation involve the use of column switching. Here, a standard is injected onto one HPLC column and the retention time of the analyte noted. In subsequent sample injections, a valve is toggled just before the analyte elutes, resulting in a “heart-cut” that contains the peak of interest. The resulting ”slug” of sample flows onto a second “trapping” column or sample loop, and finally the valve is toggled back to its original position after the analyte elutes. The “slug” containing the analyte is transferred onto a third analytical column and then finally into a coulometric detector (Loft et al. (1992, 1995); Verhargen et al. (1995)). This method is extremely complex; requires two detectors, two or three columns, and a switching valve; and has as an extremely long run time, thereby limiting sample throughput to only a few samples per day. Other researchers have combined column-switching techniques with an SPE procedure prior to injecting the sample onto the first column (Lagorio et al. (1994); Tagesson et al. (1995)). This technique speeds up the analytical run time because the lipophilic compounds that elute for several hours remain on the disposable SPE cartridge. A different approach to column switching was recently been developed and used to measure 8OH2’dG in a variety of body fluids including urine, sweat, plasma and CSF (Bogdanov et al. (1998, 1999, 2003). This approach uses in-line porous graphite columns, made from carbon chosen for its purine-binding properties, to selectively clean urine samples before analysis by ECD. Urine diluted in basic buffer first passes through a C18 column. The band containing 8OH2’dG is then trapped onto a carbon column. The carbon column is first washed to remove interfering analytes and then exposed to a mobile phase containing a competitive non-EC active compound (adenosine) to displace bound 8OH2’dG. 8OH2’dG and the few compounds also bound to the carbon column are finally resolved on a second C18 column and measured using ECD. This approach is reproducible, highly selective and sensitive (~500fg on column), routine and allows up to forty samples to be analyzed each day (Figure 3.12). During method development Matson (1998) observed that the precipitate that sometimes forms in urine samples over time is capable of binding 8OH2’dG. This precipitate (probably composed of uric acid and other small molecules) is readily solubilized in the basic dilution buffer. Procedures that ignore this precipitate may therefore not be measuring “true” urinary 8OH2’dG levels. This carbon column-based approach has great applicability to a variety of other assays. All that is required is a graphite column showing selective binding for the analyte of interest and a nonEC active displacer molecule. Currently this approach is being expanded to measure other proposed markers of oxidative stress. For example, with minor modification to the chromatographic conditions and by using different displacing molecules, the method can be adapted for the measurement of 5-hydroxycytidine, 8-hydroxyguanine, 8-hydroxyadenine, 7-methylguanine, or 8-nitroguanine. Plasma 3-nitrotyrosine and 3-chlorotyrosine can also be determined but require 3-nitrobenzoic acid as the displacing molecule, and a different HPLC-chemistry (see Bogdanov et al. (2003) for greater detail). WWW.ESAINC.COM 212 DNA Damage in Health and Disease. There have been many reports measuring steady state (DNA levels e.g., Table 3.6) and total DNA damage (urine e.g., Table 3.7). Loft and Poulsen (1996) noted that while “there is good agreement between different laboratories regarding the values of urinary excretion of repair products, the values obtained from DNA isolated from tissues or cells differ by several orders of magnitude, some of which may be due to the choice of analytical method”. The possible contribution of sample preparation and analysis to DNA adduct levels is still undergoing evaluation (see ESCODD above). Urinary 8OH2’dG I - 8OH2’dG standard, 10ng/ml II - urine from an ALS patient III-urine from a control subject Figure 3.12 The Measurement Of 8OH2’dG In Control Urine (III) And In Urine Obtained From An ALS Patient (II) Using The Carbon Column Switching Procedure (Bogdanov et al. (1999, 2003). There appears to be a direct correlation between 24hr oxygen consumption and the urinary excretion rate of 8OH2’dG and thymidine glycol (Loft and Poulsen (1996) and references therein). This is probably due to increased production of pro-oxidants by mitochondria associated with increased metabolic rate. If this is true, elevated urinary adduct levels should be seen in other conditions where the basal metabolic rate is increased, such as with exercise. Data from the few exercise studies available suggest that this is indeed the case. It is still not clear whether urinary adduct levels increase following short periods of exercise (Loft WWW.ESAINC.COM 213 and Poulsen (1996) and references therein), but they are definitely elevated following severe activity such as marathon running (Alessio (1993)) or prolonged and repetitive bouts of exercise (Poulsen et al. (1996)). Conversely, short-term caloric restriction which leads to a reduced metabolic rate is found to be associated with lowered steady state adduct levels (Chung et al. (1992); Djuric et al. (1992); McCarter (1995); McCarter and McGee (1989)). Furthermore, at least one study reported a lowered urinary excretion rate of 8hydroxy-2’deoxyguanosine by 40-50% following energy restriction for ten days (Simic and Bertgold (1991)). However, energy restriction by 20% was without affect (Loft et al. (1995); Wicric et al. (1995)). Aging is also associated with decreased rates of metabolism. Although there have been numerous reports on the association of DNA damage with aging so far there has not been a true systematic study (Ames et al. (1993); Holmes et al. (1992); Lee and Wei (1997); Perez-Camp et al. (1998); Randerath et al. (1992); Wei (1998)). Evidence to date suggests that the rate of damage decreases with age but that steady state levels appear to increase, possibly the result of failing repair mechanisms (Loft and Poulsen (1996) and references therein). Many studies have measured oxidative DNA modifications in relation to a variety of disorders including autoimmune diseases (e.g., rheumatoid arthritis and systemic lupus erythematosus), cancer, chronic hepatitis, cystic fibrosis, inflammatory bowel disease, metal storage diseases (e.g., Wilson’s disease), and Fanconi anemia (reviewed by Loft and Poulsen (1996); Marnett and Burcham (1993); Wiseman and Halliwell (1996)). In general, many of these diseases are associated with an increased rate of oxidative DNA modification or, in some cases, deficient repair. In most cases a causal relationship between DNA damage and cancer in humans still remains elusive. Increasing evidence, however, suggests a role for a mutant p53 tumor suppressor gene in some human cancers (Greenblatt et al. (1994); Husgafvel-Pursiainen et al. (1995); Semenza and Weasel (1997); Soussi (1996)). AMINO ACIDS AND PROTEINS. Introduction. Amino acids are the basic building block of proteins. Approximately 22 amino acids are commonly found in living organisms. They differ in chemical reactivity, charge, shape, size, and hydrogen bonding capacity. Free amino acids play several important roles in the body. They take part in intermediary metabolism (e.g., glycine, alanine, aspartate and glutamate), act as neurotransmitters (e.g., glutamate, aspartate and glycine), and are the precursor of monoamine neurotransmitters (tyrosine is converted to catecholamines, histidine to histamine, and tryptophan to serotonin), hormones (e.g., thyroxin), peptides (e.g., GSH, substance P, and insulin) and proteins. Amino acids possess a chiral WWW.ESAINC.COM 214 center. All amino acids used by eukaryotes are in the L-conformation (Figure 3.13). Figure 3.13 Stereo Pair of L-Tyrosine. In proteins, the α-carboxyl group of one amino acid is joined via an amide (peptide) bond to the α-amino group of another amino acid. Many amino acid units can be joined together to form a polypeptide chain. Most proteins typically contain between 50 and 200 amino acid residues (5-22 kDa). Proteins are a diverse family of molecules, but they are all composed from the basic set of 22 amino acids (remember that there may be some post-translational modifications). The sequence of amino acids in a protein is ultimately determined by the sequence of DNA bases in the DNA molecule. It is the sequence of amino acids that gives a protein its biochemical and physical properties. Proteins play many important roles including: enzymatic catalysis, transport, storage, coordinated motion, mechanical support, immune protection, generation and transmission of nerve impulses and control of growth and differentiation (Stryer (1988)). Proteins are very susceptible to oxidative damage that can affect their physiological function. To better understand how pro-oxidants can damage proteins, we must first examine their structure. Protein Molecular Structure. The physical (e.g., shape, solubility, and strength) and biochemical (e.g., enzyme activity, antigen recognition, and biomechanical contraction) properties of a protein are dependent upon its structure. It is interesting to note that although a protein initially exists as a linear code in DNA, transcription and translation WWW.ESAINC.COM 215 produces a molecule with three-dimensional structure. The folding of the polypeptide backbone and stability of the resulting form is dependent upon the electrostatic and hydrophobic interactions between amino acid residues, and stabilization by disulfide (cystine) bridges. There are at least four levels of structure applied to proteins. • • • • The primary structure refers to the amino acid sequence and location of disulfide bridges. Secondary structure refers to the spatial arrangement of amino acid residues close to each other in the linear sequence. These often are repeated and can give rise to periodic structures such as the α-helix, the β-pleated sheet and the collagen helix. Tertiary structure refers to the spatial arrangement of amino acids far apart in the linear sequence but which may ultimately locate close to each other when the protein is correctly folded. Some proteins are made of more than one independent polypeptide chain each folded into a subunit. Quaternary structure refers to the spatial arrangement of such subunits. These subunits can either be identical (e.g., all 180 coat proteins of the tomato bushy stunt virus are identical) or different (e.g., hemoglobin consists of four dissimilar subunits). The interfaces between subunits are important in transmission of information such as substrate binding. Higher structural levels have now been recognized and include super secondary structure (clusters of secondary structures, e.g., βαβ repeats) and domains (compact folded structures linked together by flexible polypeptide segments). The correct folding of a protein is essential for it to function properly. This is because the first step in the action of a protein is its binding to another molecule.13 This ability is only possible because proteins can form complementary surfaces and clefts. These structures are produced from the wide selection of amino acid side chains permitting a protein to form hydrogen, electrostatic and van der Waal’s bonds with its substrate. The correct conformation, therefore, permits all essential residues, regardless of location in the linear sequence, to play a role in the three-dimensional shape of the surface or cleft (active site) of the protein molecule. Consequently, no other macromolecule group is capable of recognizing and interacting with so many diverse molecular structures. The formation of the active site is essential for the recognition of a substrate by an enzyme. Correct folding also influences the ability of an enzyme to bind a cofactor or metal, regulation of its activity by phosphorylation, and binding of allosteric modulators. It should not be surprising that oxidative damage, either resulting in incorrect folding or alteration of the structure of essential amino acids acid residues lining the active site or other key sites in the protein molecule, can markedly effect protein function. In order to 13 Examples of protein binding include control of gene expression, determination of self from non-self, assembly of viral protein coats, binding of a substrate by a receptor, and the binding of a substrate and cofactor by an enzyme. WWW.ESAINC.COM 216 maintain cellular homeostasis damaged proteins must either be repaired or destroyed. Numerous in vitro experiments on protein folding show that all the information required for the formation of the native, three-dimensional structure of a protein is encoded in its amino acid sequence. For example, Alfinsen (1973) reported that a denatured protein is capable of regaining its tertiary structure. For many years in vivo folding was also assumed to be a similar autonomous process unaffected by other cellular components such as proteins. However, this idea was challenged with the discovery of some new proteins, the molecular chaperones (see Schwarz et al. (1996) and references therein). Chaperones play an active role in protein folding and prevention of aggregation, both in vivo and in vitro. Chaperones help to direct proteins towards repair or degradation processes thereby ensuring cell survival. Unlike enzymes that are actively involved in folding (e.g., peptidyl-prolyl cis-trans isomerases and disulfide oxidoreductase) molecular chaperones affect folding processes nonspecifically and can react with a large number of proteins that expose non-native structures. Many chaperones were originally identified as heat shock proteins (Hsps). Hsps or, more correctly, stress proteins, are produced when cells are exposed to stressors including heat stress (~5oC above normal temperature), oxidative stress, reperfusion-ischemia injury, heavy metals, mutant proteins, anticancer drugs, and apoptotic agents, and are increased in bacterial and viral infections (Arrigo (1998); Benjamin and McMillan (1998); Freeman et al. (1999); Lappa and Sistonen (1997)). This Hsp response has been implicated in the protection of cells from different forms of injury and to the improvement of cell survival following injury. To date the induction of heat shock response has been reported for a variety of diseases including inflammation, myocardial ischemia, and cystic fibrosis (Leppa and Sistonen (1997); Strickland et al. (1997); Thomas et al. (1995)). Hsps have also been proposed to: 1) Transiently bind and delay folding of nascent polypeptide chains until synthesis is complete; 2) Maintain these chains in a suitable conformation for passage across organelle membranes; 3) Prevent aggregation; 4) Actively disassemble clathrin-coated vesicles; 5) Hold steroid aporeceptor complexes in ligand competent states; and 6) Assist in degrading damaged proteins by promoting ubiquitinylation and proteasome lysis (from Benjamin and McMillan (1998)). The pathway by which damaged proteins are removed is discussed below. So far six families of Hsps exist, classified according to their molecular weights: Hsp100 (100-110 kDa), Hsp90, Hsp70, Hsp60 (the chaperonin system), Hsp40 and the small Hsps (15-30 kDa) (including heme oxygenase and α,β-crystallin). The different Hsps families appear to play different roles in the protein folding process. For example, Hsp70, the most abundant group in eukaryotic cells, is an WWW.ESAINC.COM 217 ATP-dependent chaperone that is responsible for binding to the nascent polypeptide chain before its release from the ribosome thereby preventing incorrect folding of the incomplete polypeptide. Interestingly, small Hsps, which are characterized by an in vitro ATP-independent chaperone activity, not only enhance the survival of cells exposed to oxidative stress by decreasing ROS levels in a glutathione-dependent manner but also interfere with apoptosis (Arrigo (1998) and references therein). Readers interested in a more in depth discussion of the interaction and roles of the different Hsps families are referred to Benjamin and McMillan (1998) and Buchner (1996). Pro-oxidants and Protein Damage. Pro-oxidants can damage proteins by both indirect and direct mechanisms. The effects of protein oxidation on protein function and enzyme activity are summarized in Table 3.8. The Indirect Pathway. This (mutation) pathway does not involve oxidative damage to the protein per se. Rather this process involves oxidative damage to the DNA molecule encoding the protein. Thus pro-oxidants can cause changes in the base sequence of the DNA molecule. If such base modification is in a coding region of DNA (exon) and not corrected, the DNA molecule may be transcribed incorrectly. Translation of the mutant mRNA can result in a mutant protein containing a wrong amino acid in its primary sequence. If this modified amino acid occurs in an essential part of the protein (e.g., the active site of an enzyme or a portion that alters folding), the function of that protein may be impaired. Fortunately, unlike modified DNA that can pass from cell to cell during mitosis thereby continuing the production of mutant protein, damage to a protein is non-replicating and stops with its destruction. Enzyme/Protein Aconitase, branchedchain amino acid dehydrogenase, complex 1, dehydratases, and 6phosphogluconate dehydrogenase Alcohol Dehydrogenase Reactive Species/ Treatment Hydrogen peroxide/ superoxide Peroxynitrite Modification/ Comments Reference Damage to iron-sulfur clusters leads to enzyme inhibition Bunik and Sievers (2002); Liochev and Fridovich (1994) Disruption of zincthiolate center leads to release of zinc and inactivation of the enzyme Crow et al. (1995) WWW.ESAINC.COM 218 D-Amino acid oxidase Chlorination Angiotensin II Peroxynitrite Bovine serum albumin, collagen, hemoglobin, myoglobin ROS generating systems Ca2+-ATPase (sarcoplasmic reticulum) ROS/RNS Catalase Singlet oxygen Hypochlorous acid Creatine kinase Superoxide Peroxynitrite Cu/Zn superoxide dismutase ROS Cytochrome P450 Radical intermediates Fructose bisphosphatase Galactose oxidase Formation of 3chlorotyrosine in the active site leads to inhibition Formation of essential 3nitrotyrosine residue reduces vasoconstrictive properties Protein fragmentation, usually at glycine sites Dityrosine Thiol oxidation and 3nitrotyrosine formation lead to inhibition Uncertain modification leads to inhibition Ronchi et al. (1980) Ducrocq et al. (1998) Dean et al. (1997) and references therein. Giulivi and Davies (1994) Klebl et al. (1998); Viner et al. (1996, 1999) Gantchev and van Lier (1995) Aruoma and Halliwell (1987) Heme degradation and inhibition Uncertain modification leads to inhibition Modification of tryptophan and tyrosine residues Formation of 2oxohistidine from histidine inactivates enzyme Self-inactivation. Mechanism to be defined RNS 3-Nitrotyosine formation inhibits enzyme Hydrogen peroxide Hydrogen peroxide/HRP Oxidation of essential thiol causes inhibition Formation of dityrosine activates the enzyme Halliwell and Gutteridge (1999) Stachowiak et al. (1998) Lewisch and Levine (1995) and references therein Dean et al. (1997) Daiber et al., (2000); Lin et al., (2003); Vernia et al., (2001) Halliwell and Gutteridge (1999) Verweij et al. (1982) Ozone Formation of dityrosine activates the enzyme WWW.ESAINC.COM 219 Glucose-6-phosphate dehydrogenase 4-hydroxynonenal Schiff base/Michael addition leads to inactivation Oxidation of ironsulfur clusters lead to inactivation Uchida and Stadtman (1993) Glutamine phosphoribosylpyrophosphate amidotransferase Glutamine synthetase Metal catalyzed oxidation Formation of 2oxohistidine from histidine in metal binding region causes inactivation Deactivated but only in the absence of GSH Farber and Levine (1986); Lewisch and Levine (1995) and references therein Halliwell and Gutteridge (1999) Glutathione peroxidase Superoxide 4-Hydroxynonenal Inhibits by binding to an essential lysine Bosch-Morell et al. (1999) Hypochlorous acid Mechanism leading to inhibition remains to be clarified Aruoma and Halliwell (1987) Peroxynitrite Essential selenol residue oxidized to selenocysteine 3-nitrotyrosine formation leads to inhibition Oxidation of essential thiol causes inhibition Briviba et al. (1998); Padmaja et al. (1998) Francescutti et al. (1996) Schiff base/Michael addition with lysine inhibits enzyme Uchida and Stadtman (1993) Peroxynitrite S-nitrosylation of essential thiol residues 4-hydroxynonenal Schiff base/Michael addition with histidine Galli et al. (1998); Mohr et al. (1994); Souza and Radi (1998) Uchida and Stadtman (1992) ROS Dityrosine formation Giulivi and Davies (1994) Ozone Dityrosine formation ROS o-Tyrosine, dityrosine Verweij et al. (1982) Wells-Knecht et al. ROS Glutathione reductase Peroxynitrite Glyceraldehyde-3phosphate dehydrogenase Hydrogen peroxide 4-hydroxynonenal Dean et al. (1997) and references therein Halliwell and Gutteridge (1999) Hydroxyl free radical, singlet oxygen Insulin Lens proteins WWW.ESAINC.COM 220 Lipoxygenase Low density lipoprotein Mn-superoxide dismutase Lipid peroxyl radicals/hydro peroxides Peroxynitrite Hypochlorous acid Peroxynitrite Phosphatidylinositol 3kinase Peroxynitrite Prostaglandin endoperoxide synthase Tetranitromethane Inactivation in the presence of iron Nitration and oxidation. 3nitrotyrosine formation α1-Proteinase inhibitor Hydrogen peroxide Leeuwen-burgh et al. (1997) Hazen and Heinecke (1997) 3-Chlorotyrosine Nitration and oxidation of critical tyrosine residues inactivates enzyme Nitration of the p85 regulatory subunit of this enzyme Inhibition by 3nitrotyrosine formation Prostacyclin synthase Peroxynitrite (1993) Cucurou et al. (1991) Inhibition by 3nitrotyrosine formation Formation of methionine sulfoxide from critical methionine residue leads to inactivation MacMillan-Crow et al. (1996; 1998, 1999b) Hellberg et al. (1998) Goodwin et al. (1998); Mehl et al., (1999); Schmidt et al., (2003); Shimokawa et al. (1990); Zou et al. (1997, 1999) Dean et al. (1997) Hydroxyl free radical A variety of amino acids are damaged leading to inactivation Kwon et al. (1990) Tetranitromethane Formation of 3nitrotyrosine leads to inactivation Kinase cannot act on -substrates containing 3nitrotyrosine Fragmentation and inhibition Fetse and Gan (1981) Protein kinase substrates Peroxynitrite Ribulose-1,5bisphosphate carboxylase Sedoheptulose bisphosphatase Serpin (neutrophil cytosolic serineproteinase inhibitor Oxygen species Superoxide Dismutase Peroxynitrite Hydrogen peroxide Hydrogen peroxide Oxidation of essential thiol inhibits enzyme Formation of methionine sulfoxide from critical methionine residue leads to inactivation 3-Nitrotyrosine formation and WWW.ESAINC.COM Gow et al. (1996a) Ishida et al. (1999) Halliwell and Gutteridge (1999) Thomas et al. (1991) Macmillan-Crow and Cruthirds 221 inhibition Surfactant protein A α-Synuclein Tyrosine hydroxylase Peroxynitrite RNS Peroxynitrite 3-Nitrotyrosine 3-Nitrotyrosine and possibly dityrosine may be causative or protective factor in Parkinson’s disease Nitration and oxidation products lead to enzyme inactivation. This has now been shown to be oxidation of an essential thiol group (2001); Quijano et al., (2001) Greis et al. (1996) Duda et al. (2000); Ischiropoulis (2003) Yamin et al. (2003). Ischiropoulos et al. (1995); Kuhn et al. (1999) Table 3.8 Oxidation Can Affect Enzyme Activity And Other Protein Function. The Direct Pathway. This (post-translational) pathway involves the action of a pro-oxidant on a protein resulting in modification of amino acid residues, the formation of carbonyl adducts, cross-linking and polypeptide chain fragmentation. Such changes often result in altered protein conformation and/or activity. Dakin first studied protein oxidation and showed that it resulted in the formation of carbonyl compounds such as carboxylic acids, or aldehydes with the same or one less carbon atom as the parent amino acid e.g., glycine produces glyoxal, glyoxylic acid, formaldehyde and formic acid (Dakin (1906, 1908)). This finding now appears to be true for most amino acids. Consequently, proline and arginine are converted into glutamic semialdehyde, histidine into 2-oxohistidine, and lysine into lysyl carbonyl. Stadtman (1990, 1991, 1993) observed findings similar to Dakin and reported that proteins will produce a variety of carbonyl products when exposed to metal-based systems (metal/ascorbate and metal/hydrogen peroxide) in vitro. For example, histidine yields aspartate, asparagine and 2oxoimidazoline, while proline produces glutamate, pyroglutamate, 4hydroxyproline isomers, 2-pyrrolidone and γ-aminobutyric acid (Stadtman (1993) and references therein). Metal-based systems and other pro-oxidant conditions can oxidize methionine to its sulfoxide (Brot and Weissbach (1991); Chao et al. (1997)). Carbonyls can also be formed by the action of hypohalous acids on αamino acids (Chapter 2). Carbonyl formation is not the only oxidative modification of amino acids and many other reactions can take place forming a wide variety of modified amino acid residues including tyrosine adducts and amino acid hydroperoxides (Table 3.9) (Figure 3.14). In the presence of oxygen proteins can undergo radical chain reactions (Dean et al. (1997) and references therein). Alkoxyl radicals are more WWW.ESAINC.COM 222 effective at promoting protein “peroxidation” chain reactions, than peroxyl radicals; the latter play a more important role in lipid peroxidation processes. Amino acid hydroperoxides once formed can then react with metals producing free radicals thereby propagating protein chain reactions or they can be reduced to non-reactive hydroxides. Residue Amino Acids Product Carbonyls Arginine Arginine Glutamic semialdehyde 5-Hydroxy-2aminovaleric acid Mono- and di-chlorinated Cystine, oxy acids Arginine Cysteine Cysteine Cysteine Glutamate Glycine Histidine Cysteine/4hydroxynonenal adduct S-nitrosylation Glutamic acid hydroperoxide Aminomalonic acid 2-Oxohistidine Methionine Aspartate, asparagine Histidine/4hydroxynonenal adduct Isoleucine hydroperoxides, isoleucine hydroxides, carbonyl compounds Leucine hydroperoxides, Leucine hydroxides, αketoisocaproic acid, isovaleric acid, isovaleraldehyde, carbonyl compounds Nε-(carboxymethyl)lysine 2-Aminoadipicsemialdehyde Lysine/4-hydroxynonenal adduct Lysine hydroperoxides, lysine hydroxides and carbonyl compounds Methionine sulfoxide Phenylalanine o- and m-Tyrosine Phenylalanine 4-Nitrophenylalanine Histidine Isoleucine Leucine Lysine Lysine Lysine Lysine Reference Hazen et al. (1996, 1998a,b); Nosworthy and Allsop (1956); Rowbottom (1995) Amici et al. (1989); Climent et al. (1989) Ayala and Cutler (1996a,b) Zhang et al., (2001) Armstrong (1990); Takahashi and Goro (1990); von Sonntag (1990) Uchida and Stadtman (1994) Galli et al. (1998); Mohr et al. (1994) Gebicki and Gebicki (1993); Simpson et al. (1992) Copley et al. (1992); Van Buskirk et al. (1984) Lewisch and Levine (1995); Uchida and Kawakishi (1990, 1993) Farber and Levine (1986); Creeth et al. (1983) Uchida and Stadtman (1994) Gebicki and Gebicki (1993); Simpson et al. (1992) Dean et al. (1996); Fu et al. (1995a); Gebicki and Gebicki (1993); Simpson et al. (1992); Stadtman and Berlett (1991) Dunn et al. (1990); Glomb and Monnier (1995) Szweda and Stadtman (1992) Uchida and Stadtman (1994) Gebicki and Gebicki (1993); Simpson et al. (1992); Trelstad et al. (1981) Levine et al. (1996); Li et al. (1995c,d); Vogt (1995) Ishimitsu et al. (1986); Kaur and Halliwell (1994); Liu (1993); Leeuwenburgh et al. (1997); Nair et al. (1995); Ramezian et al. (1996); Sontag et al. (1997); van del Vliet (1995) Huggins et al. (1993); van der Vleit et al. (1994) WWW.ESAINC.COM 223 Proline Tyrosine Cis/trans-4hydroxyproline γ-Aminobutyric acid Glutamate, pyroglutamate Glutamic semialdehyde 5-Hydroxy-2-amino valeric acid, proline hydroperoxides, proline hydroxides and carbonyl compounds 2-Pyrrolidone Protein/4hydroxynonenal adducts Glycolaldehyde 2-Amino-3-ketobutyric acid 2-Hydroxypropanal and acrolein 5- and 6-Nitrotryptophan N-Formylkynurenine Kynurenine oxindole3alanine 3-, 4, 5-, 6- and 7Hydroxy-tryptophan 3-Chlorotyrosine Tyrosine Tyrosine L-DOPA Dityrosine Tyrosine 3-Nitrotyrosine Tyrosine Tyrosine 3,5-Dinitrotyrosine p-Hydroxyphenylacetaldehyde Valine hydroperoxides, valine hydroxides and carbonyl compounds Proline Proline Proline Proline Proline Protein Serine Threonine Threonine Tryptophan Tryptophan Tryptophan Valine Uchida and Kawakishi (1989) Uchida et al. (1990) Cooper et al. (1985); Creeth et al. (1983); Uchida et al. (1990) Amici et al. (1989) Ayala and Cutler (1996); Gebicki and Gebicki (1993); Simpson et al. (1992); Trelstad et al. (1981) Uchida et al. (1990) Uchida and Stadtman (1994) Anderson et al. (1997) Taborsky (1973) Anderson et al. (1997) Alvarez et al. (1996); Padmaja et al.(1996) Guptasarma et al. (1992); Maskos et al. (1992); Neuzil and Stocker (1993) Armstrong and Swallow (1969); Guptasarma et al. (1992); Maskos et al. (1992) Hazen et al. (1996a); Kettle (1996); Leeuwenburgh et al. (1997) Gieseg et al. (1993) Giulivi and Davies (1994); Heinecke et al. (1993); Huggins et al. (1993); Ischiropoulos et al. (1992); Leeuwenburgh et al. (1997); van der Vleit (1995); Vissers and Winterbourne (1991) Beal et al. (1995); Fukuyama et al. (1996); Hensley et al. (1997); Kamisaki et al. (1996); Kaur and Halliwell (1994); Leeuwenburgh et al. (1997); Maruyama et al. (1996); SalmanTabcheh et al. (1995); Schulz et al. (1995); Shigenaga et al. (1997); Skinner et al. (1997) Lin et al., (2000); Yi et al. (1997) Hazen et al. (1996b) Fu et al. (1995a,b) Table 3.9 Many Amino Acids Can Be Modified By ROS, RNS And Other Reactive Species. Amino acid residues can also be modified following their reaction with carbohydrates or other carbonyl compounds that can be produced when lipids, and proteins are attacked by pro-oxidants. For example, malondialdehyde and 4hydroxynonenal produced by lipid peroxidation can readily form Schiff bases and WWW.ESAINC.COM 224 H N H O H2 N CO2H R1 R1 N H S=O CH3 H2N H CO2H 2-Oxohistidine OH NO2 Methionine Sulfoxide m-Tyrosine 4-Nitrophenylalanine R1 R1 R1 R1 R1 OH OH NO2 OH OH OH OH o-Tyrosine Dityrosine 3-Nitrotyrosine 2,4-Dihydroxy phenylalanine R1 R1 O2N R1 HO R1 HO OH NH OH HO NH NH HO 3,4-Dihydroxy phenylalanine 5-Nitrotryptophan CO2H Hydroxytryptophan Dihydroxytryptophan O R R R1 R1 NH O NH Oxindolylalanine Hydroxyhexahydro pyrroloindolecarboxylic acid COOH HO CH3 H2N HOCH2 H2N O COOH OH O HN O R1 = NH H Thymine tyrosine dimer CO2H H2N O H2 N H CO2H Glutamic acid semialdehyde Lysylcarbonyl NH HN CHO OHC NH CytosineTyrosine dimer "3"-Hydroxyvaline H COOH OH O H "2"-Hydroxyvaline H COOH CH3 CH3 "1"-Hydroxyvaline NH2 N H COOH H Indole derivatives NH2 H HOCH2 H CH3 N-Formylkynurenine/ kynurenine NH2 NH2 NH NHR NH H2N O OH H2N Thymine lysine dimer NH2 H CO2H Figure 3.14 A Variety Of Modifications Can Be Formed When Amino Acids Or Proteins Are Exposed To ROS, RNS Or Other Reactive Compounds. WWW.ESAINC.COM 225 Michael additions with the amine side chain of lysine residues and can lead to inter- and intra-molecular protein cross-links (Chapter 3 and below) (e.g., Uchida et al. (1997)). Such modification can lead to enzyme inactivation e.g., glucose-6phosphate dehydrogenase and glyceraldehyde-3-phosphate dehydrogenase are inhibited by 4-hydroxy-nonenal (Table 3.8). CO2H H2N CO2H H2 N CO2H CO2H H2N H2N CO2H CO2H H2N H2N CO2H H2 N H2N O OH OH OH OH OH OH CO2H OH DITYROSINE TRITYROSINE PULCHEROSINE CO2H CO2H CO2H CHO H2 N H2 N H2N H2N O CO2H NO Cl OH OH 3,4-L-DOPA O2H Cl Cl Br NO2 OH OH CO2H H2N H2 N H2N H2N 3-NITROSOTYROSINE CO2H CO2H CO2H CO2H H2 N 3-CHLOROTYROSINE 4-HYDROXYPHENYLACETALDEHYDE ISODITYROSINE OH OH O OH OH OH 3-NITROTYROSINE 3-BROMOTYROSINE 3,5-DICHLOROTYROSINE TYROSINE1-PEROXIDE CO2H CO2H OH CO2H HO H2 N NH2 NH O2H O TYROSINE3-PEROXIDE NO2 OH O CYCLOTYROSINE PEROXIDE ADDUCT 3-NITRO-4-HYDROXY PHENYLACETIC ACID CO2H NO2 OH 3-NITRO-4-HYDROXY PHENYLETHYLAMINE NO2 OH 3-NITRO-4-HYDROXY PHENYLLACTIC ACID Figure 3.15 A Variety Of Modified Tyrosine Residues Can Be Formed Under Oxidizing Conditions. Oxidative Damage to Tyrosine. Protein oxidation can lead to chain fragmentation. Garrison developed his “peptide α-amidation” pathway to help explain how protein oxidation can result in polypeptide chain breakage and protein fragmentation (Garrison (1987) and references therein). Tyrosine is very susceptible to pro-oxidant modification. A variety of metabolites can be produced depending upon which pro-oxidant is present and the reaction conditions (Figure 3.15). As many modified tyrosine WWW.ESAINC.COM 226 residues are currently being used as markers of pro-oxidant activity, it is worth exploring their production and importance more fully. 3-Nitrotyrosine, both free and protein-bound, are often used as indicators of increased nitric oxide activity. However, this can be erroneous, as nitric oxide does not react particularly well with non-radical species (Chapter 2). Nitric oxide does react extremely rapidly with other radicals e.g., with tyrosyl radical (formed when tyrosine reacts with oxidants such as the hydroxyl free radical and peroxynitrite) producing both carbon- and oxygen-nitrosotyrosines.14 Although the conversion of 3-nitrosotyrosine to 3-nitrotyrosine might possibly be promoted by ROS, it still remains to be proven. Current evidence suggests that 3nitrotyrosine is a better indicator of peroxynitrite production (Table 2.10; Figure 2.19) but even this has been recently challenged (Pfeiffer and Mayer (1998)). This situation is further complicated as 3-nitrotyrosine can also be formed by several other, albeit minor, pathways. These include the reactions between: • • • • • nitrogen dioxide and the tyrosyl radical; acidified nitrite, hydrogen peroxide and tyrosine; nitryl chloride and tyrosine; nitrite, hydrogen peroxide, myeloperoxidase and the tyrosine; and nitrite, hypochlorous acid and tyrosine (see Chapter 2) (See Brennan et al., (2002); Dalber et al. (1998); Eiserich et al. (1996); Halliwell (1997); van der Vliet et al. (1997)). Thus 3-nitrotyrosine is probably best regarded as a biomarker for nitrating species in general rather than for any one specific RNS. 3-Nitrotyrosine is now commonly used as a marker of oxidative stress, its free and bound levels being increased in a variety of disease states (Tables 3.10). Remember though that both free and protein-bound 3-nitrotyrosine can react with hypochlorous acid and this may lead to an underestimation of 3-nitrotyrosine at sites of chronic inflammation and possibly explain the discrepancies in the level of this analyte reported in literature (Whiteman and Halliwell (1999b)). Protein-bound levels may also be underestimated due to the action of protein nitrases (Kamisaki et al. (1998); Kuo et al. (1999)). Protein nitration is a fascinating area of research with many questions still yet to be answered. It is not clear why protein-bound tyrosine residues are nitrated by peroxynitrite more efficiently than free tyrosine molecules (Crow (1999)). Why is it that only a small fraction of the total protein pool is susceptible to nitration? 14 This reaction can also take place with an essential tyrosyl radical in the active site of ribonucleotide reductase and results in inhibition of this enzyme (Lepoivre et al. (1994)). Many enzymes contain such an intrinsic radical essential to their catalytic process (e.g., on tyrosine, tryptophan, glycine or thiol residues) (Pedersen and Finazzi (1993)). Another example is pyruvate dehydrogenase which catalyses the conversion of pyruvate to acetyl-CoA by and uses both carbonand sulfur-centered radicals (Halliwell and Gutteridge (1993)). Unfortunately, under some conditions the reaction of protein radicals with molecular oxygen (and possibly other species) can lead to cleavage of the polypeptide chain, resulting in enzyme inactivation (Dean et al. (1997) and references therein). WWW.ESAINC.COM 227 Although many proteins have several tyrosine residues, why are only a few of them capable of being nitrated (e.g., neurofilament L has twenty tyrosines only four of which are nitrated; manganese superoxide dismutase has nine tyrosine residues but only three are nitrated) (Crow (1999) and references therein)? Interestingly, it takes the nitration of just one, key tyrosine residue to disrupt function (Crow et al. (1997); MacMillan-Crow et al. (1996, 1998)). Disease/Condition Adult respiratory distress syndrome Aging Alzheimer’s disease Amyotrophic lateral sclerosis (sporadic and familial) Atherosclerosis Autoimmune uveitis (experimental) Bronchopulmonary dysplasia Carbon monoxide poisoning Celiac disease Diabetes Endotoxemia Huntington’s disease model Idiopathic pulmonary fibrosis Inclusion-body myositis Inflammation – experimental allergic encephalomyelitis Inflammation – myocardial Inflammation – rheumatoid arthritis Inflammatory bowel disease Ischemia Multiple sclerosis Organ preservation and transplantation Organ rejection, acute and chronic Parkinson’s disease Perennial nasal allergy Pneumonia (influenza virus-induced) Preeclampsia Septic shock/renal failure Smoking Ulcerative colitis Reference Haddad et al. (1994); Kooy et al. (1995) Uttenthal et al. (1998) Good et al. (1996); Smith et al. (1997); Su et al. (1997) Abe et al. (1997); Beal et al. (1997); Bruijn et al. (1997); Chou et al. (1996a,b); Ferrante et al. (1997); Toghi et al. (1999) Beckmann et al. (1994); Buttery et al. (1996) Wu et al. (1997) Banks et al. (1998) Gow et al. (1996b); Ischiropoulos et al. (1996); Thom et al. (1997) Ter Steeg et al. (1998) Suarez-Pinzon et al. (1997) Kristof et al. (1998);Wizemann et al. (1994) Beal et al. (1995) Saleh et al. (1997) Yang et al. (1996) Cross et al. (1997); Okuda et al. (1997) Ishayama et al. (1997); Kooy et al. (1997) Halliwell (1995); Kaur and Halliwell (1994) Miller et al. (1995); Singer et al. (1996) Forman et al. (1998); Ischiropoulos et al. (1995) Bagasra et al. (1995) Skinner et al. (1997) MacMillan-Crow et al. (1996) Hantraye et al. (1996) Sato et al. (1998) Akaike et al. (1996) Myatt et al. (1996) Fukuyama et al. (1997) Petruzzelli et al. (1997) Kimura et al. (1998) Table 3.10 Altered Levels Of 3-Nitrotyrosine Are Found In A Variety Of Diseases And Conditions. Although free 3-nitrotyrosine is often regarded as the final product of RNSinduced damage in vivo, this may be true only for certain biological compartments (e.g., cells). In other locations free 3-nitrotyrosine, whether it is WWW.ESAINC.COM 228 formed by nitration of free tyrosine or released from protein following proteolysis, can be further metabolized to 3-nitrophenylacetic acid and 3-nitrophenyllactic acid. For example, both free 3-nitrotyrosine and its metabolite 3-nitrophenylacetic acid were found to be elevated in patients with ALS (Beal et al. (1996)). Urine does not contain any appreciable amount of 3-nitrotyrosine, and only contains its metabolites (Ohshima et al. (1990, 1991); Shuker et al. (1993); Tabrizi-Fard et al. (1999); Wishnol et al. (1993)). Urinary levels of 3-nitrophenylacetic acid are primarily derived from the nitration of circulating 4-hydroxyphenylacetic acid and not from 3NT metabolism (Mani et al., (2003)). Urinary 3-nitrophenylacetic acid should not be used as an indicator of 3NT production. 3-Chlorotyrosine is currently being used as an indicator of the production of reactive chlorine species. Although hypochlorous acid, formed by the action of myeloperoxidase15 on hydrogen peroxide and chloride, is the main chlorinating agent produced in vivo, several others (e.g., chlorine radicals, nitryl chloride and trans-chlorine nitrite) may also be involved, but to a much lesser extent (Chapter 2). Thus, while 3-chlorotyrosine is thought to be a reasonable marker of hypochlorous acid production, care must be exercised in always assuming that all chlorination results from endogenous production of hypochlorous acid.16 Elevated 3-chlorotyrosine levels are associated with atherosclerotic lesions and inflammation (Hazen and Heinecke (1997); Hazen et al. (1997); Heinecke et al. (1998)). 3-Chlorotyrosine is not the only product when chlorinating species react with tyrosine as 3,5-dichlorotyrosine and p-hydroxy-phenylacetaldehyde can also be produced (Figure 2.25). p-hydroxy-phenylacetaldehyde is a very reactive carbonyl and can readily damage proteins by forming a Schiff base with the εamine moiety of lysine residues (Chapter 2). Dityrosine (3,3’-bityrosine, m,m’-bityrosine) is formed when two tyrosyl radicals combine (Figure 2.9) and was first described following the oxidation of tyrosine with peroxidase and hydrogen peroxide (Gross and Sizer (1959)). This approach is specific for the production of dityrosine and will not cause the formation of oand m-tyrosine isomers. Dityrosine protein cross-link formation was later found to occur normally and was shown to be responsible for the insolubility and elastic properties of some proteins (Aesbach et al. (1976)). Dityrosine and other tyrosine polymers (e.g., trityrosine and pulcherosine) are also found in lower organisms where they help to strengthen structural proteins (e.g., in the hardened fertilization envelope of sea urchins and the cuticle collagen of Ascaris) (Nomura et al. (1990)). 15 Myeloperoxidase can directly react with tyrosine by two mechanisms. First, it reacts with free tyrosine to form tyrosine radicals that can then combine to form dityrosine and tyrosine polymers (Jacob et al. (1996)). Secondly, it can form tyrosine peroxides that are metastable and may contribute to neutrophil- or monocyte-mediated tissue injury (Winterbourn et al. (1997)). 16 It is estimated that about 2% of hypochlorous acid generated by neutrophils leads to the production of 3-chlorotyrosine (Kettle (1996)). WWW.ESAINC.COM 229 Both free and protein-bound dityrosine residues can be formed in reactions involving the hydroxyl free radical, peroxynitrite, hydrogen peroxide/metal, and irradiation (UV and gamma), while free dityrosine can also be formed by the action of certain enzymes (e.g., myeloperoxidase) (Eiserich et al. (1998); Giulivi and Davies (1994) and references therein; Heinecke et al. (1993); Huggins et al. (1993); Lymar et al. (1996); Michon et al. (1997); Savenkova et al. (1994); Sharma and Jane (1998); Winterbourn et al. (1997); Yasmin et al. (1997)). The production of tyrosyl radicals will not automatically lead to protein dityrosine formation. Protein dityrosine cross-links will only be produced if two tyrosyl radicals are allowed to encounter and not be formed if protein tyrosyl radicals are located too far apart from each other. Dityrosine confers stability to a protein making it less susceptible to proteolysis and acid hydrolysis and, as it does not undergo further metabolism can be used as a quantitative index of protein oxidation. Free dityrosine is more reactive and may, under special circumstances be further metabolized. For example, it is readily oxidized by both compounds I and II of peroxidases (rate constant with compound I is 1 x 105 M-1s-1) producing trityrosine, polytyrosine and other oxidation products (Marquez and Dunford (1995) and references therein). Both free and protein-bound dityrosine are found in a variety of human tissues. Changes in their levels are currently being used as a marker for metal catalyzed oxidation in vivo and in vitro, as a measure of total index of oxidative stress and as an indicator of oxidative damage involving phagocytes (Giulivi and Davies (1993); Heinecke et al. (1993); Huggins et al. (1993); Leeuwenburgh et al.(1999); Salman-Tabcheh et al. (1993)). For example, the level of protein bound dityrosine is markedly elevated in LDL isolated from human atherosclerotic lesions, compared to circulating LDL levels (Leeuwenburgh et al. (1997a)). Protein bound dityrosine is found to be increased in aging (e.g., it is abundant in lipofuscin granules) while free dityrosine can be formed following ischemiareperfusion (Abdelrahim et al. (1997); Kato et al. (1998); Wells-Knecht et al. (1993); Yasmin et al. (1997)). Interestingly, dityrosine cross-linking in cardiac and skeletal muscle in aging rats is attenuated by caloric restriction (Leeuwenburgh et al. (1997b)). Protein Repair and Degradation. Proteins, like DNA, can be rendered non-functional following damage. Although affected proteins can be replaced by de novo synthesis this is energetically expensive so cells have developed repair mechanisms including: • • The restoration of a protein into its correct, active conformation (see the section on chaperones above); Enzymatic repair to directly reverse some forms of amino acid residue damage. Such mechanisms are capable of repairing proline isomerization, WWW.ESAINC.COM 230 and reversing isoaspartyl and methionine sulfoxide formation (Visick and Clarke (1995)). Two processes are important in protecting methionine. First, naturally occurring antioxidants can reduce the methionine intermediate that is initially formed when methionine is damaged by prooxidant species. Second, the enzyme methionine sulfoxide reductase can reform methionine from methionine sulfoxide in a process that probably uses NADPH and thioreductase. Methionine sulfoxide reductase plays an important role in reactivating the oxidized α1-proteinase inhibitor and preventing the formation of methionine sulfoxide in the lens of the eye. Excessive oxidative damage to the lens may be one of the many processes that can overwhelm the eye’s antioxidant defenses eventually leading to cataract formation (cataracts are found to contain significant amounts of methionine sulfoxide). The cyclic process of methionine sulfoxide formation and methionine regeneration has led to the suggestion that methionine residues located on the surface of a protein may constitute an important antioxidant defense mechanism protecting the protein from more harmful oxidation (Levine et al. (1996)). Damaged proteins that cannot be repaired undergo proteolysis (Visick and Clarke (1995)). In normal human subjects about 300g of tissue protein is catabolized daily and replaced by newly synthesized protein. Since six ATP molecules are used for each amino acid residue added to the growing polypeptide chain, this turnover, accounting for 15-20% of the basal metabolic rate, is energetically expensive. Protein turnover is biologically important and can vary enormously from protein to protein. Proteins with especially short half-lives include enzymes that are important in regulating metabolic pathways (e.g., hepatic phosphoenol-pyruvate carboxykinase). Changes in the rate of synthesis of a regulatory enzyme will rapidly alter its concentration and hence the flux through the pathway. Rapid degradation not only allows control of metabolic flux, but also prevents any chance of the enzyme being reactivated inappropriately. This explains why selective protein degradation always plays an important regulatory role in timing controls (e.g., cell cycle progression and various signal transduction pathways). A second group of proteins with short half-lives are the abnormal proteins resulting from errors in translation, and oxidative damage (including conformation changes and oxidation of amino acid residues). The rate of hydrolysis of abnormal proteins is dependent upon the amount of oxidation present. Proteins with limited oxidation are degraded at a greater rate than those that are more markedly damaged probably a consequence of marked changes in a protein’s structure rendering it poorly digestible. WWW.ESAINC.COM 231 AMP + PPi E1-SH + ATP E2-SH Ub-CO2S-E1 E1-SH Ub-CO2- nx Ub-CO2S-E2 E3 DUB Protein Ub-Protein Protein E2-SH Poly-Ub-Protein 26S Proteasome ATP Ub Oxidized Protein 20S Proteasome PA700 Peptides Oxidized Peptides Figure 3.16 Degradation Of Normal Proteins By The Ubiquitin-Proteasome (26S) System. Oxidatively Damaged Proteins Are Not Processed By The 26S Proteosome But Rather By Its Proteolytic Core, The 20S Proteosome. This Process Does Not Require Protein Ubiquitinylation Prior To Degradation. (DUB - deubiquitination enzymes; E2 - ubiquitin conjugating enzyme; E3 ubiquitin-protein ligase; Ub – ubiquitin). There are at least two pathways for protein degradation in eukaryotic cells, lysosomal and non-lysosomal. Lysosomes contain at least four proteinases (including cathepsins B, D, and E) and several peptidases (e.g., dipeptidyl peptidases) permitting complete protein degradation within this organelle (Bohley and Seglen (1992); Dean (1979); Dice and Terlecky (1990)). Lysosomes can also form autophagic vacuoles capable of engulfing and digesting whole organelles such as mitochondria. The non-lysosomal pathway is the most important proteolytic pathway for many short-lived proteins and involves tagging WWW.ESAINC.COM 232 them with ubiquitin prior to digestion by multisubunit complexes (the proteasomes) located in the cytosol and nucleus (Couz et al. (1996); Hilt and Wolf (1996)). Ubiquitin, a small protein (~8.5kDa) found in all eukaryotic cells, plays a wide variety of regulatory roles including gene expression, ribosome biosynthesis, receptor expression and ubiquitin-mediated proteolysis (Hochstrasser (1996); Schwartz and Ciechanover (1992)). The tagging of a protein by ubiquitin requires ATP hydrolysis to form an isopeptide bond between the ε-amino group of a lysine residue on the protein and the carboxyl terminal glycine of ubiquitin. Three enzymes are involved in this process. The first enzyme, E1, activates ubiquitin in a process requiring ATP. The second enzyme (ubiquitin conjugating enzyme, E2) takes activated ubiquitin from E1 and transfers it to a damaged protein in a process requiring the third enzyme (ubiquitin-protein ligase) E3 (Haas and Siepmann (1997); Hochstrasser (1995)) (Figure 3.16). A protein tagged for destruction usually acquires several molecules of ubiquitin. How a damaged protein is recognized by the ubiquitination system is unclear but may be controlled, in part, by chaperones (Benjamin and McMillan (1998); Raboy et al. (1991); Sherman and Golberg (1996)). The role of deubiquitination (DUBs) enzymes in regulation of protein turnover is still being evaluated (Hochstrasser (1995); Wilkinson (1997)). Ubiquinated proteins are degraded by the 26S proteasome complex (Figure 3.16) located in the cytoplasm, nucleus and endoplasmic reticulum, but not in the mitochondria. This complex is composed of a core proteinase known as the 20S proteasome and a pair of regulatory complexes (multisubunit proteins [ATPases] known as protein activators or PA700s) that are attached to both ends of the complex (Driscoll (1994); Tanaka (1998)). Although the exact steps in protein degradation are not fully known they include: binding of the multi-ubiquinated protein by its ubiquitin chains to the chain-binding subunits of PA700; a series of ATP-dependent unfolding and translocation steps that feed the unfolded protein into the central channel of the 20S proteasome; cleavage of substrate into small peptides; and finally disassembly of the ubiquitin chains, that can then be reused (based on Hochstrasser (1995)). Dysfunction of the ubiquitin-proteosome pathway has been implicated in the pathogenesis of several human diseases including cystic fibrosis, Angelman’s syndrome, and neurodegeneration (Scwartz and Ciechanover (1999); Alves-Rodrigues et al. (1998)). The turnover rate of a normal protein appears to be determined, in part, by its amino-terminal residue. Proteins can be categorized into three groups depending upon whether the amino terminal is stabilizing (half-life >20 hours; alanine, glycine, methionine, serine, threonine and valine), destabilizing (half-life 7-30 minutes; glutamate, glutamine, isoleucine, proline and tyrosine) or highly destabilizing (half-life <3 minutes; arginine, aspartate, leucine, lysine and phenylalanine) (Varshavsky (1997)). The exact mechanism driving the rate of WWW.ESAINC.COM 233 ubiquitination of these proteins remains elusive but research is centered upon the E3 enzyme. Oxidatively modified proteins are mainly degraded by the 20S proteosome located in the cytosol, while oxidatively damaged soluble histones and DNAbound histones are catabolized by the 20S proteosome located in the nucleus. Both of these are ATP- and ubiquitin-independent processes (Grune and Davies (1997); Grune et al. (1997); Ullrich et al. (1999)). In this way the association of damaged protein, through cross-linking and increased surface hydrophobicity, into potentially lethal protein aggregates is prevented. The 20S proteosome recognizes increased protein surface hydrophobicity (aromatic residues and bulky aliphatic residues) caused by changes in a protein’s secondary and tertiary structure caused by pro-oxidant damage (Grune et al. (1997); Pacifici et al. (1993)). The activity of 20S proteosome is greatly affected by the amount of protein damage. While the complex has little problem dealing with moderately damaged proteins, extensive protein damage can lead to inhibition of the complex and the build up of modified protein (Grune et al. (1998); Ullrich et al. (1999)). Amino Acid and Protein Damage in Aging and Disease. An open question in the field of aging is whether protein oxidation is an important aspect of aging or whether it is just one consequence. Abundant evidence shows that protein oxidation products such as protein carbonyls and protein-containing age pigments (e.g., lipofuscin) do accumulate with age (Halliwell and Gutteridge (1999); Stadtman (1988)). This is especially true for long-lived proteins, such as those in the lens, where oxidized proteins accumulate over time. Aging is also accompanied by a decrease in the activity of key metabolic enzymes such as glutamine synthetase, glucose-6-phosphate dehydrogenase and cytosolic neutral protease activity. Unfortunately, there is no direct evidence that altered activity is a consequence of protein oxidation. Treatment of rats with the spin-trap agent, PBN, was found to prevent the age-related increase in protein carbonyl production, loss of enzyme activity and loss of behavioral performance (Carney et al. (1991); Stadtman et al. (1992) and references therein). Although these findings are encouraging, Dean et al. (1997) have suggested that the levels of PBN used were too low to have any antioxidant effects. Transgenic Drosophila overexpressing catalase and superoxide dismutase lived longer and were more active than those overexpressing just one of these enzymes (Orr and Sohal (1994); Sohal et al. (1995)). The increased pool of damaged protein seen with aging can be explained either by an overproduction of oxidized protein overwhelming the proteolytic process and/or decreased activity of these enzymes. The latter can occur at several levels, including damage to genes encoding proteolytic enzymes (DNA damage also accumulates with aging), damage to the proteolytic enzymes themselves, WWW.ESAINC.COM 234 and oxidation-induced changes in substrate rendering it less susceptible to proteolytic attack. However, as the precise mechanisms governing proteolytic activity remain unresolved, it may be too soon to link changes in these systems to the accumulation of oxidized protein in aging (Stadtman (1992)). A variety of diseases including atherosclerosis, cataracts, diabetes, inflammation and neurodegeneration are also associated with increased protein oxidation. These have been reviewed elsewhere (Dean et al. (1997); Halliwell and Gutteridge (1999)). Altered levels of one protein oxidation “marker”, 3nitrotyrosine, has been reported to be increased in a variety of diseases and conditions (Table 3.10). A selection of potential markers and the effect of disease on their levels are presented in Table 4.11. The role of glycation and glycoxidation reactions in diabetes is discussed in greater detail below. Analyte 3,4-L-DOPA Species Human Tissue LDL protein 3,4-L-DOPA Rat Glial cells inculture Carbonyls (protein bound) Human Mixed tissues 3-Chlorotyrosine (protein bound) Dityrosine (Free) Human Aorta Human Ventricular fluid Level 6 adducts/104 Tyr control 14/104 – atherosclerotic 0 – control 1 adduct/103 Tyr after interleukin 1β treatment 1nmol/mg protein – control <8nmol/mg – diseased brain tissue 0.8 adducts/104 Tyr control 4.2/104 – atherosclerotic 3.5 adducts/103 Tyr control 12/103 – Alzheimer’s 0.2 adducts/103 Tyr control ~3/103 – Alzheimer’s 1-3 adducts/106 Tyr 2 adducts/106 Tyr – control 5/104 – plaque 5 adducts/105 Tyr – control (Protein bound) Dityrosine (bound) Dityrosine (bound) Human Hippocampus Lens protein Human LDL protein Dityrosine (bound) Rats Cat Human Protein (mito) Protein (cytosolic) Urine Urine Urine Human CSF Human Plasma 1.4+0.7nmol/L – control 9.0+0.7nmol/L – ALS 11.4+5.4 nmol/L – AD 31+6 nmol/L Human Brain - gray 0.285+0.26 to 0.959+0.02 (free) Dityrosine 3-Nitro-4hydroxyphenylacetic acid 3-Nitrotyrosine (free) 3-Nitrotyrosine (free) 3-Nitrotyrosine 7 adducts/105 Tyr – control 0.5nmol/mmol creatinine 3289-11,803ng/day 0-7.9 µg/24hr WWW.ESAINC.COM Reference Dean et al. (1997) Hensley et al. (1997) Levine et al. (1994); Lyras et al. (1996) Leeuwenburgh et al. (1997) Hensley et al. (1998) Wells-Knecht et al. (1993) Dean et al. (1997) Leeuwenburgh et al. (1999) Marvin et al. (2003) Ohshima et al. (1990,1991) Tohgi et al. (1999a,b) Kamisaki et al. (1996) Maruyama et al. 235 (free) nmol/g Brain - white 3-Nitrotyrosine (free) Human Serum 3-Nitrotyrosine (free) 3-Nitrotyrosine (free) 3-Nitrotyrosine (free) Human Human Synovial fluid Plasma Human Urine 3-Nitrotyrosine (free) 3-Nitrotyrosine (free) Mouse Brain Mouse Brain 3-Nitrotyrosine (free) 3-Nitrotyrosine 3-Nitrotyrosine (protein bound) Mouse Spinal cord Cat Human Urine Plasma proteins 3-Nitrotyrosine (protein bound) Human 3-Nitrotyrosine (protein bound) Human Polymorphonuclear leukocyte proteins LDL 3-Nitrotyrosine (protein bound) Human LDL 3-Nitrotyrosine (protein bound) Human Plasma protein Leukocyte protein 3-Nitrotyrosine (protein bound) Rat Plasma proteins 3-Nitrotyrosine (protein bound) Rat 3-Nitrotyrosine Human Peritoneal exudate proteins Plasma 0.276+0.25 to 0.962+0.02 nmol/g 0 – Control 0.18+0.07 to 0.49+0.27 µmol/L – arthritis 0 – Control 0.49+0.26 µmol/L – arthritis n.d. – control 28+12 µmol/L – renal failure 0 – control 0 to 5.8 µg 3-nitro-4hydroxyphenylacetic acid/ 24hr – control 0 to 7.9 µg 3-nitro-4hydroxyphenylacetic acid/ 24hr – smokers 2.0+0.1ng/mg protein 3.0+0.5 adducts/103 Tyr – control 6.0+1.5 – SOD transgenic 20+5 adducts/103 Tyr – control <58ng/day 7+1.2 adducts/103 Tyr – control 12.2+1.4 – stimulated 0 – control 21.3+1.2 adducts/103 Tyr – stimulated <10pmol/mg LDL protein – control <10pmol/mg – plaque 9+7 µmol/mol Tyr – control 840+140 – atherosclerotic intima 7+1 adducts/103 Tyr – control 12+1 – phorbol ester stimulated 0 – control 14+1 – phorbol ester stimulated 0.37+0.32 adducts/106 Tyr – control 12.46+3.13 – stimulated 0 – control 14.11+2.33 adducts/106 Tyr – stimulated WWW.ESAINC.COM (1996) Kaur and Halliwell (1994) Kaur and Halliwell (1994) Fukuyama et al. (1996) Ohshima et al. (1990) Schulz et al. (1995) Beal et al. (1995) Bruijn et al. (1997) Marvin et al. (2003) Salman-Tabcheh et al. (1995) Salman-Tabcheh et al. (1995) Dean et al. (1997) Leeuwenburgh et al. (1997) Salmen-Tabcheh et al. (1995) Shigenaga et al. (1997) Shigenaga et al. (1997) Skinner et al. 236 Free Protein bound 3-Nitrotyrosine Free Protein bound 3-Nitrotyrosine Free Protein bound 3-Nitrotyrosine Protein bound o-Tyrosine protein bound o-Tyrosine protein bound m-Tyrosine protein bound 0 2.3 adducts/106 Tyr Human Rat Ventricular fluid 2 adducts/103 Tyr control 4/103 Alzheimer’s Hippocampus Liver 0.2 adducts/103 Tyr control ~1.5/103 Alzheimer’s 15.7+0.3 adducts/106 Tyr (1997) Hensley et al. (1998) Skinner et al. (1997) 9.5+1.1 Rat Glial cells inculture <0.2 adducts/103 Tyr Hensley et al. (1997) Human Lens Human LDL 0.3 to 0.9 adducts/103 phenylalanine 62 and 35pmol/mg protein – control 105 and 175pmol/mg protein – plaques Wells-Knecht et al. (1993) Dean et al. (1997) o-Tyrosine protein bound Rat Cat 0.7 adducts/103 phenylalanine 0.6 adducts/103 phenylalanine 157-250ng/day Leeuwenburgh et al. (1999) o-Tyrosine Protein (mito) Protein (cytosolic) Urine Marvin et al. (2003) Table 3.11 A Selection Of Reports Measuring Amino Acid And Protein Oxidation Markers. AD – Alzheimer’s Disease; ALS – Amyotrophic Lateral Sclerosis. Measurement of Amino Acid and Protein Damage. Many modified amino acids can be formed during oxidation processes (Figure 3.14) but protein carbonyls and modified tyrosine residues have garnered most attention. The analytical procedures used to measure protein oxidative damage tend to fall into two categories – those that use whole proteins and those that measure amino acid residues following protein hydrolysis. Whole Protein. A variety of techniques can be used to measure amino acid modifications in whole protein either in situ or following isolation (e.g., Viera et al. (1999)). For isolated proteins the choice of technique is dependent upon the purity of the sample. For relatively clean samples (and for in vitro studies using purified proteins) the abundance of some modified residues can be determined using UV WWW.ESAINC.COM 237 detection. However, this approach is severely limited as only a few amino acid residues show UV absorbance. Fluorescence is often used as an indirect measure of protein damage (Jones and Lunec (1987)). Oxidative changes in tyrosine, tryptophan and cysteine residues are associated with protein aggregation and the induction of a characteristic fluorescence (excitation 360nm, emission 454nm). Although it is still unclear exactly which modifications are being measured, this technique is being used to study the role of ROS/RNS-induced protein modification in diseases such as diabetes and arthritis (Jones and Lunec (1987)). Some analytical approaches require a degree of sample preparation before the amount of protein damage can be quantified e.g., the use of polyclonal antibodies. First, proteins in complex biological samples can be immobilized on nitrocellulose and extensively washed prior to detection. The immobilized protein can then be exposed to polyclonal (or monoclonal) antibodies raised to a specific modified residue. Subsequent exposure to radiolabeled (or fluorogenic labeled) immunoglobulin G permits the measurement of oxidatively modified protein using beta scanning (or fluorescence scanning) (Crow and Ischiropoulos (1996); Ye et al. (1996)). This approach is sensitive and fairly selective but only measures total, not individual protein modifications. To examine which specific proteins are being modified, more advanced separation methods must be used. One-dimensional electrophoresis using a sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) can separate thiolreduced proteins based on their relative masses. Protein bands in the gel can then be visualized using Coomassie blue or silver stain. This approach is quick, sensitive (about 0.1µg with Coomassie blue and 0.02µg with silver stain) and can distinguish between proteins differing by only 2% of their mass. Some proteins such as glycoproteins and membrane proteins, however, can migrate anomalously. Specific modified residues can be determined following Western blotting and exposure of the resulting blot to radiolabeled or fluorogenically labeled antibodies specifically raised to the modified residue of interest. Boundlabel can then be visualized using autoradiography, beta scanning or fluorescence scanning. WWW.ESAINC.COM 238 Figure 3.17 A Two-Dimensional Gel Showing Resolution Of Many Proteins In A Rat Fibroblast Lysate. I would like to acknowledge Dr. M. Lopez for supplying this gel image. Perhaps the best approach to study the proteome is two-dimensional electrophoresis (see Lopez (1997, 1998a, 1998b) and references therein). Here proteins are initially separated based upon their charge (isoelectric focusing) and then in the second dimension on their molecular weight (SDS-PAGE). A typical two-dimensional gel is shown in Figure 3.17. Protein spots can be visualized using different stains (see above). Following blotting, individual protein spots can then be further characterized. For example, the protein sequence can be determined using tryptic digest followed by HPLC with Edman degradation chemistry. Protein mass can be measured using matrix-assisted laser desorption ionizing time of flight mass spectrometry (MALDI-tof-MS). Finally, modified residues can be determined using the antibody-based methods described above. WWW.ESAINC.COM 239 Protein Hydrolysates. This method is used to liberate residues from the modified protein prior to analysis by a variety of analytical techniques such as GC- and HPLC-based approaches. Two broad hydrolytic methods are used – acidic and enzymatic hydrolysis. Both procedures are currently being used although neither approach is perfect. Like with DNA adduct measurement described above, I urge researchers to pay careful consideration to the isolation and hydrolysis procedures. Acid hydrolysis typically involves heating the lyophilized protein under vacuo at 110oC in 6N hydrochloric acid for 12-24hr. Phenol and/or benzoic acid (~0.1-1%) are typically included to prevent artifactual generation of tyrosine adducts (Heinecke et al. (1998); Kettle (1998)). The advantage of acid hydrolysis is that it is straightforward and the protein is fully hydrolyzed to individual residues. Unfortunately acid hydrolysis suffers from several disadvantages. The protein must be extensively washed prior to hydrolysis in order to remove nitrite, nitrate and chloride ions. Under acidic conditions these can cause artifactual formation of tyrosine adducts (Heinecke et al. (1998); Kettle (1998); Shigenaga (1999); Shigenaga et al. (1997) and references therein). The acid used for hydrolysis must also be devoid of contaminating nitrite, nitrate and chloride. If hydrochloric acid is used for protein hydrolysis then a strong vacuum must be maintained during hydrolysis to avoid artifactual generation of 3-chlorotyrosine. This can be avoided by using hydrobromic acid. Hydrobromic acid, however, is unsuitable for measurement of bromo-tyrosine adducts. For the routine analysis of halogenated tyrosine residues methane sulfonic acid or other non-halogenated volatile acids are perhaps the best choice. Another major problem with acid hydrolysis is that this process can destroy tyrosine residues, thereby affecting the tyrosine adduct/tyrosine ratio. Furthermore, if acid hydrolyzed protein is to be analyzed using HPLC, the pH of the hydrolysate must be buffered so as not to expose the analytical column to detrimental acidic pH conditions. An alternative approach is to use a volatile acid that can be removed under a stream of air or nitrogen (Hazen (1998)). With enzymatic hydrolysis a protein sample is typically incubated with a proteolytic enzyme (e.g., proteinase K or pronase E) at 50oC for 12-16hr. This approach avoids the problems of acid hydrolysis but has several issues of its own. Enzymatic hydrolysis may not go to completion, producing tyrosine adductcontaining peptide fragments. Some proteolytic enzymes contain both tyrosine and 3-nitrotyrosine that can be liberated upon autodigestion. Care must be exercised in the correct choice and source of enzyme. It is also recommended that enzyme be extensively dialyzed before use (Shigenaga et al. (1997)). WWW.ESAINC.COM 240 Measurement of Free Modified Amino Acids and Modified Residues in Whole Proteins and Protein Hydrolysates. 