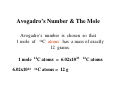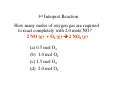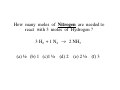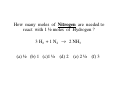* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 3 Stoichiometry STOICHIOMETRY: The chemical arithmetic
Asymmetric induction wikipedia , lookup
Radical (chemistry) wikipedia , lookup
Spinodal decomposition wikipedia , lookup
Catalytic reforming wikipedia , lookup
Chemical weapon wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Marcus theory wikipedia , lookup
Chemical Corps wikipedia , lookup
Biochemistry wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
Chemical potential wikipedia , lookup
Chemical industry wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
Water splitting wikipedia , lookup
Computational chemistry wikipedia , lookup
Chemical plant wikipedia , lookup
Safety data sheet wikipedia , lookup
Electrochemistry wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
History of chemistry wikipedia , lookup
Process chemistry wikipedia , lookup
Vapor–liquid equilibrium wikipedia , lookup
Thermometric titration wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Electrolysis of water wikipedia , lookup
Metalloprotein wikipedia , lookup
Click chemistry wikipedia , lookup
History of molecular theory wikipedia , lookup
Chemical reaction wikipedia , lookup
Rate equation wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Transition state theory wikipedia , lookup
Atomic theory wikipedia , lookup
VX (nerve agent) wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Chapter 3 Stoichiometry STOICHIOMETRY: The chemical arithmetic used to relate the amount of products and reactants to each other 1st Write Chemical Equation 2nd Balance Equation 3rd Interpret Equation 1st Write Chemical Equation REACTANTS PRODUCTS A2 + B2 A2B nd 2 Balance Equation A balanced chemical equation has the same type and number of ______ in the reactants as in the products. A2 + B2 =A= =B= A2 B 3rd Interpret Equation 2 A2 + B2 2 A2B Two units of A2 _ React with One unit of B2 __ Forming Two units of A2B__ Magnesium reacts with “air” forming Magnesium Oxide 1st Write Chemical Equation Mg(s) + O2(g) → MgO(s) 2nd Balance Equation 2 Mg + O2 → 2 MgO 2 = Mg = 2 2 = O = 2 3rd Interpret Equation 2 Mg + O2 → 2 MgO Some Balanced Chemical Reactions Combination & Decomposition reactions In a combination reaction two or more substances form a single compound. 1 2(g) + __H 3 2(g) → __NH 2 __N 3(g) In a decomposition reaction, a single compound forms two or more new substances. 2 2 3 2(g) __KClO + __O 3(s) → __KCl(s) CHEMICAL REACTIONS (YOU SHOULD KNOW) 1. COMBUSTION (Of a Hydrocarbon) 2. NEUTRALIZATION (Acid + Base) 3. ACID + ACTIVE METAL 4. FORMATION Reaction CHEMICAL REACTIONS (YOU SHOULD KNOW) 1. COMBUSTION (Of a Hydrocarbon) The combustion of a Hydrocarbon produces CO2 and H2O : CH4 + O2 → CO2 + H2O Combustion of Butane C4H10 + 6 ½ O2 → 4 CO2 + 5 H2O Is This Reaction Balanced ? With Fractions ? CHEMICAL REACTIONS (YOU SHOULD KNOW) 2. NEUTRALIZATION (ACID+ BASE) HCl (aq) + NaOH → H2O + A SALT In this case the salt is NaCl Sodium Chloride CHEMICAL REACTIONS (YOU SHOULD KNOW) 3. ACID + ACTIVE METAL HCl (aq) + Zn (s) → H2 (g) + A SALT In this case the salt is CHEMICAL REACTIONS (YOU SHOULD KNOW) 4. FORMATION Reaction Zn (s) + Cl2 (g) → ZnCl2 (s) Reactants are in their “natural” state Which of the following are Balanced ? C4H10 + 6 ½ O2 → 4 CO2 + 5 H2O 4 C4H10 + 26 O2 → 16 CO2 + 20 H2O Uniquely