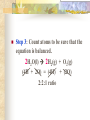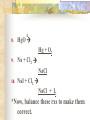* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download + H 2 (g)
Metallic bonding wikipedia , lookup
Fine chemical wikipedia , lookup
Al-Shifa pharmaceutical factory wikipedia , lookup
Chemical weapon proliferation wikipedia , lookup
Abundance of the chemical elements wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Spinodal decomposition wikipedia , lookup
Electrolysis of water wikipedia , lookup
Isotopic labeling wikipedia , lookup
Chemical potential wikipedia , lookup
Water splitting wikipedia , lookup
Chemical weapon wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Nuclear transmutation wikipedia , lookup
Chemical element wikipedia , lookup
Chemical Corps wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
Chemical bond wikipedia , lookup
Chemical plant wikipedia , lookup
Registration, Evaluation, Authorisation and Restriction of Chemicals wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Safety data sheet wikipedia , lookup
California Green Chemistry Initiative wikipedia , lookup
Chemical industry wikipedia , lookup
Click chemistry wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Drug discovery wikipedia , lookup
Extended periodic table wikipedia , lookup
Process chemistry wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Metalloprotein wikipedia , lookup
Electrochemistry wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
History of chemistry wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Chemical reaction wikipedia , lookup
George S. Hammond wikipedia , lookup
Rate equation wikipedia , lookup
Transition state theory wikipedia , lookup
History of molecular theory wikipedia , lookup
Atomic theory wikipedia , lookup
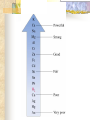
Chemical Reactions Chapter 9 Section 1 Indications of a Chemical Reaction Chemical changes alter the molecular structure of the substances involved. These observations suggest that a chemical change has occurred: 1. Evolution of heat(temp change) and light. 2. Production of a gas. 3. Formation of a precipitate (solid). 4. Color change. Chemical Equations Used to represent chemical reactions (rx). Chemical equations have the following characteristics: a. equation must represent known facts. b. equation must contain the correct formulas for the reactants and products. c. law of conservation of mass must be satisfied. Chemical Equations Let’s diagram the following equation: 2C2H2(g) + 5O2(g) 4CO2(g) + 2H2O(g) Reactants Products * is read as yield (also produce or form) *number that appears in front of a formula is called a coefficient. It represents relative number of moles of a substance. *(g) refers to the state of matter of the substance. The following is a list of symbols used in chemical equations: = reversible rx heat or Δ = reactants are heated (aq) = aqueous 2 atm = pressure at which (s) = solid phase rx took place (cr) crystalline 0˚ = temp at which rx (l) = liquid phase took place (g) = gas phase = formula of catalyst MnO2 (manganese dioxide) *add to the right of rx conditions (s) if: single metal, solid, ash, ribbon, salt, crystalline. (g) if: any “big 7” , CO2, CO. (aq) if: all acids, dissolved in water, solution. electric if electricity is added. *If substance does not fit above criteria, do not put any state of matter. Balancing Chemical Equations Balancing Steps: Step 1: Identify reactants and products. Write a formula equation by substituting correct formulas for the names of reactants and products. EX: Water breaks down into hydrogen and oxygen gases when heated. H2O(l) H2(g) + O2(g) Diatomic Molecules There are seven elements that are not found as single atoms in nature. They are found bound to other elements or to themselves in molecular compounds. They are referred to as the “Big 7” because they form the shape of a 7 on the periodic