1. Protein Carbonyls. Measurement of protein carbonyls is a commonly used method to measure oxidative damage to proteins. Protein carbonyls are usually determined using Schiff-base conjugation with 2,4-dinitrophenylhydrazine (DNPH) followed by spectrophotometric, HPLC-UV, or immunochemical techniques (Ayene et al. (1993); Fung and Grosjean (1981); Harris et al. (1994, 1995); Hensley et al. (1995); Legler et al. (1985); Levine et al. (1994); Oliver et al. (1987); Smith et al. (1991); Winterbourne and Buss (1998)). The limit of detection for the HPLC-UV approach is typically 100pmol on column but this may not be sufficient to measure the low carbonyl levels typically found under basal and even some pathological conditions.17 Typical tissue levels vary from 0.73+0.63 nmol/mg (human lumbar controls) to >4.0 nmol/mg (human brain) (see Evans et al., (1998) and references therein. Unfortunately, the DNPH approach cannot effectively distinguish between protein oxidation and post-translational modifications such as nonenzymatic glycation. Furthermore, processes not involving oxidative damage can also form protein carbonyls (Cao and Cutler (1995)). For example, α,β-unsaturated alkenals formed during lipid peroxidation can react with protein thiols forming stable covalent thioether adducts carrying carbonyl groups. The formation of Schiff bases between a lysine residue and a reducing sugar may, upon Amadori rearrangement, also yield carbonyl-containing ketamine protein conjugates. Protein carbonyl measurement, its limitations and issues, is critically reviewed by Evans et al. (1998)). 2. Methionine sulfoxide. Methionine sulfoxide can be measured in whole protein using 13C NMR (Cohen et al. (1979)) and electrophoretic methods (Amiconi et al. (1985) or in hydrolyzed protein using GC- and HPLC-based approaches (Chao et al. (1997); Maier et al. (1995)). 3. 2-Oxohistidine. 2-Oxohistidine (2-imidazolone) in proteins can be determined using automated Edman protein sequencing and mass spectrometry or in hydrolyzed proteins using HPLC-ECD and HPLC-fluorescence of the OPA derivative (Lewisch and Levine (1995, 1998); Uchida and Kawakishi (1993)). Due care must be exercised during acid hydrolysis of proteins as 2-oxohistidine is unstable and will 17 HPLC-ECD can give lower limits of detection for free carbonyls but the application of this approach to whole proteins is limited (Chiavari and Bergamini (1985); Goldring et al. (1993)). WWW.ESAINC.COM 241 decompose forming aspartate, ammonia and other products (Lewisch and Levine (1998)). This can be prevented by inclusion of the reducing agent dithiothreitol during sample processing. 4. Tyrosine Markers. Many analytical procedures are used to measure both free and protein-bound modified tyrosine residues. The extent of protein modification can be measured in situ, in whole protein or protein hydrolysates (Table 3.11). Protein hydrolysis is, however, fraught with methodological problems that can lead to artifactual production of modified tyrosine residues (see below). Out of all the oxidized residues that can be formed the measurement of modified tyrosine residues is probably one of the most common. This is due partly to the fact that they are considered to be “global” reporter molecules, capable of forming different products with ROS, RNS and oxidizing chlorine species, and partly because their measurement is relatively straightforward. 3-Nitrotyrosine. The extent of protein nitration can be determined in situ using immunohistological approaches on frozen and fixed tissues (e.g., Viera et al (1999)). Measurement of nitration of whole proteins is difficult to determine quantitatively. Current methods use immunochemical or UV detection (Beckman et al. (1994); Crow and Beckman (1995); Crow and Ischiropoulos (1996); MacMillan-Crow et al. (1999); Salman-Tabcheh et al. (1995); Viera et al. (1999); Ye et al. (1996)). Immunochemical methods are generally limited by antibody quality and visualization methods, and are often poorly reproducible, cumbersome, costly, suffer from matrix effects and slow throughput (Hensley et al. (1997); Viera et al. (1999)). Direct UV approaches are limited to relatively pure samples and are insensitive (~1µmol on column). Due to chromatographic issues, HPLC-UV detection is best performed on protein-tryptic digests (the limit of detection is ~0.1nmol on column). Protein-bound 3-nitrotyrosine is more conveniently measured following hydrolysis. 3-Nitrotyrosine, whether free or from hydrolyzed proteins, can be measured using a variety of analytical methods including GC-thermal energy analysis, GC-MS, LC-MS, and HPLC-based approaches (Althaus et al., (2000); Crowley et al. (1998); Greis et al. (1996); Herce-Pagliai et al. (1998); Leeuwenburgh et al. (1998); Ohshima et al. (1990); van der Vleit (1999); Yi et al. (1997)) (Table 3.11). Of all the HPLC-based techniques presented in Table 3.12, HPLC-UV is too insensitive for most tissue work and as 3-nitrotyrosine is not fluorogenic it must be converted to a fluorophore for HPLC-fluorescence analysis. This can be achieved by reducing 3-nitrotyrosine chemically to 3- WWW.ESAINC.COM 242 aminotyrosine (using sodium dithionite18 [sodium hydrosulfite] or sodium borohydride) (Sokolovsky et al. (1967)) which is fluorescent, or by derivatizing it with a fluorogenic agent prior to HPLC separation. HPLC Procedure UV Comments Sensitivity may be a problem especially for basal adduct measurement. Fluorescence 3-Nitrotyrosine is chemically reduced to 3-aminotyrosine using dithionite. 3-aminotyrosine can be measured directly with fluorescence. Measured following derivatization with phenylisothiocyanate. Electrochemical dual amperometric electrode detection Electrochemical single amperometric electrode detection, Measured as the 4-fluoro-7-nitrobenzo-2-oxa1,3-diazole derivative. Requires precolumn derivatization. Limit of detection 22pg on column. In samples 3NT appears to elute in a crowded area of the chromatogram. Upstream electrode reduces 3NT to 3-aminotyrosine that can then be detected at a lower oxidative potential (than is required for the measurement of 3-nitrotyrosine) at the down stream electrode. The reductive potential of – 2000mV in the presence of oxygen will generate high currents that will severely damage the working electrode. Reduction efficiency may vary over time. Although this approach can be used to measure in vitro protein nitration, it may be unsuitable for measurement of basal tissue and protein levels in vivo. This approach cannot be used to measure other tyrosine adducts (only 3nitrotyrosine can be reduced at the upstream electrode) unless a higher potential is applied to the downstream electrode. 3-Nitrotyrosine is measured directly at 1000mV on a single glassy carbon working electrode. Chromatographic issues (the References Althaus et al. (1997); Crow and Ischiropoulos (1996); Kaur and Halliwell (1994); Salman-Tabcheh et al. (1995); van der Vleit et al. (1995, 1996) Crow and Ischiropoulos (1996) Ischiropoulos and Al-Mehdi (1995) Kamisaki et al. (1996) Althaus et al. (1997) Kaur et al. (1998) 18 It should be remembered that dithionite reduction is very sensitive to pH, should preferably be used buffered, and must always be used in excess. WWW.ESAINC.COM 243 oxidative Electrochemical amperometric or coulometric detection, reductive Electrochemical amperometric or coulometric detection, OPA/βME derivatization Electrochemical photolysis followed by amperometric detection Electrochemical dual coulometric electrode detection, oxidative Electrochemical dual coulometric electrode detection, oxidative with on-line chemical reduction Electrochemical coulometric electrode array detection authors report that several endogenous compounds in brain samples co-elute with the 3NT peak) makes the reliable detection of 3-nitrotyrosine challenging. Although 3-nitrotyrosine can be measured using reductive potentials, this approach is not to be recommended. Unless oxygen is totally removed from the system 1) excessive noise makes routine measurement of 3-nitrotyrosine difficult, and 2) excessive current will limit the life of the working electrode. Pre-column derivatization of amino acid with OPA/βME is often used to render inert amino acids electrochemically active. Tyrosine (and its derivatives) is already electrochemically active and no increase in sensitivity is found upon derivatization. A novel approach using a stroboscopic photolytic unit to convert 3-nitrotyrosine to L-DOPA is described. Generated L-DOPA is detected on amperometric working electrodes placed downstream from the photolytic unit. Although this approach may prove useful for measurement of higher levels of 3NT, its applicability to biological samples is not clear. Photolytic units usually suffer from UVinduced fragility of the reactor coil and this still needs to be evaluated. The dead-volume of the reactor coil can also compromise chromatography. Direct measurement at +750mV on the downstream electrode, while the upstream electrode removes contaminants at +500mV. Anon* Anon* Liu et al. (1998a,b) Maruyama et al. (1996) Direct measurement at +850mV on the downstream electrode, while the upstream electrode removes contaminants at +600mV. Skinner et al. (1997) Extensive sample preparation permits sensitive measurement of 3-nitrotyrosine as its N-acetyl-3-aminotyrosine derivative. Shigenaga (1999); Shigenaga et al. (1997) Low picogram levels are typically measured using these approaches. An in-line Jone’s reductor placed prior to the analytical cell permits on-line reduction of 3-nitrotyrosine to 3-aminotyrosine and detection of the latter using electrochemical oxidation. Instability of the Jones reductor may compromise detection. Arrays of up to 16 electrodes coupled with gradient chromatography permit the sensitive, selective and simultaneous measurement of 3-nitrotyosine and other tyrosine derivatives. Analytes identified by their retention time and WWW.ESAINC.COM Matson (1998); Ohshima et al. (1999) Beal et al. (1995); Brujn et al. (1997); Crow (1999); Ferrante et al. (1997); Hensley et 244 GC/MS LC-MS/MS voltammetric behavior. See Figure 2.13. al. (1997, 1998); Maruyama et al. (1996); Schulz et al. (1995) Chemical reduction and derivatization (as developed by Shigenaga (1999)) followed by an oxidizing-reducing-re-oxidizing array permits extremely selective and sensitive detection of 3-nitrotyrosine as its N-acetylaminotyrosine derivative. Bose et al. (1999) Low picogram levels are typically measured using this approach. Free 3-nitro-, 3-chloro- and 3-bromotyrosine derivatives. Excellent sensitivity and selectivity when tyrosine isotopamer is used. Excellent sensitivity and selectivity (e.g., monitoring daughter ion (m/z 133.1) but difficult to operate and expensive. Gaut et al., (2002); Morton et al. (2003) Althaus et al. (2000); Marvin et al. (2003) Table 3.12 A Selection Of HPLC-Based Approaches Capable Of Measuring 3-Nitrotyrosine. Anon* preliminary experimentation at ESA Inc. HPLC-ECD is perhaps the most practical, straightforward method for the sensitive and routine measurement of 3-nitrotyrosine. A variety of HPLC-ECD approaches have been developed to measure 3-nitrotyrosine directly or following chemical (e.g., Figure 3.18) or electrochemical reduction (Table 3.12). Reduction by dithionite is also used to verify analyte identity – treatment of the sample with dithionite should, if the 3-nitrotyrosine peak is authentic, completely reduce the height of its peak in the chromatogram. Kaur et al. (1998) concluded that the use of dithionite to show peak authenticity can still be problematic as they found a peak that eluted close to 3-nitrotyrosine that was also capable of being reduced by dithionite. Perhaps a better approach would be the use of gradient HPLC and coulometric array detection to effect better separation and qualify analytes based on their voltammetric signature (Hensley et al. (1997, 1998)). Using this technique coupled to in vivo microdialysis, McCabe et al. (1997) reported that peripherally administered 3-nitrotyrosine was capable of passing through the blood-brain barrier and entering the brain (see Application Note 70-3993 Measurement of 3-Nitrotyrosine). Passage of 3-nitrotyosine through this protective barrier was by way of the large neutral amino acid carrier as coadministration of valine significantly blunted its passage (Acworth et al. (1987, 1997b)) (Figures 3.19 and 3.20). These findings suggest that central 3nitrotyrosine need not always be derived from activation of RNS pathways in the brain, but may be secondary to peripheral production resulting from chronic diseases. WWW.ESAINC.COM 245 O O Pronase E hydrolysis NO2 OH Protein Pellet NO2 Acetic Anhydride Extensive Washing to remove nitrite 10min, 25 oC CO 2- CO 2- O ProteinBound 3NT NH NH3+ N-, O-Diacetyl 3-Nitrotyrosine 3-Nitrotyrosine Formic Acid/ Ethyl Acetate Ethyl Acetate Evaporated to dryness, 30min, 37 oC 0.3M NaOH 30min, 37 oC OH OH HPLC-ECD Analysis NO2 NH2 Filter CO 2O NH N-Acetyl 3-Aminotyrosine 100mM Dithionite 10min, 25 oC Then HCl CO 2O NH N-Acetyl 3-Nitrotyrosine Shigenaga et al. (1997) Proc. Natl. Acad. Sci. USA, 74. Figure 3.18 Extensive Sample Clean-Up And Chemical Conversion Of 3-Nitrotyrosine To N-Acetyl-3-Aminotyrosine Leads To Improved Chromatographic Separation And Lower Detection Limits By HPLC-ECD. 3-Chlorotyrosine. Proteins containing 3-chlorotyrosine can be measured using immunostaining procedures (Hazell et al. (1996)). Free residues and those liberated from protein can be measured using MALDI-TOF-MS (Domigan et al. (1995)), GC-MS (Hazen et al. (1996, 1997); van der Vleit et al. (1999), HPLC-absorbance (Domigan et al. (1995); Eiserich et al. (1996)), HPLC-fluorescence of 1-nitroso-2-naphthol derivatized amino acids (Kettle (1996)) and HPLC-ECD (Acworth et al. (1998); Crow (1999)). To date there have been relatively few studies measuring tyrosine chlorination under conditions of oxidative stress. However, the level of 3-chlorotyrosine is elevated in proteins undergoing phagocytosis, exposed to inflammatory conditions and obtained from atherosclerotic lesions (Hazell et al. (1996); Hazen et al. (1996, 1997)). WWW.ESAINC.COM 246 Microdialysis of Rat Striatum after i.v. injection of 3-nitrotyrosine (10 mg/kg) Figure 3.19 Passage Of 3-Nitrotyrosine Through The Blood Brain Barrier Following Its Peripheral Administration (10mg/Kg. I.V.). (With permission of ESA, Inc.) 3NT Concentration (ng/mL) Passage of 3NT through the BBB and Inhibition by Valine 120 3NT i.v. (n=3) 100 3NT i.v. & Valine i.p. (n=2) 80 60 40 20 0 -60 -40 -20 0 20 40 60 80 100 120 140 160 180 200 Time (min) Figure 3.20 The Passage Of 3-Nitrotyrosine Through The Blood-Brain Barrier Is Blocked By Valine A Competitive Inhibitor At The LNAA Transporter. (With permission of ESA, Inc.) WWW.ESAINC.COM 247 The isocratic HPLC system consisted of a pump, an autosampler, a thermal chamber and an eight channel CoulArray detector. LC Conditions: Column: TSKgel ODS-80TM (TosoHaas) (4.6 x 250mm: 5µm) Mobile Phase: 20mM Sodium phosphate buffer, 8% methanol (v/v), pH3.2 Flow Rate: 1.0mL/min Temperature: 31oC Injection Volume 20µL Applied Potentials: +400, +450, +500, +570, +630, +670, +810, +830mV vs. Pd reference. See Application Note 70-3993 Measurement of 3-Nitrotyrosine for further details. Dityrosine. Dityrosine, free or liberated from proteins, can be measured using a variety of approaches including TLC, GC-MS and HPLC with either UV, fluorescence or ECD (Abdelrahim et al. (1997); Acworth et al. (1998); Aesbach et al. (1976); Leeuwenburgh et al. (1997a,b); Malencik et al. (1996)). See Figure 2.13. Other Tyrosine Oxidation Products. Tyrosine isomers are readily measured using HPLC-ECD (Chapter 2) or GC-MS (van der Vleit (1999)). p-Hydroxyphenylacetaldehyde can be measured directly using HPLC-UV absorbance (Hazen et al. (1996)) but due to its extreme reactivity is best trapped using a Schiff base. The Schiff base p-hydroxyphenylacetaldehyde adduct can then be measured using GC-MS (Hazen et al. (1997)). The measurement of dityrosine and other polymers is discussed above. Tyrosine peroxides can be measured using HPLC-UV detection (Winterbourn et al. (1997)). Brominated tyrosine derivatives can be measured using GC-MS, LC-MS and HPLC-ECD (Wu et al. (1999)). LIPIDS. Introduction. Lipids are water-insoluble (hydrophobic) biomolecules that are highly soluble in organic (lipophilic) solvents. Lipids consist of a wide variety of organic compounds showing great structural diversity, from the simple long chain fatty acids, through terpenes, to the more complex steroids and waxes. Lipids have a variety of biochemical roles: They act as highly concentrated energy stores (the triacylglycerols or fats), fuel molecules (e.g., fatty acids), signal molecules (e.g., prostaglandins) and components of membranes. WWW.ESAINC.COM 248 Structure of Biological Membranes. Membranes serve to define a cell’s shape and separate it from the extracellular environment, and have been reviewed elsewhere (Halliwell and Gutteridge (1999); Stryer (1998)). They are fluid-like structures that act as highly selective permeability barriers. Lipophilic compounds tend to pass through the membrane unimpeded while hydrophilic compounds require specific protein gates and channels. Membranes have been likened to a sea of lipids with protein islands floating in (intrinsic proteins) or on (extrinsic proteins) that sea. Membrane lipids are generally regarded as being inert and play merely a structural role while proteins are more active acting as gates, channels, receptors, energy transducers and enzymes. However, it is now clear that lipids can also play a more active role: some membrane lipids are the reservoir of arachidonic acid, the precursor of prostaglandins and other bioactive molecules. In actuality, membrane lipids are far from being inert and are of great interest to researchers in the field of redox biochemistry. Membranes readily undergo lipid peroxidation processes that can affect membrane fluidity and, in turn, membrane protein function, and can give rise to several cytotoxic species (see below). The three major kinds of membrane lipids are phospholipids, glycolipids, and cholesterol. Phospholipids are either based on glycerol or sphingosine (Figures 3.21). Phosphoglycerides consist of a glycerol backbone with its C1 and C2 alcohol groups esterified with fatty acids and its C3 alcohol group esterified with phosphoric acid. The phosphoric acid head group is also esterified with one of a number of small aliphatic alcohols. These alcohols include serine, choline inositol, ethanolamine and glycerol. The structural diversity of phosphoglycerides is a result of their fatty acid esters and alcoholic head group. Fatty acids tend to be between 14 and 24 carbon atoms long and can be saturated or unsaturated (usually in the cis isomer). The unsaturated fatty acid is usually attached to C2 of glycerol. The chain length and degree of saturation affect membrane fluidity, while the charge and size of the head group affect binding of extrinsic proteins. Sphingomyelin is the only phospholipid found in membranes that is not derived from glycerol. It consists of a sphingosine backbone esterified with a fatty acid (via an amine-alcohol ester) and a phosphorylcholine head group (Figure 3.21). Animals also contain glycolipids (sugar-containing lipids) and cholesterol. Glycolipids are derived from sphingosine (Figure 3.22). Cerebroside consists of a sphingosine backbone, a fatty acid amine-alcohol ester and a glucose or galactose head group directly attached to the primary alcohol group of sphingosine. Gangliosides have the same basic structure but can have a branched-chain of as many as seven sugar residues. Glycolipids are located on the extracellular side of the plasma membrane and are involved in intercellular recognition, an important aspect of the immune system. The sterol cholesterol is only found in eukaryotes and then primarily in the plasma (not organelle) membrane (Figure 3.22). Cholesterol affects membrane fluidity and architecture. WWW.ESAINC.COM 249 O CH 3CH 2 CH 2(CH 2)6CO 2H OH Palmitic acid Linolenic acid O OH O OH Oleic acid (cis) OH Trans 9-Octadecanoic acid Fatty Acids OH NH 2 OH HO HO OH N(CH 3)3 Choline Ethanolamine OH OH HO NH 2 O Serine OH Inositol Headgroups CH 2OH HO CH 3(CH 2)12 NH 3+ HO OH HO Glycerol Sphingosine Backbone O O O P O O N(CH 3)3 CH 2O(CH 2)2NH 3+ CH 3(CH 2)12 N OH O O O O O A Sphingomyelin Phosphatidylcholine Phospholipids O O O HO O O Diacyl glycerol O N(CH 3 )3 O O O P O HO O O O O O O O P Diacyl phosphatidate O Lysophosphatidylcholine Other Figure 3.21 The Basic Building Blocks Of Phospholipids And Related Species. WWW.ESAINC.COM 250 C8H17 (or Galactose) CH2O-Glucose CH3(CH2)12 N OH HO Cholesterol O O R Cerebroside (A Glycolipid) Figure 3.22 The Structures Of Cholesterol And Cerebroside. All phospholipids are amphipathic (containing both hydrophobic and hydrophilic regions) and, when exposed to water, will spontaneously form a bimolecular sheet in a self-assembly process. The reason that phospholipids readily form sheets rather than micelles is that their two fatty acid side chains are too bulky to fit into the interior of a micelle. The formation of a sheet over a micelle is biologically very important. Micelles are limited in size to <200µm, whereas bilayers can form much larger structures (typically millimeters). The sheet consists of a hydrophobic core composed of fatty acid side chains along with the bulk of the cholesterol molecule held together by hydrophobic interactions (the driving force for self-assembly). The hydrophilic head groups and the 3-hydroxyl group of cholesterol face the aqueous phase and are held together by electrostatic charges and hydrogen bonding. Lipid Damage. Lipid damage is probably not a familiar topic to most people but the consequences have been known for years. Foods high in fats (e.g., meats and dairy products) undergo oxygen-dependent deterioration leading to rancidity.19 Lipid peroxidation, the primary form of lipid damage found in biological systems, can broadly be defined as “oxidative deterioration of polyunsaturated lipids” (PUFAs) (Tappel (1979)). Lipid peroxidation is a particular problem for biological membranes as they contain high levels of PUFAs. Lipid peroxidation causes a number of problems for the cell. It decreases membrane fluidity, increases membrane porosity, inactivates membrane-bound enzymes and produces a range of toxic breakdown products (e.g., Chen et al., (1995) and references therein). Normally membrane lipid peroxidation is prevented by a variety of antioxidant mechanisms (Chapter 4) but under certain conditions, cell “rancidity” does occur. Indeed increased lipid peroxidation is associated with a variety of human diseases. 19 Removal of oxygen (e.g., canning), refrigeration and the inclusion of antioxidants prevent food rancidity (see Chapter 4). WWW.ESAINC.COM 251 Like any free radical-based reaction, lipid peroxidation has three phases: initiation, propagation and termination (Chapter 1). Initiation under peroxide free conditions starts with the abstraction of a hydrogen atom from a methylene group contained within a polyunsaturated molecule resulting in the formation of a carbon-centered radical (Eqn 3.1) (typical steady-state levels in vivo are 10-17 to 10-18M). The greater the number of double bonds in the system, the greater the chance that a hydrogen atom will be abstracted. —CH2— - H• → —CH•— Eqn 3.1 HO2• + L—H → L• + H2O2 Eqn 3.2 HO2• + L—OOH → H2O2 + LO2• Eqn 3.3 Initiation can be induced by irradiation or exposure to a variety pro-oxidant species. Some pro-oxidants are without activity. • • • • • • • Irradiation (one reason why irradiation of foods high in fats is not recommended); Exposure to the hydroxyl free radical; Exposure to iron/oxygen complexes (e.g., iron/ATP, iron/DNA, hemoglobin, myoglobin and cytochrome c); Exposure to peroxynitrite (Halliwell and Chirico (1993); Radi et al. (1991)); Superoxide cannot enter the membrane so does not directly initiate lipid peroxidation. Superoxide can, however, lead to formation of hydroxyl free radicals following its dismutation to hydrogen peroxide (which can also take part in the Fenton reaction) or it can stimulate hydroxyl free radical production by reducing Fe (III) to Fe (II) (the Haber-Weiss reaction) (Chapter 3). Under acidic conditions superoxide forms the lipophilic hydroperoxyl radical that can promote lipid peroxidation in isolated PUFAs (Eqn 3.2). Whether this reaction also occurs in membranes is unclear at present (Aikens and Dix (1991); Halliwell and Chirico (1993)). The hydroperoxyl radical can react with lipid hydroperoxides forming the lipid hydroperoxyl radical that can then attack other PUFAs, thereby stimulating the lipid peroxidation process (Eqn 3.3). Hydrogen peroxide can enter the membrane but does not appear to initiate lipid peroxidation directly. Singlet oxygen does not initiate lipid peroxidation by hydrogen abstraction. Rather it reacts directly with PUFAs, forming lipid hydroperoxides. WWW.ESAINC.COM 252 • • Ozone adds across carbon-carbon double bonds that can result in a diradical capable of hydrogen abstraction or decompose to produce reactive aldehydes (Chapter 2). Initiation can also be promoted by a variety of toxic compounds. For example, carbon tetrachloride (CCl4) can be converted to a trichloromethyl radical by cytochrome P450. The trichloromethyl radical and its peroxyl radical are very reactive and can readily abstract a hydrogen atom from a PUFA (Huie and Neta (1999)). Chloroform (CHCl3) is much less reactive than carbon tetrachloride, probably because it requires more energy to undergo homolytic fission. Once formed, the carbon-centered radical can suffer several fates, but the most likely under aerobic conditions is molecular rearrangement followed by reaction with oxygen to give lipid peroxyl radicals (Figure 3.23) (typical steady-state levels in vivo ~2 x 10-9M). These can react with a variety of molecules such as proteins, DNA and even other lipid peroxyl radicals (see Huie and Neta (1999) for an excellent review on the chemistry of organic peroxyl radicals) but in the membrane they are most likely to encounter other PUFAs from which they can abstract a hydrogen atom, thereby propagating the lipid peroxidation chain reaction. Thus just one initiation can lead to the formation of over one hundred lipid hydroperoxides. After initiation, the length of propagation is dependent upon the fatty acid composition, oxygen concentration, the amount of protein in the membrane and the presence of antioxidants. As the rate of formation of lipid peroxides increases, the chance that a lipid peroxyl radical encounters a protein increases. Such lipid-protein interaction will lead to termination of the chain reaction. The presence of chain breaking antioxidants will also end the lipid peroxidation chain reaction (Chapter 4). As discussed in Chapter 2 nitric oxide is a potent terminator of the lipid peroxidation process and reacts to form a variety of oxidized products that can be measured using HPLC-UV and LC-MS (Figure 3.249) (Freeman et al. (1995); O’Donnell et al. (1999a, b); Rubbo et al. (1994)). Some of these species have biological activity (e.g., cell signaling and anti-inflammatory properties) (Coles et al., (2002); Freedman (2002); Lim et al., (2002)). Finally, lipid hydroperoxides can decompose to yield a variety of decomposition products including reactive carbonyls (e.g., malondialdehyde and 4hydroxynonenal), polymers and alkanes (Figure 3.24 and below). WWW.ESAINC.COM 253 POLYUNSATURATED FATTY ACID (FREE OR PART OF TRIGLYCERIDE, DIGLYCERIDE OR PHOSPHOLIPID) CO 2H R HYDROGEN ABSTRACTION -H (e.g., HO, ONO2-, RO2) R CO 2H LIPID CARBON-CENTERED RADICAL R CO 2H LIPID CARBON-CENTERED RADICAL MOLECULAR REARANGEMENT O2 OXYGEN UPTAKE CYCLIZATION-POLYMERIZATION O O CO 2H R LIPID PEROXY RADICAL CYCLIZATION- FRAGMENTATION (e.g. MALONDIALDEHYDE 4-HYDROXY-2-NONENAL AND OTHER ALDEHYDES) PUFA Eqn 3.5 ABSTRACTION OF A HYDROGEN ATOM FROM OTHER FATTY ACIDS CAUSES AUTOCATALYTIC CHAIN-REACTION H H α-TOCOPHEROL CHAIN BREAKING ANTIOXIDANTS (e.g., TOCOPHEROL) REACT HERE TO STOP LIPID PEROXIDATION FROM SPREADING. α-TOCOPHERYL RADICAL PUFA H O O REDOX-ACTIVE METALS CO 2H R LIPID HYDROPEROXIDE ENZYMATIC LIPID REPAIR (FIGURE 3.29) Eqn 3.4 OH CO 2H R EXCRETED LIPID HYDROXIDE HYDROGEN ATOM ABSTRACTION FROM PUFA PROTECTION BY CHAIN BREAKING ANTIOXIDANTS O CO 2H R LIPID ALKOXYL RADICAL Figure 3.23 Initiation, Propagation And Termination Reactions Of Lipid Peroxidation. (Based on Acworth et al. (1997) and Halliwell and Gutteridge (1999)). WWW.ESAINC.COM 254 OONO OH (CH2)n COOH HYDROXYLNITROSOPEROXO FATTY ACID OONO O2H (CH2)n COOH HYDROPEROXONITROSOPEROXO FATTY ACID ONO (CH2)n COOH NITRITO FATTY ACID O2H (CH2)n COOH HYDROXPEROXO FATTY ACID OONO (CH2)n COOH NITROSOPEROXO FATTY ACID OTHER ALKENES RNS (CH2)nCOOH PUFA R CHO H O=C H C=O OH 4-HYDROXY ALKENAL MALONDIALDEHYDE OTHER ALDEHYDES Figure 3.24 Lipid Peroxidation Of PUFAs Can Lead To A Variety Of Products. WWW.ESAINC.COM 255 The Role of Metals in Lipid Peroxidation. Metals can stimulate lipid peroxidation through decomposition of lipid peroxides.20 Pure lipid peroxides are stable, but in the presence of iron or copper ions their decomposition is greatly increased producing both alkoxyl (RO•) and peroxyl (ROO•) radicals (Eqns 3.4 and 3.5). The reaction shown in Eqn 3.4 is equivalent to the Fenton reaction and the alkoxyl radical is analogous to the hydroxyl free radical. Interestingly, Fe (II) reacts with lipid hydroperoxides 20 times more rapidly than with hydrogen peroxide, and much more rapidly than Fe (III). This difference in reactivity between Fe (II) and Fe (III) salts may explain the variable effects of chelating agents on lipid peroxidation (Halliwell and Gutteridge (1988)). Both alkoxyl and peroxyl radicals are unstable and can abstract a hydrogen atom from a PUFA, thereby initiating lipid peroxidation. Alkoxyl radicals can also undergo β-scission producing a variety of products including carbonyl compounds, alkanes and alkenes, or rearrangement and oxygenation to give epoxyallylic peroxyl radicals (OROO•). The latter is far more favorable than hydrogen abstraction or β-scission so that OROO• and not RO•, as widely assumed, would promote free radical-mediated lipid peroxidation (Girotti (1998) and references therein). ROOH + “Fe2+” → “Fe3+” + OH- + RO• Eqn 3.4 ROOH + “Fe3+” → “Fe2+”+ H+ + ROO• Eqn 3.5 The role of iron in initiating lipid peroxidation has been covered extensively elsewhere (Halliwell and Gutteridge (1999); Sergent et al. (1999)). Iron can theoretically initiate lipid peroxidation by production of hydroxyl free radicals by the Fenton reaction (Chapter 2). However, it is difficult to conceive that hydroxyl free radicals, with a half-life of only 1ns, can diffuse from the site of production into the interior of the membrane to initiate lipid peroxidation. Furthermore, abundant evidence shows that initiation by iron does not have to involve the production of the hydroxyl free radical (Minotti and Aust (1989, 1992)). Other forms of iron have also been suggested as initiators including ferryl and perferryl species (Chapter 2) but their role in the process is not conclusive (Halliwell and Gutteridge (1999)). Recent evidence once more suggested a role for an unknown “Fe2+ + O2” species, possibly ferryl or perferryl in nature, that is readily capable of initiating lipid peroxidation in unsaturated fatty acid-enriched L1210 leukemia cell cultures (Qian and Buettner (1999)). Some evidence suggests a role for an undefined Fe (II)-Fe (III)-oxygen complex (or the ratio of Fe (II)/Fe (III) levels) (Minotti and Aust (1989, 1992)) but again this was challenged by Halliwell and 20 Commercially available lipid preparations always contain lipid peroxides and this is a major problem when using them to study lipid peroxidation processes. If these lipids are exposed to iron or copper ions during the experimental procedure then the contaminating lipid peroxides will form alkoxyl and peroxyl radicals that will stimulate lipid peroxidation thereby frustrating the experiment (Halliwell and Gutteridge (1999)). WWW.ESAINC.COM 256 Gutteridge (1999). Unfortunately, the exact nature of the iron/oxygen complex that is required for initiation of lipid peroxidation still remains elusive. Unlike the production of the hydroxyl free radical where only “free” iron can take part in the Fenton reaction, lipid peroxidation can be activated by both free and bound iron too. Thus iron stored in ferritin or located in heme, hemoglobin, cytochromes and peroxidases can all promote lipid peroxidation (Halliwell and Gutteridge (1999)). The mechanism by which these compounds promote lipid peroxidation may or may not involve free iron and is dependent upon assay conditions. For example, ferritin can promote liposome lipid peroxidation in a process that is inhibited by the chelating agent desferrioxamine suggesting that peroxidation is mediated by free iron ions released during the assay. Heme appears to promote peroxidation by both free iron ions and the production of radical species when lipid peroxides react with the heme ring. Lipid Peroxidation Products. The decomposition of lipid hydroperoxides can yield a variety of products including hydroxylated fatty acids, alkanes, alkenes and reactive carbonyl (aldehyde) compounds (Table 3.13) (Chapter 2). Carbonyls are regarded as secondary toxic messengers that can travel and cause damage at sites far removed from the initial point of insult. So far malondialdehyde, and the hydroxyalkenals (4-hydroxynonenal and 4-hydroxyhexenal) are the most intensively studied (Esterbauer et al. (1990)). Several other reactive aldehydes can also be formed in vivo and these are presented in Figure 2.25. Compound 4-Hydroxy-2nonenal 4-Hydroxy-2nonenal 4-Hydroxy-2nonenal 4-Hydroxy-2nonenal 4-Hydroxy-2nonenal Species Tissue Human Plasma Concentration/ Range 0.28+0.34 µmol/L Human Plasma 0.68+0.41 µmol/L Human 0.14+0.17 nmol/mg Human Low density lipoprotein Monocytes Human Plasma Synovial fluid 4-Hydroxy-2nonenal Human Plasma CSF 3.9+0.8 nmol/108 cells 0.34+0.09 µmol/L rheumatoid arthritis 0.54+0.19 - rheumatoid arthritis 0.66+0.06 µmol/L control 0.71 to 6.03 Parkinson’s patients 0.81+0.07 HIV-1 1.24+0.18 AIDS Reference Esterbauer et al. (1990) Selley et al. (1989) Esterbauer et al. (1987) Selley et al. (1989) Selley et al. (1992) Selley (1997) 0.2 to 3.14 – Parkinson’s patients WWW.ESAINC.COM 257 Bowel 4-Hydroxy-2nonenal 4-Hydroxy-2nonenal 4-Hydroxy-2nonenal 4-Hydroxy-2nonenal 4-Hydroxy-2nonenal 4-Hydroxy-2nonenal 4-Hydroxy-2nonenal Cholesteryl ester hydroperoxide Cholesterol ester hydroperoxide Fatty Acid hydroperoxides Isoprostane: 2,3-dinor-5,6dihydro-15-F2t-IP Isoprostane: 8-epi-IPF2α Isoprostane: 8-epi-IPF2α Isoprostane: 8-iso-PGF2α Rat Isoprostane: IPF2α Isoprostane: IPF2α Isoprostane: IPF2α Isoprostane: IPF2α-1 Lipid hydroperoxide 0.02 to 0.23 nmol/mg protein – control 1.25 to 7.99 – inflamed 2.65 nmol/mg protein Rat Hippocampal cells - culture Liver Plasma Liver 2.82+0.53 nmol/g 0.86+0.2 µmol/L 0.48+0.17 nmol/g Rat Liver 0.55+0.1 nmol/g Rat Hepatocytes 1.3+0.5 nmol/108 cells Rat Microsomes Lang et al. (1985) Rat Retina 0.03+0.01 nmol/mg protein 0.64+0.64 nmol/g Human Plasma 0.32 µmol/L Human Plasma 0.3 µmol/L Human Plasma 0.056 µmol/L Human Urine 390+180 pg/mg creatinine Yamamoto et al. (1987) Yamamoto and Ames (1987) Yamamoto et al. (1987) Morrow et al., (2003) Human 34.3+4.5 pg/mL Human Breath (condensate) Plasma Human Urine Human Urine Human Human CSF (ventricular) CSF (lumbar) Human Urine Human Plasma Lipid hydroperoxide Human Plasma 0.08 to 0.33 µmol/L – control 0.13 to 0.76 – postprandial 0.0 to 1.7 nmol/L Lipid hydroperoxide Human Plasma 4.0+1.7 µmol/L Lipid hydroperoxide Lipid hydroperoxide Human Human Plasma Serum 0 0 Malondialdehyde Human Plasma 3.8 to 4.6 µmol/L Rat 314.6+40 pg/mL 141+41 to 291+102 pg/mg creatinine dependent upon method 510+160 pg/mg creatinine 46+4 pg/ml control 72+7 Alheimer’s 23+1 pg/ml control 31+3 Alheimer’s 737+21 pg/mg creatinine WWW.ESAINC.COM Mark et al. (1997) Yoshino et al. (1986) Esterbauer et al. (1990) Norsten-Hoog and Cronholm (1990) Poli et al. (1985) Van Kuijk (1988) Baraldi et al., (2003) Vasselle et al., (2003) Tsikas et al., (2003) Tsikas et al., (1998) Morrow et al., (2003) Morrow et al., (2003) Pratico et al., (1998) Ursini et al. (1998) O’Gara et al. (1989) Cramer et al. (1991) Frei et al. (1988) Weiland et al. (1992) Hunter and 258 Malondialdehyde Human Plasma 0.94 µmol/L Malondialdehyde Malondialdehyde Human Human Plasma Plasma 0.6 µmol/L 3.74 µmol/L Malondialdehyde Human Plasma 35.1µmol/L Malondialdehyde Human Plasma 0.61 µmol/L Malondialdehyde Malondialdehyde Human Human Plasma Plasma 25 to 38 nmol/L 1.7 µmol/L Malondialdehyde Malondialdehyde Malondialdehyde Human Human Human Plasma Serum Serum 0.32 µmol/L 3.4 to 4.0 µmol/L 3.3 µmol/L Malondialdehyde Human Serum 0.9 to 1.88 µmol/L Malondialdehyde Human Serum 3.92 µmol/L Malondialdehyde Malondialdehyde Human Human Serum Urine 47.2 µmol/L 0.2 to 0.8 µmol/L Malondialdehyde Human Urine Malondialdehyde Rat Malondialdehyde Rat Malondialdehyde Rat Malondialdehyde Rat Brain microdialysis Brain microdialysis Heart perfused Liver Sperm 0.019+0.012 µmol/mmol creatinine 0.4 µmol/L Malondialdehyde Rat Phosphatidyl-choline hydroperoxide Phosphatidyl-choline hydroperoxide 0.02 to 0.16 µmol/L 0.068+0.016 µmol/L perfusate 0.7 to 0.8 nmol/g 0.4 to 3.9 pmol/mg protein Human Brain substantia nigra striatum cerebellum Plasma 3.23+0.25 nmol/mg protein 3.78+0.28 5,64+0.72 0.05 to 0.43 µmol/L Gerbil Brain ~9pmol/mg tissue Mohamed (1986) Ledwozyw et al. (1986) Wong et al. (1987) Yasaka et al. (1981) Santos et al. (1980) Francesco et al. (1985) Yeo et al. (1994) Viinikka et al. (1984) Lee (1980) Satoh (1978) Maseki et al. (1981) Suematsu et al. (1977) Nishigaki et al. (1981) Aznar et al. (1983) Tomita et al. (1990) Korchazhkina et al. (2003) Waterfall et al. (1996) Yang et al. (1997) Cordis et al. (1994) Yeo et al. (1994) Thiffault et al. (1995) Miyazawa et al. (1988) Zhang et al. (1994) Table 3.13 Levels Of Lipid Peroxidation Products Reported In The Literature. These analytes are routinely used as markers of lipid peroxidation. However, the reader should be aware that these low-molecular-mass products are probably formed as the result of multiple reactions far removed from the initial lipid peroxidation site. Consequently recent research is focusing on some of the initial compounds formed during the lipid peroxidation process. These include WWW.ESAINC.COM 259 cholesterol oxidation products (see below), some oxidation metabolites of arachidonic acid (e.g., the isoprostanes (see below), isoleukotrienes, and the levuglandins), and the derivatives of docosahexaenoic acid (the neuroprostanes). Malondialdehyde. Malondialdehyde (or malonaldehyde) [MDA] is a molecule with two aldehyde groups. In aqueous conditions it exhibits several pH- and age-dependent structural forms including keto-enol tautomers, intramolecular and intermolecular hydrogen bonded forms, dimers and trimers. For most biological experiments MDA is prepared by acid hydrolysis (e.g., 1% HCl) of commercially available bisdimethyl- or bis-diethylacetal. Dilute MDA solutions can be stored at 4oC for several days without noticeable degradation, but higher concentrations, especially if left for long periods at room temperature, undergo aldol condensation forming dimers and trimers. O O CO 2 H Arachidonic Acid Peroxyl Radical O O CO 2 H Cyclic Peroxide CO 2 H O O OOH OOH OOH Cyclic Endoperoxide Radical O 1,3-Dihydroperoxide PGH 2 Hydrolysis or heat O O Hydroperoxyepidioxide O2 O Malondialdehyde OOH OOH Polyunsaturated Aldehydes 1,4-Dihydroperoxide Figure 3.25 Possible Mechanisms For The Production Of Malondialdehyde From PUFAs. (Esterbauer et al. (1991); Frankel and Neff (1983); Halliwell and Gutteridge (1999); Pryor and Stanley (1995)). WWW.ESAINC.COM 260 As shown in Figure 3.25, MDA can be formed from several compounds including a variety of lipid peroxidation products, by the action of human platelet thromboxane synthetase on prostaglandins PGH2, PGH3 and PGG2, and by the action of polyamine oxidase and amino oxidase on spermine (Figure 2.25). MDA is found in both healthy and diseased tissues (Table 3.13) (Esterbauer et al. (1991) and references therein) but caution must be exercised when reviewing these levels as inappropriate analytical procedures were sometimes followed (see below). A) The Reaction of MDA and Lysine CHO N CHO NH CH CH CH N 1,4-Dihydropyridine3,5-dicarbaldehyde (1%) Amino-imino-propen Cross Link (77%) NH C CH CHO Aminopropenal (22%) B) The reactions of 4-Hydroxynonenal Protein R Protein R Protein + O R N O OH Cysteine S OH Cysteine R N Lysine Lysine OH Lysine Lysine S Histidine R N Histidine OH Figure 3.26 Some Reactions Of A) MDA And B) 4-HydroxyNonenal. (% Reflects The Abundance Of Modified Lysine Present In Vitro (Esterbauer et al. (1991)). WWW.ESAINC.COM 261 Under physiological conditions (pH7.4), MDA exists as an enolate anion (-O—C=C—CHO), and in this form it is not very reactive. Thus the claim often expressed in literature that MDA is an extremely reactive compound is untrue. Under more acidic conditions (pH<4), β-hydroxyacrolein (HO—C=C—CHO) (βHA) is the predominant form. Like 4-hydroxynonenal, βHA is a very reactive electrophile capable of reacting with nucleophiles (e.g., thiols) in a Michael type 1,4 addition. For example, glycine forms the monoglycine-βHA adduct, an enaminal, N-propenal-amino-acetic acid (OCHCH=CHNHCHRCO2H). Under strong acidic conditions and at a high concentration, further reaction produces the diglycine-βHA adduct. In a well-controlled study Nair et al. (1981) examined the reaction of several amino acids and reported that the product depended on the amino acid. Aromatic amino acids and arginine reacted at the α-amino group forming mono-enaminal adducts, whereas cysteine forms an adduct that contains two cysteine and 3 MDA molecules. Proteins are much more reactive with MDA than free amino acids. Although the reasons are not completely clear, it may be that proteins provide a more reactive environment and that MDA-condensation products might be the reacting species (Esterbauer et al. (1991)). MDA preferentially reacts with the ε-amine group of lysine and is capable of causing both intra- and inter-protein cross-links (Figure 3.26). MDA also reacts with histidine, tyrosine, arginine and methionine residues. Damage to essential –NH2 or –SH groups can lead to inactivation of enzymes (e.g., liver microsomal glucose-6-phosphatase is inactivated when its –SH groups are damaged). MDA can also react with DNA bases producing a variety of mutagenic compounds (Figure 3.7). Deoxyguanosine is the most reactive, adenosine and cytidine are fairly reactive, while thymidine is not reactive at all. MDA has the potential to induce amino-imino-propen cross-links between complementary strands of DNA and cause DNA-protein cross-linking. MDA is metabolized in the liver to malonic acid semialdehyde. This is unstable and spontaneously decomposes to acetaldehyde. Acetaldehyde is then converted to acetate by aldehyde dehydrogenase and acetate to carbon dioxide and water. Some MDA eventually ends up as acetyl-CoA. Mammalian urine also contains enaminals derived from the hydrolysis of MDA modified proteins (Esterbauer et al. (1991)). Urinary output of MDA in humans is typically 0.20.8µmol/L (Tomita et al. (1990)). 4-Hydroxyalkenals. These compounds were first discovered in the early 1960s and are formed as end products of lipid peroxidation and following hepatic metabolism of the hepatotoxic pyrrolizidine alkaloid senecionine (Esterbauer et al. (1991)). The WWW.ESAINC.COM 262 hydroxyalkenals are very reactive and show reactions common to unsaturated aldehydes and alcohols (Chapter 2). They readily react with thiols (e.g., glutathione), many amino acids (e.g., glycine produces a pyridinium derivative) (Figure 3.26), DNA (see above), and phospholipids that contain either ethanolamine or serine (Guichardant et al. (1998)). 4-Hydroxynonenal (CH3(CH2)4CHOHCH=CHCHO) ([trans] 4-hydroxy-2(E)nonenal) is a cytotoxic lipophilic compound (Muller et al. (1996)) and is formed by lipid peroxidation of n-6 fatty acids (e.g., arachidonic acid) (Van Kuijk et al. (1990)). It has been measured in both healthy and diseased tissues (Table 4.13) (Esterbauer et al. (1990)). For example, it is elevated by treatments that promote lipid peroxidation, in melanoma, in thalassemic subjects, in humans exhibiting toxic oil syndrome and in Parkinson’s disease (Selley (1998); Zarkovic (2003)). 4Hydroxynonenal also possesses diverse biological activity. It can affect cell proliferation, act as a chemotactic agent, potentiate platelet aggregation, and modify the expression of several genes including heat shock factor and the c-fos proto-oncogene (Esterbauer et al. (1991) and references therein; Kreuzer et al. (1998)). 4-Hydroxyhexenal (CH3CH2CHOHCH=CHCHO) is formed by lipid peroxidation of n-3 fatty acids (docosahexanoeic acids), and like 4-hydroxynonenal is also highly toxic (Van Kuijk et al. (1990)). It is capable of causing reversible structural damage to mitochondria (mitochondrial permeability transition) at doses a billion fold lower than 4-hydroxynonenal (Kristal et al. (1996)). High levels (mM range) of 4-hydroxyalkenals are acutely toxic to mammalian cells leading to depletion of GSH, decrease in protein thiols, induction of lipid peroxidation, disturbance of calcium homeostasis, inhibition of DNA, RNA and protein synthesis, inhibition of respiration and glycolysis, and morphological changes (see Esterbauer et al. (1991) and references therein). Lower levels (10200µM) were found to be less severe with the disturbances being more selective and dependent upon cell type. For example, fibroblasts die when exposed to 100µM 4-hydroxynonenal while hepatocytes readily tolerate the same exposure. This may be due to the hepatocyte’s ability to metabolize and detoxify this aldehyde. 4-Hydroxyalkenals are subjected to detoxification in vivo. 4-Hydroxynonenal is catabolized in the liver to a number of metabolites including: 1,4-dihydroxy-2nonene (following the action of NADH-dependent alcohol dehydrogenase), 4hydroxy-2-nonenoic acid (aldehyde dehydrogenase), a glutathione conjugate (GSH transferases), a mercaputurate conjugate, omega-hydroxylated products and their GSH and mercapturate adducts (Alary et al., (2003)). Both 4hydroxyhexenal and 4-hydroxynonenal can be reduced and rendered less toxic by aldose reductase (He et al. (1998)). WWW.ESAINC.COM 263 Other Reactive Carbonyls. Space is too short to explore the chemistry and biology of all of the reactive carbonyls formed in vivo in detail so this section will be limited to the other major cytotoxic carbonyls, the 2-alkenals, or α,β-unsaturated aldehydes. Acrolein (CH2=CHCHO) is the simplest and most reactive member of the series. It is a strong electrophile and can readily react with thiols and amines producing a variety of products (e.g., dysfunctional protein-acrolein adducts) (Calingasen et al. (1999); Uchida et al. (1998a,b). Acrolein can react with guanosine producing mutagenic lesions (Figure 3.7). It is highly cytotoxic showing similar actions to 4hydroxynonenal (reviewed by Esterbauer et al. (1991)). Acrolein is formed in vivo during metabolism of spermine and spermidine, by the action of hypochlorous acid on threonine, as a result of lipid peroxidation and during metabolism of allyl alcohol and allylamine (Figure 3.25) (Anderson et al. (1997)). Acrolein is a common pollutant and is formed during incomplete combustion of wood, petrol, coal and plastics. It is also found in cigarette smoke (typically 25-140µg/cigarette) and burning oil, and is a toxic agent formed by the metabolism of the anticancer drug cyclophosphamide (Esterbauer et al. (1991) and references therein). Crotonal (CH3CH=CHCHO) is formed during the metabolism of the hepatocarcinogenic cyclic nitrosamine, N-nitrosopyrrolidine while other members of the 2-alkenals (e.g., 2-pentenal, 2-heptenal, 2-octenal and 2-nonenal) are formed by peroxidized microsomes (Esterbauer et al. (1990)). Cholesterol Oxidation. Plasma membranes of eukaryotic cells are usually rich in cholesterol (typically 40-45 mol % of total lipids). Cholesterol, a neutral lipid, is involved in membrane fluidity. On the one hand it prevents the crystallization of fatty acyl chains by fitting between them, while on the other its bulk sterically blocks the motion of the fatty acyl chains making them less fluid. Although the accumulation of cholesterol in the plasma membrane of aging neurons is reported to affect nerve function, it now appears that such increases may actually protect neurons from oxidative damage (Joseph et al. (1996)). Cholesterol also exists as esters formed by the reaction between a carboxylic acid with cholesterol’s C-3 hydroxyl group. These occur at high levels in lowdensity lipoprotein (LDL), the major transporter of cholesterol in the blood. Cholesterol esters in LDL are rich in linoleate (a polyunsaturated fatty acid) while those stored in cells contain mainly oleate and palmitate (mono-unsaturated fatty acids). High-density lipoproteins (HDL) also contain cholesteryl esters formed by esterification of cholesterol which has been picked up from the plasma, resulting from cell death and membrane turnover. WWW.ESAINC.COM 264 C 8 H 17 HO CHOLESTEROL C 8 H 17 C 8 H 17 HO HO OOH OH CHOLESTEROL 5 α HYDROPEROXIDE CHOLESTEROL 5 α HYDROXIDE C 8 H 17 C 8 H 17 C 8 H 17 HO HO HO OOH HO OH OOH CHOLESTEROL 6 α HYDROPEROXIDE CHOLESTEROL 6 β HYDROPEROXIDE C 8 H 17 HO OH CHOLESTEROL 6 α HYDROXIDE HO CHOLESTEROL 7 α HYDROPEROXIDE OOH CHOLESTEROL 7 β HYDROPEROXIDE CHOLESTEROL 6 β HYDROXIDE C 8 H 17 C 8 H 17 OOH C 8 H 17 HO OH C 8 H 17 HO CHOLESTEROL 7 α HYDROXIDE OH CHOLESTEROL 7 β HYDROXIDE OH OH CH 3 CH 3 H 3C H 3C HO HO 24 α HYDROXYCHOLESTEROL 25-HYDROXYCHOLESTEROL HO HO O C 8 H 17 C 8 H 17 C 8 H 17 HO O C 8 H 17 HO O OH 5 α CHOLESTANE3 β ,5 α ,6 β TRIOL 5,6 α EPOXY-5 α CHOLESTAN-3 β ,7-DIOL 5,6 β EPOXY-5 β CHOLESTAN-3 β ,7-DIOL OH C 8 H 17 HO OH OH OH 3 β HYXDROXYCHOLEST5-EN-7-ONE 5 α CHOLESTANE3 β ,5 α ,6 β ,7 β -TETRIOL Figure 3.27 Cholesterol And Its Oxidation Products. WWW.ESAINC.COM 265 Cholesterol (and its esters) can become oxidized during lipid peroxidation giving rise to a variety of biologically active products that show atherogenic, cytotoxic, and mutagenic properties and possess enzyme inhibitory properties (Figure 4.22) (Peng and Morin (1987); Smith and Johnson (1989)). Some cholesterol oxidation products are being used as markers of oxidative stress (Geiger et al. (1997) and references therein; Girotti (1998); Patel et al. (1996); White et al. (1994)). These are gaining favor over phospholipid markers as: i) Cholesterol exists as a single molecular species in the membrane; ii) Its oxidation products can be measured directly without the need for potentially artifactual hydrolysis steps; and iii) Unlike phospholipids it can be readily transfer-radiolabeled without a requirement for transfer proteins (Girotti (1998)). Free radical-mediated reactions mainly produce 7α-hydroperoxide and 7β-hydroperoxide epimers, with lesser amounts of 7α-hydroxy, 7β-hydroxy, 7-one, epimeric 5,6-epoxides and other species. Singlet oxygen mainly yields the 5-α-hydroperoxide, 6α-hydroperoxide and 6β-hydroperoxide and these have been proposed as a potential marker for singlet oxygen activity. Several chlorinated sterols including a dichlorinated sterol, and cholesterol α- and βchlorohydrin are produced when cholesterol is exposed to the myeloperoxidasechlorinating system (Hazen et al. (1996)). Chlorination appears to proceed via chlorine rather then hypochlorous acid. The Isoprostanes. The isoprostanes have recently received a great deal of interest as possible markers of oxidative stress. Isoprostanes are prostaglandin-like compounds that are produced by free radical catalyzed lipid peroxidation, independent of the cyclooxygenase (COX) pathway (Fam and Morrow (1993); Morrow et al. (1999); Morrow and Roberts (1996); Pratico et al. (1997); Roberts and Morrow (1997)).21 Many isoprostanes can be formed and include those produced from the E, D and F series of prostaglandins, in turn produced from arachidonic acid (Figures 3.28). F-isoprostanes can be further classified as belonging to the F2, F3 or F4 series.22 It was the finding of Morrow and Roberts (1996) that F2-isoprostanes are formed in situ in phospholipids and are released by the action of phospholipases to circulate in the plasma, which has prompted measurement of these compounds as indices of oxidative stress in vivo. Up to 64 isomers of F2 can exist but probably most attention has focused on 8-epi PGF2α, due, in part, to its biological 21 Plants also form isoprostanes, called phytoprostanes, produced from linolenic acid (C18:3). These include dinor isoprostanes (PPF(1)), and the cyclic oxylipins (E1-phytoprostane) that can be further metabolized to novel cyclopentanone derivatives (A1- and B1-phytoprostanes) (Imbusch and Mueller (2000); Thoma et al., 2003)). PPF1 is particular abundant in pollen (e.g., 32 µg/g in birch pollen) and may be responsible for the some of the breathing problems associated with hayfever. 