* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download doc - Vanderbilt University
Center for Radiological Research wikipedia , lookup
Proton therapy wikipedia , lookup
Industrial radiography wikipedia , lookup
Medical imaging wikipedia , lookup
History of radiation therapy wikipedia , lookup
Radiosurgery wikipedia , lookup
Image-guided radiation therapy wikipedia , lookup
Backscatter X-ray wikipedia , lookup
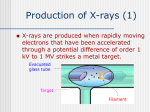
Creating a new kind of X-ray machine By David F. Salisbury Oct. 9, 2001 According to the old saying, necessity is the mother of invention. But in the case of the monochromatic X-ray project, which holds the promise for significantly improving both the quality and the safety of medical X-rays, it was not necessity but frustration that provided the impetus. For many years, Frank E. Carroll, chest radiologist and breast cancer researcher at the Vanderbilt University Medical Center Department of Radiology, has been frustrated with the poor quality of mammograms. “I’ve been reading mammograms for decades and, when I do, I still feel like I’m standing in quicksand! We find a lot of things, but miss a lot of them, too,” he says. So in 1987 Carroll and several colleagues1 studied the problem systematically and identified the quality of the X-ray beam as the major factor standing in the way of improving mammography. The standard X-ray tube used in hospitals produces a beam that contains a broad spectrum of frequencies2. It generates “soft” X-rays that barely penetrate the skin at the low end of the spectrum. At the high end, it produces “hard” X-rays that ricochet off bone and tissue, creating a fog that obscures subtle features in X-ray images. “Existing X-ray machines do not do a very good job of mammography,” Carroll says flatly. “The radiation dose is high. The accuracy is very poor. The beam that we use is simply not well suited to what we are doing.” When a group of Vanderbilt scientists banded together to submit a proposal to the Department of Defense to create a free-electron laser center on campus, Carroll joined in because he thought there might be a way to use the unusual laser to produce better X-rays. When the Strategic Defense Initiative Organization approved funding for the Vanderbilt center, however, they did not support Carroll’s proposal because they thought it was too far out. Fortunately, the researcher was able to convince The Eastman Kodak Company that his ideas had merit. Kodak funded his efforts for the first three years. That allowed Carroll and his colleagues—Charles Brau, professor of physics; Marcus Mendenhall, a research associate professor; Robert Traeger, senior research assistant; Glenn Edwards, former FEL center director now at Duke University; and research physicist James W. Waters—to create a preliminary design for the monochromatic X-ray beam line that they wanted to add to the Vanderbilt FEL. They tried several different approaches and finally hit on one that looked as if it would work. The basic idea is very simple. Generate a beam of electrons and accelerate them to nearly the speed of light. At the same time, create a high-powered beam of infrared laser light. Direct the two beams so that they collide head-on. When you do that successfully, the infrared photons should 1 Ronald Price, professor of radiology and radiological sciences; David R. Pickens III, associate professor of radiology and radiological sciences; James W. Waters research associate professor of physics; Charles A. Brau, professor of physics; and former doctoral student WeiWei Dong. 2 The wavelengths of X-rays are traditionally expressed in terms of the amount of energy that they carry. The units of these measurements are thousands of electron volts or KeV. An electron volt is the amount of energy that it takes to push an electron across an electrical potential of one volt. This is a very small unit of energy: One KeV is less than a quadrillionth of the energy required to power a 100-watt light bulb for an hour. -1- Creating a new kind of X-ray machine bounce off the electrons and gain the energy required to transform them into X-rays in a process called the Inverse Compton Effect. Making it work was another matter altogether. What interested Carroll about such a system is that it should produce monochromatic X-rays—Xrays of a single wavelength. This is very similar to an X-ray laser. Unlike laser light, however, it is not coherent. That is, the light waves are not all aligned. Despite this lack, Carroll realized that a monochromatic beam would have many advantages for medical imaging compared to current “polychromatic” X-ray sources. A great deal was already known about the characteristics of monochromatic X-rays because they are a byproduct of the massive particle accelerators that have been developed by high energy physicists to study the basic structure of subatomic matter. Originally, this “synchrotron radiation” was considered a problem because it siphoned energy from the beam lines that were central to the physics experiments being run. Then scientists realized that these X-rays were themselves a valuable resource. They found a way to control their production and have set up special laboratories, called synchrotron laboratories, specifically to give scientists access to these X-rays. Nevertheless, Department of Defense officials continued to dismiss the idea until John Madey, the inventor of the free-electron laser, stood up in a meeting and stated publicly that this was a good idea and advised them to fund it. Government support allowed Carroll to modify the Vanderbilt FEL to test the idea. The changes were made in 1998 and proved the naysayers wrong. At the same time, the experiment showed that a free-electron laser was not the ideal instrument for the purpose. For one thing, the FEL produces so much radiation that it must be operated in a heavily shielded room. Also, the strength of the X-ray beam that it generated was so low that extremely long exposure times were required to produce X-ray images. “So we thought about it and decided to get rid off all the bad things about the FEL beam and keep all of the good things: to make a new kind of machine that we had never seen before,” Carroll says. The key to the new design was a conventional type of infrared laser, a machine that can produce a beam with about 1,000 times the power of the FEL called a tabletop terawatt laser developed by Positive Light [http://www.poslight.com/index.html] in Los Gatos, CA. The researchers combined this powerful laser with a similarly sized linear accelerator for producing relativistic electrons. They estimate that this instrument could be built small enough to fit in a standard-sized X-ray room and should cost about $1 million dollars apiece when mass produced. That compares to the billion dollar price tag for building new synchrotron radiation centers. Carroll and his colleagues went to officials at the Office of Naval