* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 1st Semester Final Exam Study Guide - Mr. Dudley
Survey
Document related concepts
Density of states wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Relativistic mechanics wikipedia , lookup
Kinetic energy wikipedia , lookup
Work (thermodynamics) wikipedia , lookup
Transcript
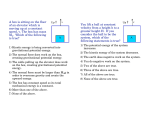
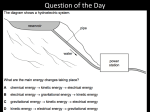
1st Semester Final Study Guide Units 1-3 Name_______________________________ Date Class 1. What is science? 2. What is an example of a small and large thing a model can represent? 3. What are some of the branches of science mentioned in Lesson 1? 4. What are the limitations of a physical model? 5. What are the different types of graphs we learned about? How is each of them useful? 6. What is the quickest way to change a liquid to a gas? 7. Why do we use scientific methods? 8. What is a controlled experiment? 9. What is a hypothesis? 10. What is the formula for finding the volume of a rectangular box like a fish tank? 11. Name two things that are not matter. 12. What are mass and weight? How are they different? 13. How would you find the volume of an irregularly shaped solid? What is the process called? 14. How would you find the density of an object? What is the formula? (Be familiar with pp. 80-81) 15. How do particles move in solids, liquids, and gases? 16. Solids have a Liquids have a definite Gases can change in volume and shape. but can and 17. 1 ml = 1 18. The opposite of freezing is The opposite of sublimation is The opposite of evaporation is 19. What happens to mass when state changes? 20. What is the density of water? 21. What is energy? shape. . . . . 22. is the energy of motion. 23. is the stored energy that an object has due to its position, condition, or chemical composition. 24. What is mechanical energy? 25. What is the law of conservation of energy? 26. What are some forms of energy? (Name at least 8, try to name 10) - - 27. Consider the way a hand-held hair dryer, similar to the illustration, dries your hair. Identify the type of energy that provides the energy source for the hair dryer. ____________________________________________________________________________________ The dryer’s source of energy is transformed into different forms of energy. Identify two of these. 1) __________________________________________________________________________________ 2) __________________________________________________________________________________ 28. When a hand-held fan is turned on, the blades spin. Which diagram shows the energy conversion that is required to make the fan work? A. B. C. D. 29. How does kinetic energy differ from potential energy? A. Kinetic energy is energy of motion, but potential energy is stored energy. B. Kinetic energy can be measured, but potential energy cannot be measured. C. Kinetic energy is in every object, but some objects do not have potential energy. D. Kinetic energy is a form of mechanical energy, but potential energy is not mechanical energy. 30. This illustration shows a bookshelf with four identical books. How does the gravitational potential energy of the four books compare? A. The books have the same gravitational potential energy. B. The gravitational potential energy increases from top to bottom. C. The gravitational potential energy decreases from top to bottom. D. The books are not moving so they do not have gravitational potential energy. 31. When a ball is thrown into the air, it follows a curved path. At which point does the ball have the greatest amount of gravitational potential energy? A. point W B. point X C. point Y D. point Z 32. Many people keep a hand-crank radio to use in case of an emergency. The radio runs on a circuit that is powered when the crank is turned. What kind of energy transformation is needed to power the radio? A. thermal to mechanical B. mechanical to electrical C. chemical to potential D. magnetic to mechanical 33. Which of these is an example of the conversion of gravitational potential energy into kinetic energy? A. a person running on a level track B. an apple falling from a tree to the ground C. a windup spring making a toy car move across the floor D. a rubber band being stretched to twice its normal length 34. A balloon can fly across the room if given enough potential energy. What action would provide that potential energy? A. dropping the balloon B. tying the balloon in a knot C. cutting the balloon into pieces D. filling the balloon with air and then releasing it 35. A ball is sitting at the top of a ramp. As the ball rolls down the ramp, the potential energy of the ball decreases. What happens to the potential energy as the ball moves? A. It is lost as gravitational energy. B. It is converted to kinetic energy. C. It is destroyed as the ball moves. D. It is used to make the ball slow down. 36. Gordon throws a baseball into the air. It rises, stops when it reaches its greatest height, and then falls back to the ground. At what point does kinetic energy convert to potential energy? A. when the baseball is rising B. when the baseball is falling C. while the baseball sits on the ground D. while the baseball is stopped in the air 37. What are some factors that scientists cannot control in a field investigation? 38. The illustration below shows an archer using a bow and arrow. What does an archer do to store potential energy in an arrow? A. raise the bow into shooting position B. fit an arrow into the notch C. pull the bowstring back D. release the bowstring to let the arrow fly 39. What was the name of the big, mega-continent that formed 225 million years ago? 40. What kind of model would you use to represent the human heart? 41. Name 3 characteristics of scientists that are important to their work, but are also found in nonscientists. *KNOW THE DEFINITIONS TO THE FOLLOWING VOCABULARY TERMS* Hypothesis Evaporation Variable Boiling Theory Condensation Science Sublimation Observation Deposition Empirical evidence Energy Model Kinetic energy Law Potential energy Experiment Mechanical energy Data Law of conservation of energy Matter Mass Weight Volume Density Solid Liquid Gas Freezing Melting Elastic potential energy Chemical potential energy Heat Light Sound Electrical energy There are 10 Lessons altogether in the first three units. The final exam will have 5 questions from each lesson.