22 F3 and F4 isoprostanes are formed by the peroxidation of eicosopentaenoic acid and docosohexaenoic acid, respectively (Nourooz-Zadeh et al. (1999) and references therein). F4 isoprostanes are sometimes refered to as neuroprostanes. WWW.ESAINC.COM 266 activity. F2 isoprostanes have been proposed as markers of oxidative stress due to their stability and presence in measurable quantities in all tissues and fluids.23 Levels of F2 isoprostanes are increased in models of oxidative stress and their levels are suppressed upon treatment with antioxidants (Morrow and Roberts (1996)). Although proposed as potentially useful oxidative stress markers, some studies have shown that small quantities of 8-epi PGF2α are formed naturally by the COX enzyme. This has raised the question as to the possibility that 8-epi PGF2α may not always be a reliable marker of oxidative stress. Other researchers have argued that the amount of 8-epi PGF2α produced by COX is too insignificant to be of concern (Morrow and Roberts (1996) and references therein). CO2 H Arachidonic Acid ROS - Hydrogen Abstraction Oxygenation Rearangement Reduction O HO OH HO A HO HO O E2-Isoprostane O HO Isothromboxane D2-Isoprostane F2-Isoprostane OH HO CO2 H HO HO CO2 H HO HO HO I CO2 H HO HO II CO2 H B OH IV III Isoprostane F2α HO CO2 H HO OH 9α, 11α HO HO CO2 H CO2 H HO OH HO 9α, 11β OH 9β, 11α HO CO2 H HO C OH 8−Epi Figure 3.28 The Formation Of Isoprostanes. Several Different Head Groups Can Be Formed (A). Each Of These Shows Different Regioisomers (B) Which Also Show Optical Isomers (C). 23 It is unclear whether urinary levels of isoprostanes reflect plasma levels or mainly result from local renal production. WWW.ESAINC.COM 267 Glutathione Peroxidase Fatty Acid O OH GSH C O OH CH 2 HC CH 2 O O C HC CH 2 CH 2 C HC O C 1/2 Native Lipid Bilayer O O C CH 2 OH HC O CH 2 Pro-oxidant O C O OH HC O HC OH CH 2 CH 2 O C CH 2 CH 2 O O C CH 2 O O O O CH 2 O O C Hydroxylated Fatty Acid OH Hydroperoxide O O Ca2+ /PLA 2 C O C O CH 2 O O C HC Acyltransferase C HC O C O O C CH 2 CH 2 O O FA-CoA Oxidized Fatty Acid Removed Oxidized Bilayer with Disrupted Structure CH 2 O O C O C Membrane Repaired EXCISION-REDUCTION REPAIR Phospholipid Hydroperoxide Glutathione Peroxidase GSH Ca2+ /PLA 2 Hydroxylated Fatty Acid OH C OH CH 2 HC O O C CH 2 HC OH CH 2 CH 2 O O C O O O C O O C Oxidized Bilayer with Disrupted Structure REDUCTION-EXCISION REPAIR Figure 3.29 Excision-Reduction And Reduction-Excision Lipid Repair Processes (Based on Van Kuijk et al. (1987) And Girotti (1998)). PLA2 – phospholipase A2; FA-CoA – fatty acyl CoA. Lipid Repair. A variety of antioxidant defenses exist in eukaryotic cells to protect against lipid peroxidation damage. These include enzymes (e.g., catalase, and superoxide dismutase) and metal binding proteins (e.g., ferritin) designed to prevent, minimize ROS formation or remove them once formed (Chapter 4). Furthermore, membranes contain the chain-breaking antioxidant, α-tocopherol that can intercept lipid peroxyl radicals and prevent lipid peroxidation chain reactions (Chapter 4). Unfortunately, protection by the antioxidant defenses is not complete WWW.ESAINC.COM 268 and lipid peroxidation can still take place. Damaged lipids must be removed if the membrane is to remain viable. The detoxification of membrane-bound lipid peroxides appears to involve the activity of membrane-bound phospholipase A2 (van Kuijck et al. (1987)) and three intracellular enzymes with peroxidatic activity, glutathione peroxidase, phospholipid hydroperoxide glutathione peroxidase, and non-seleno GSH-Stransferase type α (Figure 3.29) (Flohe (1982); Ursini and Bindoli (1984); Ursini et al. (1985)). Although both glutathione peroxidase and phospholipid hydroperoxide glutathione peroxidase reduce lipid hydroperoxides into lipid alcohols in a two-electron reaction, they differ in size, amino acid sequence, and cellular distribution (Brigelius-Flohe et al. (1994)). They also show markedly different substrate specificity: glutathione peroxidase can only act on unesterified fatty acid hydroperoxides or hydrogen peroxide, whereas phospholipid hydroperoxide glutathione peroxidase is more versatile and can act on phospholipid peroxides contained in membranes, cholesterol, cholesteryl ester hydroperoxides and hydrogen peroxide (Grossman and Wendel (1983); Thomas et al. (1990a,b); Ursini and Bindoli (1984); van Kuijk et al. (1987)). The final step in lipid repair is the re-esterification of the lysophospholipid by an acyltransferase forming a phospholipid. As shown in Figure 4.25, two possible pathways of lipid repair exist, excision-reduction and reduction-excision repair. Evidence suggests that both pathways can operate in vivo (Girotti (1998) and references therein). Lipid Damage and Disease. Lipid peroxidation is increased when cells are damaged. Thus it should come as no surprise that many diseases are associated with increased lipid peroxidation. However, the question that needs to be asked is whether lipid peroxidation causes or is just the result of a disease. The latter may still be important as, in the case for the cytotoxic aldehydes, lipid peroxidation may play a role in disease progression. Lipid peroxidation is directly involved in atherosclerosis (Halliwell and Gutteridge (1999)). Oxidative modification to lipoproteins and cholesterol can result in atherosclerosis (Chang et al. (1997); Hajjar and Haberland (1997); Morin and Peng (1989); Patel et al. (1996); Westhuyzen (1997); White et al. (1994)). In the oxidation hypothesis of atherosclerosis, oxidative damage to lipoprotein, and in particular LDL, increases its ability to cause this disease by altering receptormediated uptake by cells in the intima of blood vessels. Subsequently, oxidized LDL is then taken up by scavenger receptors on other cells such as monocytes, macrophages and smooth muscle cells. This uncontrolled process leads to the accumulation of lipids and the formation of foam cells, an early indicator of atherosclerotic plaque formation (Westhuyzen (1997)). Lipid peroxidation is also associated with brain damage resulting from reperfusion injury, and possibly neurodegenerative diseases (Acworth et al. (1998) and references therein; Cini et al. (1994) and references therein). Lipid peroxidation is also responsible for the WWW.ESAINC.COM 269 production of advanced lipid end products that accumulate with aging and disease (Sayre et al. (1997)). Measurement of Lipid Damage. Total peroxidation can be determined by the uptake of oxygen. A variety of methods are used to measure lipid peroxidation products including GC-MS, LCMS, HPLC with electrochemical, fluorescence or chemiluminescence detection (reviewed by Esterbauer et al. (1991); Frederik et al., (1987); Halliwell and Chirico (1993); Halliwell and Gutteridge (1999); Pryor (1989); Spickett (2003); Van Kuijk and Dratz (1987);) (Table 3.14). For example, carbon and oxygen centered radicals can be measured using EPR/spin trap procedures. Lipid peroxides can be measured by iodine liberation, heme degradation of the peroxide, and COX activity. These approaches vary in their selectivity, sensitivity, ease of use and extent of sample preparation. Unfortunately many of the analytical methods available are not accurate and can yield ambiguous data. For example, the iodide approach may be useful for examining the oxidation of pure lipids, but biological tissues contain other oxidants such as hydrogen peroxide that can also cause iodine production from iodide. Furthermore, two commonly used approaches, diene conjugation and the thiobarbituric acid reactivity (TBAR) test that are used to examine fatty acid oxidation and carbonyl production, respectively, also lack specificity in some systems (see below). Analyte 4-Hydroxynonenal 4-Hydroxynonenal modified proteins Carbon and oxygen centered radicals Cholesterol hydroperoxides and cholesteryl ester hydroperoxides Cholesteryl ester Technique HPLC-UV of 2,4-DNPH derivative Reference Esterbauer et al. (1991) and references therein HPLC-fluorescence of 1,3cyclohexandione derivative Esterbauer et al. (1991) and references therein HPLC-coulometric detection of DNPH derivative. Goldring et al. (1993) GC-HPLC-antibody techniques Halliwell and Chirico and references therein (1993) GC-MS Esterbauer et al. (1991) and references therein Waeg et al. (1996) Monoclonal antibodies EPR combined with spin trapping (e.g., with phenyl tbutylnitrone) HPLC-ECD Halliwell and Chirico (1993) and references therein HPLC-chemiluminescence Yasuda and Narita (1997) WWW.ESAINC.COM Arai et al. (1996), Korytowski et al. (1991) 270 hydroperoxides Hydroxy and ketocholesterols Fatty acid hydroperoxides Hydrocarbon gases GC-MS Ferrocene derivatization/ voltammetry. Vatassery et al. (1997) Mulchandani and Rudolph (1995) HPLC-ECD of GSSG production by GSHperoxidase following reaction with lipid hydroperoxides O’Gara et al. (1989) HPLC-UV Browne and Armstrong (1998) GC, GC-MS GC Isoprostanes GC-MS, LC-MS and immunochemical approaches Lipofuscin/lipopigments Fluoresence MDA HPLC-UV HPLC with pre-column derivatization with TBA, dansylhydrazine, 2,4-DNPH or methylamine + acetaldehyde. HPLC-UV, HPLC-fluorescence Halliwell and Chirico (1993) and references therein De Zwart et al.(1998); Morrow and Roberts (1996, 1999); Pratico et al. (1998) Delori and Dorey (1998); Yin and Brunk (1998) De Zwart et al. (1998) and references therein; Esterbauer et al. (1991) and references therein; Halliwell and Chirico (1993) and references therein; Korchazkina et al., (2003); Yeo et al. (1994) HPLC with post-column derivatization with TBA. HPLC-UV and HPLCfluorescence Other carbonyls Phospholipid hydroperoxide and triacylglycerol hydroperoxides GC-ECD and GC-MS GC, GC with 2,4DNPH derivatization, HPLC with 2,4-DNPH derivatization, TLC COX activity, heme degradation of peroxides, hemoglobin/methylene blue reaction, GSH peroxidase/GSH reductase (free hydroperoxides only), iodine production. De Zwart et al. (1997); Halliwell and Gutteridge (1989) and references therein Halliwell and Chirico and references therein (1993); Kuijk and Dratz (1987) and references therein; Yagi (1998) Chemiluminescence Iwaoka et al. (1987) HPLC-chemiluminescence Yamamoto et al. (1987a,b, 1990, 1994, 1998) HPLC-chemiluminescencePDA Holley and Slater (1991) WWW.ESAINC.COM 271 HPLC-UV, chemiluminescence or evaporative light scattering detectors Makinen et al. (1996) and references therein HPLC-ECD Arai et al. (1997), Yamada et al. (1987) HPLC-polarography Korytowski et al. (1995, 1999) GC-MS Total peroxidation Measurement of oxygen uptake using an oxygen electrode Halliwell and Chirico (1993) and references therein; Kuijk and Dratz (1987) and references therein Halliwell and Chirico (1993) and references therein Table 3.14 Methods used to measure lipid peroxidation. Lipid peroxidation is often measured using either diene conjugation or TBARs. However, these approaches often suffer from practical limitations: Diene Conjugates. These are formed during peroxidation of polyunsaturated fatty acids and are thought to be esters of octadeca-9,11-dienoic acid, a non-peroxide isomer of linoleic acid (Dormandy and Wickens (1987)). They absorb at 230-235nm and thus UV absorbance by these compounds is used as an indicator of the early stages of lipid peroxidation. HPLC-UV detection has been used to study diene conjugates in a variety of tissues (Cawood et al. (1993)). Double-derivative spectroscopy has improved the limit of detection of this approach but it is still limited to pure lipid samples and measurement in biological fluids remains challenging (Situnayake et al. (1990)). TBAR. This simple and cost effective test is often used to measure MDA. A biological sample is heated under acidic conditions with thiobarbituric acid, and the pink chromagen (Figure 3.30) is measured at 532nm (UV) or 553nm (fluorescence). Although this approach works well for standards and clean samples, biological samples are fraught with problems. First this method lacks selectivity and many biologically occurring carbonyls (e.g., aldehydes formed during peroxidation, bile acids, DNA bases, reducing carbohydrates and glycoproteins) can also produce a chromophore absorbing at 532nm. Second, the test does not actually measure tissue levels of MDA, rather it measures stimulated MDA levels due to WWW.ESAINC.COM 272 decomposition of lipid peroxides induced by the acid/heating stages of the test. Third, peroxide decomposition can further stimulate lipid peroxidation, thereby amplifying the response. Halliwell overcomes these problems by adding the chain-breaking antioxidant butylated hydroxytoluene to the sample before analysis and using HPLC to separate the TBA-MDA adduct from other chromagenic interferences (Halliwell and Chirico (1993)). A typical basal plasma chromatogram is shown in Figure 3.31. S N CHO N MDA HS N OH CHO OH HO 2H2O N OH OH N 2 THIOBARBITURIC ACID HS N OH PRODUCT Figure 3.30. Formation Of A Chromagen From TBA And MDA. So far there is not one method that measures all aspects of lipid peroxidation. The correct choice of method depends on what question is being asked. As some methods are not selective and some are affected by the sample preparation employed, care must be exercised when choosing between methods. If at all possible two or more different methods should be used to answer any question posed. WWW.ESAINC.COM 273 Figure 3.31 Chromatogram Showing Isolation Of The MDA-TBAR Derivative (1). See ESA Application Note 70-5033 Malondialdehyde For Further Details. The isocratic analytical system consisted of a pump, an autosampler and a fluorescence detector. LC conditions: Column: Mobile Phase: Flow Rate: Temperature: Injection Volume: Detector: Excitation Wavelength: Emission Wavelength: HR-80 (4.6 x 80mm; 3µm). 40% Methanol. 1.0mL/min. Ambient. 20µL. Fluorescence, Model 305. 515nm. 553nm. See ESA Application Note 70-5033 Malondialdehyde For Further Details. Aldehydes (e.g., 4-hydroxynonenal) can also be measured using GC-based approaches, by the measurement of their 3,5-dinitrophenylhydrazine derivatives using HPLC-ECD (Goldring et al., (1993)) or HPLC-fluorescence, or by HPLCfluorescence of an acridine derivative formed in the Hantzsch reaction (Holley et al., (1993)) (see Figures 3.32 and 3.33). See ESA Application Note 70-5041 4Hydroxynonenal And Other Aldehydes. WWW.ESAINC.COM 274 O O R O (NH4) 2SO4 + O R CHO HANTZSCH Reaction 2 1,3-Cyclohexanedione "O" N H 9-Alkyl-4,5,6,7,9,10octahydroacridine-1,8-dione O R O N 9-Alkyl-4,5,6,7-hexahydroacridine-1,8-dione Figure 3.32 Formation Of A Fluorescent Derivative When Aldehydes Undergo the Hantzsch Reaction. Figure 3.33 Chromatogram Of Aldehyde Standards (5ng on column). 1 - formaldehyde; 2 - acetaldehyde; 3 - propanal; 4 - unknown; 5 - butanal; 6 - pentanal; 7 - 4HNAL; 8 - hexanal; 9 - heptanal; 10 - octanal; 11 - nonanal; 12 - decanal. WWW.ESAINC.COM 275 The gradient analytical system consisted of two pumps, an autosampler, a fluorescence detector and a data station. LC Conditions Column: Mobile Phase A: Mobile Phase B: Gradient Conditions: HR-80 (4.6 x 80mm; 3µm). 5% Tetrahydrofuran (THF). 40% THF. Isocratic 0%B from 0 to 2min. Linear increase of phase B from 0 to 100% from 2 to 55min. Isocratic 100% phase B from 55 to 60min. Linear decrease of phase B from 100 to 0% from 60 to 65min. Isocratic 0% phase B from 65 to 70min. Flow Rate: 1.0mL/min. Temperature: Ambient. Injection Volume: 20µL. Detector: Fluorescence, Model 305. Excitation Wavelength: 380nm. Emission Wavelength: 445nm. See ESA Application Note 70-5041 4-Hydroxynonenal And Other Aldehydes. CARBOHYDRATES. Introduction. Carbohydrates are aldehyde or ketone compounds with multiple hydroxyl groups. Carbohydrates show a wide degree of structural diversity. Through the multiple hydroxyl groups single monosaccharide units (e.g., glucose, and fructose) can join together to form more complex polysaccharides (e.g., glycogen and starch). Carbohydrates are biologically very important and play multiple roles in living organisms. Carbohydrates act as energy stores, fuels and intermediates; along with phosphate they form the backbone of DNA and RNA; they act as structural elements in plants and bacteria; and as part of glycoproteins and glycolipids they are involved in cell-cell recognition. Unlike DNA, proteins and lipids, where a wealth of information has been generated, carbohydrates appear to play less of a role in redox biochemistry. For this reason, and due to space limitations, this section will concentrate on two areas of carbohydrate chemistry that are important to redox biochemistry, damage of DNA and RNA sugars, and glycation-glyoxidation reactions. WWW.ESAINC.COM 276 O O HO O OH PO O O PO PO Aerobic Cleavage From a C-5' Radical Aerobic Cleavage From a C-2' Radical O O O P O O O O Base P O O O H HO O O H P O O Base O H P O O O Phosphate Release from C-3' Oxygen Free Conditions PO Phosphate Release from C-5' Oxygen Free Conditions HO O O HO O O RO O O O O HO RO CH3 CH3 O O O O HO PO Figure 3.34 ROS-Induced Damage To 2’-Deoxyribose Causes DNA Strand Scission And The Formation Of Carbohydrate Fragments. (Based On Breen And Murphy (1995). See also von Sonntag (1984)). Ribose and Deoxyribose Damage. Radiation damage and hydroxyl free radicals can abstract a hydrogen atom from a carbohydrate molecule forming radical intermediates that can undergo further decomposition (Figure 3.1). Damage to the sugar molecule is biologically important as it can lead to DNA and RNA strand scission (von Sonntag (1984)). It must be remembered though, as discussed above, that the hydroxyl free radical WWW.ESAINC.COM 277 is much more likely to attack a base than a sugar moiety (Breen and Murphy (1995)). Numerous mechanisms have been proposed for DNA strand scission depending upon which ROS is involved which hydrogen atom is abstracted from the carbohydrate molecule and whether the reaction proceeds under aerobic or anaerobic conditions. These pathways have been extensively reviewed elsewhere (Breen and Murphy (1995); von Sonntag (1984)). Figure 3.34 presents a simplified scheme of ROS-induced deoxyribose decomposition. Carbohydrate fragments are not easily measured but several have been determined in vitro using GC/MS approaches (Breen and Murphy (1995) and references therein; Dizdaroglu (1991)). Glycation, Glyoxidation, Advanced Glycation End Products (AGEs) and Age-Related Pigments. As discussed in Chapter 2 carbonyl compounds readily form Schiff bases with amine groups located on proteins and DNA bases. One of the most abundant carbonyl compounds found in man is glucose. This reducing monosaccharide can slowly react with primary amines on proteins and DNA in a process called non-enzymatic glycation. The first step in this process is the reversible formation of a Schiff base (Figures 2.27). This can then be converted to an eneaminol before undergoing an Amadori rearrangement (effectively converting the Nglycoside of the aldose into an N-glycoside of the ketose) forming a more stable Amadori adduct (e.g., fructose-lysine) (Kikuchi et al., (2003). Non enzymatic glycation is reversible in vitro however, enolization, dehydration, cyclization, fragmentation and oxidation reactions24 form reactive intermediates that ultimately lead to stable end products, termed advanced glycation end-products (AGEs) (Fu et al. (1994); Monnier et al. (2003); Wells-Knecht et al. (1995)). Examples of AGEs include the most abundant irreversible chemical modification, Nε-(carboxymethyl)-lysine (CML), the protein cross-link, pentosidine, and pyralline (Figure 2.27) (Dunn et al. (1990); Nagaraj et al. (1996); Sell et al. (1991)). The resulting conformationally altered proteins often acquire a brown color referred to as Maillard browning. For example, human cartilage is near white at birth, but turns to dark brown in aged individuals. AGEs can be determined by immunoassays and flouresence or more accurately by GC-MS and in hydrolysates by HPLC-fluorescence (Friess et al., (2003)). 24 The exact mechanism behind the Maillard browning reaction remains elusive but several possible pathways have been proposed. For example, Wolff and colleagues have suggested a role for superoxide, hydrogen peroxide and metalinduced hydroxyl free radical formation in a process called autoxidative glycosylation (Hunt et al., (1988); Wolff et al. (1991)). WWW.ESAINC.COM 278 ADVANCED LIPID PEROXIDATION END PRODUCTS (ALPEs) SCHIFF BASE AND MICHAEL ADDUCT OH O R HYDROXYALKENAL O O MALONDIALDEHYDE R SCHIFF BASE NH2 DIHYDROPYRIDINES and IMINOENAMINES PROTEIN LYSYL GROUP OH MODIFICATIONS (CROSSLINK AND NON-CROSSLINK) O R REDUCING SUGAR SCHIFF BASE AMADORI PRODUCT ADVANCED GLYCATION END PRODUCTS (AGEs) Figure 3.35 The Formation Of AGEs And ALPs From Proteins. AGEs are found to be increased in aging and are thought to contribute to development of diabetic complications such as accelerated atherosclerosis and microvascular disease. Furthermore, AGEs may be one of the major driving forces in the development and progression of Alzheimer’s and Parkinson’s diseases (Reddy et al., (2002); Smith et al. (1994, 1995); Yan et al. (1994)). AGEs are thought to exert their cellular effects by binding to a receptor (RAGE) located on the cell’s surface. RAGE consists of two parts, a novel integral membrane protein in the immunoglobulin superfamily and a lactoferrin polypeptide (Bucciarelli et al., (2002); Yan et al. (1994)). Binding of AGE to its receptor increases the oxidative stress of the cell (as reflected by increased MDA production), induces the transcription of NF-κB and induces heme oxygenase mRNA. In this way, activation of RAGE is hypothesized to underlie diabetic vascular disease (Wendt et al., (2003); Yan at al. (1994) and references therein). Formation of age related pigment is the result of accumulated oxidative damage over time (Figure 3.35). The reaction between various primary amines and lipid peroxidation derived carbonyl compounds leads to the production of two ALPs, lipofuscin and ceroid (Kikugawa and Beppu (1987)). Lipofuscin is the classical age pigment of post-mitotic cells, whereas ceroid accumulates due to pathological or experimental processes. Lipofuscin occurs as yellow-brown irregular membrane-bound granules located in lysosomes. Lipofuscin contains about 50% (by weight) protein, a lesser amount of lipid, <1% fluorophore(s) and dolichol bound metals (iron, copper and aluminum). It has been hypothesized that normally damaged membranes and proteins, a consequence of ROS/RNS attack, are digested and recycled by lysosomes Harman (1989). However, as a WWW.ESAINC.COM 279 result of age these processes become less efficient, resulting in deposition of lipofuscin. As the disposal system becomes overloaded, ceroid is formed. REFERENCES. Abdelrahim, M., Morris, E., Carver, J., Facchina, S., White, A., and Verma, A. (1997). Liquid chromatography assay of dityrosine in human cerebrospinal fluid. J. Chromatogr. B., 696, 175-182. Abe, K., Pan, L.H., Watanabe, M., Konno, H., Kato, T., and Itoyama, Y. (1997). Upregulation of protein-tyrosine nitration in the anterior horn cells of amyotrophic lateral sclerosis. Neuronal Res., 19, 124-128. Abe, T., Isobe, C., Murata, T., Sato, C., and Tohgi, H. (2003). Alteration of 8-hydroxyguanosine concentrations in the cerebrospinal fluid and serum from patients with Parkinson’s disease. Neurosci. Lett., 336, 105-108. Abe, T., Tohgi, H., Isobe, C., Murata, T., and Sato, C. (2002). Remarkable increase in the concentration of 8hydroxyguanosine in cerebrospinal fluid from patients with Alzheimer’s disease. J. Neurosci. Res., 70, 447-450. Acworth, I.N., Bailey, B.B., and Maher, T.J. (1998). The use of HPLC with electrochemical detection to monitor reactive oxygen and nitrogen species, markers of oxidative damage and antioxidants: application to the neurosciences. In: Neurochemical Markers of Degenerative Nervous Diseases and Drug Addiction. Qureshi, G., Parvez, H., Caudy, P., and Parvez, S. (Eds.). Progress in HPLC-HPCE, 7. VSP Publications, The Netherlands. Pp. 3-56. Acworth, I.N., During, M.J., and Wurtman, R.J. (1987). Processes that couple amino acid availability to neurotransmitter synthesis. In: Amino Acid Availability and Brain Function in Health and Disease. Huether, G. (Ed.). NATO ASI Series. H: Cell Biology, 20. Springer-Verlag, New York. Pp117-136. Acworth, I.N., Maher, T.J., Chaiyakul, P., and McCabe, D. (1997b). 3-Nitrotyrosine passage through the blood-brain barrier (BBB): An in vivo microdialysis study using HPLC with coulometric array detection. Oxygen Society. San Francisco. Acworth, I.N., McCabe, D.R., and Maher, T.J. (1997). The analysis of free radicals, their reaction products, and antioxidants. In: Oxidants, Antioxidants, and Free Radicals. Baskin, S.I., and Salem, H. (Eds.). Taylor and Francis, Washington DC. Pp. 23-77. Adachi, S., Takemoto, K., Hirosue, T., and Hosogai, Y. (1993). Spontaneous and 2-nitropropane induced levels of 8hydroxy-2’deoxyguanosine in liver DNA of rats fed iron-deficient or manganese- and copper-deficient diets. Carcinogen., 14, 265-268. Adachi, S., Zeisig, M., and Moller, L. (1995). Improvements in the analytical method for 8-hydroxydeoxyguanosine in nuclear DNA. Carcinogen., 16, 253-258. Aesbach, R., Amado, R., and Neukom, H. (1976). Formation of dityrosine cross-links in proteins by oxidation of tyrosine residues. Biochem. Biophys. Acta, 439, 292-301. Agarwal, S., Wee, J.J., Hadley, M., and Draper, H.H. (1994). Identification of a deoxyguanosine-malondialdehyde adduct in rat and human urine. Lipids, 29, 429-432. Aikens, J., and Dix, T.A. (1991). Perhydroxyl radical (HOO•) initiated lipid peroxidation. J. Biol. Chem., 266, 15091-15098. Akaike, T., Noguchi, Y., Ijiri, S., Setoguchi, K., Suga, M., Zheng, Y.M., Dietzschold, B., and Maeda, H. (1996). Pathogenesis of influenza virus-induced pneumonia: Involvement of both nitric oxide and oxygen radicals. Proc. Natl. Acad. Sci. USA, 93, 2448-2453. Alam, Z.I., Jenner, A., Daniel, S.E., Lees, A.J., Cairns, N., Marsden, C.D., Jenner, P., and Halliwell, B. (1997). Oxidative DNA damage in the Parkinsonian brain: An apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J. Neurochem., 69, 1196-1203. Alary, J., Gueraud, F., and Cravedi, J.P. (2003). Fate of 4-hydroxynonenal in vivo: Disposition and metabolic pathways. Mol. Asp. Med., 24, 177-187. Allesio, H.M. (1993). Exercise-induced oxidative stress. Med. Sci. Sports Exerc., 25, 218-224. Althaus, J.S., Fici, G.J., Plaisted, F.J., Kezdy, F.J., Campbell, C.M., Hoogerheide, J.G., and VonVoigtlander., P.F., Protein nitration by peroxynitrite: A method for monitoring nitric oxide neurotoxicity. Microchem. J., 56, 155-164. Althaus, J.S., Schmidt, K.R., Fountain, S.T., Tseng, M.T., Carroll, R.T., Galatsis, P., and Hall, E.D. (2000). LC-MS/MS detection of peroxynitrite-derived 3-nitrotyrosine in rat microvessels. Free Radic. Biol. Med., 29, 1085-1095. Alvarez, B., Rubbo, H., Kirk, M., Barnes, S., Freeman, B., and Radi, R. (1996). Peroxynitrite-dependent tryptophan nitration. Chem. Res. Toxicol., 9, 390-396. Alves-Rodrigues, A., Gregori, L., and Figueiredo-Pereira, M.E. (1998). Ubiquitin, cellular inclusions and their role in neurodegeneration. Trends. Neurosci., 21, 516-520. Ames, B.N. (1989). Endogenous oxidative DNA damage, aging and cancer. Free Radic. Res. Comm., 7, 121-128. Ames, B.N., Shigenaga, M.K., and Hagen, T.M. (1993). Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci., USA, 90, 7915-7922. Amici, A., Levine, R.L., Tsai, L., and Stadtman, E.R. (1989). Conversion of amino acid residues in proteins and amino acid homopolymers to carbonyl derivatives by metal-catalyzed oxidation reactions. J. Biol. Chem., 264, 3341-3346. Amiconi, G., Scoli, F., Matarese, M., Verzili, D., and Brunori, M. (1985). Determination of methionine sulfoxide in proteins: Comparison of a gas-chromatographic and electrophoretic method. J. Biochem. Biophys. Meths., 11, 241-249. Anderson, M.M., Hazen, S.L., Hsu, F.F., and Heinecke, J.W. (1997). Human neutrophils employ the myeloperoxidasehydrogen peroxide-chloride system to convert hydroxy-amino acids into glycoaldehyde, 2-hydroxypropanal, and acrolein. J. Clin. Invest., 99, 424-432. Anfinsen, C.B. (1973). Principles that govern the folding of protein chains. Science, 181, 223-230. WWW.ESAINC.COM 280 Anon – ESCODD (2000). Comparison of different methods of measuring 8-oxoguanine as a marker of oxidative DNA damage. ESCODD (European Standards Committee on Oxidative DNA Damage). Free Radic. Res., 32, 333-341. Anon- ESCODD (2002). Inter-laboratory validation of procedures for measuring 8-oxo-7,8-dihydroguanine/8-oxo-7,8-2’deoxyguanosine in DNA. Free Radic. Res., 36, 239-245. Arai, H., Terao, J., Abdalla, D. S. P., Suzuki, T., and Takama, K. (1996). Coulometric detection in high-performance liquid chromatographic analysis of cholesteryl ester hydroperoxides. Free Radic. Biol. Med., 20, 365-371. Armstrong, D.A. (1990). In: Sulfur-centered Reactive Intermediates in Chemistry and Biology. Chatgilialoglu, C., and Asmus, K.D. (Eds.). Plenum Press, New York. Pp. 121-134. Armstrong, R.C., and Swallow, A.J. (1969). Pulse- and gamma-radiolysis of aqueous solutions of tryptophan. Radiat. Res., 40, 563-579. Arrigo, A.-P. (1998). Small stress proteins: Chaperones that act as regulators of intracellular redox state and programmed cell death. Biol. Chem., 379, 19-26. Aruoma, O.I., and Halliwell, B. (1987). Action of hypochlorous acid on the antioxidant protective enzymes superoxide dismutase, catalase and glutathione peroxidase. Biochem. J., 248, 973-976. Aruoma, O.I., and Halliwell, B. (Eds.). (1998). DNA and Free Radicals: Techniques, Mechanisms and Applications. OICA, London. Aruoma, O.I., Halliwell, B., and Dizdioglu, M. (1989). Iron ion-dependent modification of bases in DNA by the superoxide radical generating system hypoxanthine/xanthine oxidase. J. Biol. Chem., 264, 13024-13028. Ayala, A., and Cutler, R.G. (1996a). The utilization of 5-hydroxy-2-amino valeric acid as a possible marker of oxidized arginine and proline residues. Free Radic. Biol. Med., 21, 65-80. Ayala, A., and Cutler, R.G. (1996b). Comparison of 5-hydroxy-2-amino valeric acid with carbonyl group content as a marker of oxidized protein in human and mouse liver tissues. Free Radic. Biol. Med., 21, 551-558. Ayene, S.I., Al-Mehdi, A.B., and Fisher, A.B. (1993). Inhibition of lung tissue oxidation during ischemia/reperfusion by 2mercaptopropionylglycine. Arch. Biochem. Biophys., 303, 307-312. Aznar, J., Santos, M.T., Valles, J., and Sala, J. (1983). Serum malondialdehyde-like material (MDA-LM) in acute myocardial infarction. J. Clin. Pathol., 36, 712-715. Bagasra, O., Michaels, F.H., Zheng, Y.M., Broboski, L.E., Spitsin, S.V., Fu, Z.F., Tawadros, R., and Koprowski, H. (1995). Activation of the inducible form of nitric oxide synthase in the brains of patients with multiple sclerosis. Proc. Natl. Acad. Sci. USA, 92, 12041-12045. Baker, M.A., and He, S.Q. (1991). Elaboration of cellular DNA breaks by hydroperoxides. Free Radic. Biol. Med., 11, 563572. Banks, B.A., Ischiropoulos, H., McClelland, M., Ballard, P.L., and Ballard, R.A. (1998). Plasma 3-nitrotyrosine is elevated in premature infants who develop bronchopulmonary dysplasia. Pediatrics, 101, 870-874. Baraldi, E., Ghiro, L., Piovan, V., Carraro, S., Ciabattani, G., Barnes, P.J., and Montuschi, P. Increased exhaled 8isoprostane in childhood asthma. Chest, 124, 25-31. Bartsch, H., and Frank, N. (1996). Blocking the endogenous formation of N-nitroso compounds and related carcinogens. IARC Sci. Pub., 139, 189-201. Bashir, S., Harris, G., Denman, M.A., Blake, D.R., and Winyard, P.G. (1993). Oxidative DNA damage and cellular sensitivity to oxidative stress in human autoimmune diseases. Ann. Rheum. Dis., 52, 659-666. Basu, A.K., Loechler, E.L., Leadon, S.A., and Essigmann, J.M. (1989). Genetic effects of thymine glycol: Site-specific mutagenesis and molecular modeling studies. Proc. Natl. Acad. Sci. USA, 86, 7677-7681. Bashir, S., Harris, G., Denman, M.A., Blake, D.R., and Winyard, P.G. (1993). Oxidative DNA damage and cellular sensitivity to oxidative stress in human autoimmune diseases. Ann. Rheum. Dis., 52, 659-666. Beal, M.F. (1997). Oxidative damage in neurodegenerative diseases. Neuroscientist, 3, 21-27. Beal, M.F., Ferrante, R.J., Browne, S.E., Matthews, R.T., Kowall, N.W., and Brown, R.H. (1997). Increased 3-nitrotyrosine in both sporadic and familial amyotrophic lateral sclerosis. Ann. Neurol., 42, 644-654. Beal, M.F., Ferrante, R.J., Henshaw, R., Matthews, R.T., Chan, P.H., Kowall, N.W., Epstein, C.J., and Schulz, J.B. (1995). 3-Nitropropionic acid neurotoxicity is attenuated in copper/zinc superoxide dismutase transgenic mice. J. Neurochem., 65, 919-922. Beal, M.F., Ferrante, R.J., Matthews, R.T., Kowall, N.W., and Brown, R.H. (1996). 3-Nitrotyrosine in ALS. Soc. Neurosci., 22, 1943. Beckman, J.S., Ye, Y.Z., Anderson, P.G., Chen, J., Accavitti, M.A., Tarpey, M.A., and White, C.R. (1994). Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol. Chem. Hoppe Seyler, 375, 81-88. Beckman, K.B., and Ames, B.N. (1999). Endogenous oxidative damage of mt DNA. Mutat. Res., 424, 51-58. Beckman, K.B, Saljoughi, S., and Ames, B.N. (1998). Facile analysis of 8-oxoguanine in DNA with the use of Fapy glycosylase coupled to HPLC-EC. Free Radic. Biol. Med., Supplement 1, S72. Beehler, B.C., Przybyszewski, J., Box, H.B., and Kulesz-Martin, M.F. (1992). Formation of 8-hydroxydeoxyguanosine within DNA of mouse keratinocytes exposed in culture to UVB and H2O2. Carcinogen., 13, 2003-2007. Benjamin, I.J., and McMillan, D.R. (1998). Stress (heat shock) proteins. Molecular chaperones in cardiovascular biology and disease. Circ. Res., 83, 117-132. Berger, M., Cadet, J., Berube, R., Langlois, R., and van Lier, J.E. (1992). Reversed-phase high-performance liquid chromatography-thermospray mass spectrometry of radiation-induced decomposition products of thymine and thymidine. J. Chromatogr., 593, 133-138. Bergtold, D.S., Berg, C.D., and Simic, M.G. (1990). Urinary biomarkers in radiation therapy and cancer. Adv. Exp. Med. Biol., 264, 311-316. Bermudez, E., Ferng, S.F., Castro, C.E., and Mustafa, M.G. (1999). DNA strand breaks caused by exposure to ozone and nitrogen dioxide. Environ. Res., 81, 72-80. Bernstien, C. (1998). Sex as a response to oxidative DNA damage. In: DNA and Free Radicals: Techniques, Mechanisms and Applications. Aruoma, O.I., and Halliwell, B. (Eds.). OICA, London. Pp. 99-118. WWW.ESAINC.COM 281 Bessho, T., Tano, K., Kasai, H., Ohtsuka, E., and Nishimura, S. (1993). Evidence of two DNA enzymes for repair of 8hydroxyguanine (7,8-dihydroguanine) in human cells. J. Biol. Chem., 268, 19416-19421. Birnbaum, G.I., Lassota, P., and Shugar, D. (1984). 8-Chloroguanosine: Solid-state and solution conformations and their biological implications. Biochem., 23, 5048-5053. Bittrich, H., Matzig, A.K., Kraker, I., and Appel, K.E. (1993). NO2-induced DNA single strand breaks are inhibited by antioxidative vitamins in V79 cells. Chem. Biol. Interact., 86, 199-211. Bogdanov, M., Beal, M.F., Griffin, R., and Matson, W. (1998). Quantitative method for 8-hydroxy-2’deoxyguanosine in biologicals. Mitochondrial Medicine Meeting, UCSD. Bogdanov, M.B., Beal, M.F., McCabe, D.R., Griffin, R., and Matson, W. (1999). A carbon column based LCEC approach to routine 8-hydroxy-2’deoxyguanosine measurements in urine and other biological matrices. A one year evaluation of methods. Free Radic. Biol. Med., 27, 647-666. Bogdanov, M.B., Brown, R.H., Matson, W., Smart, R., Hayden, D., O-Donnell, H., Beal, M.F., and Cudkowicz, M. (2000). Increased oxidative damage to DNA in ALS patients. Free Radic. Biol. Med., 29, 652-658. Bogdanov, M.B., Matson, W.R., and Acworth, I.N. (2003). The Use of HPLC/EC for the Measurement of Oxidative DNA Damage. In: Critical Reviews of Oxidative Stress and Aging. Volume 1.Cutler, R.G., and Rodriguez, H. (Eds.). World Scientific, NJ. Pp. 203-221. Bohley, P., and Seglen, P.O. (1992). Proteases and proteolysis in the lysosome. Experientia, 48, 151-157. Bohr, V.A., Stevnsner, T., and de Souza-Pinto, N.C. (2002). Mitochondrial DNA repair of oxidative damage in mammalian cells. Gene, 286, 127-134. Bohr, V.A., Taffe, B.G., and Larminat, F. (1995). DNA repair, oxidative stress and aging. In: Oxidative Stress and Aging. Cutler, R.G., Packer, L., Bertram, J., and Mori, A. (Eds.). Birkhauser, Basel. Pp. 45-54. Boiteux, S., and Radicella, J.P. (1999). Base excision repair of 8-hydroxyguanine protects DNA from endogenous oxidative stress. Biochimie, 81, 59-67. Boiteux, S., Gajewski, E., Laval, J., and Dizdaroglu, M. (1992). Substrate specificity of the E. coli Fpg protein (formamidopyrimidine-DNA glycosylase): Excision of purine lesions in DNA produced by ionizing radiation or photosensitization. Biochem., 31, 106-110. Bose, R., Jilling, T., Caplan, M., Derrick, M., and Tan, S. (1999). Electrochemical detection of nitrotyrosine in fetal brains following repetitive uterine ischemia. Poster #207. Free Radic. Biol. Med., 27, S73. Bosch-Morell, F., Flohe, L., Marin, N., and Romero, F.J. (1999). 4-Hydroxynonenal inhibits glutathione peroxidase: Protection by glutathione. Free Radic. Biol. Med., 26, 1383-1387. Brawn, K., and Fredovich, I. (1981). DNA strand scission by enzymatically generated oxygen radicals. Arch. Biochem. Biophys., 206, 414-419. Breen, A.P., and Murphy, J.A. (1995). Reactions of oxyl radicals with DNA. Free Radic. Biol. Med., 18, 1033-1077. Brennan, M.L., Wu, W., Fu, X., Shen, Z., Song, W., Frost, H., Vadseth, C., Narin, G., Lenkiewicz, E., Borchers M.T., Lusis, A.J., Lee, J.J., Lee, N.A., Abu-soud, H.M., Ischiropoulos, H., and Hazen, S.L. (2002). J. Biol. Chem., 277, 1741517427. Brigelius-Flohe, R., Auman, K.-D., Blocker, H., Gross, G., Kiess, M., Kloppel, K-D., Maiorino, M., Roveri, A., Schuckelt, R., Ursini, F., Wingender, E., and Flohe, L. (1994). Phospholipid hydroperoxide glutathione peroxidase: Genomic DNA, cDNA, and deduced amino acid sequence. J. Biol. Chem., 269, 7342-7348. Briviba, K., Kissner, R., Koppenol, W.H., and Sies, H. (1998). Kinetic study of the reaction of glutathione peroxidase with peroxynitrite. Chem. Res. Toxicol., 11, 1398-1401. Brot, N., and Weissbach, H. (1991). Biochemistry of methionine sulfoxide residues in proteins. Biofactors, 3, 91-96. Brown, R.K., McBurney, A., Lunec, J., and Kelly, F.J. (1995). Oxidative damage to DNA in patients with cystic fibrosis. Free Radic. Biol. Med., 18, 801-806. Browne, R.W., and Armstrong, D. (1998). Separation of hydroxy and hydroperoxy polyunsaturated fatty acids by highperformance liquid chromatography. In: Methods in Molecular Biology, 108. Armstrong, D. (Ed.). Humana Press, Totowa, New Jersey. Pp. 147-155. Bruijn, L.I., Beal, M.F., Becher, M.W., Schulz, J.B., Wong, P.C., Price, D.L., and Cleveland, D.W. (1997). Elevated free nitrotyrosine levels, but not protein-bound nitrotyrosine or hydroxyl radicals, throughout amyotrophic lateral sclerosis (ALS)-like disease implicate tyrosine nitration as an aberrant in vivo property of one familial ALS-linked superoxide dismutase 1 mutant. Proc. Natl. Acad. Sci. USA, 94, 7606-7611. Bucciarelli, L.G., Wendt, T., Rong, L., lalla, E, Hoffman, M.A., Goova, M.T., Taguchi, A., Yan, S.F., Stern, D.M., and Schmidt, A.M. (2002). RAGE is a multiligand receptor of the immunoglobulin superfamily: Implications for homeostasis and chronic disease. Cell. Mol. Life Sci., 59, 1117-1128. Buchner, J. (1996). Supervising the fold: Functional principles of molecular chaperones. FASEB J., 10, 10-19. Bunik, V.I., and Sievers, C. (2002). Inactivation of the 2-oxoacid dehydrogenase complexes upon generation of intrinsic radical species. Eur. J. Biochem., 269, 5004-5015. Burney, S., Caulfield, J.L., Niles, J.C., Wishnok, J.S., and Tannenbaum, S.R. (1999). The chemistry of DNA damage from nitric oxide and peroxynitrite. Mutat. Res., 424, 37-49. Buttery, L.D., Springall, D.R., Chester, A.H., Evans, T.J., Standfield, E.N., Parums, D.V., Yacoub, M.H., and Poalk, J.M. (1996). Inducible nitric oxide synthase is present within human atherosclerotic lesions and promotes the formation and activity of peroxynitrite. Lab. Invest., 75, 77-85. Byun, J., Henderson, J.P., Mueller, D.M., and Heinecke, J.W. (1998). 8-Nitro-2’deoxyguanosine is generated by the myeloperoxidase-H2O2-nitrite generating system of activated human phagocytes. Biochem., 38, 2590-2600. Cadet, J., and Weinfeld, M. (1993). Detecting DNA damage. Anal. Chem., 65, A675-A682. Cadet, J., Delatour, T., Douki, T., Gasparutto, D., Pouget, J.P., Ravanat, J.L., and Sauvaigo, S. (1999). Hydroxyl radicals and DNA base damage. Mutat. Res., 424, 9-21. Cadet, J., D’Ham, C., Douki, T., Pouget, J.-P., Ravanat, J.-L., and Sauvaigo, S. (1998). Facts and artifacts in the measurement of oxidative base damage to DNA. Free Rad. Res., 29, 541-550. WWW.ESAINC.COM 282 Cadet, J., Douki, T., and Ravanat, J.L. (1997). Artifacts associated with the measurement of oxidized DNA bases. Environ. Health Perspect., 105, 1034-1039. Cadet, J., Odin, F., Mouret, J-F., Polverelli, M., Audic, A., Giacomono, P., Richard, M-J., and Favier, A. (1992). Chemical and biochemical postlabeling methods for singling out specific oxidative DNA lesions. Mutat. Res., 275, 343-354. 32 Cajigas, A., Gayer, M., Beam, C., and Steinberg, J.J. (1994). Ozonation of DNA forms adducts: A P-DNA labeling and thin-layer chromatography technique to measure DNA environmental biomarkers. Arch. Environ. Health, 49, 25-36. Calderon-Garciduenas, L., Osnaya, N., Rodriguez-Alcaraz, A., and Villarreal-Calderon, A. (1997). DNA damage in nasal respiratory epithelium from children exposed to urban pollution. Environ. Mol. Mutagen., 30, 11-20. Calingasan, N.Y., Uchida, K., and Gibson, G.E. (1999). Protein-bound acrolein: A novel marker of oxidative stress in Alzheimer’s disease. J. Neurochem., 72, 751-756. Cao, G., and Cutler, R.G. (1995). Protein oxidation and aging. Difficulties in measuring reactive protein carbonyls in tissues using 2,4-dinitrophenylhydrazine. Arch. Biochem. Biophys., 320, 106-114. Capella, G., Cronauer-Mitra, S., Pienado, M.A., and Peruscho, M. (1991). Frequency and spectrum mutations at codon 12 and 13 of the c-K-ras gene in human tumors. Environ. Health Perspect., 93, 125-131. Carmichael, P.L., Hewer, A., Osborne, M.R., Strain, R.J., and Phillips, D.H. (1995). Detection of bulky DNA lesions in the liver of patients with Wilson’s disease and primary haemochromatosis. Mutation Res., 326, 235-243. Carney, J.M., Starke, R.P., Oliver, C.N., Landum, R.W., Cheng, M.S., Wu, J.F., and Floyd, R.A. (1991). Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA, 88, 3633-3636. Cathcart, R., Schwiers, E., Saul, R.L., and Ames, B.N. (1984). Thymine glycol and thymidine glycol in human and rat urine: a possible assay for oxidative DNA damage. Proc. Natl. Acad. Sci. USA, 81, 5633-5637. Cattley, R.C., and Glover, S.E. (1993). Elevated 8-hydroxydeoxyguanosine in hepatic DNA of rats following exposure to peroxisome proliferators: Relationship to carcinogenesis and nuclear localization. Carcinogen., 14, 2495-2499. Cawood, P., Wickens, D.G., Iversen, S.A., Braganza, J.M., and Dormandy, T.L. (1983). The nature of diene conjugation in human serum, bile and duodenal juice. FEBS Lett., 162, 239-243. Chang, Y.H., Abdalla, D.S., and Sevanian, A. (1997). Characterization of cholesterol oxidation products formed by oxidative modification of low density lipoprotein. Free Radic. Biol. Med., 23, 202-214. Chao, C.-C., Ma, Y.-S., and Stadtman, E.R. (1997). Modification of protein surface hydrophobicity and methionine oxidation by oxidative systems. Proc. Natl. Acad. Sci. USA, 94, 2969-2974. 6 Chaudhary, A.K., Reddy, G.R., Blair, I.A., and Marnett, L.J. (1996), Characterization of an N -oxoprenyl-2’deoxyadenosine adduct in malondialdehyde-modified DNA using liquid chromatography/electrospray ionization tandem mass spectrometry. Carcinogen., 17, 1167-1170. Chen, J.J., Bertrand, H., and Yu, B.P. (1995). Inhibition of adenine nucleotide translocator by lipid peroxidation products. Free Radic. Biol. Med., 19, 583-590. Chiavari, G., and Bergamini, C. (1985). High-performance liquid chromatography of carbonyl compounds as 2,4dinitrophenylhydrazones with electrochemical detection. J. Chromatogr., 318, 427-432. Chou, S.M., Wang, H.S., and Komai, K. (1996). Colocalization of NOS and SOD1 in neurofilament accumulation within motor neurons of amyotrophic lateral sclerosis: an immunohistochemical study. J. Chem. Neuroanat., 10, 249-258. Chou, S.M., Wang, H.S., and Taniguchi, A. (1996). Role of SOD-1 and nitric oxide/cyclic GMP cascade on neurofilament aggregation in ALS/MND. J. Neurol. Sci., 139, 16-26. Chung, M.H., Kasai, H., Nishimura, S., and Yu, B.P. (1992). Protection of DNA damage by dietary restriction. Free Radic. Biol. Med., 12, 523-525. Cini, M., Fariello, R.G., Bianchetti, A., and Moretti, A. (1994). Studies on lipid peroxidation in the rat brain. Neurochem. Res., 19, 283-288. Claycamp, H.G. (1992). Phenol sensitization of DNA to subsequent oxidative damage in 8-hydroxyguanine assays. Carcinogen., 13, 1289-1292. Claycamp, H.G., and Ho, K.-K. (1993). Background and radiation-induced 8-hydroxy-2’-deoxyguanosine in γ-irradiated Escherichia coli. Int. J. Radiat. Biol., 63, 597-607. Clayton, D.A., Doda, J.N., and Friedberg, E.C. (1974). The absence of a pyrimidine dimer repair mechanism in mammalian mitochondria. Proc. Natl. Acad. Sci. USA, 71, 2777-2781. Climent, I., Tsai, L., and Levine, R.L. (1989). Derivatization of gamma-glutamyl semialdehyde residues in oxidized proteins by fluoresceinamine. Anal. Biochem., 182, 226-232. Cohen, J.S., Yariv, J., Kalb, A.J., Jacobson, L., and Shechter, Y. (1979). 13C NMR analysis of methionine sulfoxide in protein. J. Biochem. Biophys. Meths., 1, 145-151. Coles, B., Bloodsworth, A., Clark, S.R., Lewis, M.J., Cross, A.R., Freeman, B.A., and O’Donnell, V.B. (2002). Circ. Res., 91, 375-381. Collins, A.R. (1998). The comet assay: A novel approach to measuring DNA oxidation. In: DNA and Free Radicals: Techniques, Mechanisms and Applications. Aruoma, O.I., and Halliwell, B. (Eds.). OICA, London. Pp. 241-260. Collins, A.R., Dusinka, M., Gedik, C.M., and Setina, R. (1996). Oxidative damage to DNA: Do we have a reliable biomarker? Environ. Health Perspect. 104, 465-469. Collins, A.R., Duthie, S.J., and Donson, V.L. (1993). Direct enzymic detection of endogenous oxidative base damage in human lymphocyte DNA. Carcinogen.,14, 1733-1735. Collins, A.R., Gedik, C.M., Olmedilla, B., Southon, S., and Bellizzi, M. (1998a). Oxidative DNA damage measured in human lymphocytes: Large differences between the sexes and between countries, and correlations with heart disease mortality rates. FASEB J., 12, 1397-1400. Collins, A.R., Raslova, K., Somorovska, M., Petrovska, H., Ondrusova, A., Vohnout, B., Fabry, R., and Dusinska, M. (1998). DNA damage in diabetes: Correlations with a clinical marker. Free Radic. Biol. Med., 25, 373-377. Cooke, M.C., Evans, M.D., Podmore, I.D., Herbert, K.E., Mistry, N., Mistry, P., Hickenbotham, P.T., Hussieni, A., Griffiths, H.R., and Lunec, J. (1998). Novel repair action of vitamin C upon in vivo oxidative DNA damage. FEBS Letts., 363, 363-367. WWW.ESAINC.COM 283 Cooper, B., Creeth, J.M., and Donald, A.S. (1985). Studies on the limited degradation of mucus glycoproteins. The mechanism of the peroxide reaction. Biochem. J., 228, 615-626. Cooke, M.C., Mistry, N., Ahmad, J., Waller, H., Langford, L., and Lunec, J. (2003). Deoxycytidine glyoxal: Lesion induction and evidence of repair following vitamin C. Free Radic. Biol. Med., 34, 218-225. Copley, S.D., Frank, E., Kirsch, W.M., and Koch, T.H. (1992). Detection and possible origins of aminomalonic acid in protein hydrolysates. Anal. Biochem., 201, 152-157. Cordis, G.A., Bagchi, D., Maulik, N., and Das, D.K. (1994). High-performance liquid chromatographic method for the simultaneous detection of malondialdehyde, acetaldehyde, formaldehyde, acetone and propionaldehyde to monitor the oxidative stress in heart. J. Chromatogr. A., 661, 181-191. Cordis, G.A., Maulik, G., Bagchi, D., Riedel, W., and Das, D.K. (1998). Detection of oxidative DNA damage to ischemic reperfused rat hearts by 8-hydroxydeoxyguanosine formation. J. Mol. Cardiol., 30, 1939-1944. Coux, O., Tanaka, K., and Goldberg, A.L. (1996). Structure and functions of the 20S and 26S proteasomes. Ann. Rev. Biochem., 65, 801-847. Creeth, J.M., Cooper, B., Donald, B.S., and Clamp, J.R. (1983). Studies of the limited degradation of mucus glycoproteins. The effects of dilute hydrogen peroxide. Biochem. J., 211, 323-332. Cross, A.H., Manning, P.T., Stern, M.K., and Misko, T.P. (1997). Evidence for the production of peroxynitrite in inflammatory CNS demyelination. J. Neuroimmunol., 80, 121-130. Crow, J.P. (1999). Measurement and significance of free and protein-bound 3-nitrotyrosine, 3-chlorotyrosine, and free 3nitro-4-hydroxyphenylacetic acid in biological samples: A high-performance liquid chromatography method using electrochemical detection. Meth. Enzymol., 301, 151-162. Crow, J.P., and Beckman, J.S. (1995). Reactions between nitric oxide, superoxide, and peroxynitrite: Footprints of peroxynitrite in vivo. Adv. Pharmacol., 34, 17-30. Crow, J.P., and Ischiropoulos, H. (1996). Detection and quantitation of nitrotyrosine residues in proteins: In vivo marker of peroxynitrite. Meth. Enzymol., 269, 185-194. Crow, J.P., Beckman, J.S., and McCord, J.M. (1995). Sensitivity of the essential zinc-thiolate moiety of yeast alcohol dehydrogenase to hypochlorite and peroxynitrite. Biochem., 34, 3544-3552. Crow, J.P., Ye, Y.Z., Kirk, M., Barnes, S., and Beckman, J.S. (1997). Superoxide dismutase catalyzes nitration of tyrosines by peroxynitrite in the rod and head domains of neurofilament-L. J. Neurochem., 69, 1945-1953. Crowley, J.R., Yarasheski, K., Leeuwenburgh, C., Turk, J., and Heinecke, J.W. (1998). Isotope dilution mass spectrometric quantification of 3-ntriotyrosine in proteins and tissues is facilitated by reduction to 3-aminotyrosine. Anal. Biochem., 259, 127-135. Cucurou, C., Battioni, J.P., Thang, D.C., Nam, N.H., and Mansuy, D. (1991). Mechanisms of inactivation of lipoxygenases by phenidone and BW755C. Biochem., 30, 8964-8970. Culp, S.J., Cho, B.P., Kadlubar, F.F., and Evans, F.E. (1989). Structural and conformational analyses of 8-hydroxy-2’deoxyguanosine. Chem. Res. Toxicol., 2, 416-422. Cushnir, J.R., Naylor, S., Lamb, J.H., and Farmer, P.B. (1993). Tandem mass spectrometric approaches for the analysis of alkylguanines in human urine. Org. Mass Spec., 28, 552-558. Daiber, A., Harold, S., Schoneich, C., Namgaladze, D., Peterson, J.A., and Ullrich, V. (2000). Nitration of cytochrome P450BM-3 by peroxynitrite. Stopped-flow measurement prove ferryl intermediates. Eur. J. Biochem., 267, 6729-6739. Daiber, A., Mehl, M., and Ullrich, V. (1998). New aspects in the reaction mechanism of phenol with peroxynitrite: The role of phenoxy radicals. Nitric Oxide, 2, 259-269. Dakin, H.D. (1906). The oxidation of amino-acids with the production of substances of biological importance. J. Biol. Chem., 1, 171-176. Dakin, H.D. (1908). The oxidation of leucine, α-amido-n-valeric acid with hydrogen peroxide. J. Biol. Chem., 4, 63-76. Dandona, P., Thusu, K., Cook, S., Snyder, B., Makowski, J., Armstrong, D., and Nicotera, T. (1996). Oxidative damage to DNA in diabetes mellitus. Lancet, 347, 444-445. Dawson, T.M., and Dawson, V.L. (1994). Nitric oxide: Actions and pathological roles. Neurosci., Preview Issue. 9-19. Dean, R.T. (1978). Lysosomes and protein degradation. Ciba Found. Symp., 75, 139-149. Dean, R.T., Fu, S., Giesag, G., and Armstrong, S.G. (1996). In: Free Radicals: A practical Approach. Punchard, N.A., and Kelly, F.J. (Eds.). IRL Press, Oxford. Pp. 171-183. Dean, R.T., Fu, S., Stocker, R., and Davies, M.J. (1997). Biochemistry and pathology of radical-mediated protein oxidation. Biochem. J., 324, 1-18. Degan, P., Bonassi, S., De Caterina, M., Korkina, L.G., Pinto, L., Scopacasa, F., Zatterale, A., Calzone, R., and Pagano, G. (1995). In vivo accumulation of 8-hydroxy-2’-deoxyguanosine in DNA correlates with release of reactive oxygen species in Fanconi’s anemia families. Carcinogen., 16, 735-742. Degan, P., Shigenaga, M.K., Park, E.-M., Alperin, P.E., and Ames, B.N. (1991). Immunoaffinity isolation of urinary 8hydroxy-2’deoxyguanosine and 8-hydroxyguanine and quantitation of 8-hydroxy-2’deoxyguanosine in DNA by polyclonal antibodies. Carcinogen., 12, 865-871. De Flora, S., Bennicelli, C., Zanacchi, P., D’Agnostini, F., and Camoirano, A. (1989). Mutagenicity of active oxygen species in bacteria and its enzymatic or chemical inhibition. Mutat. Res., 214, 153-158. Delori, F.C., and Dorey, C.K. (1998). In vivo technique for autofluorescent lipopigments. In: Methods in Molecular Biology, 108. Armstrong, D. (Ed.). Humana Press, Totowa, New Jersey. Pp. 229-243. De Martinis, B.S., and Bianchi, M.D. (2002). Methodology for urinary 8-hydroxy-2’-deoxyguanosine analysis by HPLC with electrochemical detection. Pharmacol. Res., 46, 129-131. Demple, B., and Harrison, L. (1994). Repair of oxidative damage to DNA: Enzymology and Biology. Ann. Rev. Biochem., 63, 915-948. Denda, A., Tang, Q., Endoh, T., Tsujiuchi, T., Horiguchi, K., Noguchi, O., Mizumoto, Y., Nakae, D., and Konishi, Y. (1994). Prevention by acetylsalicylic acid of liver cirrhosis and carcinogenesis as well as generations of 8hydroxydeoxyguanosine and thiobarbituric acid-reactive substances caused by a choline-deficient, L-amino aciddefined diet in rats. Carcinogen., 15 1279-1283. WWW.ESAINC.COM 284 Deng, X.-S., Tuo, J., Poulsen, H.E., and Loft, S. (1998). Prevention of oxidative DNA damage in rats by brussel sprouts. Free Rad. Res., 28, 323-333. De Zwart, L.L., Meerman, J.H.N., Commander, J.N.M., and Vermeulen, N.P.E. (1998). Biomarkers of free radical damage applications in experimental animals and in humans. Free Radic. Biol. Med., 26, 202-226. De Zwart, L.L., Venhorst, J., Groot, M., Commandeur, J.N.M., Hermanns, R.C.A., Meerman, J.H.M., Van Baar, B.L.M., and Vermeulen, P.E. (1997). Simultaneous determination of eight lipid peroxidation degradation products in urine of rats treated with carbon tetrachloride using gas chromatography with electron-capture detection. J. Chromatogr. B, 694, 277-287. Dice, J.F., and Terlecky, S.R. (1990). Targeting of cytosolic proteins to lysosomes for degradation. Crit. Rev. Ther. Drug Carrier. Syst., 7, 211-233. Dizdaroglu, M. (1991). Chemical determination of free radical induced damage to DNA. Free Radic. Biol. Med., 10, 225242. Dizdaroglu, M. (1993). In: DNA and Free Radicals. Halliwell, B., and Aruoma, O.I. (Eds.). Ellis Horwood, Chichester. Pp. 19-39. Dizdaroglu, M. (1993b). Quantitative determination of oxidative base damage in DNA by stable isotope-dilution mass spectrometry. FEBS Lett., 315, 1-6. Dizdaroglu, M. (1994). Chemical determination of oxidative DNA damage by gas chromatography-mass spectrometry. Meth. Enzymol., 234, 3-16. Dizdaroglu, M. (1997). Measurement of oxidized DNA bases using gas chromatography/mass spectrometry. Workshop. Oxygen Society, San Francisco, CA. Dizdaroglu, M. (1998). Mechanisms of free radical damage to DNA. In: DNA and Free Radicals: Techniques, Mechanisms and Applications. Aruoma, O.I., and Halliwell, B. (Eds.). OICA, London. Pp. 3-26. Dizdaroglu, M., Jaruga, P., and Rodriguez, H. (2003). Oxidative DNA Damage to DNA: Mechanisms of Product Formation and Measurement by Mass Spectrometric Techniques. In: Critical Reviews of Oxidative Stress and Aging. Volume 1.Cutler, R.G., and Rodriguez, H. (Eds.). World Scientific, NJ. Pp. 165-189. Dizdaroglu, M., Laval, J., and Boiteux, S. (1993). Substrate specificity of the Escherichia coli endonuclease III: excision of thymine- and cytosine-derived lesions in DNA produced by radiation-generated free radicals. Biochem., 32, 1210512111 Djuric, Z., Heilbrun, L.K., Reading, B.A., Boomer, A., Valeriete, F.A., and Martino, S. (1991). Effects of low-fat diet on levels of oxidative damage to DNA in human peripheral nucleated blood cells. J. Natl. Cancer Inst., 83, 766-769. Djuric, Z., Lu, M.H., Lewis, S.M., Luongo, D.A., Chen, X.W., Heilbrun, L.K., Reading, B.A., Duffy, P.H., and Hart, R.W. (1992). Oxidative DNA damage levels in rats fed low-fat, high-fat, or caloric-restricted diets. Toxicol. Appl. Pharmacol., 115, 156-160. Djuric, Z., Luongo, D.A., and Harper, D.A. (1991b). Quantitation of 5-(hydroxymethyl)uracil in DNA by gas chromatography with mass spectral detection. Chem. Res. Toxicol., 4, 687-691. Domigan, N.M., Charlton, T.S., Duncan, M.W., Winterbourne, C.C., and Kettle, A.J. (1995). Chlorination of tyrosyl residues in peptides by myeloperoxidase and human neutrophils. J. Biol. Chem., 270, 16542-16548. Dormandy, T.L., and Wickens, D.G. (1987). The experimental and clinical pathology of diene conjugation. Chem. Phys. Lipids, 45, 353-364. 2 Douki, T., and Ames, B.N. (1994). An HPLC-EC assay for 1,N -propano adducts of 2’-deoxyguanosine with 4hydroxynonenal and other α,β-unsaturated aldehydes. Chem. Res. Toxicol., 7, 511-518. Douki, T., Delatour, T., Bianchini, F., and Cadet, J. (1996). Observation and prevention of an artifactual formation of oxidized DNA bases and nucleosides in the GC-EIMS method. Carcinogen., 17, 347-353. Douki, T., Ravanat, J.-L., Frelon, S., Bourdat, J.-P., and Cadet, J. (2003). HPLC-MS/MS Measurement of Oxidative Base Damage to Isolated and Cellular DNA. In: Critical Reviews of Oxidative Stress and Aging. Volume 1.Cutler, R.G., and Rodriguez, H. (Eds.). World Scientific, NJ. Pp. 190-202. Driscoll, J. (1994). The role of the proteasome in cellular degradation. Histol. Histopathol., 9, 197-202. Duarte, V., Muller, J.G., and Burrows, C.J. (1999). Insertion of dGMP and dAMP during in vitro DNA synthesis opposite an oxidized form of 7,8-dihydro-8-oxoguanine. Nucelic Acids Res., 27, 496-502. Ducrocq, C., Dendane, M., Laprevote, O., Serani, L., and Das, B.C. (1998). Chemical modification of the vasoconstrictor peptide angiotensin II by nitrogen oxides (NO, HNO2, HOONO) – evaluation by mass spectrometry. Eur. J. Biochem., 253, 146-153. Duda, J.E., Giasson, B.I., Chen, Q., Gur, T.L., Hurtig, H.I., Stern, M.B., Gollomp, S.M., Ischiropoulos, H., Lee, V.M., and Trojanowski, J.Q. (2000). Am. J. Pathol., 175, 1439-1445. Dunn, J.A., Ahmed, M.U., Murtiashaw, M.H., Richardson, J.M., Walla, M.D., Thorpe, S.R., and Baynes, J.W. (1990). Reaction of ascorbate with lysine and protein under autoxidizing conditions: Formation of Nε-(carboxymethyl)lysine by reaction between lysine and products of autoxidation of ascorbate. Biochem., 29, 10964-10970. Eder, E., and Hoffman, C. (1993). Identification and characterization of deoxyguanosine adducts of mutagenic β-alkylsubstituted acrolein congeners. Chem. Res. Toxicol., 6, 486-494. Eiserich, J.P., Cross, C.E., Jones, A.D., Halliwell, B., and van der Vleit, A. (1996). Formation of nitrating and chlorinating species by reaction of nitrite with hypochlorous acid. A novel mechanism for nitric oxide-mediated protein modification. J. Biol. Chem., 271, 19199-19208. Eiserich, J.P., Hristova, M., Cross, C.E., Freeman, B.A., and van der Vleit, A. (1998). Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature, 391, 393-398. Emerit, I. (1994). Reactive oxygen species, chromosome mutation, and cancer: Possible role of clastogenic factors in carcinogenesis. Free Radic. Biol. Med., 16, 99-109. Epe, B. (1993). In: DNA and Free Radicals. Halliwell, B., and Aruoma, O.I. (Eds.). Ellis Horwood, Chichester. Pp. 41-65. Epe, B., Ballmaier, D., Roussyn, I., Briviba, K., and Sies, H. (1996). DNA damage by peroxynitrite characterized with DNA repair enzymes. Nucleic Acid Res., 24, 4105-4110. WWW.ESAINC.COM 285 Erhola, M., Toyokuni, S., Okada, K., Tanaka, T., Hiai, H., Ochi, H., Uchida, K., Osawa, T., Niemmen, M.M., Alho, H., Kellokumu-Lehtinen, P. (1997). Biomarker evidence of DNA oxidation in lung cancer patients: Association of urinary 8hydroxy-2'-deoxyguanosine excretion with radiotherapy, chemotherapy and response to treatment. FEBS Lett., 409, 287-291. ESCODD (2002). Comparative analysis of baseline 8-oxo-7,8-dihydroguanine in mammalian cell DNA, by different methods in different laboratories: An approach to consensus. Carcinogen., 23, 2129-2133. ESCODD (2003). Measurement of DNA oxidation in human cells by chromatographic and enzymic methods. Free Radic. Biol. Med., 34, 1089-1099. Esterbauer, H., Eckl, P., and Ortner, A. (1990). Possible mutagens derived from lipids and lipid precursors. Mutat. Res., 238, 223-233. Esterbauer, H., Jurgens, G., Quehenberger, O., and Koller, E. (1987). Autooxidation of human low-density lipoprotein: Loss of polyunsaturated fatty acids and vitamin E and generation of aldehydes. J. Lipid. Res., 28, 495-509. Esterbauer, H., Zollner, H., and Schaur, R.J. (1990). Aldehydes formed by lipid peroxidation: Mechanisms of formation, occurrence and determination. In: Membrane Lipid Oxidation. Vigo-Pelfry, C. (Ed.). Boca Raton, FL, CRC Press, 1. Pp. 239-283. Esterbauer, H., Schaur, R.J., and Zollner, H. (1991). Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med., 11, 81-128. Evans, J., Maccabee, M., Hatahet, Z., Courcelle, J., Bockrath, R., Ide, H., and Wallace, S. (1993). Thymine ring saturation and fragmentation products: lesion bypass, misinsertion and implications for mutagenesis. Mutat. Res., 299, 147-156. Evans, P., Lyras, L., and Halliwell, B. (1998). Measurement of protein carbonyls in human brain tissues. Meths. Enzymol., 300, 145-156. Fairbairn, D.W., Olice, P.L., and O’Neill, K.L. (1995). The comet assay: A comprehensive review. Mutat. Res., 339, 37-59. Fam, S.S., and Morrow, J.D. (2003). The isoprostanes: Unique products of arachidonic acid oxidation – a review. Curr. Med. Chem., 10, 1723-1740. Farber, J.M., and Levine, R.L. (1986). Sequence of a peptide susceptible to mixed-function oxidation: Probable cation binding site in glutamine synthetase. J. Biol. Chem., 261, 4574-4578. Faux, S.P., Francis, J.E., Smith, A.G., and Chipman, J.K. (1992). Induction of 8-hydroxydeoxyguanosine in Ah-responsive mouse liver by iron and Aroclor 1254. Carcinogen., 13, 247-250. Faux, S.P., Gao, M., Aw, T.C., and Braithwaite, R.A. (1994). Molecular epidemiological studies in workers exposed to chromium-containing compounds. Clin. Chem., 40, 1454-1456. Feig, D.I., and Loeb, L.A. (1993). Mechanisms of mutation by oxidative DNA damage: Reduced fidelity of mammalian DNA polymerase beta. Biochem., 32, 4466-4473. Feinstein, E., Canaani, E., and Weiner, L.M. (1993). Dependence of nucleic acid degradation on in situ free-radical production by adriamycin. Biochem., 32, 13156-13161. Ferng, S.F., Castro, C.E., Afifi, A.A., Bermudez, E., and Mustafa, M.G. (1997). Ozone-induced DNA strand breaks in guinea pig tracheobronchial epithelial cells. J. Toxicol. Environ. Health., 51, 353-367. Ferrante, R.J.,Shinobu, L.A., Schulz, J.B., Matthews, R.T., Thomas, C.E., Kowall, N.W., Gurney, M.E., and Beal, M.F. (1997). Increased 3-nitrotyrosine and oxidative damage in mice with human copper/zinc superoxide dismutase mutation. Ann. Neurol., 42, 326-334. Fest, A., and Gan, J.C. (1981). Selective loss of elastase inhibitory activity of alpha 1-proteinase inhibitor upon chemical modification of tyrosyl residues. J. Biol. Chem., 256, 6374-6380. Fiala, E.S., Conaway, C.C., and Mathis, J.E. (1989). Oxidative DNA and RNA damage in the livers of Sprague-Dawley rats treated with the hepatocarcinogen 2-nitropropane. Cancer Res., 49, 5518-5522. Fischer-Nielson, A., Jeding, I.B., and Loft, S. (1994). Irradiation-induced formation of 8-hydroxy-2’-deoxyguanosine and its prevention by scavengers. Carcinogen., 15, 1609-1612. Fischer-Nielsen, A., Poulsen, H.E., and Loft, S. (1992). 8-Hydroxydeoxyguanosine in vitro: Effects of glutathione, ascorbate, and 5-aminosalicylic acid. Free Radic. Biol. Med., 13, 121-126. Finch, J.T., and Klug, A. (1976). Solenoidal model for superstructure in chromatin. 901 Proc. Natl. Acad. Sci. USA, 73, 1897-1901. Flohe, L. (1982). Glutathione peroxidases brought into focus. In: Free Radicals in Biology. Vol. V. Pryor, W.A. (Ed.). Academic Press, New York. Pp. 223-253. Floyd, R.A., West, M.S., Eneff, K.L., Schneider, J.E., Wong, P.K., Tingey, D.T., and Hogsett, W.E. (1990). Conditions influencing yield and analysis of 8-hydroxy-2'-deoxyguanosine in oxidatively damaged DNA. Anal. Biochem., 188, 155158. Floyd, R.A., Watson, J.J., Wong, P.K., Altmiller, D.H., and Rickard, R.C. (1986). Hydroxyl free radical adduct of deoxyguanosine: Sensitive detection and mechanisms of formation. Free Radic. Res. Comm., 1, 163-172. Foksinski, M., Bialkowski, K., Skiba, M., Ponikowska, I., Szmurlo, W., and Olinski, R. (1999). Evaluation of 8oxodeoxyguanosine, typical oxidative DNA damage, in lymphocytes of ozone-treated arteriosclerosis patients. Mutat. Res., 438, 23-27. Foksinski, M., Kotzbach, R., Szymanski, W., and Olinski, R. (2000). The level of typical biomarkers in oxidative stress 8hydroxy-2’-deoxyguanosine is higher in uterine myomas than in control tissues and correlated with the size of the tumor. Free Radic. Biol. Med., 29, 597-601. Forman, L.J., Liu, P., Nagele, R.G., Yin, K., and Wong, P.Y. (1998). Augmentation of nitric oxide, superoxide, and peroxynitrite production during cerebral ischemia and reperfusion in the rat. Neurochem. Res., 23, 141-148. Fowler, R.G., White, S.J., Moore, S.C., Dunn, R.L., and Schaaper, R.M. (2003). Interations among E. Coli mutt, mutM, and mutY damage prevention pathways. DNA Repair, 2, 159-173. Frankel, E.N., and Neff, W.F. (1983). Formation of malonaldehyde from lipid oxidation products. Biochim. Biophys. Acta, 754, 264-270. Fraga, C.G., Motchnik, P.A., Shigenaga, M.K., Helbok, H.J., Jacob, R.A., and Ames, B.N. (1991). Ascorbic acid protects against endogenous oxidative DNA damage in human sperm. Proc. Natl. Acad. Sci. USA, 88, 11003-11006. WWW.ESAINC.COM 286 Fraga, C.G., Shigenaga, M.K., Park, J.-W., Degan, P., and Ames, B.N. (1990). Oxidative damage to DNA during aging: 8Hydroxy-2’deoxyguanosine in rat organ DNA and urine. Proc. Natl. Acad. Sci. USA, 87, 4533-4537. Francesco, V., Cesare, A., Luigi, I., Stefano, F., Andrea, G., and Francesco, B. (1985). Malondialdehyde-like material and beta-thromboglobulin plasma levels in patients suffering from transient ischemic attacks. Stroke, 16, 14-16. Francescutti, D., Baldwin, J., Lee, L., and Mutus, N. (1996). Peroxynitrite modification of glutathione reductase: Modeling studies and kinetic evidence suggest the modification of tyrosine at the glutathione disulfide binding site. Protein Eng., 9, 189-194. Freedman, J.E. (2002). Nitrated lipids: Defining their bioactivity. Circ. Res., 91, 371-372. Freeman, B.A., White, C.R., Gutierrez, H., Paler-Martinez, A., Tarpey, M.M., and Rubbo, H. (1995). Oxygen radical-nitric oxide reactions in vascular disease. Adv. Pharmacol., 34, 45-69. Freeman, M.L., Borrelli, M.J., Meredith, M.J., and Lepock, J.R. (1999). On the path to the heat shock response: Destabilization, and formation of partially folded intermediates, a consequence of protein thiol modification. Free Radic. Biol. Med., 26, 737-745. Frei, B., Yamamoto, Y., Niclas, D., and Ames, B.N. (1988). Evaluation of an isoluminol chemiluminescence assay for the detection of hydroperoxides in human blood plasma. Anal. Biochem., 174, 120-130. Frenkel, K., Zhong, Z., Wei, H., Karkoszka, J., Patel, U., Rashid, K., Georgescu, M., and Solomon, J.J. (1991). Quantitative high-performance liquid chromatography analysis of DNA oxidized in vitro and in vivo. Anal. Biochem., 196, 126-136. Friedberg, E.C. (1996). Relationships between DNA repair and transcription. Ann. Rev. Biochem., 65, 15-42. Friess, U., Waldner, M., Wahl, H-G., Rainer, L., Haring, H-U., Voelter, W., and Schleicher, E. (2003). Liquid chromatography-based determination of urinary free and total N(epsilon)-(carboxymethyl)lysine excretion in normal and diabetic subjects. J. Chromatogr. B, 794, 273-280. Fu, M.X., Wells-Knecht, K.J., Blackedge, J.A., Lyons, T.J., Thorpe, S.R., and Baynes, J.W. (1994). Glycation, glyoxidation, and cross-linking of collagen by glucose. Kinetics, mechanisms, and inhibition of late stages of the Maillard reaction. Diabetes, 43, 676-683. Fu, S., Gebicki, S., Jessup, W., Gebicki, J.M., and Dean, R.T. (1995a). Biological fate of amino acids, peptide and protein hydroperoxides. Biochem. J., 311, 821-827. Fu, S., Hick, L.A., Sheil, M.M., and Dean, R.T. (1995b). Structural identification of valine hydroperoxides and hydroxides on radical-damaged amino acid, peptide, and protein molecules. Free Radic. Biol. Med., 19, 281-292. Fuciarelli, A.F., Wegher, B.J., Blakely, W.F., and Dizdaroglu, M. (1990) Yields of radiation-induced base products in DNA: Effects of DNA conformation and gassing conditions. Int. J. Radiat. Biol., 58, 397-415. Fukuyama, N., Takebayashi, Y., Hida, M., Ishida, H., Ichimori, K., and Nakazawa, H. (1996). Clinical evidence of peroxynitrite formation in chronic renal failure patients with septic shock. Free Radic. Biol. Med., 22, 771-774. Fung, K., and Grosjean, D. (1981). Determination of nanogram amounts of carbonyls as 2,4-dintrophenylhydrazones by high-performance liquid chromatography. Anal. Chem., 53, 168-171. Furuichi, M., Yoshida, M.C., Oda, H., Tajiri, T., Nakabeppu, Y., Tsuzuki, T., and Sekiguchi, M. (1994). Genomic structure and chromosome location of the human mutT homologue gene MTH1 encoding 8-oxo-dGTPase for prevention of A:T to C:G transversion. Genomics, 24, 485-490. Gajewski, E., Rao, G., Nackerdien, Z., and Dizdaroglu, M. (1990). Modification of DNA bases in mammalian chromatin by radiation generated free radicals. Biochem., 29, 7876-7882. Galli, F., Rovidati, S., Ghibelli, L., and Canestrari, F. (1998). S-nitrosylation of glyceraldehyde-3-phosphate dehydrogenase decreases the enzyme affinity to the erythrocyte membrane. Nitric Oxide, 2, 17-27. Gantchev, T.G., and van Lier, J. (1995). Catalase inactivation following photosensitization with tetrasulfonated metallophthalocyanines. Photochem. Photobiol., 62, 123-134. Garrison, W.M., (1987). Reaction mechanisms in the radiolysis of peptides, polypeptides, and proteins. Chem. Rev., 87, 381-398. Gaut, J.P., Byun, J., Tran, H.D., and Heinecke, J.W. (2002). Artifact-free quantification of free 3-chlorotyrsoine, 3bromotyrosine, and 3-nitrotyrosine in human plasma by electron capture-negative chemical ionization gas chromatography mass spectrometry and liquid chromatography-electrospray ionization tandem mass spectrometry. Anal. Biochem., 300, 252-259. Gebicki, S., and Gebicki, J.M. (1993). Formation of peroxides in amino acids and proteins exposed to oxygen free radicals. Biochem J., 289, 743-749. Gebicki, S., and Gebicki, J.M. (1999). Crosslinking of DNA and proteins induced by protein hydroperoxides. Biochem. J., 338, 629-636. Gedik, C.M., Wood, S.G., and Collins, A.R. (1998). Measuring oxidative damage to DNA: HPLC and Comet assay compared. Free Radic. Res., 29, 609-615. Geiger, P.G., Korytowski, W., Lin, F., and Girotti, A.W. (1997). Lipid peroxidation in photodynamically stressed mammalian cells: Use of cholesterol hydroperoxides as mechanistic reporters. Free Radic. Biol. Med., 23, 57-68. Germadnik, D., Pilger, A., and Rudiger, H.W. (1997). Assay for the determination of urinary 8-hydroxy-2’deoxyguanosine by high-performance liquid chromatography with electrochemical detection. J. Chromatogr. B., 689, 399-403. Gieseg, S.P., Simpson, J.A., Charlton, T.S., Duncan, M.W., and Dean, R.T. (1993). Protein-bound 3,4dihydroxyphenylalanine is a major reductant formed during hydroxyl radical damage to proteins. Biochem., 32, 47804786. Girotti, A.W. (1998). Lipid hydroperoxide generation, turnover and affector action in biological systems. J. Lipid Res., 39, 1529-1542. Giulivi, C., and Davies, K.J.A. (1993). Dityrosine and tyrosine oxidation products are endogenous markers for the selective proteolysis of oxidatively modified red blood cell hemoglobin by (the 19 S) proteasome. J. Biol. Chem., 268, 8752-8759. Giulivi, C., and Davies, K.J.A. (1994). Dityrosine: A marker of oxidatively modified proteins and selective proteolysis. Meth. Enzymol., 233, 363-371. WWW.ESAINC.COM 287 Glomb, M.A., and Monnier, V.M. (1995). Mechanism of protein modification by glyoxal and glycoaldehyde, reactive intermediates of the Maillard reaction. J. Biol. Chem., 270, 10017-10026. Goda, Y., and Marnett, L.J. (1991). High-performance liquid chromatography with electrochemical detection for determination of the major malondialdehyde-guanine adduct. Chem. Res. Toxicol., 4, 520-524. Goldring, C., Casini, A.F., Maellaro, E., Bello, B D., and Comporti, M. (1993). Determination of 4-hydroxynonenal by highperformance liquid chromatography with electrochemical detection. Lipids, 28, 141-145. Good, P.F., Werner, P., Hsu, A., Olanow, C.W., and Perl, D.P. (1996). Evidence of neuronal oxidative damage in Alzheimer’s disease. Am. J. Pathol., 149, 21-28. Goodwin, D.C., Gunther, M.R., His, L.C., Crews, B.C., Eling, T.E., Mason, R.P., and Marnett, L.J. (1998). Nitric oxide trapping of tyrosyl radicals generated during prostaglandin endoperoxide synthase turnover. Detection of the radical derivative of tyrosine 385. J. Biol. Chem., 273, 8903-8909. Gorsdorf, S., Appel, K.E., Engeholm, C., and Obe, G. (1990). Nitrogen dioxide induces singlestrand breaks in cultured Chinese hamster cells. Carcinogen., 11, 37-41. Gow, A.J., Duran, D., Malcolm, S., and Ischiropoulos, H. (1996a). Effects of peroxynitrite-induced protein modifications on tyrosine phosphorylation and degradation. FEBS Lett., 385, 63-66. Gow, A., Duran, D., Thom, S.R., and Ischiropoulos, H. (1996b). Carbon dioxide enhancement of peroxynitrite-mediated protein tyrosine nitration. Arch. Biochem. Biophys., 333, 42-48. Gowan, L.C., Avrutskaya, A.V., Latour, A.M., Koller, B.H., Leadon, S.A. (1998). BRCA1 required for the transcriptioncoupled repair of oxidative DNA damage. Science, 281, 10091012. Greenblatt, M.S., Bennett, W.P., Hollstein, M., and Harris, C.C. (1994). Mutations in the p53 tumor suppressor gene: Clues to cancer etiology and molecular pathogenesis. Cancer Res., 54, 4855-4878. Greis, K.D., Zhu, S., and Matalon, S. (1996). Identification of nitration sites on surfactant protein A by tandem electrospray mass spectrometry. Arch. Biochem. Biophys., 335, 396-402. Grollman, A.P., and Moriya, M. (1993). Mutagenesis by 8-oxoguanine: an enemy within. Trends Genet., 9, 246-249. Gross, A.J., and Sizer, I.W. (1959). The oxidation of tyramine, tyrosine, and related compounds by peroxidase. J. Biol. Chem., 234, 1611-1614. Grossman, A., and Wendel, A. (1983). Non-reactivity of selenoenzyme glutathione peroxidase with enzymatically hydroperoxidized phospholipids. Eur. J. Biochem., 135, 549-552. Grune, T., and Davies, K.J. (1997). Breakdown of oxidized proteins as a part of secondary antioxidant defenses in mammalian cells. Biofactors. 6, 165-172. Grune, T., Blasig, I.E., Sitte, N., Roloff, B., Haseloff, R., and Davies, K.J. (1998). Peroxynitrite increases the degradation of aconitase and other cellular proteins by proteosome. J. Biol. Chem., 273, 10857-10862. Grune, T., Reinheckel, T., and Davies, K.J. (1997). Degradation of oxidized proteins in mammalian cells. FASEB J., 11, 526-534. Guichardant, M., Taibi-Tronche, P., Fay, L.B., and Lagarde, M. (1998). Covalent modifications of aminophospholipids by 4-hydroxynonenal. Free Radic. Biol. Med., 25, 1049-1056. Guptasarma, P., Balasubramamian, D., Matsugo, S., and Saito, I. (1992). Hydroxyl radical mediated damage to proteins, with special reference to the crystallins. Biochem., 31, 4296-4303. Haas, A.L., and Siepmann, T.J. (1997). Pathways of ubiquitin conjugation. FASEB J., 11, 1257-1268. Haddad, I.Y., Pataki, G., Hu, P., Galliani, C., Beckman, J.S., and Matalon, S. (1994). Quantitation of nitrotyrosine levels in lung sections of patients and animals with acute lung injury. J. Clin. Invest., 94, 2407-2413. Hajjar, D.P., and Haberland, M.E. (1997). Lipoprotein trafficking in vascular cells. Molecular Trojan horses and cellular saboteurs. J. Biol. Chem., 272, 22975-22978. Halliwell, B. (1995). Oxygen radicals, nitric oxide and human inflammatory joint disease. Ann. Rheum. Dis., 54, 505-510. Halliwell, B. (1998). What nitrates tyrosine? Is nitrotyrosine specific as a biomarker of peroxynitrite in vivo? FEBS Lett., 411, 157-160. Halliwell, B., and Aruoma, O.I. (1991). DNA damage by oxygen-derived species. Its mechanism and measurement in mammalian systems. FEBS Lett., 281, 9-19. Halliwell, B., and Chirico, S. (1993). Lipid peroxidation: Its mechanisms, measurement and significance. Am. J. Clin. Nutr., 57, 715S-725S. Halliwell, B., and Gutteridge, J.M.C. (1988). Iron as a biological pro-oxidant. ISI Atlas Sci. Biochem., 1, 48-52. Halliwell, B., and Gutteridge, J.M.C. (Eds.). (1999). Free Radicals in Biology and Medicine. Clarendon Press, Oxford. Han, X., and Liehr, J.G. (1994). 8-Hydroxylation of guanine in kidney and liver DNA of hamsters treated with estradiol: Role of free radicals in estrogen-induced carcinogenesis. Cancer Res., 54, 5515-5517. Hanaoka, T., Tsugane, S., Yamano, Y., Takahashi, T., Kasai, H., Natori, Y., and Watanabe, S. (1993). Quantitative analysis of 8-hydroxyguanine in peripheral blood cells: An application for asbestos patients. Int. Arch. Occup. Environ. Health, 65, S215-S217. Hantraye, P., Brouillet, E., Ferrante, R., Palfi, S., Dolan, R., Matthews, R.T., and Beal, M.F. (1996). Inhibition of neuronal nitric oxide synthase prevents MPTP-induced Parkinsonism in baboons. Nat. Med., 2, 1017-1021. Harman, D. (1989). Lipofuscin and ceroid formation: The cellular recycling system. Adv. Exp. Med. Biol., 266, 3-15. Harris, C.C. (1995). Tumor suppressor genes: At the crossroads of molecular carcinogenesis and molecular epidemiology. In: Molecular Aspects of Carcinogenesis International Meeting. Univ. York, York. U.K. Pp. 22. Harris, C.C., and Holstein, M. (1993). Clinical implications of the p53-tumor suppressor gene. N. Engl. J. Med., 329, 13181327. Harris, M.E., Carney, J.M., and Leedle, R.A. (1994). Detection of oxidation products in individual neurons by fluorescence microscopy. Exper. Neurol., 129, 95-102. Harris, M.E., Hensley, K., Butterfield, D.A., Leedle, R.A., and Carney, J.M. (1995). Direct evidence of oxidative injury produced by the Alzheimer's beta- amyloid peptide (1-40) in cultured hippocampal neurons. Exper. Neurol., 131, 193202. WWW.ESAINC.COM 288 Hattori-Nakakuki, Y., Nishigori, C., Okamoto, K., Imamura, S., Hiai, H., and Toyokuni, S. (1994). Formation of 8-hydroxy2’deoxyguanosine in epidermis of hairless mice exposed to near-UV. Biochem. Biophys. Res. Commun., 201, 11321139. Hayakawa, H., Taketomi, A., Sakumi, K., Kuwano, M., and Sekiguchi, M. (1995). Generation and elimination of 8-oxo-7,8dihydro-2’deoxyguanosine 5’triphosphate, a mutagenic substrate for DNA synthesis in human cells. Biochem., 34, 8995. Hazell, L.J., Arnold, L., Flowers, D., Waeng, G., Malle, E., and Stocker, R. (1996). Presence of hypochlorite-modified proteins in human atherosclerotic lesions. J. Clin. Invest., 97, 1535-1544. Hazen, S.L. (1998). Personal communications. Hazen, S.L., and Heinecke, J.W. (1997). 3-Chorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low-density lipoprotein isolated from human atherosclerotic intima. J. Clin. Invest., 99, 2075-2081. Hazen, S.L., d’Avignon, A., Anderson, M.M., Hsu, F.F., and Heinecke, J.W. (1998a). Human neutrophils employ myeloperoxidase-hydrogen peroxide-chloride system to oxidize alpha-amino acids to a family of reactive aldehydes. Mechanistic studies identifying labile intermediates along the reaction pathway. J. Biol. Chem., 273, 4997-5005. Hazen, S.L., Crowley, J.R., Mueller, D.M., and Heinecke, J.W. (1997). Mass spectrometric quantification of 3chlorotyrosine in human tissues with attomole sensitivity: A sensitive and specific marker for myeloperoxidasecatalyzed chlorination at sites of inflammation. Free Radic. Biol. Med., 23, 909-916. Hazen, S.L., Gaut, J.P., Hsu, F.F., Crowley, J.R., d’Avignon, A., and Heinecke, J.W. (1997). PHydroxyphenylacetaldehyde, the major product of L-tyrosine oxidation by the myeloperoxidase-H2O2-chloride system of phagocytes, covalently modifies ε-amino groups of protein lysine residues. J. Biol. Chem., 272, 16990-16996. Hazen, S.L., Hsu, F.F., d’Avignon, A., and Heinecke, J.W. (1998b). Human neutrophils employ myeloperoxidase to convert alpha-amino acids to a battery of reactive aldehydes: A pathway for aldehyde generation at sites of inflammation. Biochem., 37, 6864-6873. Hazen, S.L., Hsu, F.F., Duffin, K., and Heinecke, J.W. (1996). Molecular-chlorine generated by the myeloperoxidasehydrogen peroxide-chloride system of phagocytes converts low-density lipoprotein cholesterol into a family of chlorinated sterols. J. Biol. Chem., 271, 23080-23088. Hazen, S.L., Hsu, F.F., and Heinecke, J.W. (1996). P-hydroxyphenylacetaldehyde is the major product of L-tyrosine oxidation by activated human phagocytes. J. Biol. Chem., 271, 1861-1867. Hazen, S.L., Hsu, F.F., Mueller, D.M., Crowley, J.R., and Heinecke, J.W. (1996). Human neutrophils employ chlorine gas as an oxidant during phagocytosis. J. Clin. Invest., 98, 1283-1289. Hazra, T.K., Hill, J.W., Izumi, T., and Mitra, S. (2001). Multiple DNA glycosylases for repair of 8-oxoguanine and their potential in vivo functions. Prog. Nucleic Acid Res. Mol. Biol., 68, 193-205. He, Q., Srivastava, S., van Kuijk, F.J., and Ansari, N.H. (1998). Reduction of 4-hydroxynonenal and 4-hydroxyhexenal by retinal aldose reductase. Biochem. Biophys. Res. Commun., 247, 719-722. Heinecke, J.W., Hsu, F.F., Crowley, J.R., Hazen, S.L., Leeuwenburgh, C., Mueller, D.M., Rasmussen, J.E., and Turk, J. (1998). Detecting oxidative modification of biomolecules with isotope dilution mass spectrometry: Sensitive and quantitative assays for oxidized amino acids in proteins and tissues. Meth. Enzymol., 300, 124-144. Heinecke, J.W., Li, W., Daehnke, H.L., and Goldstein, J.A. (1993). Dityrosine, a specific marker of oxidation, is synthesized by the myeloperoxidase-hydrogen peroxide system of human neutrophils and macrophages. J. Biol. Chem., 268, 4069-4077. Helbock, H.J., Beckman, K.B., Shigenaga, M.K., Walter, P.B., Woodall, A.A., Yeo, H.C., and Ames, B.N. (1998). DNA oxidation matters: The HPLC-electrochemical detection assay of 8-oxo-deoxyguanosine and 8-oxo-guanine. Proc. Natl. Acad. Sci. USA, 95, 288-293. Hellberg, C.B., Boggs, S.E., and Lapetina, E.G. (1998). Phosphatidylinositol 3-kinase is a target for protein tyrosine nitration. Biochem. Biophys. Res. Commun., 252, 313-317. Henle, E.S., Luo, Y., Gassmann, W., and Linn, S. (1996). Oxidative damage to DNA constituents by iron-mediated Fenton reactions. The deoxyguanosine family. J. Biol. Chem., 271, 21177-21186. Hensley, K., Hall, N., Subramaniam, R., Cole, P., Harris, M., Askenov, M., Askenova, M., Gabbita, S.P., Wu, J.F., Carney, J.M., Lovell, M., Markesbery, W.R., and Butterfield, D.A. (1995). Brain regional correspondence between Alzheimer's disease histopathology and biomarkers of protein oxidation. J. Neurochem., 65, 2146-2156. Hensley, K., Maidt, M.L., Pye, Q.N., Stewart, C.A., Wack, M., Tabatabaie, T., and Floyd, R.A. (1997). Quantitation of protein bound 3-nitrotyrosine and 3,4-dihydroxyphenylalanine by high-performance liquid chromatography with electrochemical array detection. Anal. Biochem., 251, 187-195. Hensley, K., Maidt, M.L., Yu, Z.Q., Sang, H., Markesbery, W.R., and Floyd, R.A. (1998). Electrochemical analysis of protein nitrotyrosine and dityrosine in the Alzheimer brain indicates region-specific accumulation. J. Neurosci., 18, 8126-8132. Henry, Y.A., and Singel, D. (1996). Metal-nitrosyl interactions in nitric oxide biology probed by electron paramagnetic resonance spectroscopy. In: Methods in Nitric Oxide Research. Feelisch, M., and Stamler, J.S. (Eds.). John Wiley and Sons Ltd. London. Pp. 357-372. Herbert, K.E., and Lunec, J. (1998). Progress in the immunodetection of products of oxidative damage to DNA. In: DNA and Free Radicals: Techniques, Mechanisms and Applications. Aruoma, O.I., and Halliwell, B. (Eds.). OICA, London. Pp. 271-284. Herbert, K.E., Evans, M.D., Finnegan, M.T.V., Farooq, S., Mistry, N., Podmore, I.D., Farmer, P., and Lunec, J. (1996). A novel HPLC procedure for the analysis of 8-oxoguanine in DNA. Free Radic. Biol. Med., 20, 467-473. Herce-Pagliai, C., Kotecha, S., and Shuker, D.E. (1998). Analytical methods for 3-nitrotyrosine as a marker of exposure to reactive nitrogen species: A review. Nitric Oxide, 2, 324-336. Hilt, W., and Wolf, D.H. (1996). Proteasomes: Destruction as a program. Trends Biochem. Sci., 21, 96-102. Hochstrasser, M. (1996). Ubiquitin-dependent protein degradation. Ann Rev. Genet., 30, 405-439. Hofer, T., and Moller, L. (1998). Reduction of oxidation during the preparation of DNA and analysis of 8-hydroxy-2’deoxyguanoisne. Chem. Res. Toxicol. In press. WWW.ESAINC.COM 289 Holmberg, I., Stal, P., and Hamberg, M. (1999). Quantitative determination of 8-hydroxy-2’deoxyguanosine in human urine by isotope dilution mass spectrometry: Normal levels in hemochromatosis. Free Radic. Biol. Med., 26, 129-135. Holmes, G.E., Bernstein, C., and Bernstein, H. (1992). Oxidative and other DNA damages as the basis of aging: A review. Mutat. Res., 275, 305-315. Holstein, M., Didransky, D., Vogelstein, B., and Harris, C.C. (1991). p53 mutations in human cancer. Science, 253, 49-52. Holley, A.E., and Slater, T.F. (1991). Measurement of lipid hydroperoxides in normal human blood plasma using HPLCchemiluminescence linked to a diode array detector for measuring conjugated dienes. Free Radic. Res. Comm., 15, 5163. Holley, A.E., Walker, M.K., Cheeseman, K.H., and Slater, T.F. (1993). Measurement of n-alkanals and hydroxyalkanals in biological samples. Free Radic. Biol. Med., 15, 281-289. Hsei, A.W., Recio, L., Karz, K.S., Lee, C.Q., Wagner, M., and Schenley, R.L. (1986). Evidence for reactive oxygen species inducing mutations in mammalian cells. Proc. Natl. Acad. Sci. USA, 83, 9616-9620. Huggins, T.G., Wells-Knecht, M.C., Detoria, N.A., Baynes, J.W., and Thorpe, S.R. (1993). Formation of o-tyrosine and dityrosine in proteins during radiolytic and metal-catalyzed oxidation. J. Biol. Chem., 268, 12341-12347. Huie, R.E., and Neta, P. (1999). Chemistry of reactive oxygen species. In: Reactive Oxygen Species in Biological Systems. Gilbert, D.L., and Colton, C.A. (Eds.). Kluwer Academic/Plenum Publishers, New York. Pp. 33-73. Hunt, J.V., Dean, R.T., and Wolff, S.P. (1988). Hydroxyl radical production and autoxidative glycosylation. Glucose autoxidation as the cause of protein damage in the experimental glycation model of diabetes mellitus and aging. Biochem. J., 256, 205-212. Hunter, M.I.S., and Mohamed, J.B. (1986). Plasma antioxidants and lipid peroxidation products in duchene muscular dystrophy. Clin. Chim. Acta, 155, 122-132. Husgafvel-Pursiainen, K., Ridanpaa, M., Anttila, S., and Vainio, H. (1995). p53 and ras gene mutations in lung cancer: Implications for smoking and occupational exposures. J. Occup. Environ. Med., 37, 69-76. Ide, H. (2001). DNA substrates containing defined oxidative base lesions and their application to study substrate specificities of base excision repair enzymes. Prog. Nucleic Acid Res. Mol. Biol. 68, 207-221. Ide, H., Tedzuka, K., Shimzu, Kimura, Y., Purmal, A.A., Wallace, S.S., and Kow, Y.W. (1994). Alpha-deoxyadenosine, a major anoxic radiolysis product of adenine in DNA, is a substrate for E. coli endonuclease IV. Biochem., 33, 7842-7847. Imbusch, R., and Mueller, M.J. (2000). Formation of isoprostanes F(2)-like compounds (phytoprostanes (1)) from alphalinolenic acid in plants. Free Radic. Biol. Med., 28, 720-726. Inoue, T., Mu, Z., Sumikawa, K., Adachi, K., and Okochi, T. (1993). Effect of physical exercise on the content of 8hydroxydeoxyguanosine in nuclear DNA prepared from human lymphocytes. Jpn. J. Cancer Res., 84, 720-725. Ischiropoulos, H., and Al-Mehdi, A. B. (1995). Peroxynitrite-mediated oxidative protein modifications. FEBS Letts., 364, 279-282. Ischiropoulos, H. (2003). Oxidative modifications of alpha-synuclein. Ann. N. Y. Acad. Sci., 991, 93-100. Ischiropoulos, H., al-Mehdi, A.B., and Fisher, A.B. (1995). Reactive species in ischemic rat lung injury: Contribution of peroxynitrite. Am. J. Physiol., 269, L158-164. Ischiropoulos, H., Beers, M.F., Ohnishi, S.T., Fisher, D., Garner, S.E., and Thom, S.R. (1996). Nitric oxide production and perivascular nitration in brain after carbon monoxide poisoning in rats. J. Clin. Invest., 97, 2260-2267. Ischiropoulos, H., Duran, D., and Horwitz, J. (1995). Peroxynitrite-mediated inhibition of L-DOPA synthesis in PC12 cells. J. Neurochem., 65, 2366-2372. Ishida, H., Makino, A., and Mae, T. (1999). Fragmentation of the large subunit of ribulose-1,5-bisphosphate carboxylase by reactive oxygen species occurs near Gly-329. J. Biol. Chem., 274, 5222-5226. Ishimitsu, S., Fujimoto, S., and Ohara, A. (1986). Determination of m-tyrosine and o-tyrosine in human serum by highperformance liquid chromatography with fluorometric detection. J. Chromatogr., 378, 222-225. Ishiyama, S., Hiroe, M., Nishikawa, T., Abe, S., Shimojo, T., Ito, H., Ozasa, S., Yamakawa, K., Matsuzaki, M., Mohammed, M.U., Nakazawa, H., Kasajima, T., and Marumo, F. (1997). Nitric oxide contributes to the progression of myocardial damage in experimental autoimmune myocarditis in rats. Circulat., 95, 489-496. Iwaoka, T., Tabata, F., and Takahashi, T. (1987). Lipid peroxide detected by chemiluminescence. Free Radic. Biol. Med., 3, 329-333. Jacob, J.S., Cistola, D.P., Hsu, F.F., Muzaffar, S., Mueller, D.M., Hazen, S.L., and Heinecke, J.W. (1996). Human phagocytes employ the myeloperoxidase-hydrogen peroxide system to synthesize dityrosine, trityrosine, pulcherosine, and isodityrosine by a tyrosyl radical-dependent pathway. J. Biol. Chem., 271, 19950-19956. Jajoo, H.K., Burcham, P.C., Goda, Y., Blair, I.A., and Marnett, L. A thermospray liquid chromatography/mass spectrometry method for analysis of human urine for the major malondialdehyde-guanine adduct. J. Chem. Res. Toxicol., 5, 870-875. Jaruga, P., Zastawny, T.H., Skokoswki, J., Dizdaroglu, M., and Olinski, R. (1994). Oxidative DNA base damage and antioxidant enzyme activities in human lung cancer. FEBS Lett., 341, 59-64. Jones, A.F., and Lunec, J. (1987). Protein fluorescence and its relationship to free radical activity. Br. J. Cancer, 8, 60-65. Joseph, J.A., Villalobos-Molinas, R., Denisova, N.A., Erat, S., and Strain, J. (1996). Cholesterol: A two-edged sword in brain aging. Free Radic. Biol. Med., 22, 455-462. Juedes, M.J., and Wogan, G.N. (1996). Peroxynitrite-induced mutation spectra of pSP189 following replication of bacteria and in human cells. Mutat. Res., 349, 51-61. Kamisaki, Y., Wada, K., Bian, K., Balabanli, B., Davis, K., Martin, E., Behbod, F., Lee, Y.C., and Murad, F. (1998). An activity in rat tissues that modifies nitrotyrosine-containing proteins. Proc. Natl. Acad. Sci. USA, 95, 11584-11589. Kamisaki, Y., Wada, K., Nakamoto, K., Kishimoto, Y., Kitano, M., and Itoh, T. (1996). Sensitive determination of nitrotyrosine in human plasma by isocratic high-performance liquid chromatography. J. Chromatogr. B., 685, 343-347. Kamiya, H., Miura, K., Ishikawa, H., Nishimura, S., and Ohtsuka, E. (1992). c-Ha-ras containing 8-hydroxyguanine at codon 12 induces point mutations at the modified and adjacent positions. Cancer Res., 52, 3483-3485. Kang, H., Konishi, C., Eberle, G., Rajewsky, M.E., Kuroki, T., and Huh, N-H. (1992). Highly sensitive, specific detection of 6 4 4 O -methylguanine, O -methylthymine, and O -ethylthymine by the combination of high-performance liquid 32 chromatography prefractionation, P postlabeling, and immunoprecipitation. Cancer Res., 52, 5307-5312. WWW.ESAINC.COM 290 Karakaya, A., Jaruga, P., Bohr, V., Grollman, A.P., and Dizdaroglu, M. (1997). Kinetics of excision of purine lesions from DNA by Escherichia coli Fpg protein. Nucl. Acid Res., 25, 474-479. Kasai, H. (1997). Analysis of a form of oxidative DNA damage, 8-hydroxy-2’-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutat. Res., 387, 147-163. Kato, Y., Maruyama, W., Naoi, M., Hashizume, Y., and Osawa, T. (1998). Immunohistochemical detection of dityrosine in lipofuscin pigments in the aged human brain. FEBS Lett., 439, 231-234. Kaur, H., and Halliwell, B. (1994). Evidence for nitric oxide-mediated oxidative damage in chronic inflammation. Nitrotyrosine in serum and synovial fluid from rheumatoid patients. FEBS Lett., 350, 9-12. Kaur, H., and Halliwell, B. (1996). Measurement of oxidized and methylated DNA bases by HPLC with electrochemical detection. Biochem. J., 318, 21-23. Kaur, H., Lyras, L., Jenner, P., and Halliwell, B. (1998). Artifacts in HPLC detection of 3-nitrotyrosine in brain tissue. J. Neurochem., 70, 2220-2223. Keith, G., and Dirheimer, G. (1995). Postlabeling: A sensitive method for studying DNA adducts and their role in carcinogenesis. Curr. Opin. Biotechnol., 6, 3-11. Kettle, A.J. (1996). Neutrophils convert tyrosyl residues in albumin to chlorotyrosine. FEBS Lett., 379, 103-106. Kettle, A.J. (1998). Detection of 3-chlorotyrosine in proteins exposed to neutrophil oxidants. Meth. Enzymol., 300, 111120. Kikuchi, A., Takeda, A., Onodera, H., Kimpara, T., Hisanaga, K., Nobuyuki, S., Nunomura, A., Castellani, R.J., Perry, G., Smith, M.A., and Itoyama, Y. (2002). Systemic increase of oxidative nucleic acid damage in Parkinson’s disease and multiple system atrophy. Neurobiol. Dis., 9, 244-248. Kikuchi, S., Shinpo, K, Takeuchi, M., Yamagishi, S., Makita, Z., Sasaki, N., and Tashiro, K. (2003). Glycation – a sweet tempter for neuronal death. Brain Res. Brain Res. Rev., 41, 306-323. Kikugawa, K., and Beppu, M. (1987), Involvement of lipid oxidation products in the formation of fluorescent and crosslinked proteins. Chem. Phys. Lip., 44, 277-296. Kimura, H., Hokari, R., Miura, S., Shigematsu, T., Hirokawa, M., Akiba, Y., Kurose, I., Higuchi, H., Fujimori, H., Tsuzuki, Y., Serizawa, H., and Ishii, H. (1998). Increased expression of an inducible isoform of nitric oxide synthase and the formation of peroxynitrite in colonic mucosa of patients with ulcerative colitis. Gut, 42, 180-187. Kiyosawa, H., Suko, M., Okudaira, H., Murata, K., Miyamoto, T., Chung, M.H., Kasai, H., and Nishimura, S. (1990). Cigarette smoking induces formation of 8-hydroxydeoxyguanosine, one of the oxidative DNA damages in human peripheral leukocytes. Free Radic. Res. Commun., 11, 23-27. Klebl, B.M., Ayoub, A.T., and Pette, D. (1998). Protein oxidation, tyrosine nitration and inactivation of sarcoplasmic 2+ reticulum Ca -ATPase in low-frequency stimulated rabbit muscle. FEBS Lett., 422, 381-384. Kooy, N.W., Lewis, S.J., Royall, J.A., Ye, Y.Z., Kelly, D.R., and Beckman, J.S. (1997). Extensive tyrosine nitration in human myocardial inflammation: Evidence for the presence of peroxynitrite. Crit. Care Med., 25, 812-819. Kooy, N.W., Royall, J.A., Ye, Y.Z., Kelly, D.R., and Beckman, J.S. (1995). Evidence for in vivo peroxynitrite production in human acute lung injury. Am. J. Respir. Crit. Care Med., 151, 1250-1254. Korchazhkina, O., Exley, C., and Spncer, S.A. (2003). Measurement of reversed-phase high-performance liquid chromatography of malondialdehyde in normal human urine following derivatization with 2,4-dinitrophenylhydrazine. J. Chromatogr. B, 794, 353-362. Korytowski, W., Bachowski, G., and Girotti. A.W. (1991). Chromatographic separation and electrochemical determination of cholesterol hydroperoxides generated by photodynamic action. Anal. Biochem., 197, 149-156. Korytowski, W., Geiger, P.G., and Girotti, A.W. (1995). High-performance liquid chromatography with mercury cathode electrochemical detection: Application to lipid hydroperoxide analysis. J. Chromatogr. B., 670, 189-197. Korytowski, W., Geiger, P.G., and Girotti, A.W. (1999). Lipid hydroperoxide analysis by high-performance liquid chromatography with mercury cathode electrochemical detection. Meths. Enzymol., 300, 23-33. Kozumbo, W.J., Agarwal, S., and Koren, H.S. (1992). Breakage and binding of DNA by reaction products of hypochlorous acid with aniline, 1-naphthylamine, or 1-naphthol. Toxicol. Appl. Pharmacol., 115, 107-115. Kreuzer, T., Grube, R., Wutte, A., Zarkovic, N., and Schaur, R.J. (1998). 4-Hydroxynonenal modifies the effects of serum growth factors on the expression of the c-fos proto-oncogene and the proliferation of HELA carcinoma cells. Free Radic. Biol. Med., 25, 42-49. Kristal, B.S., Park, B.K., and Yu, B.P. (1996). 4-Hydroxynonenal is a potent inducer of the mitochondrial permeability transition. 271, 6033-6038. Kristof, A.S., Goldberg, P., Laubach, V., and Hussain, S.N.A. (1998). Role of inducible nitric oxide synthase in endotoxininduced acute lung injury. Am. J. Respir. Crit. Care Med., 158, 1883-1889. Kuhn, D.M., Aretha, C.W., and Geddes, T.J. (1999). Peroxynitrite inactivation of tyrosine hydroxylase: Mediation by sulfhydryl oxidation, not tyrosine nitration. J. Neurosci., 19, 10289-10294. Kunkel, T.A. (1993). Nucleotide repeats. Slippery DNA and diseases. Nature, 365, 207-208. Kuo, W.N., Kanadia, R.N., and Shanbhag, V.P. (1999). Denitration of peroxynitrite-treated proteins by “protein nitrases” from dog prostate. Biochem. Mol. Biol. Int., 47, 1061-1067. Kvasnicova, V., Samcova, E., Jursova, A., and Jelinek, I. (2003). Determination of 8-hydroxy-2’-deoxyguanosine in untreated urine by capillary electrophoresis with UV detection. J. Chromatogr. A, 985, 513-517. Kwon, N.S., Chan, P.C., and Kesner, L. (1990). Inactivation of alpha 1-proteinase inhibitor by Cu(II) and hydrogen peroxide. Agents Actions, 29, 388-393. Lands, W.E.M., Kulmacz, R.J., and Marshall, P.J. (1994). Lipid peroxide actions in the regulation of prostaglandin synthesis. In: Free Radicals in Biology. Volume 6. Pryor, W.A. (Ed.). Academic Press, New York. Pp. 31-61. Lagorio, S., Tagesson, C., Forastiere F., Axelson, O., and Carere, A. (1994). Exposure to benzene and urinary concentrations of 8-hydroxydeoxyguanosine, a biological marker of oxidative damage to DNA. Occup. Environ. Med., 51, 739-743. Lang, J., Celetto, C., and Esterbauer, H. (1985). Quantitative determination of the lipid peroxidation product 4hydroxynonenal by high-performance liquid chromatography. Anal. Biochem., 150, 369-378. WWW.ESAINC.COM 291 Ledwozyw, A., Michalak, J., Stephen, A., and Kadziolka, A. (1986). The relationship between plasma triglycerides, cholesterol, total lipid and lipid peroxidation products during human atherosclerosis. Clin. Chim. Acta, 155, 275-284. Lee, D.M. (1980). Malondialdehyde formation in stored plasma. Biochem. Biophys. Res. Commun., 95, 1633-1672. Lee, S.W., and Wei, J.Y. (1997). Molecular interactions of aging and cancer. Clin. Geriatr. Med., 13, 69-77. Leeuwenburgh, C., Hansen, P., Holloszy, J.O., and Heinecke, J.W. (1999). Hydroxyl radical generation during exercise increases mitochondrial protein oxidation and levels of urinary dityrosine. Free Radic. Biol. Med., 27, 186-192. Leeuwenburgh, C., Hansen, P., Shaish, A., Holloszy, J.O., and Heinecke, J.W. (1998). Markers of protein oxidation by hydroxyl radical and reactive nitrogen species in tissues of aging rats. Am. J. Physiol., 274, R453-461. Leeuwenburgh, C., Hardy, M.M., Hazen, S.L., Wagner, P., Oh-ishi, S., Steinbrecher, U.P., and Heinicke, J.W. (1997). Reactive nitrogen intermediates promote low-density lipoprotein oxidation in human atherosclerotic intima. J. Biol. Chem., 272, 1433-1436. Leeuwenburgh, C., Hansen, P.A., Holloszy, J.O., and Heinecke, J.W. (1999). Oxidized amino acids in the urine of aging rats: Potential markers for assessing oxidative stress in vivo. Am. J. Physiol., 276, R128-135. Leeuwenburgh, C., Rasmussen, J.E., Hsu, F.F., Mueller, D.M., Pennathur, S., and Heinecke, J.W. (1997a). Mass spectrometric quantification of markers for protein oxidation by tyrosyl radical, copper, and hydroxyl radical in low density lipoprotein isolated from human atherosclerotic plaques. J. Biol. Chem., 272, 3520-3526. Leeuwenburgh, C., Wagner, P., Holloszy, J.O., Sohal, R.S., and Heinecke, J.W. (1997b). Caloric restriction attenuates dityrosine cross-linking of cardiac and skeletal muscle proteins in aging mice. Arch. Biochem. Biophys., 346, 74-80. Lengger, C., Schoch, G., and Topp, H. (2000). A high-performance liquid chromatographic method for the determination of 8-oxo-7,8-2’-deocyguanosine in urine from man and rat. (2000). Anal. Biochem., 287, 65-72. Lepoivre, M., Flaman, J.M., Bobe, P., Lemaire, G., and Henry, Y. (1994). Quenching of the tyrosyl free radical of ribonucleotide reductase by nitric oxide. Relationship to cytostasis induced in tumor cells by cytotoxic macrophages. J. Biol. Chem., 269, 21891-21897. Lappa, S., and Sistonen, L. (1997). Heat shock response – pathophysiological implications. Annals Med., 29, 73-79. Lesko, S.A., Lorentzen, R.J., and Ts’o P.O.P. (1980). The role of superoxide in deoxyribonucleic acid strand scission. Biochem., 19, 3023-3028. Levine, R.L., Mosoni, L., Berlett, B.S., and Stadtman, E.R. (1996). Methionine residues as endogenous antioxidants in proteins. Proc. Natl. Acad. Sci. USA, 93, 15036-15040. Levine, R.L., Williams, J.A., Stadtman, E.R., and Shacter, E. (1994). Carbonyl assays for determination of oxidatively modified proteins. Meth. Enzymol., 233, 346-357. Lewisch, S.A., and Levine, R.L. (1995). Determination of 2-oxohistidine by amino acid analysis. Anal. Biochem., 231, 440446. Lewisch, S.A., and Levine, R.L. (1998). Determination of 2-oxohistidine by amino acid analysis. Meths. Enzymol., 300, 120-124. Li, D.H., Wang, M.Y., Dhingra, K., and Hittelman, W.N. (1995a). Discovery of a 4-hydroxynonenal-induced DNA adduct in 32 human breast tissues by P-postlabeling assay. Proc. Ann. Meet. Am. Assoc. Cancer Res., 36, A826. Li, D.H., Wang, M., Liehr, J.G., and Randerath, K. (1995b). DNA adducts induced by lipids and lipid peroxidation products: Possible relationship to I-compounds. Mut. Res., 344, 117-126. Li, S., Schoneich, C., and Borchardt, R.T. (1995c). Chemical pathways of peptide degradation. VIII. Oxidation of methionine in small model peptides by prooxidant/transition metal ion systems: Influence of selective scavengers for reactive oxygen intermediates. Pharmacol. Res., 12, 348-355. Li, S., Nguyen, T.H., Schoneich, C., and Borchardt, R.T. (1995d). Aggregation and precipitation of human relaxin induced by metal-catalyzed oxidation. Biochem., 34, 5762-5772. Lieber, M.R., Grawunder, U., Wu, X., and Yaneva, M. (1997). Tying loose ends: roles of Ku and DNA-dependent protein kinase in the repair of double-strand breaks. Curr. Opin. Genet. Dev., 7, 99-104. Liehr, J.G. (1990). Genotoxic effects of estrogens. Mutat. Res., 238, 269-276. Liehr, J.G. (1994). Catechol estrogens as mediators of estrogen-induced carcinogenesis. Proc. Ann. Meet. Am. Assoc. Cancer Res., 35, 704. Lim, D.G., Sweeney, S., Bloodsworth, A., White, C.R., Chumley, P.H., Krishna, N.R., Schopfer, F., O’Donnell, V.B., Eiserich, J.P., and Freeman, B.A. (2002). Proc. Natl. Acad. Sci. USA, 99, 15941-15946. Lin, H.L., Kent, U.M., Zhang, H., Waskell, L., and Hollenberg, P.F. (2003). Mutation of tyrosine 190 to alanine eliminates the inactivation of cytochrome P450 2B1 by peroxynitrite. Chem. Res. Tox., 16, 129-136. Lin, J.K., Chen, K.J., Liu, G.Y., Chu, Y.R., and Lin-Shiau, S.Y. (2000). Nitration and hydroxylation of aromatic amino acid and guanine by the air pollutant peroxyacetyl nitrate. Chem. Biol. Interact., 127, 219-236. Lindahl, T. (1993). Instability and decay of the primary structure of DNA [see comments]. Nature, 362, 709-715. Liochev, S.I., and Fridovich, I. (1994). The role of O2• in the production of HO•: In vitro and in vivo. Free Radic. Biol. Med., 16, 29-33. Liu, H., Duda, C.T., Huang, T., Aruda, W., and Kissinger, P.T. (1998a). Optimization of post column photolysis/electrochemistry for liquid chromatographic determination of 3-nitro-L-tyrosine. J. Chromatogr. A, 818, 69-75. Liu, H., Huang, T., Kissinger, C.B., and Kissinger, P.T. (1998b). Comparison of detection methods for liquid chromatographic determination of 3-nitro-L-tyrosine. J. Chromatogr. B, 713, 289-295. Liu, R.H., and Hotchkiss, J.H. (1995). Potential genotoxicity of chronically elevated nitric oxide: A review. Mutat. Res., 339, 73-89. Lloyd, D.R., and Phillips, D.H. (1999). Oxidative DNA damage mediated by copper (II), iron (II) and nickel (II) Fenton reactions: Evidence for site-specific mechanisms in the formation of double-strand breaks, 8-hydroxydeoxyguanosine and putative intrastrand cross-links. Mutat. Res., 424, 23-36. Lodovici, M., Casalini, C., Cariaggi, R., Michelucci, L., and Dolara, P. (2000). Levels of 8-hydroxydeoxyguanosine as a marker of DNA damage in human leukocytes. Free Radic. Biol. Med., 28, 13-17. Loeb, L.A., and Preston, B.D. (1986). Mutagenesis by apurinic/apyrimidinic sites. Ann. Rev. Genet., 20, 201-230. Loft, S., and Poulsen, H.E. (1996). Cancer risk and oxidative DNA damage in man. J. Mol. Med., 74, 297-312. WWW.ESAINC.COM 292 Loft, S., and Poulsen, H.E. (1998). Markers of oxidative damage to DNA: Antioxidants and molecular damage. Meth. Enzymol., 300, 166-184.. Loft, S., Astrup, A., Buemann, B., and Poulsen, H.E. (1994). Oxidative DNA damage correlates with oxygen consumption in humans. FASEB J., 8, 534-537. Loft, S., Larsen, P.N., Rasmussen, A., Fischer-Nielsen, A., Bondesen, S., Kirkegaard, P., Rasmussen, L.S., Ejlersen, E., Tornoe, K., Bergholdt, R., and Poulsen, H.E. (1995). Oxidative DNA damage after transplantation of the liver and small intestine in pigs. Transplant., 59, 16-20. Loft, S., Vistisen, K., Ewertz, M., Tjonneland, A., Overvad, K., and Poulsen, H.E. (1992). Oxidative DNA-damage estimated by 8-hydroxydeoxyguanosine excretion in man: Influence of smoking, gender and body mass index. Carcinogen., 13, 2241-2247. Loft, S., Wierik, E.J.M.V., van der Berg, H., and Poulsen, H.E. (1995). Energy restriction and oxidative DNA damage. Cancer Epid. Biomarkers Prev., 4, 515-519. Lopez, M.F. (1997). Two-dimensional electrophoresis and mass spectrometry: Deciphering the proteome. Am. Biotech. Lab., May. Lopez, M.F. (1998a). Proteomic databases: Roadmaps for drug discovery. Am. Biotech. Lab., Accepted for publication. Lopez, M.F. (1998b). Gene products are where the biological action is. J. Chrom. B., Accepted for 1998 publication. Lovell, M.A., Gabbita, S.P., and Markesbery, W.R. (1999). Increased DNA oxidation and decreased levels of repair products in Alzheimer’s disease ventricular CSF. J. Neurochem., 72, 771-776. Lui, L., and Wells, P.G. (1995). DNA oxidation as a potential molecular mechanism mediating drug-induced birth defects: Phenytoin and structurally related teratogenesis initiate the formation of 8-hydroxy-2-’deoxyguanosine in vitro and in vivo in murine maternal hepatic and embryonic tissues. Free Radic. Biol. Med., 19, 639-648. Lunec, J., Herbert, K., Blount, S., Griffiths, H.R., and Emery, P. (1994). 8-Hydroxydeoxyguanosine: A marker of oxidative DNA damage in systemic lupus erythematosis. FEBS Lett., 348, 131-138. Luo, Y., Henle, E.S., and Linn, S. (1996). Oxidative damage to DNA by iron-mediated Fenton reactions. The deoxycytidine family. (1996). J. Biol. Chem., 271, 21167-21176. Lymar, S.V., Jiang, Q., and Hurst, J.K. (1996). Mechanism of carbon dioxide-catalyzed oxidation of tyrosine by peroxynitrite. Biochem., 35, 7855-7861. Lyras, L., Cairns, N.J., Jenner, A., Jenner, P., and Halliwell, B. (1997). An assessment of oxidative damage to proteins, lipids, and DNA in brain from patients with Alzheimer’s disease. J. Neurochem., 68, 2061-2069. Lyras, L., Evans, P.J., Shaw, P.J., Ince, P.G., and Halliwell, B. (1996). Oxidative damage and motor neuron disease. Difficulties in the measurement of protein carbonyls in human brain tissue. Free Radic. Res. Comm., 24, 397-406. Lyras, L., Perry, R.H., Perry, E.K., Ince, P.G., Jenner, A., Jenner, P., and Halliwell, B. (1998). Oxidative damage to proteins, lipids and DNA in cortical brain regions from patients with dementia with Lewy bodies. J. Neurochem., 71, 302-312. Maccabee, M., Evans, J.S., Glackin, M., Hatahet, Z., and Wallace, S.S. (1994). Pyrimidine ring fragmentation products. Effects of lesion structure and sequence context on mutagenesis. J. Mol. Biol., 236, 514-530. MacMillan-Crow, L.A., and Cruthirds, D.L. (2001). Invited Review: Manganese superoxide dismutase in disease. Free Radic. Rev., 34, 325-336. MacMillan-Crow, L.A., and Thompson, J.A. (1999). Immunoprecipitation of nitrotyrosine-containing proteins. Meth. Enzymol., 301, 135-144. MacMillan-Crow, L.A., and Thompson, J.A. (1999b). Tyrosine modifications and inactivation of active site manganese superoxide dismutase mutant (Y34F) by peroxynitrite. Arch. Biochem. Biophys., 366, 82-88. MacMillan-Crow, L.A., Crow, J.P., Kerby, J.D., Beckman, J.S., and Thompson, J.A. (1996). Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc. Natl. Acad. Sci. USA, 93, 1185311858. MacMillan-Crow, L.A., Crow, J.P., and Thompson, J.A. (1998). Peroxynitrite-mediated inactivation of manganese superoxide dismutase involves nitration and oxidation of critical tyrosine residues. Biochem., 37, 1613-1622. Maier, K.L., Lenz, A.G., Beck-Speier, I., and Costabel, U. (1995). Analysis of methionine sulfoxide in proteins. Meths. Enzymol., 251, 455-461. Makinen, M., Piironen, V., and Hopia, A. (1996). Postcolumn chemiluminescence, ultraviolet and evaporative lightscattering detectors in high-performance liquid chromatographic determination of triacylglycerol oxidation products. J. Chromatogr., 734, 221-229. Malencik, D.A., Sprouse, J.F., Swanson, C.A., and Anderson, S.R. (1996). Dityrosine: Preparation, isolation and analysis. Anal. Biochem., 242, 202-213. Mani, A.R., Pannala, A.S., Orie, N.N., Harry, D., Rice-Evans, C.A., and Moore, K.P. (2003). Nitration of endogenous parahydroxyphenylacetic acid and the metabolism of nitrotyrosine. Biochem. J., 374, 521-527. Mark, R.J., Lovell, M.A., Markesbery, W.R., Uchida, K., and Mattson, M.P. (1997). A role for 4-hydroxynonenal, an aldehydic product of lipid peroxidation, in disruption of ion homeostasis and neuronal death induced by amyloid βpeptide. J. Neurochem., 68, 255-264. Marnett, L.J. (1985). Arachidonic acid metabolism and tumor initiation. Prostagland. Leukotri. Cancer, 2, 39-82. Marnett, L.J. (1996). Identification, detection, and genetic effects of DNA adducts of endogenous aldehydes. Proc. Ann. Meet. Am. Assoc. Cancer Res., 37, 646. Marquez, L.A., and Dunford, H.B. (1995). Kinetics of oxidation of tyrosine and dityrosine by myeloperoxidase I and II. J. Biol. Chem., 270, 30434-30440. Maruyama, W., Hashizume, Y., Matsubara, K., and Naoi, M. (1996). Identification of 3-nitro-L-tyrosine, a product of nitric oxide and superoxide, as an indicator of oxidative stress in the human brain. J. Chromatogr. B., 676, 153-158. Marvin, L.F., Delatour, T., Tavvazi, I., Fay, L.B., Cupp, C., and Guy, P.A. (2003). Quantification of dityrosine, onitrotyrosine and 0-tyrosine in cat urine by LC-MS/MS. Anal. Chem., 75, 261-267. Maseki, M., Nishigaki, I., Hagihara, M., Tomoda, Y., and Yagi, K. (1981). Lipid peroxide levels and lipid content of serum lipoprotein fractions of pregnant subjects with or without pre-eclampsia. Clin. Chim. Acta, 115, 155-161. WWW.ESAINC.COM 293 Maskos, Z., Rush, J.D., and Koppenol, W.H. (1992). The hydroxylation of phenylalanine and tyrosine: A comparison with salicylate and tryptophan. Arch. Biochem. Biophys., 296, 521-529. Masuda, M., Suzuki, T., Friesen, M.D>, Ravanat, J.L., Cadet, J., Pignatelli, B., Nishino. H., and Ohshima, H. (2001). Chlorination of guanosine and other nucleosides by hypochlorous acid and myeloperoxidase of activated human neutrophils. Catalysis by nicotine and trimethylamine. J. Biol. Chem., 276, 40486-40496. Matson, W.R. (1998). Personal communications. Matthews, R.T., and Beal, M.F. (1996). Increased 3-nitrotyrosine in brains of apo E-deficient mice. Brain. Res., 718, 181184. McCabe, D.R., Acworth, I.N., Maidt, M.L., and Floyd, R.A. (1997). A sensitive and selective method for the determination th of tissue 8-hydroxy-2’deoxyguanosine using HPLC with electrochemical array detection. Presented at The 88 Annual Meeting of The American Association for Cancer Research. San Diego CA. McCarter, R.J. (1995). Role of caloric restriction in the prolongation of life. Clin. Geriatr. Med., 11, 553-565. McCarter, R.J., and McGee, J.R. (1989). Transient reduction of metabolic rate by food restriction. Am. J. Physiol., 257, E175-179. McKelvey-Martin, V.J., Green, M.H., Schmezer, P., Pool-Zobel, B.L., and De Meo, M.P. (1993). The single cell gelelectrophoresis assay (comet assay): A European review. Mutat. Res., 288, 47-63. Mecocci, P., MacGarvey, U., Kaufman, A.F., Koontz, D., Shoffner, J.M., Wallace, D.C., and Beal, M.F. (1993). Oxidative damage to mitochondrial DNA shows marked age-dependent increases in human brain. Ann. Neurol., 34, 609-616. Mehl, M., Daiber, A., Herold, S., Shoun, H., and Ullrich, V. (1999). Nitric Oxide, 3, 142-152. Mei, N., Tamae, K., Kunugita, N., Hirano, T., and Kasai, H. (2003). Analysis of 8-hydroxydeoxyguanosine 5’monophosphate (8-OH-dGMP) as a reliable marker of cellular oxidative DNA damage after γ-irradiation. Env. Mol. Mutagen., 41, 332-338. Mei, S.R., Yao, Q.H., Cai, L.S., Xing, J., Xu, G.W., Wu, C.Y. (2003). Capillary electrophoresis with end-column amperometric detection of urinary 8-hydroxy-2’-deoxyguanosine. Electrophoresis, 24, 1411-1415. Meneghini, R. (1988). Genotoxicity of active oxygen species in mammalian cells. Mutat. Res., 195, 215-230. Menon, C. (1995). Ionizing radiation induced DNA hydroperoxide damage and its repair. Diss. Abstr. Int., 56, 1291-1295. Michaels, M.L., Tchou, J., Grollman, A.P., and Miller, J.H. (1992). A repair system for 8-oxo-7,8-dihydrodeoxyguanine. Biochem., 31, 10964-10968. Michon, T., Chenu, M., Kellershon, N., Desmadril, M., and Gueguen, J. (1997). Horseradish peroxidase oxidation of tyrosine-containing peptides and their subsequent polymerization: A kinetic study. Biochem., 36, 8504-8513. Miller, M.J., Thompson, J.H., Zhang, X.J., Sadowska-Krowicka, H., Kakkis, J.L., Munshi, U.K., Sandoval, M., Rossi, J.L., Eloby-Childress, S., and Beckman, J.S. (1995). Role of inducible nitric oxide synthase expression and peroxynitrite formation in guinea pig ileitis. Gastroenterol., 109, 1475-1483. Minotti, G., and Aust, S.D. (1989). The role of iron in oxygen radical mediated lipid peroxidation. Chem. Biol. Interact., 71, 1-19. Minotti, G., and Aust, S.D. (1992). Redox cycling and lipid peroxidation. Lipids, 27, 219-226. Miyake, E., Eto, H., Takechi, Y., Kamidono, S., and Hara, I. (2003). Increased urinary 8-hydroxy-2’-deoxyguanosine excretion after ileal neobaldder replacement. BJU Int., 91, 657-660. Miyazawa, T., Yasuda, K., Fujimoto, K., and Kaneda, T. (1988). Presence of phosphatidylcholine hydroperoxide in human plasma. J. Biochem., 103, 744-749. Mo, J.Y., Maki, H., and Sekiguchi, M. (1992). Hydrolytic elimination of a mutagenic nucleotide, 8-oxodGTP by human 18kilodalton protein: Sanitization of nucleotide pool. Proc. Natl. Acad. Sci. USA, 89, 11021-11025. Mobley, J.A., Bhat, A.S., and Brueggemeier, R.W. (1999). Measurement of oxidative DNA damage by catechol estrogens and analogues in vitro. Chem. Res. Toxicol., 12, 270-277. Mohr, S., Stamler, J.S., and Brune, B. (1994). Mechanism of covalent modification of glyceraldehyde-3-phosphate dehydrogenase at its active site thiol by nitric oxide, peroxynitrite and related nitrosating agents. FEBS Lett., 348, 223227. Monnier, V.M., Sell, D.R., Saxena, A., Saxena, M., Subramaniam, R., Tessier, F., and Weiss, M. F. (2003). Glycoxidative And Carbonyl Stress In Aging And Age-Related Diseases. In: Critical Reviews of Oxidative Stress and Aging. Volume 1.Cutler, R.G., and Rodriguez, H. (Eds.). World Scientific, NJ. Pp. 413-433. Moody, C.S., and Hassan, H.M. (1982). Mutagenicity of oxygen free radicals. Proc. Natl. Acad. Sci. USA, 79, 2855-2859. Morin, R.J., and Peng, S.K. (1989). The role of cholesterol oxidation products in the pathogenesis of atherosclerosis. Ann. Clin. Lab. Sci., 19, 225-237. Moriya, M. (1993). Single-stranded shuttle phagemid for mutagenesis studies in mammalian cells: 8-Oxoguanine in DNA induces targeted C.C→T.A transversions in simian kidney cells. Proc. Natl. Acad. Sci. USA, 90, 1122-1126. Morrow, J.D., and Roberts, L.J. (1996). The isoprostanes. Current knowledge and directions for future research. Biochem. Pharmacol., 51, 1-9. Morrow, J.D., and Roberts, L.J. (1999). Mass spectrometric quantification of F2-isoprostanes in biological fluids and tissues as measure of oxidant stress. Meths. Enzymol., 300, 3-12. Morrow, J.D., Chen, Y., Brame, C.J., Yang, J., Sanchez, S.C., Xu, J., Zackert, W.E., Awad, J.A., and Roberts, L.J. (1999). The isoprostanes: Unique prostaglandin-like products of free radical-initiated lipid peroxidation. Drug Metab. Rev., 31, 117-139. Morrow, J.D., Reich, E.E., Roberts, L.J., and Montine, T.J. (2003). Quantification Of Isoprostanes As An Index Of Oxidant Stress Status In Vivo. In: Critical Reviews of Oxidative Stress and Aging. Volume 1.Cutler, R.G., and Rodriguez, H. (Eds.). World Scientific, NJ. Pp. 383-392. Morton, L.W., Puddy, I.B., and Croft, K.D. (2003). Comparison of nitration and oxidation of tyrosine in advanced human carotid plaque proteins. Biochem. J., 370, 339-344. Muller, K., Hardwick, S.J., Marchant, C.E., Law, N,S., Waeg, G., Esterbauer, H., Carpentar, K.L., and Mitchinson, M.J. (1996). Cytotoxic and chemotactic potencies of several aldehyde components of oxidized low-density lipoprotein for human monocyte-macrophages. FEBS Lett., 388, 165-168. WWW.ESAINC.COM 294 Myatt, L., Rosenfield, R.B., Eis, A.L., Brockman, D.E., Greer, I., and Lyall, F. (1996). Nitrotyrosine residues in placenta. Evidence of peroxynitrite formation and action. Hyperten., 28, 488-493. Nackerdien, Z., Olinski, R., and Dizdaroglu, M. (1992). DNA base damage in chromatin of gamma-irradiated cultured human cells. Free Radic. Res. Commun., 16, 259-273. Nagashima, M., Tsuda, H., Takenoshita, S., Nagamachi, Y., Hirohashi, S., Yokota, J., and Kasai, H. (1995). 8Hydroxydeoxyguanosine levels in DNA of human breast cancer are not significantly different from those of noncancerous breast tissue by the HPLC-ECD method. Cancer Lett., 90, 157-162. Nair, U., Nair, J., Friesen, D., Bartsch, H., and Ohshima, H. (1995). Ortho- and meta-tyrosine formation from phenylalanine in human saliva as a marker of hydroxyl radical generation during betel quid chewing. Carcinogen., 16, 1195-1198. Nair, V., Vietti, D.E., and Cooper, C.S. (1981). Degenerative chemistry of malondialdehyde. Structure, stereochemistry and kinetics of formation of enaminals from reaction with amino acids. J. Am. Chem. Soc., 103, 3030-3096. Nakajima, M., Takeuchi, T., and Morimoto, K. (1996). Determination of 8-hydroxydeoxyguanosine in human cells under oxygen-free conditions. Carcinogen., 17, 787-791. Nashimura, S. (2002). Involvement of mammalian OGG1(MMH) in excision of the 8-hydroxyguanine residue in DNA. Free Radic. Biol. Med., 32, 813-821. Nassi-Calo, L., Mello Fihlo, A.C., and Meneghini, R. (1989). O-Phenanthroline protects mammalian cells from hydrogen peroxide-induced gene mutation and morphological transformation. Carcinogen., 10, 1055-1057. 2 Nath, R.G., Ocando, J.E., Guttenplan, J.B., and Chung, F.L. (1998). 1,N -propanodeoxyguanosine adducts: Potential new biomarkers of smoking-induced DNA damage in human oral tissue. Cancer Res., 58, 581-584. Netto, L.E.S., RamaKrishna, N.V.S., Kolar, C., Cavalieri, E.L., Rogan, E.G., Lawson, T.A., and Augusto, O. (1992). 