balanced equation C4H10 + 6 ½ O2 → 4 CO2 + 5 H2O 4 C4H10 + 26 O2 → 16 CO2 + 20 H2O The ONLY Uniquely balanced equation is: 2 C4H10 + 13 O2 → 8 CO2 + 10 H2O (MOLECULAR) WEIGHTS The FORMULA WEIGHT of a substance is the sum of the atomic weights of each atom in its chemical formula. Calculate the Formula Weight of Hydrogen Sulfide Nickel II Carbonate Magnesium Acetate Ammonium Sulfate Potassium Phosphate Iron III Oxide Diphosphorus pentasulfide 1st you must know the formula Calculate the Formula Weight of H2S NiCO3 Mg(C2H3O2)2 (NH4)2SO4 K3PO4 Fe2O3 P2S5 Avogadro’s Number & The Mole Avogadro’s number is chosen so that 1 mole of 12C atoms has a mass of exactly 12 grams. 1 mole 12C atoms = 6.02x1023 6.02x1023 12C atoms = 12 g 12C atoms Mascots Lamar University University of Texas LSU Rice Oregon Nederland CARDINALS LONGHORNS TIGERS OWLS DUCKS BULLDOGS Biology Department Mascot Chemistry Department Mascot Rabbits (& other animals) If you had a mole of rabbits, how many rabbits would you have ? How many rabbit ears would you have? How many rabbit feet? Interpreting Chemical Formulas If you had a mole of water , how many molecules of water would you have ? How many Hydrogen atoms would you have? How many Oxygen atoms? • The mole is just a conversion factor that allows us to accurately work with atoms and molecules. • One mole of any substance contains 6.02 x 1023 units of that substance. Grams, Moles & Avogadro Conversion Factors Use Molecular Weight To Convert To Convert Grams to Moles or Moles to Grams Grams to Moles 3.2 Grams of Oxygen = ? Moles 1 Mole ? Grams x = ? Moles ? Grams 1 Mole 3.2 Grams x = 0.10 Moles 32 Grams Moles to Grams 1.5 Moles of Methane = ? Grams ? Grams ? Moles x = ? Grams 1 Mole 16 Grams 1.5 Moles x = 24 Grams 1 Mole Use AVOGARDRO’S Number • For NUMBER of Atoms • Or NUMBER of Molecules • 32 Grams of Oxygen = ? Molecules • 32 Grams of Oxygen = ? Atoms • 1 Molecule of Oxygen = ? Grams Interpretation of Chemical Reactions Using Stoichiometry Example 1 Nitrogen monoxide reacts with oxygen to produce Nitrogen dioxide 1st Write the reaction 2nd balanced reaction 3rd Interpret Reaction How many moles of oxygen gas are required to react completely with 2.0 mole NO? 2 NO (g) + O2 (g) 2 NO2 (g) (a) 0.5 mol O2 (b) 1.0 mol O2 (c) 1.5 mol O2 (d) 2.0 mol O2 How many moles of oxygen gas are required to react completely with 1.0 mole NO? 2 NO (g) + O2 (g) (a) 0.5 mol O2 (b) 1.0 mol O2 (c) 1.5 mol O2 (d) 2.0 mol O2 (e) 2.5 mol O2 2 NO2 (g) How many moles of oxygen gas are required to react completely with 2.50 moles NO? 2 NO (g) + O2 (g) (a) 0.5 mol O2 (b) 1.00 mol O2 (c) 1.25 mol O2 (d) 1.50 mol O2 (e) 2.00mol O2 2 NO2 (g) How many moles of oxygen gas are required to react completely with 10 moles NO? 2 NO (g) + O2 (g) (a) 5 mol O2 (b) 10 mol O2 (c) 15 mol O2 (d) 20 mol O2 (e) 25 mol O2 2 NO2 (g) If 6.0 moles of NO are reacted with 3.0 mole O2, how many moles NO2 are produced? 2 NO (g) (a) (b) (c) (d) (e) + 2.0 mol NO2 6.0 mol NO2 10.0 mol NO2 16.0 mol NO2 32.0 mol NO2 O2 (g) 2 NO2 (g) If 10.0 moles of NO are reacted with 5.0 mole O2, how many moles NO2 are produced? 2 NO (g) (a) (b) (c) (d) (e) + 2.0 mol NO2 6.0 mol NO2 10.0 mol NO2 16.0 mol NO2 32.0 mol NO2 O2 (g) 2 NO2 (g) If 10.0 moles of NO are reacted with 6.0 moles O2, how many moles NO2 are produced? 