Research (ONR), which had taken over management of the free-electron laser program, and asked for an additional $10 million in order to begin producing these monochromatic X-ray machines commercially. ONR agreed to back the project if the university was willing to invest the matching funds required to set up a commercial company for this purpose. Vanderbilt had recently set up the Office of Enterprise Development to help support the commercialization of technology based on university research. So the university agreed to use this fund to meet ONR’s conditions and set up a new company—MXISystems, Inc. [http://www.mxisystems.com/#top]—to develop these new machines. The university received the grant in July 1999 and the company began building a prototype in one of the laboratories at the FEL center. On April 10, 2001 they turned on the new machine and made X-rays for the first time. They are currently in the process of improving the machine’s performance and making it easier to produce full X-ray images. “So now we have a beam no one has ever had before. It is basically one frequency, although it is not truly monochromatic. And it is tunable. Now we have to convince people that we can use this to make better images,” says Carroll. Their initial effort is to use the new beam to produce conventional X-ray images. Because the beam can be tuned to produce X-rays ranging from 15 to 50 KeV, operators can pick the frequency that does the best job of imaging the part of the body of interest. MXISystems and -2- Creating a new kind of X-ray machine Vanderbilt scientists believe that they can produce images with greater detail than conventional X-ray machines using half the dosage. Also, the device produces X-rays in extremely short pulses3. As a result, it can take sharp pictures of subjects even if they are moving rapidly. 4 In this mode, the monochromatic X-ray machine should also go a substantial way toward meeting Carroll’s initial objective. Tumors should stand out much more clearly. He estimates that tumor tissue should be 11 percent lighter than normal tissue using monochromatic X-rays compared to the half a percent difference with conventional X-ray beams. But this is only the beginning. There are several other ways to use monochromatic X-rays that have even greater medical potential. One such approach is called “time-of-flight imaging.” In normal imaging, all the photons that make it through the body contribute to the final image. This includes photons that have bounced around in the body before emerging and so blur the image. With the extremely short pulses generated by the new X-ray source, the scientists can install electronic detectors that only measure the photons that pass through the body directly. This has the potential for reducing dosages by another factor of ten. Photons in conventional absorption X-ray imaging actually deliver very little information. Photons that pass through the body strike the X-ray plate, while those that are absorbed don’t. Physicists know that photons contain hundreds to thousands of times more information than that. As the Xray photons travel through tissue they undergo subtle changes in phase. With a monochromatic light source, scientists can tap into this information through a process called phase contrast imaging. Research done with synchrotron radiation has shown that this approach can provide valuable information about changes in tissue density, and edges between different organs and body parts show up clearly. For example, phase contrast images can show individual muscles, which are completely invisible to conventional X-rays. The monochromatic X-ray machine will also foster another technique, called K-edge imaging, that Carroll predicts will become a “whole new field of radiology.” A great deal of anatomical imaging, like angiography, 5 is done by injecting special compounds into the body that show up clearly with X-rays. One commonly used contrast agent is iodine. But it that has a greater degree of toxicity than radiologists would like, over and above the basic damage done by the X-ray beam itself.K-edge imaging provides a way to reduce both contrast agent toxicity and beam damage. Tuning the energy of the beam to a value equal to the binding energy of the electrons in the inner (K) shell of the contrast agent creates absorption effects that can greatly increase the effectiveness of such compounds. That means that they can be used at much lower dosages. Being able to tune the X-ray beam also means that researchers can choose among many more compounds. At higher energy levels, the body becomes increasingly transparent to X-rays. That means a higher percentage of the X-ray photons pass through the body without doing any damage. So, by identifying contrast agents of lower toxicity that work at higher energy levels, the adverse sideeffects of this type of radiology can be substantially reduced, Carroll says. At the same time, it should be possible to use K-edge imaging to view a new level of detail within the body. For example, an experiment performed by two of Carroll’s graduate students 6 has shown that this approach can see microcirculation channels in blood vessels that are invisible in conventional angiography while reducing both the radiation dose and the concentration of the contrast materials given to the patient. 3 Two to 10 picoseconds. A picosecond is a trillionth of a second. It is such a brief period that light only travels one hundredth of an inch in a picosecond. 4 In fact, one possible non-medical application that the researchers have identified is X-raying turbine blades in jet engines while they are running! 5 Examination of blood vessels using X-rays. 6 For an abstract go to http://www.mc.vanderbilt.edu/medschool/html/Mellon_Baughman.htm -3- Creating a new kind of X-ray machine In addition to its radiological applications, the monochromatic X-ray may have an important role in the basic research efforts made possible by the mapping of the human genome. Many of the scientists involved say that the next step is “proteomics” – that is mapping and determining the functions of the millions of proteins that act as the basic molecular machinery of living organisms. One of the key techniques for determining the structure of complex molecules like proteins is Xray crystallography. These characterization efforts are currently done primarily at the big synchrotron laboratories because monochromatic X-ray beams are far superior to polychromatic beams for this purpose. Demand for time on these beam lines is far greater than the time available, however. So researchers must prepare lengthy proposals and wait long periods of time before they can analyze their samples. “Just imagine! With this technology we will be able to put 100,000 monochromatic X-ray sources in 100,000 universities around the world for the cost of building just one centralized synchrotron lab,” says FEL center director, David Piston. - VU - -4-