8 Identification of C -methylguanine in the hydrolysates of DNA from rats administered 1,2-dimethylhydrazine. Evidence for in vivo DNA alkylation by methyl radicals. J. Biol. Chem., 267, 21524-21527. Neuzil, J., and Stocker, R. (1993). Bilirubin attenuates radical-mediated damage to serum albumin. FEBS Lett., 331, 281284. Nicotera, T.M., Munson, B.R., and Fiel, R.J. (1994). Photosensitized formation of 8-hydroxy-2'-deoxyguanosine and DNA strand breakage by a cationic meso-substituted porphyrin. Photochem. Photobiol., 60, 295-300. Nielsen, H.B., Hanel, B., Loft, S., Poulsen, H.E, Pedersen, B.K., Diamant, M., Vistisen, K., and Secher, N.H. (1995). Restricted pulmonary diffusion capacity after exercise is not an ARDS-like injury. J. Sports Sci., 13, 109-113. Nishigaki, I., Hagihara, M., Tsunekawa, H., Maseki, M., and Yagi, K. (1981). Lipid peroxide levels of serum lipoprotein fractions of diabetic patients. Biochem. Med., 25, 373-378. Nomura, K., Suzuki, N., and Matsumoto, S. (1990). Pulcherosine, a novel tyrosine-derived, trivalent cross-linking amino acid from the fertilization envelope of sea urchin embryo. Biochem., 29, 4525-4534. Norsten-Hoog, C., and Cronholm, T. (1990). Analysis of aldehydic lipid peroxidation products in rat liver and hepatocytes by gas chromatography and mass spectrometry of the oxime-t-butyldimethylsilyl derivatives. Anal. Biochem., 189, 131137. Nosworthy, J., and Allsop, C.B. (1956). The radiolysis of aqueous solutions of tyrosine. J. Colloid Sci., 11, 565-574. Nourooz-Zadeh, J., Liu, E.H.C., Yhlen, B., Anggard E.E., and Halliwell, B. (1999). F4 isoprostanes as specific markers of docosahexaenoic peroxidation in Alzheimer’s disease. J. Neurochem., 72, 734-740. O’Connor, T.R., Boiteux, S., and Laval, J. (1988). Ring-opened 7-methyl guanine residues in DNA are a block to in vitro DNA synthesis. Nucleic Acid Res., 16, 5879-5894. O’Donnell, V.B., Eiserich, J.P., Bloodsworth, A., Chumley, P.H., Kirk, M., Barnes, S., Darley-Usmar, V.M., and Freeman, B.A. (1999a). Nitration of unsaturated fatty acids by nitric oxide-derived reactive species. Meth. Enzymol., 301, 454470. O’Donnell, V.B., Eiserich, J.P., Chumley, P.H., Jablonsky, M.J., Krishna, N.R., Kirk, M., Barnes, S., Darley-Usmar, V.M., and Freeman, B.A. (1999b). Nitration of unsaturated fatty acids by nitric oxide-derived reactive nitrogen species peroxynitrite, nitrous acid, nitrogen dioxide, and nitronium ion. Chem. Res. Toxicol., 12, 83-92. O’Gara, C.Y., Maddipati, K.R., and Marnett, L.J. (1989). A sensitive electrochemical method for quantitative hydroperoxide determination. Chem. Res. Toxicol., 2, 296-300. Ohshima, H., Brouet, I., Friesen, M., and Bartsch, H. (1991). Nitrotyrosine as a new marker of endogenous nitrosation and nitration. IARC Sci. Publ., 106, 443-448. Ohshima, H., Celan, I., Chazotte, L., Pignatelli, B., and Mower, H.F. (1999). Analysis of 3-nitrotyrosine in biological fluids and protein hydrolyzates by high-performance liquid chromatography using postseparation, on-line reduction column and electrochemical detection: Results with various nitrating agents. Nitric Oxide, 3. In press. Ohshima, H., Friessen, M., Brouet, I., and Bartsch, H. (1990). Nitrotyrosine as a new marker for endogenous nitrosation and nitration of proteins. Fd. Chem. Toxicol., 28, 647-652. Okamoto, K., Toyokuni, S., Uchida, K., Ogawa, O., Takenawa, J., Kahehi, Y., Kinoshita, H., Hattori-Nakakuki, Y., Hiai, H., and Yoshida, O. (1994). Formation of 8-hydroxy-2’deoxyguanosine and 4-hydroxy-2-nonenal-modified proteins in human renal-cell carcinoma. Int. J. Cancer, 58, 825-829. Okuda, Y., Sakoda, S., Fujimura, H., and Yanagihara, T. (1997). Nitric oxide via an inducible isoform of nitric oxide synthase is a possible factor to eliminate inflammatory cells from the central nervous system of mice with experimental allergic encephalomyelitis. J. Neuroimmunol., 73, 107-116. Olinski, R., Zastawny, T.H., Foksinski, M., Barecki, A., and Dizdaroglu, M. (1995). DNA base modifications and antioxidant enzyme activities in human benign prostatic hyperplasia. Free Radic. Biol. Med., 18, 807-813. Oliver, C.N., Ahn, B., Moerman, E.J., Goldstein, S., and Stadtman, E.R. (1987). Age-related changes in oxidized proteins. J. Biol. Chem., 262, 5488-5491. Orr, W.C., and Sohal, R.S. (1994). Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science, 263, 1128-1130. Ohshima, H., and Bartsch, H. (1994). Chronic infections and inflammatory processes as cancer risk factors: Possible role of nitric oxide in carcinogenesis. Mutat. Res., 305, 253-264. WWW.ESAINC.COM 295 Pacifici, R.E., Kono, Y., and Davies, K.J. (1993). Hydrophobicity as the signal for selective degradation of hydroxyl radicalmodified hemoglobin by the multicatalytic proteinase complex, proteosome. J. Biol. Chem., 268, 15405-15411. Padmaja, S., Ramazenian, M.S., Bounds, P.L., and Koppenol, W.H. (1996). Reaction of peroxynitrite with L-tryptophan. Redox Report, 2, 173-177. Padmaja, S., Squadrito, G.L., and Pryor, W.A. (1998). Inactivation of glutathione peroxidase by peroxynitrite. Arch. Biochem. Biophys., 349, 1-6. Park, E.-M., Shigenaga, M.K., Degan, P., Korn, T.S., Kitlzer, J.W., Wehr, C.M., Kolachana, P., and Ames, B.N. (1992). Assay of excised oxidative DNA lesions: Isolation of 8-oxoguanine and its nucleoside derivatives from biological fluids with a monoclonal antibody column. Proc. Natl. Acad. Sci. USA, 89, 3375-3379. Patel, R.P., Diczfalusy, U., Dzeletovic, S., Wilson, M.T., and Darley-Usmar, V.M. (1996). Formation of oxysterols during oxidation of low-density lipoprotein by peroxynitrite, myoglobin and copper. J. Lipid Res., 37, 2361-2371. Patel, U., Bhimani, R., and Frenkel, K. (1992). Mechanism of mutagenicity by 5-hydroperoxymethyl-2’deoxyuridine, an intermediate product of ionizing radiation, in bacteria. HPMdU bacterial mutagenicity and oxidation of DNA bases. Mutat. Res., 238, 145-156. Paul, S., Bogdanov, M.B., Matson, W.R., Metakis, L., Jacobs, J., and Beal, M.F. (2003). Urinary 8-hydroxy-2’deoxyguanosine, a metabolite of oxidized DNA, is not elevated in HIV patients on combination antiretroviral therapy. Free Radic. Res., 37, 499-502. Pedersen, J.Z., and Finazzi, A.A. (1993). Protein-radical enzymes. FEBS Lett., 325, 53-58. Peng, S.K., and Morin, R.J. (1987). Effects of membrane function by cholesterol oxidation derivatives in cultured aortic smooth muscle cells. Artery, 14, 85-99. Perez-Campo, R., Lopez-Torres, M., Cadenas, S., Rojas, C., and Barja, G. (1998). The rate of free radical production as a determinant of the rate of aging: Evidence from the comparative approach. J. Comp. Physiol, B, 168, 149-158. Petruzzelli, S., Puntoni, R., Mimotti, P., Pulera, N., Baliva, F., Fornai, E., and Giuntini, C. (1997). Plasma 3-nitrotyrosine in cigarette smokers. Am. J. Crit. Care Med., 156, 1902-1907. Pfeiffer, S., and Mayer, B. (1998). Lack of tyrosine nitration by peroxynitrite generated in situ from nitric oxide and superoxide. Free Radic. Biol. Med., 25, Supplement 1, S64. Pietta, P.G., Simonetti, P., Gardana, C., Cristoni, S., Bramati, L., and Mauri, P.L. (2003). LC-APCI-MS/MS analysis of urinary 8-hydroxy=2’-deoxyguanosine. J. Pharmaceu. Biomed. Anal., 32, 657-661. Pilger, A., Ivancsits, S., Germadnik, D., and Rudiger, H.W. (2002). Urinary excretion of 8-hydroxy-2’deoxyguanosine measured by high-performance liquid chromatography with electrochemical detection. J. Chromatogr. B, 778, 393-401. Piperakis, S.M., Visvardis, E.-E., and Tassiou (1998). Comet assay for nuclear DNA damage. Meth. Enzymol, 300. 184194. Podmore, I.D., Griffiths, H.R., Herbert, K.E., Mistry, N., Mistry, P., and Lunec, J. (1998). Vitamin C exhibits pro-oxidant properties. Nature, 392, 569. Poirier, M.C., and Weston, A. (1996). Human DNA adduct measurement: State of the art. Environ. Health Perspect., 104, Suppl. 5, 883-893. Poli, G., Diianzani, M.U., Chesseman, K.H., Slater, T.F., and Lang, J., and Esterbauer, H. (1985). Separation and characterization of the aldehydic products of lipid peroxidation stimulated by carbon tetrachloride or ADP-iron in isolated rat hepatocytes and rat liver microsomal suspension. Biochem. J., 227, 629-638. Poulsen, H.E., and Loft, S. (1998). Interpretation of oxidative DNA modification: Relation between tissue levels, excretion of urinary repair products and single cell gel electrophoresis (comet assay). In: DNA and Free Radicals: Techniques, Mechanisms and Applications. O.I. Aruoma and B. Halliwell (Eds.). OICA International. In Press. Poulsen, H.E., Loft, S., Jensen, B.R., Sorensen, M., Hoberg, A,-M., and Weimann, A. (2003). HPLC-ECD, HPLC-MS/MS (Urianry Biomarkers). In: Critical Reviews of Oxidative Stress and Aging. Volume 1.Cutler, R.G., and Rodriguez, H. (Eds.). World Scientific, NJ. Pp. 233-256. Poulsen, H.E., Loft, S., and Vistisen, K. (1996). Extreme exercise and oxidative DNA modification. J Sports Sci., 14, 343346. Poulsen, H.E., Loft, S., and Weimann, A. (1998). Urinary measurement of 2-oxodG (8-oxo-2’-deoxyguanosine. In: Handbook of Clinical Analysis: In Vivo Oxidative Damage to Biomolecules. Lunec, J. (Ed.). In press. Povirk, L.F. (1996). DNA damage and mutagenesis by radiomimetic DNA-cleaving agents: bleomycin, neocarzinostatin and other enediynes. Mutat. Res., 355, 71-89. Pratico, D., Barry, O.P., Lawson, J.A., Adiyaman, M., Hwang, S.W., Khanapure, S.P., Iuliano, L., Rokach, J., and Fitzgerald, G.A. (1998). IPF2α-I: An index of lipid peroxidation in humans. Proc. Natl. Acad. Sci. USA, 95, 3449-3454. Pratico, D., Reilly, M., Lawson, J.A., and FitzGerald, G.A. (1997). Novel indices of oxidant stress in cardiovascular disease: Specific analysis of F2-isoprostanes. Prostaglandins and Control of Vascular Smooth Muscle Cell Proliferation. Birkhauser Verlag Basel. Pp. 26-41. Pryor, W.A. (1988). Why is the hydroxyl radical the only radical that commonly adds to DNA? Hypothesis: It has a rare combination of high electrophilicity, high thermochemical reactivity, and a mode of production that can occur near DNA. Free Radic. Biol. Med., 4, 219-223. Pryor, W.A. (1989). On the detection of lipid hydroperoxides in biological samples. Free Radic. Biol. Med., 7, 177-178. Pryor, W.A., and Stanley, P.A. (1975). A suggested mechanism for the production of malondialdehyde during the oxidation of polyunsaturated fatty acids. Nonenzymatic production of prostaglandin endoperoxides during autoxidation. J. Org. Chem., 40, 3615-3617. Purmal, A.A., Kow, Y.W., and Wallace, S.S. (1994). Major oxidative products of cytosine, 5-hydroxycytosine and 5hydroxyuracil, exhibit sequence context-dependent mispairing in vitro. Nucleic Acid Res., 22, 72-88. Qian, S.Y., and Buettner, G.R. (1999). Iron and dioxygen chemistry is an important route to initiation of biological free radical oxidations: An electron paramagnetic resonance spin trapping study. Free Radic. Biol. Med., 26, 1447-1456. Quijano, C., Hernandez-Saavedra, D., Castro, L., McCord, J.M., Freeman, B.A., and Radi, R. (2001). Reaction of peroxynitrite with Mn-superoxide dismutase. Role of the metal center in decomposition kinetics and nitration. J. Biol. Chem., 276, 11631-11638. WWW.ESAINC.COM 296 Raboy, B., Sharon, G., Parag, H.A., Shochat, Y., and Kulka, R.G. (1991). Effect of stress protein degradation: Role of ubiquitin system. Acta Biol. Hung., 42, 3-20. Radi, R., Beckman, J.S., Bush, K.M., and Freeman, B.A. (1991). Peroxynitrite-induced membrane lipid peroxidation: The cytotoxic potential of superoxide and nitric oxide. Arch. Biochem. Biophys., 288, 481-487. Ramezainian, M.S., Padmaja, S., and Koppenol, W.H. (1996). Nitration and hydroxylation of phenolic compounds by peroxynitrite. Chem. Res. Toxicol., 9, 232-240. Randeroth, K., Li, D.H., and Randerath, E. (1990). Age-related DNA modifications (I-compounds): Modulation by physiological and pathological processes. Mutat. Res., 238, 245-253. Randerath, K., Li, D., Nath, R., and Randerath, E. (1992). Exogenous and endogenous DNA modifications monitored by 32 P-postlabeling: Relationships to cancer and aging. Exp. Gerentol., 27, 533-549. 32 Randeroth, K., Reddy, M.V., and Gupta, R.C. (1981). P-Labeling for DNA damage. Proc. Natl. Acad. Sci. USA, 78, 6126-6129. Randeroth, K., Reddy, M.V., and Disher, R.M. (1986). Age- and tissue-related DNA modifications in untreated rats: 32 Detection by p-postlabeling assay and possible significance for spontaneous tumor induction and aging. Carcinogen., 7, 1615-1617. Raoul, S., Bardet, M., and Cadet, J. (1995). γ Irradiation of 2’-deoxyadenosine in oxygen-free aqueous solutions: Identification and conformational features of formamidopyrimidine nucleoside derivatives. Chem. Res. Toxicol., 8, 924933. Ravanat, J.L., and Cadet, J. (1995). Reaction of singlet oxygen with 2’deoxyguanosine and DNA. Isolation and characterization of the mian oxidation products. Chem. Res. Toxicol., 8, 379-388. Ravanat, J.-L., Duretz, B., Guiller, A., Douki, T., and Cadet, J. (1998). Isotope dilution high-performance liquid chromatography-electrospray tandem mass spectrometry assay for the measurement of 8-oxo-7,8-dihydro-2’deoxyguanosine in biological samples. J. Chromatogr. B, 715, 349-356. Ravanat, J.-L., Guicherd, P., Tuce, Z., and Cadet, J. (1999). Simultaneous determination of five oxidative DNA lesions in human urine. Chem. Res. Toxicol. In press. Ravanat, J.-L., Turesky, R.J., Gremaud, E., Trudel, L.J., and Stadler, R.H. (1995). Determination of 8-oxoguanine in DNA by gas chromatography-mass spectrometry and HPLC-electrochemical detection: Overestimation of the background level of the oxidized base by the gas chromatography-mass spectrometry assay. Chem. Res. Toxicol., 8, 1039-1045. Reddy, V.P., Obrenovich, M.E., Atwood, C.S., Perry, G., and Smith, M.A. (2002). Involvement of Maillard reactions in Alzheimer’s disease. Neurotox. Res., 4, 121-209. Renner, T., Fechner, T., and Scherer, G. (2000). Fast quantification of urinary marker of oxidative stress 8-hydroxy-2’deoxyguanosine using solid-phase extraction and high-performance liquid chromatography with triple-stage quadrupole mass detection. J. Chromatogr. B, 738, 311-317. Retel, J., Hoebee, B., Braun, J.E.F., Lutgerink, J.T., van den Akker, E., Wanamarta, A.H., Joneje, H., and Lafleur, M.V.M. (1993). Mutational specificity of oxidative DNA damage. Mutat. Res., 299, 165-182. Riis, B. (2002). Comparison of results from different laboratories in measuring 8-oxo-2’-deoxyguanosine in synthetic oligonucleotides. Free Radic. Res., 36, 649-659. Roberts, L.J., and Morrow, J.D. (1997). The generation and actions of isoprostanes. Biochim. Biophys. Acta, 1345, 121135. Ronchi, S., Galliano, M., Minchiotti, L., Curti, B., Rudie, N.G., Porter, D.J., and Bright, H.J. (1980). An active site-tyrosinecontaining heptapeptide from D-amino acid oxidase. J. Biol. Chem., 255, 6044-6046. Routledge, M.N., Wink, D.A., Keefer, L.K., and Dipple, A. (1994). DNA sequence changes induced by two nitric oxide donor drugs in the supF assay. Chem. Res. Toxicol., 7, 628-632. Rouzer, C.A., Chaudhary, A.K., Nokubo, M., Ferguson, D.M., Reddy, G.R., Blair, I.A., and Marnett, L.J. (1997). Analysis of malondialdehyde-2’-deoxyguanosine adduct pyrimidopurinone in human leukocyte DNA by gas chromatography/electron capture/negative chemical ionization/mass spectrometry. Chem. Res. Toxicol., 10, 181-188. Rowbottom, J. (1955). The radiolysis of aqueous solutions of tyrosine. J. Biol. Chem., 212, 877-885. Rowley, D.A., and Halliwell, B. (1983). DNA damage by superoxide-generating systems in relation to the mechanism of action of the antitumor antibiotic adriamycin. Biochim. Biophys. Acta, 761, 86-93. Rozalski, R., Gackowski, D., Roszkowski, K., Foksinski, M., and Olinski, R. (2002). The level of 8-hydroxyguanine, a possible repair product of oxidative DNA damage, is higher in urine of patients than in control subjects. Cancer, Epidemiol. Biomarkers, Prev., 11, 1072-1075. Rubbo, H., Radi, R., Trujillo, M., Telleri, R., Kalyanaraman, B., Barnes, S., Kirk, M., and Freeman, B. (1994). Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. J. Biol. Chem., 269, 26066-26075. Ryter, S.W., and Tyrrell, R.M. (1998). Singlet oxygen: A possible effector of eukaryotic gene expression. Free Radic. Biol. Med., 24, 1520-1534. Sakumi, K., Furuichi, M., Tsuzuki, T., Kakuman, T., Kawabata, S., Maki, H., and Sekiguchi, M. (1993). Cloning and expression of a cDNA for a human enzyme that hydrolyzes 8-oxo-dGTP, a mutagenic substrate for DNA synthesis. J. Biol. Chem., 268, 23524-23530. Saleh, D., Barnes, P.J., and Giaid, A. (1997). Increased production of the potent oxidant peroxynitrite in the lungs of patients with idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med., 155, 1763-1769. Salman-Tabcheh, S., Guerin, M.C., and Torreilles, J. (1995). Nitration of tyrosyl-residues from extra- and intracellular proteins of human whole blood. Free Radic. Biol. Med., 19, 695-698. Salman-Tabcheh, S., Rabgaoui, N., and Torreilles, J. (1993). Neutrophil-catalyzed dimerization of tyrosyl peptides. Free Radic. Res. Commun., 19, 217-227. Santos, M.T., Valles, J., Aznar, J., and Vilches, J. (1980). Determination of plasma malondialdehyde-like material and its clinical application in stroke patients. J. Clin. Pathol., 33, 973-976. Sato, M., Fukuyama, N., Sakai, M., and Nakazawa, H. (1998). Increased nitric oxide in nasal lavage fluid and nitrotyrosine formation in nasal mucosa – indices of severe perennial nasal allergy. Clin. Exp. Allergy, 28, 597-605. WWW.ESAINC.COM 297 Satoh, K. (1978). Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin. Chim. Acta, 90, 37-43. Satoh, M.S., Jones, C.J., Wood, R.D., and Lindahl, T. (1993). DNA excision-repair defect of xeroderma pigmentosum prevents removal of a class of oxygen free radical-induced base lesions. Proc. Natl. Acad. Sci. USA, 90, 6335-6339. Savenkova, M.I., Mueller, D.M., and Heinecke, J.W. (1994). Tyrosyl radical generated by myeloperoxidase is a physiological catalyst for the initiation of lipid peroxidation in low density lipoproteins. J. Biol. Chem., 269, 2092-2097. Sayre, L.M., Zelasko, D.A., Harris, P.L.R., Perry, G., Salomon, R.G., and Smith, M.A. (1997). 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer’s disease. J. Neurochem., 68, 2092-2097. Schmidt, P., Youhnovski, N., Daiber, A., Balan, A., Arsic, M., Bachschmidt, M., Przybylski, M., and Ullrich, V. (2003). Specific nitration of tyrosine 430 revealed by high resolution mass spectrometry as basis for redox regulation of bovine prostacyclin synthase. J. Biol. Chem., 278, 12813-12819. Schulz, J.B., Matthews, R.T., Muqit, M.M.K., Browne, S.E., and Beal, M.F. (1995). Inhibition of neuronal nitric oxide synthase by 7-nitroindazole protects against MPTP-induced neurotoxicity in Mice. J. Neurochem., 64, 936-939. Schwartz, A.L., and Ciechanover, A. (1992). Ubiquitin-mediated protein modification and degradation. Am. J. Respir. Cell Mol. Biol., 7, 463-468. Schwartz, A.L., and Ciechanover, A. (1999). The ubiquitin-proteasome pathway and pathogenesis of human diseases. Ann. Rev. Med., 50, 57-74. Seeburg, P.H., Colby, W.W., Capon, D.J., Goeddel, D.V., and Levinson, A.D. (1984). Biological properties of human cHA-ras1 genes mutated at codon 12. Nature, 312, 71-75. Seeberg, E., Eide, L., Bjoras, M., and Bjras, M. (1995). The base excision repair pathway. Trends Biochem. Sci., 20, 391397. Sell, D.R., Nagaraj, R.H., Grandhee, S.K., Odetti, P., Lapolla, A., Fogarty, J., and Monnier, V.M. (1991). Pentosidine: A molecular marker for cumulative damage to proteins in diabetes, aging and uremia. Diabetes Metab. Rev., 7, 239-251. Selley, M.L. (1997). Determination of the lipid peroxidation product (E)-4-hydroxy-2-nonenal in clinical samples by gas chromatography-negative-ion chemical ionization mass spectrometry of the O-pentafluorobenzyl oxime. J. Chromatogr., 691, 263-268. Selley, M.L. (1998). (E)-4-Hydroxy-2-nonenal may be involved in the pathogenesis of Parkinson’s disease. Free Radic. Biol. Med., 25, 169-174. Selley, M.L., Bartlett, M.R., McGuiness, J.A., Hapal, A.J., and Ardlie, N.G. (1989). Determination of the lipid peroxidation product trans-4-hydroxy-2-nonenal in biological samples by high performance liquid chromatography and combined capillary gas chromatography/negative ion chemical ionization mass spectrometry. J. Chromatogr., 488, 329-340. Selley, M.L., Bourne, D.J., Bartlett, M.R., Tymms, K.E., Brook, A.S., Duffield, A.M., and Ardlie, N.G. (1992). Occurrence of (E)-4-hydroxy-2-nonenal in plasma and synovial fluid of patients with rheumatoid arthritis and osteoarthritis. Ann. Rheum. Dis., 51, 481-484. Semenza, J.C., and Weasel, L.H. (1997). Molecular epidemiology in environmental health: The potential of tumor suppressor gene p53 as a biomarker. Environ. Health Perspect., 105, 155-163. Sergent, O., Morel, I., and Cillard, J. (1999). Involvement of metal ions in lipid peroxidation: Biological implications. In: Metal Ions in Biological System, 36. Sigel, A., and Sigel, H. (Eds.). Marcel Dekker, Inc., New York. Pp. 251-287. Sharma, M., Box, H.C., and Kelman, D. (1990). Fluorescence postlabeling assay of cis-thymidine glycol monophosphate in X-irradiated calf-thymus DNA. Chem. Biol. Interact., 74, 107-117. Sharma, M., and Jane, R. (1998). Isolation and analysis of dityrosine from enzyme-catalyzed oxidation of tyrosine and Xirradiated peptide and protein. Chem. Biol. Interact., 108, 171-185. Sherman, M.Y., and Goldberg, A.L. (1996). Involvement of molecular chaperones in intracellular protein breakdown. EXS., 77, 57-78. Shen, Z., Mitra, S.N., Wu, W., Chen, Y., Yang, Y., Qin, J., and Hazen, S.L. (2001). Eosinophil peroxidase catalyzes bromination of free nucleosides and double-stranded DNA. Biochem., 40, 2041-2051. Shibutani, S. (1993). Quantitations of base substitutions and deletions induced by chemical mutagens during DNA synthesis in vitro. Chem. Res. Toxicol., 6, 625-629. Shibutani, S., Takeshita, M., and Grollman, P. (1991). Insertion of specific bases during DNA synthesis past the oxidation damaged base 8-oxodG. Nature, 349, 431-434. Shigenaga, M.K. (1999). Quantitation of protein-bound 3-nitrotyrosine by high-performance liquid chromatography with electrochemical detection. Meth. Enzymol., 301, 27-40. Shigenaga, M.K., and Ames, B.N. (1991). Assays for 8-hydroxy-2’-deoxyguanosine: A biomarker of in vivo oxidative DNA damage. Free Radic. Biol. Med., 10, 211-216. Shigenaga, M.K., Aboujaoude, E.N., Chen, Q., and Ames, B.N. (1994). Assays of oxidative DNA damage biomarkers 8oxo-2’-deoxyguanosine and 8-oxoguanine in nuclear DNA and biological fluids by high performance liquid chromatography and electrochemical detection. Meth. Enzymol., 234, 16-33. Shigenaga, M.K., Gimeno, C.J., and Ames, B.N. (1989). Urinary 8-hydroxy-2’deoxyguanosine as a biological marker of in vivo oxidative DNA damage. Proc. Natl. Acad. Sci. USA, 86, 9697-9701. Shigenaga, M.K., Lee, H.H., Blount, B.C., Christen, S., Shigeno, E.T., Yip, H., and Ames, B.N. (1997). Inflammation and NOx-induced nitration: Assay for 3-nitrotyrosine by HPLC with electrochemical detection. Proc. Natl. Acad. Sci. USA, 94, 3211-3216. Shigenaga, M.K., Park, J.-W., Cundy, K.C., Gimeno, C.J., and Ames, B.N. (1990). In vivo oxidative DNA damage: Measurement of 8-hydroxy-2’-deoxyguanosine in DNA and urine by high-performance liquid chromatography with electrochemical detection. Meth. Enzymol., 186, 521-530. Shimoda, R., Nagshima, M., Sakamoto, M., Yamaguchi, M., Hirohashi, S., Yokota, J., and Kasai, H. (1994). Increased formation of oxidative DNA damage, 8-hydroxydeoxyguanosine in human liver with chronic hepatitis. Cancer Res., 54, 3171-3172. WWW.ESAINC.COM 298 Shimoi, K., Kasai, H., Yokota, N., Toyokuni, S., and Kinae, N. (2002). Comparison between high-performance liquid chromatography and enzyme-linked immunosorbent assay for the determination of 8-hydroxy-2’-deoxyguanosine in human urine. Cancer Epidemiol. Biomark. Prev., 11, 767-770. Shimokawa, T., Kulmacz, R.J., DeWitt, D.L., and Smith, W.L. (1990). Tyrosine 385 of prostaglandin endoperoxide synthase is required for cyclo-oxygenase catalysis. J. Biol. Chem., 265, 20073-20076. Shin, C.S., Moon, B.S., Park, K.S., Kim, S.Y., Park, S.J., Ching, M.H., and Lee, H.K. (2001). Serum 8-hydroxy-guanine levels are increased in diabetic patients. Diabetes Care, 24, 733-737. Shirname-More, L., Rossman, T.G., Troll, W., Teebor, T.W., and Frenkel, K. (1987). Genetic effects of 5-hydroxymethyl2’-deoxyuridine, a product of ionizing radiation. Mutat. Res., 178, 177-186. Shuker, D.E., Prevost, V., Friesen, M.D., Lin, D., Ohshima, H., and Bartsch, H. (1993). Urinary markers for measuring exposure to endogenous and exogenous alkylating agents and precursors. Environ. Health Perspect., 99, 33-37. Schwarz, E., Lilie, H., and Rudolph, R. (1996). The effect of molecular chaperones on in vivo and in vitro folding processes. Biol. Chem., 377, 411-416. Simic, M.G., and Bergtold, D.S. (1991). Dietary modulation of DNA damage in humans. Mut. Res., 250, 17-24. Simons, A.M., Phillips, D.H., and Coleman, D.V. (1993). Damage to DNA in cervical epithelium related to smoking tobacco. Brit. Med. J., 306, 1444-1448. Simpson, J.A., Narita, S., Gieseg, G., Gebicki, S., Gebicki, J.M., and Dean, R.T. (1992). Long-lived reactive species on free-radical-damaged proteins. Biochem. J., 282, 621-624. Singer, B., and Hang, B. (1998). Role of chemical structure in determination of repair enzyme substrate specificity and mechanism. In: DNA and Free Radicals: Techniques, Mechanisms and Applications. Aruoma, O.I., and Halliwell, B. (Eds). OICA, London. Pp. 26-61. Singer, I.I., Kawka, D.W., Scott, S., Weidner, J.R., Mumford, R.A., Riehl, T.E., and Stenson, W.F. (1996). Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory disease. Gastroent., 111, 871885. Situnayake, R.D., Crump, B.J., Zezulka, A.V., Davis, M., McConkey, B., and Thurnham, D.I. (1990). Measurement of conjugated diene lipids by derivative spectroscopy in heptane extracts of plasma. Ann. Clin. Chem., 27, 258-266. Skinner, K.A., Crow, J.P., Skinner, H.B., Chandler, R.T., Thompson, J.A., and Parks, D.A. (1997). Free and proteinassociated nitrotyrosine formation following rat liver preservation and transplantation. Arch. Biochem. Biophys., 342, 282-288. Smith, C.D., Carney, J.M., Starke-Reed, P.E, Olive, C.N., Stadtman, E.R., Floyd, R.A., and Markesbery, W.R. (1991). Excess brain protein oxidation and enzyme dysfunction in normal aging and Alzheimer’s disease. Proc. Natl. Acad. Sci. USA, 88, 10540-10543. Smith, L.L., and Johnson, B.H. (1989). Biological activities of oxysterols. Free Radic. Biol. Med., 7, 285-332. Smith, M.A., Sayre, L.M., Monnier, V.M., and Perry, G. (1995). Radical AGEing in Alzheimer’s disease. Trends Neurosci., 18, 172-176. Smith, M.A., Richey Harris, P.L., Sayre, L.M., Beckman, J.S., and Perry, G. (1997). Widespread peroxynitrite-mediated damage in Alzheimer’s disease. J. Neurosci., 17, 2653-2657. Smith, M.A., Taneda, S., Richey, P.L., Miyata, S., Yan, S.D., Stern, D., Sayre, L.M., Monnier, V.M., and Perry, G. (1994). Advanced Maillard reaction end products are associated with Alzheimer’s disease pathology. Proc. Natl. Acad. Sci. USA, 91, 5710-5714. Sodum, R.S., and Fiala, E.S. (2001). Analysis of peroxynitrite reactions with guanine, xanthine, and adenine nucleosides by high-pressure liquid chromatography with electrochemical detection: C-8nitration and –oxidation, Chem. Res. Toxicol., 14, 438-450. Sohal, R.S., Agarwal, A., Agarwal, S., and Orr, W.C. (1995). Simultaneous overexpression of copper- and zinc-containing superoxide dismutase and catalase retards age-related oxidative damage and increases metabolic potential in Drosophila melanogaster. J. Biol. Chem., 270, 15671-15674. Sokolovsky, M., Riordan, J.F., and Vallee, B.L. (1967). Conversion of 3-nitrotyrosine to 3-aminotyrsosine in peptides and proteins. Biochem. Biophys. Res. Comm., 27, 20-25. Sontag, G., Bernweiser, I., and Krach, C. (1997). HPLC with electrode array detection and its application in analytical food chemistry. In: Coulometric Electrode Array Detectors for HPLC. Progress in HPLC-HPCE (6), Acworth, I.N., Naoi, M., Parvez, S., and Parvez, H. (Eds.). VSP Press, The Netherlands. Pp. 75-126. Soussi, T. (1996). The p53 tumor suppressor gene: A model for molecular epidemiology of human cancer. Mol. Med. Today, 2, 32-37. Souza, J.M., and Radi, R. (1998). Glyceraldehyde-3-phosphate dehydrogenase inactivation by peroxynitrite. Arch. Biochem. Biophys., 360, 187-194. Spencer, J.P.E., Jenner, A., Aruoma, O.I., Evans, P.J., Kaur, H., Dexter, D.T., Jenner, P., Lees, A.J., Marsden, D.C., and Halliwell, B. (1994). Intense oxidative DNA damage promoted by L-DOPA and its metabolites: Implications for neurodegenerative disease. FEBS Letts., 353, 246-250. Spencer, J.P.E., Wong, J., Jenner, A., Aruoma, O.I., Cross, C.E., and Halliwell, B. (1996). Base modification and strand breakage in isolated calf thymus DNA and in DNA from human skin epidermal keratinocytes exposed to peroxynitrite or 3-morpholinosydnonimine. Chem. Res. Toxicol., 9, 1152-1158. Spickett, C.M. (2003). Analysis Of Phospholipid Oxidation By Electrospray Mass Spectrometry. In: Critical Reviews of Oxidative Stress and Aging. Volume 1.Cutler, R.G., and Rodriguez, H. (Eds.). World Scientific, NJ. Pp. 393-409. Stachowiak, O., Dolder, M., Wallimann, T., and Richter, C. (1998). Mitochondrial creatine kinase is a prime target of peroxynitrite-induced modification and inactivation. J. Biol. Chem., 273, 16694-16699. Stadtman, E.R. (1988). Protein modification in aging. J. Gerentol., 43, B112-120. Stadtman, E.R. (1990). Metal ion-catalyzed oxidation of proteins: Biochemical mechanisms and biological consequences. Free Radic. Biol. Med., 9, 315-325. Stadtman, E. (1991). Ascorbic acid and oxidative damage of proteins. Am. J. Clin. Nutr., 54, 1125S-1128S. Stadtman, E. (1992). Protein oxidation and aging. Science, 257, 1220-1224. WWW.ESAINC.COM 299 Stadtman, E. (1993). Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metalcatalyzed reactions. Ann. Rev. Biochem., 62, 797-821. Stadtman, E.R., and Berlett, B.S. (1991). Fenton chemistry: Amino acid oxidation. J. Biol. Chem., 266, 17202-17211. Stadtman, E., Starke-Reed, P.E., Oliver, C.N., Carney, J.M., and Floyd, R.A. (1992). Protein modification in aging. EXS. 62, 64-72. Strickland, E, Qu, B.H., Millen, L., and Thomas, P.J. (1997). The molecular chaperone Hsc70 assists the in vitro folding of the N-terminal nucleotide-binding domain of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem., 272, 25421-25424. Su, J.H., Deng, G., and Cotman, C.W. (1997). Neuronal DNA damage precedes tangle formation and is associated with up-regulation of nitrotyrosine in Alzheimer’s disease brain. Brain Res., 774, 193-199. Suarez-Pinzon, W.L., Szabo, C., and Rabinovitch, A. (1997). Development of autoimmune diabetes in NOD mice is associated with the formation of peroxynitrite in islet beta-cells. Diabetes, 46, 907-911. Suematsu, T., Kamada, T., Abe, H., Kikichi, S., and Yagi, K. (1977). Serum lipoperoxide level in patients suffering from liver diseases. Clin. Chim. Acta, 79, 267-270. Suter, M., and Rachter, C. (1999). Fragmented mitochondrial DNA is the predominant carrier of oxidized DNA bases. Biochem., 38, 459-464. Suzuki, J., Inoue, Y., and Suzuki, S. (1995). Changes in the urinary excretion level of 8-hydroxyguanine by exposure to reactive oxygen-generating substances. Free Radic. Biol. Med., 18, 431-436. Szabo, C. (1996). DNA strand breakage and activation of poly-ADP ribosyltransferase: A cytotoxic pathway triggered by peroxynitrite. Free Radic. Biol. Med., 21, 855-869. Szweda, L.I., and Stadtman, E.R. (1992). Iron-catalyzed oxidative modification of glucose-6-phosphate dehydrogenase from L. mesenteroides. Structural and functional changes. J. Biol. Chem., 267, 3096-3100. Taborsky, G. (1973). Oxidative modifications of proteins in the presence of ferrous ion and air. Effect of ionic composition of the reaction medium on the nature of the oxidation products. Biochem., 12, 1341-1348. Tabrizi-Fard, M.A., Maurer, T.S., and Fung, H.-L. (1999). In vivo disposition of 3-nitro-L-tyrosine in rats: Implications on tracking systemic peroxynitrite exposure. Drug Met. Disp., 27, 429-431. Taddei, F., Hayakawa, H., Bouton, M.-F., Cirinesi, A.-M., Matic, I., Sekiguchi, M., and Radman, M. (1997). Counteractions by MuT protein of transcriptional errors caused by oxidative damage. Science, 278, 128-130. Tagesson, C., Kallberg, M., Klintenberg, C., and Startkhammer, H. (1995). Determination of urinary 8hydroxydeoxyguanosine by automated coupled-column high performance liquid chromatography: A powerful technique for assaying in vivo oxidative DNA damage in cancer patients. Eur. J. Cancer., 31A, 934-940. Tagesson, C., Kallberg, M., and Leanderson, P. (1992). Determination of urinary 8-hydroxydeoxyguanosine by coupledcolumn high-performance liquid chromatography with electrochemical detection: A non-invasive assay for in vivo oxidative DNA damage in humans. Toxicol. Meth., 1, 242-251. Tajiri, T., Maki, H., and Sekiguchi, M. (1995). Functional cooperation of MutT, MutM and MutY proteins in preventing mutations caused by spontaneous oxidation of guanine nucleotide in E. coli. Mutat. Res., 336, 257-267. Takahashi, R., and Goto, S. (1990). Alteration of aminoacyl-tRNA synthetases with age: Heat-labilization of the enzyme by oxidative damage. Arch. Biochem. Biophys., 277, 228-233. Takeuchi, T., and Morimoto, K. (1993). Increased formation of 8-hydroxy-deoxyguanosine, an oxidative DNA damage, in lymphoblasts from Fanconi’s anemia patients due to possible catalase deficiency. Carcinogen., 14, 1115-1120. Takeuchi, T., Nakajima, M., Ohta, Y., Mure, K., Takeshita, T., and Morimoto, K. (1994). Evaluation of 8hydroxydeoxyguanosine, a typical oxidative DNA damage, in human leukocytes. Carcinogen., 15, 1519-1523. Tan, B.H., Bencsath, F.A., and Gaubatz, J.W. (1990). Steady-state levels of 7-methylguanine increase in nuclear DNA of postmitotic mouse tissues during aging. Mut. Res., 237, 229-238. Tanaka, K. (1998). Molecular biology of the proteasome. Biochem. Biophys. Res. Commun., 247, 537-541. Tappel, A.L. (1979). Measurement of and protection from in vivo lipid peroxidation. In: Biochemical And Clinical Aspects of Oxygen. Caughey, W.S. (Ed.). Academic Press, New York. Pp. 137-154. Taylor, A., Lipman, R.D., Jahngen-Hodge, J., Palmer, V., Smith, D., Padhye, N., Dallal, G.E., Cyr, D.E., Laxman, E., and Shepard, D. (1995). Dietary calorie restriction in the Emory mouse: Effects of life span, eye lens cataract prevalence, and progression, levels of ascorbate, glutathione, glucose, and glycohemoglobin, tail collagen break time, DNA and RNA oxidation, skin integrity, fecundity, and cancer. Mech. Aging Dev., 79, 33-57. Teixeira, A., Ferreira, M., van Dijk, W., van de Werken, G., and de Jong, A. (1995). Analysis of 8-hydroxy2’deoxyguanosine in rat urine and liver DNA by stable isotope dilution gas chromatography/mass spectrometry. Anal. Biochem., 226, 307-319. Ter Steeg, J.C.A., Koster-Kamphuis, L., Van Straatan, E.A., Forget, P.P., and Buurman, W.A. (1998). Nitrotyrosine in plasma of celiac disease patients as detected by a new sandwich ELISA. Free Radic. Bio. Med., 25, 953-963. Thiffault, C., Aumont, N., Quirion, R., and Poirier, J. (1995). Effect of MPTP and L-deprenyl on antioxidant enzymes and lipid peroxidation levels in mouse brain. J. Neurochem., 65, 2725-2733. Thom, S.R., Xu, Y.A., and Ischiropoulos, H. (1997). Vascular endothelial cells generate peroxynitrite in response to carbon monoxide exposure. Chem. Res. Toxicol., 10, 1023-1031. Thoma, I., Loeffler, C., Sinha, A.K., Gupta, M., Krischke, M., Steffan, B, and Mueller, M.J. (2003). Cyclopentanone isoprostanes induced by reactive oxygen species trigger defense gene activation and phytoalexin accumulation in plants. Plant J., 34, 363-375. Thomas, J.P., Maiorino, M., Ursini, F., and Girotti, A.W. (1990a). Protective action of phospholipid hydroperoxide glutathione peroxidase against membrane-damaging lipid peroxidation. J. Biol. Chem., 265, 454-461. Thomas, J.P., Geiger, P.G., Maiorino, M., Ursini, F., and Girotti, A.W. (1990b). Enzymatic reduction of phospholipid and cholesterol hydroperoxides in artificial bilayers and lipoproteins. Biochim. Biophys. Acta, 1045, 252-260. Thomas, P.J., Qu, B.H., and Pedersen, P.L. (1995). Defective protein folding as a basis of human disease. Trends Biochem. Sci., 20, 456-459. WWW.ESAINC.COM 300 Thomas, R.M., Nauseef, W.M., Lyer, S.S., Peterson, M.W., Stone, P.J., and Clark, R.A. (1991). A cytosolic inhibitor of human neutrophil elastase and cathepsin G. J. Leukocyte Biol., 50, 568-579. Tohgi, H., Abe, T., Yamazaki, K., Murata, T., Ishizaki, E., and Isobe, C. (1999a). Remarkable increase in cerebrospinal fluid 3-nitrotyrosine in patients with sporadic amyotrophic lateral sclerosis. Ann. Neurol., 46, 129-131. Tohgi, H., Abe, T., Yamazaki, K., Murata, T., Ishizaki, E., and Isobe, C. (1999b). Alterations of 3-nitrotyrosine concentrations in the cerebrospinal fluid during aging and in patients with Alzheimer’s disease. Neurosci. Letts., 269, 52-54. Tomita, M., Okuyama, T., Hatta, Y., and Kawaj, S. (1990). Determination of free malondialdehyde by gas chromatography with an electron-capture detector. J. Chromatogr., 526, 174-179. Toraason, M., Hayden, C., Marlow, D., Rinehart, R., Mathias, P., Werren, D., DeBord, D.G., and Reid, T.M. (2001). DNA strand breaks, oxidative damage, and 1-OH pyrene in roofers with coal-tar pitch dust and/or asphalt fume exposure. Int. Arch. Environ. Health, 74, 396-404. Trelstad, R.L., Lawley, K.R., and Holmes, L.B. (1981). Nonenzymatic hydroxylations of proline and lysine by reduced oxygen derivatives. Nature, 289, 310-322. Tsikas, D., Schwedhelm, E., Fauler, J., Gutzki, F., Mayatepek, E., and Frolich, J.C. (1998). Specific and rapid quantification of 8-iso-prostaglandin F2α in urine of healthy humans and patients with Zellweger syndrome by GC-MS. J. Chromatogr., B, 716, 7-17. Tsikas, D., Schwedhelm, E., Suchy, M.T., Niemann, J., Gutzki, F.M., Erpenbecl, V.J., Hohfield, J.M., Surdacki, A., and Frolich, J.C. (2003). Divergence in urinary 8-iso-PGF(2alpha) levels. Possible methodological, mechanistic and clinical implications. J. Chromatrogr. B, 794, 237-255. Uchida, K., and Kawakishi, S. (1989). Bioorg. Chem., 17, 330-343. Uchida, K., and Kawakishi, S. (1990). Site-specific oxidation of angiotensin 1 by copper(II) and L-ascorbate: Conversion of histidine into 2-imidazolones. Arch. Biochem. Biophys., 283, 20-26. Uchida, K., and Kawakishi, S. (1993). 2-Oxohistidine as a novel biological marker for oxidatively modified proteins. FEBS Lett., 332, 208-210. Uchida, K., and Stadtman, E.R. (1992). Modification of histidine residues in proteins by reaction with 4- hydroxynonenal. Proc. Natl. Acad. Sci. USA, 89, 4544-4548. Uchida, K., and Stadtman, E.R. (1994). Quantitation of 4-hydroxynonenal protein adducts. Meth. Enzymol., 233, 371-382. Uchida, K., Kanematsu, M., Mortimitsu, Y., Osawa, T., Noguchi, N., and Niki, E. (1999a). Acrolein is a product of lipid peroxidation: Formation of free acrolein and its conjugate with lysine residues in oxidized low density lipoproteins. J. Biol. Chem., 273, 16058-16066. Uchida, K., Kanematsu, M., Sakai, K., Matsuda, T., Yasuda, Y., Miyata, T., Noguchi, N., Niki, E., and Osawa, T. (1998b). Protein-bound acrolein: Potential markers for oxidative stress. Proc. Natl. Acad. Sci. USA, 95, 4882-4887. Uchida, K., Kato, Y., and Kawakishi, S. (1990). A novel mechanism for oxidative cleavage of prolyl peptides induced by the hydroxyl radical. Biochem. Biophys. Res. Commun., 169, 265-271. Uchida, K., Sakai, K., Itakura, K., Osawa, T., Toyokuni, S. (1997). Protein modification by lipid peroxidation products: Formation of malondialdehyde-derived N(epsilon)-(2-propenol)lysine in proteins. Arch. Biochem. Biophys., 346, 45-52. Ueta, K., and Hayashi, O. (1985). ADP-ribosylation. Ann. Rev. Biochem., 54, 73-100. Ullrich, O., Reinheckel T., Sitte, N., and Grune, T. (1999). Degradation of hypochlorite-damaged glucose-6-phosphate dehydrogenase by the 20S proteosome. Free Radic. Biol. Med., 27, 487-492. Ullrich, O., Sitte, N., Sommerburg, O., Sandig, V., Davies, K.J., and Grune, T. (1999). Influence of DNA binding on the degradation of oxidized histones by the 20S proteosome. Arch. Biochem. Biophys., 362, 211-216. Ursini, F., and Bindoli, A. (1984). The role of selenium peroxidases in the protection against oxidative damage of membranes. Chem. Phys. Lipids, 44, 255-276. Ursini, F., Maiorino, M., Gregolin, C. (1985). The selenoenzyme phospholipid hydroperoxide glutathione peroxidase. Biochim. Biophys. Acta, 839, 62-70. Ursini, F., Zamburlini, A., Cazzolato, G., Maiorino, M., Bon, G.B., and Sevanian, A. (1998). Postprandial plasma lipid hydroperoxides: A possible link between diet and atherosclerosis. Free Radic. Biol. Med., 25, 250-252. Uttenthal, L.O., Alonso, D., Fernandez, A.P., Campbell R.O., Moro, M.A., Leza, J.C., and Rodrigo, J. (1998). Neural and inducible nitric oxide synthase and nitrotyrosine immunoreactivities in the cerebral cortex of the aging rat. Microsc. Res. Tech., 43, 75-88. 32 Vaca, C.E., Fang, J.L., Mutanen, M., and Valsta, L. (1995). P-postlabelling determination of DNA adducts of malonaldehyde in humans: Total white blood cells and breast tissue. Carcinogen., 16, 1847-1851. Van Buskirk, J.J., Kirsch, W.M., Kleyer, D.L., Darklen, R.M., and Koch, T.H. (1984). Aminomalonic acid: Identification in E. coli and atherosclerotic plaque. Proc. Natl. Acad. Sci. USA, 81, 722-725. Van Delft, J.H.M., van Winden, M.J.M., van den Ende, A.M.C., and Baan, R.A. (1993). Determining N7-alkylguanine adducts by immunochemical methods and HPLC with electrochemical detection. Applications in animal studies and in monitoring human exposure to alkylating agents. Environ. Health Perspect., 99, 25-32. Van der Vliet, A., Eiserich, J.P., Halliwell, B. and Cross, C.E. (1997). Formation of reactive nitrogen species during peroxidase-catalyzed oxidation of nitrite. A potential additional mechanism of nitric oxide-dependent toxicity. J. Biol. Chem., 272, 7617-7625. Van der Vliet, A., Eiserich, J.P., Kaur, H., Cross, C.E., and Halliwell, B. (1996). Nitrotyrosine as biomarker for reactive nitrogen species. Meth. Enzymol., 269, 175-184. Van der Vliet, A., Eiserich, J.P., O’Niell, C.A., Halliwell, B., and Cross, C.E. (1995). Tyrosine modification by reactive nitrogen species: A closer look. Arch. Biochem. Biophys., 319, 341-349. Van der Vleit, A., Jenner, A., Eiserich, J.P., Cross, C.E., and Halliwell, B. (1999). Analysis of aromatic nitration, chlorination, and hydroxylation by gas chromatography-mass spectrometry. Meth. Enzymol., 301, 471-483. Van der Vliet, A., O’Neill, C.A.O., Halliwell, B., Cross, C.E., and Kaur, H. (1994). Aromatic hydroxylation and nitration of phenylalanine and tyrosine by peroxynitrite. FEBS Letts., 339, 89-92. WWW.ESAINC.COM 301 Van den Akker, E., Lutgerink, J.T., Lafleur, M.V.M., Joenje, H., and Retel, J. (1994). The formation of one-G deletions as a consequence of single-oxygen-induced DNA damage. Mutat. Res., 309, 45-52. Vane, J.R., Mitchell, J.A., Appleton, I., Tomlinson, A., Bishop-Bailey, D., Croxtall, J., and Willoghby, D.A. (1994). Inducible isoforms of cyclo-oxygenase and nitric oxide synthase in inflammation. Proc. Natl. Acad. Sci. USA, 91, 2046-2050. Van Houten, B. (1990). Nucleotide excision repair in Escherichia coli. Microbiol. Rev., 54, 18-51. Van Houten, B. (1998). Damage and Repair of Mitochondrial DNA. Oxygen Society, Washington DC. Van Kuijk, F.J., (1987). Sevanian, A., Handelman, G.J., and Dratz, E.A. (1987). A new role for phospholipase A2: Protection of membranes from lipid peroxidation damage. Trends Biochem. Sci., 12, 31-34. Van Kuijk, F.J., (1988). Thesis. See Esterbauer, H., Schaur, R.J., and Zollner, H. (1991). Chemistry and biochemistry of 4hydroxynonenal, malondialdehyde and related aldehydes. Free Radic. Biol. Med., 11, 81-128. Van Kuijk, F.J., and Dratz, E.A. (1987). Detection of phospholipid peroxides in biological samples. Free Radic. Biol. Med., 3, 349-354. Van Kuijk, F.J., Holte, L.L., and Dratz, E.A. (1990). 4-Hydroxyhexenal: A lipid peroxidation product derived from oxidized docosahexaenoic acid. Biochim. Biophys. Acta, 1043, 116-118. van Poppel, G., Poulsen, H.E., Loft, S., and Verhagen, H. (1995). No influence of beta-carotene on oxidative DNA damage in male smokers. J. Natl. Acad. Sci. USA, 90, 310-311. Varshavsky, A. (1997). The N-end rule pathway of protein degradation. Genes Cells, 2, 13-28. Vassalle, C., Botoo, N., Andreassi, M.G., Berti, S., and Biagini, A. (2003). Evidence for enhanced 8-isoprostane plasma levels, as index of oxidative stress in vivo, in patients with coronary heart disease. Coron. Artery Dis., 14, 213-218. Vatassery, G.T., Quach, H.T., Smith, W.E., Krick, T.P., and Ungar, F. (1997). Analysis of hydroxy and keto cholesterols in oxidized brain synaptosomes. Lipids, 32, 101-107. Verhagen, H., Poulsen, H.E., Loft, S., van Poppel, G., Willems, M.I., and van Bladeren, P.J. (1995). Reduction of oxidative DNA-damage in humans by brussel sprouts. Carcinogen., 16, 969-970. Vernia, S, Sanz-Gonzalez, S.M., and Lopez-Garcia, M.P. (2001). Involvement of peroxynitrite on early loss of p450 in short-term hepatocytes cultures. Adv. Exp. Med. Biol., 500, 209-212. Verweij, H., Christianse, K., and Van Steveninck, J. (1982). Ozone-induced formation of O,O’-dityrosine cross-links in proteins. Biochem. Biophys. Acta, 701, 180-184. Viera, L., Zu, Y., Estevez, A.G., and Beckman, J.S. (1999). Immunohistochemical methods to detect nitrotyrosine. Meth. Enzymol., 301, 373-381. Vigue, C.A., Frei, B., Shigenaga, M.K., Ames, B.N., Packer, L., and Brooks, G.A. (1993). Antioxidant stress and indexes of oxidative stress during consecutive days of exercise. J. Appl. Physiol., 75: 566-573. Viinikka, L., Vuori, J., and Ylikorkala, O. (1984). Lipid peroxides, prostacyclin and thromboxane A2 in runners during acute exercise. Med. Sci. Sport Exer., 16, 275-277. Viner, R.I., Ferrington, D.A., Williams, T.D., Bigelow, D.J., and Schoneich, C. (1999). Protein modification during biological 2+ aging: Selective tyrosine nitration of the SERCA2a isoform of sarcoplasmic reticulum Ca -ATPase in skeletal muscle. Biochem. J., 340, 657-669. Viner, R.I., Huhmer, A.F., Bigelow, D.J., and Schoneich, C. (1996). The oxidative inactivation of sarcoplasmic reticulum 2+ Ca -ATPase by peroxynitrite. Free Radic. Res., 24, 243-259. Visick, J.E., and Clarke, S. (1995). Repair, refold, recylce: How bacteria can deal with spontaneous environmental damage to proteins. Mol. Microbiol., 16, 835-845. Vissers, M.C., and Winterbourne, C.C. (1991). Oxidative damage to fibronectin. II. The effects of H2O2 and the hydroxyl radical. Arch. Biochem. Biophys., 285, 357-364. Vogt, W. (1995). Oxidation of methionyl residues in proteins: Tools, targets, and reversal. Free Radic. Biol. Med., 18, 6173. Von Sonntag, C. (1990). In: Sulfur-centered Reactive Intermediates in Chemistry and Biology. Chatgilialoglu, C., and Asmus, K.D. (Eds.). Plenum Press, New York. Pp. 359-366. Von Sonntag, C. (1991). The chemistry of free-radical-mediated DNA damage. In: Physical and Chemical Mechanisms in Molecular Radiation Biology. Glass, W.A., and Varma, M.N. (Eds.). Plenum Press, New York. Pp. 287-321. Waeg, G., Dimsity, G., and Esterbauer, H. (1996). Monoclonal antibodies for detection of 4-hydroxynonenal modified proteins. Free Rad. Res., 25, 149-159. Wagner, J.R., Hu, C.-C., and Ames, B.N. (1992). Endogenous oxidative damage of deoxycytidine in DNA. Proc. Natl. Acad. Sci. USA, 89, 3380-3384. Wallace, D.C. (1992). Diseases of the mitochondrial DNA. Ann. Rev. Biochem., 61, 1175-1212. Wang, M.L., and Liehr, J.G. (1991). Detection of DNA adducts of unsaturated fatty acids (UF) hydroperoxides (UF-OOH) 32 by P-postlabeling analysis. Proc. Ann. Meet. Am. Assoc. Cancer Res., 32, A864. Wang, Z.Q., Auer, B., Stingl, L., Berghammer, H., Haidacher, D., Schweiger, M., and Wagner, E.F. (1995). Mice lacking ADPRT and poly(ADP-ribosyl)ation develop normally but are susceptible to skin disease. Genes Dev., 9, 510-520. Wani, G., and D’Ambrosia, S.M. (1995). Cell type-specific expression of human 8-oxo-7,8-dihydroguanosine triphosphate in normal breast and skin tissues in vivo. Carcinogen., 16, 277-283. Ward, J.F., Blakely, W.F., and Joner, E.I. (1985). Mammalian cells are not killed by DNA single-strand breaks caused by hydroxyl radicals from hydrogen peroxide. Radiat. Res., 103, 383-392. Waterfall, A.H., Singh, G., Fry, J.R., and Marsden, C.A. (1996). Acute acidosis elevates malondialdehyde in rat brain in vivo. Brain Res., 712, 102-106. Wei, H., Cai, Q., Rahn, R., and Zhang, X. (1997). Singlet oxygen involvement in ultraviolet (254nm) radiation-induced formation of 8-hydroxy-deoxyguanosine in DNA. Free Radic. Biol. Med., 23, 148-154. Wei, Y.H. (1998). Mitochondrial DNA mutations and oxidative damage in aging and diseases: An emerging paradigm of gerontology and medicine. Proc. Natl. Acad. Sci Counc. Repub. China, B., 22, 55-67. Weiland, E., Schettler, V., Diedrich, F., Schuff-Werner, P., and Oellerich, M. (1992). Determination of lipid hydroperoxides in serum iodometry and high performance liquid chromatography compared. Eur. J. Clein. Chem. Clin. Biochem., 30, 363-369. WWW.ESAINC.COM 302 Weimann, A., Belling, D., and Poulsen, H.E. (2001). Measurement of 8-oxo-2’-deoxyguanosine and 8-oxo-2’deoxyadenosine in DNA and human urine by high performance liquid chromatography-electrospray tandem mass spectrometry. Free Radic. Biol. Med., 30, 757-764. Weinberg, R.A. (Ed.). (1989). Oncogenes and The Molecular Origins of Cancer. Cold Spring Harbor Press, New York. Weitzman, S.A., Turk, P.W., Milkowaki, D.H., Kozlowski, K. (1994). Free radical adducts induce alterations in DNA cytosine methylation. Proc. Natl. Acad. Sci. USA, 91, 1261-1264. Wells-Knecht, M.C., Huggins, T.G., Dyer, D.G., Thorpe, S.R., and Baynes, J.W. (1993). Oxidized amino acids in lens protein with age. Measurement of o-tyrosine and dityrosine in the aging human lens. J. Biol. Chem., 268, 12348-12352. Wells-Knecht, M.C., Zyzak, D.V., Litchfield, J.E., Thorpe, S.R., and Baynes, J.W. (1995). Mechanism of autoxidative glycosylation: Identification of glyoxal and arabinose as intermediates in the autoxidative modification of proteins by glucose. Biochem., 34, 3702-3709. Wendt, T., Tanji, N., Guo, J., Hudson, B.I., Bierhaus, A., Ramasamy, R., Arnold, B., Nawroth, P.P., D’Agati, V., and Scmidt, A.M. (2003). Glucose, glycation and RAGE: Implications for amplification of cellular dysfunction in diabetic nephropathy. J. Am. Soc. Nephrol., 14, 1383-1395. Westhuyzen, J. (1997). The oxidation hypothesis of atherosclerosis: An update. Ann. Clin. Lab. Sci., 27, 1-10. White, C.R., Brock, T.A., Chang, L.Y., Crapo, J., Briscoe, P., Ku, D., Bradley, W.A., Gianturco, S.H., Gore, J., and Freeman, B.A. (1994). Superoxide and peroxynitrite in atherosclerosis. Proc. Natl. Acad. Sci. USA, 91, 1044-1048. Whiteman, M., and Halliwell, B. (1999b). Loss of 3-nitrotyrosine on exposure to hypochlorous acid: Implications for the use of 3-nitrotyrosine as a biomarker in vivo. Biochem. Biophys. Res. Commun., 258, 168-172. Whiteman, M., Hong, H.S., Jenner, A., and Halliwell, B. (2002). Loss of oxidized and chlorinated bases in DNA treated with ROS. Biochem. Biophys. Res. Commun., 296, 883-889. Whiteman, M., Jenner, A., and Halliwell, B. (1997). Hypochlorous acid –induced base modifications in isolated calf thymus DNA. Chem. Res. Toxicol., 10, 1240-1246. Whiteman, M., Spencer, J.P., Jenner, A., and Halliwell, B. (1999a). Hypochlorous acid-induced DNA base modification: Potentiation by nitrite: Biomarkers of DNA damage by reactive oxygen species. Biochem. Biophys. Res. Commun., 257, 572-576. Wicric, E.J.M.V., Leeuwen, R.E.W., van Hendricks, H.F.J., Verhagen, H., Loft, H., and Poulsen, H.E., van der Berg, H. (1995). Moderate energy restriction does not affect parameters of oxidative stress and genotoxicity in man. J. Nutr., 125, 2631-2639. Wilkinson, K.D. (1997). Regulation of ubiquitin-dependent processes by deubiquitinating enzymes. FASEB J., 11, 12451256. Wilson, V.L., Taffe, B.G., Shields, P.G., Powey, A.C., and Harris, C.C. (1993). Detection and quantification of 8hydroxydeoxyguanosine adducts in peripheral blood of people exposed to ionizing radiation. Environ. Health Prosp., 99, 261-263. Winterbourne, C.C., and Buss, I.H. (1998), Protein carbonyl measurement by an enzyme-linked immunosorbent assay. Meth. Enzymol., 300, 106-111. Winterbourn, C.C., Pichorner, H., and Kettle, A.J. (1997). Myeloperoxidase-dependent generation of a tyrosine peroxide by neutrophils. Arch. Biochem. Biophys., 338, 15-21. Wiseman, H., and Halliwell, B. (1996). Damage to DNA by reactive oxygen and nitrogen species: Role in inflammatory disease and progression to cancer. Biochem. J., 313, 17-29. Wishnok, J.S., Tannedbaum, S.R., Stillwell, W.G., Glogowski, J.A., and Leaf, C.D. (1993). Urinary markers for exposure to alkylating or nitrosating agents. Environ. Health Perspect., 99, 155-159. Witherell, H.L., Hiatt, R.A., Replogle, M., and Parsonnet, J. (1998). Helicobacter pylori infection and urinary excretion of 8hydroxy-2-deoxyguanosine, and oxidative DNA adduct. Cancer Epidemiol. Biomarkers Prev., 7, 91-96. Witt, E.H., Reznick, A.Z., Viguie, C.A., Starke-Reed, P., and Packer, L. (1992). Exercise, oxidative damage and effects of antioxidant manipulation. J. Nutr., 122, 766-773. Wizemann, T.M., Gardner, C.R., Laskin, J.D., Quinones, S., Durham, S.K., Goller, N.L., Ohnishi, S.T., and Laskin, D.L. (1994). Production of nitric oxide and peroxynitrite in the lung during acute endotoxemia. J. Leukoc. Biol., 56, 759-768. Wolff, S.P., Jiang, Z.Y., and Hunt, J.V. (1991). Protein glycation and oxidative stress in diabetes mellitus and aging. Free Radic. Biol. Med., 10, 339-352. Wong, S.H.Y., Knight, J.A., Hopfer, S.M., Zaharia, O., Leach, C.N., and Sunderman, F.W. (1987). Lipoperoxides in plasma as measured by liquid-chromatographic separation of malondialdehyde thiobarbituric acid adduct. Clin. Chem., 33, 214-220. Wood, M.L., Dizdaroglu, M., Gajewski, E., and Essigmann, J.M. (1990). Mechanistic studies of ionizing radiation and oxidative mutagenesis: Genetic effects of a single 8-hydroxyguanine (7-hydro-8-oxoguanine) residue inserted at a unique site in a viral genome. Biochem., 29, 7024-7032. Wu, G.S., Zhnag, J., and Rao, N.A. (1997). Peroxynitrite and oxidative damage in experimental autoimmune uveitis. Invest. Ophthalmol. Vis. Sci., 38, 1333-1339. Wu, W., Chen, Y., d’Avignon, A., and Hazen, S.L. (1999). 3-Bromotyrosine and 3,5-dibromotyrosine are major products of protein oxidation by eosinophil peroxidase: Potential markers for eosinophil-dependent tissue injury in vivo. Biochem., 38, 3538-3548. Yagi, K. (1998). Simple procedure for specific assay of lipid hydroperoxides in serum and plasma. In: Methods in Molecular Biology, 108. Armstrong, D. (Ed.). Humana Press, Totowa, New Jersey. Pp. 107-110. Yakes, F.M., and Van Houten, B. (1997). Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc. Natl. Acad. Sci. USA, 94, 514-519. Yamada, K., Terao, J., and Matsushita, S. (1987). Electrochemical detection of phospholipid hydroperoxides in reversedphase high-performance liquid chromatography. Lipids, 22, 125-128. Yamamoto, F., Frei, B., and Ames, B.N. (1990). Assay of lipid hydroperoxides using HPLC with post-column chemiluminescence detection. Meth. Enzymol., 186, 371-380. WWW.ESAINC.COM 303 Yamamoto, T., Hosokawa, K., Tamura, T., Kanno, H., Urabe, M., and Honjo, H. (1996). Urinary 8-hydroxy-2’deoxyguanosine (8-OhdG) levels in women with and without gynocological cancer. J. Obstet. Gynaecol. Res., 22, 359363. Yamamoto, Y. (1994). Chemiluminescence-based high-performance liquid chromatography assay of lipid hydroperoxides. Meth. Enzymol., 233, 319-324. Yamamoto, Y., and Ames, B.N. (1987). Detection of lipid hydroperoxides and hydrogen peroxide at picomole levels by an HPLC and isoluminol chemiluminescence assay. Free Radic. Biol. Med., 3, 359-361. Yamamoto, Y., Brodsky, M.H., Baker, J.C., and Ames, B.N. (1987). Detection and characterization of lipid hydroperoxides at picomole levels by high-performance liquid chromatography. Anal. Biochem., 160, 7-13. Yamamoto, Y., Kambayashi, Y., and Ueda, T. (1998). Assay of phospholipid hydroperoxides by chemiluminescencebased high-performance liquid chromatography. In: Methods in Molecular Biology, 108. Armstrong, D. (Ed.). Humana Press, Totowa, New Jersey. Pp. 63-70.. Yamim, G., Uversky, V.N., and Fink, A.L. (2003). Nitration inhibits fibrillation of human alpha-synuclein in vitro by formation of soluble oligomers. FEBS Letts., 542, 147-152. Yan, S.D., Chen, X., Schmidt, A.M., Brett, J., Godman, G., Zou, Y.S., Scott, C.W., Caputo, C., Frappier, T., Smith, M.A., Perry, G., Yen, S.H., and Stern, D. (1994). Glycated tau protein in Alzheimer’s disease: A mechanism for induction of oxidative stress. Proc. Natl. Acad. Sci. USA, 91, 7787-7791. Yan, S.D., Schmidt, A.M., Anderson, G.M., Zhang, J., Brett, J., Zou, Y.S., Pinsky, D., and Stern, D. (1994). Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptor/binding proteins. J. Biol. Chem., 269, 9889-9897. Yanez, L., Groffen, J., and Valenzuela, D.M. (1987). C-K-ras mutations in human carcinomas occur preferentially in codon 12. Oncogene, 1, 315-318. Yang, C.C., Alvarez, R.B., Engel, W.K., and Askanas, V. (1996). Increase of nitric oxide synthases and nitrotyrosine in inclusion-body myositis. Neuroreport, 8, 153-158. Yang, C.-S., Tsai, P.-J., Wu, J.-P., Lin, N.-N., Chou, S.T., and Kuo, J.-S. (1997). Evaluation of extracellular lipid peroxidation in brain cortex of anesthetized rats by microdialysis perfusion and high-performance liquid chromatography with fluorometric detection. J. Chromatogr. B., 693, 257-263. Yang, M.H., and Schaich, K.M. (1996). Factors affecting DNA damage caused by lipid hydroperoxides and aldehydes. Free Radic. Biol. Med., 20, 225-236. Yasaka, T., Ohya, I., Matsumoto, J., Shiramizu, T., and Sasaguri, Y. (1981). Acceleration of lipid peroxidation in human paraquat poisoning. Arch. Intern. Med., 141, 1169-1173. Yasmin, W., Strynadka, R., and Schulz, R. (1997). Generation of peroxynitrite contributes to ischemia-reperfusion injury in isolated rat hearts. Cardiovasc. Res., 33, 422-432. Yasuda, M., and Narita, S. (1997). Simultaneous determination of phospholipid hydroperoxides and cholesteryl ester hydroperoxides in human plasma by high-performance liquid chromatography with chemiluminescence detection. J. Chromatogr. B., 693, 211-217. Ye, Y.Z., Strong, M., Huang, Z-Q., and Beckman, J.S. (1996). Antibodies that recognize nitrotyrosine. Meth. Enzymol., 269, 201-209. Yeo, H.C., Helbock, H.J., Chyu, D.W., and Ames, B.N. (1994). Assay of malondialdehyde in biological fluids by gas chromatography-mass spectrometry. Anal. Biochem., 220, 391-396. Yermilov, V., Rubio, J., and Ohshima, H. (1995). Formation of 8-nitroguanine in DNA treated with peroxynitrite in vitro and its rapid removal from DNA by depurination. FEBS Lett., 376, 207-210. Yermilov, V., Yoshie, Y., Rubio, J., and Ohshima, H. (1996). Effects of carbon dioxide/bicarbonate on induction of DNA single-strand breaks and formation of 8-nitroguanine and base-propenal mediated by peroxynitrite. FEBS Lett., 399, 6776. Yi, D., Smythe, G.A., Blount, B.C., and Duncan, M.W. (1997). Peroxynitrite-mediated nitration of peptides: Characterization of products by electrospray and combined gas chromatography-mass spectrometry. Arch. Biochem. Biophys., 344, 253-259. Yin, B., Whyatt, R.M., Perera, F.P., Randall, M.C., Cooper, T.B., and Santella, R.M. (1995). Determination of 8hydroxydeoxyguanosine by an immunoaffinity chromatography-monoclonal antibody-based ELISA. Free Radic. Biol. Med., 18, 1023-1032. Yin, D., and Brunk, U. (1998). Autofluorescent ceroid/lipofuscin. In: Methods in Molecular Biology, 108. Armstrong, D. (Ed.). Humana Press, Totowa, New Jersey. Pp. 217-227.. Yoshie, Y., and Ahshima, H. (1998). Synergistic induction of DNA strand breakage by catechol-estrogen and nitric oxide: Implications for hormonal carcinogenesis. Free Radic. Biol. Med., 24, 341-344. Yoshino, K., Sano, M., Fujita, M., and Tomita, I. (1986). Formation of aliphatic aldehydes in rat plasma and liver due to vitamin E deficiency. Chem. Pharm. Bull., 34, 5184-5187. Zarkovic, N. (2003). 4-Hydroxynonenal as a bioactive marker of pathophysiological processes. Mol. Aspects Med., 24, 281-291. Zhang, C., Reiter, C., Esiserich, J.P., Boersa, B., Parks, D.A., Beckman, J.S., Barnes, S., Kirk, M., Baldus, S., DarleyUsmar, V.M., and White, C.R. (2001). L-Arginine chlorination inhibits endotheilial nitric oxide production. J. Biol. Chem., 276, 27159-271-65. Zhang, J.-R., Andrus, P.K., and Hall, E.D. (1994). Age-related phospholipid hydroperoxide levels in gerbil brain measured by HPLC-chemiluminescence and their relation to hydroxyl radical stress. Brain Res., 639, 275-282. Zou, M., Martin, C., and Ullrich, V. (1997). Tyrosine nitration as a mechanism of selective inactivation of prostacyclin synthase by peroxynitrite. Biol. Chem., 378, 707-713. Zou, M., Yesilkaya, A., and Ullrich, V. (1999). Peroxynitrite inactivates prostacyclin synthase by heme-thiolate-catalyzed tyrosine nitration. Drug. Metab. Rev., 31, 343-349. WWW.ESAINC.COM 304 Appendix 3.1 Typical DNA Extraction and Hydrolysis With Thanks to M.L. Maidt and R. Floyd (Oklahoma Medical Research Foundation, OK). DNA Extraction Procedure (non-chaotropic). I. Reagents. Homogenizing buffer: 0.3 M sucrose, 0.025 M TRIS buffer, 0.002 M EDTA. Adjust pH to 7.2 with 1.0 M HCl. Filter solution through a 0.2 µm Nylon filter into a sterile, autoclaved bottle. Store at 4oC for up to 6 months. RES (RNA extraction solution): 1.0 M lithium chloride, 2 M urea, 0.04 M sodium citrate, 0.005 M EDTA, 2% SDS Adjust to pH 6.8 with 1.0 M HCl. Filter solution through 0.2 µm Nylon filter before addition of SDS. Store at room temperature for up to 6 months. DNAse RNAse Proteinase K TE solution: 0.010 M TRIS buffer, 0.001 M EDTA CIA solution: Chloroform:isoamyl alcohol (24:1) 3 M sodium acetate solution (pH 7.0) 95% ethanol II. Procedural Notes. 1. 2. 3. Adjust pH of solutions with 1.0 M HCl or 1.0 M NaOH. In order to minimize contamination; wear gloves during all procedures. Homogenizing solution must be filtered through a 0.2 µm Nylon-66 filter into a sterile bottle and stored at 4oC for up to 6 months. Discard solution if bacterial growth is observed. All bottles should be clean, RNA/DNA free, and stored with caps/covers on. 4. WWW.ESAINC.COM 305 III. Procedure. 