table. These elements are: Hydrogen, Nitrogen, Oxygen, Fluorine, Chlorine, Bromine, and Iodine. Balancing Chemical Equations Step 2: Balance the formula equation according to the law of conservation of mass. a. Balance the different types of atoms one at a time changing only the coefficients. b. Balance unique atoms first. c. Balance polyatomic ions if they appear on both sides as single units. d. Balance H atoms and O atoms last. Write after d. **If an odd # on one side and even on another, make odd even with a 2** EX: H2O(l) H2(g) + O2(g) *Is this balanced? Why or Why not? Not balanced. Because there are more oxygen atoms on the product side. This violates the law of conservation of mass. Balancing Chemical Equations H2O(l) H2(g) + O2(g) How to balance: 1. There are no unique atoms to balance first. 2. H atoms are already balanced. 3. Balance the O atoms using only coefficients. 2H2O(l) H2(g) + O2(g) 4. By adding a coefficient of 2 to H20 to balance the O atoms, the H atoms became unbalanced. Balance the H atoms. 2H2O(l) 2H2(g) + O2(g) Step 3: Count atoms to be sure that the equation is balanced. 2H2O(l) 2H2(g) + O2(g) (4H + 2O) = (4H) + (2O) 2:2:1 ratio Avoid the following when balancing: 1. Writing incorrect chemical formulas. Balancing equations by changing the subscripts. Coefficients do not represent the smallest whole number ratio. 2. 3. EX: 4H2O(l) 4H2(g) + 2O2(g) 4:4:2 2:2:1 * Factor out the LCM. Hint to balancing hydrocarbons: -hydrocarbons are CxHx or CxHxOH + O2 -place a coefficient of 2 in front of hydrocarbon and then balance. -may have to factor 2 out when finished. Practice Balancing 1. Zn(s) + HCl(aq) ZnCl2(aq) + H2(g) Zn(s) + 2HCL(aq) ZnCl2(aq) + H2(g) 2. HNO3(aq) + Mg(OH)2(s) Mg(NO3)2(aq) + H2O(l) 2HNO3(aq) + Mg(OH)2(s) Mg(NO3)2(aq) + 2H2O(l) Types of Chemical Reactions There are 5 basic types of chemical rxs. 1. Synthesis rx 2. Decomposition rx 3. Single replacement (displacement) rx 4. Double replacement (displacement) rx 5. Combustion rx Synthesis Rxs In a synthesis rx, two or more reactants combine to form a single new product. Represented by: A + X AX EX: 2Mg(s) + O2(g) 2MgO(s) 4Fe(s) + 3O2(g) 2Fe2O3(s) 2Na(s) + F2(g) 2NaF(s) Decomposition Rxs In a decomposition rx, a single reactant undergoes a rx that produces two or more simpler products. Represented by: AX A + X EX: 2H2O(l) 2H2(g) + O2(g) 2KClO3(s) 2KCl(s) + 3O2(g) H2CO3(aq) CO2(g) + H2O(l) Decomposition Rxs A metal carbonate breaks down to form a metal oxide and carbon dioxide gas. CaCO3(s) CaO (s) + CO2(g) A metal hydroxide (except those metals in Group 1) break down to form a metal oxide and water. Ca(OH)2(s) CaO(s) + H2O A metal chlorate breaks down to form a metal chloride and oxygen gas. 2KClO3(s) 2KCl(s) + 3O2(g) Single Replacement Rxs In a single replacement rx, one elemental reactant replaces a similar type element in a reactant compound to form a new product compound and a new elemental product. Represented by: A + BX AX + B EX: 3Fe(s) +4H2O(l) Fe3O4(s) + 4H2(g) 2Al(s) + 3Pb(NO3)2(aq) 3Pb(s) + 2Al(NO3)3(aq) Wow! He’s the bomb. Mg(s) + 2HCl(aq) Stupid Jerk! MgCl2(aq) + H2(g) Single Replacement Rxs The ability of an element to react is referred to as the element’s activity. The more readily an element reacts with other substances, the greater its activity is. An activity series is a list of elements organized according to the ease with which the elements undergo single replacement reactions. Activity Series of Elements In an activity series, the most active metals are placed at the top. An active metal can replace each of the elements below it but not above it. Activity series are used to help predict whether certain rxs will occur. Using the Activity Series of Elements Aluminum is more reactive than Zinc so the following rx will occur: 2Al(s) + 3ZnCl2(aq) 3Zn + 2AlCl3(aq) Cobalt is less reactive than Sodium so the following rx will not occur: Co(s) + 2NaCl(s) CoCl2(s) + Na2(s) This rx should be written as: Co(s) + NaCl(s) no reaction Using the Activity Series of Elements Use the activity series to predict whether each of the following rxs will occur: 1. Zn(s) + H2O(l) Will Occur 2. Cu(s) + HCl(aq) No Reaction 3. Cd(s) + Pb(NO3)2(aq) Will Occur Single Replacement Rxs Indicate whether the metal or nonmetal is being replaced. EX: Mg + FeCl2 Fe + MgCl2 SR-metal Cl2 + KI KCl + I2 SR-nonmetal Double Replacement Rxs In a double replacement rx, the ions of two reactant compounds exchange places in an aqueous solution to form two new product compounds. Represented by: AX + BY AY + BX EX: 2KI(aq) + Pb(NO3)2(aq) PbI2(s) + 2KNO3(aq) Combustion Rxs In a combustion rx, a hydrocarbon/alcohol (consisting of carbon and hydrogen) combines with oxygen, releasing a large amount of energy in the form of heat and light. Represented by: (CxHx OH) or CxHx + O2 CO2 + H2O EX: C3H8(g) + 5O2(g) 3CO2(g) + 4H2O(g) *combustion of hydrocarbons always result in the formation of CO2 and water. Identification of Rx Types Synthesis Combustion (combust) Double Replacement (DR) Single Replacement (SR) -Indicate whether metal or nonmetal Decomposition (decomp) -Indicate metal carbonate, metal hydroxide, or metal chlorate. -If none of the above, general. Identifying Rxs 1. 2. 3. 4. 5. 6. Use your knowledge of rx types to classify the following rxs: NH3(g) + HCl(g) NH4Cl(s) C2H5OH(l) + O2(g) CO2(g) + H2O(g) 2KClO3(s) 2KCl(s) + 3O2(g) Zn + H2SO4 ZnSO4 + H2 S(s) + O2(g) SO2(g) AgNO3(aq) + NaCl(aq) AgCl(s) + NaNO3(aq) Reaction Prediction Steps Step 1: Find the reactants and write them in formula form. Step 2: Identify the type of rx based on the reactants. Step 3: Write products -positive oxidations bond with negative oxidations. Step 4: Balance equation. Hints: 1. 2. 3. 4. 5. 6. Know the 3 types of decomp rxs and what products they produce. Combustion products, CO2 + H2O H2O as a reactant, HOH 2 single elements always synthesis SR rxs, use Activity Series If you need oxidation for metals, darker number on periodic table is most common ion so use that. Predicting Products 1. 2. 3. Use your knowledge of rx types to predict the products of the following rxs: Na + H2O [HOH] NaOH + H2 HNO3 + Ca(OH)2 Ca(NO3)2 + H2O Mg + O2 MgO Predicting Products 4. 5. 6. 7. Ca + Cl2 CaCl2 C6H14 + O2 CO2 + H2O Br2 + KI KBr + I2 MgO + Pb No Reaction 8. Δ HgO Hg + O2 9. Na + Cl2 NaCl 10. NaI + Cl2 NaCl + I2 *Now, balance these rxs to make them correct. In Summary Five observations that suggest a chemical reaction is taking place are the evolution of light and heat, the production of gas, a change in color, and the formation of a precipitate. Reactants are the starting substances in a reaction. Products are the substances resulting from a reaction. In Summary Physical states of substances are shown by; (g) = gas, (l) = liquid, (s) = solid, and (aq) = aqueous, which indicates substance is dissolved in water. Balancing an equation means adjusting coefficients so that there is the same number of atoms of each element on the left and right sides of the equation. In Summary There are 5 basic types of chemical reactions: 1. Synthesis Rx: A + X AX 2. Decomposition Rx: AX A + X 3. Single Replacement Rx: A + BX AX + B 4. Double Replacement Rx: AX + BY AY + BX 5. Combustion Rx: CxHx + O2 CO2 + H2O In Summary Activity series list the elements in order of their chemical reactivity and are useful in predicting whether a chemical reaction will occur. Products of reactions can be predicted by a general knowledge of the reaction types. In Summary For the exam you must be able to: 1. Make a formula equation out of a word equation. (or the opposite) 2. Identify the reaction type. 3. Predict the products for the reaction. 4. Balance the formula equation. Be familiar with the entire contents of the notepacket and chapter in book. A C6H12O6 production