2 NO (g) (a) (b) (c) (d) (e) + 2.0 mol NO2 6.0 mol NO2 10.0 mol NO2 16.0 mol NO2 32.0 mol NO2 O2 (g) 2 NO2 (g) • Chemical Reactions do not always go the way we expect them to • Using stoichiometry we can calculate the theoretical (Maximum) amount of product formed in a reaction. . If the actual amount of product formed in a reaction is less than the theoretical amount we can calculate a percentage yield. Actual product yield % yield = × 100% Theoretica l product yield The Limiting Reactant A reaction stops when one reactant is totally consumed. This is the limiting reactant. The other reactants are excess reactants. The Limiting Reactant • How many bikes can be made from 10 frames and 16 wheels ? 1 frame + 2 wheels → 1 bike What is the limiting “reagent” ? Excess “reagent” ? If 10.0 moles of NO are reacted with 6.0 moles O2, how many moles NO2 are produced? 2 NO (g) + O2 (g) (a)What LIMITS the reaction? (b)What is in excess ? 2 NO2 (g) If 10.0 moles of NO are reacted with 6.0 moles O2, how many moles of the excess reagent remain? 2 NO (g) • • • • + 1.0 mol O2 5.0 mol O2 4.0 mol NO 8.0 mol NO O2 (g) 2 NO2 (g) Example 2: Hydrogen reacts with Nitrogen to form Ammonia 1st Write Reaction Next Balance Equation Interpret Balanced Equation Interpretation of a Chemical Reaction 3 H2 + Three moles of Hydrogen 1 N2 → One mole of Nitrogen 2 NH3 Two moles of Ammonia ==================================================== Mole Ratio MUST Always be 3 : 1 : 2 3 H2 + 3/3 H2 + 3/2 H2 + 1 N2 → 1/3 N2 → 1/2 N2 → 2 NH3 2/3 NH3 1 NH3 3/4 H2 + 1/4 N2 → 2/4 NH3 3/8 H2 + 1/8 N2 → 2/8 NH3 All are 3 :1 : 2 How many moles of Nitrogen are needed to react with 3 moles of Hydrogen ? 3 H2 + 1 N2 → 2 NH3 (a) ½ (b) 1 (c)1 ½ (d) 2 (e) 2 ½ (f) 3 How many moles of Nitrogen are needed to react with 1 ½ moles of Hydrogen ? 3 H2 + 1 N2 → 2 NH3 (a) ½ (b) 1 (c)1 ½ (d) 2 (e) 2 ½ (f) 3 Chemical Reactions are Interpreted on the Mole basis but chemicals are weighed in the laboratory in Grams Use Molecular Weight To Convert To Convert Grams to Moles or Moles to Grams Units, Units, Units ___Grams 1 moles x ? Grams = ___moles Units, Units, Units ___moles ? Grams x 1 moles = ___Grams How many moles of Nitrogen are needed to react with Three grams of Hydrogen ? 3 g x 1 mole = 1 ½ moles of H2 2 g 3 H2 + 1 N2 → 2 NH3 (a) ½ (b) 1 (c)1 ½ (d) 2 (e) 2 ½ (f) 3 3 H2 + 1 N2 → 2 NH3 How many moles of Ammonia ( NH3 ) are produced from (a) 3 grams of H2 and ½ mole of N2? 1 mole = (1 mole)x(17 g/mole) grams of NH3 (b) 3 grams of H2 and 28 grams of N2? 1 mole NH3 with 14 g of Nitrogen in excess 3 H2 + 1 N2 → 2 NH3 With a 50 % Yield, How many moles of NH3 are produced from (a) 3 grams of H2 and ½ mole of N2? ½ mole = (½ mole)x(17 g/mole) grams of NH3 (b) 3 grams of H2 and 28 grams of N2? ½ mole NH3 with 14 g of Nitrogen in excess DESCRIBING COMPOUNDS Percent Composition Empirical Formulas Molecular Formulas Two Approaches to Chemical Formulas 1. Given Formula Determine Percent Composition of Each Element in Compound 2. Given Percent Composition of Each Element in Compound Determine Formula A student obtains the following data Mass of crucible and cover = 28.288 gms Mass crucible, cover & K = 28.709 gms Mass crucible, cover & = 28.793 gms potassium oxide Determine the EMPIRICAL formula First determine % by Weight Weight K = 28.709 – 28.288 = 0.421 Weight O = 28.793 – 28.709 = 0.084 Total = 0.505 0.421 % by Wt K = x 100 = 83.36633 0.505 83.4 % by wt K and 16.6 % by wt O Choose any total weight 100 grams is convenient Construct the following work sheet Element % Wt moles ratio K 83.4 83.4 g 83.4 g / 39 = 2.14 O 16.6 16.6 g 16.6 g / 16 = 1.04 = 1 Therefore formula is = 2