1. Homogenize tissue in homogenizing buffer (at a ratio of 1:4 to 1:5; e.g., 100 mg tissue to 0.4 - 0.5 mL buffer) for 10 - 15 sec. For softer tissues (i.e. brain) a Teflon plunger-type homogenizer can be used. For harder tissue (e.g., muscle, heart, intestine, etc.) a Polytron-type homogenizer can be used to shred the tissue. Add an equal volume of RES. Calculate the amount of RNAse solution that needs to be added to the sample solution in order to achieve a final concentration of 100 µg/mL. Heat this RNAse aliquot at 70oC for 10 min to inactivate DNAse. After heating, add the RNAse to the sample solution to achieve a final concentration of 100 µg/mL and incubate for 30 min at 50oC. Add sufficient proteinase K to achieve a concentration of 250 µg/mL and incubate at 50oC for 60 min. Add an equal volume of ClA and place tubes on rotary mixer. Mix slowly at 15 – 20 rotations/min for 15 min. Place tubes in centrifuge and spin for 5 min at 2,000 – 3,000 g. After centrifuging, the tube will have an aqueous portion on top that contains the DNA/RNA mixture. The bottom layer contains the ClA. The interface between the two layers contains proteins. Remove the aqueous layer into a clean tube, taking care not to remove any of the proteincontaining interface. Repeat steps 5 through 7 two more times. With each successive extraction, the protein-containing interface should become visibly smaller. Add a 1:15 aliquot (e.g. 10 µL to 150 µL sample) of 3M sodium acetate (pH 7.00) and 2.5x volume of 95% (e.g. 1,250 µL to 500 µL aqueous sample volume). Place tube into refrigerator (4oC) for 60 min. Place tube in centrifuge at 3,000 – 4,000 g for 10 min to spin out the precipitated DNA. Evaporate the sample to dryness. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. Note – Step 3 is only necessary if an RNA-free sample is desired. This step can be omitted since the RNA, DNA and adducts that will be extracted can be separated chromatographically on the HPLC-ECD system. WWW.ESAINC.COM 306 2. DNA Hydrolysis Procedure. I. Reagents. TE Solution: 0.010 M TRIS buffer, 0.001 M EDTA. 0.5 M Sodium acetate, pH 5.1 1.0 M Magnesium chloride Nuclease P1: 10 mg/mL prepared in water and stored at 4oC TRIS base: 1.0 M solution, pH 10.0 Alkaline phosphatase: 1 unit/µL solution 5.8 M acetic acid II. Procedure. 1. 2. 3. 4. If not in solution, solubilize the DNA in 0.25 mL TE solution. Add 25 µL of 0.5 M sodium acetate (pH 5.1). Add 2.75 µL of 0.1 M magnesium chloride. Heat sample in a boiling water bath (100oC) for 5 min to make the DNA single stranded. Immediately cool the sample on ice for 5-10 min. Once cooled, add 10 µL (10 µg) of nuclease P1 from a stock solution prepared at 1.0 mg/mL in water. Incubate the sample at 37oC for 1 hr. Add 8 µL of 1.0M TRIS base (pH 10.0) to adjust to pH 7.8. Add 2 µL alkaline phosphatase (equivalent to 2 units at stock concentration of 1 unit/µL). Incubate sample at 37oC for 1hr. Precipitate enzyme by adding 4 µL of 5.8 M acetic acid. Place sample in centrifuge and spin at 3,000-4,000 g for 5 min. Remove the aqueous portion that contains the hydrolysate and filter this solution through a 0.2 µm spin filter (5,000 g for 10 min). The sample is now ready for injection onto the HPLC system. 5. 6. 7. 8. 9. 10. 11. 12. 13. Note: DNA extractor kits are also available from Wako Fine Chemical Company. WWW.ESAINC.COM 307 Chapter 4 Protection Living organisms are constantly being exposed to a variety of pro-oxidant species capable of damaging many vitally important biomolecules, yet life still thrives. This is because, during evolution, the cell developed a series of defensive mechanisms designed to minimize the consequences of pro-oxidant action. We have already discussed repair and destruction of damaged macromolecules in Chapter 3. Now it is time to turn our attention to the protective role played by the antioxidants. This chapter will present an overview of endogenous and exogenous antioxidants, examine the use antioxidant therapy to treat disease, present ways of estimating the total antioxidant capacity of different systems and explore the use of antioxidants as food preservatives. WWW.ESAINC.COM 308 INTRODUCTION. Mention the word “antioxidant” and most people would probably think of vitamins C or E. However, although these compounds are vitally important antioxidants, many other molecules are used as antioxidants in vivo.1 In fact antioxidants are made up of three different classes of molecules: 1. Enzymes (e.g., catalase and superoxide dismutase). 2. Metal sequestering proteins (e.g., ferritin and ceruloplasmin). 3. Low molecular weight (small) molecules (e.g., vitamins C (ascorbic acid) and E (α-tocopherol). Perhaps the best definition of an antioxidant was put forward by Halliwell and Gutteridge (1990) who stated that an antioxidant is “any substance that, when present at low concentrations compared to those of an oxidizable substrate, significantly delays or inhibits oxidation of that substrate”. In this instance a substance could be any of the three classes of antioxidants mentioned above, while substrate refers to many biologically important molecules such as DNA, lipids and proteins. However, this definition is not perfect. For example, the hydroxyl free radical reacts with virtually every molecule it encounters, but it would be ridiculous to propose that such molecules are antioxidants (see below). Lester Packer modified Halliwell and Gutteridges’ definition and suggested that such compounds should ideally display a range of antioxidant activities, react with more than just one specific pro-oxidant, and be present in sufficient concentration in vivo. This definition is appropriate for small molecule antioxidants but does not take into account the ability for enzymes and metal sequestering proteins to act as antioxidants. The antioxidant or suite of antioxidants used to control oxidation is a very complex topic. Much is dependent upon which pro-oxidant species is involved, and where it is being produced. For example: 1) Under biological conditions, superoxide is relatively long lived. Its cellular level is readily controlled by intracellular enzymes but in the extracellular fluids, where enzyme activity is typically low, its level is probably either regulated by small molecule species, or once it has been taken up by erythrocytes, by enzymes. Control of the plasma levels of superoxide is of critical importance due to its reaction with circulating nitric oxide. Thus by influencing the level of superoxide, extracellular superoxide dismutase (ecSOD) can direct nitric oxide’s activity away from its normal physiological role towards oxidation through the formation of peroxynitrite. 2) The hydroxyl free radical is much too reactive to be controlled enzymatically. In fact, the hydroxyl free radical is just as capable of damaging enzymes as it is any other molecule it encounters. Rather than 1 Remember that for many compounds showing antioxidant activity, such reactions are often secondary to other more important biological functions. WWW.ESAINC.COM 309 dealing directly with the hydroxyl free radical, by far the most effective approach is to prevent its production in the first place. To this end, the availability of substrates involved in hydroxyl free radical production is tightly regulated: hydrogen peroxide is catabolized enzymatically, while iron is sequestered in an unreactive form. 3) Prevention of damage to the hydrophobic lipid portion of membranes is controlled by metal sequestration, enzymatic removal of pro-oxidants and through the reaction of lipid peroxyl radicals with the fat-soluble vitamin, αtocopherol. Regeneration of α-tocopherol from its radical is accomplished probably through reaction with cytosolic ascorbic acid or glutathione (GSH) at the membrane-cytosol interface. The next three sections will explore the way living organisms make use of enzymes, metal chelators and low molecular weight molecules as antioxidants. ENZYMES. Many different enzymes can act as antioxidants. Enzymes can be categorized as being either primary, reacting directly with pro-oxidants (e.g., superoxide dismutase (SOD), and catalase), or secondary, involved in regenerating low molecular weight antioxidant species (e.g., ascorbate dehydrogenase and glutathione reductase). Some background information including the basic properties of the antioxidant enzymes is presented in Table 4.1. Enzyme Ascorbate Dehydrogenase; GSH-dependent Dehydroascorbate (DHAA) Reductase; Glutaredoxin; NADH-dependent (DHAA) reductase; NADPHdependent (DHAA) Reductase Catalase (CAT) Comments Secondary. Regenerate ascorbic dehydroascorbic acid. acid from Primary. Discovered by Thernard in 1818. Named catalase by Loew in 1901. There are many forms of CAT – most contain heme but some contain manganese. E. coli contain two forms of CAT: HPI is a tetrameric protein (337,000 Daltons) containing 2 molecules of protoheme IX per tetramer and is associated with the periplasmic membrane. It is inducible by hydrogen peroxide and ascorbate. It possesses both catalytic and peroxidative activities. HPI is increased during log growth. HPII is a tetrameric WWW.ESAINC.COM protein containing Reference Maellaro et al. (1994); Park and Levine (1996); Rose and Bode (1992, 1993); Sauberlich (1994); Wells and Xu (1994) Iozzo et al. (1982); Michiels et al. (1994); Percy (1984); Radi et al. (1991); Singh et al. (1996) and references therein 2 310 molecules of protoheme IX per tetramer and is associated with the cytoplasm. It possesses peroxidatic activity only, is not inducible by either hydrogen peroxide or ascorbate and it is increased during the stationary phase of growth. Humans also contain at least two forms of CAT: Ubiquitous heme-based enzyme of molecular mass ~240,000 Daltons, consisting of four identical subunits each containing ferriprotoporphyrin. Different forms of the enzyme are found in peroxisomes and cytoplasm. CAT has also been reported to be found in the cytoplasmic granules of eosinophils and in heart mitochondria. It is abundant in liver (peroxisomes), kidney and erythrocytes (cytoplasm). In higher organisms CAT binds four molecules of NADPH. Although the function of NADPH binding is unclear it has been suggested to protect CAT from hydrogen peroxide inactivation or to act as a source of NADPH for glutathione peroxidase under conditions of stress. CAT concentrations are ~1.2 x 10-6, 3.8 x 10-8, and 7.2 x 10-7M for liver, heart and heart mitochondria, respectively. CAT is inhibited non-specifically by cyanide and azide, or specifically by aminotriazole, which interacts with compound 1 (see below). Thus aminotriazole can only inhibit CAT if hydrogen peroxide is present to generate compound 1. Glutathione Peroxidase (GPx) Manganese-CATs are found in a variety of microbes. The enzyme from Lactobacillus plantarum has a molecular weight of 172,000 Daltons and is composed of six subunits each containing one Mn3+ ion. As it does not contain heme it is not inhibited by either cyanide or azide. The enzyme is thought to be important in protecting microbes from damage by hydrogen peroxide. Primary. Discovered by Mills in 1957. This enzyme is not found in bacteria or higher plants, but is found in all eukaryotes. It shows distinct tissue distribution with high amounts in liver, moderate amounts in heart, lung and brain, and low amounts in muscle. Liver levels of GPx are reported to be ~2.7 x 10-6 and 1.2 x 10-6M for liver cytosol and mitochondria, respectively. There are five known forms: Chu et al. (1993); Michelson (1998); Michiels et al. (1994); Sies et al. (1997); Stadtman (1980, 1990); Ursini et al. (1997) GPx-1. A cytosolic enzyme of molecular mass of 76,000 to 92,000 Daltons. It consists of four WWW.ESAINC.COM 311 identical subunits each containing selenium in the form of a single selenocysteine residue. Unspecific acting on hydrogen peroxide, lipid hydroperoxides, peroxidized membranes and DNA-hydroperoxides. Specific for its cofactor, GSH. Mitochondrial GPx. It has never been isolated but may be related to thioredoxin/peroxiredoxin. Likely to occur in the matrix. Human plasma GPx. A tetrameric protein of molecular mass ~88,000 Daltons that is synthesized and secreted by the kidney. Each subunit contains a selenium atom. A novel GSHPx-1 enzyme. This has similar substrate specificities to GPx-1. This cytosolic enzyme is a tetrameric protein with a molecular weight of 88,000 Daltons. Reduces hydrogen peroxide, amino hydroperoxide, linoleic acid hydroperoxide but not phosphatidylcholine hydroperoxide. Glutathione Reductase (GR) Glutathione synthetic enzymes Glutathione-S-Transferase (GST) Phospholipid hydroperoxide glutathione peroxidase PH-GPx. This is a monomeric (~20,000 Daltons), hydrophobic, seleniumcontaining enzyme that is associated with membranes and preferentially reduces lipid hydroperoxides (Chapter 4). It occurs in two forms. The long form (23,000 Daltons) is a mitochondrial GPx and is located in mitochondrial membrane. The short form (20,000 Daltons) is found in the cytosol. The long form may play a critical role in prevention of apoptosis. Secondary. GR is a flavoprotein (molecular mass 104,800 Daltons; composed of two subunits) which reduces GSSG to GSH using NADPH produced by the pentose phosphate pathway. The enzyme is located in both cytoplasm and mitochondria. GR is responsible for removal of toxic GSSG and keeps GSH in its biologically active form. GR can also reduce mixed disulfides such as those formed between GSH and coenzyme A. Secondary. γ-Glutamylcysteine synthetase and glutathione synthetase – see below. Primary. A non-selenium containing GPx. It is a dimer composed of four different subunits (22,000 to 25,000 Daltons) which combine to produce six isozymes. The enzyme is found in liver (where it accounts for ~10% of total soluble protein), red blood cell and intestine. It is located in the cytoplasm, nucleus and on the surface of the cell, but not in mitochondria. GST does not act on WWW.ESAINC.COM Meister and Anderson (1983) Burk et al. (1978); Jenkinson et al. (1983); Ketterer and Meyer (1989); Lawrence and Burk (1976) 312 Heme Oxygenase Paraoxonase (PON 1) Peroxiredoxin (Prx) Superoxide Dismutase (SOD) hydrogen peroxide but does reduce hydroperoxides. GST may function as GPx when Se levels are low. It is also involved in detoxification of xenobiotics through the mercapturic acid pathway (see below). GST is also involved in conjugation reactions of endogenous compounds (e.g., steroids, and prostaglandins). Primary. Microsomal mono-oxygenase enzymes consisting of two homologous isozymes responsible for conversion of the pro-oxidant, heme, to the antioxidant, bilirubin. NADPH and oxygen are used as cofactors. HO-2 is constitutive while HO-1 is an inducible-enzyme. HO-1 production is stimulated up to 100 fold by cytokines, endotoxins, glutathione depletion, heat shock, heavy metals, prostaglandins, and by Alzheimer’s disease. Inhibited by zinc protoporphyrin-9 and tin-protoporphyrin. HO-1 plays a role in adaptive resistance of cells to chronic exposure of nitric oxide. Primary. An enzyme found in human serum associated with high-density lipoprotein (HDL). It protects both HDL and LDL against lipid peroxidation and is capable of hydrolyzing both peroxidized phospholipids and cholesteryl linoleate hydroperoxides. PON 1 also possesses arylesterase activity capable of hydrolyzing organophosphate insecticides and nerve gases. PON 1 has two genetic polymorphisms important in determining the capacity of LDL to protect HDL and its activity towards organophosphates. Primary. Consists of six members. Prx-3 is located in the mitochondria, Prx-1 and Prx-2 exist in the cytosol, and Prx-5 is found in both mitochondria and peroxisomes. Prx-3, also called MER5 and antioxidant protein-1 (AOP-1), scavenges hydrogen peroxide in the cooperation with thiol, and peroxynitrite by itself. Primary. Discovered by Fridovich and McCord in 1969. Consists of several unrelated enzymes differing in amino acid sequence, active metal site and cellular location. Prokaryotes have an iron-SOD (Fe-SOD) associated with their outer membrane and a manganese-SOD (Mn-SOD) located in their matrix. The Fe-SOD is a dimer with overall molecular weight of 40,000 Daltons. The Mn-SOD occurs as dimers or tetramers with overall molecular weight of 40,000 and 80,000 Daltons, respectively. E. coli also contains a hybrid SOD consisting of one subunit of MnSOD and one subunit of FeSOD. WWW.ESAINC.COM Bishop et al. (1999); Maines (1997); Smith et al. (1994) Aviram et al. (1999); Mackness et al. (1996, 1998) Hattori, et al., (2003) and references therein Adachi et al. (1992); Benov et al. (1996); Hjalmarsson et al. (1987); Fridovich (1974, 1986; 1995); Geller and Winge (1982); Gregory and Dapper (1980); Halliwell and Gutteridge (1999); Karlsson and Marklund (1988a,b); Luoma et al. (1998); 313 Eukaryotes contain: A copper-zinc enzyme (CuZn-SOD). This is an acidic, very stable protein that occurs in the cytoplasm, possibly between the inner and outer mitochondrial membranes, in lysosomes and in the nucleus. This blue-green colored dimeric enzyme has molecular weight of ~32,000 Daltons and is readily inhibited by cyanide and copper chelators such as diethyldithiocarbamate. Liver levels of this enzyme are ~2.4-3.7 x 10-5M. Marklund (1984)); Marklund et al. (1982); McCord and Fridovich (1969); Michalski (1996); Michiels et al. (1994); Sandstrom et al. (1992); Stralin et al. (1995) A manganese enzyme (Mn-SODs). This occurs in the mitochondrial matrix. This pink tetrameric enzyme has a molecular weight of ~88,000 Daltons. It is less stable than CuZn-SOD but is not affected by cyanide or copper chelators. Liver levels of this enzyme are ~0.3-1.1 x 10-5M An extracellular copper-zinc enzyme (EC-CuZnSOD). This tetrameric, slightly hydrophobic glycoprotein has a molecular weight of 135,000 Daltons. It is the major SOD found in extracellular fluid (e.g., plasma, lymph and synovial fluid). It is also found in tissues and binds to the endothelial cells of the vascular system. It is involved in inflammation. The enzyme shows heterogeneity with regard to its ability to bind heparin and therefore its ability to bind to proteoglycans found on the cell surface and connective tissue matrix. Class A lacks heparin binding, class B has intermediary binding while class C binds heparin with strong affinity. Interestingly, heparin suppresses inflammation by releasing EC-Cu,ZnSOD. This enzyme is inhibited by cyanide, azide, hydrogen peroxide and copper chelators. Thioredoxin, and Thioredoxin Reductase An extracellular manganese enzyme (EC-MnSOD). This enzyme has a molecular weight of 150,000 and occurs as a dimer or tetramer. Primary. Thioredoxin (TRX) is a small (~12,000 Daltons), heat-stable, dithiol, redox-active protein widely distributed in bacterial, plant and animal kingdoms; TRX-1 is located in the cytosol, nucleus and can be excreted while TRX-2 only occurs in the mitochondria. Thioredoxins contain cysteine residues capable of undergoing reversible oxidation. Reduced thioredoxin serves as a hydrogen donor for ribonucleotide reductase, protein tyrosine phosphatase and for enzymes involved in reducing sulfate and methionine sulfoxide. The thioredoxin/thioredoxin reductase system also acts as a peroxidase and is capable of scavenging lipid hydroperoxides. It is also involved in the up-regulation of the expression of catalase-hydroperoxidase I. Thioredoxin can also WWW.ESAINC.COM Arteel et al. (1999); Bjornstedt et al. (1997); Buchanan et al. (1994); Follman and Haberlein (1995); Gleason and Holmgren (1988); Holmgren (1985); Takemoto et al. (1998) and references therein; Zhong et al. (1998) 314 donate electrons to glutathione peroxidase thereby providing a mechanism by which human plasma glutathione peroxidase can reduce hydroperoxides under conditions of low glutathione levels. Thioredoxin Peroxidase (TPx) and peroxiredoxins (Prx) Thioredoxin reductase is an FAD-containing general-purpose protein disulfide reductase which uses NADPH as the reducing agent for efficient reduction of TRX-disulfide to TRX. Mammalian thioredoxin reductase, a dimeric protein (molecular weight 57,000 Daltons) is homologous to glutathione reductase. Interestingly, the rat enzyme contains a selenocysteine residue and in addition to its primary substrate, thioredoxin, has broad substrate specificity (e.g., selenite, organoselenium compounds, cytosolic and plasma GPx, and peroxynitrite). TPx is a newly discovered, non-selenium containing enzyme that reduces hydrogen peroxide using electrons from thioredoxin. It has two essential conserved cysteine moieties. Its reaction is analogous to GPx. TPx homologs, the Prxs are designated 1-Cys Prx as they contain only one conserved cysteine moiety. Four Prxs have been identified, all are the products of distinct genes and all are members of the thioredoxin superfold family. Prx I and II are cytosolic, Prx III is mitrochondrial, while Prx IV is extracellular. The Prxs act on hydrogen peroxide and phospholipid hydroperoxides. For many of these proteins the endogenous thiol cofactor remains unknown. These enzymes may play a role in cell proliferation and differentiation, protection of proteins from oxidative damage, and intracellular signaling. Fisher et al. (1999); Kang et al. (1998a,b); Lee et al.(1999); Lim et al. (1998); Matsumoto et al. (1999); Schroder and Ponting (1998) Table 4.1 Some Antioxidant Enzymes. Catalases. CAT is a very reactive enzyme that is responsible for the dismutation of hydrogen peroxide (Eqn 4.1). The actual mechanism of CAT activity is best described by Eqns 4.2 and 4.3. Compound I contains iron in the Protein-FeIV-O form2 (Footnote 2) (Eqn 4.4) (and see Chapter 2). It is very difficult to saturate 2 Many reactions catalyzed by heme-containing proteins involve the oxidation of heme one (compound II) or two (compound I) equivalents above the Fe (III) state. Compound I is readily formed by catalase, cytochrome P450 monooxygenases and others (Eqn 4.2). There is still great confusion in this area with several different formulae (e.g., IV IV 4+ + 4+ + Protein• -Fe =O, Protein-Fe =O, Protein-Fe =O and Protein -Fe =O) being used to describe the structure of compound I. Readers are referred to Everse (1998) for an excellent review on this topic. WWW.ESAINC.COM 315 catalase with hydrogen peroxide because its maximum velocity (Vmax) for dismutation of hydrogen peroxide is huge. H2O2 + H2O2 → H2O + O2 CAT-FeIII + H2O2 → Compound I (k=1.7 x 107 M-1s-1) Compound I + H2O2 → CAT-FeIII + H2O + O2 (k=2.6 x 107 M-1s-1) Protein-FeIII + H2O2 → Protein-FeIV-O + H2O Eqn 4.1 Eqn 4.2 Eqn 4.3 Eqn 4.4 Similar reactions are though to take pace for manganese-CAT (Eqns 4.5 and 4.6). CAT-Mn3+ + H2O2 + 2H+ → CAT-Mn (V) CAT-Mn (V) + H2O2 → CAT-Mn3+ + O2 + 2H+ Eqn 4.5 Eqn 4.6 CAT can also show peroxidative reactions in the presence of hydrogen peroxide, with compound I oxidizing methanol and ethanol to methanal and ethanal, respectively (Thurman and Handler (1989)). The peroxidative action of CAT is typically much less rapid (k=102 to 103 M-1s-1) than its catalytic activity. Spinach catalase can oxidize formic acid to carbon dioxide, and nitrite into nitrate (Halliwell and Gutteridge (1999)). CAT activity is localized mainly to peroxisomes, fragile membrane bound organelles of various shapes and sizes (Chandoga (1994)). Mammalian peroxisomes are intimately involved with redox biochemistry. They are responsible for the production of hydrogen peroxide (flavin oxidases), catabolism of hydrogen peroxide (CAT) and superoxide (SOD), lipid metabolism (biosynthesis and catabolism (oxidation of fatty acids and their derivatives)) and intermediary metabolism (transaminases and dehydrogenases) (del Rio et al. (1992); Lazarow (1987); Vamecq and Draye (1989)). Peroxisomes are essential to normal cellular function. Peroxisome dysgenesis and/or dysfunction in their enzymatic pathways are found in several inherited metabolic diseases with serious clinical ramifications (e.g., Zellweger syndrome) (Keller et al. (1993)). CAT activity can be determined using colorimetric, spectrophotometric, and polarographic assays (Aebi (1984); Cohen et al. (1996); Dempsey et al. (1975); Haining and Legan (1972); Sinah (1972); Thomson et al. (1978)). WWW.ESAINC.COM 316 Peroxidases. Peroxidases catalyze the general reaction presented in Eqn 4.7, where R represents an alkyl group. RH2 + H2O2 → R + 2H2O Eqn 4.7 Glutathione Peroxidase-1 (GPx-1) is an important antioxidant enzyme that specifically requires glutathione (GSH) as a cofactor. It is a relatively unspecific enzyme acting upon hydrogen peroxide, free lipid hydroperoxides and other peroxidized compounds (Eqns 4.8 and 4.9). In contrast to heme peroxidases (e.g., CAT) GPx produces just one oxidation product. Overall GPx is much more versatile than CAT but unlike CAT it reacts with hydrogen peroxide only slowly. 2GSH + H2O2 → GSSG (glutathione disulfide) + 2H2O 2GSH + RO2H → GSSG + ROH + H2O Eqn 4.8 Eqn 4.9 GPx-1 cannot act on lipid hydroperoxides when they are part of a membrane, unless the hydroperoxide is first freed by phospholipase A2 (see Figure 3.29). Phospholipid hydroperoxide glutathione peroxidase (PH-GPx), on the other hand, plays an important role in controlling membrane lipid peroxidation and is the only enzyme capable of reducing fatty acid hydroperoxides while they are still part of the membrane (Chapter 4). The GPxs are remarkable because they require selenium. The selenium atom is part of a selenium analog of cysteine that is covalently attached to the enzyme and is located within its active site (Figure 4.1). (Reddy and Massaro (1983); Zachara (1992)). Selenium is an essential micronutrient obtained from the diet. Humans require at least 60µg/day (typical intake for developed countries is 60200µg/day) in order to remain healthy (Burk (1989); Foster and Sumar (1997)). Selenium deficiency is associated with liver necrosis, degenerative heart disease (Keshan disease), exudative diathesis and a failure to grow and reproduce (Reddy and Massaro (1983); Zachara (1992)). Overdosing with selenium is associated with increased lipid peroxidation and cellular toxicity possibly through generation of ROS (Seko and Imura (1997)). WWW.ESAINC.COM 317 GSH GSSG + H+ E-Se-S-G E-Se- Selenosulfide Selenolate ROOH + H+ H2O GSH E-Se-OH ROH Selenenic Acid Figure 4.1 The Role Of Selenium In Glutathione Peroxidase. Glutathione peroxidase and possibly other selenoproteins that contain either selenocysteine or selenomethionine can also act as peroxynitrite reductases protecting against oxidative damage caused by this pro-oxidant (Sies et al. (1997)).3 Unfortunately, GPx can itself be inactivated by peroxynitrite probably by oxidation of the ionized selenol of the selenocysteine residue (Padmaja et al. (1998)). In order for glutathione peroxidases to function properly GSH availability must be maintained. GSSG must therefore be continuously reduced to GSH. This is achieved by glutathione reductase in a reaction using NADPH, (Eqn 4.10) (and see below). GSSG + NADPH + H+ → 2GSH + NADP+ Eqn 4.10 Several non-specific peroxidases are also found in animals. Myeloperoxidase is located in phagocytes. As discussed in Chapter 2, it is responsible for the production of hypochlorous acid, a strong pro-oxidant and bacteriocidal agent. Thyroid peroxidase is the major enzyme of the thyroxine synthesis pathway and is responsible for both the iodination of tyrosine residues of the protein thyroglobulin and also their coupling to form iodothyronines. Lactoperoxidase is found in milk and saliva and may be responsible for the oxidation of thiocyanate 3 Interestingly, derivatives of another Group 6B element, tellurium, also protects against peroxynitrite-mediated oxidation and nitration reactions (Briviba et al. (1998)). WWW.ESAINC.COM 318 (also found in milk and saliva) to hypothiocyanate, a compound toxic to some pathogens. Hypothiocyanate may offer some protection to babies against gastrointestinal tract infection. Other peroxidases include yeast cytochrome c peroxidase, bacterial NADH peroxidase, plant non-specific horseradish peroxidase, fungal non-specific chloroperoxidase and plant ascorbate peroxidase. For the purification of GPx and measurement of enzyme activity readers are referred to the excellent review by Toribio et al. (1996) and to Halliwell and Gutteridge (1999). The Biological Importance of Catalase and Glutathione Peroxidase. At first site it may appear that nature is being redundant in having two enzymes, CAT and GPx, that are both capable of catabolizing hydrogen peroxide. It should be remembered, however, that these enzymes show distinct activity and specificity. CAT acts very rapidly and specifically with hydrogen peroxide, whereas GPx reacts more slowly with hydrogen peroxide but is capable of reacting with hydroperoxides and other peroxidized compounds too. Some tissues have one enzyme present at much higher levels than the other so it becomes de facto the enzyme responsible for controlling hydrogen peroxide levels. For example, GPx is the principal enzyme for controlling hydrogen peroxide levels in brain and spermatozoa. Some tissues have both enzymes present but have them located in different compartments. For example, liver CAT is located in peroxisomes where it is responsible for handling hydrogen peroxide produced by the ROS generating enzymatic pathways located within these organelles. Liver GPx is located in the cytosol where it can destroy hydrogen peroxide produced within this compartment. Interestingly, heart mitochondria also contain a CAT located within the matrix that handles hydrogen peroxide produced within these organelles (Radi et al. (1991)). In some cases there seems to be a backup function. For example, although both enzymes are present in red blood cells, it would appear that the low levels of hydrogen peroxide produced by SOD is normally handled by glutathione peroxidase. CAT is only recruited when glutathione peroxidase is overwhelmed e.g., following administration of a drug which generates hydrogen peroxide. Interestingly, patients suffering from an inborn error of metabolism, acatalasemia, who possess a mutant CAT with low activity, show few, if any, symptoms. Presumably, in such cases, GPx compensates for the low catalase activity. WWW.ESAINC.COM 319 Glutathione-S-Transferase. Glutathione-S-transferase (GST) is a non-selenium containing enzyme that functions in a similar fashion to GPx. GST does not act on hydrogen peroxide but it will reduce a variety of hydroperoxides (Eqns 4.11 and 4.12). ROOH + GSH → GSOH + ROH GST GSOH + GSH → GSSG + H2O Nonenzymatic? Eqn 4.11 Eqn 4.12 GST is also involved in the detoxification of some xenobiotics through the mercapturic acid pathway (see below). Heme Oxygenases. Heme plays several important roles in the body (e.g., as part of hemoglobin in the red blood cell it is responsible for transporting oxygen throughout the body). Unfortunately, heme can be a major problem too. Heme released following red blood cell turnover, tissue damage, or turnover of heme-containing proteins, can initiate lipid peroxidation or react with hydrogen peroxide to release redox-active iron (Chapter 3). This iron, in turn, can take part in the Fenton reaction with additional hydrogen peroxide. It is vitally important therefore that during heme catabolism its potential pro-oxidant activity is prevented. This is the responsibility of heme oxygenase (HO). HO consists of two forms, the inducible protein HO-1 (HSP32) and the constitutive isozyme HO-2. These enzymes, which are different gene products, share limited sequence homology and show different regulation and tissue distribution. Both enzymes catalyze the first and rate-limiting step in heme degradation producing three biologically important molecules: biliverdin, carbon monoxide and iron (Figure 4.2). Iron is a gene regulator and, as discussed earlier, a pro-oxidant. Thus free iron produced during this process must be effectively bound in order to prevent it from reacting with hydrogen peroxide. Biliverdin is reduced to bilirubin by biliverdin reductase. While bilirubin is an effective antioxidant, biliverdin is not. In scavenging two hydroperoxyl radicals bilirubin is oxidized to biliverdin, and this can then be rapidly reduced back to bilirubin by biliverdin reductase leading to react with more pro-oxidants. In addition, bilirubin is an antimutagen and anti-complement agent (Marilena (1997)). Carbon monoxide plays a similar role to nitric oxide and its reaction with guanylyl cyclase has been implicated as a modulator of gene expression, in retrograde neurotransmission and a modulator of vascular tone (Dulak and Jozkowicz (2003); Durante (2002); Ingi and Ronnett (1995) and references WWW.ESAINC.COM 320 therein; Marilena (1997)). HO-2 is expressed at high levels in discrete brain regions and the carotid bodies. HEME O2 + NADPH HEME OXYGENASE CO + Fe3+ H2O + NADP+ GUANYLYL CYCLASE R' R R R" R R' O NH NH N NH O R" R BILIVERDIN NADPH + H+ BILIVERDIN REDUCTASE NADP+ R' R O NH R" R N R R" R NH R' NH O BILIRUBIN Figure 4.2 The Catabolism Of Heme. Superoxide Dismutases. Superoxide dismutase (SOD) is responsible for the dismutation of superoxide (Eqn 4.13). It is the only enzyme known that can act on a free radical. Although superoxide dismutation occurs naturally at physiological pH, it is much slower (k2=~5 x 105 M-1s-1) than the enzyme catalyzed reaction (e.g., CuZn-SODbovine k2=~1.6 x 109 M-1s-1; Mn-SODE. coli k2=1.8 x 109 M-1s-1). WWW.ESAINC.COM 321 2O2•- + 2H+ → H2O2 + O2 Eqn 4.13 The general mechanism for superoxide dismutation involves changes in the oxidation state of the metal within the enzyme (Eqns 4.14-4.16) (also see Hart et al. (1999)). In this example, M(n)+ represents Cu+ or Fe2+. The Zn atom of the CuZn-SOD appears not to take part in the reaction, but is essential in the stabilization of the enzyme. The mechanism of action of the manganesecontaining enzyme is much more complex and will not be dealt with here. E-M(n+1)+ + O2•- → E-M(n)+ + O2 Electron transfer (n)+ E-M + O2•- + 2H+ → E-M(n+1)+ + H2O2 Proton and electron transfer Overall: O2•- + O2•- + 2H+ → H2O2 + O2 Eqn 4.14 Eqn 4.15 Eqn 4.16 It is interesting to note that in certain circumstances both CuZn-SOD and FeSOD can be inactivated by their own reaction product, hydrogen peroxide. In the case of CuZn-SOD it appears that hydrogen peroxide reduces Cu2+ to Cu+ which causes reversible inactivation. At higher hydrogen peroxide levels Cu+ can then produce hydroxyl free radicals that can oxidize an essential histidine (His118) in the enzyme’s active site to an inactive 2-oxohistidine residue, thereby irreversibly inhibiting the enzyme (Chapter 3). Excessive production of hydroxyl free radicals can permanently damage the enzyme through fragmentation (see Uchida and Kawakishi (1994) and references therein). As CuZn-SOD is very effective at controlling superoxide levels the physiological significance of hydrogen peroxide inhibition still remains to be clarified. CuZn-SOD, by affecting the concentration of superoxide, plays a critical role in the regulation of the formation of peroxynitrite from its precursors, superoxide and nitric oxide (Chapter 2). This has led some researchers to suggest that the raison d’être for SOD is to enhance the biological activity of nitric oxide by preventing its diversion to peroxynitrite (Pryor and Squadrito (1995)). Thus downregulation of CuZn-SOD leads to cell death by overproduction of peroxynitrite, while over-expression or supplementation with SOD can overcome some of the problems associated with peroxynitrite (Troy et al. (1996)). To help explain the importance of SOD in controlling the production of peroxynitrite Crow and Beckman (1995) proposed the use of a “target area”. This is the probability that some species will be attacked by a pro-oxidant, and depends upon the reaction rate times the concentration of the species. Thus as the reaction rate of CuZnSOD with superoxide is ~2 x 109 M-1s-1 and CuZn-SOD’s typical cellular concentration is ~10-5M then this enzyme has a target area of 2 x 10-4 s-1. Nitric oxide reacts with superoxide at a rate of 6.7 x 109 M-1s-1 and, under physiological conditions, is present at a concentration of 10-7M. Nitric oxide therefore has a WWW.ESAINC.COM 322 target area 30 fold lower than SOD. However, under pathological conditions nitric oxide can reach concentrations of 4 x 10-6M, resulting in a target area of 2.7 x 104 s-1, exceeding the target area of SOD (Crow and Beckman (1995) and references therein). Changes in SOD activity can lead to disease and may even play a role in aging. Autosomal dominant mutations in CuZn-SOD has been linked to familial amyotrophic lateral sclerosis (ALS) (Andersen et al. (1997); Beckman (1993) and references therein; Wong and Borchelt (1995)). Interestingly transgenic animals expressing CuZn-SOD mutants also have decreased chaperone activity (proteins that are responsible for proper protein folding and targeting mutant proteins for degradation – Chapter 3) (Bruening et al. (1999)). Insufficiency of chaperones may be directly involved in loss of motor neurons in ALS. The role of SOD in aging is less clear. Genetic manipulations that increase CuZn-SOD activity have only slight, if any, effect on maximal life span of several species, even though they do show increased resistance to oxidative stress (Sohal (1997) and references therein; Warner (1994) and references therein). Interestingly increasing the activity of both CuZn-SOD and catalase does significantly increase life span (Sohal (1997) and references therein; Warner (1994) and references therein). The amount of SOD present in tissues can be determined using immunological methods (Halliwell and Gutteridge (1999)). SOD activity can be determined using both direct- and indirect methods. Direct methods include pulse radiolysis, stop flow spectroscopy, EPR-spin with spin trap, far UV detection, polography and 19F NMR (Halliwell and Gutteridge (1999); Michalski (1996)). Unfortunately these approaches are not usually practical with crude enzyme preparations and at SOD levels typically found in biological systems. For these reasons, indirect assays are far more common. In these assays, superoxide is generated by some mechanism (e.g., xanthine/xanthine oxidase, auto-oxidation reactions, or illuminated flavins) and allowed to react with a reporter molecule (e.g., cytochrome c or nitroblue tetrazolium) which can be monitored, usually by changes in absorbance (Halliwell and Gutteridge (1999); Michalski (1996)). The addition of SOD will remove superoxide from the reaction and thereby inhibit the absorbance change. Readers are referred to Halliwell and Gutteridge (1999) and Michalski (1996) for precautions when using indirect SOD assays. The Catabolism of Nitric Oxide. Unlike superoxide and hydrogen peroxide, nitric oxide is not catabolized enzymatically. Rather, as discussed in Chapter 2, nitric oxide undergoes a series of oxidation reactions eventually producing nitrite and nitrate. Excess nitric oxide produced in the plasma is also oxidized to nitrate by its reaction with oxyhemoglobin or oxymyoglobin (Figure 2.18). Nitric oxide can also be removed by its reaction with superoxide to produce peroxynitrite. WWW.ESAINC.COM 323 SEQUESTRATION OF METAL IONS. As has already been discussed, transition metals are capable of undergoing redox reactions. They therefore possess pro-oxidant activity and are capable of initiating lipid peroxidation and hydroxyl free radical production. Of all the transition metals encountered in vivo, iron and copper are the most abundant and therefore the most problematic. The amounts of nickel, manganese, chromium, vanadium and cobalt are usually incredibly low under physiological conditions and thus do not normally pose a problem.4 Adult humans typically contain about 4.5g of iron, obtain ~1mg of iron from the diet and, when in iron-balance, excrete ~1mg. About 60% of the body’s iron is found in hemoglobin, the remainder occurs in myoglobin, various enzymes and in the iron-transport protein, transferrin (Table 4.2). Other pools include iron bound to iron-storage proteins (e.g., ferritin) and to non-protein chelators (e.g., citrate, ATP, ADP and GTP). Free iron concentrations are very low (Fe2+ ~10-8M; Fe3+ ~10-18M). Consequently there is virtually no free iron ions in humans (iron is undetectable in plasma from healthy individuals but can be measured in sweat (Gutteridge and Halliwell (1999)). Human adults also contain ~80mg of copper. About 90% of circulating copper is bound to ceruloplasmin; the rest is bound to albumin, small peptides and histidine (Table 4.2). There is little free copper (ions) in humans (e.g., normal human plasma is devoid of free copper, while sweat contains 2-27µM (Gutteridge and Halliwell (1999)). One approach to prevent the pro-oxidant activity of iron and copper would be to eliminate them entirely from the biological system. Not only would this be physiologically impossible, but it would be fatal as well, for both iron and copper are essential micronutrients and play a role in the activity of several enzymes, in the immune system and the electron transport chains (Harris and Gitlin (1996); Olivares and Uauy (1996); Percival (1998); Solomon and Lowery (1993); Stryer (1988)). Instead, nature makes use of these metal ions but keeps them sequestered in redox-inactive chelation complexes. For a given metal ion, the metal’s oxidation state and the nature of its ligand affect the strength of chelation. In general chelators that use oxygen atoms in ligating the metal prefer the oxidized form of the metal; consequently the redox potential of the metal is usually decreased. Chelators that use nitrogen atoms to bind the metal prefer its reduced form; thus the redox potential is usually increased (Miller et al. (1990)). For example, the effects of chelation on the redox potential is readily apparent from Appendix 2.1. The Eo’ for the Fe3+/Fe2+ couple is +260mV, +110 and -190mV for cytochrome, aqueous ions and ferritin, respectively. The importance of binding and redox state is illustrated by iron’s interaction with ferritin and this is discussed further below. 4 However, inadvertent exposure to elevated amounts of these metals can be biologically catastrophic. WWW.ESAINC.COM 324 Metal Iron Protein Transferrin (lactoferrin; melanotransferrin; ovotransferrin [conalbumin]; plasma transferrin; and uteroferrin) Process Transport Comments Group of proteins with a molecular mass of ~80,000 Dalton capable of tightly but reversibly binding two Fe3+ ions. Requires a counter anion (usually bicarbonate) at each site for tight binding of iron. Human transferrin is usually only 30% full under normal conditions. Occurs in extracellular locations, but can enter the cell for delivery of Fe3+ to ferritin or to mitochondrial ferrochelatase for heme biosynthesis. Also includes ovotransferrin, lactotransferrin and sero-transferrin. References Aisen and Listowsky (1980); Crichton (1990) Iron Ferritin Storage A large (~500,000 Dalton) protein made up of 24 identical subunits and capable of storing up to 2300-2500 iron (Fe III) ions. The ferritin core consists of ferrihydrite (5Fe2O3.9H2O) and phosphate. The mechanisms underlying the neurotoxicity of 6-hydroxydopamine include its autooxidation (producing ROS) and the reduction Fe3+ to Fe2+ (thereby releasing active pro-oxidant Fe2+ ions). Aisen and Listowsky (1980); Crichton (1990); Double et al. (1998); Harrison and Arosio (1996); Theil (1987) Iron Hemosiderin Storage Derived from intralysosomal aggregation and proteolysis of ferritin. Thought to act as a back-up system under conditions of iron excess. Crichton (1990); Harrison and Arosio (1996) Iron Neuromelanin Storage This complex polymer bound to lipofuscin granules, abundant in the nigrostriatal neuronal pathway, is capable of acting as an iron store. See Gerlach et al. (1994) and references therein Copper Ceruloplasmin Transport An α2-glycoprotein (~134,000 Dalton), consisting of three homolgous (42-45,000 Dalton) capable of binding six copper atoms/molecule. Responsible for binding >90% of circulating copper. It may be able to donate copper intracellularly for incorporation into other copper-proteins such as superoxide dismutase and cytochrome oxidase. It has other biological roles including: mobilization of iron to transferrin; antioxidant activity; regulation of plasma biogenic amines; role in inflammatory Evans (1973); Frieden (1986); Luza and Speisky (1996) WWW.ESAINC.COM 325 response; growth promotor of certain cells; stimulation of angiogenesis in cornea. It also possesses ferroxidase activity. This protein can bind one copper atom preferentially before complexing with others. Occurs in portal and general circulatory systems. Copper Albumin Transport Copper Transcuprein Transport A 270,000 Dalton copper-containing protein occurs in plasma and portal circulation. Copper Metallothionein Storage Copper Amino acid Complexes Transport/ storage Single polypeptide chain (5000 to 6500 Dalton) containing 25-30% cysteine residues, no disulfide bonds or aromatic amino acid residues. Binds a total of 11-12 copper atoms per molecule in two sites. Under oxidative stress redox active copper may be released. Found in erythrocytes and plasma (e.g., histidine-amino acid complex). Evans (1973); Frieden (1986) Frieden (1986); Weiss and Linder (1985) Fabisiak et al. (1999); Frieden (1986); Luza and Speisky (1996) Evans (1973) Table 4.2 The Binding Of Iron And Copper. Another advantage of chelation is the ability to solubilize ions for use in biological processes. Both Cu (II) and Fe (III) ions have very limited solubility at physiological pH (10-9 and 10-18M, respectively). Thus without appropriate chelation the accumulation of sufficient amounts of Fe (III) and Cu (II) ions for normal metabolism would put excessive demands on the absorption, transport and storage processes. Before exploring the pro-oxidant role of iron and copper further, we must first understand the metabolism of these metals and how nature tries to prevent accidental release of redox active forms. The Metabolism of Iron and Copper. The mechanism of iron uptake and storage in mammals is a fascinating area of study (reviewed by Meneghini (1997) and Harrison and Arosio (1996)). Under normal conditions transferrin is in excess in relation to iron. Iron is transported in the plasma as diferric-transferrin complex that serves to solubilize iron, protect against iron’s pro-oxidant activity and act as a ligand for the transferrin-receptor (TR) located in the plasma membrane (Bali et al. (1991)). The TR allows a fine control of intracellular iron homeostasis. The Fe (III)-transferrin-TR complex is WWW.ESAINC.COM 326 encapsulated by an endosome in which Fe (III) is reduced to the more soluble Fe (II) form (Dancis et al. (1994); Watkins et al. (1992)). The emptied (apo)transferrin is then exported and released when the endosome fuses with the plasma membrane (Mengheni (1997)). Inside the cell, the Fe (II) ion can follow several routes including storage in ferritin. Ferritin chelates Fe (III) ions strongly but only forms a weak chelation complex with Fe (II) ions.5 Thus iron entering into ferritin as Fe (II) ions must be oxidized by the protein before being stored. Similarly, release of iron involves the reduction of Fe (III) to Fe (II) ions. δAminolevulinic acid, ascorbic acid, nitric oxide and superoxide are capable of reducing Fe (III) ions and releasing iron from ferritin (Oteiza et al. (1995); Reif (1992)). It is unlikely that these compounds represent the physiological mechanism by which iron release is controlled and this process still awaits elucidation (Meneghini (1997)). Iron is thus stored in cells in a safe form that cannot take part directly in the Fenton reaction. As discussed below, conditions in which chelation is weakened may permit the metal to act as a pro-oxidant. Apart from oxidation and subsequent storage in ferritin, Fe(II) ions can also be transferred to sites of protein synthesis for incorporation into iron-containing proteins, or along with Fe (III) ions, chelated by citrate, ATP, ADP (Aisen (1994). This chelatable iron pool can affect iron regulatory protein (IRP), which is responsible for posttranscriptional control of intracellular iron homeostasis (Meneghini (1997) and references therein).6 Low levels of chelatable iron change the conformation of IRP, permitting it to bind to the stem-loop structures (iron responsive elements) of both ferritin and the transferrin receptor mRNAs. Such binding stabilizes TR mRNA but inhibits the translation of the ferritin mRNA (Klausner et al. (1993); Kuhn (1994); Leibold and Munro (1988)). Thus TR synthesis is increased while ferritin synthesis is decreased in periods when intracellular iron is low. If iron levels increase, then IRP is inactivated, TR synthesis is decreased and ferritin production is increased. These two concerted mechanisms help to maintain intracellular iron levels. 5 Similarly EDTA forms a stronger complex with Fe (III) [log k=25.0] than with Fe (II) [log k=14.3] as does citrate (Fe (III) [log k=11.2], Fe (II) [log k=4.8]). 6 IRP is an interesting molecule. It is a protein with a single iron-sulfur cluster (4Fe-4S) (Figure 4.3) showing great sequence homology with mitochondrial aconitase. Removal of the iron-sulfur forms the apo-enzyme that can bind to the IRE of the mRNA of TR, ferritin and possibly other mRNAs too. WWW.ESAINC.COM 327 Cys Cys S S Fe S S S Fe S Cys S Fe Fe OH2 Cys S Fe S Cys Fe S S S Cys S Cys [2Fe-2S] Cluster [4Fe-4S] Cluster CH3 HC CH3 S Cys Cys S Fe S Cys N H3 C N Cys S OC:OCH2 CH2 SCH2 Fe Protein CH3 N N SCH2 Protein (CH2 )2 CO2 CH3 Single Iron-Sulfur Cluster Heme (Cytochrome C) Figure 4.3 Structures Of Iron-Sulfur Clusters And A Heme Molecule. Copper metabolism is different to that of iron. Copper is transported bound to ceruloplasmin. Unlike transferrin that readily releases iron and is effectively recycled, ceruloplasmin has to be degraded in order for it to release its copper load. Ceruloplasmin has ferroxidase activity, oxidizing Fe (II) to Fe (III) while simultaneously reducing oxygen to water (Eqn 4.17). This effectively competes with spontaneous Fe (II) oxidation that is capable of producing ROS. Thus ceruloplasmin is an antioxidant as it depresses the availability of Fe (II) that can take part in the Fenton reaction or Fe (II) -dependent lipid peroxidation. Although ceruloplasmin can react with hydrogen peroxide and superoxide, this is not thought to be biologically important. Ceruloplasmin can oxidize a variety of substrates in vitro, such as polyamines and polyphenols, but again the physiological relevance, if any, of these reactions is unclear at present. 4Fe2+ + O2 + 4H+ → 4Fe3+ + 2H2O WWW.ESAINC.COM Eqn 4.17 328 The intracellular trafficking of copper in mammalian cells is highly regulated. Cells have developed a number of mechanisms to ensure copper’s proper intracellular transport and compartmentalization. The passage of copper into a cell begins with its reduction by one of several plasma membrane reductases followed by its transport across the membrane by a high-affinity copper transporter (Valentine and Gralla (1997) and references therein). Once inside the cell several different proteins (or metal chaperones) bind and deliver copper to specific intracellular proteins (Valentine and Gralla (1997)). Thus intracellular free copper is undetectable (Rae et al. (1999)). Cox17 in conjunction with the mitochondrial proteins SCO1 and SCO2 specifically deliver copper to mitochondrial cytochrome oxidase (Glerum et al. (1996a,b)). Lys7 (yeast) and CCS (human) deliver copper specifically to CuZn-SOD (Cullota et al. (1997, 1999); Rothstein et al. (1999)). Atx1 (yeast) and Hah1 (human) direct copper to a post-Golgi- compartment via a protein Ccc2 (yeast) or Wilson disease protein (human) (Valentine and Gralla (1997) and references therein). Similar mechanisms are now being proposed for iron transport and compartmentalization. Iron and Copper Species as Pro-oxidants. Iron and copper can occur in several forms in vivo, but not all forms act as prooxidants. The role of these metals in the production of hydroxyl free radicals was discussed in Chapter 2, while their ability to initiate lipid peroxidation was covered in Chapter 3. In general many more forms of iron can stimulate lipid peroxidation than hydroxyl free radical formation (Halliwell and Gutteridge (1999)). This is because lipid peroxidation need not proceed through the formation of the hydroxyl free radical (Chapter 3). The importance of iron and copper participation in pro-oxidant reactions is a complex area that has become controversial because of varying assay conditions being used7, the form of the metal being studied, and which pro-oxidant activity is being measured (Halliwell and Gutteridge (1999)). Overall it appears that under normal physiological conditions, apart from the low molecular mass iron pool that is used for synthesis of ironcontaining proteins, redox active metals are tightly bound in vivo and do not act as pro-oxidants. However, problems do occur under disease conditions (e.g., metal storage diseases, hemochromatosis, and acute porphyria) or with overexposure to metals (e.g., through the diet or in the treatment of thalassemias) (Britton and Brown (1995); Evans (1973); Gerlach et al. (1994); Halliwell and Gutteridge (1999); Houglum et al. (1997); Liochev (1999); Livrea et al. (1996); Muller-Hocker et al. (1987); Olivares and Uauy (1996)). Superoxide can reduce Fe (III) to Fe (II) contained within a number of proteins or free in solution. For example, aconitase, which exists in both mitochondrial and cytosolic forms, reacts with superoxide to release iron from its 4Fe-4S cluster. This process is reversible in vivo. But it is the effect of superoxide on ferritin, 7 Laboratory reagents can be contaminated by free “reactive” iron. For example, bottles of old saline can contain dissolved iron, a form that could produce ROS under test conditions and lead to erroneous data (Halliwell and Gutteridge (1993)). WWW.ESAINC.COM 329 however, that is quantitatively the most important (McCord (1998)). The Fe (II) so formed can then take part in both Fenton reaction and lipid peroxidation. Measurement of Iron and Copper. Iron and copper can be measured using atomic absorbance and anodic stripping voltammetry. However, these approaches are usually not sufficient to measure the low levels of redox active metals in vivo. Iron can be measured using the bleomycin method developed by Halliwell and Gutteridge (1999) (see also Chevion et al. (1999); Gutteridge and Halliwell (1999)). Here the ability for the antibiotic bleomycin to damage DNA is directly proportional to the amount of labile iron ions. Other assays include EPR-based approaches and the use of fluorescent probes such as calcein (Chevion et al. (1999); Gutteridge and Halliwell (1999)). The latter is the only method for the direct determination of cellular pool of labile iron. Copper can be measured using the phenanthroline assay. Here 1,10phenathroline in the presence of oxygen, a suitable reducing agent and copper can lead to DNA degradation. Such DNA damage can then be detected using a TBAR approach (see Chapter 3) (Chevion et al. (1999); Gutteridge and Halliwell (1999)). LOW MOLECULAR WEIGHT MOLECULES. Humans utilize a wide range of antioxidants that are either synthesized de novo or obtained from the diet; many of the latter are the classical vitamins. Antioxidants have diverse chemical structures, but for convenience are normally separated based upon their solubility. This section examines the antioxidant (and pro-oxidant) activities of both water- and fat-soluble antioxidants. Many other endogenous and exogenous compounds also show antioxidant activity and these will be discussed too. Within each section we will examine the antioxidants alphabetically. This does not reflect either their level or importance. Water-Soluble Antioxidants. Albumin. Albumin is a water-soluble protein abundant in serum and plasma (380-920µM human serum 535-760µM human plasma) (Table 4.3). Albumin can bind copper (Table 4.2) thereby preventing copper-ion dependent hydroxyl free radical formation and lipid peroxidation. However, hydroxyl free radical formation can continue at the metal binding site, resulting in damage to the albumin molecule. This is probably biologically insignificant, as albumin is rapidly turned-over. WWW.ESAINC.COM 330 Copper binding also diverts copper’s reactivity away from significant targets such as essential sulfhydryl groups of enzymes, thus limiting oxidative damage. Albumin transports a variety of compounds such as tryptophan, fatty acids, bilirubin and certain drugs. It has been hypothesized that albumin protects fatty acids from lipid peroxidation not only by interfering with copper’s pro-oxidant effect but also by assimilating the antioxidant bilirubin (Halliwell and Gutteridge (1990)). Albumin is an efficacious scavenger of hypochlorous acid (Halliwell (1998); Wasil et al. (1987)). Compound Species Tissue Range Albumin Human Plasma Allantoin Allantoin Human Human Allantoin Human Ascorbic acid Ascorbic acid Human Human Plasma - control Serum - control Serum - rheumatoid Synovial fluid rheumatoid Muscle Plasma Plasma Plasma Ascorbic acid Human Plasma Ascorbic acid Human Ascorbic acid Human Plasma CSF Plasma Ascorbic acid Human Plasma 10 to 110 µmol/L 21.5+4.4 65 to 164 µmol/L neonates 27.8±1.32 µmol/L Ascorbic acid Human Plasma 23.2±17.3 µmol/L Ascorbic acid Human Plasma 10 to 90 µmol/L Ascorbic acid Human Plasma 5.68 to 85 µmol/L Ascorbic acid Ascorbic acid Human Mouse Saliva Liver 0 to 3.7 mg/L total 1.00+0.05 µmol/g Ascorbic acid Rat 9.7 to 15.4 µmol/L 20 to 40 µmol/L Ascorbic acid Rat Ascorbic acid Rat Brain - ECF Ventricularmyocardium Brain Liver Kidney Lens WWW.ESAINC.COM 370 to 530 µmol/L neonates, 535 to 760 adults 12.4 to 20.6 µmol/L 14.1 to 25.4 µmol/L 20.3 to 45.2 7.2 to 31.3 0.03 µmol/g 11.9 µmol/L 50+20 µmol/L 61.8 µmol/L (2.3 µmol/L DHAA) 40 to 140 µmol/L 1.8 µmol/g 1.6 0.4 30.3±7.7 ng/mg Reference Gopinathan et al. (1994) Lux et al. (1992) Grootveld and Halliwell (1987) Hellsten et al. (1997) Koh et al. (1980) Schorah et al. (1996) Koch et al. (1980) Nagy and Degrell (1989) Gopinathan et al. (1994) Lykkesfeldt (1995) Nagy and Degrell (1989) Levine et al. (1996) Capellmann et al. (1994) Karp (1990) Barja de Quiroga et al. (1991) Tsai et al. (1996) Schell and Bode (1993) Mitton and 331 Ascorbic acid Rat Liver Kidney Pancreas Colon Bilirubin Human Plasma α-Carotene Human Plasma (ascorbate + DHAA) 5.4±0.65 nmol/g 3.2±0.41 0.32±0.58 4.1±0.19 20 to 126 µmol/L – neonates, <20 – adults 0.055+0.053 µmol/L α-Carotene Human Plasma 0.02 to 0.22 µmol/L α-Carotene Human Plasma 0.08 to 0.19 µmol/L β-Carotene Human Plasma 0.13 to 0.33 µmol/L β-Carotene Human Plasma 0.3 to 0.6 µmol/L β-Carotene Human Plasma 0.13 to 1.3 µmol/L β-Carotene β-Carotene Human Human Plasma - neonate Plasma 0.02 to 0.05 µmol/L 0.182+0.084 µmol/L β-Carotene Human Plasma 0.07 to 0.88 µmol/L β-Carotene Human Plasma 1.81+1.57 µmol/L β-Carotene (trans) β-Carotene (13-cis) β-Carotene (trans) Human Serum 0.322+0.259 µmol/L Human Serum 0.016+0.011 µmol/L Human β-Carotene (9-cis) Human β-Carotene (13-cis) Human γ-Carotene Human Serum Liver Adrenal Serum Liver Adrenal Serum Liver Adrenal Plasma 131 to 703 nmol/L 1.4 to 7.3 nmol/g 1.8 to 4.4 nmol/g n.d. 0.4 to 2.1 nmol/g 0.1 to 0.3 nmol/g 8 to 38 nmol/L 0.1 to 0.9 nmol/g 0.2 to 1.1 nmol/g 144 to 277 nmol/L α-Cryptoxanthin Human Plasma 39 to 130 nmol/L β-Cryptoxanthin Human Plasma 149 to 371 nmol/L β-Cryptoxanthin Human Plasma 0.05 to 0.52 µmol/L β-Cryptoxanthin Cysteine Human Human Plasma - neonate Plasma 0.51 to 0.63 µmol/L 9.0+1.1 µmol/L Cysteine Human Plasma 2.7±1.8 µmol/L WWW.ESAINC.COM Trevithick (1994) Rose and Bode (1995) Gopinathan et al. (1994) Milne and Botnen (1986) Sowell et al. (1994) Khachik et al. (1992) Khachik et al. (1992) Motchnik et al. (1994) Winklhofer-Roob et al. (1997) Finckh (1995) Milne and Botnen (1986) Sowell et al. (1994) Yamashita and Yamamoto (1997) Stahl et al. (1993) Stahl et al. (1993) Stahl et al. (1993) Stahl et al. (1993) Stahl et al. (1993) Khachik et al. (1992) Khachik et al. (1992) Khachik et al. (1992) Sowell et al. (1994) Finckh (1995) Andersson et al. (1995) Velury and Howell (1988) 332 Cyst(e)ine Human Blood 42.0 to 123 µmol/L Cysteine Rat Liver 0.050±0.032 µmol/g DHAA Human Plasma Glutathione Human Blood Plasma Glutathione Human Brain Glutathione Human Brain Glutathione Human Erythrocytes Glutathione Human Erythrocyte Glutathione Human Hair Glutathione Human Plasma Glutathione Human Plasma neonate Glutathione Human Whole blood 5.8+2.7 µmol/L 5.8+5.1 849+63 µmol/L – GSH 3.39+1.04 – GSH 0.3+0.04 µmol/g – GSH 3.75+0.98 nmol/g – GSSG 0.19+0.04 µmol/g – GSH 3.26+0.65 nmol/g – GSSG 2.02+0.1 µmol/mL cell – GSH 6.8+0.8 – GSSG 19.6 to 23.2 nmol/million RBC – GSH 5+3.6 nmol/µg DNA – GSH 0.096+0.08 – GSSG 1.0±0.8 µmol/L – GSH 2.4 to 3.4 µmol/L GSH 0.4 to 0.6 GSSG 0.9 to 1.7 mmol/L Glutathione Cow Eye - lens Glutathione Guinea pig Brain Liver Glutathione Horse Hemolysate Glutathione Horse Bronchial lavage Hemolysate Glutathione Mouse Bronchial lavage Liver Glutathione Mouse Trachea Glutathione Rat Bile WWW.ESAINC.COM 6.79+0.4 µmol/g GSH <0.048 GSSG 0.96+0.03 PSSG 1.68±0.03 nmol/mg protein – GSH 6.69±0.23 nmol/mg protein – GSH 720 to 1190 µmol/L – GSH 0.9 to 1.2 – GSH 33 to 114 µmol/L – GSSG 0.06 to 0.1 – GSSG 10+1 µmol/g – GSH 200+110 nmol/g – GSSG 2.71±0.71 nmol/mg protein – GSH 2.9+0.54 µmol/g – GSH Richie et al. (1996) Demaster et. al. (1984) Nagy and Degrell (1989) Michelet et al. (1995) Sofic et al. (1992) Sofic et al. (1991) Kuninori and Nishiyama (1991) Rabenstein and Saetre (1978) Krien et al. (1992) Velury and Howell (1988) Smith et al. (1996) Rabenstein and Saetre (1978) Ozcimder et al. (1991) Mefford and Adams (1978) Smith et al. (1995) Smith et al. (1995) Carro-Ciampi et al. (1988) Lakritz et. al. (1997) Carro-Ciampi et al. (1988) 333 Glutathione Rat Brain Glutathione Rat Lens 325+168 nmol/g – GSSG 650+205 µmol/L – GSH 218+70 – GSSG 5.67+0.6 µmol/g – GSH 0.284+0.03 – GSSG 1.77+0.4 µmol/g – GSH 0.149+0.05 – GSSG 2.25±0.03 nmol/mg protein – GSH 3.9 µmol/g – GSH Glutathione Rat Lens 5.2 µmol/g – GSH Glutathione Rat Liver Glutathione Rat Liver Glutathione Rat Liver Kidney Pancreas Colon Glutathione Rat Lung Homocysteine Human Plasma Homocysteine Human Plasma 4.07 ± 0.21 µmol/g – GSH 4 to 6 µmol/g – GSH 20 to 650 nmol/g – GSSG 6.5±0.39 nmol/g 3.8±0.22 0.61±0.02 0.26±0.01 1.31+0.6 µmol/mg – GSH 0.04+0.02 – GSSG 11.3±2.96 µmol/L – males 8.8±2.7 µmol/L – females 1.8±1.2 µM Homocysteine Human Plasma 0.14+0.03 µmol/L Homocysteine Human CSF 0.210+0.028 µmol/L Homocyst(e)ine Homocyst(e)ine Human Human Plasma Plasma 13.4+4.8 µmol/L 12.4+2.9 µmol/L Homocysteine Rat Liver 0.087±0.006 µmol/g Hypoxanthine Human CSF 5.94+0.74 µmol/L Hypoxanthine Human CSF Hypoxanthine Human Hypoxanthine Human Plasma Erythrocytes Urine Serum 1.8 to 5.5 µmol/L – neonate 0.6 to 5.1 µmol/L – adult 2.5+1 µmol/L 8.0+6.2 µmol/L 48+26 µmol/24h 1.7 to 16.9 µmol/L Glutathione Rat Blood Liver Kidney WWW.ESAINC.COM Asensi et al. (1994) Mefford and Adams (1978) Iriyama et al. (1986) Mitton and Trevithick (1994) Demaster et. al. (1984) Harvey et al. (1989) Rose and Bode (1995) Martin and White (1991) Wu et. al. (1994) Velury and Howell (1988) Andersson et al. (1995) Quinn et al. (1997) Wu et al. (1994). Fermo et al. (1992) Demaster et. al. (1984) Castro-Gago et al. (1986) Harkness and Lund (1983) Boulieu et al. (1984) Kock et al. 334 Hypoxanthine Rat CSF 100 to 840 nmol/L Hypoxanthine Rat CSF 80 to 310 nmol/L Lipoic acid Human Plasma hydrolysates Lutein/ zeaxanthin Lutein Human Lens 58 to 208 nmol/L (ox) 160 to 703 (red) 0.02+0.001 nmol/g Human Plasma 135 to 265 nmol/L Lycopene Human Plasma 0.65+0.36 µmol/L Menaquinone-4 Human Milk 2.9+2.3 nmol/L Menaquinone-4 Human Phylloquinone Human Plasma maternal Plasma umbilical Placenta Milk 0.11 nmol/L 0.09 nmol/L 2.66 pmol/g 4.6+2.0 nmol/L Phylloquinone Human Phylloquinone Human Plasma Liver Plasma 1.19+0.16 nmol/L 28+4 pmol/g 0.29-2.64 nmol/L Phylloquinone Human Phylloquinone Human Plasma maternal Plasma umbilical Placenta Serum 3.4 nmol/L 0.02 nmol/L 2.62 pmol/g 20+11 nmol/L Phylloquinone Human Serum 0.09-1.96 nmol/L Retinol Human Lens 0.133+0.014 nmol/g Retinol Human Plasma 0.3 to 3.0 µmol/L Retinol Human Plasma 1.29 to 2.99 µmol/L Retinol Retinyl esters Mousehairless Human Plasma Liver Lens 0.67+0.05 µmol/L 14+3.5 nmol/g 21 to 25 ng/g Retinyl esters Human Plasma 0.03 to 0.38 µmol/L Retinyl palmitate Liver 240+62 nmol/g Cardiac mitochondria α-Tocopherol Mousehairless Cow Mouse Rat Chicken Tocopherols Human 0.02+0.01 0.37+0.02 0.33+0.04 19+4 pmol/mg* 4+0.8 79+18 35 to 99 10 to 40 µmol/L α-Tocopherol Plasma Erythrocyte Liver Muscle Plasma WWW.ESAINC.COM (1994) Walter et al. (1988) Polasek et al. (1989) Teichert and Preiss (1992) Yeum et al. (1995) Khachik et al. (1992) Yamashita and Yamamoto (1997) Isshiki et al. (1988) Hiraike et al. (1988) Isshiki et al. (1988) Usui et al. (1990) Sadowski et al. (1989) Hiraike et al. (1988) Sann et al. (1985) Moussa et al. (1989) Yeum et al. (1986) Winklhofer-Roob et al. (1997) Khachik et al. (1992) Savoure et al. (1995) Yeum et. al. (1995) Sowell et al. (1994) Savoure et al. (1995) Lass and Sohal (1999) Murphy and Kehrer (1987) Farrell et al. 335 α-Tocopherol Human Erythrocyte 3.92 to 6.99 µmol/L α-Tocopherol Human Lens α-Tocopherol Human Plasma 3.65+0.39 nmol/g 3.6 to 5.9 nmol/g 10 to 34 µmol/L α-Tocopherol Human Plasma 28 to 49 µmol/L α-Tocopherol α-Tocopherol Human Human Plasma - neonate Serum 0.65 to 1.23 µmol/L 9.98 to 22.5 µmol/L α-Tocopherol α-Tocopherol Human Human α-Tocopherol Mouse hairless α-Tocopherol Rat 27.9±10 µmol/L 20.3±0.5 µmol/L 13.4±0.3 µmol/L 5.4+0.1 nmol/g 24.2+1.1 21.9+0.6 21.2+2.9 5.4+0.2 5.6+0.1 µmol/L α-Tocopherol Rat Serum Plasma Blood Brain Heart Kidney Liver Skin Erythrocyte membrane Lens 0.3+0.05 nmol/g α-Tocopherol Rat α-Tocopherol Rat Liver Muscle Plasma 78.5±2.3 nmol/g 21.7±0.5 10+0.1 µmol/L β-Tocopherol γ-Tocopherol Human Chicken γ-Tocopherol Human Plasma - neonate Plasma Erythrocyte Liver Muscle Lens γ-Tocopherol Human Plasma 8.47 to 19.0 µmol/L 2.3+1.0 pmol/mg* 0.64+0.18 19+4 8 to 25 0.62 to 1.20 nmol/g 0.85+0.14 nmol/g 0.2 to 1.3 µmol/L γ-Tocopherol Human Plasma 5 to 15 µmol/L γ-Tocopherol Mouse hairless δ-Tocopherol Human Brain Heart Kidney Liver Skin Erythrocyte 0.01+0.02 nmol/g 0.19+0.05 0.35+0.06 0.29+0.03 0.04+0.00 0.05 to 0.13 µmol/L α-Tocopherylquinone Chicken α-Tocopherylquinone α-Tocopherylquinone Human Plasma Erythrocyte Liver Muscle Erythrocyte 0.21+0.08 nmol/g* 0.82+0.12 1.47+1.1 0.07 to 0.49 0.04 to 0.07 µmol/L Rat Plasma 0.053+0.011 µmol/L WWW.ESAINC.COM (1978) Vatassery et al. (1993) Yeum et al. (1995) Winklhofer-Roob et al. (1997) Khachik et al. (1992) Finckh (1995) Chou et al. (1985) Edlund (1988) Lang et. al. (1986) Pods et al. (1996) Takeda et al. (1996) Mitton and Trevithick (1994) Lang et. al. (1986) Takeda et al. (1996) Finckh (1995) Murphy and Kehrer (1987) Yeum et. al. (1995) Winklhofer-Roob et al (1997) Khachik et al. (1992) Pods et al. (1996) Vatassery et al. (1993) Murphy and Kehrer (1987) Vatassery et al. (1993) Takeda et al. (1996) 336 α-Tocopherylquinone γ-Tocopherylquinone Rat Chicken α-Tocotrienol Mouse hairless γ-Tocotrienol Mouse hairless Ubiquinone total Human Ubiquinol-8 Rat Ubiquinol-8 Rat Ubiquinone-8 Rat Ubiquinol-9 Human Ubiquinol-9 Mouse hairless Ubiquinol-9 Rat Ubiquinol-9 Rat Ubiquinol-9 Rat Ubiquinone-9 Cow Mouse Pig Erythrocyte membrane Plasma Erythrocyte Liver Muscle Brain Heart Kidney Liver Skin Brain Heart Kidney Liver Skin Plasma - control endurance athletes hyperthyroid hypothyroid hypercholesterolemic Brain Liver Kidney Plasma Liver Heart Kidney Brain Heart Kidney Liver Serum Serum Brain Heart Kidney Liver Skin Brain Heart Kidney Liver Serum Liver Muscle Plasma Liver Heart Kidney Cardiac mitochondria WWW.ESAINC.COM 0.36+0.14 µmol/L 0.16+0.06 pmol/mg* 0.21+0.08 0.09+0.03 0.004 to 0.49 n.d. 0.08+0.01 nmol/g 0.06+0.04 0.10+0.04 0.24+0.20 n.d. 0.19+0.05 nmol/g 0.15+0.07 0.19+0.16 0.76+0.71 0.80+0.20 mg/L 0.58+0.17 0.27+0.13 0.62+0.11 1.15+0.15 3.02+0.9 nmol/g 13.2+2.9 1.5+0.27 82.5+13.8nmol/L 3.02+0.8 nmol/L 1.0+0.12 0.61+0.04 2.88+0.41 nmol/g 11.5+2.6 2.33+0.27 14.6+3 101+22 46.4+7.8 nmol/L 1.6+0.1 nmol/g 19+4 81+29 42+16 2.2+0.3 24.2+5.8 nmol/g 26.2+4.8 80+18 85+24 44+9 nmol/L 121.5±11.6 nmol/g 4.9±0.4 0.46+0.07 µmol/L 84.4+0.5 nmol/g 21+0.6 23+5 0.12+0.01 µmol/g 6.01+0.05 0.18+0.01 Takeda et al. (1996) Murphy and Kehrer (1987) Pods et al. (1996) Pods et al. (1996) Grossi et al. (1992) Wakabayashi et al. (1994) Okamoto et. al. (1988) Wakabayashi et al. (1994) Wakabayashi et al. (1994) Pods et al. (1996) Wakabayashi et al. (1994) Lang et. al. (1986) Okamoto et. al. (1988) Lass and Sohal (1999) 337 Ubiquinone-9 Rabbit Rat Human Brain Heart Kidney Liver Brain Heart Kidney Liver Skin Muscle 0.10+0.01 5.18+0.01 1.25+0.13 µmol/g 3.14+0.4 4.15+0.25 2.26+0.3 10.2+0.5 nmol/g 245+22 302+124 46+18 7.6+1.9 nmol/L 31.9±3.2 nmol/g 24+5.8 nmol/g 240+44 126+36 152+44 593+94 nmol/L 47+4 nmol/g 254+23 156+14 165+18 0.4 to 1.0 µmol/L Ubiquinone-9 Mouse hairless Ubiquinone-9 Rat Ubiquinone-9 Rat Ubiquinone-9 Rat Ubiquinol-10 Human Brain Heart Kidney Liver Serum Brain Heart Kidney Liver Plasma Ubiquinol-10 Human Serum 1.59+0.2 µmol/L Ubiquinol-10 Ubiquinol-10 Human Human Plasma - neonate Plasma 0.48 to 0.60 µmol/L 0.927+0.214 µmol/L Ubiquinol-10 Human Plasma 1.14 µmol/L (male) 0.56 (female) 50.86+16 nmol/g creatinine (male) 57.9+24 (female) 0.6+0.1 nmol/g 2.8+0.7 11+6 1.7+0.3 0.4+0 15.2±1.4 nmol/g Urine Ubiquinol-10 Mouse hairless Ubiquinol-10 Rat Ubiquinol-10 Rat Plasma Liver Heart Kidney Ubiquinone-10 Cow Mouse Pig Rabbit Rat Human Human Cardiac mitochondria Ubiquinone-10 Ubiquinone-10 Brain Heart Kidney Liver Skin Liver Plasma - neonate Plasma WWW.ESAINC.COM 0.11±0.011 µmol/L 13.3±1.7 nmol/g 2.2±0.09 5.1±0.9 6.51+0.02 µmol/g 0.71+0.03 5.79+0.05 4.78+0.08 0.65+0.02 0.03 µmol/L 0.041+0.012 µmol/L Aberg et al. (1992) Pods et al. (1996) Lang et. al. (1986) Wakabayashi et al. (1994) Aberg et al. (1992) Motchnik et al. (1994) Wakabayashi et al. (1994) Finckh (1995) Yamashita and Yamamoto (1997) Okamata et. al. (1988) Pods et al. (1996) Lang et. al. (1986) Okamata et. al. (1988) Lass and Sohal (1999) Finckh (1995) Yamashita and Yamamoto 338 Ubiquinone-10 Human Serum 0.82+0.5 µmol/L Ubiquinone-10 Human Serum 0.68 to 1.68 µmol/L Ubiquinone-10 Ubiquinone-10 Human Human Ubiquinone-10 Mouse hairless Ubiquinone-10 Rat Ubiquinone-10 Rat Uric acid Human 0.28 to 0.95 µmol/L 15.5+1.2 nmol/g 132+10.7 77+7.7 64+4.8 3.4+0.5 nmol/g 21+8 31+14 n.d. n.d. 2.7±0.5 nmol/g 2.2±0.4 21+1.7 nmol/g 19.5+2 25.5+2 25+2 258 to 621 µmol/L 273 to 485 123 to 351 Uric acid Human Uric acid Human Serum Brain Heart Kidney Liver Brain Heart Kidney Liver Skin Liver Muscle Brain Heart Kidney Liver Serum - control Serum - rheumatoid Synovial fluid rheumatoid Plasma Urine Plasma Uric acid Human Uric acid Human Uric acid Human Uric acid Human Muscle Plasma Plasma Uric acid Mouse Liver Uric acid Rat Liver Kidney Pancreas Colon Uric acid Rat CSF 0.13±0.01 nmol/g 0.40±0.01 0.24±0.01 1.1±0.06 0.1 to 3.6 µmol/L Uric acid Rat CSF 3.55 to 19.0 µmol/L Xanthine Human CSF 5.2+0.87 µmol/L Xanthine Human CSF 0.9 to 9.1 µmol/L neonate Plasma - control Plasma premenopausal females Serum WWW.ESAINC.COM 120 to 360 µmol/L 1500 to 3600 140 to 600 µmol/L 160 to 450 µmol/L 120 to 340 185 to 486 µmol/L 0.26 µmol/g 305 µmol/L 191 to 413 µmol/L neonates, 180 to 420 adults 130+5 nmol/g (1997) Wakabayashi et al. (1994) Ikenoya et. al. (1979) Edlund (1988) Aberg et al. (1992) Pods et al. (1996) Lang et. al. (1986) Aberg et al. (1992) Grootveld and Halliwell (1987) Boulieu et al. (1984) Benzie and Strain (1996) Wyngaarden and Kelly (1976) Kock et al. (1994) Hellsten et al. (1997) Gopinathan et al. (1994) Barja de Quiroga et al. (1991) Rose and Bode (1995) Walter et al. (1988) Polasek et al. (1989) Castro-Gago et al. (1986) Harkness and Lund (1983) 339 Rat Serum Plasma Erythrocytes Urine CSF 0.6 to 4.7 µmol/L adult 0.2 to 6.2 µmol/L 1.4+0.7 µmol/L <0.5 µmol/L 68+42 µmol/24h 2.6 to 8.6 µmol/L Xanthine Rat CSF 0.21 to 2.9 µmol/L Xanthophylls Human Lens 11 to 25ng/g Zeaxanthin Human Plasma 35 to 50 nmol/L Xanthine Xanthine Human Human Xanthine Kock et al.(1994) Boulieu et al. (1984) Walter et al. (1988) Polasek et al. (1989) Yeum et. al. (1995) Khachik et al. (1992) Table 4.3 Levels Of Antioxidants Reported In The Literature. Ascorbic Acid. Most animals can synthesize ascorbic acid (vitamin C) from glucose in the liver (Banhegyi et al. (1997)). Man, primates, guinea pigs and fruit bats, however, do not possess L-gulonolactone oxidase, the terminal enzyme in the pathway of ascorbic acid biosynthesis. For these mammals, ascorbic acid must be derived from the diet. Thus diets deficient in ascorbic acid will lead to disease (e.g., scurvy) in these organisms. Ascorbic acid plays several important metabolic roles (reviewed by Levine (1986); Levine et al. (1996)). For example, it is a cofactor for enzymes involved in both post-translational modification of collagen (prolyl and lysyl hydroxylases) and catecholamine synthesis (dopamine-β-hydroxylase) (Sauberlich (1994), Udenfriend et al. (1954)). Furthermore, it may act as a neuromodulator in the brain (Grunewald (1993)). Ascorbic acid plays key roles in the regulation of absorption of iron and its cellular metabolism (Dorey et al. (1993); Hoffman et al. (1991); Toff and Bridges (1995)). It may also prevent stomach cancer by helping eliminate nitrosamines derived from the diet or formed in the stomach from secondary amines (Chapter 2) (Block (1991); Cohen and Bhagavan (1995); Dyke et al. (1994)). Antioxidant Properties. Ascorbic acid is a very important antioxidant (Halliwell (1996); Rose and Bode (1993)). Halliwell (1996) has summarized the various in vitro antioxidant properties of ascorbic acid and an updated version is presented in Table 4.4. Although data supporting the antioxidant role of ascorbic acid in vitro is overwhelming, in vivo data are much more scarce. Ascorbic acid does fulfill the WWW.ESAINC.COM 340 criteria used to establish whether a compound is a suitable antioxidant candidate (see above and Rose and Bode (1993)): • It is present in adequate amounts in the body (Table 4.3)); • It is versatile and readily oxidized; • It is compartmentalized (e.g., it is particularly abundant in adrenal chromaffin granules and neuronal monoamine vesicles where it prevents oxidation of the monoamines); • It is readily available; • It is conserved by the kidneys; and • It has tolerable toxicity. Comments Scavenges peroxynitrite (rate constant 2.4 x 102 M-1.s-1) Scavenges superoxide and HO2• (rate constants 1.0 x 104 to >105 M-1.s-1) Scavenges hydroxyl free radicals (rate constant >109 M-1.s-1) Scavenges thiyl and sulphenyl radicals Scavenges hypochlorous acid. Protects against chloramine-dependent modifications to LDL Scavenges ozone and nitrogen dioxide Scavenges some flavonoid radicals but may be oxidized by other flavonoids Scavenges and quenches singlet oxygen May regenerate α-tocopherol from α-tocopheroxyl radical in membranes (and lipoproteins) Protects plasma lipids from peroxidation induced by activated neutrophils Inhibits lipid peroxidation induced by hemoglobin (or myoglobin)-H2O2 mixtures and prevents peroxide-induced release of iron from heme Protects neutrophils from self inflicted oxidative stress Reference Bartlett et al. (1995); Vasquez-Vivar et al. (1996); Whiteman and Halliwell (1996) Cabelli and Bielski (1983); Halliwell and Gutteridge (1999); Nandi and Chatterjee (1987); Nishikimi (1975); Radi et al. (1991) Anbar and Neta (1987); Bartlett et al. (1994) Asmus (1987); Sevilla et al. (1989) Carr et al., (2000); Folkes et al. (1995); Halliwell et al. (1987) Cross et al. (1994) Bors et al. (1995) Chou and Khan (1983) Beyer (1994); Bisby and Parker (1995); Buettner (1993); Cadenas et al. (1996); Chan (1993); Esterbauer et al. (1989); Kagan et al. (1992a,b); Liebler et al. (1986); Mehlhorn et al. (1989); Muckai et al. (1992); Niki et al. (1984); Packer (1994); Packer et al. (1979); van den Berg et al. (1990); Wefers and Sies (1988). But see Glascott and Farber (1998) and references therein. Frei et al. (1989) Rice-Evans et al. (1989) Wang et al. (1997) Table 4.4 Evidence Supporting The Role For Ascorbic Acid As An Antioxidant In Vitro. WWW.ESAINC.COM 341 HO O O -H+ OH HO HO HO O O O O -e - OH OH OH HO O O- SEMIDEHYDRO-LASCORBIC ACID L-ASCORBATE ANION L-ASCORBIC ACID O -e - HO H OH H OH O O O O O O HO OH OH +H2O O O OH O OH -H2O O O DEHYDRO-L-ASCORBIC DEHYDRO-L-ASCORBIC ACID ACID HYDRATE +H2 O GLUCOSE-6PHOSPHATE + H O LACTIC ACID OH HO O O OH CH 2 OH H 2,3-DIKETO-L-GULONIC ACID L-THREONIC ACID + OXALIC ACID Figure 4.4 Chemical Structure Of Ascorbate And Relationship To Its Oxidation Products. (Antioxidant pathway is presented in red; metabolism in blue). Furthermore, evidence suggests that ascorbic acid is depleted during oxidative stress, e.g., in patients with rheumatoid arthritis (Blake et al. (1981); Lunec and Blake (1985)), adult respiratory distress syndrome (Cross et al. (1990)) and preeclampsia (Huber et al. (1997)). Recently Wang et al. (1997) reported that ascorbic acid recycling in stimulated neutrophils is an important antioxidant defense mechanism protecting them from damage by their own pro-oxidant molecules. When ascorbic acid reacts with a more aggressive radical the result is the production of an intermediate radical (ascorbyl) of low reactivity (Figure 4.4). The lower activity comes from the ability of ascorbate to delocalize the radical electron throughout its π-system (Chapter 1). As can be seen from Table 2.1.1, WWW.ESAINC.COM 342 the redox potential (Eo) of ascorbic acid is low and it is therefore a good reducing agent (antioxidant). Consequently ascorbic acid will quench the hydroxyl free radical, lipid peroxyl radical, uric acid radical and tocopheroxyl radical. Ascorbic acid may be involved in the regeneration of tocopherol from the tocopheroxyl radical formed during the prevention of lipid peroxidation (Figure 4.5). Although there is abundant in vitro evidence for this interaction (see Table 4.4) little in vivo evidence exists. In fact recent data suggest that ascorbic acid acts as an antioxidant independent of α-tocopherol and reacts with radicals prior to their reaction with α-tocopherol (Glascott and Farber (1998) and references therein). In this way ascorbic acid prevents the loss of α-tocopherol in an indirect manner in vivo. MEMBRANE CYTOSOL CH3 LIPID-O2 O O HO CH(OH)CH2 OH R O H3 C Lipid Peroxyl Radical O CH3 O Semidehydroascorbate Radical CH3 α-Tocopherol CH3 O LIPID-O2H Lipid HydroPeroxide R O H3 C CH3 O O CH(OH)CH2 OH CH3 α-Tocopheroxyl Radical HO OH Ascorbic Acid Figure 4.5 Proposed Interaction Between Ascorbic Acid And Tocopherol At The Cytosol-Membrane Interface. As shown in Figure 4.4, the ascorbyl radical can undergo a single electron oxidation to form dehydroascorbic acid (DHAA). This reaction probably involves disproportionation (Eqn 4.18). DHAA can then suffer three fates, hydration with irreversible ring opening and the formation of 2,3-diketo-L-gulonic acid with the loss of ascorbic acid, dehydration with cyclization, or reduction of DHAA with the regeneration of ascorbic acid. As 2,3-diketo-L-gulonic acid produces oxalic acid which is toxic to both animals and plants, the favored pathway is regeneration. This also helps to conserve ascorbic acid. There has been some debate as to how ascorbic acid is regenerated from the DHAA (Rose and Bode (1993) and references therein). Evidence suggests that ascorbic acid may be regenerated either in an enzyme-independent process using GSH (Varma and Richards (1988); Winkler (1992)) or an enzyme-dependent process using either NADH, WWW.ESAINC.COM 343 NADPH or GSH (Halliwell (1996); Maellero et al. (1994); Park and Levine (1996); Rose and Bode (1992, 1993); Sauberlich (1994); Wells and Xu (1994)) (see Table 5.1). 2 Ascorbyl• → Ascorbate + Dehydroascorbate Eqn 4.18 Pro-oxidant Properties. Several studies suggest that ascorbic acid may be toxic. However, these reports usually use extremely high doses of ascorbic acid, or involve diseased patients (e.g., iron-overloaded individuals) (Halliwell, (1996)). Although in vitro evidence suggests that, under certain circumstances, ascorbic acid may act as a prooxidant (Baysal et al. (1989); Girotti et al. (1985); Giulivi and Cadenas (1993); Herbert (1996); Skakagami and Satoh (1997)) there is little evidence that it may act as a pro-oxidant in vivo. Ascorbic acid’s pro-oxidant activity comes from the finding that it can reduce Fe (III) to Fe (II) (Eqn 4.19) that can then take part in the Fenton reaction (Udenfriend et al. (1954)). Bendich et al. (1986) found that, under certain conditions, ascorbate is capable of reducing oxygen to superoxide that can then reduce Fe (III) to Fe (II) (Eqns 4.20 and 4.21). However, based upon thermodynamic principles, Halliwell (1996) concluded that it is unlikely that ascorbic acid can reduce oxygen to superoxide. Whether acting directly or through the superoxide radical ascorbate can both mobilize bound iron and influence the Fe3+/Fe2+ redox status. This effect on iron metabolism has been proposed to promote lipid peroxidation (Minotti and Aust (1992)). For example, Andorn et al. (1996) reported that ascorbate could promote iron-dependent lipid peroxidation in human brain samples. However, others could find no pro-oxidant activity even in the presence of iron overload (Chen et al., (2000)). Whether this pro-oxidant effect of ascorbic acid is important in vivo, however, is still a matter of conjecture. Fe3+ + ascorbate → Fe2+ + ascorbyl• O2 + Ascorbate → Ascorbyl• + O2•- + H+ O2•- + Fe3+ → O2 + Fe2+ Eqn 4.19 Eqn 4.20 Eqn 4.21 Ascorbate can also react with copper ions producing hydroxyl free radicals (Buettner (1996); Aruoma et al. (1991)). Some have even warned of the possibility that the production of ROS could lead to gastric problems when ascorbic acid, iron and copper are consumed as part of a multivitamin pill (Maskos and Koppenol (1991)). Indeed, many multivitamin supplements now come with a warning on their labels. However, recent evidence could not find any WWW.ESAINC.COM 344 pro-oxidant activity of ascorbic acid to either plasma proteins or lipids when subjected to the “Undenfriend system” (hydrogen peroxide + copper II + iron II) (Suh et al., (2003)). The consumption of ascorbic acid (>500mg/day) was reported to promote the formation of a potentially mutagenic lesion in human DNA (8-oxoadenine), probably through ascorbic acid’s reaction with DNA-bound metals (Podmore et al., (1998)). Similarly, co-supplementation of human subjects with ascorbic acid and iron was also found to promote DNA adduct formation (Rehman et al. (1998)). Whether ascorbic acid can actually promote DNA damage in vivo has been severely challenged by several groups (Frei (1998); Levine et al. (1998); Poulsen et al. (1998)). However, recent evidence suggests that ascorbic acid can promote the decomposition of lipid hydroperoxides thereby producing genotoxic aldehydes (e.g., 4-oxo-3-nonenal, 4,5-epoxy-2(E)-decanal and 4-hydroxynonenal) that can form mutagenic DNA lesions such as etheno2’deoxyadenosine (Lee et al., (2001)). This may explain why ascorbic acid lacks efficacy as an anticarcinogenic agent. Measurement. The unstable nature of ascorbic acid makes it difficult to analyze accurately unless certain precautions are undertaken. Although a multitude of assays have been published for the analysis of ascorbate and dehydroascorbate, many suffer from the “four-S syndrome” - resulting from inattention to stability, sensitivity, specificity and substance interference (Washko et al. (1992)). These issues have been extended by Lykkesfeldt et al. (1995) to include analyte recovery, reproducibility, choice of detection principle and column durability. It should be remembered that ascorbic acid, the ascorbyl radical and dehydroascorbic acid are all in equilibrium with each other, so in order to measure true analyte levels reliably, the correct choice of analytical method should minimally disturb these equilibria. For example, many methods include a variety of antioxidants during sample processing and analysis (e.g., dithiothreitol, homocysteine); however, these substances may affect the ascorbate/dehydro-ascorbate ratio and lead to erroneous data. This situation is even more complex as dehydroascorbic acid is relatively unstable (half-life of 6 minutes at pH 7.0 and 37oC) and is rapidly and irreversibly lost as 2,3-diketogulonic acid (Schell and Bode (1993)). Several analytical approaches have been used to measure ascorbic acid and include spectrophotometric, gas chromatographic and HPLC-based techniques and these have been critically reviewed (Lykkesfeldt et al. (1995), Washko et al. (1992)). These authors examined the use of HPLC-ECD in detail and concluded that coulometric detection was more reliable and offered marked improvement in sensitivity over amperometric approaches. Several groups have used HPLCcoulometric detection to measure ascorbate either alone (Lykkesfeldt et al. (1995); Schell and Bode (1993); Xu and Wells (1996)) or with other antioxidants WWW.ESAINC.COM 345 (e.g., glutathione, glutathione disulfide, uric acid) simultaneously (Rose and Bode (1995), Sofic et al. (1991)). Ascorbic acid is also routinely determined as part of a global method for metabolic profiling using gradient HPLC and coulometric array detection (Gamache et al. (1993); Rizzo et al. 1991)). Tissue levels of ascorbic acid in a variety of species are presented in Table 4.1. Dehydroascorbic acid can be measured one of three ways: directly, following derivatization, or after reduction to ascorbic acid (Washko et al. (1992)). One of the most promising assays is the use of HPLC-coulometric detection following chemical reduction of dehydroascorbic acid to ascorbic acid (Dhariwal et al. (1990); Lykkesfeldt et al. (1995)). The ascorbyl radical has been measured directly using a spectrophotometric approach with an absorbance wavelength of 360nm or indirectly using semidehydroascorbate reductase and monitoring changes in the NAD+/NADH ratio at 340nm. The ascorbyl radical can also be measured using EPR approaches (Washko et al. (1992) and references therein). Thiols. The term thiol (or sulfhydryl) refers to a compound that contains an –SH group. The chemistry of thiols and their corresponding disulfides is extensive and has been briefly described in Chapter 2. Numerous thiols can be found in biological systems but due to space constraints this review will be limited to the aminothiols cysteine, homocysteine and glutathione. 1. Glutathione. The tripeptide, glutathione, (γ-glutamyl-cysteinyl-glycine) [GSH] was first discovered by J. de Rey-Pailhade over 100 years ago and is the most ubiquitous peptide found in cells. In this text GSH will be used to designate glutathione and GSSG will be used for glutathione disulfide. Often in literature GSH is inaccurately referred to as reduced glutathione and GSSG as oxidized glutathione (the latter should actually refer to glutathione sulfenic, sulfinic and sulfonic acids). GSH can be obtained from the diet or can be synthesized de novo in the liver (Anderson (1998); Flagg et al. (1994); Jones (1995); Lu (1999)). Synthesis primarily occurs in the cytoplasm from non-essential amino acid constituents according to Figure 4.6. GSH is degraded by membrane bound γ-glutamyl transpeptidase producing glutamate and cysteinyl-glycine that is then hydrolyzed to cysteine and glycine by cysteinyl-dipeptidase. The enzymes involved in the synthesis and catabolism of GSH are linked to form the γ-glutamyl cycle (see below). WWW.ESAINC.COM 346 O O OH OH O O H2 N O SH NH2 H2 N Cysteine NH Glycine H2 N O SH NH OH H2 N OH SH OH OH O NH O ATP ADP, Pi O O OH ATP γ-Glutamyl-cysteine Glutamate O ADP, Pi HO Glutathione (GSH) γ-Glutamyl-cysteine Synthetase Glutathione Synthetase Figure 4.6 Synthesis Of GSH. Mammalian tissue concentrations of GSH are typically 0.5 to 10mM, but considerably less is found in plasma (Table 4.1). GSH is readily oxidized to GSSG (see below). In most tissues the level of GSSG is kept low, a consequence of glutathione reductase activity. For example, in brain the GSSG level is typically <1% that of GSH (Cooper (1997); Cooper et al. (1980)). Some GSH also exists as mixed disulfides (with cysteine, coenzyme A and protein thiolates). The tissue levels of GSH are tightly regulated. For example, it is difficult to deplete hepatic GSH below 30% of control values even following xenobiotic challenge or prolonged starvation. On the other hand, even with supplementation it is difficult to exceed hepatic GSH stores. Such nutritional and hormonal regulation of glutathione homeostasis was reviewed recently (Taylor et al. (1996)). Biological Roles of Glutathione. GSH plays several important roles in biological systems. It protects against the action of some pro-oxidants and is involved in detoxification of harmful compounds. GSH acts as a cofactor for numerous enzymes and represents a safe storage form of cysteine. It is involved in the transport of amino acids across membranes and in the regulation of cellular metabolism. Protection. GSH can readily be oxidized to its disulfide (GSSG).(Eqn 4.22). With an Eo’=240mV (Table 2.1.1), the GSH/GSSG couple is one of the most reducing reactions found for endogenous small molecule antioxidants. The reductive WWW.ESAINC.COM 347 capacity of GSH is utilized by glutathione peroxidase (and other peroxidases) in the destruction of peroxides (Figure 4.7).8 The reduction of GSSG back to GSH is energetically unfavorable so it is catalyzed by an enzyme, glutathione reductase, that requires a strong reducing agent (NADPH) (NADP+/NADPH: Eo’=320mV). Glutathione reductase consists of two subunits, each containing FAD at the active site. Electrons are passed from NADPH through FAD to a cystine disulfide bridge located in the active site. The resulting two active cysteine thiols then take part in reducing GSSG. 2GSH → GSSG + 2H+ + 2e- Glucose SH 2NAD Pentose Phosphate Pathway H2O2 (RO2 H) γ -Glu-Cys-Gly (GSH) + + 2H + 2NADPH Eqn 4.22 2 Glutathione Peroxidase Glutathione Reductase γ-Glu-Cys-Gly S Ribulose-5Phosphate S γ -Glu-Cys-Gly 2H2O (ROH + H2 O) (GSSG) Figure 4.7 The Use And Regeneration Of GSH. The GSH/GSSG ratio is usually kept high in cells (typically >10:1 to >100:1), maintaining a reducing environment. Keeping cellular GSSG levels low is important as it can affect protein synthesis and inhibit several enzymes possibly by forming mixed disulfides with essential protein thiols. Enzymes affected include adenylate cyclase, chicken hepatic fatty acid synthetase, rabbit muscle phospho-fructokinase, and phosphorylase phosphatase.9 Under periods of oxidative stress, the liver and heart can actively transport GSSG out of their cells, thereby preventing deleterious action. The GSH/GSSG ratio is also high in mitochondria. Here it serves to keep transport proteins and enzymes (e.g., ATPases and dehydrogenases) active by maintaining essential thiol groups in a reduced form. 8 GSH also reacts directly with a variety of free radicals producing reactive thiyl radicals that must be further metabolized (Chapter 2). 9 Conversely, GSH can protect certain enzymes from oxidative stress through tightly regulated S-thiolation (e.g., glyceraldehyde-3-phosphate dehydrogenase) (Grant et al. (1999)). WWW.ESAINC.COM 348 GSH also plays a role in the regeneration of other antioxidants. For example, it can regenerate ascorbic acid from dehydoascorbic acid and membrane bound α-tocopherol from the α-tocopheroxyl radical formed during inhibition of lipid peroxidation (see below). R-X GSH GSH-R GSH Transferase -Glutamate Glycine-Cysteine-R Glutamyltranspeptidase NHCOCH3 R S O OH A Mercapturic Acid Cysteinylglycinase -Glycine N-Acetylase Cysteine-R Figure 4.8 The Detoxification Of A Xenobiotic (R-X) By The Mercapturic Acid Pathway. Detoxification and Bioactivation. GSH is important in the detoxification of potentially harmful endogenous compounds and xenobiotics (e.g., α-oxoaldehydes10 and redox active compounds such as monoamines, catecholestrogens, polyphenols and some drugs) (Figure 2.24). GSH is a nucleophile and readily forms S-conjugates with electrophilic compounds in a reaction catalyzed by glutathione-S-transferases. Glutathione-S-conjugates are further metabolized through the mercapturate pathway (Figure 4.8) and are usually excreted in the bile. The mercapturic pathway is also important for the metabolism of endogenous substances such as the leukotrienes. Unfortunately many S-conjugates retain (and some may even be bioactivated to products that can even exceed) the electrophilic and redox properties of the parent compound (Anders and Dekant (1998); Monks and Lau (1998)). For example, dibromoethane can produce a highly reactive episulfonium intermediate capable of damaging DNA (Anders and Dekant (1998) and references therein). 10 A number of potentially toxic α-oxoaldehydes (e.g., glyoxal, methylglyoxal and 4,5-dioxovalerate) are formed in vivo during lipid peroxidation, glycation and as part of normal metabolism (e.g., metabolism of ketone bodies and triosephosphates, and threonine catabolism). These compounds readily react with amine groups found in DNA (leading to mutagenesis and apoptosis), RNA and proteins (leading to protein degradation and cytokine-mediated immune response (Thornalley (1998) and references therein). They are detoxified by oxidation to aldonic acids catalyzed by cytosolic enzymes (glyoxylase I and II) using GSH as cofactor. WWW.ESAINC.COM 349 Excretion of the GSH-conjugate may protect the cell from the toxic effect of these compounds, but once in the circulation, other cells can then accumulate these toxic metabolites leading to tissue damage. Another unfortunate consequence of GSH-mediated detoxification is the deactivation of anticancer drugs (Zhang et al. (1998a)). Inhibition of this detoxification pathway is currently being explored as a strategy to modulate drug resistance. Consumption of excessive amounts of the painkiller acetaminophen (paracetamol) can lead to hepatotoxicity. The mechanism for this action involves the conversion of acetaminophen (and its derivative 4-ethoxyacetanilide) to a highly reactive quinoneimine by the action of the cytochrome P450 system. The quinoneimine exerts its toxicity by reacting with protein-thiol groups (adducts) and by depleting GSH stores due to the formation of acetaminophen-GSH adducts. Treatment includes supplementation with N-acetylcysteine and methionine that act by maintaining GSH levels. H2O2 Catabolism Ascorbate Dehydroascorbate Glutaredoxin 2H2O H2O2 GSH GSSG Peroxidases NADPH GSH Reductase Figure 4.9 The Relationship Between Glutathione And Ascorbic Acid (see Meister (1994) for greater details). Cofactor. GSH is a cofactor for many enzymes including glutathione peroxidase, and other peroxidases, dehydrochlorinase, formaldehyde dehydrogenase, glyoxalase, maleyl-acetoacetate isomerase, and prostaglandin endoperoxidase isomerase (Meister (1989); Thornally (1998)). GSH is also used by dehydroascorbate reductase, the enzyme responsible for the regeneration of ascorbic acid from its potentially toxic metabolite dehydroascorbate (Eqns 4. 23 and 4.24) (Figure 4.9) (Meister (1994)). WWW.ESAINC.COM 350 Dehydroascorbate + 2GSH → Ascorbate + GSSG Or Eqn 4.23 2Semidehydroascorbate + 2GSH → 2Ascorbate + GSSG Eqn 4.24 Storage of Cysteine in a Non-toxic Form. Cysteine is excitotoxic probably through its action on the NMDA receptor. Its sulfhydryl group readily forms hemithioacetals with aldehydes and hemithioketals with α-ketoacids. For example, following its reaction with pyridoxal 5’-phosphate, the hemithioacetal adduct cyclizes to form a thiazolidinone that can effectively inhibit any enzyme using pyridoxal 5’-phosphate as a cofactor. The sulfhydryl group of GSH is much less reactive, thus permitting cysteine to be stored in mammalian cells at 10-100 times the level of the free amino acid (Cooper (1997)). Amino Acid Transport. The enzymes for GSH synthesis and catabolism are linked forming the γ-glutamyl cycle that may function in amino acid transport (Orlowski and Meister (1970)). The location of γ-glutamyltranspeptidase on the cell surface is thought to enable the translocation of amino acids across the cell membrane. The γ-glutamyl cycle is found to be most active in tissues where amino acid transport is high (e.g., the kidney). However, as it is energetically very expensive (requiring the hydrolysis of 3 ATP molecules) and other less energetic amino acid transporters are available, the importance of the γ-glutamyl cycle in amino acid translocation remains to be elucidated (Meister (1994) and references therein). The γ-glutamyl cycle also plays a role in the metabolism of estrogens, leukotrienes and prostaglandins and in the detoxification of xenobiotics (see Cooper and Kristal (1997) and references therein). Regulation. The regulation of cellular metabolism was first proposed in the 1950s and 1960s when it was shown that key metabolic enzymes could be regulated by thioldisulfide exchange. More recently in vitro studies have reported that signal transduction (protein/protein interactions) and gene transcription (protein/DNA interactions) are dependent on the redox status of critical sulfhydryl groups which, in turn, could be affected by GSH levels (Taylor et al. (1996) and references therein). WWW.ESAINC.COM 351 Compartmentalization. GSH is primarily synthesized in the cytoplasm, yet many of its physiological functions occur in other compartments including the endoplasmic reticulum11, mitochondrial matrix, nucleus and extracellular space. The availability of GSH in these different compartments is complex and dependent upon several factors including transport, utilization, synthesis and reduction of GSSG and GSH-mixed disulfides (Cooper and Kristal (1997); Smith et al. (1996)). For example, the uptake of GSH by mitochondria is an energy dependent transport process coupled to the efflux of anions, whereas the passage of GSH into the nucleus appears to be by passive diffusion (Smith et al. (1996)). Conditions and Diseases Affecting Glutathione. The GSH/GSSG ratio is affected by a number of conditions and diseases including aging, AIDS, arthritis, cancer, cardiovascular disease, Crohn’s disease, diabetes, exercise, gluten sensitivity, nephrotoxicity, neurodegenerative disease, oxidative stress, pre-eclampsia, pulmonary disease, and Wilson’s disease (Boda and Nemeth (1992); Harding et al. (1994); Iantomasi et al. (1993, 1994); Lomaestro and Malone (1995); Navarro et al. (1999); Reed (1990); Samiac et al. (1998); Staal (1998); Summer and Esenburg (1985); Vina et al. (1996)). Measurement of Glutathione and its Disulfide. Many methods exist for the measurement of glutathione. There are two major considerations when choosing a method. Firstly, not all methods differentiate between GSH and GSSG. For example, the most widely used technique, enzyme recycling, measures total GSH (GSH and GSSG) in a reaction involving NADPH, 5,5’-dithiobis-(2-nitrobenzoic acid) and glutathione reductase (Tietze (1969)). In order to measure GSSG alone the alkylating agent N-ethylmaleimide (NEM) is added, but this can also lead to inactivation of glutathione reductase. Excess NEM must therefore be completely removed for the assay to function. A less laborious modification uses 2-vinylpyridine that does not inhibit the enzyme (Griffith (1980)). This technique may not be suitable for the measurement of low GSH tissue levels. Brigelius et al. (1983) developed an assay using 1-chloro-2,3dinitrobenzene and glutathione-S-transferase with a limit of detection of 300nM. Secondly, extreme care must be exercised when collecting, storing and analyzing samples as it is relatively easy to artificially alter the GSH/GSSG ratio (e.g., Jones et al. (1998)). Precautions include the use of serine borate to inhibit γglutamyltransferase activity, not rupturing red blood cells, and sampling at a specific time of day to avoid possible circadian rhythms). 11 Where it is involved in protein-disulfide isomerase-dependent protein folding (Walker and Gilbert (1997)). WWW.ESAINC.COM 352 Analyte GSH, GSSG Electrode Material Glassy carbon Eye lens GSH Gold amalgam Brain GSH Gold amalgam GSH, GSSG Gold amalgam Liver microdialysis perfusion Blood GSH, GSSG Gold amalgam Red blood cells GSH, GSSG Gold amalgam Eye-lens GSH, ascorbic acid Flow-through graphite Flow-through graphite Flow-through graphite Human brain Liver and peripheral tissues Plasma Rose and Bode (1995) Acworth and Bailey (1995) Flow-through graphite Plasma Melnyk et al. (1999) Flow-through graphite Bronchoalveolar lavage, plasma Smith et al. (1995) Flow-through graphite Flow-through graphite Flow-through graphite Flow-through graphite Lung Lakritz et al. (1997) Human substantia nigra Human hair Sofic et al. (1992) Krien et al. (1992) Bile, kidney, lens, liver Carro-ciampi et al. (1988) Flow-through graphite Bile, liver Harvey et al. (1989) Flow-through graphite Bile, urine Hill et al. (1992) GSH, ascorbic acid, uric acid, cysteine, allantoin GSH, GSSG, cysteine, homocysteine, methionine, Nacetylcysteine GSH, GSSG, cysteine, cystine, cystathionine, homocysteine, cysteinylglycine, methionine, homocystine GSH, GSSG, cystathionine, cysteine, cystine, homocysteine, homocystine, methionine GSH, GSSG GSH, GSSG GSH, GSSG GSH, GSSG, methionine, N-acetylcysteine GSH, GSSG, cysteine, homocysteine, cystathionines GSH-conjugates Tissue Reference Ozcimder et al. (1991). Pileblad and Magnusson (1989) Yang et al. (1995) Allison and Shoup (1983) Yamashita and Rabenstein (1989) Mitton and Trevithick (1994) Sofic et al. (1991) Table 4.4 HPLC-ECD Methods For The Measurement Of Glutathione, Glutathione Disulfide etc. From Acworth et al. (1998) and updated. A variety of HPLC techniques have also been developed. HPLC-UV requires derivatization (e.g., with Sanger’s reagent) (Fariss and Reed (1987)) but as it possesses poor limit of detection it may not be sensitive enough for many WWW.ESAINC.COM 353 biological applications. HPLC-fluorescence detection requires derivatization with OPA (Keller and Menzel (1985); Michelet et al. (1995)), monobromobimane (Fahey and Newton (1987); Newton et al. (1981)) or dansyl chloride (Martin and White (1991)). HPLC-ECD uses either amperometric or coulometric electrodes to measure GSH and GSSG directly, thus avoiding typical problems associated with derivatization procedures (e.g., derivatization efficiency, ghost peaks) (Table 4.4).12 In general, coulometric detection offers superior sensitivity and selectivity to the dual-amperometric approach and furthermore avoids the use of toxic, unstable mercury amalgams and the problem of complete oxygen removal prior to reductive determination of GSSG. A typical chromatogram showing the use of coulometric detection to measure a variety of thiols and disulfides is presented in Figure 4.10. See also ESA Application Notes – 70-5343 Total Glutathione and 70-5043 Total Thiols. Hill et al. (1993) used a gradient coulometric array approach to study the metabolism of a variety of S-substituted GSH conjugates formed when animals were exposed to hydroquinone. Their method offered excellent resolution of the conjugates, even in complex biological matrices such as urine. Figure 4.10 Separation Of Thiol And Disulfide Standards Using HPLC-ECD (20ng On Column). (Reproduced with permission of Achilli and Cellerino (1996)). 12 Although this approach is exquisitely sensitive it is not without problems. The low amount of organic modifiers typically used with reversed-phase HPLC can result in microbial growth in the system leading to noise and ghost peaks in the chromatogram. Also, biological materials (lipids, proteins etc) can build up on the column and foul the working electrode, causing poor chromatography and loss of sensitivity. This can be overcome by routine cleaning. Finally, trace transition metal contamination can result in auto-oxidation of GSH. Only biologically compatible HPLC-systems should be used. WWW.ESAINC.COM 354 2. Homocysteine. Homocysteine is a key metabolite in both sulfur amino acid biochemistry and the transfer of “activated” methyl groups (in regeneration of the “methyl carrier” - Sadenosylmethionine). Homocysteine is formed from methionine, and can react with serine for the synthesis of cysteine, or converted back to methionine by transfer of a methyl group from N5-methyltetrahydropteroyl-tri-L-glutamate (a folate derivative) in a reaction that involves a vitamin B12 (cobalamin) derivative, 5-methyltetrahydrofolate and the enzyme methionine synthase (or tetrahydropteroylglutamate methyltransferase) (Figure 4.11). It is not surprising therefore that plasma levels of homocysteine are used clinically to monitor folate and cobalamin function. NH2 O Methionine Adenosyl Transferase Adenosyl R CH3 Pi + PPi ATP Methyltransferase S OH R-CH3 S-Adenosyl-Methionine (SAM) NH2 NH2 O S O S Adenosyl OH CH3 OH Methionine S-Adenosyl-Homocysteine (SAH) + Methylcobalamin + 5-Methyltetrahydrofolate Methionine Synthase H2O NH Adenosine 2 SAH Transferase O HS OH Homocysteine Serine Cystathionine β-synthase H2O NH2 NH2 O S O OH OH Cystathionine H2O Cystathioninase NH4+ NH2 HS O + H3C CH2 CO.CO2H α-Ketoglutarate OH Cysteine Figure 4.11 The Metabolism Of Homocysteine. WWW.ESAINC.COM 355 Several forms of homocysteine occur in plasma, including the reduced thiol (<0.25µM), the free disulfide (homocystine and homocysteine-cysteine mixed disulfides; <2µM) and the protein bound disulfide (<8µM) (Ueland (1995)). The plasma homocysteine pool is in dynamic equilibrium with the other aminothiols found in the plasma compartment. For example, an increase in plasma homocysteine also increases the level of free cysteine by liberating it from its protein-bound form. Furthermore, changes in plasma homocysteine redox status rapidly affect and are related to the redox status of other aminothiols (Ueland et al. (1996)). Although homocysteine can act as an antioxidant through its oxidation to its disulfide (Baker et al. (1996); Ueland et al. (1996); Zappacosta et al., (2001)) it can also act as a pro-oxidant capable of promoting lipid peroxidation and protein damage (Halverson et al. (1996); Olszewski and McCully (1993)) and as a neurotoxin (Kim and Pae (1996)). The possible physiological relevance of its prooxidant status still awaits clarification. Homocysteine can be converted enzymatically to an intramolecular thioester, or thiolactone. Homocysteine thiolactone readily undergoes nucleophilic addition with primary amines to form biologically relevant homocystamide adducts (see Ferguson et al. (1999) and references therein). For example, LDL reacts with homocysteine thiolactone to produce LDL-homocystamide adducts that have been implicated to increase atherogenicity of LDL (Naruszewicz et al. (1994)). LDL-homocystamide adducts may also serve as markers of plasma homocysteine levels and can be measured using polyclonal antibodies (Ferguson et al. (1998)). Contrary to earlier reports, LDL-homocystamide adducts may serve as a local antioxidant making the LDL molecule more resistant to prooxidant damage (Ferguson et al. (1999)). Moderate hyperhomocysteinemia (plasma levels <30µM) is an important cardiovascular risk factor (Jacobsen (1998)). Consequently, there is now a growing interest in the possible roles of homocysteine in control of the plasma redox thiol status in disease and oxidative stress (Frishman (1998); Jacobson (1998); Selhub and D’Angelo (1998); Ueland (1995)). As it is the “total” (free + free disulfide + protein-bound disulfide) level of homocysteine that has been determined to be clinically significant, sample pretreatment includes the use of urea to denature proteins and a reducing agent (e.g., dithiothreitol, sodium borohydride, or tributylphosphine) to reduce disulfides. Such sample treatment may be problematic (e.g., incomplete reduction) or cause problems for the subsequent analytical procedure. Homocysteine and its metabolites have been determined using a variety of techniques, including antibody based procedures, GC-MS, radioenzymatic methods and HPLC with UV or fluorescence detection following derivatization with reagents such as OPA, monobromobimane, 2-chloro-1-methylpyridinium iodide, or 7-fluorobenzo-2-oxa-1,3-diazole-4-sulfonate (Andersson et al. (1995); WWW.ESAINC.COM 356 I.S. Penicillamine Homocysteine Daskalakis et al. (1996); Fermo et al. (1992, 1998); Fiskerstrand et al. (1993); Frantzen et al. (1998); Hyland and Bottiglieri (1992); Imai et al (1983); Jacobsen et al. (1994); Kaniowska et al. (1998); Santhosh-Kumar et al. (1994); Shipchandler and Moore (1995)). Homocysteine has also been measured using HPLC with coulometric (Achilli and Cellerino (1996); Martin et al. (1999)) (Figure 5.8) or amperometric (D’Ermo et al. (1999)) electrochemical detection or pulsed amperometric detection on a gold working electrode (Evrovski et al. (1995); Wu et al. (1994)). Recently, Bailey (1998) has developed a simplified yet highly sensitive HPLC-ECD-based method capable of measuring the total plasma level of homocysteine (Figure 4.12). The addition of the novel reducing agent tris(2carboxyethyl)-phosphine (a stable, water soluble and easy to handle reagent) directly to plasma completely liberates homocysteine from all of its disulfides without the need for the use of urea or extensive sample preparation. See ESA Application Notes – 70-3994 Total Plasma Homocysteine and 70-4989 An Alternate Plasma Homocysteine Method. Figure 4.12 Chromatogram Of A Human Plasma Sample. The isocratic system consisted of a pump, an autosampler and a Coulochem® III detector. LC Conditions: Column: Guard Column: Mobile Phase: HR-80 C18 (4.6 x 80mm; 3 µm). C18 0.15M Sodium Dihydrogen Phosphate, 1.0mM SDS, 10% Acetonitrile, final pH = 2.80 (With Phosphoric Acid). Flow Rate: 1.2mL/min. Temperature: Ambient. Injection Volume: 20µL. Cell Potentials: EGC = +850mV. E1 = +450mV. E2 = +750mV. See Application Notes – 70-3994 Total Plasma Homocysteine or 70-4989 Alternative Homocysteine, for further details. WWW.ESAINC.COM 357 As with GSH, when attempting to measure fractionated homocyst(e)ines, regardless of the analytical approach being used, care must be taken so as not to artificially alter the redox status of the tissue sample (Fermo et al. (1997)). 3. Miscellaneous Endogenous Sulfur-Containing Compounds. A variety of sulfur containing compounds including coenzyme A, cysteamine, cysteic acid, hypotaurine, S-adenosyl-L-methionine, pantothenic acid and aminoethylcysteine ketimine decarboxylated dimer have been proposed as antioxidants (Aruoma et al. (1988); Evans et al. (1997); Fontana et al. (1998); Matarese et al. (1998); Slyshenkov et al. (1995)). However, whether these compounds occur at sufficient levels in vivo to act as antioxidants still remains to be clarified. It seems unlikely that taurine functions as an antioxidant in vivo as it does not react rapidly with ROS, and its reaction product with HOCl still shows pro-oxidant activity (Aruoma et al. (1988)). Lipoic acid, a strong antioxidant, is discussed below. NH2 O N N N HN N NH NH H2 N ADENINE GUANINE H2O 1 NH3 H2O + O2 2H + + O2.- N HN N 4 - XANTHINE DEHYDROGENASE H2O 2 NH3 O 1 - ADENINE DEAMINASE 2 - GUANINE DEAMINASE 3 - XANTHINE OXIDASE NH O N HN NH 3 O NH NH 4 O NH HN 3 O O NH NH 4 O2, NAD+/NADH HYPOXANTHINE 2H + + O2.- H2O + O2 O2, NAD+/NADH XANTHINE URIC ACID Figure 4.13 The Conversion Of Purines Into Uric Acid. WWW.ESAINC.COM 358 Uric Acid. Formation. The purine catabolic pathway shown in Figure 4.13 is found in all animals. Uric acid represents a metabolic branch-point and the final end product of purine catabolism is species dependent. In man and primates, who lack uricase, uric acid is the final product and is excreted in the urine. Mammals other than man and primates excrete allantoin. Teleost fish produce allantoic acid; other fish and reptiles produce urea; some marine invertebrates hydrolyze urea to ammonia and carbon dioxide. O N HN O2 NH N HYPOXANTHINE Mo VI FeII Mo IV FeIII O2.- O N HN O NH NH O2 XANTHINE O FeII Mo IV FeIII O2.- NH HN O Mo VI O NH NH URIC ACID Figure 4.14 The Role Of Metals In Superoxide Production By Xanthine Oxidase. As presented in Figure 4.13, a single enzyme, xanthine oxidase, is responsible for the conversion of hypoxanthine to xanthine and xanthine to uric acid with the simultaneous production of superoxide as a byproduct. The mechanism of action of xanthine oxidase is shown in Figure 4.14. This enzyme is a molybdenum- WWW.ESAINC.COM 359 containing flavin-hydroxylase and is capable of existing as both an oxidase (using oxygen as a cofactor and producing superoxide) and dehydrogenase (using NAD+ and not producing superoxide). Xanthine dehydrogenase is the naturally occurring form of the enzyme but is readily converted into the oxidase form either irreversibly (by proteolysis) or reversibly (by sulfhydryl oxidation) (Hille and Nishino (1995); Parks et al. (1999)). As this can occur during sample preparation due care should be exercised when purifying the enzyme. Apart from its role in uric acid formation, xanthine oxidase can also catalyze other reactions. It possesses nitrite reductase activity and can reduce nitrite to nitric oxide (Zhang et al. (1998c)). Although the physiological importance of this activity is at present unknown, the generation of nitric oxide by xanthine oxidase may serve as a supplement to NOS and help to redistribute blood flow following ischemia (Zhang et al. (1998c)). Xanthine oxidase can decompose dibromoacetontrile (a product of water disinfection) to cyanide a problem for potable water (Mohamadin and Abdel-Naim (2003)). Xanthine oxidase also promotes decomposition of nitrosothiols forming nitric oxide (anaerobic conditions) and peroxynitrite (aerobic conditions) (Trujillo et al. (1998)). Xanthine oxidase can be inhibited by analogs of uric acid, e.g., allopurinol. This compound is called a “suicide inhibitor” because once it is oxidized by xanthine oxidase it is converted to oxypurinol, a compound that binds tightly to molybdenum-containing active site of the enzyme. Allopurinol is commonly used to treat gout. Other inhibitors include pterinaldehyde, the FAD-site inhibitor, diphenyleneiodonium and some flavonoids (Cos et al. (1998)). Xanthine Oxidase and Tissue Injury. There is considerable interest in the role that xanthine oxidase may play in the damage associated with reperfusion injury (Hille and Nishino (1995); Nishino (1994); Saugstad (1996)). It has been hypothesized that during hypoxia ATP is depleted by conversion to hypoxanthine, while xanthine dehydrogenase (the principle form of the enzyme) is slowly converted to the oxidase. Upon reperfusion, xanthine oxidase aggressively converts hypoxanthine to uric acid while simultaneously reducing oxygen to superoxide which also dismutates to hydrogen peroxide. The resulting burst of ROS damages tissue, facilitating the release of xanthine oxidase with the possibility of tissue damage far removed from the site of the initial insult. Although this hypothesis is attractive it has been challenged. For example, there is some question as to whether xanthine dehydrogenase exists in the organs affected by reperfusion injury (see Nishino (1994) and references therein). Another issue is whether the enzyme actually is converted to xanthine oxidase during ischemia (see Nishino (1994) and references therein). However, it now appears that xanthine dehydrogenase conversion to xanthine oxidase may not be essential for ROS production, as xanthine dehydrogenase can lead to ROS production too (Zhang et al. (1998b)). WWW.ESAINC.COM 360 Antioxidant and Pro-oxidant Activities. Uric acid is often erroneously regarded as a waste product. This does not make good biochemical sense as the production of purines is an energy-dependent process and their destruction is physiologically costly. Furthermore, >90% of uric acid is reabsorbed from urine by the kidney. In man the circulating level of uric acid is typically 120-450µM (see Table 4.1) which approaches the solubility limit for this analyte. A variety of diseases are associated with uric acid’s poor solubility. For example, when urine is unusually acidic calcium urate stones can form in the kidney and bladder. Conditions where the solubility limit of uric acid (~450µM) is exceeded, either by its increased production or failure of the kidney to effect its removal, can result in the deposition of monosodium urate crystals in joints, leading to painful inflammation and gout. In fact, a uric acid concentration >600µM is virtually certain to cause gout. Interestingly, a phenomenal concentration of uric acid is achieved by blood sucking insects where hemolymph levels can reach as high as 5mM. This high level of uric acid protects the insect from the pro-oxidant effects of digested hemoglobin (Souza et al. (1997)). So what is the evolutionary significance of uric acid? It seems strange that evolution has not enabled man to produce a more soluble and less problematic product. One possible answer is that uric acid is a very good antioxidant. It has been hypothesized that an important step in human evolution was the replacement of ascorbic acid with uric acid as the principal circulating antioxidant (Ames et al. (1981); Becker (1993); Cutler (1991)). Consequently, the increased plasma concentration of uric acid enabled man to live longer and avoid the cancers commonly associated with short-lived species. In support of this theory it is found that man and primates live longer than prosimians where the circulating level of uric acid is ten-fold lower. It appears that uric acid now contributes up to 60% of the measured total antioxidant capacity of plasma in healthy subjects (Benzie (1996); Wayner et al. (1997)). Uric acid is an effective antioxidant. Uric acid reacts with singlet oxygen, nitrogen dioxide, alkyl peroxyl radicals and peroxynitrite13 (Halliwell and Gutteridge (1993); Hooper et al. (1998); Skinner et al. (1998)). Uric acid, like most other compounds, will react with hydroxyl free radicals if present at sufficient concentration at the site of production of this pro-oxidant. The one-electron oxidation of uric acid produces potentially damaging urate radicals (Aruoma and Halliwell (1989); Kitteridge and Wilson (1984); Maples and Mason (1988)). Fortunately, the uric acid radical can be converted back to uric acid by its interaction with ascorbic acid (Maples and Mason (1988)). Uric acid can also bind iron and copper in forms that do not react with hydrogen peroxide, and it protects against damage from heme intermediates containing iron in IV and V valencies 13 Interestingly, peroxynitrite reacts with uric acid to produce a nitrated derivative that possesses vasoactive properties through its release of nitric oxide (Skinner et al. (1998)). WWW.ESAINC.COM 361 (Ames et al. (1981); Halliwell and Gutteridge (1990); Halliwell et al. (1992); Maples and Mason (1988); Vasquez-Vivar et al. (1996)). Interestingly, uric acid does not react with superoxide and only plays a limited protective role against hypochlorous acid (Hu et al. (1993)). Following reaction with pro-oxidants uric acid can decompose to give a variety of metabolites, some of which are potentially toxic (Figure 4.15) (Hicks et al., (1993); Kaur and Halliwell (1990)). Possible reaction mechanisms for uric acid breakdown have been studied using coulometric electrochemical detection coupled to mass spectrometry (Volk, et al. (1989)). Uric acid breakdown products can also be used as oxidative stress markers. For example, Hillered and Persson (1995) measured parabanic acid as an indicator of oxidative stress in microdialysis perfusates obtained from patients with severe acute brain injuries. Similarly, the ratio of allantoin/uric acid levels have also been used as a potential index of free radical reactions in vivo and have shown to be increased with disease (Grootveld and Halliwell (1987); Lux et al. (1992); Moison et al. (1997)), exercise (Hellsten et al. (1996)) and during development (Moison et al. (1997)). H2NCONH2 NH O HN UREA CO2 H N HO NH OH N O OH O OXONIC ACID CYANURIC ACID NH HN O N O NH NH URIC ACID O NH O O NH2 CONH O NH ALLANTOIN NH O H2NCONHCOCO2H NH PARABANIC ACID OXALURIC ACID Figure 4.15 Uric Acid Can Decompose To Give A Variety Of Products. Even though uric acid can show some pro-oxidant activity this is insignificant when compared to its ability to act as an antioxidant. The question arises as to WWW.ESAINC.COM 362 why do biological systems have both uric acid and ascorbic acid present to react with radicals. This can be answered by looking at the electrode potential of the various reactions. The Eo for uric acid is +590mV which is markedly higher than that of ascorbic acid (see Table 2.1.1). Using the Nernst equation there will be 29.9 Kjmol-1 less energy if urate, rather than ascorbate, reacts with the hydroxyl free radical (under standard conditions and a pH=7). Thus there is definitely a major advantage when energy is released in more controllable small steps (hydroxyl free radical/urate; urate radical/ascorbate) rather than all at once (Benzie and Strain (1996)). Under some conditions, excessive demands on dietary ascorbic acid for uric acid recycling may lead to ascorbic acid depletion which will, in turn, interfere with tocopherol regeneration (see below). This and other problems associated with uric acid pro-oxidant activity (e.g., stimulated secretion of superoxide from leukocytes; its ability to release iron from oxyhemoglobin) led Benzie and Strain (1996) to question uric acid’s true biological role. Measurement. Uric acid can be measured using GC-MS (Chen et al. (1998)). It can also be measured using HPLC-UV absorbance (Ames et al. (1981), Tang-Liu and Riegelman (1982); Yang (1998)) or with better selectivity and sensitivity, by HPLC-ECD. HPLC-ECD approaches either measure it alone (Aoki et al. (1984), Iwamoto et al. (1983), Roch-Ramel et al. (1980)) or in conjunction with ascorbate (Honegger et al. (1989), Irayama et al. (1984), Shirachi and Omaye (1992)) or other metabolites (Gogia et al. (1998); Rose and Bode (1995)). Uric acid has also been determined as part of a global method for metabolic profiling using gradient HPLC with coulometric array detection (Rizzo et al. (1991)). Fat-Soluble Antioxidants. Carotenoids. Carotenoids are primarily a group of lipophilic C40 polyisoprenoid compounds that possess an extensive conjugated double bond system which enables them to strongly absorb UV and/or visible light, act as antioxidants and renders them electrochemically active. A consequence of electromagnetic radiation absorption is that many of the carotenoids are brilliantly colored. For example, lycopene is red (tomatoes), lutein and zeaxanthin are yellow (sweet corn), and α- and βcarotene are orange (carrots). Carotenoids can be subdivided into the xanthophylls (oxygenated carotenoids) and carotenes (hydrocarbons). The carotenoids are synthesized in plants and microorganisms (where they aid photosynthesis or act as photo-protectants) and are essential nutrients for animals. The consumption of large amounts of astaxanthin producing organisms WWW.ESAINC.COM 363 is responsible for the pink coloration of flamingo feathers and salmon flesh. It is also responsible for the red coloration of boiled shellfish. Over 600 carotenoids have been identified and some of the more biologically important ones are presented in Figure 4.16. CH3 CH3 CH3 CH3 H3 C CH3 CH3 CH3 CH3 CH3 CH3 CH3 CH3 CH3 CH3 α-CAROTENE CH3 CH3 CH3 CH3 CH3 H3 C CH3 CH3 CH3 CH3 CH3 CH3 CH3 CH3 LUTEIN CH3 CH3 CH3 CH3 CH3 CH3 H3 C CH3 CH3 CH3 CH3 CH3 CH3 CH3 CH3 H3 C CH3 CH3 CH3 ZEAXANTHIN CH3 CH3 CH3 CH3 γ-CAROTENE CH3 OH H3 C CH3 CH3 HO CH3 CH3 OH H3 C CH3 CH3 HO CH3 CH3 OH H3 C CH3 CH3 CH3 CH3 CH3 CH3 CH3 CH3 H3 C CH3 O O CH3 CH3 CH3 CH3 CH3 CH3 CANTHAXANTHIN δ-CAROTENE CH3 CH3 CH3 CH3 CH3 CH3 CH3 CH3 CH3 CH3 CH3 H3 C CH3 O CH3 CH3 HO OH O CH3 CH3 CH3 CH3 CH3 ASTAXANTHIN LYCOPENE Figure 4.16 The Structures Of Some Biologically Important Carotenoids. Carotenoids and Disease. The role of the carotenoids in health and disease prevention has been reviewed extensively elsewhere (Canfield et al. (1993); Mayne (1996); Krinsky (1993); Omaye et al. (1997)) so will not be dealt with in detail here. Mayne (1996) critically reviewed the role for β-carotene (and some other carotenoids) in cancer prevention (including lung, oral, gastrointestinal, breast, prostate, cervical and skin cancers) but concluded, “supplemental β-carotene is unlikely to be beneficial in reducing the major cancers occurring in westernized populations”. In fact, supplemental β-carotene was without effect (Hennekens et al. (1996)) or even promoted cancer in subjects that smoked (De Luca and Ross (1996); Heinonen et al. (1994); Omenn (1998); Omenn et al. (1996); Paolini et al., (2003)). Other WWW.ESAINC.COM 364 studies associate β-carotene levels with either decreased incidence or little effect on cardiovascular disease (reviewed by Palace et al. (1999)). For example, βCarotene did not prevent cardiovascular disease and was potentially harmful (Lonn and Yusuf (1997); Rimm and Stampfer (1997)). Supplemental β-carotene and canthaxanthin have been used to successfully treat certain photosensitivity diseases such as erythropoietic protoporphyria (Mayne (1996)). The consumption of β-carotene rich foods has been associated consistently with decreased risk of cardiovascular disease, yet β-carotene supplementation failed to reduce the incidence of this disease (Mayne (1996)). Finally, dietary carotenoids have been found to be protective against various forms of cataract (Taylor (1993)) and lutein and zeaxanthin can reduce the risk of macular degeneration (Seddon et al. (1994)). The potential roles for lycopene in human health and disease still awaits further evaluation (Clinton (1998); Gerster (1997)). Antioxidant and Pro-oxidant Activities of Carotenoids. The antioxidant role of the carotenoids has been reviewed extensively elsewhere (Bast et al. (1998); Byers and Perry (1992); Krinsky (1989, 1993), Paloza and Krinsky (1992), Rousseau et al. (1992)). The antioxidant activity of carotenoids is usually determined in vitro using solutions or model membrane systems such as liposomes. Many of these studies measure the ability of carotenoids to inhibit lipid peroxidation. In general, although many reports imply that carotenoids can also act as antioxidants in vivo, there is still little direct evidence for this capability (Krinsky (1993)). Some have even challenged the fact that carotenoids are antioxidants (Crabtree and Adler (1997)). The antioxidant activity of a carotenoid depends upon its structure (e.g., the number and degree of conjugation of double bonds, degree of steric hindrance, and presence of functional groups on the terminal rings) (Terao (1989), Miki (1991)). For example, Miller et al. (1996) showed that lycopene (11 conjugated double bonds, no terminal cyclohexene rings) was nearly three-times more efficient at scavenging radicals than α-carotene (9 conjugated double bonds, a higher degree of steric hindrance, and two terminal cyclohexene rings) and 100times more efficient than astaxanthin (11 conjugated double bonds, and electron withdrawing carbonyl and hydroxyl groups on cyclohexene rings). Thus the hydroxyl free radical and peroxyl radical scavenging abilities of the carotenoids are found to be lycopene > β,β-carotene = zeaxanthin > isozeaxanthin > astaxanthin (Woodall et al. (1997)). Carotenoids are electron rich compounds and can readily react with electron deficient compounds such as the ROS. The resulting charge on the carotenoid product is delocalized through hyperconjugation, thus inferring stability and rendering it less reactive. There have been several studies on the reaction WWW.ESAINC.COM 365 mechanisms of carotenoids and different ROS and RNS (Liebler and McClure (1996); Rice-Evans et al. (1997)) (Figure 4.17). For example, β-carotene reacts with peroxyl radicals to form the peroxyl radical-β-carotene adduct [β-carotene......RO2]• (Burton and Ingold (1984)), while reaction with NO2• forms the β-carotene radical cation [β-carotene]•+ (Everett et al. (1996)). Under certain conditions, both the carotenoid adduct and carotenoid radical cation can be formed simultaneously. The resulting carotenoid adduct and the carotenoid radical cation both undergo slow bimolecular decay to non-radical products (e.g., epoxides and carbonyl-containing chain cleavage products). The carotenoid radical cation can also be rapidly scavenged by tocopherol (Mortensen and Skibsted (1997)). Interestingly, some RNS appear to be capable of nitrating β-carotene’s rings producing 4-nitro-β-carotene. However, as this appears to only take place in the gas phase, formation of this adduct is biologically unimportant. β-Carotene R R [β-Carotene]+ [β-Carotene] R R β-Carotene-R SUBSTITUTION [β-Carotene-R] R O2 R-β-Carotene-R R-β-Carotene-O2 ADDITION AUTOOXIDATION Figure 4.17 Possible Reaction Mechanisms Of β-Carotene. β-Carotene shows several antioxidant activities in vitro. It is a chain breaking antioxidant and scavenges lipid peroxyl, nitrogen dioxide, thiyl, RSO2•, and the trichloromethylperoxyl radicals (e.g., typical rate constants for the scavenging of carbon-centered and peroxyl radicals by β-carotene are ~1.0 x 104 and ~1 to 50 x 105 M-1s-1, respectively (Appendix 2.2) (Iannone et al. (1998); Ozhogina and Kaisikinia (1995)). It scavenges and quenches singlet oxygen (Frei and Ames (1991); Miller et al. (1996) and references therein; Mortensen et al. (1997); Ozhogina and Kaisikinia (1995), Rice-Evans and Diplock (1993)) and readily reacts with peroxynitrite (Pannala et al. (1999) and references therein). WWW.ESAINC.COM 366 Lycopene is one of the most abundant carotenoids in the Western diet (Gerster (1997)). It is the most effective singlet oxygen quencher of all the carotenoids, can effectively scavenge peroxyl radicals and has been reported to protect against some human cancers (Di Macio et al. (1989); Gerster (1997); Klebanov et al. (1998); Stahl and Sies (1996)). Lycopene is also more effective than β-carotene at scavenging peroxynitrite (Pannala et al. (1998b)) and hypochlorous acid (Panasenko et al. (1997)). The antioxidant chemistry of carotenoids is intimately dependent upon the oxygen tension (pO2). At the low pO2 typically found in tissues carotenoids act as antioxidants but at high pO2 they can auto-oxidize and show pro-oxidant behavior (Burton and Ingold (1984)). The exact mechanism is unclear but may involve the addition of oxygen to the lipid peroxyl-carotene radical intermediate forming a peroxyl radical adduct (Eqns 4.25 and 4.26) capable of promoting lipid peroxidation. Furthermore, the formation of auto-oxidation products (e.g., epoxides) increases with pO2 and these can decompose to produce alkoxyl radicals capable of promoting lipid peroxidation (Kennedy and Liebler (1992); McClure and Liebler (1994)). The pro-oxidant actions of carotenoids in biological systems have recently been reviewed (Palozza (1998)). β-Carotene + ROO• → ROO-β-Carotene• Eqn 4.25 ROO-β-Carotene• + O2 → ROO-β-Carotene-OO• Eqn 4.26 Retinoids. Of the 600 or so naturally occurring carotenoids, about 50 have vitamin A activity. Provitamin A carotenoids can be converted enzymatically in the intestinal mucosa to produce retinal and finally retinol (vitamin A1). Over 90% of the total body reserve of vitamin A1 is stored in the liver of well nourished individuals, primarily in stellate (Ito or fat-storing) cells. The principal storage form of vitamin A1 is as retinyl palmitate, with oleate and stearate occurring as the next most prevalent esters (Blomhoff et al. (1992)). Unlike the carotenoids which are relatively safe, the retinoids are toxic and excessive consumption can lead to a variety of diseases (Meyers et al. (1996)). Vitamin A1 is transported by specific binding-proteins and its circulating levels are strictly regulated (Olson (1993)). WWW.ESAINC.COM 367 The Biological Activity of the Retinoids. The retinoids (including retinal, retinol and retinoic acid and their isomers) (see Figure 4.18) have a variety of important biological roles including signal transduction in the eye (Wald (1968), Wolken, (1966)), maintenance of epithelial tissue (Argiles et al. (1989)), and regulation of the immune system response (West et al. (1991)). Vitamin A1 is also involved in the regulation of proliferation and differentiation of many cell types (Blomhoff et al. (1991, 1992)), probably through the action of retinoic acid on nuclear retinoic acid receptors (Kastener et al. (1994); Kliewer et al. (1994)). Many retinoids have teratogenic activity (Kamm, (1982)). For example, retinoic acid can cause developmental anomalies in prenatal systems and may play a role in coordinating cellular development (Thaler et al. (1993); Tickle et al. (1985); Wagner et al. (1990)). Retinoids are also involved in the establishment of the development axis of the central nervous system (Durston et al. (1989); Sundin and Eichele (1992); Wagner et al. (1990)). Finally, retinoic acid may also play a role in melatonin synthesis by activating HOMT (Bernard and Klein (1995)). Vitamin A may play a protective role in cardiovascular disease (Palace et al. (1999)). CH2OH CH2 OH ALL-TRANS RETINOL 13-CIS RETINOL CHO ALL-TRANS RETINAL 11-CIS RETINOL CH2 OH CO2 H CO2H ALL-TRANS RETINOIC ACID O O 9-CIS RETINOIC ACID 4-OXO-ALL-TRANS RETINOIC ACID 4-OXO-13-CIS RETINOIC ACID CO2 H CO2H Figure 4.18 The Structure Of Biologically Important Retinoids. WWW.ESAINC.COM 368 Antioxidant and Pro-oxidant Activities of the Retinoids. Like the carotenoids, the retinoids have a conjugated double bond system and can act as antioxidants. The retinoids have half the number of double bonds of the carotenoids and are therefore less capable of delocalizing charge. One consequence of this is that retinoids oxidize electrochemically at a higher potential than the carotenoids (see below). Thus, retinoids are chemically less effective antioxidants. This may be one of the reasons why nature uses them for functions other than as antioxidants. In fact vitamin A1 is protected from oxidation in vivo by a variety of antioxidants including ascorbic acid, GSH and vitamin E, thereby keeping it in its active form (Olson (1996)). Vitamin A acts as a pro-oxidant sensitizer, exciting oxygen into destructive singlet oxygen (Halliwell and Gutteridge (1999)). This may be a problem for the eye which not only contains vitamin A (as rhodopsin) but is also particularly abundant in poly-unsaturated fatty acids that can readily undergo singlet oxygen-induced lipid peroxidation. Several studies now suggest that lipid peroxides and cytotoxic breakdown products may lead to severe retinal damage (Halliwell and Gutteridge (1999)). It should not be surprising, therefore, that the eye is usually well protected by GSH and other antioxidants capable of acting against ROS-induced damage. Measurement of Carotenoids and Retinoids. The tissue levels for some carotenoids and retinoids are presented in Table 4.1 and the various analytical methods used to measure them in Table 4.5. Unfortunately, many carotenoids are extremely light and oxygen sensitive, and can undergo rapid decomposition so due care must be exercised during sample preparation (Wyss (1995)). Analytical methods differ in sensitivity, selectivity, complexity and limitations. HPLC-UV is usually adequate when tissue levels are high as in plant material. However, when tissue levels are low (as in animal samples) concentration steps may be required. For example, Gunderson et al. (1997) used a complex HPLC-UV method with on-line solid phase extraction (consisting of five valves and three columns) to measure low levels of retinoic acid in plasma. GC procedures can result in the decomposition of more labile analytes (e.g., retinol and its esters are converted to anhydroretinol) and require derivatization to make the analytes volatile. Although not as common as spectrophotometric approaches, HPLC-ECD has proven very useful. Gamache et al. (1997a) used HPLC with gradient elution and coulometric electrode array detection to measure a variety of retinoids, carotenoids, ubiquinone and vitamin K simultaneously in human plasma (see Figure 4.19). Human plasma data have been verified as part of a NIST/NCI micronutrients measurement quality assurance program: measurement reproducibility, repeatability, stability, and relative accuracy for fat-soluble vitamin-related compounds in human sera. See Table 4.6. Compounds with conjugated double bonds (e.g., carotenoids, WWW.ESAINC.COM 369 retinoids, tocopherols, vitamin D2 and D3) are all electrochemically active, a direct result of their ability to delocalize charge. Thus, the very chemical characteristics that make a compound a good antioxidant also render it measurable by electrochemical detection. Gamache’s method has now been extended to include astaxanthin, β-cryptoxanthin, zeaxanthin and all-trans retinoic acid. With a limit of detection of <10pg (on column) for most analytes, this approach enables the direct measurement of many carotenoids and retinoids in animal and human tissues. When coupled to the greater resolution obtained by the use of a C30 column, the abundance of various carotene isomers in processed carrots, human plasma and cervical tissue samples can be determined (Ferruzzi et al. (1998); Gamache et al. (1997b; 2003)). See ESA Application Note 70-4927 Carotenoid Isomers and Figure 4.20. Analyte Apocarotenoids, retinoids Carotenoids Carotenoids Carotenoids Carotenoids Carotenoids Retinoic acids, retinal, retinol Retinoic acids Retinoic acids Retinoid radicals Retinoids Retinoids Retinoids, carotenoids, and tocopherols, ubiquinone, vitamin K Retinyl esters, retinol Vitamin A1 Vitamin A1 Vitamin A1, β-carotene, tocopherol Vitamin A1, carotenoids, tocopherol(s) Method GC-MS HPLC-ECD C30 column HPLC-UV HPLC-UV, HPLC-MS HPLC-UV, C30 column HPLC-UV, HPLC-IR Capillary-LC-ECD HPLC-PDA HPLC-UV HPLC-EPR, HPLCECD HPLC-ECD HPLC-UV HPLC-ECD HPLC-UV HPLC-ECD HPLC-UV HPLC-UV, HPLCECD HPLC-UV Reference Furr et al. (1992) Gamache et al. (1997a); Ferruzzi et al. (1998) Barua and Furr (1992); Handelman et al. (1992); Nurdin (1991); Parker et al. (1993); Schmitz et al. (1993) van Breemen (1996) Bell et al. (1997); Emenhiser et al. (1995,1996) Stanchar et al. (1988) Hagen et al. (1996) Gundersen et al. (1997) Dimitrova et al. (1996) Iwahashi et al. (1987) Bryan et al. (1991); MacCrehan and Schonberger (1987b); Sakhi et al. (1998) Noll (1996); Stanchar and Zonta (1984) Gamache et al. (1997b; 2003) Got et al. (1995) Wring and Hart (1989); Wring et al. (1988) Peng et al. (1987) MacCrehan and Schonberger (1987a) Barua and Olson (1998); Barua et al. (1993); Kaplan et al. (1987, 1990); Miller et al. (1984, 1985); Steghens et al. (1997); Talwar et al. (1998); Xu et al. (1996) Table 4.5 A Variety Of GC- And HPLC-Based Methods Can Be Used To Measure The Carotenoids And Retinoids. WWW.ESAINC.COM 370 γ-Tocopherol Retinol Response (µA) 0.80 Retinyl Acetate 0.60 α-Tocopherol Lutein Retinyl Palmitate δ-Tocopherol CoQ10 Lycopene β-Carotene 0.40 0.20 -0.00 0.0 5.0 10.0 15.0 20.0 25.0 Retention time (minutes) Figure 4.19 HPLC-CoulArray Chromatogram Showing Simultaneous Measurement Of Several Fat Soluble Vitamin And Antioxidant Standards (only 6 channels are shown for clarity. See ESA Application Note 10-1176 Fat Soluble Vitamins for further details. See also ESA Application Note – 70-4935 Multivitamins in Tablets, Infant Formula and Milk. Trans-Retinol γ-Tocopherol 1 1 α-Tocopherol 1 1 Trans-β-Carotene 2 Trans-Lutein 2 Trans-Lycopene 3 Retinyl Palmitate 3 CoQ10 *Units: µg/L NIST Categories: 1 NIST SRM 968c Level I Level II Range* Range* Level I Mean* RSD % Level II Mean* RSD % 814-868 472-496 861 3.1 489 3.9 3770-4030 1460-1660 4013 7.5 1565 7.1 7000-7940 16030-17750 8040 2.6 17666 4.2 141-173 344-438 153 10.9 365 12.6 40-54 61-75 44 13.7 69 8.0 100-160 140-200 148 8.3 191 9.7 30 80 27 21.0 72 15.3 520 - 535 3.2 - - 2 Certified Values; Reference Values; ESAL Data 3 Information Values Table 4.6 Fat Soluble Vitamin And Antioxidant Levels In Plasma Using ESA’s CoulArray Method As Part Of NIST/NCI Micronutrients Measurement Quality Assurance Program. (Brown and Sharpless (1995); Duewer et al., (1997 and 1999)). WWW.ESAINC.COM 371 Carotenoid Isomers Trans-ß-carotene Trans-ß-carotene Trans-∝-carotene Trans-∝-carotene 2.0 1.0 0.0 0.0 9-cis-ß-carotene* 9-cis-ß-carotene* 1.0 3.0 Lutein 2.0 4.0 Response (µA) Trans-∝-carotene Trans-∝-carotene 3.0 Lutein Lutein Response (µA) 4.0 9-cis-∝-carotene 9-cis-∝-carotene Cooked Carrot Trans-ß-carotene Trans-ß-carotene Raw Carrot 0.0 5.0 10.0 15.0 20.0 0.0 Retention time (minutes) 5.0 10.0 15.0 20.0 Retention time (minutes) Figure 4.20 Analysis Of A Thermally Processed Carrot. The isocratic analytical system consisted of a pump, an autosampler, a thermostatic chamber, a twelve-channel CoulArray® detector and a UV/vis detector placed prior to the array. LC Conditions: Column: Mobile Phase: ESA Carotenoid C30 (4.6 x 250mm; 5µm). Methanol – Methyl-tert-butyl Ether (MTBE) – 1.0M Ammonium Acetate, pH 4.4 (63:35:2) (v/v/v). Flow Rate: 1.0 mL/min. Temperature: 28 oC. Injection Volume: 10 µL. Applied Potentials: 100, 160, 220, 280, 340, 400, 460 and 520mV vs. Pd. Wavelength: 450nm. See ESA Application Note 70-4927 Carotenoid Isomers for further details. Quinones and Hydroquinones. Coenzyme Q (Ubiquinone, ubiquinol). Coenzyme Q10 (CoQ10), also called ubiquinone-50 (2,3-dimethoxy-5-methyl-6decaprenylbenzoquinone), was first discovered by Crane et al. (1957), and is one of a number of important quinones found in biological systems (Figure 4.21) (Crane and Navas (1997); Andree et al. (1999)). Although coenzyme Q was earlier named vitamin Q, this was deemed inappropriate as ubiquinone can be WWW.ESAINC.COM 372 synthesized de novo in all animal tissues. Coenzyme Q exists in three biologically relevant forms: the fully oxidized quinone, the partially reduced semiquinone radical (semiubiquinone) and the fully reduced ubiquinol (see Figure 4.22 for the relationship between these redox forms). The term coenzyme Q will be used where the indeterminate form is most applicable and/or to discuss both the oxidized and reduced forms together. In addition to serving as an electron and proton carrier in the respiratory chain of mitochondria, evidence suggests that ubiquinone can also, under certain conditions, act as a pro-oxidant. Ubiquinol is a very important lipid soluble antioxidant. O O CH3O CH3 CH3 CH3 CH3 CH3O (CH2CH=CCH2)10H CH3 (CH2CH=CCH2)n H O O PLASTOQUINONE (n= 6 to 10) UBIQUINONE-50 O OH O O NH CH3 CH3 CH3 CH2CH=CCH2(CH2CH2CHCH2)3H HO HO N O O PHYLLOQUINONE (VITAMIN K1) O O PYRROLOQUINOLINE QUINONE Figure 4.21 Some Quinones Found In Biological Systems. Biology of Coenzyme Q. The biosynthesis of coenzyme Q in mammalian cells involves the interplay between two metabolic pathways: the 4-hydroxybenzoate pathway (using tyrosine or phenylalanine) for synthesis of the quinone moiety and the mevalonate pathway for production of polyprenyl side-chain (Appelkvist et al. (1994)).14 The mevalonate pathway also produces cholesterol, dolichol, and two cytoplasmic intermediates (farnesyl-PP and gereanylgeranyl-PP) which are capable of isoprenylating proteins. In humans the major form of coenzyme Q has 14 A variety of drugs have been used to manipulate CoQ10’s levels. The HMG-CoA reductase inhibitors, pravastatin, lovastatin and related cholesterolemic drugs, have been used to decrease CoQ10’s levels in a variety of tissues (Appelkvist et al. (1993); Goldstein and Brown (1990); Low et al. (1992); Willis et al. (1990)). On the other hand, the cholesterol synthesis inhibitor squalestatin, or the peroxisome proliferators clofibrate and di(2-ethylhexyl)phthalate lead to elevation in ubiquinone levels (Aberg et al. (1994); Thelin et al. (1994)). The importance of such CoQ10 manipulations on the effect of oxidative stress still awaits clarification. WWW.ESAINC.COM 373 ten isoprenyl subunits (CoQ10), whereas rats have nine (CoQ9). Under normal conditions enough coenzyme Q is produced in the cell to satisfy its needs. However, pathological conditions have been associated with disturbances in coenzyme Q levels. Decreased coenzyme Q levels have been associated with cardiomyopathy (Folkers et al. (1985); Mortensen (1993)), encephalomyopathy (Ogasahara et al. (1989)), degenerative muscle disease (Karlsson et al. (1990)) and hepatocellular carcinoma (Eggens et al. (1989)). Elevated levels have been found in Alzheimer’s disease (Soderberg et al. (1992)), prion disease in mice (Ericsson and Dalner (1993)) and hyperplastic liver nodules in rats (Olsson et al. (1991)). Whether a change in ubiquinone levels is the cause or an effect of the disease is still under investigation. A OH CH3O CH3 CH3 CH3O (CH2CH=CHCH2)10H OH -H+ - e - UBIQUINOL-50 O CH3O CH3 2H + + 2e - CH3 CH3O O CH3O (CH2CH=CHCH2)10H OH CH3 SEMIQUINONE -H+ - e CH3 CH3O (CH2CH=CHCH2)10H O UBIQUINONE-50 B OH CH3O CH3 CH3 CH3O O2 O2 - (CH2CH=CHCH2)10H OH UBIQUINOL-50 O2 O CH3O H2O2 CH3 CH3 CH3O O OH CH3 CH3O SEMIQUINONE O2 CH3 CH3O (CH2CH=CHCH2)10H (CH2CH=CHCH2)10H O2 - O UBIQUINONE-50 Figure 4.22 The Redox Relationship Between Ubiquinone, Ubisemiquinone And Ubiquinol (A), And The Involvement Of Ubiquinol In ROS Production (B). Current evidence suggests that coenzyme Q synthesis begins in the endoplasmic reticulum and is completed in the Golgi body from where coenzyme Q is transported to other cellular locations (Ernster and Dallner (1995) and WWW.ESAINC.COM 374 references therein). Some coenzyme Q passes through the plasma membrane to the blood where it is bound to serum lipoproteins. The amount of ubiquinone and ubiquinol in rat and human is tissue dependent, with the heart showing the greatest abundance of ubiquinone and the lung the least (Table 5.1). Furthermore, the ubiquinol/ubiquinone ratio is also tissue specific approaching 100% in the pancreas, liver and intestine, but only 25% in the lung (Aberg et al. (1992)). Although it is not clear by which mechanism(s) coenzyme Q is reduced in membranes (other than the mitochondrial inner membrane), several possible enzymes have been suggested, including microsomal NADH- (and NADPH) cytochrome reductases as well as the NADH dehydrogenases associated with the outer mitochondrial and plasma membranes (Ernster and Dallner (1995) and references therein; Kishi et al.(1997); Takahashi et al. (1995, 1996a,b). Coenzyme Q’s primary role is as part of the electron-transport (respiratory) chain of the inner mitochondrial membrane, where it acts as an obligatory two-electron and two-proton carrier molecule and forms a redox link between flavin dehydrogenases and cytochromes (Chapter 2). Coenzyme Q is also found in extra-mitochondrial redox chains where it plays a similar role (Crane et al. (1993)). Antioxidant and Pro-oxidant Activities of Coenzyme Q Abundant evidence shows that ubiquinol is an antioxidant (Table 4.7) (Crane and Navas (1997); Nohl et al.(1997)). It appears that ubiquinol acts directly to inhibit both the initiation (by reduction of the perferryl radical) and propagation (by reducing the lipid peroxyl radical) phases of lipid peroxidation (Beyer (1990, 1991); Ernster and Dallner (1995); Ernster and Forsmark-Andree (1993)). Another possible antioxidant function of coenzyme Q cycle is the regeneration of α-tocopherol formed during inhibition of the propagation phase of lipid peroxidation. Interestingly the isoprenoid side chain can affect antioxidant activity in membrane preparations with short chain homologs showing the greatest activity (Kagan et al (1990)). The fact that the coenzyme Q redox cycle involves electron transfer raises the possibility that ROS could be generated as byproducts, therefore suggesting a pro-oxidant role for this redox couple (Figure 4.22B) (Nohl et al. (1996)). For measurement of the oxidation activity of coenzyme Q see Kagan et al. (1994)). WWW.ESAINC.COM 375 Evidence for Antioxidant Activity IN VITRO – SUBCELLULAR SYSTEMS • Reduced CoQ6 is four times more effective than the oxidized form in reducing the stable free radical diphenylp-picrylhydrazyl • Reduced CoQ6 is much more effective than the oxidized form in preventing hemoglobin induced peroxidation of arachidonic acid emulsions • Reduced CoQ10 inhibits ascorbate-Fe2+ -induced peroxidation of phosphatidylcholine liposomes • CoQ2 to CoQ10 homologues protect lipid vesicles from peroxidation. CoQ3 protects phospholipid vesicles from HO•-radical damage • Reduced and oxidized CoQ6 protects mitochondrial lipids from light induced peroxidation • CoQ10 protects submitochondrial particles from lipid peroxidation • Reduced CoQ10 prevents lipid peroxidation directly and spares α-tocopherol in liposomal membranes exposed to pro-oxidant conditions • Reduced CoQ10 is more effective at inhibiting liver microsome lipid peroxidation • Submitochondrial particles isolated from heart of exercise-trained animals had higher levels of CoQ10 and following succinate (to reduce oxidized CoQ10) showed less lipid peroxidation than sedentary (age-matched) controls • CoQ10 protects against ROS inactivation of respiratory chain components and inhibits membrane lipid peroxidation • Reduced CoQo and reduced CoQ2 readily react with nitric oxide. Under anaerobic conditions ubiquinones and the nitroxyl anion are formed. Under aerobic conditions the reaction proceeds with the formation of peroxynitrite CELLULAR SYSTEMS • CoQ10 protects cultured cells against free radical damage during one-electron reduction of antitumor quinones IN VIVO SYSTEMS (intact animal and clinical studies) • Conditions where the metabolic rate and ROS production are increased (e.g., following endurance exercise) are associated with elevated CoQ levels in highly aerobic tissues • Administration of CoQ10 protects animals against the effects of ischemia • Administration of CoQ10 to humans increases circulating CoQ10 levels in lipoproteins and protects LDL from lipid WWW.ESAINC.COM References Mellors and Tappel (1966) Mellors and Tappel (1966) Booth et al. (1982) Landi et al. (1984, 1985, 1989) Mellors and Tappel (1966) Takeshige et al. (1980) Frei et al. (1990) Beyer (1988); Ernster and Nordenbrand (1967) Beyer (1990) and references therein Solaini et al. (1987) Poderosa et al. (1999) Powis (1989); Takahashi et al. (1988) Beyer (1990); Beyer et al. (1962, 1984); Boveris et al. (1969); Davies et al. (1982); Lang et al. (1987); Ogasahara et al. (1989); Pederson et al. (1963); Salminen and Vihko (1983a,b); Saran and Bors (1989) Katagiri et al. (1986); Kawasaki et al. (1986); Shibata et al. (1986); Sugiyama et al. (1980) Mohr et al. (1992) 376 • • peroxidation. Administration of CoQ10 protects against oxidative damage resulting from the administration of carbon tetrachloride or ethanol Administration of CoQ to humans has been used to treat a variety of diseases associated with altered oxidative metabolism such as congestive heart failure and certain neurological diseases (e.g., Kearns-Sayre syndrome) Bertelli et al. (1986); Brattin et al. (1985); Quinn et al. (1980); Therman et al. (1973); Uysal et al. (1985) Bresolin et al. (1988); Fisher et al. (1986); Folkers and Yamamura (1977, 1980, 1984, 1986); Karlsson et al. (1986); Lenaz (1985); Mortensen et al. (1986); Ogasahara et al. (1985, 1986); Takahashi et al. (1986); Trumpower (1982); Yamamura et al. (1980); Zeirz et al. (1989) Table 4.7 In Vitro And In Vivo Evidence That Coenzyme Q10 Acts As An Antioxidant. Measurement of Coenzyme Q. Many different approaches have been used to measure tissue levels of ubiquinols, ubiquinones, and their derivatives including GC, GC-MS, and chemiluminescence (Frei et al. (1988); Gurtler and Blomstrand (1971); Morimoto et al. (1973)).15 By far the most common approach, however is HPLC. Aberg et al. (1992) used HPLC-UV to measure the redox status of CoQ9 and CoQ10 in human and rat tissues. Andersson (1992) used HPLC-PDA to measure the redox status of CoQ9 and CoQ10 and the metabolite ubichromenol in pharmaceutical preparations. Some researchers have used a combination of HPLC with both UV and EC detectors for the simultaneous measurement of ubiquinone and tocopherols (Lang and Packer (1987); Lang et al. (1986); Poddo et al. (1996)). This combination of detectors was necessary as ubiquinones cannot be measured oxidatively with ECD. To overcome the unnecessary expense of having to use two different detector modalities, some approaches used chemical reduction with either sodium borohydride (Okamoto et al. (1988)) or platinum (Wakabayashi et al. (1994)) to convert the ubiquinones to their corresponding electrochemically oxidizable ubiquinols. Thus ubiquinones can be indirectly measured using HPLC-ECD. However, the use of chemical reducing agents not only increases sample preparation time but also often leads to chromatographic issues, especially when attempting to measure low analyte levels in biological samples. Yamashita and Yamamoto (1997) simplified the chemical reduction step by using an on-line (unspecified) reactor column placed before the electrochemical cell. Coulometric-based assays offer a much simpler approach to the measurement of ubiquinones. Instead of requiring chemical reduction, ubiquinones are electrochemically reduced before being measured oxidatively. This method permits the quantitation of both reduced and oxidized CoQ10 15 As with many antioxidants, regardless of the approach used, due care must be exercised to prevent changes in analyte redox status during isolation and analysis. WWW.ESAINC.COM 377 simultaneously. With two serially placed coulometric electrodes, the upstream electrode can electrochemically reduce ubiquinones to ubiquinols with 100% conversion efficiency. Thus the naturally occurring ubiquinols, as well as those formed from coulometric reduction of the ubiquinones, can all be measured at the downstream “oxidizing” electrode. Several researchers have taken advantage of this approach including Edlund (1988); Finckh et al. (1995; 1999); Gamache et al. (1997; 2003); Grossi et al. (1992); Kaikkonen et al. (1997); Lagendijk et al. (1996); Motchnik et al. (1994); Tang et al., (2001, 2002). Plastoquinone. Plastoquinone is structurally related to ubiquinone (Figure 4.21) and, like ubiquinone, is also involved in redox reactions. Plastoquinone is primarily found in chloroplasts of higher plants (and is also present in other photosynthetic organisms) where it acts as an electron acceptor and forms part of the photosystem II complex. This complex is responsible for light-driven transfer of electrons from water to plastoquinone forming oxygen and plastoquinol. Thus the energy from two photons is stored in the reducing potential of plastaquinol. Plastoquinol can then feed these electrons into a proton-pumping electron transport chain that is linked to the photosystem I complex. The proton gradient is ultimately used to produce ATP (Stryer (1988)). Plastaquinol can be involved in single electron redox reactions producing semiplastoquinone or two electron redox reactions producing plastoquinone (cf Figure 4.22). The ability for these reactions to be coupled to ROS production is but one of the oxidative stressors occurring in chloroplasts. Vitamin K. Vitamin K consists of two groups of naphthoquinones, the phylloquinones (vitamin K1) (Figure 4.21 and the menaquinones (vitamin K2). The members of each group differ in the length of their phytyl side-chain. Menadione (vitamin K3) is a synthetic vitamin K analog. Vitamin K is essential in mammals and its daily requirement is met by a combination of dietary intake (phylloquinone) and microbial synthesis in the large intestine (menaquinone). Vitamin K is needed for the post-translational carboxylation of certain glutamate residues in proteins to γcaboxyglutamate. This modification is extremely important in the activation of certain blood-clotting factors (factors II (prothrombin), VII, IX and X). The exact roles of the phylloquinones and menaquinones in oxidative metabolism still remains unclear. There is some evidence that they (in their reduced forms) can act as antioxidants capable of inhibiting lipid peroxidation (Fiorentini et al. (1997); Ohyashiki et al.(1991); Tampo and Yonaha (1996); Vervoort et al. (1997)). However, there is considerable evidence that menadione acts as a prooxidant and cytotoxin in vitro. Like many quinones it appears that several WWW.ESAINC.COM 378 mechanisms can account for menadione’s toxicity. First, it can react with cellular nucleophiles such as amines and thiols (e.g., GSH) leading to the formation of aryl-conjugates (see below) (Brunmark and Cadenas (1989); Gant et al. (1988)). Second, reaction of menadione with GSH through aryl substitution and by oxidation of GSH to GSSG depletes cellular GSH levels (Bellomo et al. (1987)). Third, menadione metabolism is associated with an inhibition of GSH-reductase that also leads to the depletion of GSH (Bellomo et al. (1987)). Fourth, quinones are readily reduced under physiological conditions by either direct reactions with NADH (or NADPH) (Kukielka et al. (1990)) or through those involving enzymes such as diaphorase (NAD(P)H: [quinone acceptor] oxidoreductase) (Murphy et al. (1991); Thor et al. (1982)), in one- or two-electron processes, yielding the semiquinone and hydroquinone, respectively (Figures 2.7 and 4.22). Metaldependent auto-oxidation reforms the quinone with concomitant regeneration of ROS (Brunmark and Cadenas (1989); Comporti (1989); O’Brian (1991)). Consequently, menadione promotes DNA damage (Ngo (1993); Nutter et al. (1992)) but without production of 8OH2’dG (Fischer-Nielsen et al. (1995)). It also causes hemolysis in a variety of species (Munday et al. (1994) and references therein). Conversely, menadione has been reported to be beneficial, having anticancer, antimalarial, and antileishmanial activity, and has been used as an electron carrier in the treatment of a patient with a severe defect in complex III of the electron transport chain (Eleff et al. (1984); Munday et al. (1994) and references therein). Technique HPLC-UV HPLC-Fl HPLC-electrofluoresence HPLC-ECD (single or dual electrodes) HPLC-ECD (coulometric electrode array) References Haroon et al. (1982); Lefevere et al. (1982); Shearer et al. (1980) Abe et al. (1979) Langenberg and Tjaden (1984a,b); Moussa et al. (1989, 1994) Haroon et al.(1984); Hart et al. (1984, 1985); Hiroshima et al. (1981); Isshiki et al. (1988); Kaikkonen et al. (1997); McCarthy et al. (1997); Rawlinson et al. (1998) Gamache et al. (1997) Table 4.8 Some HPLC-Based Methods For Vitamin K Measurement. A variety of analytical approaches are used to measure vitamin K in different sample matrices including HPLC coupled with: UV, fluorescence, coulometricelectrofluoresence, amperometric, coulometric, and coulometric array detection (Table 4.8). WWW.ESAINC.COM 379 Pyrroloquinoline quinone. Pyrroloquinoline quinone (2,7,9-tricarboxypyrroloquinoline quinone; methoxatin) (PQQ) is a widely distributed redox-active cofactor and essential nutrient (Figure 4.21) (McIntire (1992)). PQQ is reported to be an essential and versatile cofactor for a variety of enzymes including dehydrogenases and oxidases, and takes part in a variety of hydroxylation, transamination, decarboxylation and hydration reactions (Duine (1989); McWhirter and Klapper (1990)). Others however, using more selective and sensitive approaches, propose that PQQ has been misidentified and that another cofactor, topaquinone, may be present instead (Harris (1992); Kumazawa et al. (1990)). Free PQQ has been found in a variety of animal tissues. It can catalyze dioxygen-superoxide interconversion, and participates in both superoxide generation (in the respiratory burst) and scavenging (acting as an antioxidant) (Bishop et al. (1994, 1995); Gallop et al. (1993)). In its role as antioxidant, PQQ scavenges ROS, may convert xanthine oxidase into xanthine dehydrogenase, spares GSH and protects neurons against the neurotoxic action of NMDA (Gallop et al. (1993) and references therein). The mammalian enzyme PQQ reductase also participates in mechanisms protecting tissues against oxidative stress (Christensen (1994)). Under certain conditions it can act as a pro-oxidant producing hydrogen peroxide during metal-induced auto-oxidation (He et al., (2003)). Tocopherols. In literature, vitamin E has become synonymous with the most abundant form found in human tissues, α-tocopherol (2,5,7,8-tetramethyl-2-(4’, 8’, 12’-trimethyltridecyl)-6-chromanol). In fact, vitamin E is not a single compound but consists of a group of eight naturally occurring, lipophilic molecules including: the tocopherols (which differ in the number of methyl groups on the chromanol ring (Figure 4.23)) and the tocotrienols (which also possess an unsaturated tail). Furthermore, each of the tocopherols (and tocotrienols) can produce a corresponding tocopheryl quinone (and tocotrienyl quinone) during oxidation processes (Figure 4.24). In the strictest sense, the use of vitamin E to represent α-tocopherol is incorrect as the other forms of tocopherol (and tocotrienols) also show varying degrees of biological activity (e.g., influencing membrane fluidity, controlling prostaglandin and leukotriene synthesis, and regulating nucleic acid synthesis and gene expression) and antioxidant capacity (Sokol (1989) and references therein). The antioxidant and biological activities of the different forms of vitamin E show great interspecies variability. For example, γ-tocopherol has about 50% the antioxidant activity of α-tocopherol (Mukai (1993)) but only 10-30% of its biological activity WWW.ESAINC.COM 380 (Bunyan et al. (1961)). On the other hand, α-tocotrienol has higher (Serbinova et al. (1993)) or equivalent (Suarna et al. (1993)) antioxidant activity to α-tocopherol but only 30% its biological activity (Bunyan et al. (1961)). HO H3 C HO CH 3 O CH 3 CH 3 4' 8' CH 3 CH 3 HO CH 3 CH 3 2 H3C O O CH 3 H3C CH 3 CH 3 O CH 3 CH 3 CH 3 RRR-β -TOCOPHEROL HO CH 3 CH 3 CH 3 CH 3 CH 3 RRR-γ -TOCOPHEROL HO CH 3 CH 3 CH 3 CH 3 CH 3 RRR-α -TOCOPHEROL CH 3 CH 3 CH 3 RRR-δ-TOCOPHEROL NO 2 CH 3 O CH 3 CH 3 CH 3 CH 3 CH 3 5-Nitro-RRR- γ -TOCOPHEROL CH 3 HO HO O H3 C CH 2CO 2H CH 3 H3C HO O CH 3 CH 3 4' 8' CH 3 CH 3 CH 3 CH 3 CH 3 O CH 3 CH 3 RRR-β -TOCOTRIENOL CH 3 CH 3 RRR-γ -TOCOTRIENOL CH 3 CH 3 CH 3 CH 3 CH 3 CH 3 HO O CH 3 H3C RRR-α -TOCOTRIENOL CH 3 CH 3 HO 2 CH 3 CO 2 H TROLOX IRF1005 CH 3 O CH 3 CH 3 HO CH 3 CH 3 CH 3 O CH 3 CH 3 CH 3 RRR-δ-TOCOTRIENOL Figure 4.23 The Structures Of Naturally Occurring Tocopherols, Tocotrienols, 5-Nitro-γ-Tocopherol And The Synthetic Water Soluble Analogs, Trolox™ (Hoffman-Laroche) And IRF1005. The tocopherols show complex stereochemistry. Although the tocopherol molecule has three centers of chirality at the 2, 4’ and 8’ carbons, it only occurs naturally in one form, the RRR isomer (Figure 4.23). The RRR form also has two ambo-forms (RRR and SRR) and each of these has four isomers (ambo-RRR has the RRR, RSR, RRS and RSS isomers; ambo-SRR has the SRR, SSR, SRS and SSS isomers). Unless otherwise stated most vitamin E supplements usually consist of a mixture of isomers and may even totally lack the natural RRR isomer. WWW.ESAINC.COM 381 CH 3 CH 3 HO -H+, CH 3 H3 C O -e - CH 3 H3 C R CH 3 O O CH 3 TOCOPHEROL R TOCOPHER(OX)YL RADICAL 2H+, +2e -H2 O, +H2 O, -H+, -e - CH 3 -2H+, -2e - HO HO H3 C OH CH 3 R CH 3 O H3 C CH 3 HO CH 3 O R CH 3 TOCOPHERYLHYDROQUINONE TOCOPHERYLQUINONE Figure 4.24 Redox Reactions Of The Tocopherols. The Biology of Tocopherols. In man, tocopherols are essential and must be obtained from the diet. Adequate consumption of vitamin E is mandatory for good health. The role of tocopherols in human health and disease has been extensively reviewed (e.g., see Diplock et al. (1989); Packer et al. (1993); Sokol (1989); Traber and Packer (1995); Traber and Sies (1996)). Being lipophilic, the tocopherols are absorbed, processed and transported like most other fats. This is a very complex field but fortunately has been excellently reviewed by Traber (1994). Unlike other fat soluble vitamins, the tocopherols have no specific plasma carrier proteins, rather they are transported by plasma lipoproteins.16 The circulating level of tocopherol, and consequently its biological activity (e.g., the amount of a particular tocopherol that is necessary to alleviate various symptoms in vitamin E deficient rats) is controlled by hepatic tocopherol binding protein (Traber (1994)). Human diets typically contain large amounts of γ-tocopherol, mostly derived from corn and soybean oils, and these usually exceed the intake of α-tocopherol considerably (Bieri and Evarts (1974)). Nonetheless, the plasma concentrations of γ-tocopherol rarely reach 20% of αtocopherol levels (Traber and Kayden (1989); Traber (1994)). The tocotrienols are present in high concentrations in palm oil. Consumption of palm oil can lead 16 Vitamins A and D are stored in the liver and can easily reach toxic levels. So far there are no reports of any toxic effects of vitamin E following supplementation (Bendich and Machlin (1988)). Tocopherols are readily excreted through bile and in the feces, thereby preventing excessive hepatic accumulation. WWW.ESAINC.COM 382 to elevated, but non-sustained, increases in circulating levels of tocotrienols, again a consequence of poor binding to tocopherol binding protein. Literature tissue levels for the tocopherols, tocotrienols and their quinones are presented in Table 4.3. There has been considerable controversy regarding the biological activity of RRR-α-tocopherol and synthetic (all rac)- α-tocopherol (Veris (1994)). Much of the debate has centered upon blood levels following consumption of experimental amounts of natural or synthetic α-tocopherol. Unfortunately, the level of tocopherol in the blood may not be the best index for biological activity as this is dependent upon several factors including: the rate of absorption, the rate of release by the liver, the rate of transfer to other tissues and the animal model being used. Knight and Roberts (1985) reported that, following administration of either natural or synthetic α-tocopherol to rabbit pups, the plasma level of tocopherol was of little value in establishing efficacy of vitamin E therapy. Traber et al. (1990) reported that humans could discriminate between natural and synthetic α-tocopherol administered as their esters. Acetate and succinate esters are often used in commercial preparations as they are more stable than the free forms. Following ingestion pancreatic enzymes hydrolyze the ester. Consequently, almost all of the vitamin E in blood and tissue is in the free form following ingestion of tocopherol esters. Following ingestion of RRR-αtocopheryl- and SRR-α-tocopheryl acetate there was little difference in the circulating forms until 11 hours post administration. The circulating RRR-/SRR-αtocopherol ratio continued to increase with time and reached 4-fold after 24 hours. These researchers concluded that this discrimination was not due to differences in absorption but rather to differences in secretion by the liver. Chung et al. (1992) experimented with pigs. These animals are often regarded to be more similar to humans in their metabolism than rats. They reported that RRR-αtocopherol had much more biological potency than the synthetic forms when using the pig rather than rat as the model. Antioxidant, Pro-Oxidant and other Reactions of the Tocopherols. A major biological role for the tocopherols is their ability to act as potent peroxyl radical scavengers and chain breaking antioxidants (Liebler (1993); Wolf et al. (1998)). α-Tocopherol is the principal lipid-soluble antioxidant of both plasma and the LDL particle (Esterbauer et al. (1990)). It occurs at low levels in membranes, typically one molecule for every 2000-3000 lipid molecules, where it acts as a chain breaking antioxidant, a singlet oxygen quencher, and a regulator of both enzyme activity and membrane fluidity (Packer and Landvik (1989)). Tocopherols can react with alkyl radicals, hydroperoxyl radicals, peroxynitrite, as well as the hydroxyl free radical. α-Tocopherol can also react with superoxide in vitro and form a variety of novel compounds (e.g., tocopherol dimers and epoxides) depending on whether protic or aprotic conditions are used (Ha and Csallany (1992)). As shown in Figures 3.23 and 4.25, tocopherol can readily intercept a WWW.ESAINC.COM 383 lipid peroxyl radical and donate a labile proton forming a lipid hydroperoxide and tocopheroxyl radical, thereby preventing the propagation of the chain reaction (Chapter 3). Readers interested in the structure-activity relationship of various natural and synthetic tocopherol species are referred to Lien et al. (1999). MEMBRANE LO 2 LO 2H TOCOPHEROL TOCOPHEROXYL RADICAL CYTOSOL DIHYDROLIPOIC ACID/α -LIPOIC ACID ASCORBATE/SEMIDEHYDROASCORBATE RADICAL GSH/GSSG ASCORBATE COUPLE/GSH COUPLE ASCORBATE COUPLE/ α -LIPOIC ACID COUPLE UBISEMIQUINONE UBIQUINOL INDIRECT TOCOPHEROL REGENERATION DIRECT TOCOPHEROL REGENERATION LO 2 LO 2H LO 2 LO 2H LO 2 LO 2H LO 2 LO 2H LO 2 LO 2H TOCOPHEROL TOCOPHEROXYL RADICAL TOCOPHEROL TOCOPHEROXYL RADICAL TOCOPHEROL TOCOPHEROXYL RADICAL TOCOPHEROL Lipoamide Dehydrogenase DIHYDROLIPOIC ACID GSSG GSH NADPH Glutathione Reductase NADP+ GSH ASCORBATE GSSG SEMIDEHYDROASCORBATE RADICAL ASCORBATE TOCOPHEROL GSSG GSH ? NAD+ SEMIDEHYDROASCORBATE RADICAL TOCOPHEROXYL RADICAL TOCOPHEROXYL RADICAL NADH α -LIPOIC ACID NADPH Glutathione Reductase NADP+ DIHYDROLIPOIC ACID NADH Lipoamide Dehydrogenase α -LIPOIC ACID DIHYDROLIPOIC ACID NAD+ NADH Lipoamide Dehydrogenase α -LIPOIC ACID NAD+ Figure 4.25 The Prevention Of Lipid Peroxidation By Tocopherol And Possible Direct And Indirect Pathways For The Regeneration Of Tocopherol By Water Soluble And Fat Soluble Antioxidants. (The Eventual Regeneration Of Ascorbate, GSH And Lipoic Acid Is Explained In Text.). Due to delocalization of charge the tocopheroxyl radical is much less energetic than the lipid peroxyl radical and is therefore less able to react with other species and cause damage (Chapter 1). Tocopherol is regenerated from the tocopheroxyl radical following reaction with endogenous reducing agents which can occur either within the membrane (e.g., ubiquinol) (Kagan et al. (1990); Maguire et al. (1992); Stoyanovski et al. (1995)) or in the cytoplasm (e.g., GSH, ascorbic acid WWW.ESAINC.COM 384 and dihydrolipoic acid) (Nohl and Gille (1998)). For this reason, a little bit of tocopherol goes a long way. This may explain why the short-term absence of vitamin E in human diet does not result in any specific deficiency disease in normal adults. However, vitamin E deficiency is problematic for premature babies who can be predisposed to hemolytic anemia (probably due to the fragility of the erythrocyte membrane) and in those with diseases that affect fat metabolism, such as abetalipo-proteinaemia and short-bowel syndrome. The redox behavior of tocopherol is presented in Figure 4.24 and shows the redox reactions found in vivo and during electrochemical measurement. Tocopherol can undergo a single electron oxidation with the production of the tocopheroxyl radical (the usual “biological” product when tocopherol reacts with an ROS). A second single electron oxidation of the tocopheroxyl radical, or a two electron oxidation of tocopherol, results in ring opening and the production of tocopheryl quinone, the final oxidation product of tocopherol. Tocopheryl quinone is a potent anticoagulant and may be responsible for some of the effects of α-tocopherol in preventing heart attacks and strokes (Dowd and Zheng (1995)). Evidence suggests that tocopheryl quinone can be “recycled” to tocopherol in vivo, albeit with very poor yields (<0.8%) (Moore and Ingold (1997)). The physiological impact of this salvage pathway has yet to be determined. Tocopheryl quinone can be reduced in a two electron process to form tocopheryl hydroquinone. Kohar et al. (1995) showed that tocopheryl hydroquinone can be formed in vivo and have suggested that the tocopheryl hydroquinone/tocopheryl quinone couple may be biologically important acting like the coenzyme Q redox system. Recently Siegel et al. (1997) demonstrated that tocopheryl form is more readily reduced by NAD(P)H:quinone oxidoreductase (NQO1) than ubiquinone. This suggests that one of the physiological functions of NQO1 is the regeneration of antioxidant forms (tocopheryl hydroquinone) of α-tocopherol. A variety of different products can be formed when tocopherols react with RNS. d’Ischia and Novellino (1996) examined the mechanism of reaction between nitric oxide and α-tocopherol. Under aprotic conditions one oxidation pathway produced some α-tocopheryl quinone and little amounts of its nitrite ester. However, under physiological conditions they reported considerable oxidation of α-tocopherol to α-tocopheryl quinone, formation of its nitrite ester (which is capable of nitrosating amines), a yellow dimer and a series of related oligomers. Similarly, nitric oxide (de Groot et al. (1993)), nitrogen dioxide (Cooney et al. (1995)) and peroxynitrite (Hogg et al. (1993), Pannala et al. (1999); Vatassery (1996)) have all been reported to rapidly oxidize α-tocopherol. Such depletion in α-tocopherol levels have been suggested to underlie the cytotoxic nature of many RNS. In support of this, Burkart et al. (1995) showed that α-tocopherol efficiently protects eukaryotic cells from nitric oxide-induced cytotoxicity. Gorbunov et al. (1996) found that nitric oxide can also lead to the production of the tocopheroxyl radical and suggested that another possible cytotoxic mechanism is the depletion of ascorbic acid resulting from α-tocopherol regeneration. WWW.ESAINC.COM 385 Recent research has focused on tocopherol nitration. While α-tocopherol can react with nitrogen dioxide to form a nitrosating agent, γ-tocopherol17 reduces nitrogen dioxide to nitric oxide without the formation of a nitrosating species (Cooney et al. (1993)). This γ-tocopherol-mediated detoxification of nitrogen dioxide may be important physiologically as it has been shown to prevent nitrogen dioxide-mediated DNA strand breaks (Bittrich et al. (1993)). γ-Tocopherol also reacts with nitrating species to form a variety of oxidation products and adducts (Hoglen et al. (1997); Pannala et al.(1999)). For example, based on the earlier work of Cooney et al. (1995), Christen et al. (1997) used HPLC-ECD to show that γ-tocopherol preferentially reacts with nitrating agents (e.g., peroxynitrite or SIN-1) forming 5-nitro-γ-tocopherol (Figure 4.23). 5-Nitro-γtocopherol is currently being used as a marker of RNS damage in vivo in a variety of biological fluids (Hensley et al., 1999, 2000); Morton et al., (2002); Williamson et al., (2002)). As expected δ-tocopherol is even more reactive than γ-tocopherol, due to it having two available positions for substitution to take place (Figure 4.23). αTocopherol cannot react in this way as its 5-position is blocked and thus unavailable for reaction with electrophiles. This suggests that γ-tocopherol’s principal role may be to trap membrane-soluble electrophilic nitrogen oxides whereas α-tocopherol’s action is as a chain breaking antioxidant (Christen et al. (1997); Wolf (1997)). Furthermore, as large doses of α-tocopherol (the form in supplements) readily displace γ-tocopherol (the form in human diet) from plasma and other tissues, Christen et al. (1997) have questioned the wisdom of only having α-tocopherol in supplements. However, Goss et al. (1999) have challenged the requirement for γ-tocopherol and reported that α-tocopherol could prevent nitration of both γ-tocopherol and tyrosine by peroxynitrite. Their data suggest that α-tocopherol alone is readily capable of removing any peroxynitritederived RNS and that γ-tocopherol probably only plays a role once α-tocopherol levels are depleted. Under certain circumstances α-tocopherol has been reported to have pro-oxidant activity (Bowery et al. (1992). For example, in LDL in the absence of regenerating antioxidants (e.g., ascorbic acid), the α-tocopheroxyl radical can abstract a hydrogen atom from a nearby PUFA molecule to form a conjugated diene that can then form a lipid peroxyl radical upon reaction with oxygen. This peroxyl radical can then react with α-tocopherol to complete the cycle of α-tocopherolmediated lipid peroxidation. As it is unlikely that regenerating antioxidants will be absent, the physiological significance of tocopherol’s pro-oxidant activity is yet to be fully explored. 17 γ-Tocopherol is also capable of undergoing Michael addition with cellular thiols (such as GSH) forming 5-substituted adducts which are potential chemotherapeutic agents (Thornton et al. (1995)). WWW.ESAINC.COM 386 Tocopherol and Disease. Several studies have addressed the importance of the tocopherols’ antioxidant properties in disease prevention. For example, tocopherol appears to play a vital role in preventing coronary diseases: there is a significant correlation between α-tocopherol concentrations and coronary artery disease (Gey (1998); Gey et al. (1992)); there is an inverse relationship between plasma vitamin E concentration and the risk of angina pectoris (Riemersma et al. (1991)); and vitamin E supplementation reduces the risk of coronary artery (Rimm et al. (1992); Stampfer et al. (1993)) and atherosclerotic heart disease (Diaz et al. (1997)). α-Tocopherol inhibits LDL oxidation both in vitro and in human subjects (Jialal and Grundy (1992); Jialal et al. (1995)). Additionally, α-tocopherol may offer additional benefits for cardiovascular disease beyond its antioxidant properties. For example, it reduces platelet adhesion (Salonen et al. (1991)); inhibits smooth muscle proliferation and protein kinase C activity (Ozer et al. (1993)); inhibits agonist-induced monocyte adhesion to cultured human endothelial cells (Faruqui et al. (1994)); and preserves endothelium-dependent vasodilation in hypercholesterolemic rabbits (Keaney et al. (1994)). In addition to cardiovascular problems, humans with suboptimal vitamin E intake are thought to be at increased risk for several aging related diseases such as cancer (Bostick et al. (1993); Gridley et al. (1992); Wald et al. (1984)), and neurological disorders (Behl et al. (1992); (Diplock (1998); Kayden (1993); Sokol (1989); Tanyel and Mancano (1997); Vatassery (1998)). The potential benefits of α-tocopherol supplementation on cancer incidence is far from clear. Although previous studies have suggested that higher intakes of α-tocopherol may be associated with a reduced risk of lung cancer, Heinonen and Albanes (1994) found no such correlation among male smokers. Recently, McAll and Frei (1999) posed the question “can antioxidant vitamins materially reduce oxidative damage in humans”. They concluded that “with the only exception of supplemental vitamin E, and possibly vitamin C, being able to lower lipid oxidative damage in both smokers and non-smokers, the current evidence is insufficient to conclude that antioxidant vitamin supplementation materially reduces oxidative damage in humans”. Measurement of Tocopherols and Their Metabolites. A variety of methods have been used to measure the tocopherols including colorimetric (Diplock et al. (1996)), spectrophotometric (Vatassery and Mortenson (1972)), fluorometric (Taylor et al. (1976); Vatassery and Mortenson (1972)), HPLC with UV (Barua et al. (1991; 1993); Miller and Yang (1985); Xu et al. (1996)), GC-MS (Liebler et al. (1996)), and tandem MS approaches (Walton et al. (1988)). Several HPLC-ECD methods exist for the measurement of the tocopherols, either alone or with other lipophilic antioxidants (Table 4.9). WWW.ESAINC.COM 387 Analyte Tocopherol Tocopherols, tocotrienols, ubiquinols, ubiquinones Tocopherols/tocopheryl quinones Tocopherols/tocopheryl quinones Tocopherol, tocopheryl quinones Tocopherol, ubiquinol, ubiquinone Tocopherol, ubiquinol, ubiquinone Tocopherols α-Tocopherol, β-carotene and retinol α-Tocopherol, CoQ10 Tocopherols, β-carotene, lycopene, ubiquinol, ubiquinone Tocopherols, carotenoids, ubiquinol, ubiquinone Tocopherols, CoQ10, retinoids, carotenoids, vitamin K Tocopherol, CoQ10 and tocopherol oxidation products Method Reference HPLC-dual coulometric electrode detection. HPLC-amperometric electrode detection for tocopherols, tocotrienols, ubiquinols; HPLCUV detection for ubiquinones. Castle and Cook (1985) Podda et al. (1996) HPLC-triple coulometric electrode detection. HPLC-dual coulometric electrode detection. HPLC-triple coulometric electrode detection. HPLC-amperometric electrode detection for tocopherol only. HPLC-amperometric electrode detection with platinum catalyst reduction. HPLC-amperometric electrode detection. HPLC-UV and amperometric electrode detection. HPLC-dual coulometric electrode detection. HPLC-dual coulometric electrode detection. Murphy and Kehrer (1987) Vatassery et al. (1993) Takeda et al. (1996) Lang and Packer (1987) Wakabayashi et al. (1994) Huang et al. (1986) MacCrehan and Schonberger (1987) Edlund (1988) Motchnik et al. (1994) HPLC-dual coulometric electrode detection. Gradient HPLC-coulometric electrode array detection. Finckh et al. (1995, 1999) Gamache et al. (1997, 2003) HPLC- dual coulometric electrode detection. Leray et al. (1998) Table 4.9 HPLC-ECD Methods Used To Measure Tocopher